Abstract
As the third part of an ongoing investigation of middle Miocene palynofloras in the Yatağan Basin (YB), southwestern Anatolia, the palynofloras of the Salihpaşalar lignite mine in the main YB were studied. Seven types of algal spores, aplanospores/zygospores or cysts, six types of lycophyte and fern spores, 12 types of gymnosperm pollen and 90 types of angiosperm pollen were identified. Of a total of ca. 140 plant taxa described from the YB, over 10% are confined to the Salihpaşalar assemblage. Differences between coeval palynofloras of the Sekköy Member might reflect changing or prograding depositional environments. A number of rare accessorial taxa reflect these local differences: Pilularia, Valeriana, Drosera and Persicaria aff. amphibia only occur at Salihpaşalar and are typical of shallow water or temporary ponds associated with a lake shore. Apart from this, all the palynofloras, originating from the lignite seams and overlying limnic limestones (uppermost Turgut and Sekköy Member), of the YB are strongly indicative of extensive woody vegetation with a dominance of diverse Fagaceae and Pinaceae. In addition, a list comparing the well-documented YB palynomorphs to morphologically similar palynomorphs of published late early to middle Miocene plant assemblages of western Anatolian was compiled. Such a comparison reveals that in many instances different taxon names have been used to denote the same taxa. Hence, resolving these synonymies is a prerequisite of any meaningful comparison of palynofloras in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High tectonic activity in western Anatolia during the late Cenozoic associated with the collision of the Arabian and Anatolian plates resulted in orogeny, volcanic activity and basin formation (Rögl 1999; Popov et al. 2004; van Hinsbergen and Schmid, 2012). In southwestern Anatolia, the Denizli, Söke and Yatağan Basin (YB) among others formed with the onset of the Miocene subsidence (Alçiçek 2010). Age correlation of these Miocene fault-bounded basins is complicated and previously suggested ages for individual basins based on different proxies have been highly controversial. Specifically, it has been demonstrated that palynological data alone are not sufficient to reliably infer the age of a particular basin (Bouchal et al. 2016). Examples from western Anatolia for conflicting ages inferred from palynological data and radiometric dating include the Soma Basin, for which Akgün et al. (2007) suggested a middle Miocene age whereas radiometric dates from Ersoy et al. (2014) suggest an early Burdigalian age, the Gördes Basin, for which Akgün et al. (2007) suggested an early to middle Serravallian age, while Purvis et al. (2005) based on radiometric dates inferred a late Aquitanian to early Burdigalian age based on radiometric dates, and the Bigadiç Basin, for which Akyol and Akgün (1990) inferred a middle to earliest late Miocene age, while Erkül et al. (2005) suggested an early Burdigalian age, based, again, on radiometric dating. Complementary data, such as vertebrate fossils or radiometrically dated layers, are hence of utmost importance for reliably inferring the age of a fossil locality.
Another issue that hampers the comparison of Neogene sedimentary basins in western Anatolia relates to the two concepts applied for the naming of dispersed fossil pollen and spores. A substantial number of studies have been using the artificial fossil nomenclature (strictly morphology based) for palynomorphs, while in more recent publications, conventional botanical names are used more often. Therefore, it maybe difficult to compare studies for a particular area, potentially leading to highly redundant taxon lists.
The present contribution is the third in a series of papers dealing with the palynology of middle Miocene (MN6–MN7/8) deposits of the upper Turgut and Sekköy Member of the Eskihisar Formation in southwestern Anatolia (Bouchal et al. 2016, 2017). Previous palynological studies have concentrated on the Eskihisar and the Tınaz lignite mines and vertebrate localities (e.g. Benda 1971; Gemici et al. 1990; Erdei et al. 2002; Akgün et al. 2007; Yavuz-Işık et al. 2011; Koç, 2017; Bouchal et al. 2016, 2017).
The present investigation focuses on the Salihpaşalar lignite mine located to the south of the main YB that was exploited for a short period between 2012 and 2015. The sections studied in previous papers and in the present paper are strongly correlated by the palynological content of the lignite seams overlying limestones and their age is well-constrained by vertebrate fossils recently identified from the main coal seam of the Eskihisar lignite mine (electronic supplementary material in Bouchal et al., 2017) and the fossil vertebrate locality Yeni Eskihisar (Sickenberg 1975; Saraç 2003; Wessels 2009; The NOW Community 2018).
In this paper, I will (i) describe palynological assemblages from a stratigraphic column at Salihpaşalar using a combined scanning electron and light microscopy approach, (ii) compare the palynological records of three lignite mines in the YB, and (iii) discuss the palaeoenvironmental signal of the palynological data from the YB palynofloras. In addition, a list comparing the well-documented YB palyno-morphs to morphologically similar pollen of other late early to middle Miocene palynoassemblages of western Anatolian strata was compiled [Supplementary Material (S) 2]. This is an attempt to accommodate previous concepts of palynological nomenclature (strictly morphology based) and modern concepts of applying botanical names to palynomorphs for this region.
Materials and methods
Geological setting
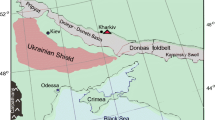
The YB, Muğla, southwestern Turkey, is a southeast-trending graben, up to 50 km long and 15 km wide (Fig. 1a, b). The basin is divided by an axial bedrock horst (metamorphic cover rocks of the Menderes Massif) in the smaller (ca 12 km long, 1 km wide, south east trending) Tınaz subbasin (Fig. 1b) and the main YB. The northern area of the main basin between Eskihisar and Turgut (west and north of Aladağ Mountain) is segregated from the southern part by a bedrock sill (Fig. 1b; Atalay 1980; Becker-Platen 1970). The Neogene basin fill is up to 600 m thick and grouped into the Eskihisar Formation (early to middle Miocene), the Yatağan Formation (late Miocene to early Pliocene), and the Milet Formation (middle to late Pliocene). The Eskihisar Formation is subdivided in Turgut Member (reddened alluvial-fan deposits followed by fluviatile deposits and lignites) and Sekköy Member (limnic marls and limestones; Fig. 1c). For the present study, the type profiles of the Turgut and Sekköy members as designated by Becker-Platen (1970, p. 22 ff. and pp. 26–27) were used as reference points and the boundary placed between the two members where the change from siliciclastic (non-carbonatic) to carbonate sedimentary rocks occurs. Five lignite fields (Eskihisar field, Tınaz field, Bağyaka field, Bayir field, Turgut field) have been prospected in the YB. All economically exploited lignite seams of the YB are confined to the transition zone of the Turgut and Sekköy members of the Eskihisar Formation (Becker-Platen 1970; Inaner et al. 2008).
Geographic and regional geologic setting of the Salihpaşalar lignite mine section, Yatağan basin. a Map showing the geographical position of the Yatağan Basin (b). b Simplified regional geological map of the Yatağan Basin based on Atalay (1980), Becker-Platen (1970) and Inaner et al. (2008); basin subdivisions main Yatağan Basin (MYB), Tınaz subbasin (TSB), Eskihisar-Turgut area (ETA); lignite mines Salihpaşalar (A), Tınaz (B), Bağyaka (C) and Eskihisar (D). Asterisk: vertebrate fossil localities (1) Salihpaşalar-Kemikalan (MN12) and Salihpaşalar-Karaağaç (MN12), (2) Yeni Eskhihisar 1 (MN7/8). c Profile of the sampled Salihpaşalar mine section. The sampled section belongs to the Eskihisar Formation and includes the uppermost part of the Turgut Member, which is here considered to be of Langhian (middle middle Miocene) age
Age of the Salihpaşalar lignite mine section
The stratigraphic chart of the mediterranean and parathetyan realm has been going through several changes since the 1970s; for stratigraphic ages and ranges of Becker-Platen (1970), the updated corresponding age is provided based on the compiled stratigraphic chart of Neubauer et al. (2015). In the overview map of the YB, Becker-Platen (1970, pl. 3) assigned an area (ca 2 km long, 0.5 km wide) east of the village of Salihpaşalar to the Sekköy Member (=Sekköy Formation in Becker-Platen). Additionally, the following age constraining fossils are listed (sample localities P419, P431, P434 in Becker-Platen 1970): pulmonate gastropods—Clausilla sp.; Ostracoda—Cytheromorpha zinndorfi, Loxoconcha granosa, Hemicythere folliculosa, Cyprideis cf. littoralis → Helvet to Pannon (=middle to late Miocene age); pharyngeal teeth of cyprinids—“Cypridopsis” biplanata → Torton (=early late Miocene). Atalay (1980) reported two vertebrate fossil localities NEE of the village Salihpaşalar (Salihpaşalar-Kemikalan, Salihpaşalar-Karaağaç; both assigned to MN12, late Tortonian to early Messinian; The NOW Community 2018) and assigned the rocks of this area to the late Miocene Yatağan Formation. So far, the stratigraphic relevant animal taxa of the Salihpaşalar lignite mine have not been investigated (Serdar Mayda, personal communication 2017). The here documented palynoflora corresponds to previously reported pollen floras and leaf assemblages of the YB lignite mines (Tables 1 and 2; Bouchal et al. 2016, 2017; Güner et al. 2017); for the macro and micro floras, an age of MN6 to MN7/8 is proposed due to their lithostratigraphic position between the vertebrate locality Eskihisar Gallery (MN6) and the younger Yeni Eskihisar locality [MN(7)/8] (Bouchal et al. 2017; Wessels 2009). Tuff layers from the upper Sekköy Member (Yeni Eskihisar II mammal locality) produced radiometric dates of 13.2 Ma ± 0.35 and 11.2 ± 0.2 (Becker-Platen et al. 1977).
The Salihpaşalar mine and other lignite mines of the YB
The Salihpaşalar open cast lignite mine is located close to the D-550 motorway, southeast of the village of Salihpaşalar (Fig. 1b) and is the southernmost part of the Bayir lignite field (Inaner et al. 2008). The main lignite seam of the Bayir lignite field is up to 18 m thick and split by several faults, leading to highly varying thickness of overburden (5–15 m at Salihpaşalar lignite mine, up to 493 m in other parts of the lignite field, see table 3 in Inaner et al. 2008). Three further opencast lignite mines are currently active in the YB, (i) the Eskihisar lignite mine located west of the Aladağ Mountain exploiting the Eskihisar and Turgut lignite fields; (ii) the Tınaz lignite mine, located in the southern part of the Tınaz subbasin; and (iii) the Bağyaka lignite mine, located in the northen part of the Tınaz subbasin (Fig. 1b). In recent years, the plant macrofossils and palynogical assemblages of the Eskihisar and Tınaz lignite mines have been the focus of a rather high number of studies (Akgün et al. 2007; Benda 1971; Bouchal et al. 2016, 2017; Erdei et al. 2002; Gemici et al. 1990; Güner et al. 2017; Koç, 2017; Yavuz-Işık et al. 2011).
Sampled stratigraphic section
A stratigraphic column comprising 25 m (hereafter Salihpaşalar section; Fig. 1c) was sampled at 1-m intervals (mainly siliciclastic and marly sedimentary rocks alternated by lignite seams). Twenty-seven samples were collected of which 16 were suitable for palynological analysis (detailed information on samples is provided in S 1). The base of the Salihpaşalar section starts below the excavated lignite seam (uppermost part of the Turgut Member) with weakly consolidated, blueish-grey to dark grey, micaceous clayey siltstones. They are followed by a section of thin lignite seams (2–20 cm) intercalated with silty claystones (1–10 cm); indeterminable plant debris is common in these layers. This section is succeeded by the main lignite layers, which have a combined thickness of three to four meters. Its lower part is intercalated with thin clayey siltstones (> 1 cm), which are replaced in the upper part by light-grey to blueish-grey marls and clayey limestones (> 1 cm). The succeeding section comprises 19 m of grey clayey limestones and blueish-grey marls (0.01–2 m) alternating with lignites (1–50 cm). Leaf and root fossils as well as gastropods and mollusc shell debris occur in this part of the section but are rare or restricted to single horizons. The following yellowish-grey to yellowish-red clayey limestones were not further investigated or sampled. In 2015, the mining activity ceased and the pit was flooded with groundwater; therefore, the profile is no longer accessible.
Sample processing and data
Sedimentary rock samples were processed following the protocol described in Grímsson et al. (2008) and the same pollen grains were investigated using LM and SEM (single grain method, Zetter 1989). LM photographs of dispersed fossil pollen were taken with an Olympus BX51 microscope equipped with an Olympus DP71 camera. Specimens were sputter coated with gold and photographed using a Hitachi S-4300 cold field emission scanning electron microscope. For the pollen diagram, 400 palynomorphs were counted per sample. The terminology for pollen morphology follows mostly Punt et al. (2007) and Hesse et al. (2009). The pollen diagram (Fig. 2) was generated in C2 vers. 1.7.6, maps and sections were drawn in Adobe Illustrator 15.0.0 and photographs were cropped in Adobe Photoshop 12.0. Sediment samples, processed samples and SEM stubs are stored at the Swedish Museum of Natural History, Stockholm, under accession numbers S153622 to S153648.
LM pollen count diagram of the Salihpaşalar mine section showing percentages of taxa. Abundance in percent. + = rare (< 2.5%); x = Botryococcus colonies present, xx = B. colonies frequently encountered. Asterisk: taxon comprises several genera or infrageneric groups discernible in SEM but not in LM. Hence, these genera are not considered in the pollen diagram. Abbreviations: S = small; L = large; SM = Smilax; AR = Arceuthobium; VA = Valeriana; CE = Centranthus. N = 400 pollen per sample. Sample numbers are indicated next to the simplified stratigraphic section
Systematic palynology
Table 1 lists all taxa identified for and their occurences through the Salihpaşalar section. A number of taxa have previously been described, discussed and figured from adjacent localities and coeval strata of the upper Turgut and lower Sekköy members (Bouchal et al. 2016, 2017). These taxa, although from different lignite mines of the YB, are not described here. Light microscopy and SEM documentation of all palynotaxa is provided in S 3.
Algae
Class Trebouxiophyceae Friedl, 1995
Family Botryococcaceae
Genus Botryococcus Kürtzing, 1849
Botryococcus cf. B. braunii Kürtzing, 1849
(Fig. 3a and S 3-Figs. 1a–c)
LM overview (a–c, e, f, h, j, l, n–p) and SEM detail (d, g, i, k, m) micrographs of Botryococcaceae, Zygnemataceae, algal cysts, Osmundaceae, Ephedraceae and spores of uncertain affinities. aBotryococcus cf. B. braunii (S153627). bSpirogyra sp. 1/Ovoidites lanceolatus (S153623). c, dSpirogyra sp. 2/Ovoidites microfoveolatus (S153635), (d) surface detail. eSpirogyra sp. 3/Ovoidites spriggii (S153623). f–iSpirogyra sp. 4/Cycloovoidites cyclus (SI153623), (g, i) suface detail. jk Algal cyst type 1 (S153622). k Surface detail. l, m Algal cyst indet. 2/Sigmopollis. pseudosetarius (S153623). m Surface detail. nOsmunda sp./Baculatisporites nanus, PV. o Monolete spore fam. et gen. indet./Laevigatosporites haardti (S153622), EV. pEphedra sp./Ephedripites (Distachyapites) tertiarius (S153637), EV. Abbreviations: polar view (PV), equatorial view (EV). Scale bars 10 μm (a–c, e, f, h, j, l, n–p), 1 μm (d, g, i, k, m)
Remarks: For remarks, see Bouchal et al. (2016, p. 17).
Class Zygnematophyceae Round, 1971
Family Zygnemataceae Kützing, 1843
Genus Spirogyra Link, 1820/Ovoidites R.Potonié, 1951 emend. Krutzsch, 1959
Spirogyra sp. 1/Ovoidites lanceolatus Takahashi et Jux, 1991 (Fig. 3b and S 3-Fig. 1d–f)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 20).
Spirogyra sp. 2/Ovoidites microfoveolatus Krutzsch et Pacltová, 1990
(Fig. 3c, d and S 3-Fig. 1g–i).
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 4).
Spirogyra sp. 3/Ovoidites spriggii (Cookson et Dettmann, 1959) Zippi, 1998
(Fig. 3e and S 3-Fig. 1j–l)
Description: Zygospore or aplanospore, outline circular to broadly ovoidal, length of axis perpendicular to fissure 65–85 μm (LM, SEM), length of axis parallel to fissure 60–80 μm (LM, SEM); mesospore 1–1.5 μm thick (SEM); psilate (LM, SEM), if ruptured gap spanning from pole to pole.
Genus Cycloovoidites Krutzsch et Pacltová, 1990
Spirogyra sp. 4/Cycloovoidites cyclus (Krutzsch, 1959) Krutzsch et Pacltová, 1990
(Fig. 3f–i and S3-Fig. 1m–o)
Description: Zygospore or aplanospore, outline nearly circular to broadly ovoidal, length of axis perpendicular to fissure 85–95 μm (LM, SEM), length of axis parallel to fissure 95–115 μm (LM, SEM); mesospore 2–2.5 μm thick (SEM); verrucate to rugulate (LM, SEM), verrucae diameter 1–2 μm, rugulae length 2–5 μm, rugulae width 1 μm (SEM), predetermined breaking fissure indicated by slightly raised ca 0.5–1 μm broad band spanning from pole to pole.
Remarks: This zygospore/aplanospore falls in the morphological range (shape, size, verrucate sculpture) of the fossil species Cycloovoidites cyclus (Krutzsch and Pacltová 1990; Worobiec 2014). Zygnemataceae zygospores/aplanospores were combined in the pollen diagram. The fossil species of Ovoidites and Cycloovoidites are commonly associated with shallow, stagnant, oxygen-rich fresh waters and lake margins (see table 1 in Worobiec 2014).
Incerta sedis
Algal cyst indet. 1(Fig. 3j–k and S 3 Fig. 2a–c)
Description: Cyst, spheroidal, outline circular, diameter 25–30 μm (LM, SEM); cyst wall 2–2.5 μm thick (LM); psilate (LM, SEM)
Remarks: Algal cysts of uncertain affinity were combined in the pollen diagram. Algal cysts indet. 1 differs by psilate surface from algal cysts previously reported from the YB (Bouchal et al. 2016, 2017).
Genus Sigmopollis Hedlung, 1965
Algal cyst indet. 2/Sigmopollis pseudosetarius (Weyland et Pflug, 1957) Krutzsch et Pacltová, 1990
(Fig. 3l–m and S 3-Fig. 2d–f).
Description: Cyst, spheroidal, outline circular, diameter 20–30 μm (LM, SEM); cyst wall 2.5–3 μm thick (LM, SEM); echinate (LM), echinus base width 0.5–0.8 μm, echinus length 2–4 μm (SEM).
Remarks: This fossil species is commonly associated with eutrophic to mesotrophic open freshwater (e.g. Worobiec 2014). S. pseudosetarius specimens previously reported from the YB by Bouchal et al. (2016, fig. 3L) differ by larger and nanoverrucate echini.
Division Tracheophyta Sinnott, 1935
Class Equisetopsida C.Agardh, 1825
Subclass Lycopodiidae Beketov, 1863
Family Lycopodiaceae Mirbel in Lamarck et Mirbel, 1802
Genus Lycopodium L., 1753/Retitriletes (Pierce, 1961) Döring, Krutzsch, Mai et Schulz in Krutzsch 1963
Lycopodium sp./Retitriletes annotinioides Krutzsch, 1963
(Fig. 4a–c)
LM overview (a, d, g, j, m), SEM overview (b, e, h, k, n) and SEM detail (c, f, i, l, o, p) micrographs of Lycopsida and Marsileaceae spores and Pinaceae pollen. a–cLycopodium sp. (S153622), (a) PV, (b, c) DV. d–fLycopodiella sp. (S153627), (d) PV, (e,f) DV. g–iSelaginella sp. (S153648), (g) EV, (h, i) DV. j–lPilularia sp. (S153623), (j) EV, (k, l) DV. m–pTsuga sp. (S153648), (m) EV, (n) DV, (o) saccus detail, (p) leptoma detail. Abbreviations: PV, polar view; EV, equatorial view; DV, distal view. Scale bars, 10 μm (a, b, d, e, g, h, j, k, m, n), 1 μm (c, f, i, l, o, p)
Description: Spore, oblate, amb convex triangular, equatorial diameter 30–45 μm (LM, SEM); exospore 1.5–2.5 μm thick without sculpture elements, 4–4.5 μm thick including sculpture elements (LM); trilete, laesurae 2/3 to 3/4 of spore radius; reticulate (LM, SEM), sculptural elements more prominent on distal face.
Remarks: The figured specimen corresponds in size and exospore ornamentation (reticulate) to extant Lycopodium (Tyron and Lugardon 1991), showing closest similarities to the Lycopodium clavatum type of Wilce (1972) and the Lycopodium annotinum type of Jones and Blackmore (1988). Spores of Lycopodium have previously been reported from middle Miocene localities of western Turkey: Retitriletes (Akgün et al. 2007, Appendix A), Lycopodium (Akkiraz 2011, fig. 7J; Akkiraz et al. 2012).
Genus Lycopodiella Holub, 1964/Camarozonosporites Pant, 1954 ex R.Potonié, 1956
Lycopodiella sp./Camarozonosporites helenensis Krutzsch 1963
(Fig. 4d–f).
Description: Spore, oblate, amb convex triangular, equatorial diameter 30–40 μm (LM, SEM); exospore 2.3–2.8 μm thick including sculpture elements (LM); trilete, laesurae 2/3 to 3/4 of spore radius; rugulate on distal face (LM, SEM).
Remarks: Spores of this type correspond in size and exospore ornamentation to extant Lycopodiella (Tyron and Lugardon 1991), with closest morphological similarities to the Lycopodiella cernuum type of Wilce (1972) and the Lycopodiella inundata type of Jones and Blackmore (1988). The term hamulate has been used to describe the rugulate sculpture of Lycopodiella spores (Krutzsch 1963; Jones and Blackmore 1988).
Family Selaginellaceae Willk. in Willk. et Lange, 1861
Genus Selaginella P.Beauv., 1804/Echinatisporites Krutzsch 1959
Selaginella sp./Echinatisporis miocenicus Krutzsch et Sontag in Krutzsch 1963
(Fig. 4g–i)
Description: Spore, oblate, amb convex triangular, length of polar axis 35–45 μm (LM, SEM), equatorial diameter 45–55 μm (LM, SEM); exospore 2.5–3 μm thick without sculpture elements (LM); trilete, laesurae 2/3 to 3/4 of spore radius; echinate (LM, SEM), echini absent on proximal face, echini length 3–5.5 μm (LM, SEM).
Remarks: Echinate spores of this type correspond in size and exospore ornamentation to extant Selaginella selaginoides (L.) Link (Stafford 1991; Tyron and Lugardon 1991).
Spores of Selaginella have previously been reported from middle Miocene localities of western Turkey: Echinatisporis (Akgün et al. 2007, Appendix A), Selaginella (Akkiraz et al. 2012, pl. 1, fig. 5).
LM overview (a–d, f, h, j–n, p, r) and SEM detail (e, f, i, o, q, s) micrographs of Cupressaceae, Pinaceae and Poaceae pollen. a Cupressaceae gen. indet. 1 “non-papillate”/Cupressacites bockwitzensis (S153627). b Cupressaceae gen. indet. 1 “non-papillate”/Inaperturopollenites dubius (S153629), PV. c Cupressaceae gen. indet. 2 “papillate”/Inaperturopollenites concedipites (S153631), PV. d–g Cupressaceae gen. indet. 3 “papillate”/Sequoiapollenites gracilis.d–e (S153631), (d) EV, (e) detail PRV. f–g (S153635), (f) EV, (g) detail PRV. h, iCathaya sp. (S153632), PV. (i) detail nanoechinate sculpture of corpus PRV. jCedrus sp. (S153623), PV. kPicea sp. (S153641), PV. lPinus subgenus Pinus sp. (S153627), PV. mPinus subgenus Strobus sp. (S153631), EV. n, o Poaceae gen. indet. 2 (S153629), (n) PV, (o) exine sculpture detail. p–q Poaceae gen. indet. 1 (S153622), (p) EV, (q) exine sculpture detail. r, s Poaceae gen. indet. 3 (S153622), (r) PV, (s) exine sculpture detail. Abbreviations: PV, polar view; EV, equatorial view; PRV, proximal view (PRV). Scale bars, 10 μm (a–d, f, h, j–n, p, r), 1 μm (e, f, i, o, q, s)
Monilophytes
Subclass Polypodiidae Cronquist, Takht. et Zimmerm., 1966
Family Marsileaceae Mirbel, 1802
Genus Pilularia L., 1753
Pilularia sp.
(Fig. 4j–l)
Description: Microspore, oblate, amb circular, length of polar axis 30–35 μm (LM, SEM), equatorial diameter 35–45 μm (LM, SEM); exospore 3.5–4.5 μm thick (LM), compact, epispore (uncondensed) three times thicker than exospore (LM); trilete, laesurae ¼ to ½ of spore radius; scabrate (LM), rugulate, fossulate (SEM) dented or folded in central area.
Remarks: The morphology (sporewall architecture, epispore sculpturing) of the available specimen corresponds to microspores of extant Pilularia (Tyron and Lugardon 1991), with closest similarities to the Pilularia globulifera type of Stafford (1995). Nakoman (1967), Anatoliensisporites ornatiturcicus Nakoman, pl. 1, fig. 14 and Akgün and Akyol (1999, indetermined form, fig. 10.41) reported morphologically similar spores from Miocene strata of Anatolian.
Family Osmundaceae Bercht. et Presl, 1820
Genus Osmunda L., 1753/Baculatisporites P.W.Thomson et Pflug, 1953
Osmunda sp./Baculatisporites nanus (Wolff, 1934) Krutzsch, 1959
(Fig. 3n and S 3-Fig. 2g–i)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 20).
Incerta sedis
Genus Laevigatosporites Ibrahim, 1933
Monolete spore fam. indet./Laevigatosporites haardti (R.Potonié et Venitz, 1934) P.W.Thomson et Pflug, 1953
(Fig. 3o and S 3-Fig. 2j–l).
Remarks: Several extant fern families (e.g. Aspleniaceae, Davalliaceae, Dryopteridaceae, Gleicheniaceae, Lomariopsidaceae, Oleandraceae, Thelypteridaceae, Vittariaceae, Polypodiaceae; see Tyron and Lugardon 1991) produce psilate, monolete spores similar to the fossil species Laevigatosporites haardti. For descriptions and further remarks, see Bouchal et al. (2016, p. 23; 2017, p. 6).
Subdivision Spermatophyta Willkomm, 1854
Gymnosperms
Subclass Gnetidae Pax in Prantl, 1894
Order Ephedrales Dumort., 1829
Family Ephedraceae Dumort., 1829
Genus Ephedra L., 1753/Ephedripites (Distachypites) Bolchovitina, 1953 emend. Krutzsch 1970
Ephedra sp./Ephedripites (Distachypites) tertiarius Krutzsch, 1970
(Fig. 3p and S 3-Fig. 2m–o).
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 10).
Subclass Pinidae Cronquist, Takht. et Zimmerm., 1966
Order Pinales Gorozh., 1904
Family Cupressaceae Rich. ex Bartling, 1830
Cupressaceae gen. indet. 1 “non-papillate”
(Fig. 5a, b and S 3-Fig. 3a–g)
Description: Pollen, shape spheroidal, outline circular with a deep split to spindle-like (folded), diameter 25–40 μm (LM), 20–38 μm (SEM); exine 1–1.5 μm thick (LM); leptoma without papilla (LM, SEM); scabrate (LM), microverrucate (SEM), microverrucae covered with blunt nanoechini, leptoma area less ornamented, orbicules covered with blunt nanoechini, orbicule diameter 0.3–0.6 μm (SEM).
Remarks: Non-papillate pollen is commonly produced by Cupressaceae. No further allocation is possible owing to poor preservation and general lack of diagnostic morphological characters of the available specimens (compare Van Campo-Duplan 1951, 1953; Kedves 1985). Spindle-shaped non-papillate, ruptured pollen (Fig. 5a and S 3-Fig. 3a–c) can be assigned to the fossil species Cupressacites bockwitzensis Krutzsch, while non-papillate, ruptured pollen with circular outline (Fig. 5b and S 3-Fig. 3d–f) fit the morphological range of the fossil species Inaperturopollenites dubius (R.Potonié et Venitz) P.W.Thomson et Pflug, (e.g. Stuchlik et al. 2002).
Genus Inaperturopollenites Pflug et P.W.Thomson in P.W.Thomson et Pflug, 1953
Cupressaceae gen. indet. 2 “papillate”/Inaperturopollenites concedipites (Wodehouse, 1933) Krutzsch, 1971
(Fig. 5c and S 3-Fig. 3h–k)
Description: Pollen, shape spheroidal, outline circular with deep split, diameter 25–40 μm (LM, SEM); exine 1–1.5 μm thick in leptoma area (LM); leptoma papillate (LM, SEM); scabrate (LM), microverrucate (SEM), microverrucae covered with blunt nanoechini, leptoma less ornamented (nano-verrucate), specimens can sporadically have a wrinkly leptoma, orbicules covered with blunt microechini, orbicule diameter 0.3–0.7 μm (SEM).
Remarks: Ruptured and unruptured papillate Cupressaceae pollen were combined in the pollen diagram. In extant Cupressaceae, papillate pollen is produced in a few subfamilies, namely Athrotaxioideae (Athrotaxis D.Don), Sequoioideae (Metasequoia Hu et W.C.Cheng, Sequoia Endl., Sequoiadendron J.Buchholz), Taiwanioideae (Taiwania Hayata) and Taxodioideae (Cryptomeria D.Don, Glyptostrobus Endl., Taxodium Rich., see Van Campo-Duplan 1951; Kvavadze 1988). Pollen of this type commonly ruptures from proximal to the papillate distal pole during the hydration process preceding pollen germination (Southworth 1988) leading to its characteristic shape (Fig. 5c). Owing to this deformed state, no further generic determination is recommended.
Genus Sequoiapollenites Thiergart, 1938 ex R.Potonié, 1958
Cupressaceae gen. indet. 3 “papillate”/Sequoiapollenites gracilis Krutzsch, 1971
(Fig. 5d–g and S 3-Fig. 3l–s)
Description: Pollen, shape spheroidal, outline circular, polar axis 23–29 μm (LM), 22–25 μm (SEM), equatorial diameter 26–33 μm (LM), 22–27 μm (SEM), leptoma diameter 15–21 μm (LM), papilla length 2.5–3.5 μm; exine 1–1.5 μm thick (LM); leptoma papillate (LM, SEM), papilla straight to bent; scabrate (LM), microverrucate (SEM), microverrucae covered with blunt nanoechini, leptoma less ornamented (nanoverrucate), orbicules covered with microechini, orbicule diameter 0.2–0.7 μm (SEM).
Remarks: Unruptured papillate Cupressaceae pollen is much rarer than ruptured pollen due to its germination modus operandi (see above). The here depicted specimens fit within the morphological (size, papilla size) range of extant Taxodioideae and Sequoioideae (Kvavadze 1988). Macrofossil remains belonging to both subfamilies have been reported from Miocene localities in Turkey (Kasapligil 1977; Mädler and Steffens 1979; Paicheler and Blanc 1981; Gemici et al. 1991, 1993; Denk et al. 2017).
Family Pinaceae Spreng. ex F.Rudolphi, 1830
Genus Cathaya Chun et Kuang, 1958
Cathaya sp.
(Fig. 5h, i and S 3-Fig. 4a–d)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 25).
Genus Cedrus Trew, 1757
Cedrus sp.
(Fig. 5j and S 3-Fig. 4e–h).
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 25).
Genus Picea A.Dietr., 1824
Picea sp.
(Fig. 5k and S 3-Fig. 4i–l)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 27).
Genus Pinus L., 1753
Subgenus Pinus L., 1753
Pinus subgenus Pinus sp.
(Fig. 5l and S 3-Fig. 4m–p)
Remarks: Near spherical sacci with narrow attachment to the corpus are characteristic for Pinus subgenus Pinus (Diploxylon) pollen (e.g. Hesse et al. 2009). For descriptions and further remarks, see Bouchal et al. (2016, p. 27).
Genus Pinus L., 1753
Subgenus Strobus Lemmon, 1895
Pinus subgenus Strobus sp.
(Fig. 5m and S 3-Fig. 4q–t)
Remarks: Half-spherical sacci with broad attachment to the corpus are characteristic of Pinus subgenus Strobus (Haploxylon) pollen type (e.g. Hesse et al. 2009). For descriptions and further remarks, see Bouchal et al. (2016, p. 27).
Genus Tsuga Carrière, 1847
Tsuga sp.
(Fig. 4m–p).
Description: Pollen, monosaccate, shape oblate, circular in polar view, equatorial diameter 55–70 μm (LM), 55–60 μm (SEM); rugulate (LM); leptoma verrucate to rugulate, echinate; monosaccus rugulate, fossulate, echinate, proximal face rugulate to verrucate, fossulate, echinate (SEM).
Remarks: Grímsson and Zetter (2011) recognised four distinct pollen morphologies in extant Tsuga: (1) bisaccate group, (2) monosaccate, nonechinate group, (3) monosaccate, echinate, narrow monosaccus group and (4) monosaccate, echinate, broad monosaccus group. The fossil Tsuga sp. can be assigned to the fourth group and has closest morphological similarities (density of echini, broad saccus) with pollen of extant Tsuga diversifolia (Maxim.) Mast. and Tsuga forresti Downie (Sivak 1978; Miyoshi et al. 2011). Tsuga pollen with narrow monosaccus and no echini has previously been reported from the Tınaz lignite mine (Bouchal et al. 2017, pl. 7.13–16).
Subclass Magnoliidae Novák ex Takht., 1967
Order Liliales Perleb, 1826
Family Smilacaceae Vent., 1799
Genus Smilax L., 1753
Smilax sp. “aspera type”
(Fig. 6a–c)
LM overview (a, d, g, j, m), SEM overview (b, e, h, k, n) and detail (c, f, i, l, o) micrographs of Smilacaceae, Ranunculaceae, Euphorbiaceae and pollen. a–cSmilax sp. (S153637), EV. d–fArceuthobium sp. (S153634). g–i Ranunculaceae gen. indet. (S153623), EV. j–lEuphorbia sp. 1 (S153636), PV. m–oEuphorbia sp. 2 (S153627), EV. Abbreviations: PV, polar view; EV, equatorial view, proximal view. Scale bars, 10 μm (a, b, d, e, g, h, j, k, m, n), 1 μm (c, f, i, l, o)
Description: Pollen, shape spheroidal, outline circular, diameter (including echini) 20–24 μm (LM), 19–22 μm (SEM); possibly atectate, exine ca 1 μm thick (LM), sexine thinner than nexine; inaperturate (LM, SEM); echinate (LM), echinate, nanoverrucate, perforate (SEM), echini base diameter 0.5–1.5 μm (SEM), echini length 0.6–1.7 μm (SEM).
Remarks: Chen et al. (2006) identified four main pollen types within extant Smilacaeae; (1) Smilax aspera type (inaperturate, echinate), (2) Heterosmilax type (inaperturate, verrucate, nanoechinate), (3) Smilax smallii type (pseudocolporate, granulate) and (4) Ripogonum type (sulcate, microreticulate to reticulate). Smilax sp. corresponds by its spheroidal shape, echinate and nanoverrucate sculpture and lack of aperture to the S. aspera type pollen. Leaves of Smilax miohavanensis Denk, D.Velitzelos, T.Güner et Ferrufino-Acosta have previously been reported from the YB (Denk et al. 2015, Güner 2016, Güner et al. 2017). Small, spheroidal, echinate pollen has previously been reported from other middle Miocene localities of western Turkey: Smilacipites Wodehouse (Takahashi and Jux 1991, pl. 6, only fig. 2a, b), possibly Echinigraminidites moravicus Krutzsch (Akgün and Akyol 1999).
Order Poales Small, 1903
Family Poaceae Barnhart, 1895
Poaceae gen. indet. 1
(Fig. 5p, q and S 3-Fig. 5a–c)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 29).
Poaceae gen. indet. 2
(Fig. 5n, o and S 3-Fig. 5d–f)
Description: Pollen, shape spheroidal, equatorial outline circular, diameter 20–30 μm (LM, SEM); eutectate, exine 1–1.5 μm thick (LM); ulcerate, ulcus diameter 1.4–1.8 μm (SEM), annulus present; scabrate (LM), areolate, nanoechinate, fossulate, 3–9 nanoechini per areola, acute nanoechini (SEM).
Remarks: Poaceae gen. indet. 1, 2 and 3 were combined in the pollen diagram. Several extant Poaceae genera (e.g. Avena L., Oryza L, Phragmites Adanson, Spartina Schreber) produce areolate, nanoechinate pollen (Page 1978; Köhler and Elsbeth 1979). Poaceae gen. indet. 2 differs from Poaceae gen. indet. 1 by its smaller size and acute nanoechini.
Poaceae gen. indet. 3
(Fig. 5r, s and S 3-Fig. 5g–i)
Description: Pollen, shape spheroidal, equatorial outline circular, equatorial diameter 40–60 μm (LM, SEM); eutectate, exine 1–1.5 μm thick (LM); ulcerate, ulcus diameter 2–3 μm (SEM), weak annulus present (LM); scabrate (LM), nanoechinate, fossulate (SEM), blunt nanoechini (SEM).
Remarks: Poaceae gen. indet. 3 differs from Poaceae gen. indet. 1 and 2 by its markedly larger size and lack of areolae. A number of extant Poaceae genera produce pollen with nonclustered, blunt nanoechini, e.g. Stipa L., Diandrolyra Stapf, Glyceria Nutt. and Secale L. (Page 1978; Köhler and Elsbeth 1979). Poaceae gen. indet. 3 shares morphological characters (aperture, spaced nanoechini) with pollen previously reported from the Tınaz section, YB (Bouchal et al. 2017, Poaceae gen. indet. 3, p. 12, pl. 5.5–7) but differs by more densely spaced nanoechini.
Family Typhaceae Juss., 1789
Genus Sparganium L., 1753
Sparganium sp.
(Fig. 7a, b and S 3-Fig. 5j–l)
LM overview (a, c, e, g–p, r, t, v, x, z, aa, cc, ee, ff). SEM overview (dd) and detail (b, d, f, q, s, u, w, y, bb) micrographs of Typhaceae, Buxaceae, Altingiaceae, Salicaceae, Betulaceae, Fagaceae and Juglandaceae pollen. a, bSparganium sp. (S153623). c, dTypha sp. (S153623), DV. e, f. Buxus sp. (S153631). gLiquidambar (S153622). h, iSalix sp., (h) EV (S153623), (i) EV (S153637). jAlnus sp./Alnipollenites verus (S153648), PV. k–lBetula sp. 1 (S153646), PV. m, nBetula sp. 2 (S153629), PV. oCarpinus sp. (S153626), PV. pCorylus sp. (S153631), PV. qOstrya sp. (S153635), PV. r–uFagus sp. (S153623), EV. v–wQuercus sp. 1 (Quercus sect. Cerris) (S153637), EV. x, yQuercus sp. 2 (Quercus sect. Ilex) (S153622), left EV, right PV. z–aaQuercus sp. 3 (Quercus sect. Quercus) (S153648), PV. bb Engelhardioideae gen. indet. 2 (S153622), PV. cc–ff Engelhardoideae gen. indet. 1, (S153622) PV, (S153627) PV, (ee, ff) pseudocolpus-like folds. ggCarya sp./Caryapollenites simplex (S153627), PV. hhJuglans sp. (S153629), PV. Abbreviations: PV, polar view; EV, equatorial view, proximal view. Scale bars, 10 μm (a, c, e, g–p, r, t, v, x, z, aa, cc–ff), 1 μm (b, d, f, q, s, u, w, y, bb)
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 12).
Genus Typha L., 1753
Typha sp.
(Fig. 7c, d and S 3-Fig. 5m–o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 29).
Eudicots
Order Ranunculales Juss. ex Bercht. et J.Presl, 1820
Family Ranunculaceae Juss., 1789
Ranunculaceae gen. indet.
(Fig. 6g–i)
Description: Pollen, shape prolate, outline elliptic in equatorial view, length of polar axis 22–28 μm (LM, SEM), equatorial diameter 18–20 μm (LM), 16–18 μm (SEM); tectate, exine 1.5–2 μm thick (LM), nexine thinner than sexine; tricolpate, colpus length 2/3 to 3/4 of polar axis; scabrate (LM), microechinate, perforate (SEM), microechini base diameter 0.2–0.4 μm (SEM), microechini height 0.2–0.4 μm (SEM).
Remarks: Ranunculaceae gen. indet. falls within the morphological range of the Ranunculus acris type of Clark et al. (1991). It is produced by several extant genera of this family (e.g. Anemone L., Ceratocephalus L., Clematis L., Pulsatilla Mill., Ranunculus L.).
Order Buxales Takht. ex Reveal, 1996
Family Buxaceae Dumort., 1822
Genus Buxus L., 1753
Buxus sp. balearica type
(Fig. 7e, f and S 3-Fig. 6a–c)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 31).
Order Saxifragales Bercht. et J.Presl, 1820
Family Altingiaceae (Horan., 1841) Lindl., 1846
Genus Liquidambar L., 1753
Liquidambar sp.
(Fig. 7g and S 3-Fig. 6d–f)
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 12).
Fabids
Order Malpighiales Juss. ex J.Presl, 1820
Family Euphorbiaceae Juss., 1789
Genus Euphorbia L., 1753
Euphorbia sp. 1
(Fig. 6j–l)
Description: Pollen, outline circular to lobate in polar view, equatorial diameter 40–45 μm (LM), 35–40 μm (SEM); eutectate, exine 3–4 μm thick (LM), nexine thinner than sexine; tricolpate, colpus length 2/3 to 3/4 of polar axis, in colpus area psilate margo present; perforate, microreticulate (LM, SEM).
Remarks:Euphorbia sp. 1 and 2 were combined in the pollen diagram. Euphorbia sp. 1 corresponds to subtype 1a.-Euphorbia villosa of El-Ghazaly and Chaudhary (1993); this type is produced by several species of Euphorbia which are not part of a natural group (El-Ghazaly and Chaudhary 1993). Highly similar Euphorbia pollen has been reported by Bouchal et al. (2016, as Euphorbia sp., p. 31, fig. 9G–J), corresponding in exine architecture and margo presence, but differing by prolate shape and perforate exine sculpture.
Euphorbia sp. 2 (Fig. 6m–o).
Description: Pollen, shape prolate, outline elliptic in equatorial view, polar axis 31–36 μm (LM), 30–34 μm (SEM), equatorial diameter 22–28 μm (LM), 21–25 μm (SEM); eutectate, exine 2–2.5 μm thick (LM), nexine thinner than sexine, thickened in aperture area; tricolporate, endoporus circular, colpus length 3/4 to 5/6 of polar axis; microreticulate (LM), microreticulate to fossulate-perforate (SEM).
Remarks:Euphorbia sp. 2 corresponds in size and sunken colpi to type 2-Euphorbia nutans of El-Ghazaly and Chaudhary (1993), which is produced by several not closely related Euphorbia species.
Family Salicaceae Mirb., 1815
Genus Salix L., 1753
Salix sp.
(Fig. 7h, i and S 3-Fig. 6g–l)
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 14).
Order Fagales Engler, 1892
Family Betulaceae Gray, 1822
Genus Alnus Mill., 1754/Alnipollenites R.Potonié, 1931 emend. Grabowska et Ważyńska, 2009
Alnus sp./Alnipollenites verus (R.Potonié, 1931) R.Potonié, 1931
(Fig. 7j and S 3-Fig. 6m–o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 33).
Genus Betula L., 1753
Betula sp. 1
(Fig. 7k, l and S 3-Fig. 7a–c)
Description: Pollen, shape oblate, outline circular in polar view, equatorial diameter 21–26 μm (LM, SEM); eutectate, exine 1–1.5 μm thick (LM), nexine thinner than sexine; triporate, pori circular, pori diameter 1–1.5 μm (LM, SEM), annulus formed by sexine, vestibulum present; scabrate (LM), microrugulate to microverrucate, nanoechinate, perforate (SEM), nanoechini positioned on microrugulae/microverrucae.
Betula sp. 2 (Fig. 7m, n and S 3-Fig. 7d–f)
Description: Pollen, shape oblate, outline convex triangular in polar view, equatorial diameter 25–32 μm (LM, SEM); eutectate, exine 1.5–2 μm thick (LM), nexine thinner than sexine; triporate, pori circular to elongated, pori diameter 1–2 μm (LM, SEM), annulus formed by sexine, vestibulum present; scabrate (LM), microrugulate, nanoechinate (SEM), nanoechini postioned on microrugulae.
Remarks:Betula sp. 1 and 2 were combined in the pollen diagram. Betula sp. 1 and 2 display a distinct vestibulum, the distinguishing character for pollen of this genus (e.g. Blackmore et al. 2003; Beug 2004; Stuchlik et al. 2009; Grímsson et al. 2016). Betula sp. 2 differs from Betula sp. 1 by larger size, thicker exine, mainly microrugulate sculpture and more spacious nanoechini. For further remarks concerning Betula, see Bouchal et al. (2016, p. 34).
Genus Carpinus L., 1753
Carpinus sp.
(Fig. 7o and S 3-Fig. 7g–i)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 33).
Genus Corylus L., 1753
Corylus sp.
(Fig. 7p and S 3-Fig. 7j–l)
Remarks: Dispersed and poorly preserved pollen of Corylus, Ostrya and Morella vel Myrica is difficult to unambiguously assign to one of these genera using only LM, owing to a high degree of morphological similarities (cf. Edwards 1981). Therefore, these three taxa were combined in the pollen diagram in the category Corylus/Ostrya/Morella vel Myrica.
So far, only macrofossils of Myrica have previously been reported from the YB (Güner 2016; Güner et al. 2017). For descriptions and remarks, see Bouchal et al. (2016, p. 34).
Genus Ostrya Scop., 1760
Ostrya sp.
(Fig. 7q and S 3-Fig. 7m–o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 34).
Family Fagaceae Dumort., 1829
Genus Fagus L., 1753
Fagus sp.
(Fig. 7r–u and S 3-Fig. 8a–f)
LM overview (a–c, e, g, i–l, o, r–w, y, aa), SEM overview (m, p, bb) and detail (d, f, h, n, q, t, y, aa) micrographs of Juglandaceae, Myricaceae, Cannabaceae, Ulmaceae, Geraniaceae, Lythraceae, Malvaceae, Amaranthaceae, Caryophyllaceae, Polygonaceae and Sapindaceae pollen. a, bCyclocarya vel Pterocarya sp. (a) exceptionally large specimen, PV (S153646), (b) PV (S153629). c, dMorella vel Myrica sp. (S153637), PV. e, fCeltis vel Pteroceltis (S153632), PV. g, hCedrelospermum sp. (S153635), PV. iUlmus sp. (S153629), PV. jZelkova sp. (S153646), PV. kErodium sp. (S153622), PV. l–nDecodon sp. 1 (S153635), EV. m–qDecodon sp. 2 (S153627), EV. rTilia sp./Intratriporopollenites instructus (S153633), PV. s, t Amaranthaceae gen. indet. 1 (S153622). u Amaranthaceae gen. indet. 2 (S153623). v, w Caryophyllaceae gen. indet. 1 (S153622). x, y Caryophyllaceae gen. indet. 2 (S153622). z, aaRumex sp. (S153623), EV. bb–ccAcer morphotype 1 (S153627), EV. dd–eeAcer morphotype 2 (S153623), EV. Abbreviations: PV, polar view; EV, equatorial view. Scale bars 10 μm (a–c, e, g, i–m, o, p, r–z, bb,dd, ee), 1 μm (d, f, h, n, q, aa, cc)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 36).
Genus Quercus L., 1753.
Remarks:
Denk and Grimm (2009) identified five types of exine sculpturing corresponding to six sections within extant Quercus (this has been incorporated in the most recent classification of oaks; Denk et al. 2017): Quercus sect. Cerris (scattered verrucate), Quercus sect. Ilex (rodlike, microrugulate), Quercus sect. Cyclobalanopsis (vertical rodlike), Quercus sect. Protobalanus (masked rodlike), Quercus sect. Lobatae and Quercus sect. Quercus (microverrucate); these distinguishing infrageneric characters are miniscule and not detectable using LM. For the pollen diagram, only size (large or small) was used to categorise oak pollen. Quercus sp. 3 was less frequent in the Salihpaşalar section than Quercus sp. 1 and 2.
Quercus sp. 1 (Quercus sect. Cerris)
(Fig. 7v, w and S 3-Fig. 8g–i)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 36, as Quercus Group Cerris).
Quercus sp. 2 (Quercus sect. Ilex)
(Fig. 7x, y and S 3-Fig. 8j–l)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 38, as Quercus Group Ilex).
Quercus sp. 3 (Quercus sect. Quercus)
(Fig. 7z, aa and S 3-Fig. 8)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 38, as Quercus Group Quercus).
Family Juglandaceae DC. ex Preleb, 1818
Subfamily Engelhardioideae Iljinsk., 1990
Engelhardioideae gen. indet. 1
(Fig. 7cc–ff and S 3-Fig. 9d–i)
LM overview (a, d, g, j, m), SEM overview (b, e, h, k, n) and SEM detail (c, f, i, l, o) micrographs of Moraceae, Rosaceae, Lythraceae and Sapindales pollen. a–c Moraceae/Urticaceae gen. indet. (S153623), PV. d–f Rosaceae gen. indet. 1. (S153627), EV. g–i Rosaceae gen. indet. 2. (S153631), polar view. j–l Lythraceae gen. indet. aff. Ammannia (S153627), PV. m–o Sapindales fam. et gen. indet. (S153623), (top) PV, (bottom left and right) EV. Abbreviations: PV, polar view; EV, equatorial view (EV). Scale bars, 10 μm (a, b, d, e, g, h, j, k, m, n), 1 μm (c, f, i, l, o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 38, as Engelhardioideae gen. indet.). Engelhardioideae pollen types were combined in the pollen diagram.
Engelhardioideae gen. indet. 2
(Fig. 7bb and S 3-Fig. 9a–c)
Description: Pollen, shape oblate, outline circular to convex triangular in polar view, equatorial diameter 18–22 μm (LM), 17–20 μm (SEM); eutectate, exine 1.0–1.5 μm thick (LM), nexine thinner than sexine; triporate, pore diameter 1–2 μm (LM), ectoporus circular to elliptic, aperture sunken; psilate (LM), nanoechinate (SEM).
Family Myricaceae Rich. ex Kunth, 1817
Genus Morella Lour., 1790 vel Myrica L., 1753
Morella vel Myrica sp.
(Fig. 8c, d and S 3-Fig. 10–i)
LM overview (a, d, g, j, m), SEM overview (b, e, h, k, n) and SEM detail (c, f, i, l, o, p) micrographs of Amaranthaceae, Droseraceae, Polygonaceae, Sapotaceae and Oleaceae pollen. a–c Amaranthaceae gen. indet. 3 (S153636). d–fDrosera sp. (S153636). g–iPersicaria sp./Persicarioipollenites pliocenicus (S153634). j–l Sapotaceae gen. indet. (S153631), EV. m–o Oleaceae gen. indet. 1 (S153623), EV. Abbreviations: PV, polar view; EV, equatorial view. Scale bars, 10 μm (a, b, d, e, g, h, j, k, m, n), 1 μm (c, f, i, l, o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 42).
Order Rosales Bercht. et J.Presl, 1820
Family Cannabaceae Martinov, 1820
Genus Celtis L., 1753 vel Pteroceltis Maxim., 1873
Celtis vel Pteroceltis sp.
(Fig. 8e, f and S 3-Fig. 10j–l)
Remarks: For descriptions and remarks, see Bouchal et al. (2017, p. 16).
Family Moraceae Gaudich., 1835/Urticaceae Juss., 1789
Moraceae/Urticaceae gen. indet.
(Fig. 9a–c)
Description: Pollen, outline circular to convex triangular in polar view, equatorial diameter 12–16 μm (LM), 11–14 μm (SEM); tectate, exine 1–1.5 μm thick (LM); triporate (LM, SEM), pores circular, pore diameter 1.3–1.8 μm (SEM); scabrate (LM), microverrucate, nanoechinate (SEM).
Remarks: Pollen of this type is morphologically (size, aperture, exine sculpture) similar to the Urticaceae Parietaria L., e.g. P. judaica L., P. officinalis L. (Diethart 2005; Halbritter 2015), and Moraceae pollen, e.g. Morus alba type of Punt and Malotaux (1984).
Family Rosaceae Juss., 1789
Rosaceae gen. indet. 1
(Fig. 9d–f)
Description: Pollen, prolate, outline elliptic in equatorial view, length of polar axis 10–12 μm (LM, SEM), equatorial diameter 7–10 μm (LM, SEM); eutectate, exine 1–1.5 μm thick (LM), tricolporate, ectocolpus length 3/4 to 5/6 of polar axis (SEM); psilate (LM), striate, (SEM), striae parallel to perpendicular to polar axis, striae 0.1–0.2 μm wide (SEM).
Remarks: Rosaceae gen. indet. 1 and 2 were combined in the pollen diagram. Assignment of tricolporate, striate perforate Rosaceae pollen to subfamily or genus level is difficult due to a high degree of morphological overlap (Hebda et al. 1988a, 1988b; Hebda and Chinnappa 1990, 1994, table 1). Reports of Rosaceae pollen from middle Miocene localities of western Turkey are rare (see S 2).
Rosaceae gen. indet. 2
(Fig. 9g–i)
Description: Pollen, prolate, outline circular to lobate in polar view, equatorial diameter 15–18 μm (LM), 13–16 μm (SEM); eutectate, exine 1–1.5 μm thick (LM), tricolporate, colpus membrane granulate, ectocolpus length 3/4 to 5/6 of polar axis (LM, SEM); psilate (LM), striate, perforate (SEM), striae mainly parallel to polar axis, striae 0.1–0.2 μm wide (SEM).
Remarks: Rosaceae gen. indet. 2 differs from Rosaceae gen. indet. 1 by its larger size and by narrower and a higher number of striae.
Family Ulmaceae Mirbel, 1815
Genus Cedrelospermum Saporta, 1889
Cedrelospermum sp.
(Fig. 8g, h and S 3-Fig. 10m–o)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 42).
Genus Ulmus L., 1753
Ulmus sp.
(Fig. 8i and S 3-Fig. 11a–c)
LM overview (a, c, e, f, g, h, j, k, m, n, p, t, v, x, z), SEM overview (l, o, q, s, u, w, y, aa) and detail (b, d, i) micrographs of Eucommiaceae, Aquifoliaceae, Asteraceae, Caprifoliaceae and Apiaceae pollen. a, bEucommia sp./Tricolpopollenites parmularius (S153623), EV. c, dIlex sp. (S153637), PV. e Asteroideae type 2 (S153623), EV. f Asteroideae type 3 (S153648), EV. g Asteroideae type 1 (S153648), EV. h, i Asteroideae type 5 (S153622), EV. j Asteroideae type 4 (S153622). k, l Cichorioideae gen. indet. (S153623). mDipsacus vel Cephalaria (S153641), (left) PV, (right) EV. n, o Apiaceae type 1 (S153623), EV. p, q Apiaceae type 2 (S153623), EV. r, s Apiaceae type 3 (S153623), EV. t, u Apiaceae type 4 (S153641), EV. v, w Apiaceae type 5 (S153623), EV. x, y Apiaceae type 6 (S153622), EV. z–aa Saniculoideae gen. indet. (S153622), EV. Abbreviations: PV, polar view; EV, equatorial view. Scale bars, 10 μm (a, c, f–aa), 1 μm (b, d, i)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 44).
Genus Zelkova Spach, 1841
Zelkova sp.
(Fig. 8j and S 3-Fig. 11d–f)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 44).
Order Geraniales Juss. ex Bercht., 1892
Family Geraniaceae Juss. 1789
Genus Erodium L’Hér., 1789
Erodium sp.
(Fig. 8k and S 3-Fig. 11g–i)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 44).
Order Myrtales Juss. ex. Bercht. et Presl, 1820
Family Lythraceae J.St.-Hilaire, 1805
Lythraceae gen. indet. aff. Ammannia L., 1753
(Fig. 9j–l)
Description: Pollen, shape prolate, elliptic in equatorial view, length of polar axis 22–27 μm (LM), 21–25 μm (SEM), equatorial diameter 17–21 μm (LM), 14–19 μm (SEM); eutectate, exine 1.5–2 μm thick (LM); tricolporate, ectocolpus with bridge, colpus length 1/2 to 2/3 of polar axis, six pseudocolpi present, pseudocolpus length 1/3 to 1/2 of polar axis (SEM), area surrounding endoporus thickened (LM); rugulate (LM), rugulate to striate, fossulate (SEM), perforate in colpus and pseudocolpus area (SEM), in equatorial area rugulae/striae partly perpendicular to polar axis.
Remarks: The Lythraceae clade containing the genera Hionanthera A.Fern. et Diniz, Ammannia L. [including Nesaea Comm. ex Kunth (Graham et al. 2005)], Lawsonia L. and Ginoria Jacq. produce pollen corresponding to the fossil pollen in size, aperture and presence of pseudocolpi (Graham et al. 1985, 1987; Graham et al. 2011). Pollen of Ammannia displays closest morphological similarities (pollen shape, length of pseudocolpi) but differs by its exine sculpture; Lythraceae gen. indet. aff. Ammannia has much broader/larger rugulae.
Identical pollen has previously been described from Karpatian sediments (late early Miocene) of the Korneuburger Basin, Austria (Hofmann et al. 2002), where this type also co-occurs with pollen of Decodon. Lythraceaepollenites bavaricus Thiele-Pfeiffer (Thiele-Pfeiffer 1980; Stuchlik et al. 2014) corresponds in size, shape, aperture (tricolporate) and presence of pseudocolpi with the present pollen; pollen of extant Lawsonia, Rotala L. and Ammannia has been compared to this fossil species.
Genus Decodon J.F.Gmel., 1791
Decodon sp. 1
(Fig. 8l–n and S 3-Fig. 11j–l)
Remarks: For descriptions and remarks, see Bouchal et al. (2016, p. 44).
Decodon sp. 2 (Fig. 8o–q and S 3-Fig. 11m–o)
Description: Pollen, shape prolate, elliptic in equatorial view, length of polar axis 14–18 μm (LM), 14–17 μm (SEM), equatorial diameter 10–14 μm (LM), 10–12 μm (SEM); eutectate, exine 1.5–2 μm thick (LM); tricolporate, in some specimens ectocolpus with bridge, colpus length 1/2 to 2/3 of polar axis (SEM), area surrounding endoporus thickened (LM); psilate (LM), rugulate, fossulate, perforate (SEM).
Remarks:Decodon sp. 1 and 2 were combined in the pollen diagram. Decodon sp. 2 differs by rugulate sculpture and absence of psilate areas from Decodon sp. 1 and corresponds to Decodon morphotype 5 of Grímsson et al., 2012, figs. 3D-L and 5J-L).
Order Malvales Juss. ex. Bercht. et Presl, 1820
Family Malvaceae Juss., 1789
Subfamily Tilioideae Arn., 1832
Genus Tilia L., 1753/Intratriporopollenites Pflug et P.W.Thomson in P.W.Thomson et Pflug, 1953
Tilia sp./Intratriporopollenites instructus (R.Potonié, 1931) P.W.Thomson et Pflug, 1953
(Fig. 8r and S 3-Fig. 12a–c)
LM overview (a, d, g, j, n), SEM overview (b, e, h, k, o) and SEM detail (c, f, i, l, m, p, q) micrographs of Oleaceae, Asteraceae, Adoxaceae and Caprifoliaceae pollen. a–cOleaceae gen. indet. 2 (S153623), PV. d–f. Oleaceae gen. indet. 3 (S153623), EV, (f) brochi with free-standing columellae, g–iArtemisia sp. (S153623), (g, h) EV. j–mViburnum sp. (S153631), EV, free-standing (j) colpus detail. n–qValeriana sp. (S153636), EV, (m) exine sculpture detail, (n) colpus detail. Abbreviations: PV, polar view; EV, equatorial view. Scale bars, 10 μm (a, b, d, e, g, h, j, k, n, o), 1 μm (c, f, i, l, m, p, q)
Description: For detailed descriptions, see Bouchal et al. (2016, p. 46, as Tilia sp.).
Remarks:Tilia sp./I. instructus falls within the morphological range of this fossil species and strongly resembles pollen of extant T. platyphyllos Scop. (Christensen and Blackmore 1988; compare striae on reticulum in S 3-Fig. 12c to Perveen et al. 2004, fig. 4g; Stuchlik et al. 2014; Zhang and Chen 1984). Güner et al. (2017) reported fossil inflorescence bracts of Tilia from the Eskihisar and Tinaz lignite mines. Worobiec et al. (2010) indicated a possible interconnection between I. instructus and the extinct species Byttneriophyllum tiliifolium Givulescu ex Knobloch et Kvaček. Unfortunately, to date, foliage of this species has not been found attached to reproductive structures with in situ pollen to verify this possible association. B. tiliifolium, though widely distributed in the Miocene of central and eastern Europe (e.g. Austria, Bulgaria, Czech Republic, Poland, Germany, Switzerland, Hungary, France; see Knobloch and Kvaček, 1965; Worobiec et al. 2010), is absent from the Neogene fossil record of Greece and Turkey (e.g. Gemici et al. 1990, 1991, Gemici and Akgün, 1992, Gemici et al., 1993; Mädler and Steffens 1979; Velitzelos et al. 2014).
Order Sapindales Juss. ex Bercht. et J.Presl, 1820
Sapindales fam. et gen. indet.
(Fig. 9m–o)
Description: Pollen, shape prolate, elliptic in equatorial view, lobate in polar view, length of polar axis 25–30 μm (LM, SEM), equatorial diameter 15–19 μm (LM, SEM); semitectate, exine 2.5–3 μm thick, sexine thicker than nexine; tricolporate, ectocolpus length 3/4 to 5/6 of polar axis (LM, SEM), endoporus rhombic to circular, costae present, costae reaching polar colpus endings (LM); scabrate (LM), rugulate to striate, fossulate, perforate (SEM).
Remarks: The morphological characteristics of this pollen type are shared by a number of families within the Sapindales (Erdtman 1952), and hence determination down to the family level is not possible.
Family Sapindaceae Juss., 1789
Genus Acer L., 1753
Acer morphotype 1
(Fig. 8bb, cc and S 3-Fig. 13g–i)
LM overview (a, e, h, k, n), SEM overview (b, f, i, l, o) and SEM detail (c, g, j, m, p) micrographs of Asteraceae, Araliaceae and undetermined pollen. a–dCentranthus sp. (S153631), PV, (c) exine sculpture detail, (d) colpus membrane detail. e–g Araliaceae gen. indet. (S153641), EV. h–j Pollen type 1 (S153623), (h, i) pollen agglomeration, (j) exine sculpture detail. k–m Pollen type 2 (S153632), EV. n–p Pollen type 3 (S153637), PV. Abbreviations: PV, polar view; EV, equatorial view. Scale bars, 10 μm (a, b, e, f, h, i, k, l, n, o), 1 μm (c, g, j, m, p)
Description: For detailed descriptions and remarks, see Bouchal et al. (2017, p. 18).
Acer morphotype 2
(Fig. 8dd, ee and S 3-Fig. 13j–l)
Description: For detailed descriptions and remarks, see Bouchal et al. (2017, p. 21, as Acer morphotype 3).
Order Santalales R.Br. ex Bercht. et J.Presl, 1820
Family Santalaceae R.Br., 1810
Genus Arceuthobium M.Bieb., 1820
Arceuthobium sp.
(Fig. 6d, f)
Description: Pollen, shape spheroidal, outline circular, diameter (including echini) 27–33 μm (LM), 21–26 μm (SEM); possibly atectate, exine ca 1 μm thick (LM), sexine thinner than nexine; heterocolpate, tricolpate with three pseudocolpi, colpi and pseudocolpi indistinct in LM, ectocolpi length 2/3 of polar axis, pseudocolpi length ½ of polar axis (SEM); echinate (LM), echinate, weakly nanoverrucate, perforate (SEM), echini base diameter 0.5–1.5 μm (SEM), echini length 0.9–2.1 μm (SEM), a number of echini have broken off, circular abscission marks visible (Fig. 6f, arrow)
Remarks: The fossil Salihpaşalar specimen corresponds by aperture, size range and exine sculpturing to extant members of Arceuthobium (Erdtmann 1952; Hawksworth and Wiens 1972), with closest similarities in exine sculpture to A. pusillum Peck. Extant members of this genus are parasitic on Pinaceae or Cupressaceae (Hawksworth and Wiens 1972).
Order Caryophyllales Takht., 1967
Family Amaranthaceae Juss., 1789
Amaranthaceae gen. indet. 1
(Fig. 8s, t and S 3-Fig. 12g–i)
Description: Pollen, shape spheroidal, outline circular, diameter 15–20 μm (LM, SEM); eutectate, exine 1.5–2 μm thick (LM), sexine thicker than nexine; pantoporate (45+), pori diameter 0.5–1 μm (SEM), pori sunken, operculate, operculum ornamented with 3–8 nanoechini; scabrate (LM), nanoechinate, perforate (SEM).
Remarks: Amaranthaceae gen. indet. 1, 2 and 3 were combined in the pollen diagram. Amaranthaceae gen. indet. 2 differs from Amaranthaceae gen. indet. 1 and 3 by smaller size, fewer pores and operculi with fewer echini.
Amaranthaceae gen. indet. 2
(Fig. 8u and S 3-Fig. 12d–f).
Description: For detailed descriptions and remarks, see Bouchal et al. (2016, p. 50, as Amaranthaceae vel Chenopodiaceae gen. indet. 2).
Amaranthaceae gen. indet. 3
(Fig. 10g–i and S 3-Fig. 12j–l)
Description: Pollen, shape spheroidal, outline circular, diameter 10–15 μm (LM, SEM); eutectate, exine 1.5–2 μm thick (LM), sexine thicker than nexine; pantoporate (18+), pori diameter 1–1.5 μm (SEM), pori sunken, operculate, operculum ornamented with 15–25 nanoechini; scabrate (LM), nanoechinate, perforate (SEM).
Remarks: Amaranthaceae gen. indet. 3 differs by its smaller size and operculi with a higher number of nanoechini from Amaranthaceae gen. indet. 1 and 2. Strong morphological similarities between the Ranunculaceae Thalictrum L. (Clarke et al. 1991) and the fossil species Thalictrumpollis thalictroides Stuchlik (Stuchlik et al. 2009) can be observed using LM. Using SEM Amaranthaceae pollen reveals nanoechinate and perforate exine sculpturing, whereas Thalictrum lacks perforations and its nanoechini are more evenly spaced (compare Clarke et al. 1991).
Family Caryophyllaceae Juss., 1789
Caryophyllaceae gen. indet. 1
(Fig. 8v, w and S 3-Fig. 12m–o)
Description: Pollen, shape spheroidal, outline polygonal to weakly circular, diameter 30–35 μm (LM, SEM); eutectate, exine 2–3 μm thick (LM), nexine thinner than sexine, pantoporate, pori diameter 4–7 μm (SEM), number of pori 15–20, pori sunken; scabrate (LM), nanoechinate, perforate (SEM), nanoechini evenly spaced (SEM).
Caryophyllaceae gen. indet. 2
(Fig. 8x, y and S 3-Fig. 13a–c)
Description: Pollen, shape spheroidal, outline polygonal to weakly circular, diameter 29–32 μm (LM, SEM); eutectate, exine 3–4 μm thick (LM), nexine thinner than sexine, pantoporate, pori diameter 3.5–4.5 μm (SEM), number of pori 10–15 (LM), pori sunken, operculate (SEM); scabrate (LM), nanoechinate, perforate (SEM), nanoechini evenly spaced, operculum with weak ornamentation (SEM).
Remarks: Caryophyllaceae gen. indet. 1 and 2 were combined in the pollen diagram. Caryophyllaceae gen. indet. 2 differs from Caryophyllaceae gen. indet. 1 by smaller pores, smaller perforations and fewer apertures. Pollen of this family has been reported previously from YB (Table 2).
Family Droseraceae Salisb., 1808
Genus Drosera L., 1753
Drosera sp.
(Fig. 10a–c)
Description: Pollen, tetrahedral tetrad, single pollen shape spheroidal to oblate, tetrad diameter 80–100 μm (LM), single pollen diameter 45–65 μm (LM); atectate, nexine 1.5–2 μm thick on distal face (LM); aperture indistinct, pores in equatorial area; echinate, microechinate (LM, SEM), echini length 2.5–3.5 μm (SEM), microechini length 0.7–1 μm (SEM).
Remarks: Extant pollen of Drosera rotundifolia L. (Halbritter et al. 2012) and the D. rotundifolia type of Punt et al. (2003) correspond the Salihpaşalar specimen by the presence of echini and microechini.
Family Polygonaceae Juss., 1789
Genus Persicaria (L., 1753) Mill., 1754/Persicarioipollenites Krutzsch 1962 emend. Krutzsch, 1966
Persicaria sp./Persicarioipollenites pliocenicus Krutzsch, 1962
(Fig. 10d–f)
Description: Pollen, shape spheroidal, outline circular, diameter 60–70 μm (LM, SEM); semitectate, exine 5–6 μm thick (LM), sexine thicker than nexine; pantoporate, pori diameter 2–3 μm (SEM), lumen containing a porus slightly smaller; reticulate, +/− homobrochate (LM, SEM), lumen with freestanding columellae (clava-like), muri duplicolumellate (SEM), porus membrane granulate (Fig. 10f)
Remarks:Persicaria sp. corresponds (reticulate, pantoporate, duplicolumellate muri) to pollen of the extant genus, with closest similarities to the Polygonum persicaria type of Van Leeuwen et al. (1988). Akgün and Akyol (1999, fig. 20.40) figured a morpholgically similar grain from the Menderes Graben, Turkey.
Genus Rumex L., 1753
Rumex sp.
(Fig. 8z, aa and S 3-Fig. 13d–f)
Description: For detailed descriptions and remarks, see Bouchal et al. (2016, p. 52).
Order Ericales Bercht. et J.Presl, 1820
Family Sapotaceae Juss., 1789
Sapotaceae gen. indet.
(Fig. 10j–l)
Description: Pollen, shape prolate, outline elliptic in equatorial view, length of polar axis 30–40 μm (LM), 24–32 μm (SEM), equatorial diameter 25–34 μm (LM), 20–28 μm (SEM); eutectate, exine 2–3.5 μm thick (LM), nexine thinner than sexine, nexine thickened in aperture area (costa), costa not reaching apical ends of colpus; tetracolporate, ectocolpus length 3/4 to 5/6 of length of polar axis, endoporus quadrangular, framed by costa; scabrate (LM), nanoverrucate, perforate (SEM), nanoverrucae and perforations evenly spaced.
Remarks: Extant Sapotaceae pollen has been studied extensively by Harley (1991); perforate, nanoverrucate (finely granulate in Harley 1991) exine sculpture, corresponds to Harley’s pollen type IA, which is found in several genera of tribus Sapoteae [e.g. Mimusops L., Payena A.de Candolle, Madhuca Hamilton ex J.F.Gmelin (Harley 1991; Swenson and Anderberg 2005)]; extant genera of this tribus have a palaeotropical distribution (Pennington 2004). Sapotaceae pollen differing in aperture has been reported from the YB (Bouchal et al. 2017, p. 21, pl. 11.8–10).
Order Garryales Mart., 1835
Family Eucommiaceae Engl., 1907
Genus Eucommia Oliv., 1895/Tricolpopollenites Pflug et P.W.Thomson in P.W.Thomson et Pflug, 1953
Eucommia sp./Tricolpopollenites parmularius (R.Potonié, 1935) P.W.Thomson et Pflug, 1953
(Fig. 11a–,b and S 3-Fig. 13m–o)
Description: For detailed descriptions and remarks, see Bouchal et al. (2016, p. 54, as Eucommia sp.).
Order Lamiales Bromhead, 1838
Family Oleaceae Hoffmanns. et Link, 1809
Remarks: Pollen of extant Oleaceae show a high degree of morphological variability and overlap [e.g. Olea (Nilsson 1988); Fraxinus L. (compare Renault-Miskovsky et al. 1976; Barento et al. 1987; Punt et al. 1991; Guo et al. 1994; Jones et al. 1995; Li et al. 2010; Miyoshi et al. 2011)]. This makes identification of dispersed Oleaceae pollen to the genus level difficult. Oleaceae gen. indet. 1, 2 and 3 were combined in the pollen diagram.
Oleaceae gen. indet. 1
(Fig. 10m–o)
Description: Pollen, shape spheroidal, outline circular in equatorial view, lobate to circular polar view, length of polar axis 20–26 μm (LM), 22–25 μm (SEM), equatorial diameter 17–23 μm (LM), 15–19 μm (SEM); atectate, exine 2–2.5 μm thick (LM), nexine thinner than sexine; tricolporate, ectocolpus length 2/3 to 3/4 of polar axis (LM, SEM); reticulate (LM, SEM), muri width 0.8–1.2 μm (SEM), muri crested with distinct ridges and nanoechini, lumina of irregular shape and size (SEM).
Remarks: Muri crested with distinct ridges and nanoechini are the defining characters of Oleaceae gen. indet. 1. Extant Olea L. and Chionanthus L. (Renault-Miskovsky et al. 1976; Barento et al. 1987; Nilsson 1988; Punt et al. 1991; Guo et al. 1994; Sachse 2001) produce pollen of similar morphology. Similar pollen has previously been reported from the YB (Bouchal et al. 2016, p. 56, fig. 22D–F, as Oleaceae gen. indet. 4; Bouchal et al. 2017, p. 25, pl. 12.7–9, as Oleaceae gen. indet. 4).
Oleaceae gen. indet. 2
(Fig. 12a–c)
Description: Pollen, shape prolate, outline elliptic in equatorial view, length of polar axis 22–27 μm (LM), 22–25 μm (SEM), equatorial diameter 17–23 μm (LM), 15–19 μm (SEM); atectate, exine 2–2.5 μm thick (LM), nexine thinner than sexine; tricolporate, ectocolpus length 2/3 to 3/4 of polar axis (LM, SEM); reticulate (LM, SEM), muri width 0.8–1.2 μm (SEM), muri with weak perpendicular ridges, lumina of irregular shape and size, lumina psilate (SEM).
Remarks: Oleaceae gen. indet. 2 can be distinguished from Oleaceae gen. indet. 1 and 3 by its perpendicular ridged reticulum. Similar pollen has previously been reported from the YB (Bouchal et al. 2016, p. 56, Fig. 21J–L, as Oleaceae gen. indet. 2) and from late Miocene localities of northern Italy (Sachse 2001). Similar pollen are produced by extant Phillyrea angustifolia L. (Renault-Miskovsky et al. 1976; Sachse 2001), Linociera obtusifolia (Lam.) H.Perrier and Noronhia linocerioides H.Perrier (Cerceau-Larrivall et al. 1984).
Oleaceae gen. indet. 3
(Fig. 12d–f)
Description: Pollen, shape spheroidal, outline circular in equatorial view, lobate to circular in polar view, length of polar axis 21–25 μm (LM), 20–23 μm (SEM), equatorial diameter 20–24 μm (LM), 19–22 μm (SEM); atectate, exine 2.5–3 μm thick (LM), nexine thinner than sexine; tricolporate, ectocolpus length 2/3 to 3/4 of polar axis (LM, SEM); reticulate (LM, SEM), muri width 0.8–1.2 μm (SEM), muri with weak nanoechini, lumina of irregular shape and size, lumina with freestanding columellae (SEM).
Remarks: Oleaceae gen. indet. 3 is defined by muri crested with weak nanoechini and wide lumina with freestanding columellae. Similar pollen has been reported from the YB (Bouchal et al. 2016, p. 56, fig. 21A–C, as Oleaceae gen. indet. 3, differing by psilate lumina; Bouchal et al. 2017, p. 23, pl. 12.4–6, as Oleaceae gen. indet. 3, freestanding columellae visible). Extant Olea, Fontanesia Labillardière and Osmanthus Loureiro (Renault-Miskovsky et al. 1976; Nilsson 1988; Guo et al. 1994; Sachse 2001; Li et al. 2010) produce pollen with nanoechinate muri.
Campanulids
Order Aquifoliales Senft, 1856
Family Aquifoliaceae Bercht. et J.Presl, 1825
Genus Ilex L., 1753
Ilex sp.
(Fig. 11c, d and S 3-Fig. 14a–c)
Remarks: For detailed descriptions and remarks, see Bouchal et al. (2017, p. 25).
Order Asterales Link, 1829
Family Asteraceae Berchthold et Presl, 1820
Subfamily Asteroideae Lindl
Asteroideae type 1
(Fig. 11g and S 3-Fig. 14d–f)
Remarks: For detailed descriptions and remarks, see Bouchal et al. (2017, p. 25, as Asteroideae type 1). Asteroideae types 1 to 5 clearly belong to this subfamily (compare Punt and Hoen 2009); due to poor preservation (folded, broken, deformed grains), the encountered echinate pollen cannot be assigned to particular genera. The here used types are purely artificial and of no taxonomic value. Artemisia and Asteroideae types 1 to 5 were combined in the pollen diagram.
Asteroideae type 2
(Fig. 11e and S 3-Fig. 14g–i)
Description: Pollen, shape spheroidal, outline circular in equatorial view, length of polar axis 15–20 μm (LM, SEM), equatorial diameter 15–20 μm (LM, SEM); exine including echini 3–4 μm thick (LM), sexine thicker than nexine; tricolporate, ectocolpus length 1/2 to 2/3 of polar axis; echinate (LM), echinate, perforate (SEM), echinus base diameter 2–2.5 μm (SEM), echini acute and short, echinus length 1–2 μm (SEM), perforations extending to upper half of echini, echini densely spaced.
Remarks: Asteroideae type 2 is defined by small size, densely spaced broad based echini and small perforations.
Asteroideae type 3 (Fig. 11f and S 3-Fig. 14j–l)
Description: Pollen, shape prolate, outline elliptic in equatorial view, length of polar axis 20–30 μm (LM, SEM), equatorial diameter 15–20 μm (LM, SEM); exine including echini 2–3 μm thick (LM), sexine thicker than nexine; tricolporate, ectocolpus length 1/2 to 2/3 of polar axis; echinate (LM), echinate, perforate (SEM), echinus base diameter 1–1.5 μm (SEM), echini acute and short, echinus length 1–1.5 μm (SEM), perforations extending to lower third of echini, echini widely spaced.
Remarks: Asteroideae type 3 differs by thinner exine and small, more widely spaced echini from the remaining four Asteroideae pollen types.
Asteroideae type 4
(Fig. 11j and S 3-Fig. 14 m–o)
Remarks: For detailed descriptions and remarks, see Bouchal et al. (2016, p. 58, as Asteroideae type 2).
Asteroideae type 5
(Fig. 11h, i and S 3-Fig. 15a–c)
Description: Pollen, shape prolate, outline elliptic in equatorial view, length of polar axis 30–35 μm (LM, SEM), equatorial diameter 25–30 μm (LM, SEM); exine including echini 3.5–5 μm thick (LM), sexine thicker than nexine; tricolporate, ectocolpus length 2/3 to 3/4 of polar axis; echinate, perforate (LM, SEM), echinus base diameter 3–4 μm (SEM), echini blunt and short, echinus length 1.5–2 μm (SEM), perforations extending to upper third of echini.
Remarks: The characters defining Asteroideae type 4 are distinctly large perforations (visible in LM, Fig. 11h), a thick exine and blunt echini, clearly setting it apart from the other Asteroideae pollen types.
Genus Artemisia L., 1753
Artemisia sp.
(Fig. 12g–i and S 3-Fig. 15d–f)
Description: Pollen, shape prolate, pollen outline circular to lobate in polar view, equatorial diameter 10–15 μm (LM, SEM), polar axis 12–20 μm (LM, SEM); tectate; tricolpate, ectocolpus length 3/4 to 5/6 of polar axis (SEM); scabrate (LM), nanoechinate, echini densely spaced (SEM).
Remarks: Pollen of Artemisia has rarely been reported from middle Miocene localities of western Turkey: Artemisia (Jiménez-Moreno 2005; Kayseri-Özer et al. 2014b).
Subfamily Cichorioideae Chevall., 1828
Cichorioideae gen. indet.
(Fig. 11k, l and S 3-Fig. 15g–j)
Description: Pollen, shape spheroidal, outline polygonal, pollen diameter 30–40 μm (LM, SEM); lophate, exine including lophae and echini 3–6 μm thick (LM), nexine thinner than sexine; tricolporate, colpus membrane granulate, lacune forming bridge over edoporus; lophate, echinate, perforate (LM, SEM), echini acute, echinus base diameter 0.8–1.3 μm (SEM, LM), echinus height 1.5–2 μm (SEM), echini without perforations, echini present only on lophae, lophae perforate, interporal lacuna perforate, abporal lacuna psilate.
Remarks: Cichorioideae gen. indet. can be assigned to the Lactuca sativa type of Blackmore (1984) owing to the position and presence of perforate lacunae.
Order Dipsacales Juss. ex Bercht. et J.Presl, 1820
Family Adoxaceae E.Mey., 1839
Genus Viburnum L., 1753
Viburnum sp.
(Fig. 12j–m)
Description: Pollen, shape prolate, outline elliptic, length of polar axis 28–33 μm (LM, SEM), equatorial diameter 18–23 μm (LM, SEM); semitectate, exine 2–3 μm thick (LM), sexine thicker than nexine; tricolporate, porus diameter 2–3 μm (SEM), endoporus circular, colpus length 2/3 to 3/4 of polar axis, costae reaching polar colpus endings; reticulate, +/− homobrochate in mesocolpium and polar areas, reticulum condensed in aperture areas (SEM), lumen with freestanding columellae (SEM), muri smooth and elevated by columellae.
Remarks:Viburnum sp. corresponds in its morphological characters (tricolporate apertures, reticulate tectum, reticulum condensed in aperture areas, lumina with freestanding columellae) to the extant pollen of this genus (Maciejewska 1997), with closest similarities to pollen “class A” of Donoghue (1985), which is commonly found in this genus.
Family Caprifoliaceae Juss., 1789
Subfamily Dipsacoideae Eaton
Genus Dipsacus L., 1753/Cephalaria Schrad. 1818
Dipsacus vel Cephalaria sp.
(Fig. 11m)
Remarks: For detailed descriptions and remarks, see Bouchal et al. (2017, p. 27).
Subfamily Valerianoideae (Valerianaceae Batsch, 1802)
Genus Valeriana L., 1753
Valeriana sp.
(Fig. 12n–q)
Description: Pollen, shape prolate, length of polar axis 35–47 μm (LM, SEM), equatorial diameter 30–38 μm (LM, SEM); eutectate, exine 3–4 μm thick including echini (LM), sexine thicker than nexine; tricolpate, (LM, SEM), ectocolpus length 2/3 to 3/4 of polar axis; microechinate (LM), microechinate (more numerous), nanoechinate, perforate (SEM), area surrounding microechini slightly raised (weak verrucae), micro- and nanoechini blunt (obtuse), colpus membrane nanoechinate (SEM).
Remarks: The available specimen corresponds by its exine architecture and microechinae situated on weak verrucae to the Valeriana officinalis type of Clarke and Jones (1977) and the Valeriana pollen type I of Clarke (1978). Previous reports of Valerianoideae pollen from middle Miocene localities of western Turkey: Valerianaceae (Yavuz-Işık 2007; Yavuz-Işık et al. 2011).
Genus Centranthus DC, 1805
Centranthus sp.
(Fig. 13a–d)
Description: Pollen, outline in polar view lobate to circular, equatorial diameter 30–37 μm (LM, SEM); eutectate, exine 2.5–3.5 μm thick (LM), sexine thicker than nexine; tricolpate, ectocolpus length 3/4 to 5/6 of polar axis, sunken, colpus ends acute; microechinate (LM), microechinate (more numerous), nanoechinate, perforate (SEM), micro- and nanoechini acute, colpus membrane covered with acute microechini.
Remarks:Centranthus sp. corresponds in several characteristics (exine architecture, exine ornamentation, aperture, microechinate colpus membrane) to extant Centranthus ruber type of Clarke and Jones (1977) and Centranthus of Clarke (1978).
Order Apiales Nakai, 1930
Family Apiaceae Lindl., 1836
Apiaceae type 1
(Fig. 11n, o and S 3-Fig. 15k–n)
Description: Pollen, shape prolate, length of polar axis 19–22 μm (LM, SEM), equatorial diameter 10–14 μm (LM, SEM); eutectate, exine 1–1.5 μm thick in apex area, 2–2.5 μm in mesocolpium area (LM), sexine thicker than nexine, outer contour convex, inner contour weakly concave; tricolporate, slit-like ectocolpus with bridge in porus area (LM, SEM), ectocolpus length 1/2 to 2/3 of polar axis, endoporus circular; scabrate (LM), microrugulate, fossulate, perforate (SEM), sculpturing in aperture area pronounced rugulate, fossulate (loosely packed), sculpturing in mesocolpium and apex area more perforate (more densly packed).
Remarks: Apiaceae types 1 to 6 were combined in the pollen diagram. For the morphological description of Apiaceae type pollen, the nomenclature of Punt (1984) is used. Apiaceae type 1 has strong morphological similarities (colpus length, exine thickened in mesocolpium) to the Conium type of Beug (2004), with closest similarities to Falcaria vulgaris Bernh. (Beug, 2004, plate 23, figs 19–20), and the F. vulgaris type of Punt (1984) but differs by its smaller size.
Apiaceae type 2
(Fig. 11p, q and S 3-Fig. 15o–r)
Description: Pollen, shape prolate, length of polar axis 17–19 μm (LM, SEM), equatorial diameter 8–10 μm (LM, SEM); eutectate, exine 1–1.5 μm thick in apex area, 2–2.5 μm in mesocolpium area (LM), sexine thicker than nexine, outer contour convex, inner contour straight; tricolporate, slit-like ectocolpus with (weakly raised) bridge in porus area (LM, SEM), ectocolpus length 3/4 to 5/6 of polar axis, endoporus circular; scabrate (LM), microrugulate, fossulate, perforate (SEM).
Remarks: This pollen type falls in the morphological range of Sison amomum type of Punt (1984) and Berula erecta group of Beug (2004), with closest affinities (size) to S. amomum L. (see pl. 25, figs. 35–36 in Beug 2004).
Apiaceae type 3
(Fig. 11r, s and S 3-Fig. 16a–d)
Description: Pollen, shape prolate, length of polar axis 24–28 μm (LM, SEM), equatorial diameter 13–17 μm (LM, SEM); eutectate, exine 2–2.5 μm thick in apex area (LM), nexine thickened in porus area, in all other areas sexine either same thickness as nexine or thicker than nexine, outer contour convex, inner contour distinctly concave curved in porus area; tricolporate, slit-like ectocolpus (LM, SEM), ectocolpus length 3/4 to 5/6 of polar axis, endoporus elliptic; scabrate (LM), microrugulate, fossulate, perforate (SEM), sculpturing in mesocolpium and aperture area loosely packed, sculpturing in apex area more densly packed (SEM).
Remarks: Apiaceae type 3 has strong morphological similarities to the Oenanthe fistulosa type of Punt (1984) and to O. fistulosa L. in Beug (2004, pl. 23, figs. 10–12).
Apiaceae type 4
(Fig. 11t, u and S 3-Fig. 16e–h)
Description: Pollen, shape prolate, length of polar axis 16–19 μm (LM, SEM), equatorial diameter 9–11 μm (LM, SEM); eutectate, exine 1.5–2 μm thick in apex area (LM), outer contour convex, inner contour not detectable; tricolporate, slit-like ectocolpus (LM, SEM), ectocolpus length 3/4 to 5/6 of polar axis; scabrate (LM), microrugulate, fossulate, perforate (SEM), sculpturing in mesocolpium and aperture area loosely packed, sculpturing in apex area more densly packed, perforate (SEM).
Remarks: Apiaceae type 4 is poorly preserved, its dimensions (size, colpus length) are similar with Apiaceae type 3 but differences in exine sculpturing set this type apart from co-occurring pollen types.
Apiaceae type 5
(Fig. 11v, w and S 3-Fig. 16i–l)
Description: Pollen, shape prolate, length of polar axis 23–26 μm (LM, SEM), equatorial diameter 11–14 μm (LM, SEM); eutectate, exine 1.5–2 μm thick (LM), sexine thicker than nexine, outer contour straight, inner contour weakly convex; tricolporate, slit-like ectocolpus with bridge in porus area (LM, SEM), ectocolpus length 3/4 to 5/6 of polar axis, endoporus elliptic; scabrate (LM), microrugulate, fossulate, perforate (SEM).
Remarks: Apiaceae type 5 has some similarities (long colpus, elliptic endoporus) with the Apium inundatum type of Punt (1984).
Apiaceae type 6
(Fig. 11x, y and S 3-Fig. 16m–p)
Description: Pollen, shape prolate, length of polar axis 19–24 μm (LM, SEM), equatorial diameter 11–14 μm (LM, SEM); eutectate, exine 1.5–2 μm thick in apex area (LM), outer contour straight, inner contour weakly concave; tricolporate, slit-like ectocolpus (LM, SEM), ectocolpus length 3/4 to 5/6 of polar axis, endoporus rectangular; scabrate (LM), microrugulate, fossulate, perforate (SEM), sculpturing in mesocolpium and aperture area loosely packed, sculpturing in apex area more densly packed (perforate).
Remarks: Apiaceae type 6 has similar dimensions (size, colpus length) with Apiaceae type 5 but differs in its exine sculpturing.
Subfamily Saniculoideae Burnett
Saniculoideae gen. indet.
(Fig. 11z, aa and S 3-fig. 16q–t)
Description: Pollen, shape prolate, length of polar axis 40–44 μm (LM, SEM), equatorial diameter 17–20 μm (LM, SEM); eutectate, exine 1.5–2 μm thick (LM), sexine thicker than nexine, outer contour convex to straight, inner contour distinctly concave in porus area; tricolporate, slit-like ectocolpus (LM, SEM), ectocolpus length >5/6 of polar axis, band-like costae around the equator (endocingulum); scabrate (LM), microrugulate, fossulate, perforate (SEM).
Remarks: Saniculoideae gen. indet. corresponds (inner and outer contour, aperture, sculpturing) to Astrantia (major) type pollen (e.g. Cerceau-Larrivall et al. 1984; Punt 1984; Beug 2004). Pollen of this subfamily has previously been reported from the YB (Bouchal et al. 2016, 2017).
Family Araliaceae Juss., 1789
Araliaceae gen. indet.
(Fig. 13e–g)
Description: Pollen, shape prolate, outline in equatorial view elliptic, length of polar axis 25–30 μm (LM, SEM), equatorial diameter 16–22 μm (LM, SEM); eutectate, exine 2.5–3.5 μm thick (LM), sexine thicker than nexine; tricolporate, pori sunken, operculate, ectocolpus length 2/3 to 3/4 of polar axis, endoporus circular, indistinct costae present; scabrate (LM), microreticulate (SEM), lumina condensed in colpus area.
Remarks: Araliaceae gen indet. has characteristics (exine sculpturing, aperture) commonly present in genera of this family, e.g. Aralia L., Panax L. (Wen and Nowicke 1999).
Incerta sedis
Remarks: Pollen of uncertain determination was not included in the pollen diagram.
Pollen type 1
(Fig. 13h–j)
Description: Pollen agglomeration, pollen outline +/− circular in polar view, equatorial diameter 10–15 μm (LM, SEM); tectate; tri- to tetra porate, porus diameter 1.5–2 μm (LM, SEM); psilate (LM), nanoechinate, perforate (SEM).
Remarks:. This pollen agglomeration consists of a single type of densely packed pollen grains and pollenkitt suggesting this is the content of an immature pollen sack. Nanoechinate, porate pollen is produced by a number of families (e.g. Betulaceae, Myricaceae, Juglandaceae, Moraceae and Urticaceae); further determination is not possible owing to the immature state of the pollen.
Pollen type 2
(Fig. 13k–m)
Description: Pollen, shape spheroidal, outline in equatorial view circular, length of polar axis 17–20 μm (LM, SEM), equatorial diameter 15–18 μm (LM, SEM); eutectate, exine 1–1.5 μm thick (LM), sexine thicker than nexine; tricolporate to tricolporidate, colpus sunken, ectocolpus length 2/3 to 3/4 of polar axis, indistinct endoporus; psialte (LM), perforate, microfossulate (SEM), fossule more frequent in colpus area (SEM).
Pollen type 3
(Fig. 13n–p)
Description: Pollen, outline circular to lobate in polar view, equatorial diameter 19–23 μm (LM, SEM); tectate, exine 1–1.5 μm thick (LM); pentacolpate, ectocolpus length 1/3 to 1/2 of equatorial diameter, colpus membrane granulate (LM, SEM); psilate (LM), granulate (SEM)
Discussion
Palynoflora and pollen zones of the Salihpaşalar lignite mine section
The most frequently observed palynomorphs of the Salihpaşalar section belong to Fagaceae (14.5–47.5%) and bisaccate gymnosperm pollen (8.75–34.5%); through the entire section arboreal pollen (AP) (73–94%) is distinctly more frequent than non-arboreal pollen (NAP).
The composition of the palynofloras of the Salihpaşalar section is rather homogenous, but two pollen zones can be distinguished.
Pollen Zone (PZ) 1 is characterised by a higher abundance of spores (7.75–14%) and azonal elements (Decodon 4.5–12.75%) and has previously been reported from the Eskihisar and Tinaz mine sections by Bouchal et al. (2016, 2017). At the Salihpaşalar mine, PZ1 is restricted to the lower part of the sampled section (S153622–S15327) comprising the thickest lignite seam, its intercalated sediments and the underlying micaceous siltstones. PZ 1 mainly concurs in its composition with PZ 2, differing only in the presence of Lycopodium, Lycopodiella, Selaginella, Pilularia, Tsuga and Drosera.
PZ 2 is confined to the upper part of the section overlying the main lignite seam consisting of clayey limestones and marls intercalated by thin lignite beds (samples S15329–S15346). In this zone, occasional abundance spikes of algal cysts (> 20%, in S15329 and S15339) and Apiaceae (9.5% in S15338) are recorded and spores are less frequent (0.5–2.75%). Pollen of Buxus, papillate Cupressaceae, Ilex, Persicaria, Sapotaceae, Valerianoideae and Viburnum have only been encountered in PZ 2.
Comparing the palynoassemblages of the YB
Palynofloras from different strata and localities in the YB, most of them belonging to the Turgut and Sekköy members, have been the focus of a high number of studies (this study; Nakoman 1967; Benda 1971; Gemici et al. 1990; Erdei et al. 2002; Jiménez-Moreno 2005; Akgün et al. 2007; Yavuz-Işık et al. 2011; Koç, 2017; Bouchal et al. 2016, 2017). Most of these studies agree that Fagaceae and Pinaceae are the most taxonomically diverse and abundant families in the palynofloras of the Turgut and Sekköy members (Table 2). This study and Bouchal et al. (2016, 2017) distinguished four pollen zones for sections of the YB lignite mines (Eskihisar, Tınaz, Salihpaşalar): (i) PZ 1 is mainly dominated by fern spores (S 4, abundances ranging from < 10–60%) and azonal elements (e.g. Alnus) and is restricted to the lignite seams and intercalated and underlying layers. Hence, in this PZ, a strong facies signal is seen rather than a stratigraphical one. Gemici et al. (1990, Table 1) report fern spore abundances of > 80% and Yavuz-Işık et al. (2011, samples E in fig. 2; spores were not investigated in this study) report Alnus pollen values of < 40% for their samples from Eskihisar; this would possibly place these samples in PZ 1. (ii) In PZ 2, zonal AP taxa are most diverse and abundant (e.g. Pinaceae, Fagaceae, Juglandaceae, Ulmaceae) while the herbaceous component is diverse but of low abundance. In the Eskihisar, Tınaz and Salihpaşalar mines, this PZ is present in the first 10–20 m of marls and limestones overlying the lignite seams. A similar signal is reported from samples from Çatakağyaka (ca 12 km southeast of Tınaz) by Jiménez-Moreno (2005, samples 1 and 2 in fig. 4.77) and Yavuz-Işık et al. (2011, samples Ç in fig. 3). Bouchal et al. (2017) reported a (iii) transitional zone between PZ 2 and PZ 3 for their Tınaz section. Here, the ratio between AP and non-AP fluctuates strongly and diversity and abundance of herbaceous taxa increases while AP taxa decrease (S 4). Possibly, the upper part of the Eskihisar section of Bouchal et al. (2016, samples S153582–S153593 in fig. 2) could belong to this PZ, because of an abundance peak of Amaranthaceae and Apiaceae. Similar increased abundance of herbaceous taxa has been reported from Çatakbağyaka (< 25% NAP, Jiménez-Moreno 2005, sample 3 in fig. 4.77) and Yeni Eskihisar 1 (< 30%, Yavuz-Işık et al. 2011, sample Y in fig. 3). It is important to note that these similarities reflect similar facies but not necessarily same age. (iv) In PZ 3, present in a single sample from the Tınaz section of Bouchal et al. (2017), AP abundance and diversity have further declined and a value of < 20% of AP is reported (S 4).
Only rare accessory elements, namely spores of Lycopodiella, Selaginella, Pilularia and pollen of Tsuga (nonechinate type), Smilax, Moraceae vel Urticaceae, Drosera, Persicaria, Artemisia, Viburnum, Valeriana, Centranthus and Araliaceae are confined to the Salihpaşalar section. Among these taxa, many are typical elements of lake shores and wetlands (e.g. Pilularia, Valeriana, Drosera and Ammania). Likewise, a number of accessory elements are only reported from Eskihisar (Succisa, Convolvulus, Erica, Ericaceae gen. indet., Apios, Ludwigia, aff. Ailanthus) and Tınaz [Ajugoideae gen. indet., Campanuloideae gen. indet., Scabiosa, Picrasma, Tsuga (echinate type)].
Noteworthy is also the presence/absence of some of these accessorial elements. While documented by Gemici et al. (1990), the presence of the rare and distinct Sapotaceae pollen type could not be verified in samples of the Eskihiar mine investigated by Benda (1971, “Eskihisar Pollenbild”) and Bouchal et al. (2016), but single ocurrences are documented for Tınaz and Salihpaşalar (this study; Bouchal et al. 2017). Another example is Buxus pollen, which is absent from the Tınaz section but documented for Eskihisar and Salihpaşalar, while leaf remains of Buxus pliocenica Saporta et Marion are reported from Tınaz and Eskihisar (Bouchal et al. 2016, 2017; Güner et al. 2017).
Depositional environment
The alternating 19-m sequence of pelitic sediment (limestones and marls) intercalated with lignites at the Salihpaşalar lignite mine shows no signs of deltaic sedimentation (absence of channelfills and coarse-grained sediments). The formation of lignite at the margin of the YB was most likely controlled by water table fluctuations within the palaeo-lake, either by transient subsidence of the basin or change in fluvial water supply. During a deepening phase (more subsidence/higher water input) of the palaeo-lake, the pellitic limestones and marls were deposited; coal swamps probably developed when the water table receded to a level that still provided a water-saturated lake margin. In the first few centimetres (1–5 cm) of limestone or marl beds following a lignite bed, poorly preserved monocotyledon root horizons (affinities to Phragmites or Typha) were observed. These probably represent the development of a reed belt that developed in response to the flooding of the shallow, swampy palaeo-lake margin areas. The overlying marls and pellitic limestones do not contain such root horizons, indicating a further deepening of the palaeo-lake.
Summarising, the deposits of the Salihpaşalar section accumulated in a marginal part of the Yatağan palaeo-lake. No clastic payload incoming from a fluviatile system affected the sedimentation. Whether cosmic cyclicities or subsidence did lead to the alternating lignite and limestone pattern of the Salihpaşalar section has not been the focus of this study. Future geological investigations on more complete sections (e.g. drill cores) of the main YB could help resolve this aspect.
Regional vegetation inferred from the palynofloras of the YB
Table 2 lists cyst, spore and pollen taxa reported from the YB palynofloras. The ecological properties of their potential modern analogues are indicated and assigned to vegetation units (VU). Only very few taxa (e.g. Engelhardioideae p.p., Sapotaceae) are today restricted to lowland forests in warm temperate to tropical climates (VU 5a). Also Carpinus, Ostrya and Tilia are nowadays commonly found in lowland forests of temperate regions. The few lianas reported here may also have been part of lowland forests (both well-drained and swamp forests). The majority of tree species reported from the YB palynofloras grow in well-drained lowland and upland forests (VU 5b–7) and comprise both angiosperms and conifers. Among conifers, it is noteworthy that coal-forming taxodiaceous taxa are not well-represented suggesting that other plants contributed to the coal in the Yatağan Basin. In contrast, Pinaceae were fairly diverse. Although modern distributions of taxa such as Cathaya, Cedrus or Picea are typically confined to high elevations, the mass occurrence of Cathaya and Cedrus leafy shoots, cones and cone scales in late Miocene deposits of northern Greece (Velitzelos et al. 2014) suggests that these elements could also have grown at lower elevation. Similar patterns of shifting of ecological niches are seen in ancestors of mountain goats (Solounias and Moelleken 1992). Among angiosperms, trees and shrubs include both deciduous and evergreen taxa and may have contributed to different types of forest vegetation. Fagaceae (Fagus, Quercus sect. Cerris, Q. sect. Ilex) most likely were the dominating forest elements in the surroundings of the YB.
Among herbaceous plants, a great number might have been associated with open lake shore vegetation including areas that temporarily fell dry. Typha and Typha-allies may have formed a reed belt in some places. Only few herbaceous taxa (e.g. the ferns Pteris and Osmunda) are characteristic elements of forests.
In summary, the regional vegetation as inferred from the extensive palynological record of the YB floras consisted of different forest types, including broadleaved thermophilous lowland forests, temperate lowland and upland forests as well as mixed broadleaved and conifer forests at mid and high elevations. In addition, rich riparian forests were present around the lake and rivers. Coal-forming swamp forests may have included taxodiaceous conifers and angiosperms such as Myrica, Alnus and Decodon. Open vegetation was restricted to disturbed and shore areas.
Attempting a standardised nomenclature for cysts, pollen and spores—comparing the palynomorphs of the YB to previously published literature
At present, no standardised correlation of palynomorphs from Turkish Neogene palynofloras exists. Such an attempt was made for the Neogene of Poland and resulted in four comprehensive pollen and spore atlases (Stuchlik et al. 2001, 2002, 2009, 2014).
Since the 1960s, many palynological studies focussed on Miocene deposits of western Anatolia (e.g. Nakoman 1967; Benda 1971; Ediger 1990; Gemici et al. 1990, 1991, 1993; Takahashi and Jux 1991; Akgün 1993; Akgün and Akyol 1999; Jiménez-Moreno 2005; Akgün et al. 2007; Kayseri 2010; Kayseri and Akgün 2010; Kayseri-Özer et al. 2014a, 2014b; Bozcu et al. 2015). Early studies used the abotanical, morphology-based nomenclature (see, e.g. Potonié 1931; Thomson and Pflug 1953) for fossil palynomorphs. Later studies increasingly used a botanical nomenclature to name palynomorphs. Regardless of which nomenclature was used, most studies indicated botanical affinities of the encountered palynomorphs.
In this and two previous studies (Bouchal et al. 2016, 2017), a combined LM and SEM approach was used to achieve best possible botanical determination for palyno-morphs for three coeval sections in the YB. At the same time, it was noticed that in the published literature for the same fossil-taxon, a variety of botanical affinities had been indicated by different authors (S 2). Using the well-documented (LM and SEM) studies on YB lignite mine sections (this study; Bouchal et al. 2016, 2017) as a taxonomical and morphological baseline, I compared 24 published accounts on (preferably figured) dispersed palynomorphs from early to middle Miocene deposits of western Anatolia. Although the palynological record of this time span and region likely is more diverse and complex than that of three sections of a single basin, a fairly high degree of homogeneity in composition (not in abundance) at regional scale has previously been noted by other authors (Benda 1971; Takahashi and Jux 1991; Akgün et al. 2007). In Table S 2, both botanical and abotanical taxa were compiled (i) to visualise the various palynological concepts used for this region since the 1960ies; (ii) to discriminate well-documented (common) taxa from those that so far lack documentation; and (iii) to narrow down the list of over 400 names that have been used in reviewed literature to the ca 170 taxa that are botanically (using LM and SEM) recognisable for the YB. The resulting redundancies of names are highly misleading when discussing past biodiversity but may also affect taxonomic interpretation and as such the inference of palaeoenvironments. Finally, (iv) they were compiled to provide a basis for further scientific discussion.
In the following, I provide a few examples to illustrate why a standardised palynomorph nomenclature with updated botanical affinities will improve regional environmental comparisons and palaeoecological inference.
The name Laevigatosporites haardti is commonly used for psilate monolete spores. This stratigraphically widespread and rather featureless spore type is found in several extant fern families (e.g. Aspleniaceae, Davalliaceae, Dryopteridaceae, Gleicheniaceae, Lomariopsidaceae, Oleandraceae, Thelypteridaceae, Vittariaceae, Polypodiaceae; see Tyron and Lugardon 1991) but has mainly been associated with Polypodiaceae in the reviewed literature (S 2). If using this name, it should be clearly stated that the exact botanical affinities of this taxon cannot be established using spore morphology.
The former Taxodiaceae, “Taxodiaceae pollen”, commonly used as synonymous of papillate cupressaceaeous pollen, are currently included within the Cupressaceae and its former members have been assigned to the subfamilies Taiwanioideae, Athrotaxoideae, Sequoioideae and Taxodioideae (Earle 2010; Farjon 2005). Several studies demonstrated the limited taxonomic value of papillate and non-papillate Cupressaceae pollen (Kedves 1985; Bortenschlager 1990; Grimm et al. 2016). For identification to subfamily level, LM and SEM investigation of exceptionally well-preserved and complete fossil material (3D preservation) is needed (Grímsson and Zetter 2011); such type of preservation has not been reported for any of the reviewed pollen floras of western Anatolia. Since modern members of the Cupressaceae are ecologically very diverse, overly specific (to genus level) identifications run the risk of great error when inferring palaeoenvironments.
The botanical affinity of the fossil species Pityopollenites libellus (R.Potonié) Nakoman has been assumed to be with the mainly southern hemispheric family Podocarpaceae or its genus Podocarpus (S 2). Under SEM, all YB specimens falling within the morphological range of this fossil-taxon show unequivocal characteristics (microechini) of the Pinaceae Cathaya (Bouchal et al. 2016, 2017). Thus, numerous reports of Podocarpus for the Neogene of western Anatolia would need to be verified or rejected by SEM investigations before any biogeographic or palaeoenvironmental conclusions can be drawn.
Tricolporopollenites megaexactus, a fossil species affiliated to Cyrillaceae, is commonly reported and figured in the reviewed literature (S 2). Combined LM and SEM investigation demonstrates that this fossil species shares morphological characteristics (size, shape, aperture) with pollen of the Lythraceae genus Decodon. A first, revised record of Decodon for Turkey shows a stratigraphic range, at least, from middle Miocene to early Pleistocene (this study; Bouchal et al. 2016, 2017; Alçiçek et al. 2017).
Likewise, dispersed pollen in Neogene western Anatolian strata has frequently been determined as Castanea, Castanopsis and/or Lithocarpus or assigned to the fossil species Tricolporopollenites cingulum, T. cingulum oviformis or T. oviformis for which affinities with Castanopsis and Lithocarpus were assumed. However, pollen of extant Castanoideae is strikingly uniform and not sufficiently distinct in LM and SEM to distinguish different genera of this polyphyletic group (see, e.g. Praglowski 1984). Furthermore, poorly preserved pollen of an extinct Castanoideae, Trigonobalanopsis, is indistinguishable in LM from extant castanoids and can only be securely determined using SEM. Trigonobalanopsis is not uncommon in YB palynofloras and hence may be hidden behind some of the names mentioned above from other localities of western Anatolia. Hence, the great diversity of castanoid Fagaceae implied by the reviewed palynological literature is highly misleading. For the reviewed LM investigations, only the botanical affiliation Castaneoideae gen. indet. ought to be used for such palynomorphs.
These are but a few examples out of many which illustrate the urgent need of a catalogue of palynomorphs from Neogene strata of western Anatolia, standardising nomenclature and taxonomic affinities and documenting cysts, spores and pollen using LM and SEM. The skills, knowledge, experience and archives of all palynologists currently working in this geographical region would need to be joined to achieve this task.
Change history
23 November 2019
Fig. 10 LM overview (a, d, g, j, m), SEM overview (b, e, h, k, n) and SEM detail (c, f, i, l, o, p) micrographs of Amaranthaceae, Droseraceae, Polygonaceae, Sapotaceae and Oleaceae pollen. <Emphasis Type="Bold">a–c</Emphasis> <Emphasis Type="BoldItalic">Drosera</Emphasis> <Emphasis Type="Bold">sp. (S153636). d–f</Emphasis> <Emphasis Type="BoldItalic">Persicaria</Emphasis> <Emphasis Type="Bold">sp./</Emphasis> <Emphasis Type="BoldItalic">Persicarioipollenites pliocenicus</Emphasis> <Emphasis Type="Bold">(S153634). g–i Amaranthaceae gen. Indet. 3 (S153636)</Emphasis>
References
Akgün, F. (1993). Palynological age revision of the Neogene Soma coal basin. Bulletin of the Geological Society of Greece, 28, 151–170.
Akgün, F., & Akyol, E. (1999). Palynostratigraphy of the coal-bearing Neogene deposits in Büyük Menderes Graben, western Anatolia. GEOBIOS, 32(3), 367–383.
Akgün, F., Kayseri, M. S., & Akkiraz, M. S. (2007). Palaeoclimatic evolution and vegetational changes during the Late Oligocene–Miocene period in western and central Anatolia (Turkey). Palaeogeography, Palaeoclimatology, Palaeoecology, 253, 56–90.
Akkiraz, M. S. (2011). Vegetation and climate in the Miocene deposits of southern side of Büyük Menderes Graben, Şahinali-2 core, SW Turkey. Bulletin of Geosciences, 86(4), 859–878.
Akkiraz, M. S., Akgün, F., Utescher, T., Wilde, V., Bruch, A. A., Mosbrugger, V., et al. (2012). Palaeoflora and climate of lignite-bearing lower–middle Miocene sediments in the Seyitömer and Tunçbilek sub-basins, Kütahya Province, Northwest Turkey. Turkish Journal of Earth Sciences, 21, 213–235 Maden Tetkik Arama Dergisi, 11.
Akyol, E., & Akgün, F. (1990). Bigadiç. Kestelek, Emet ve Kırka boratlı Neojen tortullannın palinolojisi ve karşılaştınlması. Maden Tektik ve Arama Dergisi, 111, 165–173.
Alçiçek, H. (2010). Stratigraphic correlation of the Neogene basins in southwestern Anatolia: regional palaeogeographical, palaeoclimatic and tectonic implications. Palaeogeography, Palaeoclimatology, Palaeoecology, 291, 297–318.
Alçiçek, H., Wesselingh, F. P., Alçiçek, M. C., Jiménez-Moreno, G., Feijen, F. J., van den Hoek Ostende, L. W., Mayda, S., & Tesakov, A. S. (2017). A multiproxy study of the early Pleistocene palaeoenvironmental and palaeoclimatic conditions of an anastomosed fluvial sequence from the Çameli Basin (SW Anatolia, Turkey). Palaeogeography, Palaeoclimatology, Palaeoecology, 467, 232–252.
Atalay, Z. (1980). Muğla-Yatağan ve yakın dolayı karasal Neojen’inin stratigrafi araştırması. Bulletin of the Geological Society Turkey, C23, 93–99.
Barento, C., Candau, P., Díez, M. J., Fernández, I., Fernández Paniagua, I., Godoy, C., et al. (1987). Atlas polinico de Andalucia occidental. Sevillia: Instituto de desarollo regional de la Universida de Sevilla.
Becker-Platen, J. D. (1970). Lithostratigraphische Untersuchungen im Känozoikum Südwest-Anatoliens (Türkei) (Känozoikum und Braunkohlender der Türkei, 2). Beihefte zum Geologischen Jahrbuch, 97, 1–244.
Becker-Platen, J. D., Benda, L., & Steffens, F. (1977). Litho- und biostratigraphische Deutung radiometrischer Altersbestimmungen aus dem Jungtertiär der Türkei. Geologisches Jahrbuch, B25, 139–167.
Benda, L. (1971). Grundzüge einer pollenanalytischen Gliederung des türkischen Jungtertiärs (Känozoikum und Braunkohlender der Türkei, 4). Beihefte zum Geologischen Jahrbuch, 113, 1–45.
Beug, H. J. (2004). Leitfaden der Pollenbestimmung für Mitteleuropa und angrenzende Gebiete. München: Dr. Friedrich Pfeil.
Blackmore, S. (1984). The Northwest European pollen flora, 32: Compositae-Lactuceae. Review of Palaeobotany and Palynology, 42(45–85), 45–85.
Blackmore, S., Steinmann, J. A. J., Hoen, P. P., & Punt, W. (2003). The Northwest European pollen flora, 65: Betulaceae and Corylaceae. 123(71–98), 71.
Bortenschlager, S. (1990). Aspects of pollen morphology in the Cupressaceae. Grana, 29(2), 129–137.
Bouchal, J. M., Mayda, S., Grímsson, F., Akgün, F., Zetter, R., & Denk, T. (2017). Miocene palynofloras of the Tınaz lignite mine, Muğla, southwest Anatolia: taxonomy, palaeoecology and local vegetation change. Review of Palaeobotany and Palynology, 243, 1–36.
Bouchal, J. M., Zetter, R., Grímsson, F., & Denk, T. (2016). The middle Miocene palynoflora and palaeoenvironments of Eskihisar (Yatağan Basin, southwestern Anatolia): a combined LM and SEM investigation. Botanical Journal of the Linnéan Society, 182(1), 14–79.
Bozcu, M., Akgün, F., Grüdal, G., Bozcu, A., Yeşilyurt, S. K., Karaca, Ö., et al. (2015). Evolution of Çan-Etili (Çanakkale - NW Turkey) lignite basin: sedimentology, petrology, palynology and lignite characterization. International Journal of Sediment Research, 30(3), 190–207.
Cerceau-Larrivall, M. T., Straka, H., & Friedrich, B. (1984). Palynologia Madegassica et Mascarenica: Fam. 155–166. Tropische und subtropische Pflanzenwelt, 51, 595–638.
Chen, S.-C., Zhao, X.-P., Ni, S.-F., Fu, C.-X., & Cameron, K. M. (2006). The systematic value of pollen morphology in Smilacaceae. Plant Systematics and Evolution, 259, 19–37.
Christensen, P. B., & Blackmore, S. (1988). The northwest European pollen flora, 40: Tiliaceae. Review of Palaeobotany and Palynology, 57, 33–43.
Clarke, G. C. S. (1978). Pollen morphology and generic relationships in the Valerianaceae. Grana, 17, 61–75.
Clarke, G. C. S., & Jones, M. R. (1977). The northwest European pollen flora, 16: Valerianaceae. Review of Palaeobotany and Palynology, 24, 155–179.
Clarke, G. C. S., Punt, W., & Hoen, P. P. (1991). The northwest European pollen flora, 51: Ranunculaceae. Review of Palaeobotany and Palynology, 69, 117–271.
Denk, T. (2016). Palaeoecological interpretation of the late Miocene landscapes and vegetation of northern Greece: a comment to Merceron et al., 2016. Geobios, 49, 135–146.
Denk, T., & Grimm, G. W. (2009). Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus (Fagaceae). International Journal of Plant Sciences, 170(7), 926–940.
Denk, T., Grimm, G. W., Manos P. S., Deng, M., & Hipp, A. (2017). An updated infrageneric classification of the oaks: review of previous taxonomic schemes and synthesis of evolutionary patterns. In E. Gil-Peregrin, J.J. Peguero-Pina & D. Sancho-Knapik (Eds.), Oaks physiological ecology. Exploring the functional diversity of genus Quercus. Tree physiology, 7. Springer Nature, Cham, Switzerland.
Denk, T., Velitzelos, D., Güner, T. H., & Ferrufino-Acosta, L. (2015). Smilax (Smilacaceae) from the Miocene of western Eurasia with Caribbean biogeographic affinities. American Journal of Botany, 102(3), 423–438.
Diethart, B. (2005). Parietaria officinalis. In: PalDat (2016-07-18)—a palynological database University of Vienna. Online publication. Accessed 29-11-2017. https://www.paldat.org/pub/Parietaria_officinalis/301233
Donoghue, M. J. (1985). Pollen diversity and exine evolution in Viburnum and Caprifoliaceae senus lato. Journal of the Arnold Arboretum, 66, 421–469.
Earle, C. J. (2010). The gymnosperm database. Online publication. Accessed 29-11-2017. http://www.conifers.org/.
Ediger, V. S. (1990). Paleopalynology of coal-bearing Miocene sedimentary rocks assosciated with volcanics of the Biga Peninsula (NWTurkey) and the effect of volcanism on vegetation. Neues Jahrbuch der Geologie und Paläontologie, Abhandlungen, 180(2), 259–277.
Edwards, K. J. (1981). The separation of Corylus and Myrica pollen in modern and fossil samples. Pollen et Spores, 23(2), 205–218.
El-Ghazaly, G., & Chaudhary, R. (1993). Pollenmorphology of some species of the genus Euphorbia L. Review of Palaeobotany and Palynology, 78, 293–319.
Erdei, B., Yavuz, N., Akgün, F., Hably, L., 2002. Some data to the Miocene flora of Western Turkey. 6th European Palaebotany Palynology Conference Athens, pp. 79–80.
Erdtman, G. (1952). Pollen morphology and plant taxonomy. Stockholm: Almqvist & Wiksell.
Erkül, F., Helvacı, C., & Sözbilir, H. (2005). Stratigraphy and geochronology of the Early Miocene volcanic units in the Bigadiç Borate Basin, Western Turkey. Turkish Journal of Earth Science, 14, 227–253.
Ersoy, E. Y., Çemen, I., Helvacı, C., & Billor, M. Z. (2014). Tectono-stratigraphy of the Neogene basins in Western Turkey: implications for tectonic evolution of the Aegean Extended Region. Tectonophysics, 635, 33–58.
Farjon, A. (2005). A monograph of Cupressaceae and Sciadopitys. Kew: Kew Royal Botanic Gardens.
Gemici, Y., & Akgün, F. (1992). Macro and micro fossil flora of the Akçaşehir (Tire-Izmir) Neogene basin. Turkish Journal of Botany, 16, 383–393.
Gemici, Y., Akyol, E., & Akgün, F. (1993). Şahınalı (Aydın) Neojen havzasinin fosil makro ve mikorflorasi. Doğa. Turkish Journal of Botany, 17, 91–106.
Gemici, Y., Akyol, E., Akgün, F., & Seçmen, Ö. (1991). Soma kömür havzasi fosil makro ve mikroflorasi. Maden Tektik ve Arama Dergisi, 112, 161–178.
Gemici, Y., Akyol, E., Seçmen, Ö., & Akgün, F. (1990). Macro et microflore fossile du Bassin Néogène d’Eskihisar (Yatağan-Muğla). Journal of the Faculty of Science Ege University, series B, 12, 29–41.
Graham, A., Nowicke, J. W., Skvarla, J. J., Graham, S. A., Patel, V., & Lee, S. (1985). Palynology and systematics of the Lythraceae. I. Introduction and genera Adenaria through Ginoria. American Journal of Botany, 72(7), 1012–1031.
Graham, A., Nowicke, J. W., Skvarla, J. J., Graham, S. A., Patel, V., & Lee, S. (1987). Palynology and systematics of the Lythraceae. II. Genera Haitia through Peplis. American Journal of Botany, 74(6), 829–850.
Graham, S. A., Diazgranados, M., & Barber, J. C. (2011). Relationships among the confounding genera Ammannia, Hionanthera, Nesaea and Rotala (Lythraceae). Botanical Journal of the Linnéan Society, 166, 1–19.
Graham, S. A., Hall, J. C., Sytsma, K. J., & Shi, S.-h. (2005). Phylogenetic analysis of the Lythraceae based on four gene regions and morphology. International Journal of Plant Sciences, 166(6), 995–1017.
Grimm, G. W., Bouchal, J. M., Denk, T., & Potts, A. J. (2016). Fables and foibles: a critical analysis of the Palaeoflora database and the Coexistence Approach for palaeoclimate reconstruction. Review of Palaeobotany and Palynology, 233, 216–235. https://doi.org/10.1016/j.revpalbo.2016.07.001.
Grímsson, F., Denk, T., & Zetter, R. (2008). Pollen, fruits, and leaves of Tetracentron (Trochodendraceae) from the Cainozoic of Iceland and western North America and their palaeobiogeographic implications. Grana, 47, 1–14.
Grímsson, F., Ferguson, D. K., & Zetter, R. (2012). Morphological trends in the fossil pollen of Decodon and the palaeobiogeographic history of the genus. International Journal of Plant Sciences, 173, 1–22.
Grímsson, F., Grimm, G. W., Meller, B., Bouchal, J. M., & Zetter, R. (2016). Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part IV. Magnoliophyta 2—Fagales to Rosales. Grana, 55(2), 101–163.
Grímsson, F., & Zetter, R. (2011). Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: part II. Pinophyta (Cupressaceae, Pinaceae and Sciadopityaceae). Grana, 50(4), 262–310.
Güner, H. T. (2016). The Miocene floras and vegetation of the Yatağan Basin, western Anatolia. Unpublished PhD thesis, Istanbul University, Istanbul.
Güner, H. T., Bouchal, J. M., Köse, N., Göktaş, F., Mayda, S., & Denk, T. (2017). Landscape heterogeneity in the Yatağan Basin (southwestern Turkey) during the middle Miocene inferred from plant macrofossils. Palaeontographica, Abteilung B, 296(1–6), 113–171.
Guo, S., Fujiki, T., & Miyoshi, N. (1994). Pollen morphology by means of scanning electron microscope: 13. Oleaceae (Angiospermae). Japanese Journal of Palynology, 40(2), 99–112.
Halbritter, H. (2015). Parietaria judaica. In: PalDat (2015-04-15)—a palynological database. Online publication. Accessed 29-11-2017. https://www.paldat.org/pub/Parietaria_judaica/300062
Halbritter, H., Hesse, M., & Weber, M. (2012). The unique design of pollen tetrades in Dionaea and Drosera. Grana, 51(2), 148–157148.
Harley, M. M. (1991). The pollen morphology of the Sapotaceae. Kew Bulletin, 46(3), 379–491.
Hawksworth, F. J. m., & Wiens, D. (1972). Biology and classification of dwarf mistletoes (Arceuthobium). Agriculture Handbook, 401, 228.
Hebda, R. J., & Chinnappa, C. C. (1990). Studies on pollen morphology of Rosaceae in Canada. Review of Palaeobotany and Palynology, 64, 103–108.
Hebda, R. J., & Chinnappa, C. C. (1994). Studies on pollen morphology of Rosaceae. Acta Botanica Gallica, 141(2), 183–193.
Hebda, R. J., Chinnappa, C. C., & Smith, B. M. (1988a). Pollen morphology of the Rosaceae of Western Canada. I. Agrimonia to Crataegus. Grana, 27, 95–113.
Hebda, R. J., Chinnappa, C. C., & Smith, B. M. (1988b). Pollen morphology oft he Rosaceae of Western Canada II. Dryas, Fragaria, Holodiscus. Canadian Journal of Botany, 66, 595–612.
Hesse, R. W., Halbritter, H., Zetter, R., Weber, M., Frosch-Radivo, A., & Ulrich, S. (2009). Pollenterminology—an illustrated handbook. Wien, New York: Springer.
Hinsbergen, D. J. J van & Schmid, S. M. (2012). Map view restoration of Aegean–West Anatolian accretion and extension since the Eocene. Tectonics, 31, TC5005 1–40.
Hofmann, C. C., Zetter, R., & Draxler, I. (2002). Pollen- und Sporenvergesellschaftungen aus dem Karpatium des Korneuburger Beckens (Niederösterreich). Beiträge zur Paläontologie, 27, 17–43.
Inaner, H., Nakoman, E., & Karayigit, A. I. (2008). Coal resource estimation in the Bayir field, Yatağan-Muğla, SW Turkey. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 30, 1005–1015.
Jiménez-Moreno, G. (2005). Utilización del análisis polínico para la reconstrucción de la vegetación, clima y estimación de paleoaltitudes a lo largo de arco alpino europeo durante el Mioceno (21–8 Ma). Granada: Granada.
Jones, C. A., & Blackmore, S. (1988). The northwest European pollen Flora, 38: Lycopodiaceae. Review of Palaeobotany and Palynology, 57, 1–25.
Jones, G. D., Bryant, V. M. J., Hoag Lieux, M., Jones, S. D., & Lingren, P. D. (1995). Pollen of the souteastern United States: with emphasis on melissopalynology and entomopalynology (Vol. 30, AASP Contribution Series).
Kasapligil, B. (1977). Tertiary conifer-hardwood forest from the vicinity of Güvem village, near Kizilcahamam, Ankara. Bulletin of the mineral research and exploration institute of Turkey, 88, 25–55.
Kayseri, M. S. (2010). Oligo-Miocene palynology, palaeobotany, vertebrate, marine faunas, palaeoclimatology and palaeovegation of the Oren Basin (north of the Gokova Gulf), western Anatolia. PhD Thesis, Dokuz Eylul University, Izmir. 1–552.
Kayseri, M. S., & Akgün, F. (2010). The late Burdigalian–Langhian time interval in Turkey and the palaeoenvironment and palaeoclimatic implications and correlation of Europe and Turkey: late Burdigalian-Langhian palynofloras and palaeoclimatic properties of the Muğla–Milas (Kultak). Geological Bulletin of Turkey, 53(1), 1–44.
Kayseri-Özer, M. S., Akgün, F., Mayda, S., & Kaya, T. (2014a). Palynofloras and vertebrates from Muğla-Ören region (SW Turkey) and palaeoclimate of the Middle Burdigalian-Langhian period in Turkey. Bulletin of Geosciences, 89(1), 137–162.
Kayseri-Özer, M. S., Sözbilir, H., & Akgün, F. (2014b). Miocene palynoflora of the Kocaçay and Cumaovasi basins: a contribution to the synthesis of Miocene palynology, palaeovegetation in western Turkey. Turkish Journal of Earth Sciences, 23, 233–259.
Kedves, M. (1985). LM, TEM and SEM investigations on recent inapertureate Gymnospermatophyta pollen grains. Acta Biologica Szegedinesnsis, 31, 129–146.
Koç, Ç. S. (2017). Palaeoflora and palaeoclimate of the coal bearing sediments in the Tınaz (Muğla-Yatağan) and surround. Unpublished Master’s Thesis, Ege University, Izmir.
Köhler, E., & Elsbeth, L. (1979). A contribution to distinguishing cereal from wild grass pollen grains by LM and SEM. Grana, 18, 133–140.
Knobloch, E., & Kvaček, Z. (1965). Byttneriophyllum tiliaefolium (Al.Braun) Knobloch Kvaček in den tertiären Floren der Nordhalbkugel. Sborník geologických věd Paleontologie, 5, 123–166.
Krutzsch, W. (1963). Atlas der mittel- und jungtertiären dispersen Sporen- und Pollen- sowie der Mikroplanktonformen des nördlichen Mitteleuropas II. Berlin: Deutscher Verlag der Wissenschaft.
Krutzsch, W., & Pacltová, B. (1990). Die Phytoplankton-Mikroflora aus den Pliozänen Süsswasserablagerungen des Cheb-Beckens (Westböhmen, CSFR). Acta Universitatis Carolinae - Geologica, 34(4), 345–420.
Kvavadze, E. V. (1988). The pollen of Taxodiaceae and its peculiarities. Tiblisi: Metsniereba.
Li, T. Q., Cao, H. J., Kang, M. S., Zhang, Z. X., Zhao, N., & Zhang, H. (2010). Pollen flora of China woody plants by SEM. Bejing: Science Press.
Maciejewska, I. (1997). Pollen morphology of the Polish species of the family Caprifoliaceae, part 2. Acta Societatis Botanicorum Poloniae, 66(2), 143–151.
Manchester, S. R. (1989). Attached reproductive and vegetative remains of the extinct American-European genus Cedrelospermum (Ulmaceae) from the Early Tertiary of Utah and Colorado. American Journal of Botany, 76, 256–276.
Mädler, K., & Steffens, P. (1979). Neue Blattfloren aus dem Oligozän, Neogen und Pleistozän der Türkei (Känozoikum und Braunkohlen der Türkei. 20.). Geologisches Jahrbuch, B33, 3–33.
Miyoshi, N., Fujiki, T., & Kimura, H. (2011). Pollenflora of Japan. Sapporo: Hokkaido: University Press.
Nakoman, E. (1967). Microflore des dépots tertiaires du sud-ouest de l’Anatolie. Pollen et Spores, 9(1), 121–142.
Neubauer, T. A., Harzhauser, M., Kroh, A., Georgopoulou, E., & Mandic, O. (2015). A gastropod-based biogeographic scheme for the European Neogene freshwater systems. Earth-Science Reviews, 143, 98–116.
Nilsson, S. (1988). A survey of the pollen morphology of Olea with particular reference to Olea europaea sens. lat. Kew Bulletin, 43(2), 303–315.
Page, J. S. (1978). A scanning electron microscope survey of grass pollen. Kew Bulletin, 32(2), 313–319.
Paicheler, J.-C., & Blanc, C. (1981). La flore du bassin lacustre miocène de Bes-Konak (Anatolie septentrionale, Turquie). Géologie Méditerranéenne, 8, 19–60.
Pennington, T. D. (2004). Sapotaceae. In K. Kubitzki (Ed.), The families and genera of vascular plants 6—flowering plants–Dicotyledons–Celastrales, Oxalidales, Rosales, Cornales, Ericales (Vol. 6, pp. 390–421). Berlin: Springer-Verlag.
Perveen, A., Grafström, E., & El-Ghazaly, G. (2004). World pollen and spore flora 23. Malvaceae Adams. p.p.-subfamilies: Grewioideae, Tilioideae, Brownlowioideae. Grana, 43, 129–155.
Popov, S. V., Rögl, F., Rozanov, A. Y., Steininger, F. F., Shcherba, I. G., & Kovac, M. (2004). Lithological-Paleogeographic maps of Paratethys. 10 maps. Late Eocene to Pliocene. Courier Forschungsinstitut Senckenberg, 250, 1–46.
Potonié, R. (1931). Zur Mikroskopie der Braunkohlen. Tertiäre Sporen- und Blütenstaubformen (Vierte Mitteilung). Zeitschrift für Gewinnung und Verwertung der Braunkohle, 30(27), 554–556.
Praglowski, J. (1984). Fagaceae Dumort.-Castaneae Oerst. In S. Nilsson (Ed.), World pollen and spore Flora (Vol. 13). Stockholm: The Almquist & Wiksell Periodical Company.
Punt, W. (1984). The northwest European pollen flora, 37: Umbelliferae. Review of Palaeobotany and Palynology, 42, 155–364.
Punt, W., Bos, J. A. A., & Hoen, P. P. (1991). The northwest European pollen flora, 45: Oleaceae. Review of Palaeobotany and Palynology, 69, 23–47.
Punt, W., & Hoen, P. P. (2009). The northwest European pollen flora, 70: Asteraceae-Asteroideae. Review of Palaeobotany and Palynology, 157, 22–183.
Punt, W., Hoen, P. P., Blackmore, S., Nilsson, R. H., & Le Thomas, A. (2007). Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology, 143, 1–81.
Punt, W., & Malotaux, M. (1984). The northwest European pollen flora, 31: Cannabaceae, Moraceae and Urticaceae. Review of Palaeobotany and Palynology, 42, 23–44.
Punt, W., Marks, A., & Hoen, P. P. (2003). The northwest European pollen flora, 60: Droseraceae. Review of Palaeobotany and Palynology, 123, 27–40.
Purvis, M., Robertson, A., & Pringle, M. (2005). 40Ar–39Ar dating of biotite and sanidine in tuffaceous sediments and related intrusive rocks: implications for the early Miocene evolution of the Gördes and Selendi basins, W Turkey. Geodinamica Acta, 18, 239–253.
Renault-Miskovsky, J., Girard, M., & Trouin, M. (1976). Observations de quelques pollen d'Oléacées au microscope électronique a balayage. Bulletin de l'Association française por l'Etude du Quaternaire, 13(2), 71–86.
Rögl, F. (1999). Mediterranean and Paratethys. facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geologica Carpathica, 50, 339–349.
Sachse, M. (2001). Oleaceous laurophyllous leaf fossils and pollen from European tertiary. Review of Palaeobotany and Palynology, 115, 213–234.
Saraç, G., 2003. Türkiye Omurgalı Fosil Yatakları. Scientific Report No. 10609. Ankara: General Directorate of the Mineral Research and Exploration of Turkey.
Sickenberg, O. (1975). Die Gliederung des höheren Jungtertiärs und Altquartärs in der Türkei nach Vertebraten und ihre Bedeutung für die internationale Neogen-Stratigraphie (Känozoikum und Braunkohlen der Türkei. 17.). Geologischens Jahrbuch, B15, 1–167.
Sivak, J. (1978). Obsevations nouvelles sur les grains de pollen de Tsuga. Pollen et Spores, 15, 397–457.
Solounias, N., & Moelleken, S. M. C. (1992). Dietary adaptations of two goat ancestors and evolutionary considerations. Geobios, 25, 797–809.
Southworth, D. (1988). Isolation of exines from gymnosperm pollen. American Journal of Botany, 75(1), 15–21.
Stafford, P. J. (1991). The northwest European pollen flora, 44: Selaginellaceae. Review of Palaeobotany and Palynology, 69, 1–22.
Stafford, P. J. (1995). The northwest European pollen flora, 52: Marsileaceae. Review of Palaeobotany and Palynology, 88, 3–24.
Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Słodkowska, B., Ważyńska, H., et al. (2009). Atlas of pollen and spores of the Polish Neogene. Volume 3—angiosperms (1) (Vol. Vol 3). Kraków: W. Szafer Institute of Botany, Polish Academy of Science.
Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Słodkowska, B., Worobiec, E., et al. (2014). Atlas of pollen and spores of the Polish Neogene. Volume 4—angiosperms (2). (Vol. 4). Kraków: W. Szafer Institute of Botany, Polish Academy of Science.
Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Ważyńska, H., & Sadowska, A. (2001). Atlas of pollen and spores of the Polish Neogene. Volume 1—spores (Vol. 1). Kraków: W. Szafer Institute of Botany, Polish Academy of Science.
Stuchlik, L., Ziembińska-Tworzydło, M., Kohlman-Adamska, A., Grabowska, I., Ważyńska, H., & Sadowska, A. (2002). Atlas of pollen and spores of the Polish Neogene. Volume 2—gymnosperms. . (Vol. 2). Kraków: W. Szafer Institute of Botany, Polish Academy of Science.
Swenson, U., & Anderberg, A. A. (2005). Phylogeny, character evolution, and classification of Sapotaceae (Ericales). Cladistics, 21, 101–130.
Takahashi, K., & Jux, U. (1991). Miocene palynomorphs from lignites of the Soma Basin (West Anatolia, Turkey). Bulletin of the Faculty of Liberal Arts Nagasaki University (Natural Science), 32, 7–165.
The NOW Community (2018). New and old worlds database of fossil mammals (NOW). Licensed under CC BZ 4.0 Release 2008, Accessed 28–05-2018. Published on the Internet. http://www.helsinki.fi/science/now/
Thiele-Pfeiffer, H. (1980). The Miocene microflora from the brown coal open-cast Oder mine near Wackersdorf/Oberpfalz. Palaeonto-graphica, Abteilung B, 174(4–6), 95–224.
Thomson, P. W., & Pflug, H. (1953). Pollen und Sporen des mitteleuropäischen Tertiärs. Palaeontographica, Abteilung B, 94(1–4), 1–138.
Tyron, A. F., & Lugardon, B. (1991). Spores of the Pteridophyta. New York, Berlin, Heidelberg, London, Paris, Tokyo, Hong Kong, Barcelona: Springer.
Van Campo-Duplan, M. (1951). Recherches sur la phylogénie des Taxodiacées d’après leurs grains de pollen. Travaux du Laboratoire Forestier de Toulouse, Tome II, 4, 1–14.
Van Campo-Duplan, M. (1953). Rechereches sur la phylogénie des Cupressacées d'aprés leurs grain de pollen. Travaux du Laboratoire Forestier de Toulouse, Tome II, 4, 1–20.
Van Leeuwen, P., Punt, W., & Hoen, P. P. (1988). The northwest European pollen flora, 43: Polygonaceae. Review of Palaeobotany and Palynology, 57, 81–151.
Velitzelos, D., Bouchal, J. M., & Denk, T. (2014). Review of the Cenozoic floras and vegetation of Greece. Review of Palaeobotany and Palynology, 204, 56–117. https://doi.org/10.1016/j.revpalbo.2014.02.006.
Wang, Q., Dilcher, D. L., & Lott, T. A. (2007). Podocarpium A.Braun ex Sitzenberger 1851 from the middle Miocene of Eastern China and its palaeoecology and biogeography. Acta Palaeobotanica, 47, 237–251.
Wen, J., & Nowicke, J. W. (1999). Pollen ultrastructure of Panax (the ginseng genus, Araliaceae), an eastern Asian and eastern North Anerican dijunct genus. American Journal of Botany, 86(11), 1624–1636.
Wessels, W. (2009). Miocene rodent evolution and migration—Muroidea from Pakistan, Turkey and Northern Africa. Geologica Ultraiectina, 307, 1–290.
Wilce, J. H. (1972). Lycopod spores, I. General spore patterns and the generic segregates of Lycopodium. American Fern Journal, 62(3), 65–79.
Worobiec, E. (2014). Fossil zygospores of Zygnemataceae and other microremains of freshwater algae from two Miocene palaeosinkholes in the Opole region, SW Poland. Acta Palaeobotanica, 54(1), 113–157.
Worobiec, G., Worobiec, E., & Kvaček, Z. (2010). Neogene leaf Morphotaxa sl. in Europe. International Journal of Plant Sciences, 171(8), 892–914.
Yavuz-Işık, N. (2007). Pollen analysis of coal-bearing Miocene sedimentary rocks from the Seyitömer Basin (Kütahya), Western Anatolia. Geobios, 40, 701–708.
Yavuz-Işık, N., Saraç, G., Ünay, E., & Bruijn, H. de (2011). Palynological analysis of Neogene mammal sites of Turkey—vegetational and climatic implications. Yerbilimeri, 32(2), 105–120.
Zetter, R. (1989). Methodik und Bedeutung einer routinemäßigen kombinierten lichtmikroskopischen und rasterelektronen-mikroskopischen Untersuchung fossiler Mikrofloren. Courier Forschungsinstitut Senckenberg, 109, 41–50.
Zhang, Y.-L., & Chen, Y.-S. (1984). Studies on pollen morphology in Tiliaceae of China. Acta Phytotaxonomica Sinica, 22, 367–377.
Acknowledgements
Funding by the Swedish Research Council (VR) is acknowledged. Tuncay H. Güner, Istanbul, is thanked for accompanying me during fieldwork and the staff of the Salihpaşalar lignite mine for facilitating work in the mining area. Shirley Graham is acknowledged for sharing her expertise on Lythraceae pollen. Reinhard Zetter and Thomas Denk are thanked for sharing their highly valued palaeobotanical knowledge of this region and help with the manuscript. Fridgeir Grímsson and an anonymous reviewer provided constructive comments on the first version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
The author declares that he has no conflict of interest.
Additional information
This article is a contribution to the special issue "Taking the Orient Express? The role of Anatolia in mediterranean Neogene palaeobiogeography".
Electronic supplementary material
Supplementary Information 1
(XLSX 46 kb)
Supplementary Information 2
(XLSX 84 kb)
Supplementary Information 3
(PDF 10.2 mb)
Supplementary Information 4
(XLSX 75 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bouchal, J.M. The middle Miocene palynofloras of the Salihpaşalar lignite mine (Yatağan Basin, southwest Anatolia): environmental characterisation and comparison with palynofloras from adjacent basins. Palaeobio Palaeoenv 99, 591–636 (2019). https://doi.org/10.1007/s12549-018-0345-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12549-018-0345-0