Abstract
Anderson-Fabry disease is an X-linked lysosomal storage disorder caused by mutations in the GLA gene that result in deficiency of the enzyme alpha-galactosidase A. The worldwide incidence of Fabry’s disease is reported to be in the range of 1 in 40,000–117,000, although this value may be a significant underestimate given under recognition of symptoms and delayed or missed diagnosis. Deficiency in alpha-galactosidase A causes an accumulation of neutral glycosphingolipids such as globotriaosylceramide (Gb3) in lysosomes within various tissues including the vascular endothelium, kidneys, heart, eyes, skin and nervous system. Gb3 accumulation induces pathology via the release of pro-inflammatory cytokines, growth-promoting factors and by oxidative stress, resulting in myocardial extracellular matrix remodelling, left ventricular hypertrophy (LVH), vascular dysfunction and interstitial fibrosis. Cardiac involvement manifesting as ventricular hypertrophy, systolic and diastolic dysfunction, valvular abnormalities and conduction tissue disease is common in AFD and is associated with considerable cardiovascular morbidity and mortality from heart failure, sudden cardiac death and stroke-related death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anderson-Fabry disease (AFD) is an X-linked lysosomal storage disorder caused by mutations in the GLA gene that result in deficiency of the enzyme α-galactosidase A. The worldwide incidence of Fabry’s disease is reported to be in the range of 1 in 40,000–117,000, although this value may be a significant underestimate given under recognition of symptoms and delayed or missed diagnosis (Zarate and Hopkin 2008; Mehta et al. 2004). The prevalence in selected patient cohorts is even higher and reported to be between 0.25–3.5% in male haemodialysis patients, 0.9–3.9% in male patients with hypertrophic cardiomyopathy (HCM) and 3–5% in patients with cryptogenic stroke (Linhart and Elliott 2007; Sachdev et al. 2002; Nakao et al. 2003; Kotanko et al. 2004; Kubo et al. 2017; Shi et al. 2014; Doheny et al. 2018; Elliott et al. 2011; Monserrat et al. 2007; Hagege et al. 2011).
Deficiency in α-galactosidase A causes an accumulation of neutral glycosphingolipids such as globotriaosylceramide (Gb3) in lysosomes within various tissues including the vascular endothelium, kidneys, heart, eyes, skin and nervous system (Clarke 2007). Gb3 accumulation induces pathology via the release of pro-inflammatory cytokines, growth-promoting factors and by oxidative stress, resulting in myocardial extracellular matrix remodelling, left ventricular hypertrophy (LVH), vascular dysfunction and interstitial fibrosis (Putko et al. 2015; Linhart 2006; Moon et al. 2003). Lysosomal storage deposits also reduce the activity of respiratory chain enzymes I, IV and V and reduce cellular levels of energy-rich phosphates (Lucke et al. 2004).
Cardiac involvement manifesting as ventricular hypertrophy, systolic and diastolic dysfunction, valvular abnormalities and conduction tissue disease is common in AFD and is associated with considerable cardiovascular morbidity and mortality from heart failure, sudden cardiac death and stroke-related death (O'Mahony and Elliott 2010; Patel et al. 2015).
Genetics
The GLA gene, located at Xq22.1, comprises seven exons over 12 Kb and encodes a 101 kD homodimeric glycoprotein (Linhart and Elliott 2007; Kornreich et al. 1989). In excess of 700 predominantly missense (60%), mutations in GLA have been identified (Schaefer et al. 2005; Shabbeer et al. 2006). Deletion of several exons or even the entire gene is uncommon and may result in a negative genetic test particularly in heterozygote females unless multiplex ligation-dependent probe amplification or copy number variation is analysed (Schirinzi et al. 2008). Most mutations in GLA affect protein-folding by disrupting the hydrophobic core of the GLA protein or by altering active binding sites and also affect enzyme localisation to the lysosome and result in reduced enzyme activity (Linhart and Elliott 2007; Garman 2007).
Many of the pathogenic mutations in GLA are ‘private’, and this limits the ability to undertake detailed genotype-phenotype analysis. In women, random X-chromosome inactivation (lyonization) means that α-galactosidase levels in plasma and white cells can fall within the normal range but disease expression still occurs, albeit later and generally less severe than in male hemizygotes (Dobrovolny et al. 2005). Severe disease in women may occur when there is skewed inactivation of X chromosomes in favour of the mutant allele (O'Mahony and Elliott 2010; The Human Gene Mutation Database 2018; Ries and Gal 2006; Echevarria et al. 2016). Inter- and intra-familial variation in phenotype may be modulated by other genetic modifiers, environmental factors and epigenetics although more research is being undertaken in this field (Ries and Gal 2006; Altarescu et al. 2005; Hassan et al. 2017; Cammarata et al. 2015; Rigoldi et al. 2014; Teitcher et al. 2008).
Some mutations in the GLA gene are associated with later-onset or ‘non-classical’ disease with a predominant or exclusive cardiac phenotype. These include variants such as N215S or R112H and are often referred to as ‘cardiac variants’ and the later-onset phenotype may be due to residual α-galactosidase activity (von Scheidt et al. 1991; Eng et al. 1993; Spada et al. 2006; Frustaci et al. 2001). Affected patients with ‘classical’ disease have a significantly higher rate of adverse events compared to those with ‘non-classical’ disease, and males with ‘classical’ disease tend to have a higher left ventricular mass and lower renal function than those with ‘non-classical’ forms of disease (Arends et al. 2017a). The non-classical N215S mutation is one of the most prevalent AFD mutations in Europe (Muntze et al. 2018). The later symptom onset, delayed development of LVH and the relative paucity of extra-cardiac manifestations means the cardiac phenotype often mimics non-obstructive HCM, which results in a significant delay in diagnosis in probands and delay in commencement of Fabry-specific therapy (Lavalle et al. 2018; Germain et al. 2018).
Cardiac disease in AFD
Histopathology
Histological analysis of hearts affected by AFD reveals accumulation of glycosphingolipids within cardiomyocytes, the cardiac conduction system (Fig. 1a) and the coronary vasculature (Fig. 1b). Glycosphingolipid accumulation also contributes to valve leaflet thickening (Fig. 1c, d) (Wu et al. 2010). There is marked vacuolization within the cytoplasm of cardiomyocytes (Fig. 1e), and electron microscopy demonstrates dense lamellar deposits of Gb3 within lysosomes. Myocardial disarray can be observed but is much less prominent than in patients with familial HCM (Putko et al. 2015; Sheppard 2011). Interstitial fibrosis may be evident with a subepicardial or mid-wall pattern of fibrosis that becomes more extensive in the posterolateral basal portion of the left ventricle (Fig. 1f) (Moon et al. 2003). Uncommonly, myocardial scarring is associated with aneurysm formation (Moon et al. 2003; Poulin et al. 2015).
(Histological features of Anderson-Fabry disease). a Masson trichrome stain demonstrating vacuolization within myocytes with extension into the right bundle branch of the conduction pathway. b Section through the left circumflex coronary artery showing diffuse wall thickening without significant luminal occlusion. c Section through the mitral valve apparatus demonstrating mild mitral valve leaflet thickening and ballooning of the anterior and posterior valve leaflets. d H&E staining demonstrating hyaline pink material within the spongiosa and fibrosa. e H&E staining of the ventricular myocardium with evidence of myocyte hypertrophy and marked vacuolization of the cytoplasm of myocytes. f Transverse section of the heart. There is concentric left ventricular hypertrophy associated with thinning of the posterolateral wall (blue arrow). Mild right ventricular hypertrophy is also present and prominent in the posterobasal area of the RV. Fig. 1a–f: Reprinted from Cardiovasc Pathol.; 19(5), Sheppard MN, Cane P, Florio R, Kavantzas N, Close L, Shah J, Lee P, Elliott P; “A detailed pathologic examination of heart tissue from three older patients with Anderson-Fabry disease on enzyme replacement therapy.”; pages 293–301; 2010, reproduced with permission from Elsevier (Sheppard et al. 2010)
Clinical presentation
The classical variant of AFD usually affects men with very low or absent levels of serum α-galactosidase A. Patients develop extra-cardiac signs and symptoms such as angiokeratoma, gastrointestinal disturbance, hypohidrosis and peripheral neuropathy in the first decade of life and renal involvement with proteinuria and progression to end-stage renal failure are often established by the fourth decade of life (Mehta et al. 2004; Linhart and Elliott 2007; O'Mahony and Elliott 2010; Kubo 2017). The cardiac phenotype typically develops between the 3rd and 5th decade of life (Putko et al. 2015; Havndrup et al. 2010).
In heterozygous females, cardiac and extra-cardiac manifestations are generally milder and develop later in life than in hemizygous males (Kubo 2017). Women tend to have slower disease progression and a longer median cumulative survival than affected males (Mehta et al. 2004; Linhart and Elliott 2007).
As many of 10% of patients have a cardiac event as their index presenting symptom, and more than 60% of patients experience cardiovascular symptoms such as dyspnoea, angina, palpitations, syncope and peripheral oedema (Mehta et al. 2004; Clarke 2007; Wu et al. 2010; Eng et al. 2007). Dyspnoea is associated with left ventricular hypertrophy and is caused by left ventricular outflow tract obstruction, diastolic dysfunction and systolic dysfunction. Less commonly, patients with advanced disease develop symptomatic aortic or mitral valve disease. In the Fabry outcome survey, exertional dyspnoea occurred in 23% of affected males and females and as many as 10% developed advanced heart failure symptoms (Putko et al. 2015) (Patel et al. 2015; Linhart et al. 2007).
Cardiac disease is the major cause of death in men and women with AFD, accounting for 38% of all-cause mortality (Mehta et al. 2009a). The prevalence of cardiovascular death is reported to be 3% (annual incidence of 0.52 per 100 person-years) in a large observational study of patients over a mean follow-up period of 7.1 years in which 2.4% of the overall cohort had a sudden cardiac death and 0.97% suffered a heart failure-related mortality (Patel et al. 2015).
Left ventricular hypertrophy and LV outflow tract obstruction
Left ventricular hypertrophy (LVH) is the commonest structural cardiac abnormality observed (Linhart et al. 2007). Forty percent of AFD patients have LVH at diagnosis but this proportion rises to 77% in those over the age of 75 (Linhart et al. 2000; Lidove et al. 2016). In heterozygous females, plasma α-galactosidase levels do not correlate with the severity of LVH. LVH is caused by intra-cellular accumulation of Gb3 as well as by the release of hypertrophy-inducing growth factors and extracellular matrix remodelling. This is supported by experimental studies demonstrating that plasma from affected patients induces rat vascular smooth muscle cell and neonatal mouse cardiomyocyte proliferation in culture when compared to cell culture using plasma from normal controls or from hypertensive-control populations (Barbey et al. 2006a). Another study identified the presence of a plasma proliferative factor, sphingosine-1 phosphate (S1P), and demonstrated that plasma levels of S1P correlated with left ventricular mass index and common carotid artery intima-media thickness in patients with AFD (Brakch et al. 2010). S1P-treated mice also developed cardiovascular remodelling with cardiac hypertrophy similar to that seen in affected AFD patients (Brakch et al. 2010).
ECG abnormalities indicative of LVH include large voltage QRS complexes, and repolarisation abnormalities which are present in 61% of men and 18% of women over the age of 30 (Linhart et al. 2000). ECG changes may predate abnormal LV morphology on cardiac imaging (Linhart et al. 2000).
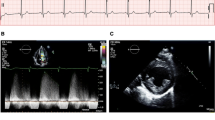
The prevalence of LVH increases with age in patients treated and untreated with enzyme replacement therapy (ERT). Concentric LV remodelling precedes overt left ventricular hypertrophy, but when hypertrophy is established, it can be clinically indistinguishable from other causes of left ventricular hypertrophy including hypertrophic cardiomyopathy (HCM) (Putko et al. 2015; Linhart et al. 2000). Other causes for concentric LV remodelling and hypertrophy include hypertension, aortic valve disease or cardiac amyloidosis. The LVH pattern in AFD can be asymmetric, eccentric, distal or concentric although a concentric pattern of hypertrophy is the most prevalent (Fig. 2a–e) (Wu et al. 2010; Kampmann et al. 2008). 2.2% of patients develop asymmetric septal hypertrophy with a septal to posterior wall dimension ratio > = 1.5 (Wu et al. 2010). Right ventricular hypertrophy (RVH) also develops in as many as 25% of patients with a similar prevalence in men and women (Wu et al. 2010; Palecek et al. 2008).
(Echocardiogram features of Anderson-Fabry disease). a Transthoracic echocardiogram (TTE) parasternal long-axis view (PLAX) demonstrating concentric LVH with an interventricular septum (IVS) and left ventricular posterior wall (LVPW) diameter of 16 mm. b Apical 4 chamber view on TTE demonstrating the presence of concentric LVH. c Moderately dilated left atrium (31 cm2) on apical 4-chamber TTE view. d TTE parasternal short-axis view (PSAX) at mid-ventricular level demonstrating concentric LVH and hypertrophied papillary muscles. e TTE subcostal view demonstrating concentric LVH. f Pulsed wave Doppler across mitral valve inflow demonstrating impaired relaxation (grade 1 diastolic dysfunction) with E:A ratio of 0.87
There is an inverse relationship between left ventricular mass and estimated glomerular filtration rate (eGFR) and a positive correlation between LV mass and the common carotid intima-medial thickness (Linhart et al. 2007; Barbey et al. 2006a). LVH is associated with an increased frequency of dysrhythmia. The presence of increased cardiovascular mass at baseline evaluation is associated with an increased event rate over follow-up (Wu et al. 2010; Arends et al. 2017b). In a multivariate model of patients naïve to ERT, the presence of LVH significantly increased the odds of cardiovascular events by a factor of 4.8 in males (odds ratio 4.8; 95% CI 1.0–22.2; p = 0.046) and 8.2 (odds ratio 8.2; 95% CI 2.6–26.0; p < 0.001) in females (Patel et al. 2011).
Left ventricular volume, LV dimension, stroke volume and LV ejection fraction (LVEF) are within the normal range in most patients, but left ventricular and papillary muscle hypertrophy can cause a reduction in LV cavity size as well as contribute to the development of left ventricular outflow tract obstruction (LVOTO) or mid-cavity obstruction (MCO) which exacerbate symptoms of chest pain, dyspnoea and syncope (Sachdev et al. 2002; Linhart et al. 2000). Resting LVOTO is rare but may be provocable in approximately 50% of patients with LVH (Calcagnino et al. 2011).
Exercise physiology
Exercise testing in affected patients demonstrates an increase in stroke volume on exercise, albeit less than predicted from normal population data (Lobo et al. 2008). In those with a more advanced cardiac phenotype (impaired diastolic function and higher LV mass), there is a reduction in end-diastolic volume and a lack of reduction in end-systolic volume on exercise resulting in evidence of abnormal stroke volume augmentation (Spinelli et al. 2008). There is also evidence that in a significant proportion of patients (46%), there is a diastolic blood pressure drop on exercise (Bierer et al. 2005). Use of ERT results in a mild improvement in anaerobic threshold value, but only over the first year of therapy (Lobo et al. 2008; Bierer et al. 2006).
Myocardial tissue characterisation with magnetic resonance imaging
Cardiac MRI with gadolinium contrast administration often identifies characteristic subepicardial basal to mid-inferolateral late gadolinium enhancement (Fig. 3a), which in the setting of advanced disease with a ‘burnt out’ phenotype of LV systolic dysfunction may be associated with hypokinesia or thinning of the basal posterior wall (Takenaka et al. 2008). Glycosphingolipid intra-myocardial accumulation can shorten the non-contrast T1 mapping parameter on cardiac magnetic resonance imaging. Septal T1 values are generally low in AFD and increased in other diseases causing LVH such as hypertension, HCM, aortic stenosis and AL cardiac amyloidosis except in regions of extensive myocardial replacement fibrosis, and consequently, it can be a useful imaging parameter in patients with unexplained LVH undergoing cardiac MRI (Sado et al. 2013; Linhart and Cecchi 2018). T1 values correlate inversely with maximal LV wall thickness in AFD (Sado et al. 2013).
(Complications of Anderson-Fabry disease). a Mid-LV short-axis view on cardiac MRI (CMRI) showing concentric LVH with dense subepicardial and mid-wall LGE in the inferolateral wall. b Apical left ventricular aneurysm (blue arrow) demonstrated on a 4-chamber view on CMRI. c TTE apical 4-chamber view demonstrating severe eccentric (posteriorly) directed jet of MR due to mitral valve thickening and dysfunction. d 12-Lead electrocardiogram of a patient with conduction disease—borderline RBBB (QRS duration 120 ms) and right axis deviation. There is also presence of large voltage QRS complexes (prominent R waves V4-6) and repolarisation abnormalities with T wave inversion inferolaterally (leads II, III, aVF and V4-6) meeting ECG criteria for LVH. e Chest X-ray demonstrating a dual chamber secondary prevention implantable cardioverter-defibrillator (ICD) in an AFD patient presenting with an out-of-hospital cardiac arrest. f CT coronary angiogram of an AFD patient symptomatic with chest pain, demonstrating a non-calcified proximal LAD plaque lesion (blue arrow) associated with severe stenosis (70–99% diameter reduction)
Recently, high-sensitivity troponin (hs-TnT) has been shown to positively correlate with late gadolinium enhancement, and elevated hs-TnT levels are associated with a reduction in LVEF over follow-up suggesting that it may be a useful biomarker for progression in Fabry’s cardiomyopathy (Seydelmann et al. 2016).
Diastolic dysfunction and restrictive physiology
Imaging modalities including pulsed wave Doppler and tissue Doppler imaging may be used in the echocardiographic assessment of diastolic function in AFD patients although more sensitive markers include global diastolic strain rate analysis. Diastolic dysfunction is common and results in impaired ventricular filling due to increased ventricular stiffness and impaired relaxation (Fig. 2f). Diastolic dysfunction is probably the commonest cause of exertional dyspnoea and reduced exercise tolerance in patients and may be present before the development of overt LVH (Pieroni et al. 2003). In general, diastolic dysfunction progresses in parallel with ventricular hypertrophy, but rarely, patients can develop severe diastolic dysfunction with a restrictive filling pattern or rising pulmonary pressures that is associated with a poor prognosis. NT-proBNP levels demonstrate a significant positive correlation with LV diastolic dysfunction and are a sensitive marker in detecting early cardiac changes (Torralba-Cabeza et al. 2011).
Diastolic dysfunction contributes to left atrial enlargement which in turn predisposes to atrial arrhythmias including atrial fibrillation (Fig. 2c) (Wu et al. 2010; Weidemann et al. 2003). Left atrial enlargement, increased left atrial stiffness index and reduced atrial compliance also occurs, even in the absence of LVH suggesting an impact on atrial myocardial function and properties early in the disease phase (Boyd et al. 2013). Left atrial volume is usually less dilated than in patients with HCM but speckle tracking demonstrates an atrial cardiomyopathy in Fabry affecting the three phasic functions of the left atrium (Saccheri et al. 2018). This impact on atrial size and function may be due to raised LV filling pressures passively transmitted to the left atrium or due to glycosphingolipid deposition in the left atrial myocardium associated with interstitial fibrosis (Chimenti et al. 2010). Over a third of patients on ERT have evidence of left atrial enlargement (Wu et al. 2010).
Systolic dysfunction
Left ventricular systolic dysfunction is a less common complication of Fabry’s cardiomyopathy. The prevalence of LV systolic dysfunction amongst patients with AFD is 6–8% which is associated with an increased overall incidence of heart failure-related mortality (Wu et al. 2010; Rosmini et al. 2017). Left ventricular systolic dysfunction may develop as the disease progresses, in particular in ERT-untreated patients, and may occur in part due to progressive LV myocardial fibrosis (Shah et al. 2005a). In a study of patients on ERT, only a small proportion of patients, all males (6.5%), developed LV systolic dysfunction and a couple of individuals also developed LV dilatation, including one with a LV aneurysm (Fig. 3b) (Wu et al. 2010).
Tissue Doppler imaging (TDI) helps to identify early subclinical cardiac involvement before the onset of left ventricular hypertrophy or systolic and diastolic impairment (Pieroni et al. 2003; Pieroni et al. 2004). Strain and strain rate analysis are also useful in identifying patients with reduced myocardial function at an early phase (Shanks et al. 2013; Gruner et al. 2012).
LV basal posterior wall thinning with progressive fibrosis is significantly associated with progression to advanced heart failure and cardiac death (Kawano et al. 2007; Kampmann et al. 2002).
Valvular heart disease
Gb3 accumulation occurs on both left- and right-sided valves although clinically relevant disease is mostly confined to the aortic and mitral valves possibly due to the greater haemodynamic stress (Putko et al. 2015; Linhart 2006). Individuals can develop valvular stenosis, regurgitation or prolapse (Desnick et al. 1976). Mild thickening of the left-sided valves is seen in as many as a quarter of patients (Sheppard 2011). Mitral valve dysfunction may be accentuated by papillary muscle hypertrophy and by systolic anterior motion of the mitral valve leaflet resulting in altered coaptation and an eccentric jet of mitral regurgitation (Fig. 3c). There is no association between the presence of valvular abnormalities and α-galactosidase levels. (Wu et al. 2010).
Although the presence of valvular disease is common, few patients develop valvular regurgitation or stenosis sufficient to warrant surgical intervention with valve repair or replacement (Wu et al. 2010; Choi et al. 2009; Fernandez et al. 2012). There are reports of successful valvular intervention including using newer transcatheter aortic valve replacements (TAVR) in patients with Fabry’s disease (Giustino et al. 2014).
Conduction disease and arrhythmias
The ECG in AFD is often abnormal. Apart from QRS voltage changes, up to 40% of affected male patients have a short PR interval typically without the presence of a concomitant delta wave suggesting accelerated AV nodal connection (Pochis et al. 1994; Jastrzebski et al. 2006). With increasing age, patients may develop conduction disease with a progressively prolonged PR interval and a broadened QRS duration. A prolonged QRS duration (> 120 ms) as well as abnormal QRS axis is seen in 9% of patients (Fig. 3d) (O’Mahony et al. 2011).
AFD is associated with atrial and ventricular arrhythmias (Frustaci and Chimenti 2007). Resting bradycardia and an impaired heart rate response on exercise are very frequent (Lobo et al. 2008). Bradyarrhythmias resulting from sinus node disease and atrioventricular block may necessitate permanent pacemaker implantation, and the rates of anti-bradycardia pacing are more than 25 times greater than that in the general population (O’Mahony et al. 2011). PR interval duration and QRS duration are independent predictors of the need for pacemaker implantation (O’Mahony et al. 2011). In those with an intra-cardiac device in situ, there is a high rate of atrial or ventricular pacing (Acharya et al. 2012). The mechanism for premature conduction disease may be related to autonomic dysfunction as well as degeneration of the cardiac conduction system with glycosphingolipid accumulation, apoptosis and vacuolation (Frustaci and Chimenti 2007; Ikari et al. 1992; Mehta et al. 2010).
Affected individuals are prone to tachyarrhythmias including atrial fibrillation (AF) due to left atrial enlargement and diastolic dysfunction, non-sustained ventricular tachycardia (NSVT) or sustained malignant ventricular arrhythmia such as ventricular tachycardia (VT) or ventricular fibrillation (VF). The frequency of AF or NSVT in studies vary and reflect differences in cohort characteristics and the method of arrhythmia monitoring. In a study of 60 Fabry patients undergoing 24-h Holter analysis, 3.9% had persistent AF and 13.3% had paroxysmal AF. Age was an independent predictor for AF (odds ratio 1.2, 95% CI 1.1–1.3, p = 0.001) (Shah et al. 2005b). Glycosphingolipid deposition in the atrial myocardium, atrial dilatation due to diastolic dysfunction and elevated LV filling pressure increase the risk of AF (Linhart et al. 2007). AF may contribute to the increased risk of stroke in addition to AFD-related small vessel cerebrovascular disease.
NSVT occurs mostly in males with moderate to severe hypertrophy (Shah et al. 2005b). The clinical significance of NSVT is yet to be determined, but it is an established risk factor for sudden cardiac death (SCD) in other hypertrophic disorders. In one study, sudden cardiac death events only occurred in patients with prior documentation of non-sustained ventricular tachycardia and with late gadolinium enhancement on cardiac MRI (Weidemann et al. 2013). When implantable loop recorder (ILR) implants are used to assess for arrhythmias instead of conventional 24-h Holter, over 30% of patients have VT episodes (NSVT or sustained VT) and a similar percentage were identified to have short runs of paroxysmal AF lasting more than 3 min (Weidemann et al. 2016). Other series have identified ventricular arrhythmias in 14% of affected males and 20% in affected females (Pinderski and Strotmann 2006).
Cases of sudden cardiac death (SCD) are attributed to malignant bradyarrhythmias or tachyarrhythmia but are rarely recorded (Frustaci and Chimenti 2007; Eckart et al. 2000; Carter et al. 1995; Sivaloganathan 1992). The incidence of SCD is difficult to accurately assess due to small patient numbers and the rarity of the condition. Single-centre experience reports an annual incidence of SCD between 0.3–1.4% (Patel et al. 2015; Shah et al. 2005b; Baig et al. 2017). In patients in whom there is felt to be a sufficiently elevated risk of sudden cardiac death, an implantable cardioverter-defibrillator may be implanted to protect against malignant ventricular arrhythmias (Fig. 3e).
A recent systematic review identified that SCD is a major cause for cardiovascular mortality in AFD patients accounting for 62% of reported deaths. In this review, the average prevalence of ventricular tachycardia was 15.3% and risk factors associated with SCD were age > 40 years, male gender, left ventricular hypertrophy, NSVT and presence of late gadolinium enhancement on CMRI (Baig et al. 2017).
Vascular disease
Glycosphingolipid deposits are found in in the endothelium and media of blood vessels. In smaller coronary vessels, there is also evidence for smooth muscle cell proliferation (Sheppard 2011; Elleder 2003). Globotriaosylceramide metabolites, including de-acylated Gb3 and globotriaosylsphingosine, appear to stimulate vascular smooth muscle cells and inhibit α-galactosidase A activity (Barbey et al. 2006a; Aerts et al. 2008).
The carotid, brachial and aortic intima-media thickness is increased compared to normal controls in the absence of atherosclerotic plaques (Barbey et al. 2006b; Kalliokoski et al. 2006). Brachial artery flow-mediated dilatation is also impaired (Kalliokoski et al. 2006). Aortic dilatation at the sinus of Valsalva is common, affecting 32.7% of males and 5.6% of females and aortic aneurysms are described (Barbey et al. 2010). Aortic root dilatation may contribute to the development of aortic valvular regurgitation due to reduced aortic leaflet coaptation.
Chest pain is a common clinical symptom in patients even in the absence of significant LVH (Chimenti et al. 2008). The prevalence of angina in the Fabry outcome survey was 23% in females and 22% in males (Linhart et al. 2007). Whilst there have been case reports of patients with AFD developing premature coronary disease (Fig. 3F), there has been no definitive evidence of increased coronary atherosclerosis in this setting (Fisher et al. 1992; Chimenti et al. 2007). Most analyses are also limited by the absence of data on age and risk factor-matched control populations. In the Fabry outcome survey, there was a similar prevalence of myocardial infarct in women (1.6%) compared to men (0.8%) at baseline study entry in untreated (naïve to ERT) Fabry patients and overall there were relatively few events of coronary revascularization or myocardial infarction (Linhart et al. 2007). This supports data from another study demonstrating that during the natural history period of Fabry, only 2.7% of male patients and 1.5% of females experienced a myocardial infarct (Patel et al. 2011).
Intravascular ultrasound (IVUS) of the coronary arteries identifies evidence of diffuse coronary plaques which are hypo-echogenic and more likely to have lipid cores compared to control patients (Kovarnik et al. 2008). The latter finding may be accounted for by the higher lipid content in plaques due to glycosphingolipid accumulation in the endothelium of coronary arteries.
In a case report of a Fabry patient undergoing coronary artery bypass grafting (CABG) for multi-vessel coronary arterial disease, the internal mammary artery was found to be occluded at 1 year post-cardiothoracic surgery with recurrence of chest pain. This occlusion of a LIMA graft is unusual as they typically have a 98% patency rate at 1 year. Histological analysis of the LIMA artery showed diffuse glycosphingolipid storage in smooth muscle cells and the presence of fibrous tissue contributing to an abnormal arterial structure (Chimenti et al. 2007). This raised the hypothesis that arterial grafts in AFD patients may be more prone to occlusion through glycosphingolipid exposure, and venous conduits may be preferred. Other cases involving LIMA grafting in Fabry however have shown graft patency at 6 and 19 months post-operatively although histological analysis intra-operatively of the resected portion of the LIMA graft did demonstrate degeneration of the outer portion of the medial smooth muscle layer with intracellular vacuoles and collagen despite a normal endothelium and internal elastic lamina (Fisher et al. 1992; Osada et al. 2016).
Apart from macrovascular coronary arterial disease, AFD patients also have abnormal coronary microvascular function demonstrated by a lower hyperaemic myocardial blood flow on positron emission tomography (PET) imaging. ERT does not improve this parameter (Elliott et al. 2006). In another study of 15 patients, there was evidence of lower myocardial perfusion reserve (Kalliokoski et al. 2005). Microvascular ischaemia is believed to be a major mechanism of cardiac chest pain in the setting of AFD.
Treatment of cardiac disease in AFD
Enzyme replacement therapy
ERT aims to compensate for the reduced α-galactosidase levels and to reduce accumulation of glycosphingolipids in tissues. Two formulations of ERT are licenced, both administered as an intravenous infusion fortnightly, agalsidase-α (Replagal, Shire) and agalsidase-β (Fabrazyme, Sanofi-Genzyme). Agalsidase-α is derived from human fibroblasts whereas agalsidase-β is derived from Chinese hamster ovarian cells. Side effects of ERT include mild infusion reactions although serious side effects can include anaphylaxis. Patients may also develop IgG antibodies which might reduce treatment efficacy, and both formulations have similar antibody cross-reactivity (Lee et al. 2003).
ERT has been shown to reduce the severity of neuropathic pain, improve body weight and stabilise or improve renal creatinine clearance (Schiffmann et al. 2001; Baehner et al. 2003; Beck et al. 2004). From a cardiological perspective, ERT is associated with a small decrease in the QRS duration. Data on the impact of ERT on changes in left ventricular hypertrophy are conflicting with some studies showing a reduction in LV mass and improved myocardial function as assessed by systolic radial strain rate whereas others fail to show significant changes in ventricular wall thickness (Schiffmann et al. 2001; Baehner et al. 2003; Koskenvuo et al. 2008; Breunig et al. 2006). The benefits of ERT on cardiovascular symptoms and complications are less clear, in particular, in patients with established myocardial fibrosis or with extensive hypertrophy or significant valvular disease (Weidemann et al. 2009).
The Fabry outcome survey had reported that treatment with agalsidase-α resulted in stability in left ventricular mass index and mid-wall fractional shortening over a period of 5 years. This study also showed a sustained reduction in LV mass index and a significant increase in mid-wall fractional shortening after 3 years of ERT in those with LV hypertrophy at baseline evaluation (Mehta et al. 2009b). These changes were also associated with a reduction in T2 relaxation times on CMRI in all myocardial regions (Imbriaco et al. 2009).
Agalsidase-β treatment slows progression to a composite clinical outcome of cardiac, renal and cerebrovascular complications or mortality, although this was predominantly driven by reduction in renal complications including deteriorating renal function (Banikazemi et al. 2007). A recent pooled analysis of data in a systematic review suggested agalsidase-β was associated with a significantly reduced incidence of cardiovascular events when compared to treatment-naïve patients (El Dib et al. 2017).
In general, most data suggest that in advanced disease, ERT is unlikely to be transformative and in many cases, the cardiac structural phenotype progresses despite ERT (Mehta et al. 2009a). The presence of late gadolinium enhancement (LGE) or fibrosis on CMRI at baseline, before ERT institution, is associated with a lack of regression of LVH and worsening segmental myocardial function despite subsequent therapy (Weidemann et al. 2009).
Chaperone therapy
More recently, oral chaperone therapy has become available for use in patients. Migalastat is a small molecule chaperone that reversibly binds to the active site of α-galactosidase and thereby stabilises mutant enzyme and promotes α-galactosidase-based catabolism of cellular products. In a study of 57 AFD patients randomised to ERT or migalastat, left ventricular mass index significantly decreased in patients on migalastat treatment (Hughes et al. 2016; Germain et al. 2016). Migalastat has been shown in case reports to reduce left ventricular hypertrophy and decrease LGE and associated cardiac serum biomarkers (TnT and NT-ProBNP) (Muntze et al. 2018). Further studies are required to assess the longer-term impact chaperone therapy has on the AFD cardiac phenotype.
General management of cardiac disease
From a cardiac perspective, the majority of medications are for symptom relief and are not prognostic. Patients should have their cardiovascular risk profile managed with control and optimisation of blood pressure to minimise the risk of progressive left ventricular hypertrophy or nephropathy from suboptimally controlled hypertension.
In a cohort of patients on ERT, 30% of the study cohort was identified to be hypertensive at the time of study enrolment (Wu et al. 2010). Systolic blood pressure was also identified to be higher in AFD patients with LGE compared to those without, and the highest systolic blood pressures were identified to occur in a subgroup of patients with rapidly increasing LGE. This suggests that systolic BP can impact the rate of progression of Fabry’s cardiomyopathy (Kramer et al. 2015). In another analysis of 2869 Fabry registry patients naïve to ERT, multivariate analysis demonstrated both hypertension and LVH as risk factors that were strongly associated with the risk of a major adverse cardiovascular event (myocardial infarct, heart failure or cardiac-related mortality) in either gender with hypertension exhibiting an odds ratio of 4.5 in females (95% CI 1.6–12.3; p = 0.004) and 7.8 in males (95% CI 2.1–28.6; p = 0.002) on outcome (Patel et al. 2011).
Hypercholesterolaemia, another cardiovascular risk factor, is more prevalent in male AFD patients with cardiovascular events (42.9%) than in those without cardiovascular events (20.3%) (Patel et al. 2011). Statins are commonly prescribed in patients to help treat the associated hyperlipidaemia.
In the Fabry Registry, a prior history of smoking was common in both genders in those with cardiovascular events occurring before the commencement of ERT (44.7% of males and 38.6% of females with cardiovascular events) (Patel et al. 2011). Similar to the general population, the presence of cardiovascular risk factors including hypertension, renal dysfunction and smoking are associated with an increased incidence of cardiovascular events (Patel et al. 2011).
There are no large-scale randomised trial data of alternative medical agents in the setting of AFD. Management of LVOTO in the setting of Fabry is similar to that of patients with sarcomeric HCM and in accordance with ESC guidelines (Elliott et al -ESC Task Force 2014). Patients may be given β-blockers or non-dihydropyridine calcium antagonists (verapamil or diltiazem) for their negative inotropic and chronotropic action, and in the case of calcium antagonists, also for their negative lusitropic action. Care must be taken with β-blockers and non-dihydropyridine calcium antagonists which may potentiate bradycardia especially in the presence of conduction disease. Disopyramide may be considered as a second-line therapy in the management of LVOTO. In those with drug-refractory LVOTO, patients may be considered for more invasive therapy including right ventricular pacing with a short AV delay (DDDR pacing), alcohol septal ablation, septal myectomy or mitral valve intervention.
Diastolic dysfunction is treated with thiazide diuretics, mineralocorticoid antagonists (e.g. spironolactone) or loop diuretics (e.g. furosemide) to reduce LV filling pressures and pulmonary pressures. Higher doses of diuretics may also be needed in patients with fluid overload in the context of pulmonary or peripheral oedema especially in the setting of significant left ventricular systolic dysfunction. Coronary disease is treated identically to the general population.
In those with atrial fibrillation (AF), no established disease-specific guidelines are present, but in general, the threshold for anticoagulation is lower even with short paroxysms of AF given the increased risk of thromboembolism in the setting of structural or valvular heart disease (January et al. 2014; Yogasundaram et al. 2017). Antiarrhythmics such as sotalol or amiodarone may be used in Fabry patients although general principles of avoiding bradycardia and care with QTc prolongation are necessary. In patients with recurrent ventricular tachycardia or ICD ‘storms’, there are reported cases of successful combined endocardial and epicardial substrate VT ablation for treatment of ventricular arrhythmia (Higashi et al. 2011).
AFD patients may require permanent pacemaker implantation for anti-bradycardia indications in those with evidence of advanced conduction disease. A primary prevention implantable cardioverter defibrillator (ICD) may also be implanted as a prophylaxis to protect against the risk of sudden cardiac death in those thought to be at higher risk of such an event. Secondary prevention ICDs are implanted in those who have survived an aborted sudden cardiac death episode with successful resuscitation from ventricular dysrhythmia or in those with haemodynamically significant ventricular tachycardia.
In individuals that develop left ventricular systolic dysfunction, conventional heart failure medication and guidelines are followed with use of ACE inhibitors (or angiotensin receptor blockers), β-blockers and spironolactone (Ponikowski et al. 2016). There are only a few case reports on the role of cardiac resynchronization therapy (CRT) in patients with AFD and a ‘burnt out’ phenotype of severe LV systolic dysfunction. Concerns in the setting of AFD include that several studies have demonstrated poor response and outcomes from CRT in patients with greater scar burden and in particular those with extensive scar in the free wall of the LV (Leyva 2010). In those with symptoms of refractory heart failure, despite optimal medical therapy, advanced heart failure strategies such as orthotopic cardiac transplantation may be considered. Fabry’s development in the donor organ is unlikely to occur as the myocardial cells in the donor heart should possess normal levels of α-galactosidase A which should prevent Gb3 accumulation within the donor organ (Cantor et al. 1998).
Potential future therapy
Gene therapy represents a potential curative treatment modality in AFD. As with various genetic disorders, research has been undertaken to assess the potential utilisation of gene therapy by recombinant adeno-associated virus-mediated gene transfer to correct the genetic defect in animal models of disease. Preliminary work has limited results but has suggested elevated α-galactosidase levels and reduced Gb3 deposition in various organs in transfected animals (Choi et al. 2010; Pacienza et al. 2012). The first in-human study of gene therapy via autologous stem cell transplantation using cells transduced via a lentivirus vector containing the ‘wild-type’ allele of GLA is currently underway (ClinicalTrials.gov 2018).
Conclusions
Anderson-Fabry disease is a genetically transmitted condition that affects patients from a young age and substantially impairs their quality and quantity of life. Cardiovascular disease accounts for considerable morbidity in patients with AFD and in affected relatives, and a collaborative multi-disciplinary model of care for affected patients is essential to minimise morbidity and to optimise survival. Timely institution of ERT or chaperone therapy prior to the development of advanced cardiac disease or end-stage irreversible complications is essential to alter the natural course of disease. Conventional cardiovascular risk factor modification, including hypertension, is also important to minimise the risk of LVH progression and to avoid adverse cardiovascular events. In individuals with an established cardiac phenotype, management involves conventional medical therapy and regular monitoring enables pre-emptive intervention with pacemakers and primary prevention ICDs.
References
Acharya D et al (2012) Arrhythmias in Fabry cardiomyopathy. Clin Cardiol 35(12):738–740
Aerts JM et al (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 105(8):2812–2817
Altarescu G, Moore DF, Schiffmann R (2005) Effect of genetic modifiers on cerebral lesions in Fabry disease. Neurology 64(12):2148–2150
Arends M et al (2017a) Characterization of classical and nonclassical Fabry disease: a multicenter study. J Am Soc Nephrol 28(5):1631–1641
Arends M et al (2017b) Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: analysis of prognostic factors. PLoS One 12(8):e0182379
Elliott et al (2014) ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 35(39):2733–2779
Baehner F et al (2003) Enzyme replacement therapy in heterozygous females with Fabry disease: results of a phase IIIB study. J Inherit Metab Dis 26(7):617–627
Baig S et al (2017) Ventricular arrhythmia and sudden cardiac death in Fabry disease: a systematic review of risk factors in clinical practice. Europace
Banikazemi M et al (2007) Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med 146(2):77–86
Barbey F et al (2006a) Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol 26(4):839–844
Barbey F et al (2006b) Increased carotid intima-media thickness in the absence of atherosclerotic plaques in an adult population with Fabry disease. Acta Paediatr Suppl 95(451):63–68
Barbey F et al (2010) Aortic remodelling in Fabry disease. Eur Heart J 31(3):347–353
Beck M et al (2004) Fabry disease: overall effects of agalsidase alfa treatment. Eur J Clin Investig 34(12):838–844
Bierer G et al (2005) Cardiopulmonary exercise testing in Fabry disease. Respiration 72(5):504–511
Bierer G et al (2006) Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease. J Inherit Metab Dis 29(4):572–579
Boyd AC et al (2013) Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J Am Soc Echocardiogr 26(12):1415–1423
Brakch N et al (2010) Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J 31(1):67–76
Breunig F et al (2006) Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int 69(7):1216–1221
Calcagnino M et al (2011) Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J Am Coll Cardiol 58(1):88–89
Cammarata G et al (2015) High variability of Fabry disease manifestations in an extended Italian family. Biomed Res Int 2015:504784
Cantor WJ et al (1998) Cardiac transplantation for Fabry’s disease. Can J Cardiol 14(1):81–84
Carter N, Milroy CM, Shepherd RT (1995) Sudden death in elderly women with Fabry’s disease. Am J Forensic Med Pathol 16(1):21–26
Chimenti C et al (2007) Coronary artery bypass grafting for Fabry’s disease: veins more suitable than arteries? Hum Pathol 38(12):1864–1867
Chimenti C et al (2008) Angina in fabry disease reflects coronary small vessel disease. Circ Heart Fail 1(3):161–169
Chimenti C, Russo MA, Frustaci A (2010) Atrial biopsy evidence of Fabry disease causing lone atrial fibrillation. Heart 96(21):1782–1783
Choi S et al (2009) Fabry disease with aortic regurgitation. Ann Thorac Surg 87(2):625–628
Choi JO et al (2010) Characterization of Fabry mice treated with recombinant adeno-associated virus 2/8-mediated gene transfer. J Biomed Sci 17:26
Clarke JT (2007) Narrative review: Fabry disease. Ann Intern Med 146(6):425–433
ClinicalTrials.gov. Autologous stem cell transplantation of cells engineered to express alpha-galactosidase A in patients with Fabry disease. [Website] 2017 10/08/2017 [cited 2018 6.5.2018]; Available from: https://clinicaltrials.gov/ct2/show/NCT02800070
Desnick RJ et al (1976) Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation 54(5):818–825
Dobrovolny R et al (2005) Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the alpha-galactosidase A gene in the Czech and Slovak population. J Mol Med 83(8):647–654
Doheny D et al (2018) Fabry disease: prevalence of affected males and heterozygotes with pathogenic GLA mutations identified by screening renal, cardiac and stroke clinics, 1995–2017. J Med Genet 55(4):261–268
Echevarria L et al (2016) X-chromosome inactivation in female patients with Fabry disease. Clin Genet 89(1):44–54
Eckart RE et al (2000) Ventricular fibrillation refractory to automatic internal cardiac defibrillator in Fabry’s disease. Review of cardiovascular manifestations. Cardiology 94(3):208–212
El Dib R et al (2017) Enzyme replacement therapy for Anderson-Fabry disease: a complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies. PLoS One 12(3):e0173358
Elleder M (2003) Sequelae of storage in Fabry disease—pathology and comparison with other lysosomal storage diseases. Acta Paediatr Suppl 92(443):46–53 discussion 45
Elliott PM et al (2006) Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 92(3):357–360
Elliott P et al (2011) Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry disease survey. Heart 97(23):1957–1960
Eng CM et al (1993) Nature and frequency of mutations in the alpha-galactosidase A gene that cause Fabry disease. Am J Hum Genet 53(6):1186–1197
Eng CM et al (2007) Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis 30(2):184–192
Fernandez J, Sigurdsson G, Farivar RS (2012) Cardiac surgery in patients with Fabry’s disease: review of literature. J Card Surg 27(4):478–480
Fisher EA et al (1992) Fabry disease: an unusual cause of severe coronary disease in a young man. Ann Intern Med 117(3):221–223
Frustaci A, Chimenti C (2007) Images in cardiovascular medicine. Cryptogenic ventricular arrhythmias and sudden death by Fabry disease: prominent infiltration of cardiac conduction tissue. Circulation 116(12):e350–e351
Frustaci A et al (2001) Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med 345(1):25–32
Garman SC (2007) Structure-function relationships in alpha-galactosidase A. Acta Paediatr 96(455):6–16
Germain DP et al (2016) Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 375(6):545–555
Germain DP et al (2018) Phenotypic characteristics of the p.Asn215Ser (p.N215S) GLA mutation in male and female patients with Fabry disease: a multicenter Fabry Registry study. Mol Genet Genomic Med. https://doi.org/10.1002/mgg3.389
Giustino G, Chieffo A, Montorfano M, Panoulas VF, Bernelli C, Spagnolo P, Latib A, Covello RD, Alfieri O, Colombo A (2014) First case reported of transcatheter aortic valve implantation in a patient affected by Fabry’s disease and severe low-flow low-gradient aortic valve stenosis. Case Reports in Internal Medicine 1(2):71–74
Gruner C et al (2012) Systolic myocardial mechanics in patients with Anderson-Fabry disease with and without left ventricular hypertrophy and in comparison to nonobstructive hypertrophic cardiomyopathy. Echocardiography 29(7):810–817
Hagege AA et al (2011) Screening patients with hypertrophic cardiomyopathy for Fabry disease using a filter-paper test: the FOCUS study. Heart 97(2):131–136
Hassan S, Sidransky E, Tayebi N (2017) The role of epigenetics in lysosomal storage disorders: uncharted territory. Mol Genet Metab 122(3):10–18
Havndrup O et al (2010) Fabry disease mimicking hypertrophic cardiomyopathy: genetic screening needed for establishing the diagnosis in women. Eur J Heart Fail 12(6):535–540
Higashi H et al (2011) Endocardial and epicardial substrates of ventricular tachycardia in a patient with Fabry disease. Heart Rhythm 8(1):133–136
Hughes DA et al (2016) Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet
Ikari Y, Kuwako K, Yamaguchi T (1992) Fabry’s disease with complete atrioventricular block: histological evidence of involvement of the conduction system. Br Heart J 68(3):323–325
Imbriaco M et al (2009) Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: a prospective long-term cardiac magnetic resonance imaging study. Heart 95(13):1103–1107
January CT et al (2014) AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130(23):2071–2104
Jastrzebski M et al (2006) Electrophysiological study in a patient with Fabry disease and a short PQ interval. Europace 8(12):1045–1047
Kalliokoski RJ et al (2005) Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis 28(4):563–573
Kalliokoski RJ et al (2006) Structural and functional changes in peripheral vasculature of Fabry patients. J Inherit Metab Dis 29(5):660–666
Kampmann C et al (2002) Cardiac manifestations of Anderson-Fabry disease in heterozygous females. J Am Coll Cardiol 40(9):1668–1674
Kampmann C et al (2008) Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int J Cardiol 130(3):367–373
Kawano M et al (2007) Significance of asymmetric basal posterior wall thinning in patients with cardiac Fabry’s disease. Am J Cardiol 99(2):261–263
Kornreich R, Desnick RJ, Bishop DF (1989) Nucleotide sequence of the human alpha-galactosidase A gene. Nucleic Acids Res 17(8):3301–3302
Koskenvuo JW et al (2008) Twenty-four-month alpha-galactosidase A replacement therapy in Fabry disease has only minimal effects on symptoms and cardiovascular parameters. J Inherit Metab Dis 31(3):432–441
Kotanko P et al (2004) Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol 15(5):1323–1329
Kovarnik T et al (2008) Intravascular ultrasound assessment of coronary artery involvement in Fabry disease. J Inherit Metab Dis 31(6):753–760
Kramer J et al (2015) Left ventricular geometry and blood pressure as predictors of adverse progression of Fabry cardiomyopathy. PLoS One 10(11):e0140627
Kubo T (2017) Fabry disease and its cardiac involvement. J Gen Fam Med 18(5):225–229
Kubo T et al (2017) Prevalence and clinical features of Fabry disease in Japanese male patients with diagnosis of hypertrophic cardiomyopathy. J Cardiol 69(1):302–307
Lavalle L et al (2018) Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS One 13(4):e0193550
Lee K et al (2003) A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology 13(4):305–313
Leyva F (2010) Cardiac resynchronization therapy guided by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 12:64
Lidove O et al (2016) Fabry in the older patient: clinical consequences and possibilities for treatment. Mol Genet Metab 118(4):319–325
Linhart A (2006) The heart in Fabry disease, in Fabry disease: perspectives from 5 Years of FOS. A Mehta, M Beck, and G Sunder-Plassmann (eds) Oxford
Linhart A, Cecchi F (2018) Common presentation of rare diseases: left ventricular hypertrophy and diastolic dysfunction. Int J Cardiol 257:344–350
Linhart A, Elliott PM (2007) The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart 93(4):528–535
Linhart A et al (2000) New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J 139(6):1101–1108
Linhart A et al (2007) Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J 28(10):1228–1235
Lobo T et al (2008) Cardiovascular testing in Fabry disease: exercise capacity reduction, chronotropic incompetence and improved anaerobic threshold after enzyme replacement. Intern Med J 38(6):407–414
Lucke T et al (2004) Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab 82(1):93–97
Mehta A et al (2004) Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Investig 34(3):236–242
Mehta A et al (2009a) Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet 46(8):548–552
Mehta A et al (2009b) Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet 374(9706):1986–1996
Mehta A et al (2010) Fabry disease: a review of current management strategies. QJM 103(9):641–659
Monserrat L et al (2007) Prevalence of Fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 50(25):2399–2403
Moon JC et al (2003) Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 24(23):2151–2155
Muntze J et al (2018) Treatment of hypertrophic cardiomyopathy caused by cardiospecific variants of Fabry disease with chaperone therapy. Eur Heart J
Nakao S et al (2003) Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int 64(3):801–807
O’Mahony C et al (2011) Incidence and predictors of anti-bradycardia pacing in patients with Anderson-Fabry disease. Europace 13(12):1781–1788
O'Mahony C, Elliott P (2010) Anderson-Fabry disease and the heart. Prog Cardiovasc Dis 52(4):326–335
Osada H, Kanemitsu N, Kyogoku M (2016) Coronary artery bypass graft in a patient with Fabry’s disease. Cardiovasc Pathol 25(4):280–283
Pacienza N et al (2012) Lentivector transduction improves outcomes over transplantation of human HSCs alone in NOD/SCID/Fabry mice. Mol Ther 20(7):1454–1461
Palecek T et al (2008) Right ventricular involvement in Fabry disease. J Am Soc Echocardiogr 21(11):1265–1268
Patel MR et al (2011) Cardiovascular events in patients with Fabry disease natural history data from the Fabry registry. J Am Coll Cardiol 57(9):1093–1099
Patel V et al (2015) Clinical and genetic predictors of major cardiac events in patients with Anderson-Fabry disease. Heart 101(12):961–966
Pieroni M et al (2003) Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 107(15):1978–1984
Pieroni M et al (2004) Tissue Doppler imaging in Fabry disease. Curr Opin Cardiol 19(5):452–457
Pinderski LJ, Strotmann J (2006) 76. The Journal of Heart and Lung Transplantation 25(2):S70
Pochis WT et al (1994) Electrophysiologic findings in Fabry’s disease with a short PR interval. Am J Cardiol 74(2):203–204
Ponikowski P et al (2016) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18(8):891–975
Poulin MF et al (2015) Advanced Anderson-Fabry disease presenting with left ventricular apical aneurysm and ventricular tachycardia. World J Clin Cases 3(6):519–524
Putko BN et al (2015) Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev 20(2):179–191
Ries M, Gal A (2006) Genotype–phenotype correlation in Fabry disease. In: Beck M, Mehta A, Sunder-Plassmann G (eds) Fabry Disease: perspectives from 5 Years of FOS, Oxford PharmaGenesis, Oxford
Rigoldi M et al (2014) Intrafamilial phenotypic variability in four families with Anderson-Fabry disease. Clin Genet 86(3):258–263
Rosmini S et al (2017) Relationship between aetiology and left ventricular systolic dysfunction in hypertrophic cardiomyopathy. Heart 103(4):300–306
Saccheri MC, et al (2018) Comparison of left atrial size and function in hypertrophic cardiomyopathy and in Fabry disease with left ventricular hypertrophy. Echocardiography
Sachdev B et al (2002) Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 105(12):1407–1411
Sado DM et al (2013) Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging 6(3):392–398
Schaefer E, Mehta A, Gal A (2005) Genotype and phenotype in Fabry disease: analysis of the Fabry Outcome Survey. Acta Paediatr Suppl 94(447):87–92 discussion 79
Schiffmann R et al (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285(21):2743–2749
Schirinzi A et al (2008) Identification of GLA gene deletions in Fabry patients by multiplex ligation-dependent probe amplification (MLPA). Mol Genet Metab 94(3):382–385
Seydelmann N et al (2016) High-sensitivity troponin: a clinical blood biomarker for staging cardiomyopathy in Fabry disease. J Am Heart Assoc 5(6)
Shabbeer J et al (2006) Fabry disease: identification of 50 novel alpha-galactosidase a mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum Genomics 2(5):297–309
Shah JS et al (2005a) The natural history of left ventricular systolic function in Anderson-Fabry disease. Heart 91(4):533–534
Shah JS et al (2005b) Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry disease. Am J Cardiol 96(6):842–846
Shanks M et al (2013) Systolic and diastolic function assessment in Fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J Am Soc Echocardiogr 26(12):1407–1414
Sheppard MN (2011) The heart in Fabr’s disease. Cardiovasc Pathol 20(1):8–14
Sheppard MN et al (2010) A detailed pathologic examination of heart tissue from three older patients with Anderson-Fabry disease on enzyme replacement therapy. Cardiovasc Pathol 19(5):293–301
Shi Q et al (2014) Prevalence of Fabry disease in stroke patients—a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 23(5):985–992
Sivaloganathan S (1992) Fabry’s disease—a rare cause of sudden death. Med Sci Law 32(3):263–266
Spada M et al (2006) High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet 79(1):31–40
Spinelli L et al (2008) Cardiac performance during exercise in patients with Fabry’s disease. Eur J Clin Investig 38(12):910–917
Takenaka T et al (2008) Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol 51(1):50–59
Teitcher M et al (2008) Genetic polymorphisms of vitamin D receptor (VDR) in Fabry disease. Genetica 134(3):377–383
The Human Gene Mutation Database. [cited 2018 10/03/2018]; Available from: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GLA
Torralba-Cabeza MA et al (2011) Cystatin C and NT-proBNP as prognostic biomarkers in Fabry disease. Mol Genet Metab 104(3):301–307
von Scheidt W et al (1991) An atypical variant of Fabry’s disease with manifestations confined to the myocardium. N Engl J Med 324(6):395–399
Weidemann F et al (2003) Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation 108(11):1299–1301
Weidemann F et al (2009) Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation 119(4):524–529
Weidemann F, Niemann M, Störk S et al (2013) Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 274(4):331–341
Weidemann F et al (2016) Usefulness of an implantable loop recorder to detect clinically relevant arrhythmias in patients with advanced Fabry cardiomyopathy. Am J Cardiol 118(2):264–274
Wu JC et al (2010) Cardiovascular manifestations of Fabry disease: relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur Heart J 31(9):1088–1097
Yogasundaram H et al (2017) Clinical features, diagnosis, and management of patients with Anderson-Fabry cardiomyopathy. Can J Cardiol 33(7):883–897
Zarate YA, Hopkin RJ (2008) Fabry’s disease. Lancet 372(9647):1427–1435
Funding
M Akhtar is supported by unrestricted educational grant from Sanofi Genzyme. The UCL Centre for Heart Muscle Disease is partially funded by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Perry Elliott has received speaker honoraria from Shire HGT and consultancy and speaker honoraria from Amicus, MyoKardia, Pfizer Inc., Sanofi Genzyme Idorsia and Alnylam.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of a Special Issue on ‘Heart Failure Due to Non-Myofibrillar Defects’ edited by Elisabeth Ehler and Katja Gehmlich.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Akhtar, M.M., Elliott, P.M. Anderson-Fabry disease in heart failure. Biophys Rev 10, 1107–1119 (2018). https://doi.org/10.1007/s12551-018-0432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-018-0432-5