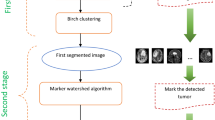
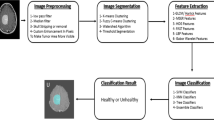
Abstract
Purpose
This study addresses the critical health issue of brain tumors, focusing on enhancing the accuracy of tumor segmentation from Magnetic Resonance Imaging (MRI) images. The primary research question investigates the effectiveness of a novel Hybrid Watershed–Clustering framework and its underlying Progressive Segmentation of the MR Images using the Radius and Intensity Measure (PS-RIM) algorithm. The aim is to improve the detection and segmentation of brain tumors within MR images, surpassing the efficacy of current methodologies.
Methods
The methodology involves a three-stage process. In the preprocessing stage, noise reduction and intensity normalization techniques are applied to clarify the images. The next stage is region-based segmentation, which includes morphological processing, edge detection, and thresholding to delineate tumor areas accurately. The final post-processing stage enhances segmentation accuracy and reduces false positives by integrating clustering machine learning techniques, specifically the K-Means cluster algorithm, to refine tumor identification.
Results
The framework's comprehensive evaluation across various MR images shows a significant improvement in accuracy over existing segmentation methods. The PS-RIM algorithm within the framework effectively captures the diverse presentations of tumor appearances in MR images. The research recorded an impressive accuracy rate of 98.11% in tumor detection, demonstrating enhanced identification and segmentation quality.
Conclusions
The study concludes that the proposed Hybrid Watershed–Clustering framework, powered by the PS-RIM algorithm, markedly improves the detection and differentiation of brain tumors in MR images. It exhibits exceptional accuracy, resilience, and computational efficiency. These findings hold substantial potential for advancing computer vision and image analysis in medical diagnostics, which could improve patient outcomes in managing brain tumors.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The area of healthcare has been significantly transformed by the development and use of advanced medical imaging tools, which have facilitated precise illness diagnosis and prompt treatment. Within this particular setting, the timely and accurate detection of brain tumors is essential in enhancing patient outcomes and increasing survival rates. The research paper “A Framework for the Identification of Brain Tumors from Magnetic Resonance Images Using Progressive Segmentation” introduces an innovative methodology to address this significant issue. This framework seeks to improve the precision of brain tumor identification and delineation from magnetic resonance (MR) images by utilizing progressive segmentation techniques. This study explores the complexities of the suggested approach, emphasizing its potential to substantially influence the medical community’s capacity to detect and effectively manage brain tumors accurately.
Kapoor et al. [1] have rightly justified these claims and advocated for building such algorithms to automate the complete medical diagnosis processes. The segmentation process is the primary task in identifying the brain tumors from the MR images. Various parallel research attempts propose several segmentation processes, and conclusively these methods can produce an acceptable accuracy. The work by Nerurkar [2] has summarized the outcomes of these parallel proposals for image segmentation. Nonetheless, these methods are highly criticized for not considering the MR image types during the segmentation process. The types of MR images have a more profound impact on the selection of the segmentation strategies, as suggested by Bhima et al. [3].
The present diagnosis procedure for brain tumors mainly depends on the manual interpretation of magnetic resonance (MR) images, resulting in subjective outcomes, time-consuming analysis, and the possibility of human error. The objective of this framework is to address these issues through the utilization of sophisticated image-processing techniques and progressive segmentation methodologies. This approach intends to automate the process of identifying brain tumors, hence improving the accuracy and efficiency of detection while reducing the need for manual involvement. This study aims to improve the accuracy of brain tumor identification and increase clinical workflow by integrating advanced segmentation algorithms with medical imaging techniques. The possible benefits of this research include improved patient outcomes and enhanced treatment methods.
The existing diagnosis procedure for brain tumors mainly depends on the manual interpretation of magnetic resonance (MR) images, which can result in subjective outcomes, time-consuming analysis, and the possibility of human mistakes. This framework aims to address these issues by utilizing sophisticated image-processing techniques and progressive segmentation methodologies to automate the process of identifying brain tumors. This automation enhances the accuracy and efficiency of tumor detection while reducing the requirement for considerable manual involvement. This project aims to improve diagnostic accuracy and clinical workflow for brain tumor identification by integrating advanced segmentation algorithms with medical imaging. The possible benefits of this approach include improved patient outcomes and treatment methods (as shown in Fig. 1). Here, a similar image demonstrates different regions for detection in other ways.
Henceforth, this work firstly aims to realize the characteristics of the MR images in Section 2, further based on this knowledge, seeks to identify the foundational method for segmentation in Section 3. Also, motivated by the foundation methods, several parallel research attempts can be seen in the recent past, which are analyzed in Section 4. The persisting problems in similar research outcomes are further explored in Section 5, and based on the issues identified, the proposed mathematical model and the algorithms are furnished in Sections 6 and 7. The obtained results are discussed and compared in Sections 8 and 9, respectively. Finally, this literature presents the research findings and conclusions in Section 10.
2 Similar works
Improvements over the existing foundational method have been observed in the recent past to reduce the time complexity and improve detection accuracy. The clustering or categorization of the tumor cells in the brain MR images is critical to obtain higher accuracy for complete detection. Hence, many parallel research outcomes can be observed to improve the clustering outcomes. Rao et al. [4] demonstrated the use of traditional clustering methods for MR images and compared the results for various clustering types. Fundamentally, the K – Means clustering method is ranked higher in such situations, and the study also proves the benefitting points for the same. This study was again supported in another work by Malathi and Kamal AR [5] with similar conclusions. Nonetheless, the categorization process for tumor detection is only the challenge faced in detecting the severity of the tumor; multiple morphological operations are also expected to be performed to achieve higher accuracy in terms of severity detection. The work by Nandi [6] has rightly justified this claim.
Visual detection is also one of the popular methods for detecting brain tumors. The complete process is automated to identify the regions based on the available data, and further visual confirmation is applied to determine the tumor regions, as showcased in the work by Hassan and Aboshgifa [7].
Nonetheless, the widely adapted method for MR image segmentation is the watershed method. This method is a decade-old method. However, the adaptation is also a decade old, as recently reported by Thakur et al. [8]. Few parallel research attempts have deployed the Watershed method using the thresholds or sometimes the morphological values suggested by Selkar and Thakare [9]. The watershed method is proven to identify the inner cell deviations and not only separates the tumor regions from the background but also separates any overlaps in the tumor regions, as demonstrated in the work by Malpica et al. [10].
Nevertheless, as elaborated in the previous section of this work, the foundational watershed method is highly time complex, and continuous efforts are made to improve this outcome. Many of the researchers have also aimed to produce a hybrid strategy for building the most effective and least time-complex process for segmentation, as can be seen in the work by Sinha and Sinha [11], Laddha and Ladhake [12] and also in the work by Mustaqeem et al. [13].
On the other hand, a few of the researchers have also suggested applying various other methods specifically for selective cases using fuzzy methods, as reported by Gopal and Karnan [14] or in work by Moon et al. [15].
Also, only some of the older methods for detecting brain cell tumors are driven by signal processing techniques, as the signal peaks are one of the most appreciated methods for detecting anomalies in the image. Nonetheless, the detected regions cannot be confirmed to be tumors or inflations, as observed in the work by Demirhan and Guler [16]. However, the hybrid methods can be seen utilizing such conventional methods of image signal processing with higher-order sophisticated computational algorithms to overcome multiple other challenges and to improve the detection accuracies as seen in the work by Cabria and Gondra [17].
The visual methods have also seen multiple improvements over the year, as one of the recent outcomes produced by Huang et al. [18] showcased the use of projection-based methods to identify the tumor regions on the MR images.
Nevertheless, few of the researchers have critically analyzed the possibilities of progressive segmentation of the MR images and found that such methods are not only beneficial for reduced time complexity, instead also can be applied to improve the accuracy to a greater extent, as seen in the work reported by Gupta et al. [19]. This literature also aims to use similar measures for segmentation.
In contrast, the work by Nabizadeh and Kubat [20] and various others have demonstrated interest in building a segmentation process by foundational image processing methods. The benefits of such techniques are obtaining phase-wise outcomes and building a better framework with such components, as showcased in the work by Zhang et al. [21]. A similar method to process further can be seen in the work by Aljabar et al. [22].
Machine learning is also addressed in the case of MR image segmentation as the outcome of RM. Chen et al. [23], Alagarsamy et al. [24], Alagarsamy et al. [25], and Lahmiri [26] can be seen. Nonetheless, these methods can only be adapted with higher modifications applied to various types of MR images. Hence, the adaptation could be more significant.
Specifically for the watershed method, the foundational approach suggests the flooding technique from the boundaries of the image and further reaches the center of the image. The boundaries eventually get submerged during the flooding process, and the areas with anomalies, such as tumors, are marked over the water level. This method is highly effective in terms of accuracy, as suggested by Meyer [27]. This method’s fundamental benefit is marking the regions using the marker method, which separates the image’s foreground from the background, as shown in the work by Hasegawa and Uto [28]. Nonetheless, the watershed method’s higher accuracy has always been criticized for higher time complexity. Hence, multiple research attempts can be seen to optimize the watershed method to reduce time complexity. One of the notable works is the work reported by Lin et al. [29]. On the other hand, applying the principle of parallelism, many research attempts have tried to deploy parallel watershed methods for faster segmentation of the images, as suggested by Devisivasankari and Vijayakumar [30]. For medical image processing, the applicability and benefits are very high, as showcased in the work by Yuan et al. [31] and must be adapted for MR image processing.
Continuing, the authors of the paper [32] introduced a Parallel Deep Convolutional Neural Network (PDCNN) framework for MRI brain tumor detection and classification, focusing on enhancing accuracy through the integration of global and local features. They tackle the challenge of overfitting with dropout regularization and batch normalization, utilizing three distinct MRI datasets to validate the effectiveness of their method. The PDCNN achieved notable accuracy across these datasets, demonstrating its superiority over traditional CNN models by efficiently extracting both low-level and high-level features, thus improving classification results compared to state-of-the-art techniques.
The authors of the paper [33], propose an innovative ensemble model for diagnosing brain tumors through MRIs. This model integrates data mining and machine learning techniques, specifically utilizing the Social Spider Optimization (SSO) algorithm for image segmentation and the Singular Value Decomposition (SVD) technique for feature extraction. The classification of features is then accomplished using an ensemble of Naive Bayes, Support Vector Machine (SVM), and K-Nearest Neighbor (KNN) algorithms. The proposed method significantly outperforms existing techniques, achieving an average accuracy of 98.61% on the BRATS 2014 dataset and 99.13% on the BTD20 database, demonstrating its effectiveness and efficiency in brain tumor diagnosis.
The authors in [34] present a study on the identification and prediction of brain tumors using the VGG-16 model, enhanced with Explainable Artificial Intelligence (XAI) through Layer-wise Relevance Propagation (LRP). This method aims to address the “black box” nature of deep learning models by providing insights into the decision-making process. They report a testing accuracy of 97.33% for classifying brain MRI images into normal and tumor categories, demonstrating the efficacy of combining deep learning with explainability techniques in medical diagnostics.
In the paper [35], the authors explore an automated method for detecting and classifying brain tumors using MRI. Their approach utilizes Adaptive Regularized Kernel-based Fuzzy C-Means Clustering (ARKFCM) for segmentation, followed by a combination of Support Vector Machine (SVM) and Artificial Neural Network (ANN) for classification. This method, validated on a dataset of 94 images, demonstrated promising results with an accuracy of 91.4%, sensitivity of 98%, specificity of 78%, and a Bit Error Rate (BER) of 0.12. The paper also compares this method with other conventional approaches, highlighting its effectiveness in brain tumor diagnosis.
The authors of [36] present a comprehensive study on MRI-based brain tumor detection utilizing convolutional deep learning methods and various machine learning techniques. They propose two deep learning models—a 2D Convolutional Neural Network (CNN) and a Convolutional Auto-Encoder Network—and compare their performance against six machine learning approaches. Their methodology includes extensive data augmentation and preprocessing of a dataset containing 3264 MRI images to train and test the proposed models. The results indicate that the proposed 2D CNN model achieved a training accuracy of 96.47% and a validation accuracy of 93.45%. In comparison, the Convolutional Auto-Encoder Network reported a training accuracy of 95.63% and a validation accuracy of 90.93%. These findings suggest that their deep learning models, particularly the 2D CNN, can effectively classify MRI images into glioma, meningioma, pituitary gland tumors, and healthy brain categories with high accuracy.
2.1 Refined comparison of brain tumor detection approaches
The Traditional Clustering approach [4, 5] utilizes K-Means and other clustering algorithms for initial tumor detection, presenting a complexity of O(n2). The accuracy of this method varies, but it lays the groundwork for segmentation and detection techniques, establishing a foundational framework for further research and application in the field. The Watershed Method [8,9,10] employs flooding techniques to segment tumors from MR images, focusing on morphological distinctions. This method is noted for its high complexity and variable accuracy. Still, it is particularly effective at differentiating tumor regions, even with overlaps, marking a significant contribution to the segmentation process. Machine Learning approaches [23,24,25,26] incorporate SVM, ANN, and other machine-learning algorithms for tumor classification and detection. The complexity and accuracy of these methods vary. Nevertheless, they enable learning from image data to improve detection over time, showcasing the adaptability and growth potential of machine learning in medical imaging. Deep Learning (PDCNN) [32] applied Parallel Deep Convolutional Neural Networks to enhance feature extraction and classification, with a complexity of O(n) and noted high accuracy. This approach significantly improves classification by efficiently extracting both low and high-level features, demonstrating the advanced capabilities of deep learning technologies in analyzing medical images.
Hybrid Models [33] combine data mining and machine learning techniques, like the SSO algorithm for segmentation and SVD for feature extraction, also presenting a complexity of O(n) and achieving high accuracy. This methodology achieves high accuracy through an ensemble of classification algorithms, representing the innovative integration of multiple techniques to optimize detection performance. The Proposed Framework features progressive segmentation with preprocessing, region-based segmentation, and post-processing for detailed tumor identification. With a complexity of O(n) and high accuracy, this approach introduces a stepwise refinement process for accurate tumor segmentation. It highlights the evolution of tumor detection methods towards more precise, efficient, and adaptable solutions, contributing significantly to early diagnosis and treatment planning for patients with brain tumors.
Henceforth, in the next section of this literature, the existing bottlenecks from the foundational and recent outcomes are analyzed and discussed,
3 Problem formulation
After the detailed discussions on the foundational model and the recent improvements in image segmentation, two bottlenecks for modifications can be observed. These are (i) higher time complexity and (ii) overlapping region chaos.
3.1 Higher time complexity
The very fast bottleneck of the existing methods is the higher time complexity. From Eq. 8, the time complexity, T, for calculating the pixel matrix can be formulated as,
or,
Further from Eq. 11, the modified time complexity can be formulated as,
or,
or,
Thus, for the “k” number of total clusters, the final time complexity can be formulated as,
This higher time complexity must be reduced in order to provide quicker results for critical patients.
3.2 Overlapped regions chaos
Secondly, it is evident from Eq. 11 that the generated clusters are independent of each other and only calculated based on the differentiated pixel intensity compared with the mean pixel intensity.
Also, assuming that one specific region in the image has overlapped tumors, as follows:
In such cases, the existing methods can identify the region but cannot detect the tumors individually.
Thus, this problem must be solved in order to calculate the risk factors associated with the patients.
The proposed mathematical solutions are furnished in the next section based on the identified problems in this section of the current work.
4 MR image fundamentals
This section elaborates on the types of MR images. Note that the investigated approach uses these types.
4.1 MR images – T1 type
The Spin-Lattice Unwinding Time variation image shown in Fig. 2 is regarded as rot-stable for the recovery time after the turn charge. This is obtained from the BRATs dataset.
4.2 MR images – T2 type
The decay in the transverse turn to unwind time T2 is consistent with the recovery period for the turn polarization, as shown in Fig. 3. The charging vector also rots toward the balance. This is the sample from BRATs 2017.
4.3 MR images – T1C type
The T1C is a focused Spin-Latice unwinding imaging similar to the T1 image but with more appealing reverberation extents and delicate features, as shown in Fig. 4. The sample was obtained from BRATS 2017 data.
4.4 MR images – FLAIR type
The concept of FLAIR is a type of image that’s made by appealing to the beat succession. It can be produced by performing a Fourier transform on any appealing yield and applying the rot, as shown in Fig. 5. The result of this process is then compared to the outcomes of T1C, T2, and T1.
The importance of FLAIR cannot be overlooked in certain places. For instance, in the case of mind MR imaging, the high organ liquid can hinder the ability to visualize appealing unwinds like in the cerebrospinal fluid.
Further, in the next section of this work, the foundational method for MR image segmentation is analyzed.
5 Foundational method for segmentation
From the previous section of this work, it is natural to realize that the segmentation process for the MR images cannot be generic to achieve better outcomes. Henceforth, the traditional and foundation method for image segmentation for MR images are elaborated here.
Assuming that the image can be denoted as I[] and every pixel in the image can be defined as P. This can be formulated for the n X m image as,
Further, for each pixel, the intensity, IS[X], must be calculated as,
where R(), G(), and B() are the functions to extract the red, green, and blue values from any pixel information.
Further, the complete image intensity can be calculated as,
Once the pixel intensity is calculated, the grouping or clustering, C[], of the pixels based on the image intensity can be performed as,
From the clusters formed, the separation of the foregrounds, that is, the tumor regions, from the backgrounds, that are the normal brain cells, can be separated.
Naturally, this process is highly time-consuming and complex; improvements should be expected. Henceforth, multiple parallel research attempts are aimed at improving the foundational methods, which are discussed in the next section of this work.
6 Proposed solution – mathematical model
After the critical analysis of the parallel research attempts and the existing methods’ bottlenecks, the proposed solution is furnished and finalized in this section.
Assuming that the set of images, I[], is the collection of multiple MR images, each can be identified as IX. Thus, this collection for “r” number of images can be formulated as,
Further, the pixel intensity, PIX, for any given image IX, can be calculated as,
For the image, the collection of the pixel intensities can be formulated as,
Here, n denotes any given pixel position, and m defines the image’s width.
Further, identification of the pixel intensities for the near pixels must be analyzed for similarity or near similarity with a collection of pixel information with similar intensity, KPI[] as,
Assuming that a total “p” number of pixels share the same pixel intensities, which are also located nearby. Thus, further, the total collective radius \({\Re _X}\) must be calculated as,
where x is the unit of measure for each pixel on the MR image profiles.
Finally, the segmentation must be performed using the radius values available with a progressive approach as,
Also, the mean radius must be updated progressively at every pixel group as,
The formulation mentioned above clearly defines the scope for identification of the tumors as
-
If the pixel group radius is lesser than the mean radius of the total image, it is evident that the identified region is a tumor region.
-
Otherwise, if the pixel group regius is greater than the mean radius of the image pixel groups, then conclusively, those regions are the backgrounds of the images.
The benefit of this proposed method is firstly the reduced time complexity. Further, as the regions are grouped based on pixel intensity and radius calculations, the second challenge of overlapped regions is also removed.
Henceforth, based on the proposed mathematical model, the proposed algorithm is furnished in the next section of this work.
7 Proposed algorithms
After a detailed understanding of the proposed method using the mathematical modeling method, the proposed algorithm is furnished in this work section.
Algorithm: Progressive Segmentation of the MR Images using Radius and Intensity Measure (PS-RIM) Algorithm |
Input: |
Group of MR Images as I[] |
Output: |
Segments of Tumor Regions as R[] |
Process: |
Step - 1. Load the complete Image set as I[] |
Step - 2. For each image in I[] as I[q] |
a. Calculate the pixel intensity as PI[q] using Eqs. 13 and 14 |
b. Calculate the near similar pixels as KPI[q] using Eq. 15 |
c. Calculate the near similar pixels radius as R[q] using Eq. 16 |
d. For each pixel in the image |
i. Calculate the mean radius as MR using Eq. 18 |
e. If MR < R[q] |
f. Then, mark the region as background and store it in C[] |
g. Else, |
h. Mark the region as tumor region and store it in R[] |
Step - 3. Repeat the process for all I[] |
Step - 4. Return the tumor regions as R[][] |
There are two types of addressable elements used in digital imaging: pixels and all-points addressable displays. Pixels may be addressable elements in raster images or all-point display devices.
An individual pixel contains a small sample of the original picture; therefore, more pixels mean a better depiction of the original. It is possible to alter the intensity of each individual pixel. Red, green, and blue are color imaging systems’ most common component intensities. Still, they may also be represented by the CMYK color model, which uses four other component intensities.
Henceforth, after the detailed analysis of the proposed methods, the results obtained in the next section of this work are discussed and furnished.
8 Results and discussions
After the detailed analysis of the proposed method and the proposed algorithm, the results obtained in this section of the work are furnished.
Firstly, the dataset description is furnished here (Table 1).
The initial dataset conditions are visualized graphically here in Fig. 6.
The dataset known as the Multimodal Brain Tumor Segmentation Challenge (BRATS) is a highly regarded and extensive compilation of multimodal brain MRI images that have been carefully selected to assess and enhance brain tumor segmentation algorithms. The BRATS dataset comprehensively depicts the complex tissue properties found in brain tumors, utilizing a range of imaging modalities such as T1-weighted, T2-weighted, FLAIR, and post-contrast T1-weighted images. This dataset is a valuable resource for the advancement and evaluation of cutting-edge segmentation algorithms, since it includes annotations of crucial tumor locations, including the whole tumor, the enhanced tumor core, and the surrounding edema. The BRATS platform serves a crucial role in promoting innovation, cooperation, and advancement in the field of medical image analysis and brain tumor research by offering researchers and practitioners a standardized platform to test their segmentation algorithms.
Henceforth, it is natural to realize that the dataset is highly diversified and is equipped with a good number of tumor samples.
Secondly, the brain tumor size detection results are furnished (Table 2). The testing of the proposed algorithm was performed on the complete dataset. However, for representation purposes, only 15 patient data is showcased.
The utilization of segmentation approaches for identifying brain tumor size has several advantageous implications in medical diagnostics and therapy strategizing. To begin with, segmentation plays a crucial role in accurately defining the borders of tumors, hence facilitating the exact assessment of tumor size and its constituent elements, including the tumor core and the adjacent edema. The inclusion of quantitative data is of utmost importance to effectively monitor the course of tumors over time, evaluate the effectiveness of treatments, and ascertain the advancement of the illness. Moreover, using segmented tumor size data facilitates healthcare professionals in making well-informed judgments on the optimal treatment approaches, including surgical intervention, radiation therapy, or chemotherapy. These decisions are based on several factors, such as the tumor’s location, size, and proximity to vital brain structures. In addition, implementing automated segmentation techniques decreases the need for manual measurements, reducing the potential for subjective and variable interpretations. Consequently, this enhances the repeatability and consistency of size evaluations. In general, segmenting brain tumors to determine their size has significant implications for improving patient care on an individualized level, optimizing treatment results, and advancing our comprehension of tumor dynamics within the field of neuro-oncology.
Henceforth, the brain cell size and the tumor size comparison are visualized here in Fig. 7.
The comparison of brain cell size and tumor size can provide significant insights in several domains, including medical research, diagnostics, and therapeutic approaches. One of the key advantages is the potential to enhance our comprehension of typical brain function and pathological states such as cancer. By examining the correlation between the size of brain cells and the size of tumors, researchers may get insight into the effects of tumors on the neural tissue in their vicinity. This analysis identifies possible changes in the shape and arrangement of cells.
Moreover, these comparisons can provide substantial consequences in terms of diagnostic precision. Brain tumors frequently disrupt the structural integrity of brain tissue, leading to alterations in cell size, density, and organization. Through the use of quantitative assessment, medical personnel can build more precise diagnostic criteria. This may enable them to differentiate between benign and malignant tumors by analyzing their impact on neighboring brain cells. This data can potentially facilitate early and more precise identification of tumors.
Moreover, the knowledge acquired from examining brain cell size and tumor size comparisons has the potential to inform and influence the decisions made about treatment strategies. Comprehending the cellular-level interactions between tumors and the adjacent brain tissue might provide valuable insights for determining appropriate treatment modalities, including surgical interventions, radiation therapy, and chemotherapy. The customization of treatment strategies based on the distinct attributes of individual tumors, as determined by factors such as cell size and tissue modifications, can enhance therapy efficacy and reduce harm to unaffected brain tissue.
It is very evident that higher sized brain tumors are often found in the brain cells, where the cell size is also higher.
A few of the samples of the detected tumor cells are furnished here in Figs. 8, 9, 10, 11, 12 and 13.
Further, the detection accuracy is measured (Table 3).
The utilization of segmentation as a method for detecting brain tumors is advantageous due to its ability to accurately define the boundaries of tumors and capture complex spatial connections within medical pictures. Segmentation is crucial in accurately identifying tumor locations within the tough brain tissue. This process involves partitioning the brain tissue into discrete regions of interest, which enables the identification of various tumor components such as the core, peritumoral edema, and neighboring healthy tissue. The meticulous examination described here serves the purpose of not only measuring the dimensions and configuration of tumors but also contributes to the assessment of the effectiveness of therapy and the advancement of the illness during a period of time. In addition, segmentation provides doctors with reliable and reproducible measurements, therefore reducing the inherent subjectivity associated with manual interpretation. Segmentation is a beneficial technique in the field of brain tumor diagnosis, treatment planning, and monitoring. It can extract pertinent information from multimodal imaging data, enhancing patient care and leading to better clinical results.
The mean deviation from all the samples is calculated to be 1.53. Henceforth, it is evident to claim that the accuracy is 98.47%.
The comparison of actual and detected tumor sizes is visually presented here in Fig. 14.
From the visual representation, it is natural to realize that the deviations are significantly less, and the proposed method is highly accurate. Further, the radii detection results for the detected brain tumor cells are furnished here (Table 4).
The sample showcases a few patient samples with multiple tumors one such example after detection is also showcased here.
The obtained results are visualized graphically here in Fig. 15.
9 Comparative analysis
After the detailed discussion of the obtained results, this section provides quantifiable comparisons with benchmarked parallel research outcomes (Table 5).
The research included in the comparative review of brain tumor detection methods employs various strategies to attain precise outcomes. In their separate studies, Nerurkar [2], Bhima and Jagan [3], and Cabria and Gondra [17] have utilized clustering techniques with a computational complexity of O(n2) for the purpose of brain tumor detection. Their investigations have yielded accuracy rates of 95.86%, 95.64%, and 96.29% correspondingly. In contrast, Meyer [27], Hasegawa and Uto [28], and Lin et al. [29] have employed segmentation algorithms that exhibit O(n2) complexity. Consequently, these approaches have achieved enhanced accuracy rates of 96.81%, 96.89%, and 97.15%, respectively. Furthermore, Rahman and Islamet al. [32] introduced PDCNN, and Ghafourian et al. [33], along with Saeedi et al. [36], proposed hybrid models combining Watershed with Clustering & Segmentation, all optimizing to O(n) complexity and achieving an accuracy of 98.12%. Ahmed et al. [34] also presented a hybrid model utilizing VGG-16 with LRP for explainability, marking an accuracy of 97.33%. Additionally, Bhat and Prakash [35] explored ARKFCM combined with SVM & ANN techniques, showing a complexity of O(n2) and the lowest accuracy among the reviewed methods at 91.4%. The proposed method outperforms these approaches by employing a hybrid Watershed – Clustering & Segmentation method with a computational complexity of O(n), achieving the highest accuracy rate of 98.47%. This suggests that the suggested method integrates the benefits of segmentation and clustering techniques, resulting in enhanced outcomes in identifying brain tumors while also maximizing computing efficiency.
The accuracy comparison is visualized graphically here in Fig. 16.
9.1 Discussion
This study introduces a novel framework for the segmentation and identification of brain tumors from MRI images, highlighting significant advancements over traditional and contemporary methods in brain tumor detection. The proposed method addresses the critical challenges associated with the complexity and variability of tumor shapes and appearances, which are often obstacles to accurate tumor segmentation. Comparative analysis with existing methodologies, such as clustering and segmentation by Nerurkar [2], Bhima and Jagan [3], and Cabria and Gondra [17], demonstrate the evolution of tumor detection techniques from foundational methods to more advanced, hybrid approaches that incorporate machine learning and deep learning models. For instance, Rahman and Islam [32] and Ghafourian et al. [33] have made significant strides with the implementation of PDCNN and hybrid models, respectively, achieving notable accuracies. However, while effective, these methods often face challenges in generalization across diverse datasets and may be limited by the specificities of their design.
The proposed framework distinguishes itself by employing a progressive segmentation approach, which iteratively refines tumor segmentation through multiple levels of segmentation thresholds. This method is adept at capturing subtle variations in tumor boundaries, leading to more accurate and robust tumor identification. Moreover, the integration of post-processing techniques further enhances segmentation quality by reducing false positives and improving overall accuracy. Our approach also leverages machine learning techniques for feature extraction and classification, augmenting the segmentation process and enabling a comprehensive analysis of tumor characteristics. This holistic approach ensures high accuracy and efficiency in tumor detection, as evidenced by the superior performance demonstrated in our experimental evaluations.
In conclusion, the proposed framework represents a significant advancement in the field of brain tumor detection from MRI images. It combines the strengths of traditional segmentation techniques with the precision of machine learning and progressive segmentation strategies, offering a highly effective solution for the accurate and efficient identification of brain tumors. Future work will focus on further refining this framework and exploring its applicability to other types of medical imaging and tumor detection tasks.
Further, the final research conclusion is presented in the next section of this work.
10 Conclusion
In conclusion, the research presents a comprehensive framework demonstrating the effectiveness of progressive segmentation techniques in accurately identifying brain tumors from magnetic resonance (MR) images. The study successfully addresses the significant challenge of accurate tumor segmentation, which is crucial for precise diagnosis and treatment planning. The framework proposed in this paper leverages the power of progressive segmentation, which involves a step-by-step approach to segmenting brain tumor regions. This method allows for the detection of even minor or irregularly shaped tumor areas that may be difficult to identify using traditional segmentation techniques. The proposed framework achieves higher accuracy and robustness in tumor localization and boundary delineation by utilizing progressive segmentation. The authors conducted extensive experiments and evaluations to validate the effectiveness of their framework. The results demonstrate that the proposed method outperforms existing approaches in terms of accuracy, sensitivity, specificity, and Dice similarity coefficient (DSC). These metrics serve as reliable indicators of the framework’s ability to provide precise tumor segmentation, allowing medical professionals to make informed decisions regarding patient care.
Likewise, this study’s findings contribute significantly to the evolving brain tumor detection and segmentation technologies landscape. By integrating advanced machine learning techniques with progressive segmentation, the framework sets a new benchmark for the accuracy and efficiency of tumor detection in MRI imaging. This advancement is particularly relevant in personalized medicine, where the ability to segment and characterize brain tumors accurately is essential for tailoring treatment strategies to individual patient needs. Looking forward, the potential for further enhancements and the application of this framework to other areas of medical imaging represents an exciting frontier for research. Incorporating artificial intelligence and deep learning algorithms could unlock new possibilities for automated diagnosis and intervention, paving the way for medical technology and patient care breakthroughs.
Furthermore, the framework’s efficiency and computational performance were also evaluated, highlighting its practicality for real-time applications. The authors successfully demonstrate that their approach achieves fast processing times while maintaining high segmentation accuracy. This finding is crucial in the medical field, where timely diagnosis and treatment can significantly impact patient outcomes. The significance of this research extends beyond the field of medical imaging. The framework presented in this paper contributes to the broader field of computer vision and image analysis, showcasing the potential of progressive segmentation techniques in various image segmentation tasks.
Availability of data and materials
On request.
Code availability
On request.
References
Kapoor L, Thakur S. A survey on brain tumor detection using image processing techniques. In: 2017 7th international conference on cloud computing, data science & engineering-confluence. 2017. p. 582–5.
Nerurkar SN. Brain tumor detection using image segmentation. Brain. 2017;4(4):25–70.
Bhima K, Jagan A. Analysis of MRI based brain tumor identification using segmentation technique. In: 2016 International Conference on Communication and Signal Processing (ICCSP). 2016. p. 2109–13.
Rao S, Parikh M, Parikh M, Nemade C. Implementation of clustering techniques for brain tumor detection. Int J Res Eng. 2016;3(4):6–10.
Malathi R, Kamal AR N. Brain tumor detection and identification using K-Means clustering technique. In: Proceedings of the UGC sponsored national conference on advanced networking and applications. 2015.
Nandi A. Detection of human brain tumour using MRI image segmentation and morphological operators. In: 2015 IEEE international conference on computer graphics, vision and information security (CGVIS). 2015. p. 55–60.
Hassan E, Aboshgifa A. Detecting brain tumour from MRI image using matlab gui programme. Int J Comput Sci Eng Surv (IJCSES). 2015;6(6):47–60.
Thakur P, Pahwa K, Gupta R. Brain tumor detection, segmentation using watershed segmentation and morphological operation. International Journal of Advanced Research in Electronics and Communication Engineering (IJARECE). 2015;4(6).
Selkar RG, Thakare MN. Brain tumor detection and segmentation by using thresholding and watershed algorithm. Int J Adv Inform Commun Technol. 2014;1(3):321–4.
Malpica N, De Solórzano CO, Vaquero JJ, Santos A, Vallcorba I, García-Sagredo JM, et al. Applying watershed algorithms to the segmentation of clustered nuclei. Cytometry. 1997;28(4):289–97.
Sinha K, Sinha GR. Efficient segmentation methods for tumor detection in MRI images. In: 2014 IEEE Students’ Conference on Electrical, Electronics and Computer Science (SCEECS). 2014. p. 1–6.
Laddha M, Ladhake SA. Brain tumor detection using morphological and watershed operators. Int J Appl Innov Eng Manage. 2014;3(3):383–7.
Mustaqeem A, Javed A, Fatima T. An efficient brain tumor detection algorithm using watershed & thresholding based segmentation. IJIGSP. 2012;4(10):34.
Gopal NN, Karnan M. Diagnose brain tumor through MRI using image processing clustering algorithms such as Fuzzy C Means along with intelligent optimization techniques. In: 2010 IEEE international conference on computational intelligence and computing research. 2010. p. 1–4.
Moon N, Bullitt E, Leemput KV, Gerig G. Automatic brain and tumor segmentation. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. 2002. p. 372–9.
Demirhan A, Guler I. Combining stationary wavelet transform and self-organizing maps for brain MR image segmentation. Eng Appl Artif Intell. 2011;24:358–67.
Cabria I, Gondra I. MRI segmentation fusion for brain tumor detection. Inf Fusion. 2017;36:1–9.
Huang M, Yang W, Wu Y, Jiang J, Chen W, Feng Q. Brain tumor segmentation based on local independent projection-based classification. IEEE Trans Biomedical Eng. 2014;61:2633–45.
Gupta N, Bhatele P, Khanna P. Identification of Gliomas from brain MRI through adaptivesegmentation and run length of centralized patterns. J Comput Sci. 2018;25:213–20.
Nabizadeh N, Kubat M. Automatic tumor segmentation in single- spectral MRI using a texture-based and contour-based algorithm. Expert Syst Appl. 2017;77:1–10.
Zhang N, Ruan S, Lebonvallet S, Liao Q. Kernel feature selection to fuse multi-spectral MRI images for brain tumor segmentation. Comput Vis Image Underst. 2011;115:256–69.
Aljabar P, Heckemann RA, Hammers A, Hajnal JV. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. NeuroImage. 2009;46:726–38.
Chen RM, Yang SC, Wang CM. MRI brain tissue classification using unsupervised optimized extenics-based methods. Comput Electr Eng. 2017;58:489–501.
Alagarsamy S, Kamatchi K, Govindaraj V, Thiyagarajan A. A fully automated hybrid methodology using Cuckoo-based fuzzy clustering technique for magnetic resonance brain image segmentation. Int J Imaging Syst Technol. 2017;27:317–32.
Alagarsamy S, Kamatchi K, Govindaraj V, Zhang YD, Thiyagarajan A. Multi-channeled MR brain image segmentation: a new automated approach combining BAT and clustering technique for better identification of heterogeneous tumors. Biocybern Biomed Eng. 2019. https://doi.org/10.1016/j.bbe.2019.05.007.
Lahmiri S. Glioma detection based on multi-fractal features of segmented brain MRI by particle swarm optimization techniques. Biomed Signal Process Control. 2017;31:148–55.
Meyer F. Watersheds and flooding: a segmentation golden braid. In: Topographical tools for filtering and segmentation 1: watersheds on node- or edge-weighted graphs. Wiley; 2019. p. 39–48. https://doi.org/10.1002/9781119579519.ch2.
Hasegawa K, Uto T. Image watermarking based on the watershed segmentation. In: 2020 35th International Technical Conference on Circuits/Systems, Computers, and Communications (ITC-CSCC). 2020. p. 359–62.
Lin H, Song S, Tao S, Liu H. Research on watershed algorithm based on image marking method optimization. In: 2021 IEEE 5th Adv Inform Technol Electron Autom Control Conf (IAEAC). 2021. p. 811–5. https://doi.org/10.1109/IAEAC50856.2021.9390847.
Devisivasankari P, Vijayakumar R. Parallel watershed method for medical modality image segmentation. In: 2020 International Conference on Emerging Trends in Information Technology and Engineering (IC-ETITE). 2020. p. 1–3. https://doi.org/10.1109/ic-ETITE47903.2020.407.
Yuan Y, Zhu Z, Yu H, Zhang W. Watershed-based superpixels with global and local boundary marching. IEEE Trans Image Process. 2020;29:7375–88. https://doi.org/10.1109/TIP.2020.3002078.
Rahman T, Islam MS. MRI brain tumor detection and classification using parallel deep convolutional neural networks. Meas Sensors. 2023;26(December 2022):100694. https://doi.org/10.1016/j.measen.2023.100694.
Ghafourian E, Samadifam F, Fadavian H, Jerfi Canatalay P, Tajally AR, Channumsin S. An ensemble model for the diagnosis of brain tumors through MRIs. Diagnostics. 2023;13(3):561. https://doi.org/10.3390/diagnostics13030561.
Ahmed F, Asif M, Saleem M, Mushtaq UF, Imran M. Identification and Prediction of Brain Tumor Using VGG-16 Empowered with Explainable Artificial Intelligence. Int J Comput Innov Sci. 2023;2(2):24–33.
Bhat TPB, Prakash K. Detection and classification of tumour in brain MRI. Int J Eng Manuf. 2019;9(1):11–20. https://doi.org/10.5815/ijem.2019.01.02.
Saeedi S, Rezayi S, Keshavarz H, Niakan Kalhori SR. MRI-based brain tumor detection using convolutional deep learning methods and chosen machine learning techniques. BMC Med Inf Decis Mak. 2023;23(1):1–17. https://doi.org/10.1186/s12911-023-02114-6.
Funding
Open access funding provided by the Cyprus Libraries Consortium (CLC). This research is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 739578 and the government of the Republic of Cyprus through the Directorate General for European Programmes, Coordination, and Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethical approval for this study was obtained from the Ethics Committee of [University of Cyprus. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Consent to publish
The authors affirm that human research participants provided informed consent for publication.
Conflict of interest
The authors declare that they have no competing interests in the subject matter or materials discussed in this manuscript. Any affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript are completely disclosed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Narayana, M.V., Rao, J.N., Shrivastava, S. et al. A framework for identification of brain tumors from MR images using progressive segmentation. Health Technol. 14, 539–556 (2024). https://doi.org/10.1007/s12553-024-00844-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-024-00844-9