Abstract
Purpose
Boron Neutron Capture Therapy (BNCT) represents an advanced radiation therapy capable of selectively killing tumor cells. It operates on a dual therapy approach, utilizing boronated agents that preferentially deliver boron-10 to tumors, followed by neutron irradiation. This leads to the emission of two ionizing particles. These particles expend all their energy over a distance comparable to the diameter of a cell and can cause irreparable damage to DNA when passing through the nucleus.
Methods
This paper outlines the approach taken by the Italian National Cancer Center for Oncological Hadrontherapy (CNAO) to achieve BNCT certification for clinical applications within the EU regulatory framework.
Results
The existing literature reports excellent outcomes for many unresectable and recurrent tumors, especially in head and neck cancer (HNC).
Conclusions
The paper seeks to clarify the rationale and methods for formulating a clinical trial design to meet these objectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
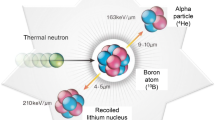
In the oncological landscape, researchers continue to explore and develop innovative therapies that are more effective and less toxic to improve the therapeutic ratio. BNCT is an advanced form of radiotherapy with a distinctive capability for selectively irradiating tumor cells with heavy particles [1, 2]. It is based on irradiation with epithermal neutrons when the target has been loaded with 10B. Initially, 10B is delivered to the cancer cell by a targeted drug, e.g., boronophenylalanine (BPA) and sodium borocaptate (BSH). Boron compounds are actively transported into the cells, and the biologic mechanisms were described more than two decades ago [3]. Subsequently, neutrons are generated by the BNCT therapy system and delivered to the patient’s tumor region [1, 2]. Neutrons captured by 10B then produce two high-LET, short-range (close to one cellular size) heavy ions which can directly damage the DNA double helix of cancer cells, causing irreparable harm while minimizing damage to surrounding normal cells [1, 2]. The underlying principle is that a considerable radiation dose gradient between the tumor and the normal tissues is created, and the 10B is highly selectively distributed to tumor cells, with less damage occurring to normal tissues and a potential very high antitumor effect [1, 2].
Overall, this technique integrates the principles of systemic therapy (infusion of a drug with the potential of accumulating in cancer cells) with the cornerstone of RT (the capability of targeting tumor volume on a spatial basis).
Thus far, BNCT has been applied in over 2000 cases globally, with reported effectiveness in treating head and neck cancer (HNC), malignant meningioma, glioblastoma, cutaneous melanoma, and hepatocellular carcinoma. However, prospective BNCT data are scarce, and only 17 clinical trials have been registered with the National Institutes of Health. Only HNC, glioblastomas, and melanomas have been studied prospectively in these trials. Of the 17 trials, 4 were terminated, 4 stopped updating, and 2 never published their results, leaving 7 completed studies [4,5,6,7,8,9,10]. In addition, many countries have conducted retrospective research on BNCT for HNC. Since June 2021, BNCT for locally unresectable recurrent or unresectable advanced HNC has been covered by national health insurance in Japan [11].
Overall, L-BPA and BSH were the most commonly compounds used although neutrons were mainly sourced from a reactor. A BPA formulation is currently marketed in Japan [12]. The recent development of compact accelerator-based neutron sources, replacing nuclear reactors, represents the most critical innovation in BNCT, and allows the deployment and the application of.
this technique in a hospital environment.
In November 2020, TAE Life Sciences and CNAO Foundation agreed to establish a new facility with a specialized tandem accelerator designed for BNCT treatments. The purpose of this collaboration is to cultivate the ideal conditions within the clinical and research setting of CNAO, with the ultimate objective of advancing the treatment of patients using this innovative modality. This paper aims to outline the approach adopted by CNAO to achieve BNCT certification for clinical use within the EU regulatory framework. Additionally, the paper aims to provide details on the rationale and methods employed in developing a clinical trial design to attain these objectives.
2 General considerations for study development
A clinical investigation has to be designed when applying for a BNCT EU medical device certification. Its planning represents arguably the central part of the application dossier. In addition to the general contents required to run an interventional clinical study (study identification number, sponsor, legal representative, principal investigator) it will have to contain the design, its rationale for that choice, endpoints, variables to be collected, information on the device and any other comparator or other device used, information on any medications used. Being BNCT a binary form of treatment, the study will have to address aspects both of the device and the borate compounds and treatment-related potential biomarkers. The study has to identify the target population, risks and benefits of the device in the given population, the study’s relevance, the investigation’s objective, and statistics for meeting study endpoints. The risk-benefit analysis describes, on the one hand, the proportion of the potential treatment efficacy and, on the other hand, the proportion of all the possible risks to the study patient, whether due to quality, safety, or efficacy. The proportion in the risk-benefit analysis is crucial for study approval. By plotting the risks of the investigational therapy and risks of the disease tackled by the study, the optimal ratio will have to be identified, considering a risk minimization approach. Standard criteria do not exist, and the risk-benefit analysis is considered provisional when the study is conceptualized and approved. In this scenario, close monitoring of these aspects is required during the study conduction, and in case of an unexpected unfavorable ratio, the study will have to be discontinued.
3 Study background
A phase II study is being considered within this operational framework to certify BNCT at CNAO. This approach is suitable for a clinical investigation under the medical device regulation required by the European Union (EU), potentially leading to BNCT device approval. In essence, by comprehensively reviewing the literature already reported with BNCT procedures, we identified the HNC population as the most promising target population to show a favorable risk-benefit ratio [4,5,6,7,8,9,10,11].
Although very limited, experiences with BNCT in recurrent HNC patients reported a high response rate (75%) with a significant number of complete remissions (CR) of 45%. The adverse events were mild, essentially related to local toxicity, which is expected with any form of radiation, and grade > 3 toxicity was reported in 85%. Still, these numbers mainly include hyperamylasamia without any clinical impact.
At CNAO, the experience in hadrontherapy for HNC is deeply consolidated since it represents 40% of all clinical activities. CNAO counts on expert radiation oncologists fully dedicated to the disease and on a national referral of cancer patients candidate to hadrontherapy. At CNAO, HNC patients are offered hadrontherapy as a curative primary approach or as salvage treatment such as re-irradiation where both protons and carbon ions RT may be considered and offered according to histological type and recurrence tumor extension.
This long-term experience will ensure adequate subject accrual and identification and optimal selection, thus supporting the feasibility and success of a monocentric study. In addition, the experience acquired in this disease ensures an appropriate and evidence-based approach to supportive care of accrued subjects. This is vital for making milder and/or reducing expected treatment-related toxicities; thus, study-associated risks contribute to maintaining a favorable ratio throughout the investigation.
CNAO can count on a clinical trial center used to run interventional studies under Good Clinical Practice (GCP) and General Data Protection Regulation (GDPR) rules.
The HNC population still represents a very high-risk population, which has been cited together with pancreatic cancer to be considered as a poor prognostic disease in the European Beating Cancer Plan. This recognition puts HNC at the top priority for cancer research in the EU. By designing a study on this specific population, CNAO pursues its mission to complete the offer of a diversified array of treatment options suitable to fill more personalized treatment gaps, especially for those subjects who are not candidates for salvage surgery and/or curative re-irradiation for whom solutions are still lacking. Clinical recommendations exist to define the population that might benefit from reirradiation [13]. In this setting, re-irradiation procedures may achieve long term tumor control, which is considered the standard of care especially in a selected patient population where treatment results might be anticipated according to a recent recursive partitioning analysis (RPA) on more than 400 patients [14].
At present, subjects for whom salvage surgery and/or reirradiation is excluded or not appropriate to the scope are preferably treated with a systemic approach. Either with immunotherapy alone (pembrolizumab) or chemoimmunotherapy (cisplatin, 5FU, pembrolizumab) alone in the presence of positive PDL1 CPS (Combined Positive Score) that represents the predictive marker for immunotherapy response [15]. The PDL1 CPS-negative population will receive standard chemotherapy. This reflects the standard of care in many EU countries [16]. The response rate in this population ranges from 19% with immunotherapy to 36% with chemo or chemoimmunotherapy. The progression-free survival (PFS) for immunotherapy alone is 3.2 months, and for chemoimmuno, the median PFS is 5 months. Median overall survival (mOS) is generally more favorable with chemoimmunotherapy ranging from 14.7 in CPS ≥ 20 population, 13.6 in CPS ≥ 1, and 13 months in all comers. At the same time, mOS for pembro alone ranges from 14.9, 12.3, and 11.5 in the 3 CPS populations, respectively. Noteworthy, the subgroup analysis for both treatment arms comparing subjects with metastatic disease vs. loco-regional recurrences revealed less benefit for the latter subgroup with an HR crossing 1. Although the majority of patients have metastatic disease, only 1/3 have only loco-regional recurrence and apparently will not benefit from immunotherapy, underlining the peculiarity of this specific advanced HNC population, which still represents a hard-to-treat treatment and for whom new approaches are eagerly needed. Any adverse events of subjects treated with pembro alone occurred in approximately 60% and 96% of the chemopembro group, while grade > 3 was observed in 17% and 70%, respectively.
4 Study design
Patients will be treated with the neutron source manufactured by TAE Life Sciences [17] and boronophenylalanine (BPA), using the two-step infusion protocol.
The trial will include untreated locally recurrent histologically proven squamous cell HNC patients (HNSCC) not candidate for surgery and/or reirradiation with curative intent defined according to the American Radium Society as re-irradiation > 60 Gy (SBRT 35–50 Gy) to tissues that have previously received 45–75 Gy, the target lesion must be measurable according to RECIST (Response Evaluation Criteria in Solid Tumors) v1.1, tumours should preliminary capture BPA at a positron emission tomography (PET) using the tracer 4-borono-2-(18) F-fluoro-phenylalanine (FBPA) F-BPA-PET (T/N > 2.5).
The main exclusion criteria will be:
-
absence of loco-regional recurrence.
-
presence of metastatic disease at distant sites.
-
poor performance status.
-
life expectancy shorter than 100 days.
-
patients with organ dysfunction due to the local presence of disease.
-
severe comorbidities.
Response rate will be the primary endpoint. A closely monitored safety run-in phase is foreseen. Patients will be accurately selected and prehabilitated based on standard practice guidelines. A nutritional and geriatric assessment (if applicable) will be performed among the study protocol procedures. A specific protocol section will be devoted to supportive measures after treatment delivery. Patients will be regularly followed up with clinical and imaging procedures. Data on survival, progression-free survival, and acute and late toxicity will be collected.
The BNCT schedule, in terms of photon- equivalent total dose and overall treatment time, as well as planning goals to target volumes and organs at risk are under investigation.
5 Statistical considerations
The primary endpoint of the clinical study is the objective response rate (ORR) assessed according to RECIST version 1.1. The null hypothesis (H0) is that the ORR with BNCT is 35%, which is equal to the ORR observed in recurrent/metastatic HNSCC patients (unselected for PDL1) treated with platinum-based systemic chemotherapy. The alternative hypothesis (H1) is that the ORR with BNCT is 65%, meaning a percentage similar (with an absolute reduction of -10%) to the one observed in the literature on HNSCC patients treated with BNCT (75–76% [5, 6]).
Using a single-stage design according to A’Hern [18] and considering a type I α = 0.05, and a power (1-β) = 0.8, the requested sample size would be 19 patients. Given this study design, the primary endpoint would be considered met in case at least 11 objective responses will be observed.
To limit the risk of severe acute toxicities, a safety run-in phase will be conducted as follows:
-
6 patients will be initially enrolled (in the meantime, no further patients will be included). A patient and disease assessment of those patients will be performed at least 8 weeks after BNCT end:
-
If G ≥ 3 AEs = 6/6 (100%) the study will be stopped;
-
If G ≥ 3 AEs ≤ 5/6 (83%, similar to the published literature) the study will be continued, so that step 2 will follow;
-
-
Further 6 patients will be enrolled (in the meantime, no further patients will be included). A patient and disease assessment of those patients will be performed at least 8 weeks after BNCT end:
-
If G ≥ 3 AEs ≥ 11/12 (92%) the study will be stopped;
-
If G ≥ 3 AEs ≤ 10/12 (83%, similar to the published literature) the study will be continued, so that step 3 will follow;
-
-
The remaining 7 patients will be enrolled and the disease assessment will be performed at least 8 weeks after BNCT end.
6 Study objectives
The primary objective of the clinical study is activity.
Secondary objectives include:
-
Early and late toxicity.
-
Survival.
7 Study endpoints
The primary endpoint of the clinical study is objective response rate (ORR) according to RECIST1.1.
Secondary endpoints include:
-
Adverse events assessed according to CTCAE v.5.0 within and after 100 days from BNCT (early and late toxicity, respectively).
-
Loco-regional control.
-
Progression-free survival.
-
Overall survival.
8 Statistical analysis
Statistical analysis for primary endpoint: the precision of the ORR estimate will be presented with its 95% confidence interval.
Statistical analysis for secondary endpoints: the proportion of patients with acute (within 3 months) and late (after 9 months follow-up) toxicity and loco regional control will be presented with theirs binomial 95% confidence interval. PFS and OS will be estimated with Kaplan-Meier product limit method.
The relationship between selected clinical and treatment variables and time to event outcomes (PFS and OS) will be investigated with univariable proportional hazard Cox regression models and Log-rank test. Hazard risks will be presented with their 95% binomial confidence interval. P-values will be as well reported.
9 Conclusions
This article outlines the path taken by the National Italian Center for Oncological Hadrontherapy (CNAO) to certify an accelerator-based BNCT as a clinical application within the regulatory framework of the UE. We propose a phase II study including HNC patients not eligible for surgery or curative re-irradiation. The studied approach promises to significantly contribute to the development and the implementation of BNCT as an innovative therapy for the treatment of locally recurrent HNCs.
Data availability
No new data are included in the present paper.
References
He H, Li J, Jiang P, Tian S, Wang H, Fan R, Liu J, Yang Y, Liu Z, Wang J. The basis and advances in clinical application of boron neutron capture therapy. Radiat Oncol. 2021;16:1–8.
Suzuki M. Boron neutron capture therapy (BNCT): a unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int J Clin Oncol. 2020;25:43–50.
Wittig A, Sauerwein WA, Coderre JA, Coderre JA. Mechanisms of Transport of p-Borono-Phenylalanine through the Cell Membrane In Vitro. https://doi.org/10.1667/0033-7587(2000)153[0173:MOTOPB]2.0.CO;2 2000, 153, 173–180.
Chadha M, Capala J, Coderre JA, Elowitz EH, Iwai JI, Joel DD, Liu HB, Wielopolski L, Chanana AD. Boron Neutron-capture therapy (BNCT) for Glioblastoma Multiforme (GBM) using the Epithermal Neutron Beam at the Brookhaven National Laboratory. Int J Radiat Oncol. 1998;40:829–34.
Hirose K, Konno A, Hiratsuka J, Yoshimoto S, Kato T, Ono K, Otsuki N, Hatazawa J, Tanaka H, Takayama K, et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother Oncol. 2021;155:182–7.
Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Välimäki P, et al. Boron Neutron capture Therapy in the treatment of locally recurred Head-and-Neck Cancer: final analysis of a phase I/II trial. Int J Radiat Oncol. 2012;82:e67–75.
Kawabata S, Miyatake SI, Nonoguchi N, Hiramatsu R, Iida K, Miyata S, Yokoyama K, Doi A, Kuroda Y, Kuroiwa T, et al. Survival benefit from boron neutron capture therapy for the newly diagnosed glioblastoma patients. Appl Radiat Isot. 2009;67:S15–8.
Wang LW, Chen YW, Ho CY, Hsueh Liu YW, Chou FI, Liu YH, Liu HM, Peir JJ, Jiang SH, Chang CW, et al. Fractionated Boron Neutron capture Therapy in locally recurrent Head and Neck Cancer: a prospective phase I/II trial. Int J Radiat Oncol. 2016;95:396–403.
Kawabata S, Suzuki M, Hirose K, Tanaka H, Kato T, Goto H, Narita Y, Miyatake SI. Accelerator-based BNCT for patients with recurrent glioblastoma: a multicenter phase II study. Neuro-oncology Adv 2021, 3.
Kankaanranta L, Seppälä T, Koivunoro H, Saarilahti K, Atula T, Collan J, Salli E, Kortesniemi M, Uusi-Simola J, Mäkitie A, et al. Boron Neutron capture Therapy in the treatment of locally recurred Head and Neck Cancer. Int J Radiat Oncol. 2007;69:475–82.
Clinical Centres in Japan - ISNCT Available online. https://isnct.net/bnct-clinical-centers/clinical-centres-in-japan/ (accessed on Jan 15, 2024).
Boron agents | Japanese Society of Neutron Capture Therapy Available online. http://www.jsnct.jp/e/about_nct/houso.html (accessed on Feb 23, 2024).
Ward MC, Koyfman SA, Bakst RL, Margalit DN, Beadle BM, Beitler JJ, Chang SSW, Cooper JS, Galloway TJ, Ridge JA, et al. Retreatment of recurrent or second primary head and Neck Cancer after Prior Radiation: executive summary of the American Radium Society Appropriate Use Criteria. Int J Radiat Oncol. 2022;113:759–86.
Ward MC, Riaz N, Caudell JJ, Dunlap NE, Isrow D, Zakem SJ, Dault J, Awan MJ, Vargo JA, Heron DE, et al. Refining patient selection for Reirradiation of Head and Neck Squamous Carcinoma in the IMRT era: a multi-institution cohort study by the MIRI Collaborative. Int J Radiat Oncol. 2018;100:586–94.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, Psyrri A, Basté N, Neupane P, Bratland Å, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28.
Machiels JP, Leemans CR, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2020;31:1462–75.
Home | TAE Life Sciences Available online. https://taelifesciences.com/ (accessed on Feb 23, 2024).
A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66.
Funding
The authors declare that they have no funding and no financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Lisa Licitra, Stefano Cavalieri, Jessica Franzetti and Ester Orlandi. Statistical analyses were performed by Stefano Cavalieri and Carmine Tinelli. The first draft of the manuscript was written by Lisa Licitra and Stefano Cavalieri and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Institutional review board statement / Ethical approval
Not applicable.
Informed consent statement / Consent to participate / Consent to publish
Not applicable.
Disclosures of competing interests
Lisa Licitra declares travel expenses received by TAE. The remaining Authors declare no conflict of interest with regard to the current article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Licitra, L., Cavalieri, S., Tinelli, C. et al. Developing a trial design to establish BNCT as clinical application: insight for the national italian center for oncological hadrontherapy (CNAO). Health Technol. (2024). https://doi.org/10.1007/s12553-024-00839-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00839-6