Abstract
Eighty-five samples of cocoa products sampled in Canada were analysed for ochratoxin A (OTA) and aflatoxins in 2011–2012. Inclusion of the aflatoxins in this survey required additional method development. Chocolate was extracted with methanol–water plus NaCl, while for cocoa two successive extractions with methanol and methanol–water were made. Extracts were cleaned on an AflaOchra immunoaffinity column (IAC). Determination was by reversed phase high performance liquid chromatography (HPLC). Detection of the aflatoxins was with a post-column photochemical reactor and of OTA by fluorescence detection. Mean limits of quantification (LOQ) of chocolate and cocoa powders were 0.16 ng/g (OTA) and 0.07 ng/g (aflatoxin B1), respectively. Survey results showed that the incidences of OTA above the LOQ in natural cocoa were 15/15 (mean 1.17 ng/g), 20/21 for alkalized cocoa (mean 1.06 ng/g), 9/9 for baking chocolate (mean 0.49 ng/g), 20/20 for dark chocolate (mean 0.39 ng/g), 7/10 for milk chocolate (mean 0.19 ng/g), 5/5 for cocoa liquor (mean 0.43 ng/g), and 0/5 for cocoa butter. These results confirm our previous work with OTA. In the same samples, incidences of aflatoxin B1 above the LOQ were 14/15 for natural cocoa (mean 0.86 ng/g), 20/21 for alkalized cocoa (mean 0.37 ng/g), 7/9 for baking chocolate (mean 0.22 ng/g), 16/20 for dark chocolate (mean 0.19 ng/g), 7/10 for milk chocolate (mean 0.09 ng/g), 4/5 for cocoa liquor (mean 0.43 ng/g), and 0/5 for cocoa butter. Both aflatoxins and OTA were confirmed by HPLC-MS/MS when OTA or aflatoxin levels found were above 2 ng/g in cocoa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochratoxin A (OTA; L-phenylalanylcarbonyl-5-chloro-8-hydroxy-3,4-dihydro-3-R-methylisocoumarin) is a mycotoxin formed mainly by some species of Aspergillus and Penicillium (Amézqueta et al. 2012). OTA has been shown to be carcinogenic, nephrotoxic, teratogenic, immunotoxic, and hepatotoxic in various experimental animal models, and the International Agency for Research on Cancer (IARC) has classified it as possibly carcinogenic to humans (group 2B) (IARC 1993). The main fungal sources of OTA in cocoa beans in African countries and in South America are black aspergilli (A. carbonarius and A. niger aggregate) (Amézqueta et al. 2008; Mounjouenpou et al. 2008; Sánchez-Hervás et al. 2008; Copetti et al. 2010; Teixeira de Magalhães et al. 2011).
OTA is found in grains and many other kinds of foodstuffs (Bayman and Baker 2006; Clark and Snedeker 2006); these include cocoa and cocoa products (reviewed by Codex Alimentarius Commission 2007, 2008; Tabata et al. 2008; Chung et al. 2009; Aoyama et al. 2010; Brera et al. 2011; Jayeola et al. 2011; Nwagu and Ire 2011; Turcotte and Scott 2011; Copetti et al. 2012a, 2013; Mounjouenpou et al. 2012). The European Commission (EC) has stated that it does not appear necessary to set a maximum level for OTA in cocoa and cocoa products (European Commission 2010). Similarly, based on the data available, neither cocoa nor cocoa products were identified by Health Canada as products for which a maximum OTA level should be considered (Health Canada 2010).
The aflatoxins are formed by Aspergillus flavus, A. parasiticus, and other Aspergillus spp. (Basappa 2009). The most important aflatoxin, in terms of toxicity and occurrence, is aflatoxin B1 ((6aR,9aS)-2,3,6a,9a-tetrahydro-4-methoxycyclopenta[c]furo-(3′,2′:4,5)furo[2,3-h][l]benzopyran-1,11-dione), which is classified as carcinogenic to humans (group 1) (IARC 2002). There was some early work on the use of thin layer chromatographic and HPLC analysis in cocoa beans for the determination of aflatoxins (and OTA) (Scott and Przybylski 1971; Scott 1973; Czerwiecki 1974; Hurst et al. 1982), but in recent years, the presence of aflatoxins in cocoa and cocoa products has received less attention than OTA. However, there have been reports from Germany (Raters and Matissek 2000), Turkey (Dogan et al. 2006; Ulca et al. 2010), Japan (Kumagai et al. 2008; Kawamura and Hamada 2009; Sugita-Konishi et al. 2010), and Brazil (Copetti et al. 2011, 2012a, b) on the natural occurrence of aflatoxins in cocoa and cocoa products. Neither the EC nor Health Canada has developed maximum limits for aflatoxins in cocoa or cocoa products.
Co-occurrence of OTA and aflatoxins in cocoa products has been reported (Raters and Matissek 2000; Kumagai et al. 2008; Tabata et al. 2008; Copetti et al. 2012a), so analysis for both of these mycotoxins in the same sample is desirable. The commercial availability of an IAC column (AflaOchra) for cleanup of both toxins in foods simplifies this analysis. This column has been used for various foods (Chan et al. 2004; Trucksess et al. 2007, 2008; Leeman and Marley 2010).
The present paper describes the use of the AflaOchra cleanup column in the analysis of cocoa products, such as cocoa and chocolate for OTA and aflatoxins, by surveying these foods sold in Canada. A previous Canadian survey of cocoa and chocolate was for OTA only (Turcotte and Scott 2011). For detection by fluorescence, OTA and aflatoxins B2 and G2 are naturally fluorescent, but aflatoxins B1 and G1 require derivatization. The UVE™ reactor (LCTech, Germany) uses a 254-nm UV light for post-column photochemical derivatization (hydroxylation). The reactor is placed in the flow path, between the HPLC column and fluorescence detector, and no chemicals are necessary for derivatization.
Materials and methods
Sample preparation
The Canadian Food Inspection Agency (CFIA) collected three 250-g sub-samples of each lot of cocoa product from different manufacturers nationally. For local purchases, our laboratory (Ottawa, Canada) collected two sub-samples of each lot. All the samples were stored at −18 °C prior to analysis. For cocoa powder, a minimum of 1/3 of each sub-sample was combined and thoroughly mixed prior to sampling. For other cocoa products (chocolates, liquors, and butters), the frozen samples were ground with a direct drive food processor, coarse grinding blade and knife. The food processor bowl and blades were frozen prior to grinding. For chocolates purchased locally, sample size was limited and a coffee grinder was necessary for complete grinding. Finally, the ground sample was sieved through a 2-mm screen and the fines were composited and mixed thoroughly.
If deemed necessary to identify a natural or alkali cocoa, a 2-g sample was mixed with 40 ml deionized water and filtered. If the pH of the filtrate was above 6, the cocoa was and labeled as alkalized cocoa.
Chemicals and reagents
Ochratoxin A, >98 %, was purchased from Sigma-Aldrich Canada (Oakville, Ontario) and stored at −20 °C. The aflatoxins—AFB1, AFB2, AFG1, and AFG2 (separate vials), ≥ 98 %—were obtained from Enzo Life Sciences International (Farmingdale, NY, USA) and stored at 4 °C. Ultrapure water had a resistivity of >18 megohm-cm. Toluene, methanol, and acetonitrile were HPLC grade (99.9+ %). Glacial acetic acid, orthophosphoric acid, 0.1 M HCl, 0.1 M NaOH, disodium hydrogen orthophosphate, potassium dihydrogen phosphate, potassium chloride, and sodium chloride were all ACS grade. Phosphate-buffered saline (PBS) was prepared by dissolving 1.16 g disodium hydrogen orthophosphate, 0.2 g potassium dihydrogen phosphate, 0.2 g potassium chloride, and 8 g sodium chloride in 1 L ultrapure water. Final pH was 7.4.
Equipment
The following equipment was used: Kinematica Polytron Homogenizer, Model PT 10/35GT; solid phase extraction (SPE) vacuum manifold (Supelco) with pump; Agilent model 1100 HPLC with an Agilent fluorescence detector; derivatization module for the analysis of aflatoxins—UVE photochemical detector with 1 ml loop (LCTech); AflaOchra cleanup columns, stored at room temperature, were from Vicam (Watertown, MA, USA), with 60 ml polypropylene reservoirs and adapters for IAC chromatography; silanized 4-ml amber glass vials and 2-ml amber deactivated autosampler vials, with Teflon (PTFE) lined septa and threaded caps; and silanized 400-μl flat bottom vial inserts. Nitrogen gas was Ultra High Purity.
Standards
HPLC calibration standards—0, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 ng/ml OTA + each aflatoxin (AFB1, AFB2, AFG1, AFG2) at ¼ of OTA concentration (0, 0.025, 0.05, 0.125, 0.25, 0.5, 1.25 ng/ml)—were prepared from an aliquot of AFLA-OTA intermediate standard transferred to a volumetric flask, evaporated to dryness under nitrogen at 40 °C, made up to volume with injection solvent [water–methanol (60:40 v/v)] and filtered through a 0.45-μm PTFE filter. They were stored in silanized amber vials or silanized glass inserts at −18 °C for up to 6 months. Good linearity was obtained for the calibration curves as the coefficient of determination (R2) was above 0.999 for each mycotoxin.
Fortified (spiked) samples of cocoa products were prepared by adding an aliquot of each spiking solution (50 ng/ml OTA in methanol and 20 ng/ml AFB1, AFB2, AFG1, and AFG2 in toluene) to 5-g ground sample, and extracting after 15 min.
Extraction and cleanup of chocolate (also applicable to cocoa liquor and cocoa butter)
A 5-g ground sample was extracted using a Polytron for 3 min with 100 ml methanol–water (80:20, v/v) plus 0.5 g NaCl, centrifuged (2,000 rpm for 10 min), and filtered (Whatman #1). Five ml of the filtered extract and 20 ml PBS were loaded onto an AflaOchra IAC, previously conditioned with 5 ml PBS; the column was washed with 10 ml water then the toxins were eluted with 2 × 750 μl methanol using gravity flow. The eluate was evaporated to dryness under nitrogen at 40 °C, 500 μl of injection solvent [water–methanol (60:40, v/v)] were added, and the solution was vortexed then filtered into a silanized autosampler vial by syringe pressure through a 0.45-μm Teflon filter. Sample concentration in the final extract was 0.5 g/ml.
Extraction and cleanup of cocoa powder
Each 5-g sample was extracted (Polytron, 3 min) with 50 ml methanol and centrifuged (2,000 rpm, 10 min). After filtration (typically not necessary), 2.5 ml (taken before a precipitate formed) was added to 20 ml PBS. Methanol was decanted and extraction of the residual solids was made with 50 ml methanol–water (60:40, v/v) using an ultrasonic bath; after centrifuging, a 2.5-ml aliquot was added to 20 ml PBS and the 2.5-ml aliquot taken earlier. The combined extracts were cleaned up on an AflaOchra IAC as described earlier for chocolate except the elution was with 2 × 750 μl methanol–1 % acetic acid (80:20, v/v).
HPLC analysis
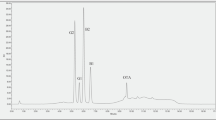
OTA and aflatoxins were separated by reversed-phase HPLC on a Supelcosil LC18, 4.6 × 150 mm × 5 μ column (Supelco) for chocolate and a Gemini C18, 4.6 × 250 mm × 5 μ column (Phenomenex) for cocoa, with an Opti-guard C18 guard column (Optimize Technologies). The column heater was set at 40 ± 0.5 °C and 50 μl was injected into the HPLC. The flow rate was 1.2 ml/min. Detection of the aflatoxins was with a post-column photochemical reactor and of OTA by fluorescence detection with excitation wavelengths (λex) of 360 nm for aflatoxins and 225 nm for OTA and emission wavelengths (λem) of 440 nm (aflatoxins) and 470 nm (OTA). The HPLC gradient program is summarized in Table 1. A typical chromatogram showed the following mycotoxin retention times: AFG2 (7.6 min), AFG1 (8.8 min), AFB2 (10.2 min), AFB1 (12.0 min), and OTA (26.5 min) (Fig. 1).
Traces of OTA, equivalent to 0.2 ng/g sample concentration, were detected in some lots of IAC columns. These levels were subtracted to obtain the values reported for cocoa and chocolate.
The longer 250-mm HPLC column should be used for subsequent surveys of cocoa products, including chocolate. It offers adequate sensitivity, better resolution between each aflatoxin, and less potential for interferences from sample matrices.
Confirmation of results
Sample confirmation was performed when OTA or aflatoxin levels found were above 2 ng/g in cocoa. For chocolate, no sample was found above 1 ng/g OTA or 2 ng/g aflatoxin. Both OTA and aflatoxins were confirmed by HPLC-MS/MS. An Agilent 1100 series HPLC with a thermostated autosampler/column compartment, binary pump, and in-line degasser was coupled to a Waters Quattro Ultima tandem mass spectrometer with Z-Spray API source. The diverter valve was a Rheodyne MX9900 (column effluent was diverted to waste for first 4 min of each analysis—controlled via MassLynx software). The HPLC column was a Gemini C18, 100 × 2.00 mm × 3 μm (Phenomenex) and mobile phases were A: 0.1 % formic acid + 5 mM ammonium formate and B: 0.1 % formic acid + 5 mM ammonium formate in acetonitrile-methanol (1:1, v/v) with a gradient: 30 % B, hold for 2 min; 30 to 100 % B by 15 min; 100 % B, hold for 8 min; 30 % B from 23.01 to 28 min. The flow rate was 300 μl/min. Column temperature was 30 °C, injector temperature was 20 °C and injection volume was 100 μl.
Negative ion electrospray ionization MS/MS conditions included: capillary voltage 3.0 kV, cone voltage 25 V, source temperature 120 °C, desolvation temperature 350 °C, cone gas (N2) flow 50 L/h, desolvation gas (N2) flow 500 L/h, and collision gas (Ar) pressure 3.00 × 10−3 mbar. Interchannel delay time, inter-scan time, and dwell time were 0.020, 0.050, and 0.09 s, respectively. The span was 0.2 Da. Mutiple reaction monitoring (MRM) transitions and collision energies are shown in Table 2. Peaks were positively identified if they eluted within ±5 % of the retention time of the standard and if the qualifier ion ratio was within ±25 % of that of the standard. We observed signal suppression for aflatoxins B2 and B1 and signal enhancement of OTA due to matrix effects. It would be necessary to use labeled internal standards or matrix matched standards for quantitative measurements.
OTA confirmation was also possible by methyl ester formation after derivatization with 14 % BF3 in methanol at 60 °C for 20 min. The aflatoxins could also be confirmed by shutting off the UVE, suppressing responses of aflatoxins G1 and B1.
Results and discussion
Method development
Inclusion of the aflatoxins in this survey of OTA in cocoa products required additional method development. The main challenges were matrix adsorption, interferences, and low aflatoxin recoveries, especially for G1 and G2. The aflatoxin B isomers have one lactone ring and the aflatoxin G isomers have two lactone rings that may be cleaved and potentially bind to the matrix (Diaz et al. 2012).
Although we used a 50-mm HPLC column for our first survey (Turcotte and Scott 2011), it was not feasible with the photochemical reactor in the flow path. The reactor uses a braided coil with an internal volume of 1 ml, and a flow rate of at least 1 ml/min flow is required for acceptable peak width. Unfortunately, the operating pressures were too high with the 50-mm column.
Although an acidic mobile phase is typically not necessary for the analysis of aflatoxins by reverse phase HPLC, it was necessary to start the gradient with an acidic mobile phase to avoid dissociation of the carboxyl group of OTA. Furthermore, the addition of acetonitrile at the start of the gradient improved the response of the aflatoxins. The HPLC parameters were first optimized using 1 % acetic acid in the mobile phase, but after a few months substantial signal suppression (quenching) occurred. We changed to 0.1 % phosphoric acid in the mobile phase and ran over 1,000 injections with excellent signal stability and sensitivity.
Initially, we selected a 150-mm C18 HPLC column for the aflatoxins and OTA. During method development, we found the sample extracts were quite dirty in spite of the IAC cleanup. Initially, we tested different solid phase extraction (SPE) columns to pre-clean the extract. A non-retentive approach, where only the contaminants were retained on a LC-NH2 aminopropyl silica SPE column (Supelco, Bellefonte, PA, USA), resulted in a cleaner extract (less pigment), with ∼40–60 % aflatoxin recovery and 70 % recovery of OTA. An acidic methanol wash was necessary to elute the residual OTA from the LC-NH2, but matrix interferences were also eluted. At this point, it was evident that more work was necessary to improve the aflatoxin and OTA recoveries from cocoa. Low aflatoxin recoveries from the cocoa matrix, particularly for aflatoxins G1 and G2, have been reported previously (Kawamura and Hamada 2009).
For method validation, the recoveries of aflatoxins and OTA from fortified chocolate were acceptable. The analyses of chocolate samples (baking, dark, and milk chocolates, and cocoa liquors) were completed using a 150-mm HPLC column with IAC cleanup only. The detection limit for OTA was further improved by using an excitation wavelength of 225 nm for the fluorescence detector (Amézqueta et al. 2004).
For cocoa powders, it was necessary to use a 250-mm HPLC column due to matrix interferences. During optimization of HPLC parameters, the main challenges were interferences eluting near the aflatoxins (large peak at the front of the chromatogram) and a ghost peak eluting near OTA. The ghost peak was eliminated by using a methanol:phosphoric acid 0.1 % (70:30, v/v) wash near the end of the run, after OTA elution. Figure 1 shows the chromatograms of a calibration standard and a naturally contaminated cocoa powder (expanded scale between 5 and 30 min).
During method validation, we found low aflatoxin recoveries likely due to the binding of aflatoxins to the cocoa powder matrix. The recoveries of aflatoxins G2 and G1 were below 50 % using the same methodology as for chocolate. Recoveries improved when the aflatoxin spiking solution was prepared in toluene. We tried different approaches to further improve aflatoxin recovery such as pre-wetting the cocoa, using other extraction solvents, extraction additives [sodium chloride, silver nitrate, polyvinyl-pyrrolidone (PVP)], and PBS additives [Tween 20 and polyethylene glycol (PEG 8000)]. Finally, we obtained optimal aflatoxin recovery using 100 % methanol for solvent extraction, but only 55 % of OTA was recovered from a fortified cocoa. A second aqueous methanol extraction was necessary for optimal OTA recovery. Although the first methanol extraction solution was clear with a slight tint, the second extraction (methanol/water) was quite pigmented. It was necessary to take an aliquot of the first methanol extract before a precipitate came out of solution. The precipitation time was sample-dependant, anywhere from 15 min to a few hours. A significant loss of the aflatoxins (especially G2 and G1) coincided with formation of this precipitate. The precipitate was identified as theobromine (3,7-dimethylxanthine) by gas chromatography and mass spectrometry (GC-MS). The amount of theobromine is typically ∼2.6 % in cocoa powder but can vary with each variety. The recoveries of aflatoxins and OTA from fortified cocoa improved when using acidic methanol for IAC elution. Although IAC cleanup is very selective, the final extract was pigmented, proving that non-selective adsorption can occur on the antibodies.
For method validation, it is necessary to use matrix fortification, but the addition of mycotoxin in a solvent is not always representative of a natural contamination. With the optimized method, the extent of improvement of aflatoxin recovery for the fortified cocoa was higher than for a naturally contaminated cocoa sample.
Other challenges were associated with aflatoxin recovery during method validation. It is necessary to minimize light exposure during sample extraction and to use deactivated amber glass vials. Good stability of calibration standards was obtained with a 400-μl glass insert (deactivated) placed in an amber autosampler vial. Many batches of calibration standards can be prepared and stored at −18 °C for up to 6 months. This is not the case for the final sample extracts, as ∼20–50 % losses of aflatoxin G1 and G2 can occur on storage at −18 °C within a few days or weeks, depending on the matrix. The instability of the G aflatoxins has been reported previously (Diaz et al 2012).
For health risk assessment purposes, IAC column specificity helps achieve optimal sensitivity. We obtained several lots of clean IAC columns with no detectable aflatoxins or OTA in blanks. However, we did receive one lot of AflaOchra IACs contaminated with traces of OTA (50 pg).
Survey
Method recoveries for aflatoxins and OTA from chocolate and cocoa powder are shown in Table 3. Generally, the recoveries are acceptable considering the spiking levels are less than 5 ppb. The RSDs are below 5 % for chocolate and cocoas at the higher spike level and below 12 % at the lower spike level. The instability of the G aflatoxins contributes to the higher standard deviation of the mean recovery for the three matrices at both spiking levels.
The limits of detection (LOD) and limits of quantitation (LOQ) are reported in Table 4. The LOD was based on three times the signal-to-noise ratio (S/N) and the LOQ was calculated as ten times the S/N ratio, both calculated for each matrix at the lowest spike level. The mean LOQs were used to measure incidence of each mycotoxin in the different cocoa products.
The survey showed the presence of both OTA and aflatoxins at high incidence in cocoa and chocolate at concentrations generally <2 ng/g (Table 5), with only the occasional sample of natural cocoa containing >2 ng/g. Generally, the overall mean concentrations of the aflatoxins and OTA were proportional to the total cocoa solids content of each commodity, increasing from cocoa butter, milk chocolate, dark chocolate, baking chocolate, and cocoa liquor to cocoa powder. The organic cocoa products did not always show less contamination than the regular products. However, from nine baking chocolates, the three that showed the least contamination were organic and originated from Peru.
There was no correlation between OTA and the aflatoxin content for each commodity, as the most contaminated samples for OTA did not coincide with higher aflatoxin contamination. Co-occurrence of the four aflatoxins was found in 1/10 milk chocolate, 1/20 dark chocolate, 1/5 cocoa liquor, and 2/15 natural cocoa samples.
The very low levels of OTA and absence of the aflatoxins in cocoa butter agree with previous surveys on cocoa by-products (Copetti et al. 2012b; Mounjouenpou et al. 2012). Copetti et al. (2012a) reported co-occurrence of OTA and aflatoxins in chocolate marketed in Brazil, where the overall mean concentrations of OTA and AFB1 were equivalent in powdered raw chocolate (0.39 and 0.43 ng/g) and dark chocolate (0.34 and 0.43 ng/g). In our survey (Table 5), the overall mean concentration of OTA was often twice that of AFB1 for most commodities.
Results confirm our previous work with OTA (Table 6) and the findings of others (e.g., Codex Alimentarius Commission 2007, 2008; Copetti et al. 2012a, b). Table 6 shows that the OTA concentration was generally higher in the 2008–2009 survey, except for natural cocoa, where a higher mean concentration was found in 2011–2012. In 2008–2009, the highest concentration of OTA was in alkalized cocoa (7.75 ng/g) with 5 of 16 alkali cocoa above 2 ng/g. The current survey (2011–2012) found higher concentrations and incidence of OTA in natural cocoa; however, only 2 of 15 natural cocoa samples were above 2 ng/g and no alkalized cocoa samples were above 2 ng/g.
Currently, there are no Canadian standards (maximum limits) for OTA or the aflatoxins in cocoa or chocolate products. In 2003, an expert committee of the European Commission (EC) was considering a maximum limit of 1 ng/g OTA in chocolate and 2 ng/g OTA in cocoa powder; however, the EC now considers that, on the basis of available information, it does not appear necessary for the protection of public health to set a maximum level for OTA in cocoa and cocoa products. For our 2011–2012 survey, only 2/80 cocoa products (excluding cocoa butter) were greater than the previously considered EC limits, and 7/60 in 2008–2009, for a total of 9/140. Although the overall mean levels are low, the concentration range can vary from 0.07 to 7.8 ng/g OTA (2-year survey) and nd-3.52 ng/g total aflatoxins (1-year survey) in cocoa powder.
References
Amézqueta S, González-Peñas E, Murillo M, Lopez de Cerain A (2004) Validation of a high-performance liquid chromatography analytical method for ochratoxin A quantification in cocoa beans. Food Addit Contam 21(11):1096–1106
Amézqueta S, González-Peñas E, Dachoupakan C, Murillo-Arbizu M, de Cerain AL, Guiraud JP (2008) OTA-producing fungi isolated from stored cocoa beans. Lett Appl Microbiol 47:197–201
Amézqueta S, Schorr-Galindo S, Murillo-Arbizu M, González-Peñas E, López de Cerain A, Guiraud JP (2012) OTA-producing fungi in foodstuffs: a review. Food Control 26:259–268
Aoyama K, Nakajima M, Tabata S, Ishikuro E, Tanaka T, Norizuki H, Itoh Y, Fujita K, Kai S, Tsutsumi T, Takahashi M, Tanaka H, Iizuka S, Ogiso M, Maeda M, Yamaguchi S, Sugiyama K, Sugita-Konishi Y, Kumagai S (2010) Four-year surveillance for ochratoxin A and fumonisins in retail foods in Japan. J Food Prot 73:344–352
Basappa SC (2009) Aflatoxins: formation, analysis and control. Alpha Science International, Oxford
Bayman P, Baker JL (2006) Ochratoxins: a global perspective. Mycopathologia 162:215–223
Brera C, Debegnach F, De Santis B, Iafrate E, Pannunzi E, Berdini C, Prantera E, Gregori E, Miraglia M (2011) Ochratoxin A in cocoa and chocolate products from the Italian market: occurrence and exposure assessment. Food Control 22:1663–1667
Chan D, MacDonald SJ, Boughtflower V, Brereton P (2004) Simultaneous determination of aflatoxins and ochratoxin A in food using a fully automated immunoaffinity column clean-up and liquid chromatography-fluorescence detection. J Chromatogr A 1059:13–16
Chung SW, Kwong KP, Tang ASP, Yeung STK (2009) Ochratoxin A levels in foodstuffs marketed in Hong Kong. J Food Compos Anal 22:756–761
Clark HA, Snedeker SM (2006) Ochratoxin A: its cancer risk and potential for exposure. J Toxicol Environ Health B 9:265–296
Codex Alimentarius Commission (2007) Discussion paper on ochratoxin A in cocoa. Joint FAO/WHO Food Standards Programme. Codex Committee on Contaminants in Foods. First Session, Beijing, China. CX/CF 07/1/19 ftp://ftp.fao.org/codex/cccf1/cf01_19e.pdf and CX/CF 07/1/19 Add.2 ftp://ftp.fao.org/Codex/cccf1/cf0119be.pdf
Codex Alimentarius Commission (2008) Discussion paper on ochratoxin A in cocoa. Joint FAO/WHO Food Standards Programme. Codex Committee on Contaminants in Foods. Second Session, The Hague, the Netherlands. CX/CF 08/2/15 ftp://ftp.fao.org/codex/cccf2/cf02_15e.pdf
Copetti MV, Pereira JL, Iamanaka BT, Pitt JI, Taniwaki MH (2010) Ochratoxigenic fungi and ochratoxin A in cocoa during farm processing. Int J Food Microbiol 143:67–70
Copetti MV, Iamanaka BT, Pereira JL, Fungaro MH, Taniwaki MH (2011) Aflatoxigenic fungi and aflatoxin in cocoa. Int J Food Microbiol 148:41–44
Copetti MV, Iamanaka BT, Pereira JL, Lemes DP, Nakano F, Taniwaki MH (2012a) Co-occurrence of ochratoxin A and aflatoxins in chocolate marketed in Brazil. Food Control 26:36–41
Copetti MV, Iamanaka BT, Pereira JL, Lemes DP, Nakano F, Taniwaki MH (2012b) Determination of aflatoxins in by-products of industrial processing of cocoa beans. Food Addit Contam A 29:972–978
Copetti MV, Iamanaka BT, Nester MA, Efraim P, Taniwaki MH (2013) Occurrence of ochratoxin A in cocoa by-products and determination of its reduction during chocolate manufacture. Food Chem 136:100–104
Czerwiecki L (1974) Detection of aflatoxin B1 in cocoa beans. Rocz Państw Zakł Hig 25:663–666 (in Polish)
Diaz GJ, Cepeda S, Martos P (2012) Stability of aflatoxins in solution. JAOAC Int 95:1084–1088
Dogan M, Liman BC, Sagdic O (2006) Total aflatoxin levels of instant foods in Turkey. Arch Leb 57:56–59
European Commission (2010) Commission Regulation (EU) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off J Eur Union 35:7–8
Health Canada website (2010) Summary of comments received on Health Canada’s proposed maximum limit for ochratoxin A in certain foods. http://www.hc-sc.gc.ca/fn-an/securit/chem-chim/toxin-natur/summary-resume-eng.php
Hurst WJ, Lenovich LM, Martin RA Jr (1982) Liquid chromatographic determination of aflatoxins in artificially contaminated cocoa beans. J Assoc Off Anal Chem 65:888–891
International Agency for Research on Cancer (IARC) (1993) Monographs on the evaluation of carcinogenic risks to humans Number 56. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC, Lyon, pp 489–521
International Agency for Research on Cancer (IARC) (2002) Monographs on the evaluation of carcinogenic risks to humans. Number 82. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC, Lyon, pp 171–300
Jayeola CO, Oluwadun A, Yahaya LE, Dongo LN, Ajao AA, Mokwunye FC (2011) Comparative analysis of detecting ochratoxin A in cocoa powder samples using high performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA). Afr J Food Sci 5:513–521
Kawamura O, Hamada A (2009) Determination of aflatoxins in cacao products by immunoaffinity column-HPLC methods. Kagawa Daigaku Nogakubu Gakujutsu Hokoku 114:85–88 (in Japanese); Chem Abstr 150:350521n (2009)
Kumagai S, Nakajima M, Tabata S, Ishikuro E, Tanaka T, Norizuki H, Itoh Y, Aoyama K, Fujita K, Kai S, Sato T, Saito S, Yoshiike N, Sugita-Konishi Y (2008) Aflatoxin and ochratoxin A contamination of retail foods and intake of these mycotoxins in Japan. Food Addit Contam A 25:1101–1106
Leeman D, Marley EC (2010) Analysis using AflaOchra Prep in combination with fluorescence HPLC for the determination of total aflatoxins and ochratoxin A [in Italian]. Rapporti ISTISAN 10/32:219-222. Chem Abstr 154:457928k (2011)
Mounjouenpou P, Gueule D, Fontana-Tachon A, Guyot B, Tondje PR, Guiraud JP (2008) Filamentous fungi producing ochratoxin a during cocoa processing in Cameroon. Int J Food Microbiol 121:234–241
Mounjouenpou P, Mbang JAA, Guyot B, Guiraud J-P (2012) Traditional procedures of cocoa processing and occurrence of ochratoxin - A in the derived products. J Chem Pharm Res 4:1332–1339
Nwagu TNT, Ire FS (2011) Ochratoxin in cocoa, health risks and methods of detoxification. Int J Agric Res 6:101–118
Raters M, Matissek R (2000) Vorkommen der Mykotoxine Aflatoxin B1, B2, G1, G2 und Ochratoxin A in Kakao und Kakaoprodukten. Projekt Nr. 9 der Stiftung der Deutschen Kakao- und Schokoladenwirtschaft, Hamburg www.kakao-stiftung.de/pdf/Projekt_9.pdf www.kakao-stiftung.de/project9_e.html (English summary)
Sánchez-Hervás M, Gil JV, Bisbal F, Ramón D, Martínez-Culebras PV (2008) Mycobiota and mycotoxin producing fungi from cocoa beans. Int J Food Microbiol 125:336–340
Scott PM (1973) Modified method for the determination of mycotoxins in cocoa beans. J Assoc Off Anal Chem 56:1028–1030
Scott PM, Przybylski W (1971) Collaborative study of a method for the analysis of cocoa beans for aflatoxins. J Assoc Off Anal Chem 54:540–544
Sugita-Konishi Y, Sato T, Saito S, Nakajima M, Tabata S, Tanaka T, Norizuki H, Itoh Y, Kai S, Sugiyama K, Kamata Y, Yoshiike N, Kumagai S (2010) Exposure to aflatoxins in Japan: risk assessment for aflatoxin B1. Food Addit Contam A 27:365–372
Tabata S, Iida K, Kimura K, Iwasaki Y, Nakazato M, Kamata K, Hirokado M (2008) Investigation of ochratoxin A, B and citrinin contamination in various commercial foods [in Japanese]. Shokuhin Eiseigaku Zasshi 49:111–115
Teixeira de Magalhães H, Sodré GA, Viscogliosi H, Grenier-Loustalot M-F (2011) Occurrence of ochratoxin A in Brazilian cocoa beans. Food Control 22:744–748
Trucksess MW, Weaver CM, Oles CJ, Rump LV, White KD, Betz JM, Rader JI (2007) Use of multitoxin immunoaffinity columns for determination of aflatoxins and ochratoxin A in ginseng and ginger. J AOAC Int 90:1042–1049
Trucksess MW, Weaver CM, Oles CJ, Fry FS Jr, Noonan GO, Betz JM, Rader JI (2008) Determination of aflatoxins B1, B2, G1, and G2 and ochratoxin A in ginseng and ginger by multitoxin immunoaffinity column cleanup and liquid chromatographic quantitation: collaborative study. J AOAC Int 91:511–523
Turcotte AM, Scott PM (2011) Ochratoxin A in cocoa and chocolate sampled in Canada. Food Addit Contam A 28:762–766
Ulca P, Evcimen MK, Senyuva HZ (2010) Surveys of aflatoxin B1 contamination of retail Turkish foods and of products intended for export between 2007 and 2009. Food Addit Contam B 3:120–125
Acknowledgments
We thank the Canadian Food Inspection Agency for collection of samples. We also thank our co-workers Cathie Ménard and Tara Hierlihy for their valuable assistance.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Turcotte, AM., Scott, P.M. & Tague, B. Analysis of cocoa products for ochratoxin A and aflatoxins. Mycotoxin Res 29, 193–201 (2013). https://doi.org/10.1007/s12550-013-0167-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-013-0167-x