Abstract
Eocene amber is an important window into the past about 35 million years ago. The large quantities of resin produced by this forest of the past, resulting in amber, triggered the idea of a forest under stress. Recent findings of higher abundances of hoverfly larvae in Eocene amber, in the modern fauna often associated with wood-borer larvae, provided a hint that wood-borer larvae may have contributed to this stress. Yet, so far only few such larvae have been reported. We have compiled a dozen additional wood-borer larvae in amber, including a giant one of at least 35 mm length in Rovno amber. Heavily damaged fossils furthermore indicate that larger larvae of this type were prone to oxidation and that, at least some, enigmatic tube-like tunnels in larger amber pieces may represent remains of large wood-borer larvae. This find strongly indicates that wood-borer larvae were not rare, but common in the Eocene amber forest, which is compatible with the high abundances of hoverfly larvae and further supports the idea of a forest under stress. Whether the possible higher abundances of wood-borer larvae were the cause of the stress or a symptom of an already stressed forest remains so far unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amber provides a unique view into past ecosystems, preserving animals in an almost life-like manner. Therefore, fossils preserved in amber are an ideal source for any comparison with the modern fauna. Still, many aspects of the amber forest remain unclear, for example the factors leading to the large amount of resin production in the amber forests (Penney 2016). It has been suggested that the extensive production of resin at least in some amber forests was a result of stress (McKellar et al. 2011; Seyfullah et al. 2018). More precisely, water stress of the resin-producing trees might have been the cause (Martínez-Delclòs et al. 2004; Labandeira 2014; Seyfullah et al. 2018), possibly mediated by drought, flood diseases, wildfires, or plant parasites (though the impact of plant parasites has been questioned: Peris et al. 2016; Peris and Rust 2020; but see also Peris 2020 and Peris et al. 2021).
Baranov et al. (2021) recently reported a large number of hoverfly larvae (Syrphidae) of the group Volucellini preserved in Baltic amber. These larvae resemble modern hoverfly larvae that co-occur with wood-borer moth larvae (Cossidae, Lepidoptera), feeding on the plant juices that seep after the moth larvae have damaged the plants (Speight 2010). Other extant larvae of Volucellini are associated with nests of eusocial hymenopterans (Marshall 2012). Such hymenopterans are rare in ambers, making it unlikely that the fossil larvae of Volucellini had such a life style (Baranov et al. 2021). Presumably, the hoverfly larvae represent an indirect indication of the presence of a specific type of plant parasites, namely wood-borer larvae in the Eocene amber forest; in fact, also larger abundances of them might be expected (see discussion in Baranov et al. 2021). Although in the modern fauna these seem mostly wood-borer larvae of the group Lepidoptera, also other types of wood-borer larvae could be expected to co-occur with the hoverfly larvae.
Actual fossils of solid-wood-borer larvae (mostly larvae of Buprestidae and Cerambycidae; for details see discussion in Haug et al. 2021) have so far only been occasionally reported in amber (e.g. Larsson 1978; Klausnitzer 2003; Nel et al. 2004; Peris 2020; Peris and Rust 2020). Larvae associated with rotting, i.e. dead wood seem to be more common (e.g. Micromalthidae: Kirejtshuk and Azar 2008; Perkovsky 2016; Eucnemidae: Chang et al. 2016; Scraptiidae: Haug and Haug 2019; Zippel et al. 2022a,b). Furthermore, larvae boring in solid wood have only rarely been figured (one specimen of Buprestidae in Cretaceous New Jersey amber: Grimaldi and Engel 2005, fig. 10.36, p. 381; two specimens of Cerambycidae in Eocene Baltic amber: Bachofen-Echt 1949; Gröhn 2015). Only recently, some larvae clearly representing solid-wood borers (Buprestidae and Cerambycidae) have been described with some detail from the Cretaceous, based on four specimens in 99 million-year-old Myanmar amber (Haug et al. 2021).
Overall, these finds provide a conflicting impression of the situation in amber forests. We have indirect indicators that the trees were under stress; this stress might have (partly) been caused by wood-borer larvae (but see also contradictory studies above), which then should have been a rather common component of the fauna. However, such larvae have so far only very rarely been found, indicating that they were not a common component.
Here we report new larvae of the beetle groups Buprestidae and Cerambycidae, representing solid-wood borers, from Eocene ambers (Baltic and Rovno amber; c. 35 million years old; for age determination, see, e.g. Perkovsky et al. 2010; Sukhomlyn et al. 2021; Matalin et al. 2021). We demonstrate that solid-wood-borer larvae seem to be much more common in ambers than so far anticipated.
Material and Methods
Material
In total, 13 specimens preserved in twelve pieces of amber were investigated. Ten amber pieces with eleven specimens are from Baltic amber, possibly some from the Yantarny mine, Kaliningrad Oblast, Sambian Peninsula (Russia), but the exact origin is unclear: SNSB-BSPG 2018 III 80 (Staatliche Naturwissenschaftliche Sammlungen Bayerns—Bayerische Staatssammlung für Paläontologie und Geologie, München), NHMD-157126 (Natural History Museum of Denmark, Copenhagen), SMF Be 8018, SMF Be 10595 (both Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt/Main), PED 1393, PED 1394 (with two specimens), PED 1410, PED 1477, PED 1601, PED 1602 (all publicly accessible at the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München). All PED specimens were legally purchased from various traders: Marius Veta, Vilnius, ambertreasure4u.com; AmberTreeLab via the platform etsy.com; Jonas Damzen, Vilnius, amberinclusions.eu; Rafiq A. Bhat, Pahalgam, Stars Magic Gems.
Two additional amber pieces with two specimens are Ukrainian Rovno amber, both from Varash district, Rovno Oblast: SIZK L-957 (Schmalhausen Institute of Zoology, Kiev) and PED 1479. The PED piece was legally purchased from a private collector and officially exported.
For comparison, an extant solid-wood-borer beetle larva of Cerambycidae was documented. The specimen is part of the collection of the Centrum für Naturkunde (CeNak), Leibniz-Institut zur Analyse des Biodiversitätswandels (LIB) Hamburg (ZMH 828298).
Some newly prepared volume renders of specimens of hoverfly larvae reported by Baranov et al. (2021) are shown for background information (Fig. 1a–e). For details about these specimens and methods, see Baranov et al. (2021).
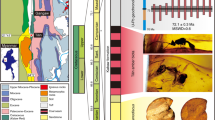
Volume renders of SRμCT and μCT scans of fossils from Eocene amber. a–e Hoverfly larvae of the group Volucellini. a, b Dip-00889. a Latero-ventral view. b Ventral view. c, d Dip-00895. c Oblique frontal view. d Terminal view. e Dip-00894, lateral view. f–i PED 1479, Rovno amber, giant larva of longhorn beetle (Cerambycidae). f Habitus in ventral view. g Close-up on anterior body region. h Close-up on head region in dorsal view. i Close-up on head region in ventral view. Abbreviations: at antenna; cl clypeus; hc head capsule; li labium; lr labrum; md mandible; mx maxilla
Methods
Amber specimens were mostly documented with optical methods. Most specimens were documented on a Keyence VHX-6000 digital microscope with standardised settings (Haug et al. 2019). All images are composite images (Haug et al. 2011), including imaging under different exposure times (Haug et al. 2013a). Some images are stereo anaglyphs based on virtual surfaces (Haug et al. 2013b, 2019). Larger amber pieces were scanned entirely with a flat-bed scanner (Haug et al. 2013c).
X-ray micro-computed tomography (μCT) of PED 1479 was performed using an XRadia MicroXCT-200 (Carl Zeiss Microscopy GmbH, Jena, Germany) equipped with switchable scintillator-objective lens units. Scanning parameters were: (1) overview: 0.39x objective, x-ray source settings: 40 kV, 8 W, exposure time 5.5 s, binning 2, image stack properties: 1024 x 1024 px, system-based calculated pixel size of 37.25 μm; (2) head: 0.39 x objective, x-ray source settings: 40 kV, 8 W, exposure time 5 s, binning 2, image stack properties: 1024 x 1024 px, system-based calculated pixel size of 25.96 μm. Tomographic images were reconstructed using XMReconstructor (Carl Zeiss Microscopy GmbH, Jena, Germany), resulting in image stacks (TIFF format). Tiff image stacks were postprocessed in Fiji (Schindelin et al. 2012). Volume renderings based on μCT data were generated using Amira 6 (FEI, Thermo Fisher Scientific) and Drishti 2.7 (Limaye 2012).
The extant specimen was documented with a super-macro-photographic setup (Canon EOS 650D + MP-E 65 mm) in its original storage liquid (70% ethanol). Lighting was provided by two Yongnuo Digital Speedlite YN560EX II flashes equipped with polarisation filters. Another polarisation filter was placed in front of the lens and arranged perpendicular to those on the flashlights, providing cross-polarized light (Haug et al. 2011). Also these images were recorded as composite images.
The larvae of Volucellini were scanned at the PETRA III (DESY Hamburg). For details of the methods, see Baranov et al. (2021).
Results
The screening of the above mentioned collections revealed an unexpected wealth of wood-borer larvae. Among the discovered specimens is a very large larva of at least 35 mm in length (Figs. 1f, g, 2b) preserved in a large piece of Rovno amber (Fig. 2a). Details of the head are not well accessible with light microscopy, but with μCT. Antennae short, clypeus and labrum well developed, prominent mandibles, other mouthparts small without prominent endites, palps only short (Fig. 1h, i). The head is distinctly surrounded by the anterior trunk (prothorax). The body is rather soft with distinct folds, but without sclerites. Ventrally, the anterior trunk segments (thorax) bear three pairs of tiny legs (Fig. 2c). Overall this arrangement is very similar to that in extant larvae of Cerambycidae (longhorn beetles; Fig. 2d).
Very similar appearing larvae occur in different body sizes. These reach from tiny ones (Fig. 3b), probably representing early stage larvae as mentioned in Larsson (1978) and Klausnitzer (2003), over slightly larger ones (Fig. 3e–h) and some of almost one centimetre in size (Fig. 3c, d) up to further specimens reaching into the centimetre range (Figs. 3a, i, 4a, b, h).
Additional specimens of wood-borer larvae in Eocene ambers. a–e Cerambycidae. a PED 1601, partial specimen. b SMF Be 10595. c–e Backsides of some larvae showing their damaged condition. c PED 1393. d, e Stereo anaglyphs (please use red-cyan glasses to view). d PED 1477. e SIZK L-957. f, g PED 1602, stereo anaglyphs, with tube-like tunnel in large amber piece, possibly representing remain of a wood-borer larva. h Overview of large amber piece, PED 1601 (in a)
An important detail of the larger specimens is that many of them are incomplete (Fig. 4). Larger animals are often not fully embedded into the amber as the resin flow was only partly able to cover them (e.g. birds: Xing et al. 2017, 2019, 2020; lizards: Daza et al. 2016; Čerňanský et al. 2022). After the carcass is rotten, this leaves a large cavity in the amber. While well preserved in the area where the specimen is embedded inside the amber, the other side is often heavily damaged (compare two sides of the same specimen: Fig. 4c vs. Fig. 3d; Fig. 4d vs. Fig. 3i; Fig. 4e vs. Fig. 3a). The inclusion is exposed here and appears to oxidise and simply crumble away.
There are rather large amber pieces with tube-like tunnels through them with a heavily oxidised and entirely destroyed surface (Fig. 4f, g). These tubes are in the size range of some of the larger larvae reported here. We suggest that one possible origin of these tubes is that originally a wood-borer larva was embedded, but not fully. It then rotted and the surface of the inclusion became heavily oxidised, leaving no detail of the original larva.
Discussion
Identity of the specimens
Convergent specialisation to wood boring seems to lead to similar morphologies in beetle larvae and other larvae of Holometabola. The larvae reported here are interpreted either as larvae of the group Buprestidae (jewel beetles; Fig. 3e, f, h) or Cerambycidae (longhorn beetles; Figs. 2, 3a–d, g, i). Many modern jewel beetle larvae have a distinct V-shaped fold on the prothorax, well apparent in some of the fossils (Fig. 3e, f, h)
Longhorn beetle larvae may look on a first glance very similar to moth larvae of the group Castniidae; yet in the latter the first pair of legs is much further anterior (Sarto i Monteys and Aguilar 2005a p. 2) than in longhorn beetle larvae, similar to the condition in many of the fossils (Fig. 3b, d, i). Also, in longhorn beetle larvae there is a distinct fold separating the posterior part of the prothorax; such a fold is well apparent in most of the fossils (Fig. 3b, c, g), but absent in extant larvae of Castniidae. Furthermore, in larvae of Castniidae only four pairs of prominent creeping processes are present in the posterior trunk (abdomen; Alario 2005 fig. 5 p. 245; Sarto i Monteys and Aguilar 2005a p. 2, 2005b fig. 11a p. 72; Lopez-Vaamonde and Lees 2010 p. 990 right) with the corresponding segments being larger, while in the fossils and modern longhorn beetle larvae more segments bear creeping welts and are more similar in size (Figs. 2, 3a–d, g, i). All these characters therefore support that the fossils are indeed larvae of Buprestidae and Cerambycidae. The assumed relationship of the larvae and their morphology strongly indicate that these are solid-wood borers.
Wood-borers seem not to be rare in Eocene amber
So far, few sources have mentioned solid-wood-borer beetle larvae in Eocene ambers (Larsson 1978; Klausnitzer 2003; Nel et al. 2004) and in only two cases specimens have been figured (Bachofen-Echt 1949 p. 115 fig. 102, specimen not found in museum collections in Munich and Vienna; Gröhn 2015 p. 272). We were able to compile no less than twelve additional solid-wood-borer beetle larvae from Eocene ambers, including rather small specimens of about 2 mm (Fig. 3b), to a specimen of at least 35 mm in length (Fig. 2). Ambers seem to tend to preserve smaller specimens (though the situation seems to be more complex and also includes different preservation potential due to differences in behaviour, e.g. Penney 2002; Penney and Langan 2006; Solórzano Kraemer et al. 2015, 2018). A larva of several centimetres in size is therefore quite unusual. The specimen might indeed represent the so far largest beetle larva (or even holometabolan larva) preserved in amber.
These finds demonstrate that solid-wood-borer larvae may be not as rarely preserved in ambers as assumed. Larval forms preserved in amber seem to be less intensely studied than adults (examples for adults in Eocene amber: Larsson 1978; Bellamy 1995; Vitali 2009a, b, 2014; Vitali and Perkovsky 2022) as these are less easy to be interpreted in a phylogenetic framework. Adults of solid wood-borer beetle larvae have been found in Eocene ambers quite often in comparison to the occurrence of such adults in modern resins (Zherikhin et al. 2009).
The dominance of studying adults also applies to the fossils here. Despite the fact that further reaching phylogenetic interpretations are difficult for beetle larvae, it remains important to emphasise that in holometabolans such as beetles often major parts of the lifetime are spent as larvae and that the major interactions with other components of the ecosystem occur in this phase. Hence, the ecological impact of larvae should not be underestimated. Furthermore, it is important that presence of adults is not a good direct indicator for presence of larvae of distinct eco-types (see discussion in Baranov et al. 2019), but the direct finding of these larvae clearly allows to draw conclusions about their ecological role. Yet, biased search for adults may only be one factor leading to the impression that wood-borer larvae would be rare.
Another factor may indeed be preservation. The often damaged condition of the larger larvae hints to the possibility that at least some, if not many, of the heavily oxidised tubes preserved in larger pieces of amber may represent remains of larger wood-borer larvae, which would make these even more common. In other words, solid-wood-borer larvae seem not to have been rare in the Eocene amber forest fauna.
This possibility might fit with the rather large abundances of hoverfly larvae of the group Volucellini, which also co-occur with plant parasites in the modern fauna (see above). Together, both factors would fit into a picture of an amber forest under stress, causing the trees to produce larger amounts of resins, which ultimately led to amber (McKellar et al. 2011; Baranov et al. 2021; but see also above). However, it remains difficult to draw causal conclusions here. It currently remains unclear whether such a possibly higher abundance of solid-wood-borer larvae was the stressor for the trees or just a symptom for an already stressed forest due to another stressor (see also discussion in Peris 2020). Some modern larvae of Buprestidae and Cerambycidae also have slightly different ecological roles as leaf miners or root feeders (e.g. Bellamy and Volkovitsh 2005; Grebennikov 2013; see discussion in Haug et al. 2021). If the fossil larvae had such roles, at least in part, they would have still represented stressors for the trees, but would not have had an influence on the density of hoverfly larvae.
Data availability statement
The SRμCT and μCT datasets analysed during the current study are available in the repository www.morphdbase.de under the following links:
Dip-00889 (Fig. 1a, b): https://www.morphdbase.de/?V_Baranov_20210130-S-7.1
Dip-00895 (Fig. 1c, d): https://www.morphdbase.de/?V_Baranov_20210130-S-5.1
Dip-00894 (Fig. 1e): https://www.morphdbase.de/?V_Baranov_20220922-M-56.1
PED 1479 (Fig. 1f–i): https://www.morphdbase.de/?V_Baranov_20220927-S-17.1 and https://www.morphdbase.de/?V_Baranov_20220927-S-18.1
References
Alario, S. M. (2005). Paysandisia archon (Burmeister, 1880) (Lepidoptera, Castniidae), nuevas localizaciones en la Península Ibérica y su gestión. Boletín de la Sociedad Entomológica Aragonesa, 34, 237–236.
Bachofen-Echt, A. (1949). Der Bernstein und seine Einschlüsse. Springer (reprint by Jörg Wunderlich Verlag 1996).
Baranov, V., Hoffeins, C., Hoffeins, H.-W., & Haug, J. T. (2019). More than dead males: reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bulletin of Geosciences, 94, 187–199. https://doi.org/10.3140/bull.geosci.1739
Baranov, V. A., Engel, M. S., Hammel, J., Hörnig, M. K., van de Kamp, T., Zuber, M., & Haug, J.T. (2021). Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest. Palaeontologia Electronica, 24(1), a07. https://doi.org/10.26879/1129
Bellamy, C. L. (1995). Buprestidae (Coleoptera) from amber deposits: A brief review and family switch. The Coleopterists’ Bulletin, 49, 175–177.
Bellamy, C. L., & Volkovitsh, M. G. (2005) Chapter 17. Buprestoidea Crowson, 1955. In R. G. Beutel, & R. A. B. Leschen (Eds.), Handbuch der Zoologie/Handbook of Zoology, Volume IV, Arthropoda: Insecta, Part 38, Coleoptera, Beetles, Volume 1: Morphology and systematics (pp. 461–468). Berlin: de Gruyter.
Čerňanský, A., Stanley, E. L., Daza, J. D., Bolet, A., Arias, J. S., Bauer, A. M., Vidal-García, M., Bevitt, J. J., Peretti, A. M., Aung, N. N., & Evans, S. E. (2022). A new Early Cretaceous lizard in Myanmar amber with exceptionally preserved integument. Scientific Reports, 12, 1660. https://doi.org/10.1038/s41598-022-05735-5
Chang, H., Muona, J., Hanyong, P., Li, X., Chen, W., Teräväinen, M., Dong, R., Qiang, Y., Xingliao, Z., & Songhai, J. (2016). Chinese Cretaceous larva exposes a southern Californian living fossil (Insecta, Coleoptera, Eucnemidae). Cladistics, 32, 211–214. https://doi.org/10.1111/cla.12124
Daza, J. D., Stanley, E. L., Wagner, P., Bauer, A. M., & Grimaldi, D. A. (2016). Mid-Cretaceous amber fossils illuminate the past diversity of tropical lizards. Science Advances, 2(3), e1501080. https://doi.org/10.1126/sciadv.1501080
Grebennikov, V. V. (2013) Life in two dimensions or keeping your head down: Lateral exuvial splits in leaf-mining larvae of Pachyschelus (Coleoptera: Buprestidae) and Cameraria (Lepidoptera: Gracillariidae). European Journal of Entomology, 110, 165–172. https://doi.org/10.14411/eje.2013.024
Grimaldi, D., & Engel, M. S. (2005). Evolution of the Insects. Cambridge: Cambridge University Press.
Gröhn, C. (2015). Einschlüsse im Baltischen Bernstein. Kiel: Wachholtz Verlag.
Haug, C., Shannon, K. R., Nyborg, T., & Vega, F. J. (2013a). Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Bolétin de la Sociedad Geológica Mexicana, 65, 273–284. https://doi.org/10.18268/bsgm2013v65n2a9
Haug, C., Kutschera, V., Ahyong, S. T., Vega, F. J., Maas, A., Waloszek, D., & Haug, J. T. (2013c). Re-evaluation of the Mesozoic mantis shrimp Ursquilla yehoachi based on new material and the virtual peel technique. Palaeontologia Electronica, 16, art. 16.2.5T. https://doi.org/10.26879/340
Haug, C., Haug, G. T., Zippel, A., van der Wal, S., & Haug, J. T. (2021). The earliest record of fossil solid-wood-borer larvae—immature beetles in 99 million-year-old Myanmar amber. Palaeoentomology, 4, 390–404. https://doi.org/10.11646/palaeoentomology.4.4.14
Haug, J. T., & Haug, C. (2019). Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera). PeerJ, 7, e7871. https://doi.org/10.7717/peerj.7871
Haug, J. T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E. N. K., & Waloszek, D. (2011). Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244, 259–272. https://doi.org/10.1111/j.1365-2818.2011.03534.x
Haug, J. T., Müller, C. H. G., & Sombke, A. (2013b). A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity & Evolution, 13, 425–432. https://doi.org/10.1007/s13127-013-0129-3
Haug, J. T., Müller, P., & Haug, C. (2019). A 100-million-year old predator: a fossil neuropteran larva with unusually elongated mouthparts. Zoological Letters, 5, 29. https://doi.org/10.1186/s40851-019-0144-0
Kirejtshuk, A .G., & Azar, D. (2008). New taxa of beetles (Insecta, Coleoptera) from Lebanese amber with evolutionary and systematic comments. Alavesia, 2(15), e46.
Klausnitzer, B. (2003). Käferlarven (Insecta: Coleoptera) in Baltischem Bernstein—Möglichkeiten und Grenzen der Bestimmung. Entomologische Abhandlungen, 61, 103–108.
Labandeira, C. C. (2014). Amber. In M. LaFlamme, J. D. Schiffbauer, & S. A. F. Darroch (Eds.), Reading and Writing of the Fossil Record: Preservational Pathways to Exceptional Fossilization: Presented as a Paleontological Society Short Course at the Annual Meeting of the Geological Society of America, Vancouver, British Columbia, October 18, 2014 (pp. 163–215). Paleontological Society Papers, 20. Paleontological Society.
Larsson, S. G. (1978). Baltic Amber: a Palaeobiological Study. Klampenborg: Scandinavian Science Press.
Limaye, A. (2012). Drishti: a volume exploration and presentation tool. Developments in X-ray Tomography VIII, Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE), 8506, 85060X. https://doi.org/10.1117/12.935640
Lopez-Vaamonde, C., & Lees, D. (2010). Paysandisia archon (Burmeister, 1879) - The castniid palm borer (Lepidoptera, Castniidae). Chapter 14: Factsheets for 80 representative alien species. Alien terrestrial arthropods of Europe, 4, 990–991. Sofia: Pensoft Publishers.
Marshall, S. A. (2012). Flies: the Natural History and Diversity of Diptera. Firefly Books.
Martínez-Delclòs, X., Briggs, D. E., & Peñalver, E. (2004). Taphonomy of insects in carbonates and amber. Palaeogeography, Palaeoclimatology, Palaeoecology, 203, 19–64. https://doi.org/10.1016/S0031-0182(03)00643-6
Matalin, A. V., Perkovsky, E. E., & Vasilenko, D. V. (2021). First record of tiger beetles (Coleoptera, Cicindelidae) from Rovno amber with the description of a new genus and species. Zootaxa, 5016(2), 243–256. https://doi.org/10.11646/zootaxa.5016.2.5
McKellar, R. C., Wolfe, A. P., Muehlenbachs, K., Tappert, R., Engel, M. S., Cheng, T., & Sánchez-Azofeifa, A. G. (2011). Insect outbreaks produce distinctive carbon isotope signatures in defensive resins and fossiliferous ambers. Proceedings of the Royal Society B: Biological Sciences, 278, 3219–3224. https://doi.org/10.1098/rspb.2011.0276
Nel, A., De Ploëg, G., Milliet, J., Menier, J. J., & Waller, A. (2004). The French ambers: a general conspectus and the Lowermost Eocene amber deposit of Le Quesnoy in the Paris Basin. Geologica Acta, 2(1), 3–8.
Penney, D. (2002). Paleoecology of Dominican amber preservation: spider (Araneae) inclusions demonstrate a bias for active, trunk-dwelling faunas. Paleobiology, 28(3), 389–398.
Penney, D. (2016). Amber Palaeobiology: Research Trends and Perspectives for the 21st Century. Siri Scientific Press.
Penney, D., & Langan, A. M. (2006). Comparing amber fossil assemblages across the Cenozoic. Biology Letters, 2(2), 266–270. https://doi.org/10.1098/rsbl.2006.0442
Peris, D. (2020). Coleoptera in amber from Cretaceous resiniferous forests. Cretaceous Research, 113, 104484. https://doi.org/10.1016/j.cretres.2020.104484
Peris, D., & Rust, J. (2020). Cretaceous beetles (Insecta: Coleoptera) in amber: the palaeoecology of this most diverse group of insects. Zoological Journal of the Linnean Society, 189, 1085–1104. https://doi.org/10.1093/zoolinnean/zlz118
Peris, D., Ruzzier, E., Perrichot, V., & Delclòs, X. (2016). Evolutionary and paleobiological implications of Coleoptera (Insecta) from Tethyan-influenced Cretaceous ambers. Geoscience Frontiers, 7, 695–706. https://doi.org/10.1016/j.gsf.2015.12.007
Peris, D., Delclòs, X., & Jordal, B. (2021). Origin and evolution of fungus farming in wood-boring Coleoptera – a palaeontological perspective. Biological Reviews, 96, 2476–2488. https://doi.org/10.1111/brv.12763
Perkovsky, E. E. (2016). A new species of Micromalthidae (Coleoptera) from the Rovno Amber: 1. Adult morphology. Paleontological Journal, 50, 293–296. https://doi.org/10.1134/S0031030116030047
Perkovsky, E. E., Zosimovich, V. Yu., & Vlaskin, A. P. (2010). Rovno amber, In D. Penney (Ed.), Biodiversity of Fossils in Amber from the Major World Deposits (pp. 116–136). Rochdale: Siri Scientific Press.
Sarto i Monteys, V., & Aguilar, L. (2005a). El cástnido barrenador de las palmeras, Paysandisia archon, una nueva plaga en Europa. XXII Reunió anual del Grupo de trabajo fitosanitario de forestales, parques y jardines, Lloret de Mar, 7–10 de Novembre de 2005, pp. 1–11.
Sarto i Monteys, V., & Aguilar, L. (2005b). The castniid palm borer, Paysandisia archon (Burmeister, 1880), in Europe: comparative biology, pest status and possible control methods (Lepidoptera: Castniidae). Nachrichten des Entomologischen Vereins Apollo, 26(1/2), 61–94.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9, 676–682. https://doi.org/10.1038/nmeth.2019
Seyfullah, L. J., Roghi, G., Dal Corso, J., & Schmidt, A. R. (2018). The Carnian Pluvial Episode and the first global appearance of amber. Journal of the Geological Society, 175, 1012–1018. https://doi.org/10.1144/jgs2017-143
Solórzano Kraemer, M. M., Kraemer, A. S., Stebner, F., Bickel, D. J., & Rust, J. (2015). Entrapment bias of arthropods in Miocene amber revealed by trapping experiments in a tropical forest in Chiapas, Mexico. PLoS ONE, 10(3), e0118820. https://doi.org/10.1371/journal.pone.0118820
Solórzano Kraemer, M. M., Delclòs, X., Clapham, M. E., Arillo, A., Peris, D., Jäger, P., Stebner, F., & Peñalver, E. (2018). Arthropods in modern resins reveal if amber accurately recorded forest arthropod communities. Proceedings of the National Academy of Sciences, 115(26), 6739–6744. https://doi.org/10.1073/pnas.1802138115
Speight, M. C. D. (2010). Species accounts of European Syrphidae (Diptera). In M. C. D. Speight, E. Castella, J.-P. Sarthou, & C. Monteil (Eds.), Syrph the Net, the database of European Syrphidae, 44, Syrph the Net Publication, Dublin. Accessed at 15 September (2020). https://www.semanticscholar.org/paper/the-syrph-the-net-database-of-european-syrphidaeSpeight/a9a7f071a4a8d6756fc9fcf113485c4f563fd5ed
Sukhomlyn, M. M., Heluta, V. P., Perkovsky, E. E., Ignatov, M. S., & Vasilenko, D. V. (2021). First record of fungus of the family Mycocaliciaceae in Rovno amber (Ukraine). Paleontological Journal, 55, 684–690. https://doi.org/10.1134/s0031030121060125
Vitali, F. (2009a). The cerambycids included in Baltic amber: current knowledge status with the description of new taxa (Coleoptera, Cerambycidae). Denisia, 26, 231–242.
Vitali, F. (2009b). About some interesting fossil and sub-fossil cerambycids of the collection Velten (Coleoptera, Cerambycidae). Lambillionea, CIX, 352–357.
Vitali, F. (2014). New fossil cerambycids (Coleoptera: Cerambycidae) from Baltic amber belonging to the collection Hoffeins. Baltic Journal of Coleopterology, 14(1), 103–112.
Vitali, F, & Perkovsky, E. E. (2022). Poliaenus europaeus n. sp., the first cerambycid from Rovno amber (Coleoptera Cerambycidae). Historical Biology. https://doi.org/10.1080/08912963.2022.2082295
Xing, L., O'Connor, J. K., McKellar, R. C., Chiappe, L. M., Tseng, K., Li, G., & Bai, M. (2017). A mid-Cretaceous enantiornithine (Aves) hatchling preserved in Burmese amber with unusual plumage. Gondwana Research, 49, 264–277. https://doi.org/10.1016/j.gr.2017.06.001
Xing, L., McKellar, R. C., O’Connor, J. K., Niu, K., & Mai, H. (2019). A mid-Cretaceous enantiornithine foot and tail feather preserved in Burmese amber. Scientific Reports, 9(1), 15513. https://doi.org/10.1038/s41598-019-51929-9
Xing, L., McKellar, R. C., & O'Connor, J. K. (2020). An unusually large bird wing in mid-Cretaceous Burmese amber. Cretaceous Research, 110, 104412. https://doi.org/10.1016/j.cretres.2020.104412
Zherikhin, V. V., Sukacheva, I. D., & Rasnitsyn, A. P. (2009). Arthropods in contemporary and some fossil resins. Paleontological Journal, 43, 987–1005. https://doi.org/10.1134/s0031030109090019.
Zippel, A., Haug, C., Müller, P., & Haug, J. T. (2022a). The first fossil false click beetle larva preserved in amber. PalZ. https://doi.org/10.1007/s12542-022-00638-2.
Zippel, A., Haug, C., Hoffeins, C., Hoffeins, H.-W., & Haug, J. T. (2022b). Expanding the record of larvae of false flower beetles with prominent terminal ends. Rivista Italiana di Paleontologia e Stratigrafia, 128, 81–104. https://doi.org/10.54103/2039-4942/17084
Acknowledgements
We thank Peter Koenigshof (Frankfurt/Main) for handling the manuscript, and David Peris (Universitat de Barcelona) and one anonymous reviewer for helpful comments, which improved the manuscript. We are grateful to Mónica M. Solórzano-Kraemer (Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt am Main), Lars Vilhelmsen (Natural History Museum of Denmark, Copenhagen), Mike Reich (formerly Staatliche Naturwissenschaftliche Sammlungen Bayerns—Bayerische Staatssammlung für Paläontologie und Geologie, München, now Staatliches Naturhistorisches Museum Braunschweig), and Martin Husemann and Thure Dalsgaard (CeNak, Leibniz-Institut zur Analyse des Biodiversitätswandels LIB Hamburg) for providing access to the material. We thank the students from LMU Munich for assisting with imaging during the excursion to Hamburg in 2019. We furthermore thank the Imaging Center of the Department of Biology, University of Greifswald and the German Research Foundation (DFG INST 292/119-1 FUGG; DFG INST 292/120-1 FUGG). Our thanks go to J. Matthias Starck, Munich, for long-time support. We are grateful to all people providing low-cost, open-access or open-source software. This is LEON publication #33.
Funding
Open Access funding enabled and organised by Projekt DEAL. JTH is supported by the Volkswagen Foundation (Lichtenberg professorship) and by the German Research Foundation (DFG Ha 6300/6-1). Lehre@LMU and the Faculty of Biology of the LMU funded the student excursion to Hamburg. The German Research Foundation supported part of the major research instrumentation at the University of Greifswald used for imaging (DFG INST 292/119-1 FUGG; DFG INST 292/120-1 FUGG). The larvae of Volucellini were scanned at beamline p05. Beamtime was allocated for proposal BAG-20210019 “Scanning the past - Reconstructing the diversity in million years old fossil amber specimens using SRμCT” at PETRA III.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haug, C., Baranov, V.A., Hörnig, M.K. et al. 35 million-year-old solid-wood-borer beetle larvae support the idea of stressed Eocene amber forests. Palaeobio Palaeoenv 103, 521–530 (2023). https://doi.org/10.1007/s12549-022-00552-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12549-022-00552-0