Abstract
Neurological disorders, including spinal cord injury, peripheral nerve injury, traumatic brain injury, and neurodegenerative diseases, pose significant challenges in terms of diagnosis, treatment, and understanding the underlying pathophysiological processes. Label-free multiphoton microscopy techniques, such as coherent Raman scattering, two-photon excited autofluorescence, and second and third harmonic generation microscopy, have emerged as powerful tools for visualizing nervous tissue with high resolution and without the need for exogenous labels. Coherent Raman scattering processes as well as third harmonic generation enable label-free visualization of myelin sheaths, while their combination with two-photon excited autofluorescence and second harmonic generation allows for a more comprehensive tissue visualization. They have shown promise in assessing the efficacy of therapeutic interventions and may have future applications in clinical diagnostics. In addition to multiphoton microscopy, vibrational spectroscopy methods such as infrared and Raman spectroscopy offer insights into the molecular signatures of injured nervous tissues and hold potential as diagnostic markers. This review summarizes the application of these label-free optical techniques in preclinical models and illustrates their potential in the diagnosis and treatment of neurological disorders with a special focus on injury, degeneration, and regeneration. Furthermore, it addresses current advancements and challenges for bridging the gap between research findings and their practical applications in a clinical setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The central nervous system (CNS) and the peripheral nervous system (PNS) can be affected by traumatic and degenerative diseases, which often result in chronic neurological deficits and morbidity. While therapeutic approaches are currently being investigated, healing treatments are not always available for the PNS and are not yet possible in the CNS. Therefore, techniques that aid in understanding the mechanisms of nervous damage and characterizing therapeutic effects are highly valuable.
This review article focuses on vibrational spectroscopy and label-free multiphoton microscopy techniques for rapid and label-free histological and molecular characterization of the CNS and PNS in various pathological states. Vibrational spectroscopy encompasses two analytical techniques – infrared (IR) and Raman spectroscopy – which investigate molecular vibrations and provide spectra containing information about the overall biochemical composition. Label-free multiphoton microscopy allows for the simultaneous acquisition of both detailed structural and chemical information within biological samples. While commonly used in biomedical research for exciting and analyzing fluorescent dyes (Sanderson 2023), multiphoton microscopy can provide chemical selectivity by exploiting the simultaneous acquisition of multiple nonlinear signals originating directly from endogenous tissue constituents.
Label-free tissue imaging offers distinct advantages. First, it eliminates the need for sample sectioning. Unlike light and fluorescence microscopy, label-free microscopy does not require histological or immunohistochemical staining of tissues. This minimizes the potential for staining-related artifacts and further preserves the natural state of the sample. Immunohistochemistry, while powerful, can yield variable results due to multiple preparation factors. Label-free imaging offers improved reproducibility, making it easier to compare and replicate results across different studies. In the context of nervous tissue, this is particularly valuable for quantitative measurements like axon morphometry, which otherwise exhibits high variability depending on experimental protocols (Saliani et al. 2017).
Toward monitoring the effects of experimental regenerative and neuroprotective therapies, spectroscopy provides information on biochemical changes within the injured tissue, while label-free microscopy allows for a more comprehensive visualization of degenerating and regenerating nervous structures, axons, and cells. Importantly, label-free methods are predestinated for in vivo tissue analysis. Stained tissue sections used in conventional microscopy limit the number of endpoints available for studying disease progression or therapy effects in preclinical studies. In vivo imaging provides better time resolution, reducing the use of laboratory animals. Moreover, label-free microscopy avoids potential perturbations associated with dye intercalation, non-specific binding, compartmentalization, leakage, and spectral overlap, which can occur in conventional intravital microscopy based on fluorescence-based methods (Pittet and Weissleder 2011).
Therefore, label-free in vivo microscopy can reveal intricate processes that are challenging to elucidate with conventional histological methods. This opens up new avenues for studying axonal regeneration and the effects of innovative therapeutic approaches (Zheng et al. 2019). In the long run, label-free microscopy may facilitate the translation of in vivo histopathology into a diagnostic tool in the clinical context.
Techniques and instrumentation for label-free imaging and analysis of myelin-rich structures, cells, and extracellular matrix of the nervous tissue
Optical methods provide the opportunity for label-free tissue characterization by utilizing various processes of light interaction with endogenous tissue molecules. Several techniques have been employed to investigate the structure and composition of nervous tissue, namely: Fourier-transform infrared (FT-IR) and Raman spectroscopy, coherent Raman scattering microscopy (including stimulated Raman scattering (SRS) and coherent anti-Stokes Raman scattering (CARS)), second and third harmonic generation (SHG, THG), as well as two-photon excited autofluorescence (TPEF) microscopy. Main characteristics and applications including already realized in vivo translation are summarized in Table 1.
Spectroscopy
Infrared and Raman spectroscopy, as well as coherent Raman scattering, are all methods that explore molecular vibrations through linear and nonlinear interactions between light and matter (Geraldes 2020). However, each technique possesses its own advantages and limitations, making them non-equivalent approaches. FT-IR and Raman spectra provide valuable information regarding lipid content, type, and saturation degree, as well as the presence and quantity of carbohydrates, nucleic acids, and proteins (Barth and Zscherp 2002; Movasaghi et al. 2008; Talari et al. 2015). FT-IR absorption spectroscopy is particularly sensitive to probing the antisymmetric vibrations of polar groups, such as in proteins, while Raman spectroscopy excels in probing the symmetric vibrations of non-polar groups (Larkin 2018), such as in lipids (Czamara et al. 2015).
However, FT-IR spectroscopy is suited for thin samples like tissue cryosections and is limited by low spatial resolution due to mid-infrared excitation. Additionally, its application on hydrated biological material is hindered by the strong absorption of water (Uckermann et al. 2014). On the other hand, Raman spectroscopy, which is a reflection technique, can be performed on bulk samples and is technically compatible with both in vitro and in vivo measurements (Cordero et al. 2018; Downes and Elfick 2010). The use of visible or near-infrared (NIR) laser beams for excitation enables high spatial resolution. It is also insensitive to water. Nevertheless, the low yield significantly restricts its application for Raman mapping in living systems, as NIR Raman spectroscopy typically requires long acquisition times (Barton et al. 2019).
Spectroscopy offers the possibility to extract a comprehensive chemical information, allowing the exploration of multiple chemical parameters, a concept referred to as “spectromics” (Petibois 2017). The compositional “fingerprint” provides the capability to differentiate between tissue types through the application of chemometrics (Galli et al. 2012). To achieve this objective, both unsupervised methods, such as clustering and principal component analysis, as well as supervised classification methods, are well suited. These techniques group spectra based on similarities, enabling the creation of multiple tissue maps that highlight the local biochemical changes of interest. Nonetheless, obtaining precise information regarding biomolecule content remains challenging since most IR and Raman bands are associated with molecular groups (e.g., amide, methyl, and methylene) rather than specific molecules.
Label-free multiphoton microscopy
Coherent Raman scattering is utilized in various ways in multiphoton microscopy. Single-band CARS microscopy is specifically designed to target a particular mode of vibration in a molecular bond, making it ideal for lipid imaging (Freudiger et al. 2008) and thus predestinated for applications in the nervous tissue, whenever imaging of myelin (the lipid-rich substance insulating the axons of all vertebrates) is needed. SRS microscopy offers another approach to generate images based on vibrational contrast and enables hyperspectral imaging of both lipids (and therefore of myelin) and proteins through fast tuning of the laser source (Ozeki et al. 2012). THG microscopy takes advantage of the nonlinear signal generated by water-lipid and water-protein interfaces, providing label-free visualization of myelin sheaths surface, of intra- and extracellular membranes as well as of extracellular matrix (ECM) structures (Weigelin et al. 2016). Since nonlinear optical processes require high photon densities achievable only in the focal point of tightly focused ultrashort lasers, all multiphoton imaging modalities are inherently confocal (Dunn and Young 2006; Zipfel et al. 2003).
The combination of different nonlinear techniques for visualization of myelin, such as CARS, SRS, or THG, in conjunction with TPEF and SHG, allows for the simultaneous acquisition of co-localized information about nervous tissue at subcellular resolution, as elaborated in the following sections. TPEF is employed for imaging various cellular and extracellular endogenous fluorophores (König 2000). SHG microscopy enables the specific imaging of structural proteins lacking inversion symmetry, such as fibrillar collagen, myosin, and tubulin (Aghigh et al. 2023).
Label-free multiphoton microscopy is typically integrated into laser scanning microscopes equipped with NIR-emitting lasers, which can penetrate deeper into the tissue (Helmchen and Denk 2005). The imaging depth depends on tissue properties but generally does not exceed 100 μm in strongly myelinated nervous tissue such as peripheral nerves (Rishøj et al. 2022). Modern techniques (such as SWIR excitation or wavefront shaping) have the potential to increase the imaging depth of multiphoton microscopy up to about 1 mm (Hofer et al. 2020; Miller et al. 2017; Rowlands et al. 2019; Xiao et al. 2023). Particularly, three-photon excited fluorescence has shown remarkable potential for imaging deep brain structures, although its primary application has historically been in conjunction with fluorescent labels (Xiao et al. 2023).
In vivo imaging in animal models can be achieved by surgically exposing the region of interest or using implanted window chambers. For example, long-term in vivo imaging with Raman spectroscopy as well as multiphoton microscopy of rodent spinal cord can be achieved using an intervertebral window and biocompatible clearing methods that preserve window transparency over time (Wu et al. 2022). Endoscopic needles, employing graded-index lenses, enable access to deep tissue during in vivo imaging (Bélanger et al. 2012; Dilipkumar et al. 2019).
However, these approaches are limited to small animal models that fit the microscope stage. To expand the application to large animal models or future clinical assessments of nerve regeneration, the development of multiphoton endoscopic systems is necessary. Fiber-based flexible endoscopes, integrating miniaturized lenses for focalization and standard single-mode glass fibers for laser excitation in the picosecond regime, were initially demonstrated for CARS imaging in 2006 (Légaré et al. 2006). Subsequently, endoscopic systems capable of generating coherent Raman signals were developed, fitting within a housing with an outer diameter of less than 1 mm (Deladurantaye et al. 2014; D. T. DePaoli et al. 2018; Saar et al. 2011). Other flexible nonlinear endoscopic systems utilize photonic crystal fibers to deliver femtosecond pulses, allowing the highly efficient generation of CARS, TPEF, and SHG in biological tissues (Ducourthial et al. 2015; Lombardini et al. 2018). Typically, these devices offer a field of view of up to 450 μm and a lateral resolution of approximately 1 μm. Currently, the research focuses on improving imaging quality (Pikálek et al. 2022).
Rigid endoscopes for nonlinear microscopy employ graded-index (GRIN) lenses. Currently, GRIN lenses can be manufactured with lengths of several centimeters, enabling CARS/TPEF/SHG endoscopic systems for in vivo preclinical studies on nervous tissue (Huland et al. 2012; Zirak et al. 2018). Furthermore, endoscopic THG imaging has been achieved using GRIN lenses (Kuzmin et al. 2016), expanding the range of imaging modalities. The feasibility of an SRS endoscope has been demonstrated in principle (Saar et al. 2011), but further testing on biological tissue is still required.
Multiphoton microscopy and endoscopy use laser excitation with power levels up to a few tens of mW. By using laser beams in the NIR range (750–1000 nm), the risk of photodamage is minimized due to the low absorption of biological tissue at these wavelengths. However, the possibility of inducing photodamage with strongly focused ultrashort pulses cannot be completely ruled out when using laser power suited for high-quality label-free imaging (Bégin et al. 2011; Evans and Xie 2008; Gao et al. 2011; Meyer et al. 2013). The topic of photodamage caused by label-free multiphoton microscopy has been extensively studied by various research groups in different biological systems (Fischer et al. 2008; Fu et al. 2006; Hopt and Neher 2001; Nan et al. 2006; Saytashev et al. 2012; Tinevez et al. 2012) and tissue types (Galli et al. 2014). These studies have shown that high-quality, label-free multiphoton imaging is indeed achievable without photodamage. Additionally, an increase in background fluorescence detected by TPEF may serve as an intrinsic marker for any eventually arising photodamage (Galli et al. 2014).
The specific morphological information and chemical selectivity provided by label-free multiphoton microscopy are well suited for automated image analysis. Each nonlinear signal generates a single-channel intensity image that can be analyzed in various ways, including intensity analysis, area measurement, structure evaluation, form analysis, directional analysis, and other morphological parameters. For instance, automated collagen quantification and fiber orientation analysis based on SHG images have already been established as reliable approaches (Dudenkova et al. 2019). The average orientation and directional anisotropy of myelinated nerve fibers can be evaluated, for example, using 2D Fourier-transform algorithms, which can be useful for assessing myelin health (Bégin et al. 2013). Segmentation algorithms have been developed for evaluating morphological parameters of myelin sheaths (Bégin et al. 2014). Furthermore, software for automated axon segmentation based on CARS images is available (Zaimi et al. 2016) and has been used to create a database of spinal cord structure (Saliani et al. 2017).
Label-free analysis of nervous tissue in healthy and diseased states
The utilization of optical label-free techniques for imaging and characterizing nervous tissue has a history spanning nearly two decades. Over this time, optical spectroscopy and label-free multiphoton microscopy have been extensively employed in various studies (Fig. 1), showcasing their potential in elucidating the mechanisms of nervous tissue damage and characterizing the effects of experimental therapies.
Applications of vibrational (Raman and FT-IR) spectroscopy and label-free multiphoton microscopy techniques to study the central and peripheral nervous systems in rodent models and humans. References in green indicate in vivo studies. In the case of multimodal multiphoton microscopy (MMP), the key technique used for visualization of myelin is reported. Created with BioRender.com
Healthy nervous tissue
The CNS of vertebrates comprises several cell types, including neurons, astrocytes, oligodendrocytes, and microglia (resident immune cells). Neurons with myelinated axons transmit stimuli along white matter tracts in the brain and spinal cord. Myelin, a lipid-rich substance produced by oligodendrocytes, forms sheaths surrounding axons and enabling rapid saltatory conduction of action potentials. Thicker myelin sheaths generally result in faster conduction, and the myelin thickness is often quantified by the g-ratio, which represents the ratio of axon diameter to the myelin tube diameter. The morphology of nodes of Ranvier, as well as paranodal and juxtaparanodal regions, also correlates with conduction properties (Suminaite et al. 2019).
In the PNS of vertebrates, myelinated axons are surrounded by collagenous ECM, known as the endoneurium. Axons are further organized into fascicles that are encapsulated by the perineurium. Finally, the epineurium wraps around the entire nerve, providing protection against external stresses. Perineurium and epineurium are also made of collagenous tissue (Griffin et al. 2013).
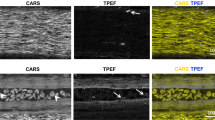
Spectroscopy has not been employed for the biochemical characterization of normal nervous tissue. Instead, it has primarily found its application in investigating biochemical alterations associated with injuries or diseases, as discussed in the relevant sections. Conversely, multiphoton microscopy has gained widespread use for examining the micro-morphology of nervous tissue in a label-free manner. Imaging techniques such as CARS allow for the visualization of lipid-rich myelin surrounding axons in spinal cord white matter and peripheral nerves with sufficient spatial resolution. SHG imaging can simultaneously visualize connective tissue and collagen fibers. TPEF imaging provides additional information about cellular and extracellular structures, contributing to the visualization of tissue morphology. Figure 2 exemplifies multimodal multiphoton microscopy of a peripheral nerve, illustrating how different structures can be imaged using these techniques.
Label-free multimodal multiphoton microscopy of a human peripheral nerve (nervus suralis). The three acquired signals are merged into an RGB image, where CARS is represented in the red channel, TPEF in the green channel, and SHG in the blue channel. A Stitched overview image of a transverse section illustrating the macrostructure of the nerve. It shows the nerve fascicles, collagenous connective tissue of the epi- and perineurium, blood vessels of various sizes, and adipocytes surrounding the nerve. B Detailed view of a single fascicle, revealing both transversely and longitudinally cut axons, as well as the collagen fibers present between the axons (endoneurium). C High-magnification image of a transverse section highlighting the structure of myelin sheaths, including incisures, and the endoneural collagen fibers
Characterization of healthy nervous tissue by label-free multiphoton microscopy
Label-free multiphoton microscopy has been extensively employed to investigate myelin sheaths in both spinal cords and peripheral nerves of rodent models. The utilization of CARS microscopy for visualizing axonal myelin and elucidating detailed structures such as the node of Ranvier and Schmidt-Lanterman incisures under physiological conditions was first demonstrated by H. Fu et al. (2005). Moreover, the response of myelin to electrical stimulation was investigated in real time using CARS imaging of myelin in spinal tissues, revealing paranodal myelin retraction during electrical stimulation of fresh spinal cord white matter strips, as demonstrated by Huff et al. (2011).
In 2007, in vivo imaging of the mouse sciatic nerve was reported in microscopy by Huff and Cheng using epi-detected CARS and SHG (Huff and Cheng 2007). This study illustrated the possibility of non-invasively imaging physiological myelinated axons and the surrounding collagen fibers following a minimally invasive surgical procedure. This visualization was achieved through CARS imaging for axons and SHG imaging for collagen fibers. In live rats, an experimental design was established to enable CARS imaging at the single axon level, allowing the imaging of myelin vesiculation, macrophage uptake of myelin debris, and spontaneous remyelination over a 3-week period (Shi et al. 2011). To achieve minimally invasive imaging of cellular processes in vivo within the mouse spinal cord, a commercially available micro-endoscopic GRIN lens was combined with a multiphoton microscope. This system enabled simultaneous CARS imaging of myelin sheaths and TPEF imaging of microglia and axons. Morphometric analysis was employed to quantify differences in myelin thickness and microglial motility (Bélanger et al. 2012).
CARS microscopy has proven to be effective in studying brain tissue in animal models (Evans et al. 2007; Fu et al. 2008; Pohling et al. 2011). Fresh unfixed ex vivo mouse brain tissue was successfully imaged using CARS microscopy to identify normal gray and white matter structures on various scales, including myelinated brain fibers. The ability to image myelinated fibers without the need for exogenous labeling allowed for white matter mapping in whole brain slices and analysis of the microstructural anatomy of brain axons. The combination of CARS with TPEF enabled multimodal imaging of myelinated axons and other cells.
Furthermore, the impact of laser beam polarization on CARS contrast in myelin imaging was investigated, revealing how the orientation of the myelin membrane relative to the laser polarization plane affects the morphometric parameters that can be extracted through image analysis, as first demonstrated by Bélanger et al. (2009). Later on, dedicated CARS systems were developed to achieve high-resolution, chemically, and orientationally sensitive imaging of myelinated tissue (Brideau et al. 2019). Additionally, a method based on rotating-polarization CARS imaging, developed by de Vito, was utilized to assess the health status of myelin and initially tested on mouse sciatic nerves (de Vito et al. 2012, 2014). Unlike other techniques, this method exploits the intrinsic molecular architecture of myelin, relying directly on its high degree of molecular order. Using this approach, the spatial anisotropy of CARS was examined, and subtle effects of fixatives on the molecular order of myelin were visualized (de Vito et al. 2020).
SRS imaging has emerged as a sensitive and quantitative tool for studying myelin and nervous diseases in recent years. SRS, in combination with rapid automated quantification of myelinated axons using a machine learning algorithm, enabled histomorphometry of peripheral nerves, as first demonstrated by Coto Hernández et al. (2020).
THG is another label-free nonlinear imaging method that allows for in vivo imaging of myelin in the vertebrate nervous system. For example, THG imaging of the mouse spinal cord enabled simultaneous visualization of myelin sheaths and of individual axons labeled with a fluorescent dye visualized by TPEF, as shown by Farrar et al. (2011). THG microscopy also facilitated label-free imaging of myelinating cells and provided insights into the structure of various myelin domains, including juxtaparanodes, Schmidt-Lanterman incisures, and Cajal bands in the murine brain cortex. Morphometry of myelin, described by the g-ratio, was determined under physiological conditions in mouse models of hypomyelination, and during postnatal development (Lim et al. 2014). By leveraging the deep penetration of IR excitation, THG imaging successfully visualized myelinated fibers in live mouse brains up to a depth of approximately 200 μm. This allowed for the visualization of fibers with different orientations and, in combination with semi-automatic axon tracing based on THG signals, revealed the three-dimensional connectivity of the nervous system (Redlich and Lim 2019).
Spinal cord injury
Spinal cord injury (SCI) is caused by a primary injury followed by a cascade of degenerative processes known as the secondary injury, which can be temporally classified into acute, sub-acute, and chronic phases. The acute phase immediately follows the trauma and involves vascular damage, ischemia/hypoxia, and subsequent damage to lipids and proteins. Necrotic cell death affects both neurons and glia. Inflammation is initiated through the recruitment of resident microglia and immune cells from the bloodstream. As the injury progresses into the sub-acute phase, additional cellular apoptosis, demyelination of surviving axons, matrix remodeling, and the formation of a glial scar at the injury site are observed. The chronic phase is characterized by ongoing degeneration, including the formation of a cystic cavity, progressive axonal die-back, and maturation of the glial scar (Alizadeh et al. 2019). Inflammation persists throughout the chronic phase and can have both beneficial and detrimental effects following SCI (Miron and Franklin 2014). Macrophages and microglia play a crucial role in the CNS wound healing process by clearing damaged cells and myelin debris (Zhou et al. 2014), as well as by expressing growth-promoting factors (David and Kroner 2011). However, the role of inflammatory cells in SCI is a topic of debate (Loane and Byrnes 2010), and chronic inflammation is generally considered detrimental to regeneration (Ankeny et al. 2006).
SCI triggers the formation of a scar around the injury epicenter, traditionally regarded as a major obstacle to axonal regeneration (Fitch and Silver 2008). Following the injury, astrocytes increase their expression of intermediate filaments and glial fibrillary acidic protein (GFAP), leading to hypertrophy. Reactive astrocytes proliferate and form a mesh-like structure of intermingled filamentous processes that isolate the injury. Within the mature glial scar, activated microglia/macrophages occupy the innermost region of the injury site, while reactive astrocytes reside in the perilesional region, forming a cellular barrier. The persistence of the glial scar throughout the chronic stage is recognized as a significant hindrance to axonal elongation (Cregg et al. 2014). In penetrating injuries where the meninges are compromised, a fibrotic scar is also observed as meningeal fibroblasts infiltrate the lesion. Similarly, in contusive lesions, perivascular fibroblasts migrate to the injury site, leading to the formation of a fibrotic scar (Soderblom et al. 2013).
The above-described pathological processes can be investigated using both FT-IR and Raman spectroscopy, as well as label-free multiphoton microscopy. Spectroscopy provides insights into tissue biochemical alterations and enables the identification of lesion-related changes in lipid and protein profiles. On the other hand, multimodal CARS microscopy offers higher lateral resolution and faster acquisition speed while still providing some chemical selectivity (see Fig. 3).
Label-free optical analysis of SCI in a rat model. Raman spectroscopy enables the biochemical characterization of tissue alterations by analyzing changes in spectral bands. Classification of spectral information provides a mapping of tissue regions that correspond to histology (L: lesion, w: white matter, g: gray matter). Multiphoton imaging, achieved through simultaneous acquisition of CARS (red channel), TPEF (green channel), and SHG (blue channel), allows visualization of tissue morphology with sufficient chemical selectivity to distinguish tissue regions and identify additional nervous structures such as axons, inflammatory (foam) cells, and collagen fibers. All images adapted from Galli et al. (2015) and assembled for illustrative purposes
Characterization of SCI by spectroscopy
In the past, Raman spectroscopy was not frequently utilized for investigating injured nervous tissue. However, there have been notable studies that employed Raman spectroscopy to explore specific aspects of SCI. For instance, Raman spectroscopy was applied to study demyelination and the upregulation of chondroitin sulfate proteoglycans in the extracellular matrix following spinal cord hemisection (Saxena et al. 2011). In a comparative study conducted by our group, we demonstrated the complementary information provided by FT-IR imaging, Raman mapping, and CARS microscopy in addressing demyelination and fibrotic scarring (Galli et al. 2012, 2015). Additionally, a further study confirmed the capability of confocal Raman spectroscopy to identify the characteristic degenerative features of SCI, such as neuron apoptosis, hemorrhage, demyelination, and upregulation of chondroitin sulfate proteoglycans (Li et al. 2019b). Raman spectroscopy has also been employed to retrieve a biochemical signature of lipid metabolism in macrophages and map inflammation within SCI (Tamosaityte et al. 2016). Furthermore, Raman spectroscopy has been used to investigate the biomedical evolution of spinal cord injury and the therapeutic outcomes of low-level laser therapy (F. Zhang et al. 2022), as well as the effects of amniotic membrane treatment in injured rat spinal cords (Coutinho et al. 2022).
Characterization of SCI by label-free multiphoton microscopy
The morphology of myelin sheath alterations and interactions with nervous and inflammatory cells has been studied using CARS microscopy in various animal models of mechanical and pharmacological spinal cord injury. Sub-micrometric resolution CARS imaging of myelin sheaths in fresh tissues revealed paranodal myelin splitting and retraction following glutamate application in both ex vivo and in vivo guinea pig spinal cords (Fu et al. 2009). Furthermore, the degradation of myelin induced by lysophosphatidylcholine was investigated in ex vivo spinal tissues using CARS imaging, where myelin swelling was monitored by a decrease in CARS intensity and loss of excitation polarization dependence (Fu et al. 2007). Immunofluorescence and CARS imaging techniques were employed to visualize myelin retraction at the nodes of Ranvier and pathological changes in ion channels in white matter strips from guinea pig spinal cords (Ouyang et al. 2010).
The combination of CARS with SHG and TPEF provides unprecedented opportunities for integrated characterization of SCI, allowing visualization of myelin damage together with inflammation and fibrotic scarring simultaneously. Specifically, our group focused on investigating the label-free detection of macrophages and activated microglia in SCI using endogenous TPEF signals. Previous reports have already indicated significant endogenous fluorescence in the cytoplasm of microglia/macrophages (Molcanyi et al. 2013; Oehmichen et al. 1986). Exploiting this endogenous information, we successfully identified activated microglia/macrophages at the single-cell level in the rodent spinal cord by correlating TPEF-positive cells with those identified through immunohistochemistry (Uckermann et al. 2015). Additionally, label-free TPEF imaging was employed to identify activated microglia and track temporal changes in their activity following injury (Galli et al. 2018; Tamosaityte et al. 2016). This information is crucial for future studies investigating inflammation modulation as a therapeutic target. For example, recent studies have shown that pharmacological inhibition of microglia proliferation after SCI in mice and nonhuman primates can lead to improved outcomes, and the combination of CARS microscopy with fluorescent imaging of stained microglia allowed for the direct correlation of improved myelination with reduced microglia proliferation (Poulen et al. 2021).
One of the main limitations of both spectroscopy and label-free multiphoton microscopy techniques is their inability to effectively address the glial scar. To date, no research group has succeeded in identifying a spectroscopic signature specific to the glial scar. In label-free multiphoton microscopy, reactive astrocytes do not exhibit autofluorescence, making it challenging to differentiate them from other structures. Moreover, the spectral resolution of many CARS systems, which use picosecond laser excitation, is insufficient to separate the signals of lipids and proteins. This limitation hinders the clear distinction between elongated axonal structures, astrocytic protein filaments, and collagen fibers that make up the fibrotic scar. However, stimulated Raman scattering (SRS) microscopy shows promise in addressing this gap by enabling the visualization of astrocytic processes through selective chemical imaging of proteins, as demonstrated in human brain tumor samples (Ji et al. 2013). Further investigation is required to determine if glial processes and collagen fibers can be effectively discerned using this technique. A multimodal approach that incorporates SHG imaging may be necessary to achieve this goal.
Spectroscopy and label-free multiphoton microscopy for studying experimental treatments of SCI
Only a few therapies for SCI are currently employed in clinical settings, primarily focusing on early injury management. These include decompression surgery and treatment approaches aimed at reducing acute damage (Ahuja et al. 2017; J. Wang and Pearse 2015). A few pharmacological neuroprotective treatments have shown promise in preclinical studies and achieved the stage of clinical trials (Badhiwala et al. 2018). Experimental approaches aimed at promoting axonal regeneration are being investigated in preclinical and early clinical trials. These include regenerative strategies involving neural scaffolds for axon guidance, scar inhibition, delivery of therapeutic drugs, and transplantation of cells (Dalamagkas et al. 2018). Therapeutic implants composed of different materials are being extensively studied. Natural materials such as collagen, chitosan, and alginate hydrogels are utilized to construct scaffolds due to their biocompatibility, biodegradability, cellular interaction, and biological functionality. Synthetic polymers are also being explored as scaffold materials in regenerative medicine, offering customizable porosity as well as physicochemical and mechanical properties (Liu et al. 2019). Rats are the preferred animal model for investigating SCI and evaluating the effects of therapeutic strategies in preclinical studies (Metz et al. 2000), benefiting from standardized evaluation of functional outcomes (Basso et al. 1995) and tailored injury models (Cheriyan et al. 2014).
Spectroscopy and multimodal multiphoton microscopy have been employed not only to study degenerative processes after SCI but also to investigate the effects of therapeutic implants. The first study, conducted in 2011, examined the successful repair of traumatically injured rat spinal cord using nanoscale block copolymer micelles. In vivo repetitive imaging in rat models was performed using CARS microscopy to measure the intra- and extra-axonal space in uninjured, compression-injured, and injured/micelle-treated white matter (Shi et al. 2011).
Afterwards, there has been a focus on studying the effects of implants, particularly alginate hydrogels, which offer the advantage of biocompatibility in clinical applications (Anker et al. 2015), as well as ease of handling and modification prior to implantation. The potential therapeutic effect of alginate hydrogels was extensively investigated using rat models of hemisection, including functional assessments. Spectroscopy was initially employed to evaluate the stability of the implants during the chronic injury phase, with FT-IR spectroscopy demonstrating that the implanted material retained its chemical structure over time (Tamosaityte et al. 2015). Spectroscopy and label-free multiphoton microscopy were then utilized to examine the effects of alginate implants and investigate potential correlations between tissue features and functional outcomes. In similar rat models of hemisection, both FT-IR spectroscopy (Tamosaityte et al. 2015) and multiphoton microscopy (Galli et al. 2018) provided consistent findings. These techniques enabled the detection and quantification of improved contralateral demyelination in implanted animals during the chronic injury phase, primarily due to the neuroprotective effects of alginate implants. Additionally, both techniques facilitated the assessment of fibrous scar extension. Multiphoton microscopy, with its superior lateral resolution compared to IR spectroscopy (0.6 μm vs. 2.7 μm), allowed for detailed morphological characterization of the fibrotic scar. SHG imaging resolved collagen fibers, enabling analysis of their orientation and the evaluation of the effects. Furthermore, CARS microscopy enabled the imaging of single-regrowing axons, while TPEF imaging provided insights into the effects of alginate implants on inflammation.
Traumatic brain injury
Label-free characterization techniques that have been applied to the study of injured nervous tissue and treatment effects in SCI can also be extended to research on traumatic brain injury (TBI), as both conditions share many common features. TBI involves a primary injury, including intracranial hemorrhage and diffuse axonal injury (Mckee and Daneshvar 2015; Su and Bell 2016), as well as a secondary injury characterized by cerebral edema and ischemia (Ghajar 2000). TBI triggers an endogenous and exogenous neuroinflammatory response that exhibits many similarities to SCI (Loane et al. 2014; Morganti-Kossmann et al. 2007). The astroglial response involves significant proliferation and hypertrophy in the lesion penumbra, leading to the formation of a glial scar. Similar to SCI, the astrocytic scar acts as a major barrier to axonal regeneration in the brain (Burda et al. 2016). However, the complex processes underlying brain injury are not yet fully understood, highlighting the need for methods that enable the probing of the molecular signature of injured brain tissue with high spatial resolution (Ercole et al. 2017).
Spectroscopy in experimental studies of TBI
FT-IR spectroscopy imaging has been successfully employed to map diffuse axonal injury (Wang et al. 2019a; Yang et al. 2014) and investigate its temporal evolution after the initial insult (Zhang et al. 2017). The sensitivity of the Amide I band to protein conformation has been utilized to identify alterations in the protein profile between injured and normal axons in rat models of TBI, potentially serving as a marker for diffuse axonal injury (Zhang et al. 2015a; Zhang et al. 2015b). Besides changes in protein content, also alterations in lipids’ content have been identified by FT-IR spectroscopy in rodent models (Kawon et al. 2021). Raman spectroscopy was also applied to various TBI models, allowing for the detection of hemoglobin presence and alterations in lipid and protein profiles in preclinical pilot studies (Jyothi Lakshmi et al. 2002; Surmacki et al. 2016; Tay et al. 2011). Changes in the Amide I band were observed as well, but their interpretation is more complex compared to FT-IR spectroscopy due to the substantial overlap between lipid and protein signals in Raman spectra. The application of multivariate methods for analyzing Raman spectra may be more effective in evaluating neuronal injury and drug effects, as demonstrated in the study of cerebral ischemic stroke (Jung et al. 2018). While CARS and SRS microscopy have not yet been utilized in the study of TBI, their ability to visualize lipids and proteins may be effectively employed to investigate axonal damage in this context.
Peripheral nerve injury
Peripheral nerve injury (PNI) can occur as a result of stretch, compression, or laceration (Burnett and Zager 2004). Unlike the spinal cord, peripheral nerves have the ability to repair themselves. However, the restoration of function depends on the severity of the injury. In less severe injuries where the epineurium and perineurium are intact, axons can regrow guided by the mesenchymal nerve structures, leading to a high likelihood of full functional recovery. In more severe injuries where the nerve mesenchymal structures are damaged or the nerve is severed, surgical intervention is necessary for recovery (Beris et al. 2019).
Following PNI, a series of degenerative processes primarily occur in the distal part of the nerve, depending on the severity of the injury. Wallerian degeneration initiates within hours of the injury and primarily affects the distal region. This process involves the swelling and fragmentation of axons and myelin, followed by the disintegration of nerve conductive structures. After the clearance of axonal and myelin debris by Schwann cells and macrophages, nerve regeneration begins a few weeks after the injury and, in most cases, leads to the reinnervation of the organ. In more severe injuries, retraction of the severed nerve fiber ends is observed, and a dense fibrous scar forms at the injury site, hindering the regrowth of axons to reach the distal nerve stump (Wang et al. 2019b). Chronic injuries characterized by scarring and delayed or failed reinnervation remain unresolved problems (López-Cebral et al. 2017). Consequently, various strategies are being investigated to reduce scarring and promote axonal growth after surgical reconnection of the nerve stumps. These strategies include nerve entubulation, graft implantation, and molecular therapies (Carvalho et al. 2019; Duraikannu et al. 2019; Modrak et al. 2020). Rat models are commonly employed for testing new treatments and gaining new insights into degenerative and regenerative nerve processes to identify novel therapeutic targets (Vela et al. 2020).
Label-free multiphoton microscopy in experimental studies of PNI
In a rat model of sciatic nerve crush injury, myelin changes were monitored using functional assessments and CARS imaging both ex vivo and in vivo for up to 4 weeks after injury to assess the nerve microenvironment (Henry et al. 2009). Histomorphometry of myelinated axons enabled the quantification of demyelination proximal to the crush site (ex vivo) and remyelination distal to the crush site (in vivo) (Bélanger et al. 2011). The evaluation of myelination in injured human nerves has taken the first steps toward translation to human diagnostics using human nerve biopsies (Wilson et al. 2022). SRS microscopy with combined imaging of lipids and proteins displayed the morphology of fascicles and the amount of myelin. These findings may pave the way for intraoperative assessments of the nerve stump’s quality, overcoming the need for frozen section sequences.
Furthermore, label-free multiphoton microscopy has been employed to investigate nerve injury and regeneration in the octopus, which serves as a fully regenerative model (Imperadore et al. 2017). In experimental neuroscience, the omission of markers allows for better comparison of preclinical data across different animal species, as the obtained information is independent of staining protocols specific to each model. For example, multimodal label-free multiphoton microscopy combining CARS, TPEF, and SHG imaging has been used in octopus PNS injury to examine the mechanisms of degeneration processes, scarring and subsequent regeneration of the pallial nerve and of the whole arm (Imperadore et al. 2018, 2022).
Neurodegeneration
The application of new optical techniques can provide valuable insights into the research on neurodegenerative diseases, particularly multiple sclerosis (MS). MS is an autoimmune disease that affects the brain and spinal cord, leading to inflammatory processes, demyelination, and axonal degeneration. The complex processes involved in MS pathogenesis have not yet been fully elucidated (Lemus et al. 2018; Rodríguez Murúa et al. 2022). Intensive experimental studies have been conducted using animal models, such as experimental autoimmune encephalomyelitis (EAE), which exhibits several features resembling MS, including neuroinflammation, astrocytic reactivity, demyelination, and axonal damage (Constantinescu et al. 2011).
CARS microscopy was used to investigate the processes of demyelination and remyelination in animal models of EAE (Bégin et al. 2013; Fu et al. 2011; Imitola et al. 2011). In our studies, we demonstrated the ability to visualize plaques and associated inflammatory cells in EAE mouse models using label-free CARS and TPEF imaging, respectively (Uckermann et al. 2015). Additionally, a combination of CARS, TPEF, and SHG imaging showed promise in measuring myelin outcomes in a rodent model of Charcot-Marie-Tooth disease, which is a chronic inflammatory demyelinating neuropathy (Hajjar et al. 2018).
SRS microscopy was employed to monitor degeneration in mouse models of amyotrophic lateral sclerosis (ALS) and ALS autopsy materials. Serial imaging enabled long-term observation of disease progression and the effects of drugs in living animals. Very interestingly, three-dimensional imaging of pre-symptomatic mouse models revealed peripheral nerve degeneration coinciding with the earliest signs of muscle denervation, preceding motor function decline (Tian et al. 2016).
Conclusions and outlook
The utilization of new optical techniques in the field of neuroscience has provided significant advancements in our understanding of various neurological conditions. From spinal cord injury to peripheral nerve injury, traumatic brain injury, and neurodegenerative diseases like multiple sclerosis, these optical techniques have demonstrated their potential in unraveling the complex processes underlying these conditions and evaluating the efficacy of therapeutic interventions.
FT-IR spectroscopy and Raman spectroscopy have proven to be effective in characterizing molecular changes associated with nervous tissue injury, such as axonal damage, protein alterations, and demyelination. These spectroscopic techniques offer valuable information about the molecular signatures of injured tissues, contributing to our understanding of the pathological processes and providing potential markers for diagnostic purposes. Especially Raman spectroscopy is now on the verge of routine application in oncological neurosurgery (D. DePaoli et al. 2020; Galli et al. 2023). Advanced Raman devices and endoscopes designed for medical applications, allowing sterilization protocols, are readily available (Daoust et al. 2021; Desroches et al. 2015, 2019; Lakomkin and Hadjipanayis 2019; Mowbray et al. 2021). Furthermore, the development of disposable Raman probes may further enhance the convenience and accessibility of these diagnostic tools (Shu et al. 2021). Additionally, it is worth noting that confocal Raman spectroscopy can be paired with Brillouin spectroscopy, allowing for the simultaneous investigation of both biochemistry and biomechanics at the subcellular level (Rix et al. 2022). Brillouin imaging spectroscopy has already demonstrated its potential in characterizing the viscoelastic properties of nervous tissue, as evidenced by recent studies (Cheburkanov et al. 2023; Rad et al. 2022; Ryu et al. 2021), while few pioneering applications have addressed the study of neurodegenerative diseases and spinal cord injuries in animal models (Palombo et al. 2018; Schlüßler et al. 2018).
Label-free multiphoton microscopy techniques, including CARS, SRS, TPEF, SHG, and THG microscopy, have emerged as powerful tools for visualizing and assessing neural tissue in a non-invasive and high-resolution manner. These techniques have enabled researchers to investigate the microenvironment of injured nerves, evaluate myelin changes, study neuroinflammatory responses, and monitor disease progression in preclinical models. Exciting new possibilities could come for the development of three-photon excited fluorescence imaging applied to unlabeled tissue, which counts to date only limited applications (Boppart et al. 2019; You et al. 2018). It offers higher penetration depth compared to the above techniques (Xiao et al. 2023), and its endoscopic application on unstained tissue has already been reported using a GRIN lens-based device (Huland et al. 2013).
Lastly, it is important to highlight that within the range of label-free imaging tools available to neuroscientists, there exists a whole family of well-established time-resolved techniques. Notably, time-resolved fluorescence spectroscopy and multiphoton fluorescence lifetime imaging are among them (Marcu and Hartl 2012). While delving into these techniques exceeds the scope of this review, they have the potential to offer specific insights into cell metabolism and neuroinflammation (Cleland et al. 2021; Sagar et al. 2020).
With further advancements and refinement, all these optical methods hold great potential for enhancing our understanding of neurological disorders and facilitating the development of novel therapeutic approaches. Indeed, there are still several technical and medical challenges that need to be addressed in future research on multiphoton imaging of nervous structures. Improving needle-like endoscopic systems and their imaging depth is a critical aspect, particularly in the context of studying deep-seated brain structures or spinal cord injury. From a medical perspective, the translation of these optical techniques to clinical applications remains a key challenge. The development of robust and user-friendly imaging systems, along with standardized protocols for data acquisition and analysis, is crucial for their adoption in clinical settings. The first steps for integration of label-free multiphoton microscopy in medical devices have been done (Li et al. 2019a), but further development is still required. Additionally, validation studies using human tissue samples and clinical trials are necessary to establish the diagnostic and prognostic value of optical imaging in neurological disorders. Furthermore, there is a need to explore the potential of optical techniques for guiding surgical interventions and monitoring treatment responses.
In conclusion, while optical techniques have already contributed to our understanding of nervous tissue injury and repair, further advancements are needed to overcome technical challenges and bridge the gap between research and clinical applications. Addressing these issues will pave the way for the development of novel diagnostic tools, therapeutic strategies, and personalized medicine approaches for nervous systems’ injury, degeneration, and regeneration.
Data availability
No new data or materials were presented in this review.
Code of availability
Not applicable.
References
Aghigh A, Bancelin S, Rivard M, Pinsard M, Ibrahim H, Légaré F (2023) Second harmonic generation microscopy: a powerful tool for bio-imaging. Biophys Rev 15(1):43–70. https://doi.org/10.1007/s12551-022-01041-6
Ahuja CS, Schroeder GD, Vaccaro AR, Fehlings MG (2017) Spinal cord injury-what are the controversies? J Orthop Trauma 31(Suppl 4):S7–S13. https://doi.org/10.1097/BOT.0000000000000943
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10. https://doi.org/10.3389/fneur.2019.00282
Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG (2006) Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem 99(4):1073–1087. https://doi.org/10.1111/j.1471-4159.2006.04147.x
Anker SD, Coats AJS, Cristian G, Dragomir D, Pusineri E, Piredda M, Bettari L, Dowling R, Volterrani M, Kirwan B-A, Filippatos G, Mas J-L, Danchin N, Solomon SD, Lee RJ, Ahmann F, Hinson A, Sabbah HN, Mann DL (2015) A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J 36(34):2297–2309. https://doi.org/10.1093/eurheartj/ehv259
Badhiwala JH, Ahuja CS, Fehlings MG (2018) Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine 30(1):1–18. https://doi.org/10.3171/2018.9.SPINE18682
Barth A, Zscherp C (2002) What vibrations tell us about proteins. Q Rev Biophys 35(4):369–430. https://doi.org/10.1017/s0033583502003815
Barton SJ, O’Dwyer K, Butler M, Dignam A, Byrne HJ, O’Neill L, Hennelly BM (2019) Improved performance of near infrared excitation Raman spectroscopy using reflective thin-film gold on glass substrates for cytology samples. Anal Methods 11(47):6023–6032. https://doi.org/10.1039/C9AY01672D
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21. https://doi.org/10.1089/neu.1995.12.1
Bégin S, Bélanger E, Laffray S, Aubé B, Chamma E, Bélisle J, Lacroix S, De Koninck Y, Côté D (2013) Local assessment of myelin health in a multiple sclerosis mouse model using a 2D Fourier transform approach. Biomed Opt Express 4(10):2003–2014. https://doi.org/10.1364/BOE.4.002003
Bégin S, Burgoyne B, Mercier V, Villeneuve A, Vallée R, Côté D (2011) Coherent anti-Stokes Raman scattering hyperspectral tissue imaging with a wavelength-swept system. Biomed Opt Express 2(5):1296–1306. https://doi.org/10.1364/BOE.2.001296
Bégin S, Dupont-Therrien O, Bélanger E, Daradich A, Laffray S, De Koninck Y, Côté DC (2014) Automated method for the segmentation and morphometry of nerve fibers in large-scale CARS images of spinal cord tissue. Biomed Opt Express 5(12):4145–4161. https://doi.org/10.1364/BOE.5.004145
Bélanger E, Bégin S, Laffray S, De Koninck Y, Vallée R, Côté D (2009) Quantitative myelin imaging with coherent anti-Stokes Raman scattering microscopy: alleviating the excitation polarization dependence with circularly polarized laser beams. Opt Express 17(21):18419–18432. https://doi.org/10.1364/OE.17.018419
Bélanger E, Crépeau J, Laffray S, Vallée R, De Koninck Y, Côté D (2012) Live animal myelin histomorphometry of the spinal cord with video-rate multimodal nonlinear microendoscopy. J Biomed Opt 17(2):021107. https://doi.org/10.1117/1.JBO.17.2.021107
Bélanger E, Henry FP, Vallée R, Randolph MA, Kochevar IE, Winograd JM, Lin CP, Côté D (2011) In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed Opt Express 2(9):2698–2708. https://doi.org/10.1364/BOE.2.002698
Beris A, Gkiatas I, Gelalis I, Papadopoulos D, Kostas-Agnantis I (2019) Current concepts in peripheral nerve surgery. Eur J Orthop Surg Traumatol 29(2):263–269. https://doi.org/10.1007/s00590-018-2344-2
Boppart SA, You S, Li L, Chen J, Tu H (2019) Simultaneous label-free autofluorescence-multiharmonic microscopy and beyond. APL Photonics 4(10):100901. https://doi.org/10.1063/1.5098349
Brideau C, Poon KWC, Colarusso P, Stys PK (2019) Excitation parameters optimized for coherent anti-Stokes Raman scattering imaging of myelinated tissue. J Biomed Opt 24(4):1–8. https://doi.org/10.1117/1.JBO.24.4.046502
Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315. https://doi.org/10.1016/j.expneurol.2015.03.020
Burnett MG, Zager EL (2004) Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus 16(5):E1. https://doi.org/10.3171/foc.2004.16.5.2
Carvalho CR, Oliveira JM, Reis RL (2019) Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Frontiers in Bioengineering and Biotechnology 7:337. https://doi.org/10.3389/fbioe.2019.00337
Cheburkanov V, Du J, Brogan DM, Berezin MY, Yakovlev VV (2023) Toward peripheral nerve mechanical characterization using Brillouin imaging spectroscopy. Neurophotonics 10(3):035007. https://doi.org/10.1117/1.NPh.10.3.035007
Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, Kirsch T, Errico TJ (2014) Spinal cord injury models: a review. Spinal Cord 52(8):588–595. https://doi.org/10.1038/sc.2014.91
Cleland NRW, Al-Juboori SI, Dobrinskikh E, Bruce KD (2021) Altered substrate metabolism in neurodegenerative disease: new insights from metabolic imaging. J Neuroinflammation 18(1):248. https://doi.org/10.1186/s12974-021-02305-w
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164(4):1079–1106. https://doi.org/10.1111/j.1476-5381.2011.01302.x
Cordero E, Latka I, Matthäus C, Schie I, Popp J (2018) In-vivo Raman spectroscopy: from basics to applications. J Biomed Opt 23(7):1–23. https://doi.org/10.1117/1.JBO.23.7.071210
Coto Hernández I, Yang W, Mohan S, Jowett N (2020) Label-free histomorphometry of peripheral nerve by stimulated Raman spectroscopy. Muscle Nerve 62(1):137–142. https://doi.org/10.1002/mus.26895
Coutinho EST, Medeiros Neto LP, Bhattacharjee T, Arisawa EALS, Sant’Anna, L. B. (2022) Raman spectroscopy of healthy, injured and amniotic membrane treated rat spinal cords. Spectrochim Acta A Mol Biomol Spectrosc 265:120323. https://doi.org/10.1016/j.saa.2021.120323
Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197–207. https://doi.org/10.1016/j.expneurol.2013.12.024
Czamara K, Majzner K, Pacia MZ, Kochan K, Kaczor A, Baranska M (2015) Raman spectroscopy of lipids: a review. J Raman Spectrosc 46(1):4–20. https://doi.org/10.1002/jrs.4607
Dalamagkas K, Tsintou M, Seifalian A, Seifalian AM (2018) Translational regenerative therapies for chronic spinal cord injury. Int J Mol Sci 19(6). https://doi.org/10.3390/ijms19061776
Daoust F, Nguyen T, Orsini P, Bismuth J, de Denus-Baillargeon M-M, Veilleux I, Wetter A, Mckoy P, Dicaire I, Massabki M, Petrecca K, Leblond F (2021) Handheld macroscopic Raman spectroscopy imaging instrument for machine-learning-based molecular tissue margins characterization. J Biomed Opt 26(2):022911. https://doi.org/10.1117/1.JBO.26.2.022911
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399. https://doi.org/10.1038/nrn3053
de Vito G, Bifone A, Piazza V (2012) Rotating-polarization CARS microscopy: combining chemical and molecular orientation sensitivity. Opt Express 20(28):29369–29377. https://doi.org/10.1364/OE.20.029369
de Vito G, Parlanti P, Cecchi R, Luin S, Cappello V, Tonazzini I, Piazza V (2020) Effects of fixatives on myelin molecular order probed with RP-CARS microscopy. Appl Opt 59(6):1756–1762. https://doi.org/10.1364/AO.384662
de Vito G, Tonazzini I, Cecchini M, Piazza V (2014) RP-CARS: label-free optical readout of the myelin intrinsic healthiness. Opt Express 22(11):13733–13743. https://doi.org/10.1364/OE.22.013733
Deladurantaye P, Paquet A, Paré C, Zheng H, Doucet M, Gay D, Poirier M, Cormier J-F, Mermut O, Wilson BC, Seibel EJ (2014) Advances in engineering of high contrast CARS imaging endoscopes. Opt Express 22(21):25053–25064. https://doi.org/10.1364/OE.22.025053
DePaoli D, Lemoine É, Ember K, Parent M, Prud’homme M, Cantin L, Petrecca K, Leblond F, Côté DC (2020) Rise of Raman spectroscopy in neurosurgery: a review. J Biomed Opt 25(5):5. https://doi.org/10.1117/1.JBO.25.5.050901
DePaoli DT, Lapointe N, Messaddeq Y, Parent M, Côté DC (2018) Intact primate brain tissue identification using a completely fibered coherent Raman spectroscopy system. Neurophotonics 5(3):035005. https://doi.org/10.1117/1.NPh.5.3.035005
Desroches J, Jermyn M, Mok K, Lemieux-Leduc C, Mercier J, St-Arnaud K, Urmey K, Guiot M-C, Marple E, Petrecca K, Leblond F (2015) Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed Opt Express 6(7):7. https://doi.org/10.1364/BOE.6.002380
Desroches J, Lemoine É, Pinto M, Marple E, Urmey K, Diaz R, Guiot M-C, Wilson BC, Petrecca K, Leblond F (2019) Development and first in-human use of a Raman spectroscopy guidance system integrated with a brain biopsy needle. J Biophotonics 12(3):e201800396. https://doi.org/10.1002/jbio.201800396
Dilipkumar A, Al-Shemmary A, Kreiß L, Cvecek K, Carlé B, Knieling F, Gonzales Menezes J, Thoma O-M, Schmidt M, Neurath MF, Waldner M, Friedrich O, Schürmann S (2019) Label-free multiphoton endomicroscopy for minimally invasive in vivo imaging. Adv Sci (Weinh) 6(8):1801735. https://doi.org/10.1002/advs.201801735
Downes A, Elfick A (2010) Raman spectroscopy and related techniques in biomedicine. Sensors (Basel, Switzerland) 10(3):1871–1889. https://doi.org/10.3390/s100301871
Ducourthial G, Leclerc P, Mansuryan T, Fabert M, Brevier J, Habert R, Braud F, Batrin R, Vever-Bizet C, Bourg-Heckly G, Thiberville L, Druilhe A, Kudlinski A, Louradour F (2015) Development of a real-time flexible multiphoton microendoscope for label-free imaging in a live animal. Sci Rep 5:18303. https://doi.org/10.1038/srep18303
Dudenkova VV, Shirmanova MV, Lukina MM, Feldshtein FI, Virkin A, Zagainova EV (2019) Examination of collagen structure and state by the second harmonic generation microscopy. Biochemistry Biokhimiia 84(Suppl 1):S89–S107. https://doi.org/10.1134/S0006297919140062
Dunn KW, Young PA (2006) Principles of multiphoton microscopy. Nephron Exp Nephrol 103(2):e33–e40. https://doi.org/10.1159/000090614
Duraikannu A, Krishnan A, Chandrasekhar A, Zochodne DW (2019) Beyond trophic factors: exploiting the intrinsic regenerative properties of adult neurons. Front Cell Neurosci 13:128. https://doi.org/10.3389/fncel.2019.00128
Ercole A, Magnoni S, Vegliante G, Pastorelli R, Surmacki J, Bohndiek SE, Zanier ER (2017) Current and emerging technologies for probing molecular signatures of traumatic brain injury. Front Neurol 8:450. https://doi.org/10.3389/fneur.2017.00450
Evans CL, Xie XS (2008) Coherent anti-Stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu Rev Anal Chem (Palo Alto, Calif) 1:883–909. https://doi.org/10.1146/annurev.anchem.1.031207.112754
Evans CL, Xu X, Kesari S, Xie XS, Wong STC, Young GS (2007) Chemically-selective imaging of brain structures with CARS microscopy. Opt Express 15(19):12076–12087. https://doi.org/10.1364/oe.15.012076
Farrar MJ, Wise FW, Fetcho JR, Schaffer CB (2011) In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys J 100(5):1362–1371. https://doi.org/10.1016/j.bpj.2011.01.031
Fischer F, Volkmer B, Puschmann S, Greinert R, Breitbart W, Kiefer J, Wepf R (2008) Risk estimation of skin damage due to ultrashort pulsed, focused near-infrared laser irradiation at 800 nm. J Biomed Opt 13(4):041320. https://doi.org/10.1117/1.2960016
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209(2):294–301. https://doi.org/10.1016/j.expneurol.2007.05.014
Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, Tsai JC, Kang JX, Xie XS (2008) Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science (New York, NY) 322(5909):1857–1861. https://doi.org/10.1126/science.1165758
Fu Y, Frederick TJ, Huff TB, Goings GE, Miller SD, Cheng J-X (2011) Paranodal myelin retraction in relapsing experimental autoimmune encephalomyelitis visualized by coherent anti-Stokes Raman scattering microscopy. J Biomed Opt 16(10). https://doi.org/10.1117/1.3638180
Fu Y, Huff TB, Wang H-W, Wang H, Cheng J-X (2008) Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt Express 16(24):19396–19409. https://doi.org/10.1364/oe.16.019396
Fu Y, Sun W, Shi Y, Shi R, Cheng J-X (2009) Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One 4(8):e6705. https://doi.org/10.1371/journal.pone.0006705
Fu Y, Wang H, Huff TB, Shi R, Cheng J-X (2007) Coherent anti-Stokes Raman scattering imaging of myelin degradation reveals a calcium-dependent pathway in lyso-PtdCho-induced demyelination. J Neurosci Res 85(13):2870–2881. https://doi.org/10.1002/jnr.21403
Fu Y, Wang H, Shi R, Cheng J-X (2006) Characterization of photodamage in coherent anti-Stokes Raman scattering microscopy. Opt Express 14(9):3942–3951. https://doi.org/10.1364/oe.14.003942
Galli R, Juratli TA, Uckermann O (2023) Clinical Raman spectroscopy of brain tumors from an interdisciplinary perspective. Clin Neuropathol 42(1):2–14. https://doi.org/10.5414/NP301522
Galli R, Sitoci-Ficici KH, Uckermann O, Later R, Marečková M, Koch M, Leipnitz E, Schackert G, Koch E, Gelinsky M, Steiner G, Kirsch M (2018) Label-free multiphoton microscopy reveals relevant tissue changes induced by alginate hydrogel implantation in rat spinal cord injury. Sci Rep 8(1):10841. https://doi.org/10.1038/s41598-018-29140-z
Galli, R., Tamosaityte, S., Koch, M., Sitoci-Ficici, K. H., Later, R., Uckermann, O., Beiermeister, R., Gelinsky, M., Schackert, G., Kirsch, M., Koch, E., & Steiner, G. (2015). Raman-based imaging uncovers the effects of alginate hydrogel implants in spinal cord injury. Advanced Microscopy Techniques IV; and Neurophotonics II (2015), Paper 95360Y, 95360Y. https://doi.org/10.1364/ECBO.2015.95360Y
Galli R, Uckermann O, Andresen EF, Geiger KD, Koch E, Schackert G, Steiner G, Kirsch M (2014) Intrinsic indicator of photodamage during label-free multiphoton microscopy of cells and tissues. PLoS One 9(10):e110295. https://doi.org/10.1371/journal.pone.0110295
Galli R, Uckermann O, Winterhalder MJ, Sitoci-Ficici KH, Geiger KD, Koch E, Schackert G, Zumbusch A, Steiner G, Kirsch M (2012) Vibrational spectroscopic imaging and multiphoton microscopy of spinal cord injury. Anal Chem 84(20):8707–8714. https://doi.org/10.1021/ac301938m
Gao L, Zhou H, Thrall MJ, Li F, Yang Y, Wang Z, Luo P, Wong KK, Palapattu GS, Wong STC (2011) Label-free high-resolution imaging of prostate glands and cavernous nerves using coherent anti-Stokes Raman scattering microscopy. Biomed Opt Express 2(4):915–926. https://doi.org/10.1364/BOE.2.000915
Geraldes CFGC (2020) Introduction to infrared and Raman-based biomedical molecular imaging and comparison with other modalities. Molecules (Basel, Switzerland) 25(23):5547. https://doi.org/10.3390/molecules25235547
Ghajar J (2000) Traumatic brain injury. Lancet (London, England) 356(9233):923–929. https://doi.org/10.1016/S0140-6736(00)02689-1
Griffin JW, Hogan MV, Chhabra AB, Deal DN (2013) Peripheral nerve repair and reconstruction. J Bone Joint Surg Am 95(23):2144–2151. https://doi.org/10.2106/JBJS.L.00704
Hajjar H, Boukhaddaoui H, Rizgui A, Sar C, Berthelot J, Perrin-Tricaud C, Rigneault H, Tricaud N (2018) Label-free non-linear microscopy to measure myelin outcome in a rodent model of Charcot-Marie-Tooth diseases. J Biophotonics 11(12):e201800186. https://doi.org/10.1002/jbio.201800186
Helmchen F, Denk W (2005) Deep tissue two-photon microscopy. Nat Methods 2(12):932–940. https://doi.org/10.1038/nmeth818
Henry FP, Côté D, Randolph MA, Rust EAZ, Redmond RW, Kochevar IE, Lin CP, Winograd JM (2009) Real-time in vivo assessment of the nerve microenvironment with coherent anti-Stokes Raman scattering microscopy. Plast Reconstr Surg 123(2 Suppl):123S–130S. https://doi.org/10.1097/PRS.0b013e318191c5b8
Hofer M, Shivkumar S, El Waly B, Brasselet S (2020) Coherent anti-Stokes Raman scattering through thick biological tissues by single-wavefront shaping. Phys Rev Appl 14(2):024019. https://doi.org/10.1103/PhysRevApplied.14.024019
Hopt A, Neher E (2001) Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophys J 80(4):2029–2036. https://doi.org/10.1016/S0006-3495(01)76173-5
Huff TB, Cheng J-X (2007) In vivo coherent anti-Stokes Raman scattering imaging of sciatic nerve tissue. J Microsc 225(Pt 2):175–182. https://doi.org/10.1111/j.1365-2818.2007.01729.x
Huff TB, Shi Y, Sun W, Wu W, Shi R, Cheng J-X (2011) Real-time CARS imaging reveals a calpain-dependent pathway for paranodal myelin retraction during high-frequency stimulation. PLoS One 6(3):e17176. https://doi.org/10.1371/journal.pone.0017176
Huland DM, Brown CM, Howard SS, Ouzounov DG, Pavlova I, Wang K, Rivera DR, Webb WW, Xu C (2012) In vivo imaging of unstained tissues using long gradient index lens multiphoton endoscopic systems. Biomed Opt Express 3(5):1077–1085. https://doi.org/10.1364/BOE.3.001077
Huland DM, Charan K, Ouzounov DG, Jones JS, Nishimura N, Xu C (2013) Three-photon excited fluorescence imaging of unstained tissue using a GRIN lens endoscope. Biomed Opt Express 4(5):652–658. https://doi.org/10.1364/BOE.4.000652
Imitola J, Côté D, Rasmussen S, Xie XS, Liu Y, Chitnis T, Sidman RL, Lin CP, Khoury SJ (2011) Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J Biomed Opt 16(2). https://doi.org/10.1117/1.3533312
Imperadore P, Galli R, Winterhalder MJ, Zumbusch A, Uckermann O (2022) Imaging arm regeneration: label-free multiphoton microscopy to dissect the process in Octopus vulgaris. Front Cell Dev Biol 10:814746. https://doi.org/10.3389/fcell.2022.814746
Imperadore P, Shah SB, Makarenkova HP, Fiorito G (2017) Nerve degeneration and regeneration in the cephalopod mollusc Octopus vulgaris: the case of the pallial nerve. Sci Rep 7:46564. https://doi.org/10.1038/srep46564
Imperadore P, Uckermann O, Galli R, Steiner G, Kirsch M, Fiorito G (2018) Nerve regeneration in the cephalopod mollusc Octopus vulgaris: label-free multiphoton microscopy as a tool for investigation. J R Soc Interface 15(141). https://doi.org/10.1098/rsif.2017.0889
Ji M, Orringer DA, Freudiger CW, Ramkissoon S, Liu X, Lau D, Golby AJ, Norton I, Hayashi M, Agar NYR, Young GS, Spino C, Santagata S, Camelo-Piragua S, Ligon KL, Sagher O, Xie XS (2013) Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med 5(201):201ra119. https://doi.org/10.1126/scitranslmed.3005954
Jung GB, Kang SW, Lee G-J, Kim D (2018) Biochemical characterization of the brain hippocampal areas after cerebral ischemia-reperfusion using Raman spectroscopy. Appl Spectrosc 72(10):1479–1486. https://doi.org/10.1177/0003702818776627
Jyothi Lakshmi R, Kartha VB, Murali Krishna C, Solomon JGR, Ullas G, Uma Devi P (2002) Tissue Raman spectroscopy for the study of radiation damage: brain irradiation of mice. Radiat Res 157(2):175–182. https://doi.org/10.1667/0033-7587(2002)157[0175:trsfts]2.0.co;2
Kawon K, Setkowicz Z, Drozdz A, Janeczko K, Chwiej J (2021) The methods of vibrational microspectroscopy reveals long-term biochemical anomalies within the region of mechanical injury within the rat brain. Spectrochim Acta A Mol Biomol Spectrosc 263:120214. https://doi.org/10.1016/j.saa.2021.120214
König K (2000) Multiphoton microscopy in life sciences. J Microsc 200(Pt 2):83–104. https://doi.org/10.1046/j.1365-2818.2000.00738.x
Kuzmin NV, Wesseling P, de Witt Hamer PC, Noske DP, Galgano GD, Mansvelder HD, Baayen JC, Groot ML (2016) Third harmonic generation imaging for fast, label-free pathology of human brain tumors. Biomed Opt Express 7(5):1889–1904. https://doi.org/10.1364/BOE.7.001889
Lakomkin N, Hadjipanayis CG (2019) The use of spectroscopy handheld tools in brain tumor surgery: current evidence and techniques. Front Surg 6:30. https://doi.org/10.3389/fsurg.2019.00030
Larkin PJ (2018) Chapter 2—Basic Principles. In: Larkin PJ (ed) Infrared and Raman spectroscopy (second edition). Elsevier, pp 7–28. https://doi.org/10.1016/B978-0-12-804162-8.00002-1
Légaré F, Evans CL, Ganikhanov F, Xie XS (2006) Towards CARS endoscopy. Opt Express 14(10):4427–4432. https://doi.org/10.1364/oe.14.004427
Lemus HN, Warrington AE, Rodriguez M (2018) Multiple sclerosis: mechanisms of disease and strategies for myelin and axonal repair. Neurol Clin 36(1):1–11. https://doi.org/10.1016/j.ncl.2017.08.002
Li A, Hall G, Chen D, Liang W, Ning B, Guan H, Li X (2019a) A biopsy-needle compatible varifocal multiphoton rigid probe for depth-resolved optical biopsy. J Biophotonics 12(1):e201800229. https://doi.org/10.1002/jbio.201800229
Li J, Liang Z, Wang S, Wang Z, Zhang X, Hu X, Wang K, He Q, Bai J (2019b) Study on the pathological and biomedical characteristics of spinal cord injury by confocal Raman microspectral imaging. Spectrochim Acta A Mol Biomol Spectrosc 210:148–158. https://doi.org/10.1016/j.saa.2018.11.022
Lim H, Sharoukhov D, Kassim I, Zhang Y, Salzer JL, Melendez-Vasquez CV (2014) Label-free imaging of Schwann cell myelination by third harmonic generation microscopy. Proc Natl Acad Sci U S A 111(50):18025–18030. https://doi.org/10.1073/pnas.1417820111
Liu S, Xie Y-Y, Wang B (2019) Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res 14(8):1352–1363. https://doi.org/10.4103/1673-5374.253512
Loane DJ, Byrnes KR (2010) Role of microglia in neurotrauma. Neurotherapeutics 7(4):366–377. https://doi.org/10.1016/j.nurt.2010.07.002
Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI (2014) Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol 73(1):14–29. https://doi.org/10.1097/NEN.0000000000000021
Lombardini A, Mytskaniuk V, Sivankutty S, Andresen ER, Chen X, Wenger J, Fabert M, Joly N, Louradour F, Kudlinski A, Rigneault H (2018) High-resolution multimodal flexible coherent Raman endoscope. Light Sci Appl 7(1):10. https://doi.org/10.1038/s41377-018-0003-3
López-Cebral R, Silva-Correia J, Reis RL, Silva TH, Oliveira JM (2017) Peripheral nerve injury: current challenges, conventional treatment approaches, and new trends in biomaterials-based regenerative strategies. ACS Biomater Sci Eng 3(12):3098–3122. https://doi.org/10.1021/acsbiomaterials.7b00655
Marcu L, Hartl BA (2012) Fluorescence lifetime spectroscopy and imaging in neurosurgery. IEEE J Sel Top 18(4):1465–1477. https://doi.org/10.1109/JSTQE.2012.2185823
Mckee AC, Daneshvar DH (2015) The neuropathology of traumatic brain injury. Handb Clin Neurol 127:45–66. https://doi.org/10.1016/B978-0-444-52892-6.00004-0
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V (2000) Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma 17(1):1–17. https://doi.org/10.1089/neu.2000.17.1
Meyer T, Chemnitz M, Baumgartl M, Gottschall T, Pascher T, Matthäus C, Romeike BFM, Brehm BR, Limpert J, Tünnermann A, Schmitt M, Dietzek B, Popp J (2013) Expanding multimodal microscopy by high spectral resolution coherent anti-Stokes Raman scattering imaging for clinical disease diagnostics. Anal Chem 85(14):6703–6715. https://doi.org/10.1021/ac400570w
Miller DR, Jarrett JW, Hassan AM, Dunn AK (2017) Deep tissue imaging with multiphoton fluorescence microscopy. Curr Opin Biomed 4:32–39. https://doi.org/10.1016/j.cobme.2017.09.004
Miron VE, Franklin RJM (2014) Macrophages and CNS remyelination. J Neurochem 130(2):165–171. https://doi.org/10.1111/jnc.12705
Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC (2020) Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res 98(5):780–795. https://doi.org/10.1002/jnr.24538
Molcanyi M, Bosche B, Kraitsy K, Patz S, Zivcak J, Riess P, El Majdoub F, Hescheler J, Goldbrunner R, Schäfer U (2013) Pitfalls and fallacies interfering with correct identification of embryonic stem cells implanted into the brain after experimental traumatic injury. J Neurosci Methods 215(1):60–70. https://doi.org/10.1016/j.jneumeth.2013.02.012
Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T (2007) Modulation of immune response by head injury. Injury 38(12):1392–1400. https://doi.org/10.1016/j.injury.2007.10.005
Movasaghi Z, Rehman S, ur Rehman, Dr. I. (2008) Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 43(2):134–179. https://doi.org/10.1080/05704920701829043
Mowbray M, Banbury C, Rickard JJS, Davies DJ, Goldberg Oppenheimer P (2021) Development and characterization of a probe device toward intracranial spectroscopy of traumatic brain injury. ACS Biomater Sci Eng 7(3):1252–1262. https://doi.org/10.1021/acsbiomaterials.0c01156
Nan X, Potma EO, Xie XS (2006) Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-Stokes Raman scattering microscopy. Biophys J 91(2):728–735. https://doi.org/10.1529/biophysj.105.074534
Oehmichen M, Eisenmenger W, Raff G, Berghaus G (1986) Brain macrophages in human cortical contusions as indicator of survival period. Forensic Sci Int 30(4):281–301. https://doi.org/10.1016/0379-0738(86)90136-2
Ouyang H, Sun W, Fu Y, Li J, Cheng J-X, Nauman E, Shi R (2010) Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma 27(6):1109–1120. https://doi.org/10.1089/neu.2010.1271
Ozeki Y, Umemura W, Otsuka Y, Satoh S, Hashimoto H, Sumimura K, Nishizawa N, Fukui K, Itoh K (2012) High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat Photonics 6(12):845. https://doi.org/10.1038/nphoton.2012.263
Palombo F, Masia F, Mattana S, Tamagnini F, Borri P, Langbein W, Fioretto D (2018) Hyperspectral analysis applied to micro-Brillouin maps of amyloid-beta plaques in Alzheimer’s disease brains. Analyst 143(24):6095–6102. https://doi.org/10.1039/C8AN01291A
Petibois C (2017) 3D quantitative chemical imaging of tissues by spectromics. Trends Biotechnol 35(12):1194–1207. https://doi.org/10.1016/j.tibtech.2017.08.002
Pikálek T, Stibůrek M, Simpson S, Čižmár T, Trägårdh J (2022) Suppression of the non-linear background in a multimode fibre CARS endoscope. Biomed Opt Express 13(2):862–874. https://doi.org/10.1364/BOE.450375
Pittet MJ, Weissleder R (2011) Intravital imaging. Cell 147(5):983–991. https://doi.org/10.1016/j.cell.2011.11.004
Pohling C, Buckup T, Pagenstecher A, Motzkus M (2011) Chemoselective imaging of mouse brain tissue via multiplex CARS microscopy. Biomed Opt Express 2(8):2110–2116. https://doi.org/10.1364/BOE.2.002110
Poulen G, Aloy E, Bringuier CM, Mestre-Francés N, Artus EVF, Cardoso M, Perez J-C, Goze-Bac C, Boukhaddaoui H, Lonjon N, Gerber YN, Perrin FE (2021) Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics 11(18):8640–8659. https://doi.org/10.7150/thno.61833
Rad MA, Mahmodi H, Filipe EC, Cox TR, Kabakova I, Tipper JL (2022) Micromechanical characterisation of 3D bioprinted neural cell models using Brillouin microspectroscopy. Bioprinting 25:e00179. https://doi.org/10.1016/j.bprint.2021.e00179
Redlich MJ, Lim H (2019) A method to measure myeloarchitecture of the murine cerebral cortex in vivo and ex vivo by intrinsic third-harmonic generation. Front Neuroanat 13:65. https://doi.org/10.3389/fnana.2019.00065
Rishøj L, Hernández IC, Ramachandran S, Jowett N (2022) Multiphoton microscopy for label-free multicolor imaging of peripheral nerve. J Biomed Opt 27(5):056501. https://doi.org/10.1117/1.JBO.27.5.056501
Rix J, Uckermann O, Kirsche K, Schackert G, Koch E, Kirsch M, Galli R (2022) Correlation of biomechanics and cancer cell phenotype by combined Brillouin and Raman spectroscopy of U87-MG glioblastoma cells. J R Soc Interface 19(192):20220209. https://doi.org/10.1098/rsif.2022.0209
Rodríguez Murúa S, Farez MF, Quintana FJ (2022) The immune response in multiple sclerosis. Annu Rev Pathol 17:121–139. https://doi.org/10.1146/annurev-pathol-052920-040318
Rowlands CJ, Bruns OT, Franke D, Fukamura D, Jain RK, Bawendi MG, So PTC (2019) Increasing the penetration depth of temporal focusing multiphoton microscopy for neurobiological applications. J Phys D 52(26):264001. https://doi.org/10.1088/1361-6463/ab16b4
Ryu S, Martino N, Kwok SJJ, Bernstein L, Yun S-H (2021) Label-free histological imaging of tissues using Brillouin light scattering contrast. Biomed Opt Express 12(3):1437–1448. https://doi.org/10.1364/BOE.414474
Saar BG, Johnston RS, Freudiger CW, Xie XS, Seibel EJ (2011) Coherent Raman scanning fiber endoscopy. Opt Lett 36(13):2396–2398. https://doi.org/10.1364/OL.36.002396
Sagar MAK, Cheng KP, Ouellette JN, Williams JC, Watters JJ, Eliceiri KW (2020) Machine learning methods for fluorescence lifetime imaging (FLIM) based label-free detection of microglia. Front Neurosci 14:931. https://doi.org/10.3389/fnins.2020.00931
Saliani A, Perraud B, Duval T, Stikov N, Rossignol S, Cohen-Adad J (2017) Axon and myelin morphology in animal and human spinal cord. Front Neuroanat 11:129. https://doi.org/10.3389/fnana.2017.00129
Sanderson J (2023) Multi-photon microscopy. Curr Protoc 3(1):e634. https://doi.org/10.1002/cpz1.634
Saxena T, Deng B, Stelzner D, Hasenwinkel J, Chaiken J (2011) Raman spectroscopic investigation of spinal cord injury in a rat model. J Biomed Opt 16(2):027003. https://doi.org/10.1117/1.3549700
Saytashev I, Arkhipov SN, Winkler N, Zuraski K, Lozovoy VV, Dantus M (2012) Pulse duration and energy dependence of photodamage and lethality induced by femtosecond near infrared laser pulses in Drosophila melanogaster. J Photochem Photobiol B 115:42–50. https://doi.org/10.1016/j.jphotobiol.2012.06.009
Schlüßler R, Möllmert S, Abuhattum S, Cojoc G, Müller P, Kim K, Möckel C, Zimmermann C, Czarske J, Guck J (2018) Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by Brillouin imaging. Biophys J 115(5):911–923. https://doi.org/10.1016/j.bpj.2018.07.027
Shi Y, Zhang D, Huff TB, Wang X, Shi R, Xu X-M, Cheng J-X (2011) Longitudinal in vivo coherent anti-Stokes Raman scattering imaging of demyelination and remyelination in injured spinal cord. J Biomed Opt 16(10):106012. https://doi.org/10.1117/1.3641988
Shu C, Zheng W, Wang Z, Yu C, Huang Z (2021) Development and characterization of a disposable submillimeter fiber optic Raman needle probe for enhancing real-time in vivo deep tissue and biofluids Raman measurements. Opt Lett 46(20):5197–5200. https://doi.org/10.1364/OL.438713
Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci Off J Soc Neurosci 33(34):13882–13887. https://doi.org/10.1523/JNEUROSCI.2524-13.2013
Su E, Bell M (2016) Diffuse Axonal Injury. In: Laskowitz D, Grant G (eds) Translational research in traumatic brain injury. CRC Press/Taylor and Francis Group http://www.ncbi.nlm.nih.gov/books/NBK326722/
Suminaite D, Lyons DA, Livesey MR (2019) Myelinated axon physiology and regulation of neural circuit function. Glia 67(11):2050–2062. https://doi.org/10.1002/glia.23665
Surmacki JM, Ansel-Bollepalli L, Pischiutta F, Zanier ER, Ercole A, Bohndiek SE (2016) Label-free monitoring of tissue biochemistry following traumatic brain injury using Raman spectroscopy. Analyst 142(1):132–139. https://doi.org/10.1039/c6an02238c
Talari ACS, Movasaghi Z, Rehman S, Rehman I, ur. (2015) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 50(1):46–111. https://doi.org/10.1080/05704928.2014.923902
Tamosaityte S, Galli R, Uckermann O, Sitoci-Ficici KH, Koch M, Later R, Schackert G, Koch E, Steiner G, Kirsch M (2016) Inflammation-related alterations of lipids after spinal cord injury revealed by Raman spectroscopy. J Biomed Opt 21(6):61008. https://doi.org/10.1117/1.JBO.21.6.061008
Tamosaityte S, Galli R, Uckermann O, Sitoci-Ficici KH, Later R, Beiermeister R, Doberenz F, Gelinsky M, Leipnitz E, Schackert G, Koch E, Sablinskas V, Steiner G, Kirsch M (2015) Biochemical monitoring of spinal cord injury by FT-IR spectroscopy—effects of therapeutic alginate implant in rat models. PLoS One 10(11):e0142660. https://doi.org/10.1371/journal.pone.0142660
Tay L-L, Tremblay RG, Hulse J, Zurakowski B, Thompson M, Bani-Yaghoub M (2011) Detection of acute brain injury by Raman spectral signature. Analyst 136(8):1620–1626. https://doi.org/10.1039/c0an00897d
Tian F, Yang W, Mordes DA, Wang J-Y, Salameh JS, Mok J, Chew J, Sharma A, Leno-Duran E, Suzuki-Uematsu S, Suzuki N, Han SS, Lu F-K, Ji M, Zhang R, Liu Y, Strominger J, Shneider NA, Petrucelli L et al (2016) Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging. Nat Commun 7:13283. https://doi.org/10.1038/ncomms13283
Tinevez J-Y, Dragavon J, Baba-Aissa L, Roux P, Perret E, Canivet A, Galy V, Shorte S (2012) A quantitative method for measuring phototoxicity of a live cell imaging microscope. Methods Enzymol 506:291–309. https://doi.org/10.1016/B978-0-12-391856-7.00039-1
Uckermann O, Galli R, Anger M, Herold-Mende C, Koch E, Schackert G, Steiner G, Kirsch M (2014) Label-free identification of the glioma stem-like cell fraction using Fourier-transform infrared spectroscopy. Int J Radiat Biol 90(8):710–717. https://doi.org/10.3109/09553002.2014.899447
Uckermann O, Galli R, Beiermeister R, Sitoci-Ficici K-H, Later R, Leipnitz E, Neuwirth A, Chavakis T, Koch E, Schackert G, Steiner G, Kirsch M (2015) Endogenous two-photon excited fluorescence provides label-free visualization of the inflammatory response in the rodent spinal cord. Biomed Res Int 2015:859084. https://doi.org/10.1155/2015/859084
Vela FJ, Martínez-Chacón G, Ballestín A, Campos JL, Sánchez-Margallo FM, Abellán E (2020) Animal models used to study direct peripheral nerve repair: a systematic review. Neural Regen Res 15(3):491–502. https://doi.org/10.4103/1673-5374.266068
Wang F, Yang T, Li J, Zhou X, Liu L (2019a) Histopathology mapping of biochemical changes in diffuse axonal injury by FTIR micro-spectroscopy. Leg Med (Tokyo, Japan) 37:76–82. https://doi.org/10.1016/j.legalmed.2019.02.001
Wang H, Fu Y, Zickmund P, Shi R, Cheng J-X (2005) Coherent anti-Stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J 89(1):581–591. https://doi.org/10.1529/biophysj.105.061911
Wang J, Pearse DD (2015) Therapeutic hypothermia in spinal cord injury: the status of its use and open questions. Int J Mol Sci 16(8):16848–16879. https://doi.org/10.3390/ijms160816848
Wang ML, Rivlin M, Graham JG, Beredjiklian PK (2019b) Peripheral nerve injury, scarring, and recovery. Connect Tissue Res 60(1):3–9. https://doi.org/10.1080/03008207.2018.1489381
Weigelin B, Bakker G-J, Friedl P (2016) Third harmonic generation microscopy of cells and tissue organization. J Cell Sci 129(2):245–255. https://doi.org/10.1242/jcs.152272
Wilson TJ, Toland A, Cayrol R, Vogel H (2022) Initial experience with label-free stimulated Raman scattering microscopy for intraoperative assessment of peripheral nerves. Clin Neurol Neurosurg 214:107180. https://doi.org/10.1016/j.clineuro.2022.107180
Wu W, He S, Wu J, Chen C, Li X, Liu K, Qu JY (2022) Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window. Nat Commun 13(1):1959. https://doi.org/10.1038/s41467-022-29496-x
Xiao Y, Deng P, Zhao Y, Yang S, Li B (2023) Three-photon excited fluorescence imaging in neuroscience: from principles to applications. Front Neurosci 17:1085682. https://doi.org/10.3389/fnins.2023.1085682
Yang T, He G, Zhang X, Chang L, Zhang H, Ripple MG, Fowler DR, Li L (2014) Preliminary study on diffuse axonal injury by Fourier transform infrared spectroscopy histopathology imaging. J Forensic Sci 59(1):231–235. https://doi.org/10.1111/1556-4029.12290
You S, Tu H, Chaney EJ, Sun Y, Zhao Y, Bower AJ, Liu Y-Z, Marjanovic M, Sinha S, Pu Y, Boppart SA (2018) Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat Commun 9(1):2125. https://doi.org/10.1038/s41467-018-04470-8
Zaimi A, Duval T, Gasecka A, Côté D, Stikov N, Cohen-Adad J (2016) AxonSeg: open source software for axon and myelin segmentation and morphometric analysis. Front Neuroinformatics 10:37. https://doi.org/10.3389/fninf.2016.00037
Zhang F, Liang Z, Song D, Wang Z, Wang K, Wang S (2022) Raman spectroscopic investigation on the biomedical evolution of spinal cord injury and the therapeutic outcomes of its low-level laser therapy. Vib Spectrosc 118:103337. https://doi.org/10.1016/j.vibspec.2022.103337
Zhang J, Huang P, Wang Z, Dong H (2017) Application of FTIR spectroscopy for traumatic axonal injury: a possible tool for estimating injury interval. Biosci Rep 37(4). https://doi.org/10.1042/BSR20170720
Zhang J, Liu L, Mu J, Yang T, Zheng N, Dong H (2015a) Chemical analysis in the corpus callosum following traumatic axonal injury using Fourier transform infrared microspectroscopy: a pilot study. J Forensic Sci 60(6):1488–1494. https://doi.org/10.1111/1556-4029.12871
Zhang J, Niu F, Dong H, Liu L, Li J, Li S (2015b) Characterization of protein alterations in damaged axons in the brainstem following traumatic brain injury using Fourier transform infrared microspectroscopy: a preliminary study. J Forensic Sci 60(3):759–763. https://doi.org/10.1111/1556-4029.12743
Zheng B, Lorenzana AO, Ma L (2019) Understanding the axonal response to injury by in vivo imaging in the mouse spinal cord: a tale of two branches. Exp Neurol 318:277–285. https://doi.org/10.1016/j.expneurol.2019.04.008
Zhou X, He X, Ren Y (2014) Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res 9(20):1787–1795. https://doi.org/10.4103/1673-5374.143423
Zipfel WR, Williams RM, Webb WW (2003) Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21(11):1369–1377. https://doi.org/10.1038/nbt899
Zirak P, Matz G, Messerschmidt B, Meyer T, Schmitt M, Popp J, Uckermann O, Galli R, Kirsch M, Winterhalder MJ, Zumbusch A (2018) Invited article: a rigid coherent anti-Stokes Raman scattering endoscope with high resolution and a large field of view. APL Photonics 3(9):092409. https://doi.org/10.1063/1.5027182
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
RG and OU performed the literature search and wrote the review.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galli, R., Uckermann, O. Vibrational spectroscopy and multiphoton microscopy for label-free visualization of nervous system degeneration and regeneration. Biophys Rev 16, 219–235 (2024). https://doi.org/10.1007/s12551-023-01158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-023-01158-2