Abstract
Chalicotheres are enigmatic perissodactyls that had large claws instead of hooves. The present study concerns the material of Chalicotheriidae from the Late Miocene hominid locality of Hammerschmiede in Germany. The HAM 5 fossil site (11.62 Ma) consists of six isolated dental and postcranial chalicothere elements. Based on the morphology and dimensions of the dentition, the material can be assigned to the chalicotheriine Anisodon sp. This genus is the most common representative of Chalicotheriidae in Central Europe during the Middle Miocene but becomes much rarer during the Late Miocene. The HAM 4 fossil site (11.44 Ma) has yielded a patella belonging to a schizotheriine and a skull fragment that could possibly also belong to a schizotheriine. Thus, the schizotheriine and the chalicotheriine occur in different horizons in Hammerschmiede. Both taxa probably had a rather similar diet but different locomotion, and their disparate occurrences are most plausibly associated with environmental differences among the two fossiliferous levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Late Miocene locality of Hammerschmiede is situated in the Allgäu region of southwestern Bavaria (Germany) and is known since the 1970s for its vertebrate fossils (Mayr and Fahlbusch 1975). The locality became internationally renowned after the description of the new hominid species Danuvius guggenmosi Böhme et al. 2019 and its potential bipedalism (Böhme et al. 2020; Williams et al. 2020). In recent years, the fauna has been further investigated, with the detailed descriptions of some ruminants, carnivores, proboscideans, beavers, and birds (Fuss et al. 2015; Hartung et al. 2020; G. Mayr et al. 2020b; G. Mayr et al. 2020a; Kargopoulos, Valenciano, et al. 2021; Kargopoulos, Kampouridis, et al. 2021a; Kargopoulos, Kampouridis, et al. 2021b; Hartung and Böhme 2022; Kargopoulos et al. 2022; Lechner and Böhme 2022; Konidaris et al. 2023; Lechner and Böhme 2023; G. Mayr et al. 2023). The most common macrovertebrate representatives are turtles and some artiodactyls, like the tragulid Dorcatherium naui Kaup 1833a, whereas perissodactyls, including equids, rhinocerotids, and chalicotheriids are rather rare.
The family Chalicotheriidae Gill 1872 is a peculiar group of Perissodactyla Owen 1848, characterized by prominent claws, which is a unique feature of this family among ungulates (Coombs 1982, 1983, 1989; Heissig 1999). The Chalicotheriidae consists of two subfamilies: the Schizotheriinae Holland and Peterson 1914, cursorial quadrupeds with relatively equal arm–leg proportions, and the Chalicotheriinae Gill 1872, potential knuckle-walking orthogrades with much longer forelimb than hindlimbs (Tassy 1978; Zapfe 1979). Both groups are a very rare faunal elements in most fossil sites in Eurasia. One of the best-known members of the Chalicotheriinae is the genus Anisodon Lartet 1851, from the Middle to Late Miocene of Europe. In particular, the species Anisodon grande (de Blainville 1849) is known from very abundant material, including complete specimens from Middle Miocene localities, such as the Devínska Nová Ves fissure fillings of Slovakia (Zapfe 1979) and the type locality Sansan in France (Anquetin et al. 2007; Guérin 2012). Anisodon grande is the geologically oldest representative of the subfamily Chalicotheriinae in Europe and its earliest record is presumably from the early Middle Miocene (MN5) of the Loire Basin in France (Ginsburg 1990, 2001). It represents the most common chalicothere in Europe during the Middle Miocene (Coombs and Göhlich 2019), whereas in the Late Miocene, the fossil record of chalicotheriines is sparse in Europe (Bonis et al. 1995; Geraads et al. 2001; Fahlke et al. 2013; Tsoukala 2022). In contrast to Anisodon, the chalicotheriine genus Chalicotherium Kaup 1833b is known with the species Chalicotherium goldfussi Kaup 1833b from the late Middle to early Late Miocene of Europe almost exclusively from dental and cranial remains (Anquetin et al. 2007). Anisodon macedonicus Bonis et al. 1995 from the Late Miocene of the Balkan Peninsula is the last European chalicotheriine (Bonis et al. 1995).
The Schizotheriinae are also well-represented in Europe during the Middle and Late Miocene, with the genera Metaschizotherium Koenigswald 1932 and Ancylotherium Gaudry, 1863. Metaschizotherium is known from its type locality Steinheim, but also from several other Middle Miocene localities in Europe like Petersbuch and Sandelzhausen in Germany (Koenigswald 1932; Coombs 2009; Fahlke and Coombs 2009). The genus is the typical schizotheriine representative in Europe during the Middle Miocene, possibly surviving into the early Late Miocene (Mottl 1966). Initially, the genus included two species: Metaschizotherium fraasi Koenigswald 1932 and Metaschizotherium bavaricum Koenigswald 1932. Later, the Oligocene to Early Miocene species Metaschizotherium wetzleri Kowalevsky 1873 was tentatively assigned to this genus, though the inclusion of this species remains debated (Heissig and Fejfar 2013; Li et al. 2022). Ancylotherium is known mainly from the Late Miocene of the Balkan Peninsula. Its most common representative is Ancylotherium pentelicum (Gaudry and Lartet, 1856) from the Turolian. Another species, Ancylotherium hellenicum Koufos 2012 is known from the Vallesian of Greece and may also be present in the Iberian Peninsula, during this time (Koufos 2012). During the Middle Miocene, another schizotheriine was present in La Grive St.-Alban. This material was initially thought to belong to the chalicotheriine taxon but was then identified as belonging to a schizotheriine and was attributed to Metaschizotherium fraasi by Koenigswald (1932). Since then, it has been variably attributed to Metaschizotherium, Phyllotillon, and potentially Ancylotherium by different authors (Koenigswald 1932; Viret 1961; Mein and Ginsburg 2002; Coombs 2009). Mein and Ginsburg (2002) erected a new species for this material: Phyllotillon grivensis (Mein and Ginsburg 2002). However, Coombs (2009), Fahlke and Coombs (2009), and Heissig and Fejfar (2013) suggested that the material differs both metrically and morphologically from Phyllotillon and Metaschizotherium and might in fact be an early representative of Ancylotherium. Thereby, it seems that during the later part of the Middle Miocene two distinct schizotheriine genera were present in Europe.
The locomotion and ecology of these enigmatic animals, just like their systematic position, has been debated for a long time (Cuvier 1823; Kaup 1833c; Gaudry 1862–67). Originally, chalicothere remains were associated with burrowing mammals, due to their large, peculiar claws (Kaup 1833c). Later, chalicotheres were interpreted as pure browsers, due to their tooth morphology and general body plan (Schaub 1943; Zapfe 1979; Coombs 1982, 1989; Heissig 1999). More recent investigations, that were based on micro- and mesowear analysis of the teeth (Schulz et al. 2007; Schulz and Fahlke 2009; Semprebon et al. 2011), suggested that members of this group, including A. grande and Metaschizotherium spp., were browsers with a relatively tough diet consisting of leaves and fruit, but also tree bark and twigs. However, different locomotor adaptations have been suggested for the different subfamilies, with a more orthograde knuckle-walking locomotion for chalicotheriines like A. grande (Zapfe 1979) and a more quadruped locomotion during which the claws might have been retracted (Coombs 1983). These very different ecological adaptations probably led to very different preferred habitats for members of the two subfamilies.
The aim of this study is the description of the material of Chalicotheriidae from the Late Miocene hominid locality of Hammerschmiede (Germany) and the assessment of the separated occurrences of a schizotheriine and a chalicotheriine. The material consists of a patella and potentially a nasal bone from HAM 4 and three mandibular teeth and three phalanges from HAM 5. The material is taxonomically and morphologically compared with other closely related members of Chalicotheriidae from the Middle and Late Miocene of Europe and the internal morphology of the nasal bone was described using micro-computed tomography.
Geological setting
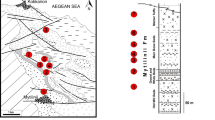
The herein studied material was excavated from the early Late Miocene deposits of the Hammerschmiede claypit, which is situated in southwestern Bavaria (Germany), in the municipality of Pforzen, only a few kilometers northwest of the city Kaufbeuren. This active claypit comprises a 27 m thick sedimentary succession of the Upper Freshwater Molasse (UFM). The sediments belong to the youngest part of the UFM, the “Obere Serie” (Kirscher et al. 2016). The profile of the claypit comprises fluvial channel-fills alternating with horizons of horizontally bedded clays, silts and fine sands with pedogenic overprints, deposited as overbank sediments by the river system (for details, see Kirscher et al. 2016). The two main vertebrate bearing horizons in the Hammerschmiede claypit are the levels HAM 4 and HAM 5. The HAM 4 fossil site, a major fluvial channel, is dated magnetostratigraphically to 11.44 Ma (Kirscher et al. 2016). The slightly older channel-fill HAM 5 has been dated to 11.62 Ma (Kirscher et al. 2016). Its sediments represent a riffle-pool sequence of a small rivulet of local origin, and it comprises mostly clays and silts, with alternating lenses rich in reworked pedogenic carbonates.
Material and methods
The material available for this study was collected during excavations from 2012 to 2021 at the fossiliferous horizons HAM 4 and HAM 5 of the Hammerschmiede claypit, with ages of 11.44 Ma and 11.62 Ma, respectively. The material is stored in the Palaeontological Collection of the University of Tübingen (GPIT). The two specimens collected during the 2021 excavation are long-term loans at the GPIT that will be deposited in the collection of the Bayerische Staatssammlung für Paläontologie und Geologie (BSPG), in Munich, Germany.
The dental terminology follows Fahlke et al. (2013). The dental measurements (Table 1) follow Coombs and Göhlich (2019) and the postcranial measurements (Table 2) follow Roussiakis and Theodorou (2001). All measurements were taken with a digital caliper with a precision of 0.1 mm.
To study the internal morphology of the cranial element GPIT/MA/17503, micro-computed tomography (µCT) scans were acquired with a Nikon XTH 320 μCT scanner operated by the staff of the 3D Imaging Lab of the Senckenberg Centre for Human Evolution and Palaeoenvironment (SHEP) and the Eberhard Karls University of Tübingen, Germany. An X-ray tube containing a multi-metal reflection target with a maximum acceleration voltage of 225 kV was used. The specimen (GPIT/MA/17503) was scanned at 205 kV and 85 μA with a voxel size of 0.06187 mm, using a copper filter of 0.1 mm thickness. The μCT-scan is available at MorphoSource (https://www.morphosource.org/) under the Project ID: 000545298.
To avoid confusion, only the genera Anisodon, Chalicotherium, and Metaschizotherium are abbreviated.
Institutional abbreviations. BSPG, Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany; GPIT, Geologisch-Paläontologisches Institut in Tübingen, Germany; MGL, Geological Museum of Lausanne, Switzerland; SNSB, Staatliche Naturwissenschaftlische Sammlungen Bayerns, Munich, Germany.
Systematic Paleontology
Class Mammalia Linnaeus, 1758
Order Perissodactyla Owen, 1848
Family Chalicotheriidae Gill, 1872
Subfamily Chalicotheriinae Gill, 1872
Tribe Anisodontini Coombs and Göhlich, 2019
Genus Anisodon Lartet, 1851
Anisodon sp.
Dental material of Anisodon sp. from HAM 5. A-C: right p2 (GPIT/MA/19652) in occlusal (A), lingual (B), and ventral views (C). D–G left p4 (GPIT/MA/10321 in occlusal (D, E), lingual (F), and buccal view (G). H–K Left m3 (GPIT/MA/09873) in occlusal (H, I), lingual (J), and buccal view (K). Black arrow indicates mesial and distal orientation for A–C, D, E, and H, I. Scale bar equals 2 cm
Phalanges of Anisodon sp. from HAM 5. A-D: middle phalanx of the manus (GPIT/MA/09543) in left side (A), right side (B), distal (C), and proximal views (D); E–H: middle phalanx of the pes (GPIT/MA/10322) in left side (E), right side (F), distal (C), and proximal views (D; and I-L: middle phalanx of the pes (SNSB-BSPG 2020 XCV-485) in left side (I), right side (J), distal (K), and proximal views (L). Scale equals 2 cm
Referred Material. HAM 5: a right p2 (GPIT/MA/19652), a left p4 (GPIT/MA/10321), a left m3 (GPIT/MA/09873), a middle phalanx of the manus (GPIT/MA/09543), and two middle phalanges of the pes (GPIT/MA/10322 and SNSB-BSPG 2020 XCV-485).
Remarks. The first chalicothere material from the Middle Miocene of Sansan (France) was described by de Blainville (1849) and assigned to Anoplotherium grande. Only two years later Lartet (1851) recognized that this material represents a new genus and consequently erected the new genus Anisodon for this species. For many years (e.g., Coombs 1975; Zapfe 1979, 1989), the genera Anisodon and Chalicotherium were regarded as synonyms, indicating their close affinity and their inclusion into the subfamily Chalicotheriinae. Geraads et al. (2001) re-established the use of the genus Anisodon by showing that it has priority for the species Anisodon grande from Sansan and that this species is distinct from Chalicotherium goldfussi from Eppelsheim. Later, Anquetin et al. (2007) reviewed the Middle Miocene Chalicotheriinae from Europe and based on their phylogenetic analysis redefined the genus Anisodon. They further included in the genus the species Macrotherium macedonicum Bonis et al. 1995 from southeastern Europe, Chalicotherium sivalense Falconer 1868 and Chalicotherium wuduensis Xue and Coombs 1985. Coombs and Göhlich (2019) erected the new tribe Anisodontini to include the taxa previously included in Anisodon (= “Anisodon-clade” sensu Anquetin et al. 2007).
Description and Comparison
p2. Specimen GPIT/MA/19652 is an almost complete right p2 lacking only the roots; nonetheless, two distinct root canals are well visible in ventral view. The tooth crown of the p2 is very simple, because the single tip of the tooth is exclusively formed by the protoconid. At its distal end, a faint crest runs from the tip of the p2 toward its hypoconid. Mesially, a weak enamel fold that may represent the paraconid is exposed.
The simple morphology of the rounded p2 from HAM 5, with a single tip and a slight distal crest, is very similar to that of A. grande (NHMW-C39) illustrated by Zapfe (1979, fig. 13). However, as seen in the p2s illustrated by Zapfe (1979, figs. 12, 14) and single p2 of Anisodon sp. illustrated by Fahlke et al. (2013, fig. 2o–p), the morphology of this tooth can vary significantly. Even the tooth roots can be either divided into two well-separated parts or fused to form a single continuous root (Zapfe 1979, fig. 14).
The European schizotheriines seem to have less reduced lower premolars, with the p2 being mesiodistally more elongated and not as rounded as the p2 of Chalicotheriinae (Coombs 2009; Fahlke and Coombs 2009; Koufos 2012), including also the HAM 5 specimen (Table 1). Metrically, the p2 from HAM 5 is slightly above the size range documented for A. grande from Devínska Nová Ves (Zapfe 1979, p. 40). More specifically the width/length ratio of M. bavaricum (0.61–0.65, N = 3; Coombs 2009), M. fraasi (0.56–0.60, N = 4; Fahlke and Coombs 2009) and Ancylotherium pentelicum (0.57–0.67, N = 3; Geraads et al. 2006; Koufos 2012) are notably lower than that of the HAM 5 p2 (0.83; GPIT/MA/19652). On the contrary, in A. grande the p2 seems to be much more mesiodistally compressed with a ratio range between 0.74 and 0.99 (N = 12; Zapfe 1979, p. 40). Thus, the proportions of the HAM 5 specimen lie well within the range of A. grande.
p4. GPIT/MA/10321 is a complete p4 with its roots intact. Only the trigonid is slightly worn, while the talonid is unworn. The tooth is molariform, with the lophids being U-shaped. The trigonid is smaller, but taller than the talonid (Table 1). Mesially and distally weak cingulids are exposed, and in the distal part of the buccal side an extremely weak cingulid is visible. The metaconid is strong and tall. The hypoconid is well-developed and slightly protrudes from the metalophid and hypolophid. The entoconid is weak, low placed within the talonid, and faintly separated from the hypolophid by a shallow mesiodistal valley.
The p4 of derived Chalicotheriidae is usually submolariform (Anquetin et al. 2007; Fahlke et al. 2013; Coombs and Göhlich 2019). In the p4 of the Middle to Late Miocene schizotheriine M. bavaricum from Sandelzhausen (Coombs 2009, tab. 2), M. fraasi from Petersbuch (Fahlke and Coombs 2009, appendix 3), Ancylotherium pentelicum from Pikermi (Greece), Hadjidimovo (Bulgaria) and Karaslari (North Macedonia), and Ancylotherium hellenicum from Pentalophos (Greece) (Koufos 2012, tab. 2), the trigonid is either equal to or wider than the talonid. However, in the p4 of Anisodon, the width of the trigonid is smaller than that of the talonid (Zapfe 1979; Anquetin et al. 2007; Fahlke et al. 2013; Coombs and Göhlich 2019), similar to the p4 from HAM 5. The entoconid itself is weakly developed in GPIT/MA/10321, as also occurs in Anisodon sp. and A. grande (Zapfe 1979; Fahlke et al. 2013; Coombs and Göhlich 2019). The dimensions of the p4 from Hammerschmiede (L = 23.9 mm, Wtrig = 15.8 mm and Wtal = 16.8 mm) fit well within the value range of the p4 of A. grande (L = 21–24.8 mm, Wtrig = 14.5–16.1 mm and Wtal = 16.1–18.1 mm, N = 10; Zapfe 1979, p. 38).
m3. GPIT/MA/09873 is a complete left m3, with most of its roots preserved. The tooth has a relatively short crown that is moderately worn, with notable Hunter-Schreger bands exposed on the occlusal wear-facets. The tooth bears a small wear facet on its mesial side due to attrition with the neighboring tooth and no respective wear facet on its distal side. Both the trigonid and talonid are V-shaped, with the talonid being larger than the trigonid (Table 1). The metaconid is strongly developed but worn, and next to it no distinct “metastylid” is visible. A small metacristid does exist in GPIT/MA/09873, and a weak entoconid seems to be present, although its tip is broken. A strong cingulid is present in the posterior part of the m3, but it gets weaker on the buccal side and disappears in the mesial part.
The morphology of the metaconid with the absence of a “metastylid” is comparable to the morphology of A. grande (Zapfe 1979; Anquetin et al. 2007). In schizotheriines, a “metastylid” is more commonly developed, as seen in M. bavaricum from Sandelzhausen (Coombs 2009), whereas in anisodonts, the reduced “metastylid” in the lower molars represents an autapomorphic feature (Anquetin et al. 2007). However, a similarly reduced “metastylid” is also known in other chalicotheriines, such as C. goldfussi (Fahlke et al. 2013; Coombs and Göhlich 2019). Based on the value ranges provided by Liu and Zhang (2012) for the width-to-length ratio of the m3, schizotheriines have a ratio of 47–55.9% (except for the geologically older schizotheriine Schizotherium priscum, which has a ratio of 61.6%). Thus, the HAM 5 molar, with a ratio of 59% is slightly above the schizotheriine value range and closer to the values of the chalicotheriine material of Liu and Zhang (2012). Therefore, based on both the morphology and the proportions, the tooth can be assigned to a chalicotheriine.
The dimensions of the molar from HAM 5 (L = 44.6 mm, Wtrig = 26.5 mm and Wtal = 25.5 mm) are larger than in M. bavaricum from Sandelzhausen (L = 33.6–35.5 mm, Wtrig = 15.6–17.3 mm and Wtal = 18.1–19.8 mm, N = 4; Coombs 2009, tab. 2). Although, the length of the HAM 5 specimen is comparable to that of M. fraasi from Petersbuch (L = 41–46.2 mm, Wtrig = 20.4–21.2 mm and Wtal = 21.6–23.2 mm, N = 4; Fahlke and Coombs 2009), its width (especially the width of the trigonid) is greater. Furthermore, its length is smaller than in the m3 of the type specimen of Ancylotherium hellenicum (L = 50.2–51.2 mm, Wtrig = 22.8 mm and Wtal = 25.0–25.3 mm; Koufos 2012, tab. 2), while its width is greater. The dimensions for both m2 and m3 of Ancylotherium pentelicum (L = 59.4–59.5 mm, Wtrig = 27–28.6 mm and Wtal = 29.5 mm, N = 1–2; Koufos 2012, tab. 2) are greater than the ones of the HAM 5 specimen. Overall, the molar from HAM 5 is slightly smaller than the m3 of C. goldfussi (L = 48–51 mm and Wtal = 26–28, N = 4; Zapfe 1989, tab. 2), but larger than the holotype of A. macedonicus (L = 40–40.8 mm and Wmax = 23.2–23.3; de Bonis et al. 1995, tab. 3). However, it fits well within the size range of the m3 of A. grande from the Middle Miocene of Sansan (L = 38–40 mm and Wmax = 21–23.5, N = 3; Guérin 2012) and Devínska Nová Ves (L = 40.3–46 mm and Wtal = 22–26.3, N = 10; Zapfe 1979).
Phalanges. Three chalicothere middle phalanges are present in the studied material (Fig. 2). Specimen GPIT/MA/09543 is larger than the other two specimens from Hammerschmiede (see Table 2). It is metrically larger than the phalanges of the pes of A. grande from Devínska Nová Ves but fits very well with size range of the manual middle phalanges (Zapfe 1979). Therefore, GPIT/MA/09543 is assigned to the manus. It is somewhat mediolaterally compressed. The morphology of the articular facet for the articulation with the respective proximal phalanx cannot be assessed, due to deformation. Only the palmar portion of the proximal articular facet is undeformed, allowing the approximate measurement of its DTprox (~ 30 mm) (sensu Roussiakis and Theodorou 2001), which is lower than the lowest value given for the second middle phalanx of A. grande from Devínska Nová Ves (Zapfe 1979, p. 165). Thus, GPIT/MA/09543 most likely represents the third or fourth digit of the manus.
The other two phalanges from HAM 5, GPIT/MA/10322 and SNSB-BSPG 2020 XCV-485, are smaller than GPIT/MA/09543 (see Table 2), fit metrically well into the range given for the middle phalanges of the pes of A. grande (Zapfe 1979), and are therefore assigned to the pes and possibly either to the third or fourth digit. Both are complete, but SNSB-BSPG 2020 XCV-485 is deformed, being transversally compressed. In proximal view, in the center of the articular facet for the articulation with the respective proximal phalanx, a proximodistal indentation is present in GPIT/MA/10322, similar to the middle phalanges of the third digit of the pes of A. grande in Zapfe (1979, fig. 140), whereas in SNSB-BSPG 2020 XCV-485, no such incision is visible. Metrically both resemble the middle phalanx of the third (L = 31–41 mm, DTprox = 24.5–30.5 mm, DAPprox = 31.5–39.5 and DTdist = 21.5–26 mm, N = 10; Zapfe 1979, p. 246) and fourth (L = 29–38 mm and DTprox = 23.2–28.5 mm, N = 8; Zapfe 1979, p. 247) digit of the pes of A. grande. The assignment to a specific digit of the HAM 5 phalanges is very difficult because they were isolated finds.
No middle phalanges are known from C. goldfussi, and comparisons are therefore impossible. This is unfortunate because this species is known from the late Middle to early Late Miocene (La Grive-St. Alban, Saint-Gaudens, Eppelsheim, Charmoille, Höwenegg; Schaefer and Zapfe 1971; Zapfe 1989; Anquetin et al. 2007), a chronologic period overlapping the Hammerschmiede stratigraphy.
Subfamily Schizotheriinae Holland and Peterson, 1914
Schizotheriinae indet.
Figure 3
Referred Material. HAM 4: a right patella (SNSB-BSPG 2020 XCIV-3834) and possibly a skull fragment, including the nasal bones and part of the frontal bone (GPIT/MA/17503).
Description and Comparison
Patella. Specimen SNSB-BSPG 2020 XCIV-3834 is a patella that was found in the fossiliferous horizon HAM 4. The anterior side of the bone is very much damaged, especially on the lateral side and proximally. The preservation of the rest of the bone is very good, with only two small cracks being visible in posterior view. The patella is very robust and proximodistally thick (DAP = 36 mm, Table 3). In posterior view, the patella is widely tear-shaped, 52.4 mm high, 46 mm wide (Table 3), and bears the articular facets for the patellar groove of the distal condyles of the femur. The two condylar facets on the posterior side of the patella form an obtuse angle and are separated by a ridge. The two facets are almost equal in size, with the lateral one being mediolaterally about 12% wider (DTmedial = 24.2 mm and DTlateral = 27.1 mm, Table 3). The ventral end of the femoral facet is weakly pointed, forming the apex. In proximal view, the base of the patella is visible, though very damaged, that acts as an attachment area for the quadriceps tendon. Centrally, a flattened transversally oriented stripe is visible. In anterior view, the surface of the patella is rough, due to its purpose as an attachment area for tendons, and it is very asymmetrical. Despite the damage to this portion of the bone, it is visible that the lateral part is more strongly developed, with a prominent ridge being visible in the distal part of the lateral side.
The patella is the largest sesamoid bone and is usually of limited value for taxonomic investigations. The large size (52.4 mm high and 46 mm wide) of the bone alone, however, precludes its attribution to any small to medium sized mammal found in the Hammerschmiede fauna. It is too large to belong to the largest artiodactyl in the fauna, the bovid Miotragocerus monacensis (Fuss et al. 2015; Hartung et al. 2020), but too small to belong to any proboscidean of the fauna (Konidaris et al. 2023). Therefore, the comparison of this bone can be limited to the perissodactyls. Among the perissodactyls present during the time period of the Hammerschmiede locality, this patella stands out as it is rather symmetrical, and its’ outline is widely tear-shaped. This contrasts with the shape of the patellae of horses and rhinos which are usually asymmetrical and rather angular. They are also wider than SNSB-BSPG 2020 XCIV-3834 and feature a lateral process, which does not exist in the Hammerschmiede specimen. Therefore, the HAM 4 patella cannot be associated with any other mammal group during this time period except chalicotheres. In the case of chalicotheriids, the patella seems to be highly variable, as it is probably also related to the differing modes of locomotion of chalicotheriines and schizotheriines. The only chalicotheriine from which the patella is known is A. grande. In posterior and anterior view, it has a rounded square-shaped outline in contrast to the schizotheriine patellae (Zapfe 1979; Guérin 2012). The most prominent feature separating the patellae of A. grande from those of schizotheriines is that in A. grande, there is no keel that separates the medial from the lateral articular condylar surface of articulation with the femoral patellar groove. In A. grande, this condylar surface is broad and only slightly transversally convex, corresponding to the shallow concave patellar groove and agreeing with the non-cursorial locomotion suggested for A. grande (Zapfe 1979). The described patellae for Moropus elatus Marsh 1877, Tylocephalonyx skinneri Coombs 1979, Ancylotherium pentelicum, A. grande, and Metaschizotherium spp. seem to differ from each other to an important degree (Gaudry 1862; Holland and Peterson 1914; Coombs 1978, 1979, 2009; Zapfe 1979; Fahlke and Coombs 2009). In Moropus elatus, the patella seems to be proximodistally more elongated than in the other taxa, as well as narrowing distally much more abruptly and the medial and lateral condylar facets form an obtuse angle (Holland and Peterson 1914; Coombs 1978). In Tylocephalonyx skinneri, the patella seems more rounded, with the width and height being about equal, and the medial and lateral condylar facets are well-separated by a keel, forming an almost right angle (Coombs 1979). In Ancylotherium pentelicum, the patella is subtriangular, narrowing distally, and the medial articular facet is much narrower than the lateral one, meeting at an obtuse angle (Gaudry 1863). The patellae of M. bavaricum and M. fraasi differ from those of the other schizotheriine genera. They are generally smaller, but much thicker anteroposteriorly, with a suboval outline. The two condylar facets for the femoral patellar groove are similarly wide mediolaterally. The patellae of the two species seem to exhibit some differences, though. For instance, in M. bavaricum, the two facets meet at a right angle, whereas in M. fraasi, they form an obtuse angle. Furthermore, the patella of M. bavaricum is more proximodistally elongated than the patella of M. fraasi. In the HAM 4 patella the ridge is blunt and forms an obtuse angle, similar to that of Moropus elatus, Ancylotherium pentelicum, and Metaschizotherium spp.; however, it exhibits a very well-developed area for the attachment of the quadriceps tendon. Moropus and Ancylotherium pentelicum do feature a prominent area for the attachment of this tendon. However, this portion of the bone is not as anteroposteriorly thickened as on the HAM 4 patella. The only patellae, which are anteroposteriorly as developed as the HAM 4 patella are those of M. fraasi and M. bavaricum, making them very thick in this dimension. The height-to-width index of the condylar surface (sensu Zapfe 1979) is also very similar between these three patellae: 114% in the HAM 4 specimen, 138% in M. bavaricum from Häder, and ~115% in M. fraasi from Petersbuch 71. Therefore, it is clear that the HAM 4 patella (SNSB-BSPG 2020 XCIV-3834), which is also rather thick anteroposteriorly and exhibits a keeled condylar surface forming an obtuse angle, should be most closely associated with the genus Metaschizotherium and probably M. fraasi, which is also stratigraphically closer to the geological age of Hammerschmiede. The two Metaschizotherium species are rather similar but can be separated based on some characters in their dentition and with more difficulty based only some postcranial features (Coombs 2009; Fahlke and Coombs 2009). Nonetheless, based only on the two existing patellae of this genus and the fact that no dental remains are known from HAM 4 it is preferred to assign the specimen to Schizotheriinae indet.
Potential schizotheriine
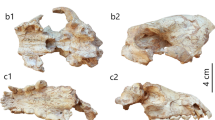
Skull fragment of a potential schizotheriine from HAM 4 (GPIT/MA/17503) in dorsal (A), ventral (B), left side (D) and right side views (E) and the possible placement of specimen GPIT/MA/17503 (F) on a line drawing of the skull of Ancylotherium pentelicum from the Late Miocene of Thermopigi in Greece (Geraads et al. 2007, fig. 2A). Scale bar is 5 cm for A-B, D-E. C is not in scale
Virtual depiction of the skull fragment of a potential schizotheriine from HAM 4 (GPIT/MA/17503) and the position of the frontal sinus: 3D surface model of specimen GPIT/MA/17503 in ventral view (A); as well as transversal (B–C) and axial (D) virtual orthoslices of the μCT-scan of specimen GPIT/MA/17503. Scale bar is 5 cm for A and D and 10 cm for B–C. The positions of the virtual orthoslices are noted in A
Description and Comparison
Nasal bones. Specimen GPIT/MA/17503 is a cranial fragment mainly including the nasal bones that are completely fused together, without any visible suture (Figs. 4, 5B). On the posterior part of the specimen, a portion of the frontal bone is preserved on the left side. The bone itself is rather thin and compact. Even though, the ventral portion of the nasal and the connection to the maxillary bones are not preserved, it is clear that the nasal bones are transversally very narrow. Also, they are very elongated, and the anterior end forms a smooth, overhanging edge. The nasal notch seems to have been slightly concave based on the preserved part. In dorsal view in the middle of the nasal bones, a notable longitudinal groove is present (Figs. 4A, 5B). The preserved portion of the frontal bone shows that at least the anterior part is raised dorsally when compared to the nasal bones, which are almost completely straight (Figs. 4D–E, 5D). In posterior-ventral view, a rather deep cavity with a diameter of up to 1 cm is visible in the anterior part of the frontal bone, extending into the nasal bones (Fig. 5D). It most plausibly represents the anterior end of the frontal sinus. The nasal bones themselves are thin and no distinct nasal sinus exists in the specimen (Fig. 5B).
The complete fusion of the nasal bones is a rather rare feature. In most mammals the nasal bones preserve the internasal suture even in adult individuals. Taking into account the large size of the specimen and the known European large mammals during this time, it could only belong to an extremely large carnivoran, or a large ungulate. To our knowledge, there are no carnivorans exhibiting such fused nasal bones. Among ungulates fused nasal bones are only known from animals such as horned rhinoceroses, and giraffes, which have a specialized function for this part of the skull (e.g., Kollmann 1920; Singer and Boné 1960; Spinage 1968; Fortelius 1983; Hieronymus et al. 2006; Farke 2008; Gerard et al. 2018). The morphology of the nasal bones in horned rhinoceroses is very distinct, with the development of enlarged nasal bones that function as horn bosses (e.g., Antoine 2002). They are easily recognizable, and completely differ from the Hammerschmiede specimen in having a central horn boss on the nasal bones, being usually rugose with many nutrient foramina for the development of the nasal horn, lacking the longitudinal groove, and being greatly pneumatized with a large nasal sinus, in comparison to the thin nasal bones (Gerard et al. 2018). In hornless rhinos, where the nasal bones are not as hugely developed, due to the lack of a large nasal horn, the nasal bones are usually triangular in dorsal view, thicker than in the Hammerschmiede specimen, and rarely completely fuse without leaving any visible suture (e.g., Ringström 1924). In extant giraffes, the nasal bones tend to completely fuse in old male individuals due to the dermal ossification on the dorsal surface of the skull, including the supra-occipital, parietal, frontal, lachrymal, and nasal bones, which is associated with intraspecific fighting, called ‘necking’, during which the fighting males use their heads as clubs to hit each other (e.g., Spinage 1968). The nasal bones of giraffes differ from the Hammerschmiede specimen in being transversally convex, lacking a longitudinal groove in the middle of the dorsal side, and in extant giraffes often exhibiting a rough, flake-like bone layer, due to the extra-ossification. Additionally, in some fossil giraffes the nasal bones had a different appearance; for instance, in Sivatherium the nasal bones were rather short, curved, and transversely convex (Geraads 1985). Other extinct genera like Palaeotragus, Decennatherium, Schansitherium, and Samotherium had similarly elongated nasal bones as the extant giraffe, but they do not seem to have been fused (e.g., Athanassiou 2014; Hou et al. 2019; Ríos and Morales 2019). Moreover, in all these genera, the nasal bones are convex, and none show a longitudinal groove in the middle of the nasal bones. Therefore, it is impossible to associate specimen GPIT/MA/17503 with either rhinos, or giraffes.
Another group in which the nasal bones seem to fuse are the chalicotheres and more specifically the subfamily Schizotheriinae as exemplified by Tylocephalonyx (Coombs 1979) from the Miocene of North America and Ancylotherium from the Late Miocene of Southeastern Europe, for which rather complete skulls are known (Garevski 1974; Coombs 1979; Munthe and Coombs 1979; Geraads et al. 2007). In general, the snout, including the nasal bones, is more elongated in schizotheriines like Moropus (Coombs 1978, fig. 3), Tylocephalonyx (Coombs 1979, fig. 6), and Ancylotherium (Geraads et al. 2007, fig. 2). Whereas skulls of chalicotheriines like A. grande (Anquetin et al. 2007, fig. 4), A. macedonicus (Bonis et al. 1995, fig. 1, pl. 1–3), and C. ?goldfussi (Anquetin et al. 2007, fig. 6), have less elongated snouts, as also testified by their less elongated molars and generally shorter toothrows, and feature shorter nasal bones that are not fused. Some schizotheriinae exhibit fused nasal bones, which are elongated and rather narrow just as in the specimen GPIT/MA/17503, including also the notable longitudinal groove in the middle of the nasal bones. Unfortunately, no adequately preserved schizotheriine skull is known from central Europe to evaluate the morphology of the nasal bones in taxa like M. fraasi and M. bavaricum. Nonetheless, the overall shape of the nasal bones from HAM 4 fits very well to the Ancylotherium pentelicum skull from the Late Miocene locality of Thermopigi in Greece (Fig. 4C), including the uplift of the anterior portion of the frontal bone compared to the straight nasal bones. Wagner (1857) described a skull fragment from Pikermi that he attributed to Rhinoceros pachygnathus (Wagner 1848), which was later assigned to Ancylotherium pentelicum (Holland and Peterson 1914; Schaub 1943). Wagner (1857) mentioned that the nasal bones are 'strongly arched’ on the sides, as in the Hammerschmiede specimen. In a juvenile skull attributed to Ancylotherium sp. from the Pliocene of China (Chen et al. 2012) and a subadult skull of Ancylotherium pentelicum from Karaslari in North Macedonia (Spassov et al. 2018), the internasal suture seems to remain unfused, possibly, due to the early ontogenetic stage. In another juvenile skull element of Ancylotherium pentelicum from Samos in Greece (MGL S61), the nasal bones bear a prominent longitudinal groove in the middle, but also remain unfused. However, the Hammerschmiede specimen is much smaller than the respective portion in the adult skull of Ancylotherium pentelicum. It seems to be only a little smaller than the respective portion in the skull of Tylocephalonyx skinneri from the Miocene of North America (Coombs 1979; Munthe and Coombs 1979). Based on the lengths of their teeth, Tylocephalonyx skinneri should be a bit larger than the Middle Miocene schizotheriine M. fraasi and M. bavaricum (Coombs 1979, 2009; Fahlke and Coombs 2009). Therefore, the size of the HAM 4 specimen possibly fits that of Metaschizotherium spp.; though, an identification, even on the generic level, is herein avoided due to the fragmentary nature of the specimen.
The frontal sinus in specimen GPIT/MA/17503 extends into the anterior-most part of the frontal bone and even into the nasal bone (Fig. 5). Large frontal sinuses are well-known in ungulates, especially those with specialized functions of the frontonasal region, like bovids, giraffes, horses and rhinos (Farke 2007, 2008, 2010; Márquez 2008; O’Brien et al. 2016), where the frontal sinus can be very large and, in some animals, extend into the nasal bone. In modern horses the frontal sinus is also well-developed and extends into the posterior-most part of the nasal bones (El-Gendy et al. 2014). Rhinos, on the other hand, are an extreme case, exhibiting greatly enlarged and pneumatized nasal bones (Gerard et al. 2018). In this group, the large frontal sinus continues into the extensive nasal sinus, without any separation. In ruminants the frontal sinus is generally different, while being very large in bovids, sometimes extending deep into their horncores, but very rarely extend into the nasal bones (e.g., Farke 2008, 2010; O’Brien et al. 2016; Hartung et al. 2020; Liakopoulou et al. 2022). In giraffes, the frontal sinus is similarly well-developed as in bovids, but seems to also partially extend into the nasal bones, similar to the condition in horses (Badlangana et al. 2011). In the HAM 4 specimen, the sinus reaches somewhat further anteriorly into the nasal bones than in equids and giraffids (Fig. 5C). This might suggest that the frontal sinus of the skull when complete was rather large, possibly larger than in equids, bovids, and giraffes. The exact function of the frontal sinus remains elusive, with many different hypotheses having been suggested. Some suggestions include function as a shock absorber in intraspecific combats, or as cooling mechanisms for the brain (e.g., Farke 2008; Badlangana et al. 2011). Accordingly, it is impossible to determine the function of the frontal sinus in this specimen.
Discussion
Taxonomic assignment
The HAM 5 material is represented by three isolated dental elements and three phalanges. The reduced “metastylid” in the molar from HAM 5 is a common feature in Anisodontini (Anquetin et al. 2007; Coombs and Göhlich 2019). Furthermore, the low (or weak) entoconid in the p4 from HAM 5 is one of only two autapomorphic characters of A. grande (Anquetin et al. 2007; Coombs and Göhlich 2019). However, the material from HAM 5 is very fragmentary and therefore it is preferable to refer it to Anisodon sp.
The HAM 4 has yielded a patella and a fragment of a skull that can be assigned to a chalicotheriid. The patella exhibits all features seen in the patellae of M. bavaricum and M. fraasi, while differing from the chalicotheriine Anisodon and from the schizotheriines Moropus, Tylocephalonyx, and Ancylotherium. Nonetheless, due to the existence of only a single patella it is referred to Schizotheriinae indet. The cranial fragment from HAM 4 includes the fused nasal bones and a small portion of the frontal bone. Based on its morphology and its similarity with Tylocephalonyx and Ancylotherium, the specimen could only be associated with a schizotheriine chalicothere. Due to its fragmentary nature, however, it is not assigned to any genus and instead is only tentatively referred to a schizotheriine. Thereby, the presence of the chalicotheriine Anisodon sp. in the HAM 5 level and a schizotheriine, closely affiliated with Metaschizotherium spp., in the HAM 4 level is herein confirmed.
European chalicotheriids
Chalicotheres are very rare faunal elements in the Miocene of Eurasia. During the Middle Miocene, A. grande is the most common representative of the family Chalicotheriidae in Europe (e.g., Zapfe 1979; Anquetin et al. 2007; Fahlke et al. 2013; Coombs and Göhlich 2019), whereas members of the Schizotheriinae, such as M. fraasi and M. bavaricum, are rarer faunal components (Coombs 2009; Fahlke and Coombs 2009). During the early Late Miocene (Vallesian), the chalicotheriine material from central Europe is mainly attributed to C. goldfussi, despite its taxonomic issues (Zapfe 1979; Bonis et al. 1995; Anquetin et al. 2007; Fahlke et al. 2013). In the Eastern Mediterranean, where the Turolian faunas are well-represented at many extraordinarily rich localities (e.g., Spassov 2002; Koufos 2006, 2017; Kostopoulos 2009), the most common chalicothere was the schizotheriine Ancylotherium pentelicum (Roussiakis and Theodorou 2001; Geraads et al. 2007; Giaourtsakis and Koufos 2009; Koufos 2012; Coombs 2013; Koufos and Kostopoulos 2016; Codrea et al. 2019; Kampouridis et al. 2022, 2023). This schizotheriine coexisted in some localities with different chalicotheriine taxa like Kalimantsia bulgarica Geraads et al. 2001, Anisodon sp. and indeterminate chalicotheriines (Geraads et al. 2001; Saraç and Sen 2005; Geraads et al. 2006; Kampouridis et al. 2023).
The genus Anisodon is present in Europe from the base of the Middle Miocene, with the report of A. grande in the Loire Basin in France, until the latest Miocene, with A. macedonicus in Dytiko 3 in Greece (Symeonidis 1973; Bonis et al. 1995; Fahlke et al. 2013). The exact phylogenetic relationship of A. grande, A. macedonicus and the other Anisodontini remains unclear (Bonis et al. 1995; Geraads et al. 2001; Anquetin et al. 2007; Fahlke et al. 2013). During the Middle Miocene A. grande is known from well-sampled localities, such as Sansan (Anquetin et al. 2007; Guérin 2012) and the Devínska Nová Ves fissure fillings (Zapfe 1979). In Europe, Anisodon becomes much rarer during the Late Miocene; the Vallesian age is especially underrepresented, with very little fossil evidence from the Middle to Late Miocene transition.
The genus Metaschizotherium includes two distinct species, M. fraasi and M. bavaricum, which are separated mainly based on their size and some dental features. Recent studies provided more elaborate diagnoses for the two species based on the comparisons of large collections of dental and postcranial material (Coombs 2009; Fahlke and Coombs 2009). Metaschizotherium bavaricum seems to be present mainly in the first half of the Middle Miocene, whereas M. fraasi is more common in the second half (Coombs 2009, tab 3) and the stratigraphical distribution of the genus may extend into the early Late Miocene (Mottl 1966). The genus Ancylotherium, is the best-known chalicothere during the Late Miocene in Europe, with Ancylotherium hellenicum present during the Vallesian and Ancylotherium pentelicum being dominant during the Turolian age (Koufos 2012; Koufos and Kostopoulos 2016; Kampouridis et al. 2023).
Co-occurrence of schizotheriines and chalicotheriines
The co-occurrence of a schizotheriine and a chalicotheriine chalicothere in the same fossil site is a very rare phenomenon. Chalicotheriines have never been reported from North America (e.g., Coombs 1978, 1979, 1989, 2004) and the two subfamilies appear stratigraphically separated in Africa (Coombs and Cote 2010). The only region where chalicotheriines and schizotheriines have overlapping stratigraphical ranges is Eurasia (Coombs 1989; Kampouridis et al. 2023). However, even there it is very rare to find material of both subfamilies in the same locality. Some authors have suggested that their potential to coexist was restricted, due to their similar dietary needs (Coombs 1989; Koufos 2012), while others, have suggested that the two chalicothere subfamilies were unable to coexist and that all reported co-occurrences represent mixing of material that comes from different horizons (Bonis et al. 1992). A recent review of the reported co-occurrences suggested that many of them cannot be taken as certain (Kampouridis et al. 2023), due to the lack of knowledge about the stratigraphical coherence and the fact that historical collections of localities like La Grive St.-Alban in France (Mein and Ginsburg 2002), Tunggur in China (Colbert 1934; Liu and Zhang 2012), and Pikermi in Greece (Gaudry 1862; Roussiakis and Theodorou 2001; Böhme et al. 2017) are known to be a result of mixing of different fossil sites and fossiliferous horizons. Accordingly, only nine localities can be considered certain cases of co-occurrence of schizotheriines and chalicotheriines at the moment, six of which are found in the Upper Miocene deposits of the Balkan-Iranian zoogeographical province (Kampouridis et al. 2023). The other three are found in the Early Miocene of the Bugti Hills (Pakistan), Pleistocene of Huangjiawan (China) (Kampouridis et al. 2023) and in the early Late Miocene of Holzmannsdorf (Styrian Basin, Austria) (Mottl 1966).
Hammerschmiede is the only locality in Central Europe with adequate stratigraphical data where both a schizotheriine and a chalicotheriine have been retrieved. The stratigraphy of the locality is well-established with the existence of six different fossiliferous layers (Kirscher et al. 2016). The richest horizons are HAM 4 and HAM 5, which include very diverse vertebrate faunas. The excavations at Hammerschmiede are stratigraphically controlled without the mixing of the different horizons. Therefore, we can state with certainty that the two chalicothere taxa were found in different horizons: the schizotheriine material comes from HAM 4 (11.44 Ma) and the chalicotheriine material comes from HAM 5 (11.62 Ma).
The fact that the two species are found in different horizons could be explained through differing preferred environments of the two subfamilies. In recent years, several studies have tried to investigate the diet using meso- and microwear analysis on their teeth and based on that the preferred environment of chalicotheres (Schulz et al. 2007; Schulz and Fahlke 2009; Semprebon et al. 2011; Fahlke et al. 2013). They found that all studied chalicotheres can be regarded as browsers and that their diet did not include a significant amount of grass. Semprebon et al. (2011, tab. 4, fig. 7) compared the microwear patterns of chalicotheres to each other and found that the results of the schizotheriine Metaschizotherium are most similar to those of the contemporaneous chalicotheriine A. grande, though the latter probably had a more abrasive diet.
During the whole Middle Miocene, no fossil site has provided convincing evidence for the coexistence of a chalicotheriine and a schizotheriine, despite the presence of members of both groups in many Middle Miocene localities in Europe. This fact, along with the data presented herein, might support the idea that the two taxa were unable to share the same ecological niche and would have preferred to inhabit different environments, to avoid competition over food resources. Considering also their rather different body plans, with schizotheriines having fore- and hindlimbs that have more equal proportions and were possibly more cursorial than the orthograde chalicotheriines with their very short hindlimbs, it would be plausible for schizotheriines to have lived in more open environments, and chalicotheriines like A. grande with an intermembral index (Zapfe 1979, p. 269) that is closer to the extant gorilla preferring dense forests, as suggested by Coombs (1983). Though no specific data is available yet about the environment of each fossiliferous level in the Hammerschmiede claypit, it is likely that the environments of HAM 5 and HAM 4 differed, with the HAM 5 fossil site possibly representing a more suitable habitat for Anisodon, while the HAM 4 fossil site might have been more appropriate for the schizotheriine. This could explain the disparate occurrences of the two subfamilies in different horizons in Hammerschmiede.
Data availability
The μCT-scan of specimen GPIT/MA/17503 is available on MorphoSource (morphosource.org) under the Project ID: 000545298. The metrical data are provided in the published article.
References
Anquetin, J., P.-O. Antoine, and P. Tassy. 2007. Middle Miocene Chalicotheriinae (Mammalia, Perissodactyla) from France, with a discussion on chalicotheriine phylogeny. Zoological Journal of the Linnean Society 151: 577–608. https://doi.org/10.1111/j.1096-3642.2007.00327.x.
Antoine, P.-O. 2002. Phylogénie et évolution des Elasmotheriina (Mammalia, Rhinocerotidae). Mémoires Du Muséum National D’histoire Naturelle 188: 1–359.
Athanassiou, A. 2014. New giraffid (Artiodactyla) material from the Lower Pleistocene locality of Sésklo (SE Thessaly, Greece): evidence for an extension of the genus Palaeotragus into the Pleistocene. Zitteliana B 32. Universitätsbibliothek der Ludwig-Maximilians-Universität München: 71–89. https://doi.org/10.5282/UBM/EPUB.22388.
Badlangana, N.L., J.W. Adams, and P.R. Manger. 2011. A Comparative Assessment of the Size of the Frontal Air Sinus in the Giraffe (Giraffa camelopardalis). The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 294: 931–940. https://doi.org/10.1002/ar.21401.
de Blainville, H.M.D. 1849. Des Anoplotheriums. In Ostéographie ou descriptions iconographique compare du squelette et du système dentaire des Mammifères récents et fossils pour server base à la zoologie et à la géologie, 4:66–70. Paris: J. B. Ballière et Fils.
Böhme, M., N. Spassov, M. Ebner, D. Geraads, L. Hristova, U. Kirscher, S. Kötter, et al. 2017. Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. Edited by Roberto Macchiarelli. PLoS ONE 12: e0177347. https://doi.org/10.1371/journal.pone.0177347.
Böhme, M., N. Spassov, J. Fuss, A. Tröscher, A.S. Deane, J. Prieto, U. Kirscher, T. Lechner, and D.R. Begun. 2019. A new Miocene ape and locomotion in the ancestor of great apes and humans. Nature. https://doi.org/10.1038/s41586-019-1731-0.
Böhme, M., N. Spassov, J.M. Desilva, and D.R. Begun. 2020. Reply to: Reevaluating bipedalism in Danuvius. Nature 586: E4–E5. https://doi.org/10.1038/s41586-020-2737-3.
Chen, S.-K., T. Deng, L. Pang, W. He, and S.-Q. Chen. 2012. A juvenile skull of Ancylotherium (Mammalia, Perissodactyla, Chalicotheriidae) from the Pliocene of China. Geobios 45: 527–534. https://doi.org/10.1016/j.geobios.2012.06.002.
Codrea, V.A., M. Bordeianu, and A.A. Solomon. 2019. The first record of the chalicothere Ancylotherium pentelicum in Romania. Muzeul Olteniei Craiova Oltenia. Studii Şi comunicări. Ştiinţele Naturii. 35: 37–44.
Colbert, E.H. 1934. Chalicotheres from Mongolia and China in the American Museum. Bulletin of the American Museum of Natural History 67: 353–387.
Coombs, M.C. 1975. Sexual dimorphism in chalicotheres (Mammalia, Perissodactyla). Systematic Zoology 24: 55. https://doi.org/10.2307/2412697.
Coombs, M.C. 1978. Reevaluation of the early Miocene North American Moropus (Perissodactyla, Chalicotheriidae, Schizotheriinae). Bulletin of Carnegie Museum of Natural History 4: 1–62.
Coombs, M.C. 1979. Tylocephalonyx, a new genus of North American dome-skulled chalicotheres (Mammalia, Perissodactyla). Bulletin of the American Museum of Natural History 164: 1–68.
Coombs, M.C. 1982. Chalicotheres (Perissodactyla) as large terrestrial mammals. Third American Paleontological Convention, Proceedings 1: 99–103.
Coombs, M.C. 1983. Large mammalian clawed herbivores: A comparative study. Transactions of the American Philosophical Society 73: 1. https://doi.org/10.2307/3137420.
Coombs, M.C. 1989. Interrelationship and diversity in the Chalicotheriidae. In The evolution of perissodactyls. New York: Oxford University Press.
Coombs, M.C. 2004. Chapter 15: Moropus merriami in the Early Barstovian Lower Snake Creek Fauna of Nebraska, with Comments on Biogeography of North American Chalicotheres. Bulletin of the American Museum of Natural History 285: 191–208. https://doi.org/10.1206/0003-0090(2004)285<0191:C>2.0.CO;2.
Coombs, M.C. 2009. The chalicothere Metaschizotherium bavaricum (Perissodactyla, Chalicotheriidae, Schizotheriinae) from the Miocene (MN5) Lagerstätte of Sandelzhausen (Germany): Description, comparison, and paleoecological significance. Paläontologische Zeitschrift 83: 85–129. https://doi.org/10.1007/s12542-009-0004-x.
Coombs, M.C. 2013. A juvenile mandible with deciduous teeth of Ancylotherium pentelicum (Perissodactyla, Chalicotheriidae, Schizotheriinae), collected by Barnum Brown from the late Miocene of Samos (Greece). Journal of Vertebrate Paleontology 33: 233–238. https://doi.org/10.1080/02724634.2012.710281.
Coombs, M.C., and S.M. Cote. 2010. Chalicotheriidae. In Cenozoic mammals of Africa, ed. L. Werdelin and W.J. Sanders, 659–668. Berlin: University of California Press. https://doi.org/10.1525/california/9780520257214.003.0033.
Coombs, M.C., and U.B. Göhlich. 2019. Anisodon (Perissodactyla, Chalicotheriinae) from the middle Miocene locality Gračanica (Bugojno Basin, Gornji Vakuf, Bosnia and Herzegovina). Palaeobiodiversity and Palaeoenvironments. https://doi.org/10.1007/s12549-018-0357-9.
Cuvier, G. 1823. Sur une phalange ongueale fossile qui annonce a elle seule un Edente inconnu, probablement du genre des pangolins, et de taille giantesque. Recherches Sur Les Ossemens Fossils 5: 193–195.
de Bonis, L., G. Bouvrain, D. Geraads, and G.D. Koufos. 1992. Diversity and paleoecology of Greek late Miocene mammalian faunas. Palaeogeography, Palaeoclimatology, Palaeoecology 91: 99–121. https://doi.org/10.1016/0031-0182(92)90035-4.
de Bonis, L., G. Bouvrain, G.D. Koufos, and P. Tassy. 1995. Un crane de chalicothere (Mammalia, Perissodactyla) du Miocene superieur de Macedoine (Grece): Remarques sur la phylogenie des Chalicotheriinae. Palaeovertebrata 24: 135–176.
El-Gendy, S.A.A., M.A.M. Alsafy, and A.A. El Sharaby. 2014. Computed tomography and sectional anatomy of the head cavities in donkey (Equus asinus). Anatomical Science International 89: 140–150. https://doi.org/10.1007/s12565-013-0209-7.
Fahlke, J.M., and M.C. Coombs. 2009. Dentition and first postcranial description of Metaschizotherium fraasi Koenigswald, 1932 (Perissodactyla: Chalicotheriidae) and its occurrence on a karstic plateau—new insights into schizotheriine morphology, relationships, and ecology. Palaeontographica Abteilung A 290: 65–129. https://doi.org/10.1127/pala/290/2009/65.
Fahlke, J.M., M.C. Coombs, and G.M. Semprebon. 2013. Anisodon sp. (Mammalia, Perissodactyla, Chalicotheriidae) from the Turolian of Dorn-Dürkheim 1 (Rheinhessen, Germany): Morphology, phylogeny, and palaeoecology of the latest chalicothere in Central Europe. Palaeobiodiversity and Palaeoenvironments 93: 151–170. https://doi.org/10.1007/s12549-013-0119-7.
Falconer, H. 1868. On Chalicotherium sivalense. In Palaeontological memoirs and notes of the late Hugh Falconer, ed. C.A. Murchison, 208–226. London: Robert Hardwicke.
Farke, A.A. 2007. Morphology, constraints, and scaling of frontal sinuses in the hartebeest, Alcelaphus buselaphus (Mammalia: Artiodactyla, Bovidae). Journal of Morphology 268: 243–253. https://doi.org/10.1002/jmor.10511.
Farke, A.A. 2008. Frontal sinuses and head-butting in goats: A finite element analysis. Journal of Experimental Biology 211: 3085–3094. https://doi.org/10.1242/jeb.019042.
Farke, A.A. 2010. Evolution, homology, and function of the supracranial sinuses in ceratopsian dinosaurs. Journal of Vertebrate Paleontology 30: 1486–1500. https://doi.org/10.1080/02724634.2010.501436.
Fortelius, M. 1983. The morphology and paleobiological significance of the horns of Coelodonta antiquitatis (Mammalia: Rhinocerotidae). Journal of Vertebrate Paleontology 3: 125–135. https://doi.org/10.1080/02724634.1983.10011964.
Fuss, J., J. Prieto, and M. Böhme. 2015. Revision of the boselaphin bovid Miotragocerus monacensis Stromer, 1928 (Mammalia, Bovidae) at the Middle to Late Miocene transition in Central Europe. Neues Jahrbuch Für Geologie Und Paläontologie - Abhandlungen 276: 229–265. https://doi.org/10.1127/njgpa/2015/0481.
Garevski, R. 1974. Beitrag zur Kenntnis der Pikermifauna Mazedoniens. Fossilreste der Chalicotheriiden. Fragmenta Balcanica 9: 201–209.
Gaudry, A. 1862-1867. Animaux fossiles et géologie de l’Attique. Paris: F. Savy.
Gaudry, A., and E. Lartet. 1856. Sur les résultats des recherches paléontologiques entreprises dans l’Attique sous les auspices de l’Académie. Comptes Rendus Des Séances De L’académie Des Sciences 43: 271–274.
Geraads, D. 1985. Sivatherium maurusium (Pomel) (Giraffidae, Mammalia) du Pléistocène de la République de Djibouti. Paläontologische Zeitschrift 59: 311–321. https://doi.org/10.1007/BF02988816.
Geraads, D.N. Spassov., and D. Kovachev. 2001. New Chalicotheriidae (Perissodactyla, Mammalia) from the Late Miocene of Bulgaria. Journal of Vertebrate Paleontology 21: 596–606. https://doi.org/10.1671/0272-4634(2001)021[0596:NCPMFT]2.0.CO;2.
Geraads, D., N. Spassov, and D. Kovachev. 2006. The Bulgarian Chalicotheriidae (Mammalia): An update. Revenue De Paléobiologie, Genève 25: 429–437.
Geraads, D., E. Tsoukala, and N. Spassov. 2007. A skull of Ancylotherium (Chalicotheriidae, Mammalia) from the late Miocene of Thermopigi (Serres, N. Greece) and the relationships of the genus. Journal of Vertebrate Paleontology 27: 461–466. https://doi.org/10.1671/0272-4634(2007)27[461:ASOACM]2.0.CO;2.
Gerard, M.P., Z.G. Glyphis, C. Crawford, A.T. Blikslager, and J. Marais. 2018. Identification of a nasoconchal paranasal sinus in the white rhinoceros (Ceratotherium simum). Journal of Zoo and Wildlife Medicine 49: 444–449. https://doi.org/10.1638/2017-0185.1.
Giaourtsakis, I., and G.D. Koufos. 2009. The Late Miocene Mammal Faunas of the Mytilinii Basin, Samos Island, Greece: New Collection 10. Chalicotheriidae. Beiträge Zur Paläontologie 31: 189–205.
Gill, T. 1872. Arrangements of the families of mammals with analytical tables. Smithsonian Miscellaneous Collections 11: 1–98.
Ginsburg, L. 2001. Les faunes de mammifères terrestres du Miocène moyen des Faluns du bassin de Savigné-sur-Lathan (France). Geodiversitas 23: 381–394.
Ginsburg, L. 1990. The Faunas and Stratigraphical Subdivisions of the Orleanian in the Loire Basin (France). In European Neogene mammal chronology, 157–175. New York: Plenum Press.
Guérin, C. 2012. Anisodon grande (Perissodactyla, Chalicotheriidae) de Sansan. In Mammifères de Sansan. Mémoires du Muséum national d’Histoire naturelle 203. Paris: Muséum national d’Histoire naturelle.
Hartung, J., and M. Böhme. 2022. Unexpected cranial sexual dimorphism in the tragulid Dorcatherium naui based on material from the middle to late Miocene localities of Eppelsheim and Hammerschmiede (Germany). Edited by Julien Louys. PLoS ONE 17: e0267951. https://doi.org/10.1371/journal.pone.0267951.
Hartung, J., T. Lechner, and M. Böhme. 2020. New cranial material of Miotragocerus monacensis (Mammalia: Bovidae) from the late Miocene hominid locality Hammerschmiede (Germany). Neues Jahrbuch Für Geologie Und Paläontologie - Abhandlungen 298: 269–284.
Heissig, K. 1999. Family Chalicotheriidae. In The Miocene Land Mammals of Europe, 189–192. Munich: Pfeil.
Heissig, K., and O. Fejfar. 2013. Die Säugetiere aus dem Untermiozän des Chomutov Beckens-I. Chalicotheriidae (Mammalia, Perissodactyla). Acta Musei Nationalis Pragae, Series b, Historia Naturalis 69: 7–64. https://doi.org/10.14446/AMNP.2013.007.
Hieronymus, T.L., L.M. Witmer, and R.C. Ridgely. 2006. Structure of white rhinoceros (Ceratotherium simum) horn investigated by X-ray computed tomography and histology with implications for growth and external form. Journal of Morphology 267: 1172–1176. https://doi.org/10.1002/jmor.10465.
Holland, W., and O. Peterson. 1914. The osteology of the Chalicotheroidea with special reference to a mounted skeleton of Moropus elatus Marsh, now installed in the Carnegie Museum. Mem Carn Mus 3: 189–406.
Hou, S., M. Cydylo, M. Danowitz, and N. Solounias. 2019. Comparisons of Schansitherium tafeli with Samotherium boissieri (Giraffidae, Mammalia) from the Late Miocene of Gansu Province, China. Edited by Suzannah Rutherford. PLoS ONE 14: e0211797. https://doi.org/10.1371/journal.pone.0211797.
Kampouridis, P., S. Roussiakis, I. Giaourtsakis, N. Kargopoulos, G. Svorligkou, and G.E. Theodorou. 2022. Ancylotherium pentelicum (Mammalia, Chalicotheriidae) from the late Miocene of Kerassia (Greece) and remarks on its intraspecific variability. Palaeobiodiversity and Palaeoenvironments 102: 193–203. https://doi.org/10.1007/s12549-021-00497-w.
Kampouridis, P., B. Ratoi, and L. Ursachi. 2023. New evidence for the unique coexistence of two subfamilies of clawed perissodactyls (Mammalia, Chalicotheriidae) in the Upper Miocene of Romania and the Eastern Mediterranean. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-023-09657-5.
Kargopoulos, N., P. Kampouridis, T. Lechner, and M. Böhme. 2021a. A review of Semigenetta (Viverridae, Carnivora) from the Miocene of Eurasia based on material from the hominid locality of Hammerschmiede (Germany). Geobios. https://doi.org/10.1016/j.geobios.2021.07.001.
Kargopoulos, N., P. Kampouridis, T. Lechner, and M. Böhme. 2021b. Hyaenidae (Carnivora) from the Late Miocene hominid locality of Hammerschmiede (Bavaria, Germany). Historical Biology. https://doi.org/10.1080/08912963.2021.2010193.
Kargopoulos, N., A. Valenciano, P. Kampouridis, T. Lechner, and M. Böhme. 2021c. New early late Miocene species of Vishnuonyx (Carnivora, Lutrinae) from the hominid locality of Hammerschmiede, Bavaria, Germany. Journal of Vertebrate Paleontology. https://doi.org/10.1080/02724634.2021.1948858.
Kargopoulos, N., A. Valenciano, J. Abella, P. Kampouridis, T. Lechner, and M. Böhme. 2022. The exceptionally high diversity of small carnivorans from the Late Miocene hominid locality of Hammerschmiede (Bavaria, Germany). Edited by Giorgio Carnevale. PLoS ONE 17: e0268968. https://doi.org/10.1371/journal.pone.0268968.
Kaup, J. 1833a. Mitteilungen an Professor Bronn. Neues Jahrbuch Für Mineralogie, Geognosie, Geologie Und Petrefaktenkunde 1833: 419–420.
Kaup, J. 1833b. Description d’ossements fossiles de mammifères inconnus jusqu’à-présent, qui se trouvent au muséum grand-ducal de Darmstadt. Second cahier. Darmstadt: J. G. Heyer.
Kaup, J. 1833c. Der Krallen-Phalanx von Eppelsheim, nach welchem Herr von Cuvier seinen Riesen-Pangolin, Manis gigantea, aufstellte, gehört zu Dinotherium. Neues Jahrbuch Für Mineralogie, Geologie Und Palaontologie 1833: 172–176.
Kirscher, U., J. Prieto, V. Bachtadse, H. Abdul Aziz, G. Doppler, M. Hagmaier, and M. Böhme. 2016. A biochronologic tie-point for the base of the Tortonian stage in European terrestrial settings: Magnetostratigraphy of the topmost Upper Freshwater Molasse sediments of the North Alpine Foreland Basin in Bavaria (Germany). Newsletters on Stratigraphy 49: 445–467. https://doi.org/10.1127/nos/2016/0288.
Kollmann, M. 1920. Etude anatomiqueet systématique d’un specimen remarquable de Giraffa camelopardalis tippelskirchi Matschie. Bulletin De La Société Zoologique De France 45: 191–204.
Konidaris, G.E., T. Lechner, P. Kampouridis, and M. Böhme. 2023. Deinotherium levius and Tetralophodon longirostris (Proboscidea, Mammalia) from the Late Miocene hominid locality Hammerschmiede (Bavaria, Germany), and their biostratigraphic significance for the terrestrial faunas of the European Miocene. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-023-09683-3.
Kostopoulos, D.S. 2009. The Pikermian Event: Temporal and spatial resolution of the Turolian large mammal fauna in SE Europe. Palaeogeography, Palaeoclimatology, Palaeoecology 274: 82–95. https://doi.org/10.1016/j.palaeo.2008.12.020.
Koufos, G.D. 2006. The Neogene mammal localities of Greece: Faunas, chronology and biostratigraphy. Hellenic Journal of Geosciences 41: 183–214.
Koufos, G.D. 2012. New material of Chalicotheriidae (Perissodactyla, Mammalia) from the Late Miocene of Axios Valley, Macedonia (Greece) with the description of a new species. Annales De Paléontologie 98: 203–224. https://doi.org/10.1016/j.annpal.2012.06.002.
Koufos, G.D. 2017. Neogene and Quaternary continental biostratigraphy of Greece based on Mammals. Bulletin of the Geological Society of Greece 50: 55. https://doi.org/10.12681/bgsg.11701.
Koufos, G.D., and D.S. Kostopoulos. 2016. Chalicotheriidae. Geobios 49: 75–83. https://doi.org/10.1016/j.geobios.2016.01.014.
Kowalevsky, W. 1873. Monographie der Gattung Anthracotherium Cuv. und Versuch einer natürlichen Classification der fossilen Hufthiere. Palaeontographica 22: 131–346.
Lartet, E. 1851. Notice sur la colline de Sansan. In Extrait de l’Annuaire du Département du Gers, année 1851. Auch: J.-A. Portes.
Lechner, T., and M. Böhme. 2022. The beaver Steneofiber depereti from the early Late Miocene hominid locality Hammerschmiede and remarks on its ecology. Acta Palaeontologica Polonica. https://doi.org/10.4202/app.00997.2022.
Lechner, T., and M. Böhme. 2023. The largest record of the minute beaver Euroxenomys minutus (Mammalia, Castoridae) from the early Late Miocene hominid locality Hammerschmiede (Bavaria, Southern Germany) and palaeoecological considerations. Historical Biology 15: 1–16. https://doi.org/10.1080/08912963.2023.2215236.
Li, Z., T. Mörs, Y. Zhang, K. Xie, and Y. Li. 2022. New Material of Schizotheriine Chalicothere (Perissodactyla, Chalicotheriidae) from the Xianshuihe Formation (Early Miocene) of Lanzhou Basin, Northwest China. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-022-09619-3.
Liakopoulou, D., M. Georgitsis, and S. Roussiakis. 2022. Frontal bone pneumatisation in Tragoportax and Miotragocerus (Mammalia, Bovidae) from the Late Miocene of Greece. Historical Biology 34: 1019–1028. https://doi.org/10.1080/08912963.2021.1959578.
Linnaeus, C. 1758. Systema Naturae. Stockholm: Laurentii Salvii.
Liu, Y., and Z. Zhang. 2012. New materials of Chalicotherium brevirostris (Perissodactyla, Chalicotheriidae) from the Tunggur Formation, Inner Mongolia. Geobios 45: 369–376. https://doi.org/10.1016/j.geobios.2011.10.011.
Márquez, S. 2008. The paranasal sinuses: The last frontier in craniofacial biology. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 291: 1350–1361. https://doi.org/10.1002/ar.20791.
Marsh, O.C. 1877. Notice of some new vertebrate fossils. American Journal of Science 14: 249–256.
Mayr, H., and V. Fahlbusch. 1975. Eine unterpliozäne Kleinsäugerfauna aus der Oberen Süßwasser-Molasse Bayerns. Mitteilungen Der Bayerischen Staatssammlung Für Paläontologie Und Historische Geologie 15: 91–111.
Mayr, G., T. Lechner, and M. Böhme. 2020a. The large-sized darter Anhinga pannonica (Aves, Anhingidae) from the late Miocene hominid Hammerschmiede locality in Southern Germany. Edited by Jun Liu. PLoS ONE 15: e0232179. https://doi.org/10.1371/journal.pone.0232179.
Mayr, G., T. Lechner, and M. Böhme. 2020b. A skull of a very large crane from the late Miocene of Southern Germany, with notes on the phylogenetic interrelationships of extant Gruinae. Journal of Ornithology 161: 923–933. https://doi.org/10.1007/s10336-020-01799-0.
Mayr, G., T. Lechner, and M. Böhme. 2023. Nearly complete leg of an unusual, shelduck-sized anseriform bird from the earliest late Miocene hominid locality Hammerschmiede (Germany). Historical Biology 35: 465–474. https://doi.org/10.1080/08912963.2022.2045285.
Mein, P., and L. Ginsburg. 2002. Sur l’âge relatif des différents dépôts karstiques miocènes de La Grive-Saint-Alban (Isère). Cahiers Scientifiques Du Muséum D’histoire Naturelle De Lyon - Centre De Conservation Et D’étude Des Collections 5: 7–47. https://doi.org/10.3406/mhnly.2002.1328.
Munthe, J., and M.C. Coombs. 1979. Miocene Dome-Skulled Chalicotheres (Mammalia, Perissodactyla) from the Western United States: A Preliminary Discussion of a Bizarre Structure. Journal of Paleontology 53: 77–91.
O’Brien, H.D., J.T. Faith, K.E. Jenkins, D.J. Peppe, T.W. Plummer, Z.L. Jacobs, B. Li, et al. 2016. Unexpected Convergent Evolution of Nasal Domes between Pleistocene Bovids and Cretaceous Hadrosaur Dinosaurs. Current Biology 26: 503–508. https://doi.org/10.1016/j.cub.2015.12.050.
Owen, R. 1848. Description of teeth and portions of jaws of two extinct anthracotherioid quadrupeds (Hyopotamus vectianus and Hyop. bovinus) discovered by the Marchioness of Hastings in the Eocene deposits on the N.W. coast of the Isle of Wight; with an attempt to develop Cuvier’s idea of the classification of pachyderms by the number of their toes. Quarterly Journal of the Geological Society of London 4: 103–141.
Ringström, T. 1924. Nashörner der Hipparion-Fauna Nord-Chinas. Palaeontologia Sinica 1: 1–156.
Ríos, M., and J. Morales. 2019. A new skull of Decennatherium rex Ríos, Sánchez and Morales, 2017 from Batallones-4 (upper Vallesian, MN10, Madrid, Spain). Palaeontologia Electronica 22.2.pvc_1: 1–16. https://doi.org/10.26879/965.
Roussiakis, S., and G. Theodorou. 2001. Ancylotherium pentelicum (Gaudry and Lartet, 1856) (Perissodactyla, Mammalia) from the classic locality of Pikermi (Attica, Greece), stored in the Palaeontological and Geological Museum of Athens. Geobios 34: 563–584.
Saraç, G., and S. Sen. 2005. Chalicotheriidae (Mammalia, Perissodactyla) from the late Miocene of Akkasdagi, Turkey. Geodiversitas 27: 591–600.
Schaefer, H., and H. Zapfe. 1971. Chalicotherium grande Blainv. und Chalicotherium goldfussi Kaup. Odontologische und osteologische Unterschiede. Verhandlungen Der Naturforschenden Gesellschaft in Basel 81: 157–199.
Schaub, S. 1943. Vorderestremität von Ancylotherium pentelicum Gaudry und Lartet. Schweizerische Palaeontologische Abhandlungen 64: 1–36.
Schulz, E., and J.M. Fahlke. 2009. The diet of Metaschizotherium bavaricum (Chalicotheriidae, Mammalia) from the MN 5 of Sandelzhausen (Germany) implied by the mesowear method. Paläontologische Zeitschrift 83: 175–181. https://doi.org/10.1007/s12542-009-0007-7.
Schulz, E., J.M. Fahlke, G. Merceron, and T.M. Kaiser. 2007. Feeding ecology of the Chalicotheriidae (Mammalia, Perissodactyla, Ancylopoda). Results from dental micro- and mesowear analyses. Verhandlungen Des Naturwissenschaftlichen Vereins Hamburg 43: 5–31.
Semprebon, G.M., P.J. Sise, and M.C. Coombs. 2011. Potential bark and fruit browsing as revealed by stereomicrowear analysis of the peculiar clawed herbivores known as chalicotheres (Perissodactyla, Chalicotherioidea). Journal of Mammalian Evolution 18: 33–55. https://doi.org/10.1007/s10914-010-9149-3.
Singer, R., and E.L. Boné. 1960. Modern giraffes and the fossil giraffids of Africa. Annals of the South African Museum 45: 375–548.
Spassov, N. 2002. The Turolian Megafauna of West Bulgaria and the character of the Late Miocene “Pikermian biome.” Bollettino Della Società Paleontologica Italiana 41: 69–81.
Spassov, N., D. Geraads, L. Hristova, G.N. Markov, B. Garevska, and R. Garevski. 2018. The late Miocene mammal faunas of the Republic of Macedonia (FYROM). Palaeontographica Abteilung A 311: 1–85. https://doi.org/10.1127/pala/2018/0073.
Spinage, C.A. 1968. Horns and other bony structures of the skull of the giraffe, and their functional significance. The East African Wildlife Journal 6: 53–61. https://doi.org/10.1111/j.1365-2028.1968.tb00900.x.
Symeonidis, N.K. 1973. Chalicotherium goldfussi Kaup (Perissodactyla, Mammalia) aus dem Altpliozän von Pikermi (Griechenland). Annales De Géo Pays Helléniques 25: 301–307.
Tassy, P. 1978. Chalicotherium: Le “cheval-gorille.” La Recherche 87: 283–285.
Tsoukala, W. 2022. The Fossil Record of chalicotheres (Mammalia: Perissodactyla: Chalicotheriidae) in Greece. In ) Fossil Vertebrates of Greece, vol. 2, ed. E. Vlachos, 501–517. Cham: Springer International Publishing. https://doi.org/10.1007/978-3-030-68442-6_14.
Viret, J. 1961. Catalogue critique de la faune des mammifères miocènes de La Grive-Saint-Alban. 2e partie (suite du fascicule III, 1951). Nouvelles Archives Du Muséum D’histoire Naturelle De Lyon 6: 53–81. https://doi.org/10.3406/mhnly.1961.991.
von Koenigswald, G.H.R. 1932. Metaschizotherium fraasi n. g. n. sp., ein neuer Chalicotheriidae aus dem Obermiocän von Steinheinm a. Albuch. Palaeontographica - Beiträge Zur Naturgeschichte Der Vorzeit Supplement 8: 1–24.
von Mottl, M. 1966. VIII. Eine neue unterpliozäne Säugetierfauna aus der Steiermark, SO-Österreich. Mitteilungen Des Museums Für Bergbau, Geologie Und Technik Am Landesmuseum “johanneum” Graz 28: 33–62.
Wagner, A. 1848. Urweltliche Säugetier-Überreste aus Griechenland. Abhandlungen Der Bayerischen Akademie Der Wissenschaften 5: 335–378.
Wagner, A. 1857. Neue Beiträge zur Kenntnis der fossilen Säugthier-Ueberreste von Pikermi. Abhandlungen Der Bayerischen Akademie Der Wissenschaften 8: 109–158.
Williams, S.A., T.C. Prang, M.R. Meyer, G.A. Russo, and L.J. Shapiro. 2020. Reevaluating bipedalism in Danuvius. Nature 586: E1–E3. https://doi.org/10.1038/s41586-020-2736-4.
Xue, X.-X., and M.C. Coombs. 1985. A new species of Chalicotherium from the Upper Miocene of Gansu Province, China. Journal of Vertebrate Paleontology 5: 336–344. https://doi.org/10.1080/02724634.1985.10011870.
Zapfe, H. 1979. Chalicotherium grande (Blainville) aus der miozänen Spaltenfüllung von Neudorf an der March (Devinska Nova Ves), Tschechoslowakei. Neue Denkschriften Des Naturhistorischen Museums in Wien 2: 1–282.
Zapfe, H. 1989. Chalicotherium goldfussi Kaup aus dem Vallesien vom Höwenegg im Hegau (Südwestdeutschland). Andrias 6: 117–126.
Acknowledgements
We would like to thank all the participants of the excavations in Hammerschmiede over the last years. We would like to thank S. Roussiakis, G. Theodorou (Athens, Greece), M. Rummel, J. Hendriks (Augsburg, Germany), L. Costeur (Basel, Switzerland), O. Sandrock, M. Blume, and M. Kollbacher (Darmstadt, Germany), A. Pictet (Lausanne, Switzerland), C. Argot (Paris, France), E. Amson (Stuttgart, Germany), I. Werneburg (Tübingen, Germany), and U. Göhlich (Vienna, Austria) for providing access to comparative material. We also thank P.-O. Antoine (Montpellier, France), M. Coombs (Massachusetts, USA), A. Matzke (Tübingen, Germany), and N. Spassov (Sofia, Bulgaria) for providing literature and fruitful discussions. We acknowledge the support of the 3D Imaging Lab of the Senckenberg Centre for Human Evolution and Palaeoenvironment (SHEP) and the Eberhard Karls University Tübingen, Germany, for instrument use, and scientific and technical assistance. We wish to thank G. S. Ferreira and C. Kyriakouli (SHEP) for assistance in generating and processing the μCT-scan. We also thank the Editor-in-Chief Michael Rasser, the Handling Editor Irina Ruf and two anonymous reviewers for their valuable comments. This research received support from the SYNTHESYS+ project (http://www.synthesys.info/) which is financed by European Community Research Infrastructure Action under the H2020 Integrating Activities Programme, Project number 823827 (AT-TAF-TA3-9, AT-TAF-TA4-29, and FR-TAF-TA4-71 to P.K.).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Irina Ruf.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kampouridis, P., Hartung, J., Lechner, T.S. et al. Disparate occurrences of a chalicotheriine and a schizotheriine chalicothere (Mammalia, Chalicotheriidae) at the Late Miocene hominid locality Hammerschmiede (Germany). PalZ 98, 313–329 (2024). https://doi.org/10.1007/s12542-024-00685-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-024-00685-x