Abstract
Despite two centuries of fossils collecting, no cetacean remains from the Oligocene marine deposits of the Mainz Basin (western Germany) have ever been reported. Here, we describe a possible mysticete tooth from the sand pit of Eckelsheim, which exposes high energy deposits belonging to the Rupelian Alzey Formation. The latter has yielded a rich assemblage of vertebrates and invertebrates, but so far, only one marine mammal in the form of the sirenian Kaupitherium. The whale tooth in some ways resembles the m2 of Llanocetus from the latest Eocene of Seymour Island, Antarctica. If the find from the Mainz Basin is not a regionally evolved form, Llanocetus, which is known from the South Atlantic, could have migrated through the Atlantic realm during the early Oligocene. It cannot be excluded that the tooth represents a more widely occurring lineage, neither endemic nor necessarily related to llanocetids, that—given the generally poor Rupelian record—has not been well documented yet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cetaceans of the early Oligocene
The Eocene–Oligocene transition coincides with a minor extinction event affecting both marine and terrestrial organisms. The installation of the Antarctic circumpolar coldwater currents contributed to the global cooling and the formation of the first Antarctic ice shield (Fordyce 1977; Kennett 1977; Jicha et al. 2009; Katz et al. 2011). The dramatic cooling of ocean deep waters after the Eocene–Oligocene boundary is well documented by oxygen isotopes in Paleogene benthic foraminifers (Shackleton 1986; Zachos et al. 1996) and otoliths (Ivany et al. 2000).
Cetaceans are documented by only scarce findings in the early Oligocene. The lowering of the sea level could either limited deposition of fossil cetaceans or destroyed already deposited specimens because of resulting more widespread erosion (Fordyce 2003; Uhen and Pyenson 2007; Uhen 2010).
Odontocetes, are represented besides indeterminable remains predominantly by the arising Agorophiidae (Whitmore and Sanders 1976, Fordyce 1981; Godfrey et al. 2016), Xenorophidae (Uhen 2008; Boessenecker et al. 2017a, b), Simocetidae (Fordyce 2002), and Ashleycetidae (Sanders and Geisler 2015)—as well as some genera of as yet undetermined higher ranked assignment: Mirocetus (Mchedlidze 1970, 1984), Ediscetus (Albright et al. 2018), Ankylorhiza (Boessenecker et al. 2020), and a possible early evidence of ?waipatiids (aff. Waipatia sp.; Boessenecker and Boessenecker 2019).
Mysticetes appeared in the early Priabonian with the toothed forms Mystacodon selensis, found in the sediments of the Yumaque Formation in Peru (Lambert et al. 2017; de Muizon et al. 2019), followed by Llanocetus denticreantus, also documented from the southern hemisphere (La Meseta Formation of Seymour Island, Antarctica; Mitchell 1989; Fordyce and Marx 2018).
Only poor fossil remains of a probable mysticete are known with Willungacetus aldingensis from the early Oligocene of Australia (Pledge 2005). Further fossil mysticetes were found in North America: the aetiocetid Fucaia buelli (Marx et al. 2015) from the early Oligocene of Washington State, the first toothless eomysticetid Micromysticetus rothauseni from a Rupelian lagoonal/shallow subtidal horizon in the Ashley Formation of South Carolina (Sanders and Barnes 2002), and the toothed species Coronodon havensteini from a latest Rupelian coastal horizon in the Ashley Formation (Geisler et al. 2017). From Japan, the eomysticetid Yamatocetus canaliculatus has been described from Kyushu (Okazaki 2012) and is dated to be of latest Rupelian age (Marx and Fordyce 2015).
Geology
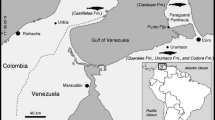
The Mainz Basin (Fig. 1) comprises the area between the Rhenish Schiefergebirge (Hunsrück and Taunus) in the north, the northern part of the Upper Rhine Graben in the east, the Haardt in the south, and the Palatinate Forest in the west (Grimm et al. 2011). It represents a remaining plateau in the overlap area of ortho- and paragraben in the intersectional zone of the Upper Rhine Graben and the Permo-Carboniferous Saar-Nahe Depression (Rothausen and Sonne 1984).
Map of central Europe showing the principal palaeogeography for the late early Oligocene (late Rupelian) with the gulf-like character of the Upper Rhine Graben with adjacent Mainz Basin at this time, after compiled data from Ziegler (1990), Berger et al. (2005), Grimm (2006), Pirkenseer et al. (2013). The point of discovery of the whale tooth is circled in red
During the early Oligocene, tectonic events created a continuous sedimentation area from the North Sea over the Hessian Depression through the Mainz Basin and Upper Rhine Graben (Grimm 2005; Grimm and Grimm 2005; Grimm et al. 2011). Sea-level rises and contemporary subsiding of the Upper Rhine Graben let to wide transgressions and the forming of an expanded embayment with islands and peninsulas at the place of the Mainz Basin (Grimm et al. 2011; Kuhn 2012; Schäfer 2012).
Sediments of the Alzey Formation (former “Unterer und Oberer Meeressand “) contain coarse-grained sands and gravels and were deposited in the later Rupelian age (second Rupelian transgression, after Hardenbol et al. 1998). They display predominantly beach sediments originating from underlying Palaeozoic rocks, such as Permian sandstones, arkoses, fine conglomerates, and also rhyolithic volcanites and tuffites (Grimm et al. 2000, 2011). Occasionally, also shell layers, carbonate, and silt are observable. Radiometric dating (87Sr/86Sr) on bivalve shells yielded an age of 30.1 ± 0.1 Ma (Grimm and Grimm 2003).
The Alzey Formation (Fig. 2) at the Eckelsheim locality, the Steigerberg, has been well investigated in the past regarding sedimentology, sequence stratigraphy, palaeoenvironment, and biostratigraphic background (Grimm and Grimm 2005). The sand pit of Eckelsheim represents a breakwater cliff containing high energy deposits, with subaqueous slump structures and erosion channels suggesting formation during tides and storms (Neuffer et al. 1978; Hartkopf and Stapf 1984; Grimm and Grimm 2005; Kuhn 2008). The bedrock surfaces here allow the interpretation of water flows (Rousse et al. 2012). Micro- (pollen and spores from adjacent core holes) and megafloral (palm trees from the Eckelsheim pit) remains suggest deposition in a subtropical humid climate resembling that of Florida today (Schaarschmidt 1982; Pross et al. 1998; Schindler and Poschmann 2001).
Fossil assemblage of the Alzey Formation
The sands of the Alzey Formation at the Steigerberg are extremely fossiliferous and contain a diverse subtropical-Mediterranean marine fossil assemblage comprising thousands of specimens in likely more than 600 species, as well as washed-in terrestrial remains (Grimm and Grimm 2005).
The Eckelsheim locality alone yielded an enormous variety of invertebrate fossils (e.g., Doebl and Sonne 1973; Neuffer et al. 1978; Vávra 1983; Gürs 1995; Nungesser 2021).
The vertebrate fauna is just as highly diverse. Generally, more than two dozens elasmobranch species are documented in the near-coastal Alzey Formation by their teeth so far (Boy 1975, von der Hocht 1978, Kuhn 2008, Schindler 2011, Nungesser 2021) including the top predator Otodus (Carcharocles) angustidens with an estimated length up to 10 m, and the largest fish, the basking shark Keasius parvus, known by many teeth (von der Hocht 1978) as well as preserved and connected gill rakers from siltic layers passing over to the deeper marine Bodenheim Formation (Reinecke et al. 2015).
Over 100 teleost species are proven in the Alzey Formation by their otoliths with alone 53 species in Eckelsheim (Syring 2015).
Reptiles are rare in the Alzey Formation. Crocodiles, addressed as marine-semiaquatic predators, are documented by teeth (Berg 1984; Schindler 2013a). One not yet published crocodile tooth was discovered from the Eckelsheim locality. A little more common remains in the Alzey Formation are marine Cheloniidae (shell, skull and scapular remains of Rupelchelys breitkreutzi) and also Trionychidae (dermal plates of Trionyx boulengeri) and possibly Emydidae, turtles, predominantly adapted to freshwater and brackish environment deposited in the coastal sediments (Lepsius 1883; Berg 1984; Karl and Tichy 1999; Schindler 2013b; Nungesser 2021).
Further amniote remains are the rarely found bones of birds. Documented in the Alzey Formation so far are one osprey claw of the Accipitridae (Schindler and Mayr 2004; Mayr 2006) and several long bones including femora and tarsometatarsus of the extinct Diomedeoididae, a kind of sea birds of the Procellariiformes (Schindler 2013c).
The marine mammals had been known up to date only by sea cows (Sirenia: Dugongidae). Isolated bones, partially articulated skeletal remains and skeletons were discovered and documented (e.g., Lepsius 1882; Bahlo and Tobien 1982; Rothausen and Sonne 1984; Voss and Hampe 2017; Nungesser 2021), and even from Eckelsheim two sympatric living species Kaupitherium gruelli and K. bronni are proven (before revision of Voss and Hampe 2017 named as “Halitherium schinzii”).
Several inwashed terrestrial mammals had been found in the Alzey Formation as well, such as hyaenodonts (Apterodon: Bahlo and Tobien 1982), carnivores (Amphicynodon: Schindler 2013b), rodents (Cricetidae: Bahlo and Tobien 1982; Theridomyidae: Schindler et al. 2009), perissodactyls (Rhinocerotidae: Schindler et al. 2009; Schindler and Schneider 2020), and artiodactyls (Anthracotherium, Bachitherium: Bahlo and Tobien 1982, Schäfer 2012).
Collecting fossils from Eckelsheim has a long history. Walchner (1850) mentioned this locality for the first time, followed by Braun (1851a, b) who began with lists and short descriptions of the fossil content. Schopp (1888) recognised the special nature of the site and presents a detailed description of the various profiles of the sand pit. During the twentieth century, sand mining was intensified and the Eckelsheim pit was enlarged under different owners. Since the second half of the twentieth century onwards, geologists of the Johannes Gutenberg-Universiät Mainz and many amateur geologists recovered large quantities of fossil material from various facies of the sand pits of the Steigerberg at Eckelsheim.
Materials and methods
The tooth presented here was discovered by Mr. Dirk Gille, an autodidact and specialist for “Meeressand” fossils, after sieving about one tonne of sediment (in fractions of 5 and 10 mm) in search of shark teeth. Prior to study the Eckelsheim tooth was prepared (post-treatment) to remove grains sand and to stabilise the root. The tooth was then measured with an electronic digital Kraftixx caliper.
Pictures were taken with a Sony alpha 7R III camera using both Canon and Sony macro lenses. To receive an optimised depth of focus, the stacking method with Novoflex Castel micro stack sleds was applied (between 9 and 21 stacking steps). Capture One and Helicon Focus image processing software were utilised for this task.
Outlines of comparative objects were drawn in ink based on published figures and original fossils.
Institutional abbreviations
CCNHM—Mace Brown Museum of Natural History, College of Charleston, U.S.A.
KMNH—Kitakyushu Museum of Natural History and Human History, Fukuoka Prefecture, Japan.
MB—Museum für Naturkunde Berlin.
MNHN—Muséum national d’Histoire naturelle, Paris.
OU—Geology Museum, University of Otago, Dunedin, New Zealand.
NHMMZ—Landessammlung für Naturkunde Rheinland-Pfalz, Naturhistorisches Museum Mainz.
UCMP—University of California Museum of Paleontology, Berkeley, U.S.A.
UWBM—Burke Museum of Natural History and Culture, Seattle, U.S.A.
USNM—United States National Museum, Smithsonian Institution, Washington, D.C.
Systematic Palaeontology
Class Mammalia Linnè, 1758.
Order Cetacea Brisson, 1762
Clade Neoceti Fordyce and de Muizon, 2001
Suborder Mysticeti Gray, 1864
Mysticeti indet.
Material. NHMMZ PW2022/5017-LS, a single, probable posterior right mandibular tooth.
Occurrence. Sand pit of Eckelsheim, breakwater cliff at Steigerberg hill, Rhineland-Palatinate State, SW-Germany; Alzey Formation, Rupelian, late early Oligocene.
Description. The tooth (Figs. 3, 4a) has a tricuspid crown with one principal cusp and two smaller posterior (distal) denticles. We use the terms ‘cusp’ and ‘denticles’ here because of uncertain homology with the cusps of other mammal teeth (proto-, metaconid, etc.).
Illustrations of selected teeth used in the comparisons. a The probable right mandibular tooth of an yet undeterminable mysticete from Eckelsheim, buccal view, NHMMZ PW2022/5017-LS. b Left m2 of Llanocetus denticrenatus (from Fordyce and Marx 2018), lingual view, USNM 183022. c Cheek tooth of ?Metasqualodon symmetricus (from Okazaki 1982), ?lingual view, KMNH VP 000.005. d Left p2 of Coronodon havensteini (from Boessencker et al. 2023), lingual view, CCNHM 108. e Left m2 of the basilosaurine Cynthiacetus peruvianus (from Martínez-Cáceres et al. 2017), lingual view, MNHN.F.PRU10. f Left penultimate postcanine mandibular tooth of the aetiocetid Salishicetus meadi (from Peredo and Pyenson 2018), lingual view, UWBM 50004. g Left p4 of the early pinniped Enaliarctos mealsi (from Poust and Boessenecker 2018), lingual view, UCMP 114474. h Left p4 of the mustelid Plesictis robustus (MB.Ma. 29,335, Oligocene/Miocene of France), lingual view. i Right mandibular cheek tooth of the platanistoid Waipatia maerewhenua (from Fordyce 1994), lingual view, OU 22095. Not to scale
Cusp and denticles are decreasing in height towards the posterior (Fig. 3a, b). The principal cusp has a broken apex, revealing a somewhat rounded to slightly lanceolate occlusal outline (Fig. 3c). The anterior denticle is complete with a nearly a right-angled triangular apex; the posterior denticle is broken in half (Fig. 3b−d). In occlusal view, cusp and denticles are roughly aligned, with only the posterior denticle deviating slightly buccally (Fig. 3d). Cusp and denticles are straight and slightly inclined posteriorly (Fig. 3a, b).
The anterior (mesial) edge of the principal cusp is straight but nodular which looks like slightly crenulated (Fig. 3a, e). The posterior cutting edge is less crenulated. Both anterior and posterior cutting edges of the lanceolate-shaped anterior denticle are finely crenulated (Fig. 3d). The posterior denticle is also lanceolate.
Both, buccal and lingual sides of the crown show a weakly pronounced ornament of vertical carinae at the principal cusp, and a combination of vertical and oblique carinae are developed on the following denticles (Fig. 3a, b). Generally, the enamel surface is smooth—there are no directional wear facets determinable. A few narrow vertical cracks are present.
The base of the crown is formed by a low cingulum, less than or equal to 1 mm, with an arched course and with ascending arch below the principal cusp in lingual and buccal view (Fig. 3a, b). Here, the ectocingulum is dorsoventrally a little higher (Figs. 3b, 4a). The entocingulum is damaged below the anterior denticle. A rhombic piece has broken away. The elevation at the mesial and distal points of the cingulum gives the impression of the presence of two tiny accessory cusplets (Fig. 3a, b, d, e).
The base is double-rooted, separated from each other and fused directly below the crown through a narrow isthmus (Fig. 3a, b). Both roots are directed nearly straight downwards—there is no curvature. The inner margins of the roots describe an angle of about 35° to each other. The very ends of the roots are broken. The pulp canals are visible in basal view (Fig. 3f). The anterior root has more or less an oval cross-section whereas the posterior root is somewhat trapezoid in outline.
The measurements are as follows:
16.15 mm: Maximum height (anterior root to principal cusp; mind the broken principal cusp).
12.47 mm: Maximum mesio-distal length at the level of the cingulum.
9.87 mm: Maximum mesio-distal length at the level of the preserved root ends.
5.27 mm: Maximum width at the lingual pronunciation of the cingulum above posterior root.
7.04 mm: Mesiodistal length of crown basis of principal cusp.
3.87 mm: Mesiodistal length of crown basis of anterior denticle.
2.94 mm: Mesiodistal length of crown basis of posterior denticle.
3.63 mm: Median distance between the roots.
Comparisons. The discovery of the tooth was a big surprise, which had been expected at some point, but which seemed unlikely due to the very good fossil record of the fossil-bearing beds in the Mainz Basin so far. Although mammal teeth are generally considered to be very characteristic, a taxonomic classification is extremely difficult in this case. It is just a single specimen that could not clearly be classified at a first glance because teeth of other mammals (terrestrial) were also found here in the coastal deposits of the Alzey Formation.
Before we talk about the similarities with representatives of the Cetacea, let us first clarify what we think it is not. We exclude a belonging to the carnivores. From the Mainz Basin, amphicyonids are documented from the younger Corbicula and Hydrobia beds (today: Rüssingen to Frankfurt Formation; late Oligocene/early Miocene) with Cynelos, Ysengrinia, Haplocyonoides and the mustelid Plesictis (Rothausen and Sonne 1984; Rothausen 1988; Morlo 1996).
When looking at the p4 of a Cynelos, however, it is noticeable that, in contrast to the tooth from Eckelsheim the anterior (mesial) edge is distinctly concave with an indicated kink at the lower third of its course and not straight (see Peigné and Heizmann 2003: fig. 6, 7, pls. 2, 3), also documented in Haplocynonoides (Peigné and Heizmann 2003: fig. 23, 24, pl. 7). Other amphicyonids, like Ysengrinia have a small anterior cusp in front of the principal cusp (Peigné and Heizmann 2003: fig. 20).
The morphology of a carnivoran p4, amphicyonids and mustelids (e.g., Tomiya and Tseng 2016: fig. 6; Wang et al. 2022: figs. 3D, 11A, B, 73C, D), is also different from the tooth from Eckelsheim by having a posterior accessory cuspulid with or without a notch very close at the distal edge of the principal cusp.
The cingulum of the posterior premolars is differently developed in such carnivores, often higher and more distinct, often stronger developed anteriorly than posteriorly. Morlo (1996: pl. 4, fig. 31), for example, described the cingulum anteriorly or anterolingually and posteriorly thickened like a bulge in the mustelid Stromeriella (= Plesictis in Rothausen and Sonne 1984: pl. 21, fig. 109). There is no ornamentation of vertical striae developed in the carnivores (Fig. 4h).
Pinnipeds are also out of the question as an assignment for the tooth from Eckelsheim. Pinnipeds have actually been known since the Rupelian (Tate-Jones et al. 2020). The early form Enaliarctos (Fig. 4g) from the northern Pacific realm has differently shaped premolars. Here, the p3 and p4 develop a prominent paraconid in form of a trenchant protuberance or a blunt cusplet anterior to the principal cusp (e.g., Berta 1991; Poust and Boessenecker 2018).
The assumption that the tooth from Eckelsheim is of cetacean origin was soon confirmed. At first glance, the multicuspid crown with a higher number of denticles is notable, a character that was already established in the archaeocete whales during the Eocene and continues in basal odontocetes and mysticetes. Basilosaurids possess also double-rooted premolars and molars but are clearly excluded because of the presence of an arched crown having a number of accessory denticles in front and behind of the principal denticle and the lack of any kind of cingulum in the upper molars (Kellogg 1936; Uhen 2004; Martínez-Cáceres et al. 2017). And the lower distal molars, m2 (Fig. 4e) and m3, which lack anterior accessory denticles are somewhat directed towards the coronoid process (e.g., Kellogg 1936: pl. 13, fig. 3f for Zygorhiza, Uhen 2004: fig. 21 for Dorudon, Martínez-Cáceres et al. 2017: figs. 28, 30, 33 for Cynthiacetus). Further differences are the lack of enamel ornament and the narrow root spacing in basilosaurids.
An assignment to the Odontoceti is also unlikely. Representatives with a multicuspid crowns clearly have a different root morphology and different ornamentation patterns. Double-rooted posterior teeth are found in at least in stem odontocetes and squalodontids, Prosqualodon, Agorophius, Simocetus, and Waipatia (de Muizon 1987; Lambert 2005; Gaetán et al. 2019).
The squalodontids, known nearly worldwide from late Oligocene and the early to middle Miocene deposits, developed no cingulum and often show a strong, rugose ornament on the enamel surface. Additionally, their teeth are characterised by distinctly lower incisions between the denticles of the crown and a high, below the crown closed root with two prongs of no more than the same height as the root base, as documented for specimens from many places of the world by, for example, Kellogg (1923: pl. 7, Squalodon calvertensis), Rothausen (1968a: pl. 11, 12, Eosqualodon langewieschei), Pilleri (1985: pl. 1–5, Squalodon bariensis; pl. 7, 10–13, 16–20, Squalodon bellunensis), Okazaki (1988: pl. 1, Squalodon sp.), Dooley (2003: fig. 2, Squalodon atlanticus), Hampe et al. (2019: fig. 1, Eosqualodon langewieschei). The middle Miocene Neosqualodon (Rothausen 1968b; Barucco 2012) from Italy shows swollen, finger-like denticles with larger interdentical spaces with no concordance with the Eckelsheim tooth. Also “Squalodon” gambierensis from the late Oligocene of South Australia (Glaessner 1955: fig. 5), recently identified as belonging to the Kekenodontidae is different to the tooth discovered from the Mainz Basin in having a more or less bilateral symmetric, fan-like crown in lingual and buccal view with a central main denticle flanked by a number of mesially and distally arranged denticles (see also Corrie and Fordyce 2022: p. 1639).
A little different is the situation regarding Waipatia. A few similarities are observable. Therefore, Waipatia, known to date with two species, W. maerewhenua and W. hectori from the late Oligocene of New Zealand (Fordyce 1994; Tanaka and Fordyce 2015), developed a strong lingual cingulum on their teeth. This cingulum is higher than that developed in the Eckelsheim tooth. And, the cingulum of Waipatia display an ornament of strong parallel cristae (Fig. 4i) in contrary to the smooth cingulum in the Eckelsheim tooth which then also runs around the entire tooth. Cheek teeth of Waipatia have the lack of anterior denticles in common. Waipatia maerewhenua has two or three posterior denticles (Fordyce 1994: fig. 13C, D), W. hectori two (Tanaka and Fordyce 2015: fig. 5A–C). However, the crown of the lower posterior teeth are recurved in Waipatia maerewhenua and even more in W. hectori. The crown surfaces of W. maerewhenua show an ornament of vertical cristae rather than carinae, in which the lingual ornament is stronger developed. The buccal ornament of Waipatia is indistinct. The double-rooted posterior teeth of Waipatia maerewhenua show a fused base for at least one third of their length (Fig. 4i; Fordyce 1994: fig. 13C, D). The teeth of W. hectori are situated in the jaw bone and their roots are not completely visible (Tanaka and Fordyce 2015: fig. 5B, C). No lower teeth are known from a yet unnamed waipatiid from the late Oligocene of Victoria, Australia (Fitzgerald 2016). Preserved upper teeth reveal waipatiid-characteristic features such as more distinct ornament of the lingual crown surface, strong lingual cingulum with strong ridges, but with blunt, worn principal denticle. Bases are double-rooted but here strongly recurved and fused about two-thirds of their length (Fitzgerald 2016: fig. 5E–L). Crenulated edges are not described at the denticles in Waipatiidae.
However, the similarities to some of the teeth of early baleen whales in particular are intriguing. The most similarities could be found out with posterior teeth of the dentary of Llanocetus denticrenatus from the latest Eocene of Seymour Island, Antarctica, the second-oldest described mysticete so far (Fordyce and Marx 2018). Llanocetus was comparable in size to an adult living minke whale (Peredo et al. 2017). The general morphology of the m2 of Llanocetus (Fig. 4b) resembles that of the Eckelsheim tooth, but is at least of more than double size (e.g., 32 mm crown length instead of 12 mm in the Eckelsheim tooth). Llanocetus has with the tooth from the Mainz Basin a low cingulum and the presence of sharp edges at the cusps/denticles in common (Fordyce and Marx 2018: figs. 2B, C, S9). The Llanocetus m2 shows also an enamel ornament of fine vertical carinae (Fig. 4b) both lingually and buccally developed. The root as preserved in the m2 is nearly identical to that seen in the Eckelsheim tooth. It is open and not fused up to the beginning of the crown. Different is the number of posterior denticles which is higher in Llanocetus (four in the m2).
Llanocetus was undoubtedly a very large mysticete. Metasqualodon, presumably a close relative of Llanocetus, was much smaller but is not well known or yet fully described (Geisler et al. 2017). We do not know about the variability of teeth in Metasqualodon but some teeth affiliated to Metasqualodon symmetricus from the lower Miocene Ashiya Group of North Kyushu in Japan show some similarities with Llanocetus resp. the tooth from Eckelsheim (Okazaki 1982: textfig. 1). A fragmentarily preserved posterior cheek tooth assigned to Metasqualodon have cusps/denticles with weakly but clearly developed edges and an ornament of vertical carinae (Fig. 4c) likely similar to that seen in the Eckelsheim tooth. However, a cingulum is not clearly determinable and not described by Okazaki. Different is again the number of accessory posterior denticles with four in the Metasqualodon tooth.
The late Eocene Mystacodon selensis has predominantly damaged teeth preserved with very limited comparison options. de Muizon et al. (2019) describe the lack of evidence of a cingulum which is different to the Eckelsheim specimen.
At the first glance, the p2 of the basal mysticete Coronodon havensteini from the latest Rupelian of South Carolina looks quite similar to the tooth from Eckelsheim (Fig. 4d). However, the Coronodon tooth in question possesses two tiny denticles anterior to the principal cusp. Other differences are the lack of a cingulum, a fused root below the crown and denticles which are standing closer together but posteriorly decreasing in height (Geisler et al. 2017: fig. 2A as p1, Boessenecker et al. 2023: figs. 26–28). There is a kind of “pseudoserration” on the edges of the principal cusp and adjacent denticles in the description of Boessenecker et al. (2023: fig. 42) which can be correlated with that we named “finely crenulated” for the Eckelsheim tooth. Two recently new described species of Coronodon are presented Boessenecker et al. (2023) by from the younger Chandler Bridge Formation (late Oligocene). All coronodontids have in difference to the Eckelsheim specimen double-rooted postcanine teeth which are characterised by a generally long root isthmus and equipped with a number of mesial denticles on premolars.
The mammalodontid Janjucetus hunderi from the late Oligocene of Victoria/Australia differ greatly from the Eckelsheim tooth in having crowns of cheek teeth with distinctly distally bended denticles and a strong ornament of coarse longitudinal ridges (Fitzgerald 2006: fig. 2). A cingulum is not developed and the two roots seem to possess here a very narrow space in between. Although poorly preserved with worn tooth crowns, it can be stated that Mammalodon colliveri, also late Oligocene of Victoria, differ also from the Eckelsheim tooth with the lack of a cingulum, probably strongly ornamented crowns as seen at its base and very narrow-spaced roots or even fused along their lengths that is emergent from the alveoli (Fitzgerald 2010: figs. 37, 38).
Teeth of Aetiocetus are quite small and generally simpler in their morphology, tending to be more homodontous (Barnes et al. 1994; Peredo et al. 2017) with no similarities to the tooth discovered from Eckelsheim.
Documented teeth of Aetiocetus cotylalveus belong probably to the maxillae. They are mostly worn and a number of denticles are not detectable (Emlong 1966: fig. 9). The figured specimen in Peredo et al. (2017: fig. 3D) shows a triangular and pointed crown with few tiny accessory denticles on a single, partly inflated root which is completely different to the Eckelsheim tooth.
Mandibular teeth of Aetiocetus weltoni, like A. cotylalveus also from the late Oligocene of Oregon, appear to be single-rooted and have mostly damaged crowns (Deméré and Berta 2008). Also here, the better preserved crowns are more or less triangular in shape with a sharply pointed apex and anterior accessory denticles that occur in the more posterior teeth.
Aetiocetus polydentatus from the late Oligocene of Hokkaido, Japan, has also single-rooted teeth with sharply pointed crowns. Accessory tiny denticles which look more like a serration occur in the middle part of the mandible (Barnes et al. 1994: figs. 19, 21).
All Aetiocetus teeth have a smooth enamel surface on the buccal aspect of the crown, while lingually there is an ornament of fine vertical ridges.
The teeth of the Fucaia buelli from the early Oligocene of Washington State could be placed in the same category. However, their characters are based on isolated teeth which presumably belong to the upper jaw (Marx et al. 2015: figs. 10, 11). The teeth are single-rooted with triangular crowns possessing denticles mostly on both anterior and posterior sides of the principal cusp. Another difference to the Eckelsheim tooth is, that all tooth crowns are curved lingually. The pattern of enamel ornament is similar to that developed in Aetiocetus (see above) with predominantly smooth buccal and carinae or ridges on the lingual side. Additionally, a papillated cingulum at the lingual side is notable on the posterior teeth.
Only Salishicetus meadi from the late Oligocene of Washington State and Morawanocetus yabukii from the late Oligocene of Hokkaido seem to have double-rooted posterior teeth (Barnes et al. 1994: p. 410, Peredo and Pyenson 2018: figs. 8, 9). As in other aetiocetid specimens, the tooth crowns of the Salishicetus mandibular teeth are distinctly triangular, here with tiny posterior denticles and a fused upper part of the root (Fig. 4f) really not corresponding to characters, seen in the Eckelsheim tooth.
Discussion
First of all, it must be noted that the Upper Rhine Graben, which at times shows a connection to the Tethys, transforms into a Upper Rhine Gulf during the late Rupelian between 32.0 and 28.5 Ma ago by a southern closure (Berger et al. 2005; Grimm 2006). This gulf reached at least a length of about 500 km starting at the level between the uplifts of the Rhenish Massif and Harz Mountains.
Several of the 80–90 species of living cetaceans (Rice 1998; Hoyt 2005; Fordyce 2018) are found in today’s gulf regions depending of geographic distribution and habitat. The Gulf of Aqaba (length: 160 km) which opens at Straits of Tiran between the Sinai and Saudi Arabia, terminating in the North at Eilat (Israel) and Aqaba (Jordan), the short Gulf of Corinth (length: 127 km), a semi-enclosed embayment in central Greece between mainland Greece in the North and the Peloponesse in the South, and the Gulf of California (length: 1,126 km) which has more than double length of that of the reconstructed Upper Rhine Graben are examples of similar structured long and narrow gulfs like the late Rupelian Upper Rhine Gulf. One can conclude, although evaluation methods varied, that the larger the extension and volume of the gulf is, the higher is the number and frequency of species in that water body (see Pardo et al. 2013; Bearzi et al. 2016; Costa et al. 2019). Therefore, cetaceans would under these premises also have been expected in the deposits of the Upper Rhine Graben.
Cetaceans are distributed worldwide today and enter all oceanic areas. In the early Oligocene, their diversity was generally lower (Uhen 2007; Uhen and Pyenson 2007; Marx et al. 2016) and their distribution was basically only pachy—fossils recorded from Oregon, Washington State, South Carolina, Mississippi, Denmark (Jutland), and Azerbaijan in the Northern Hemisphere and New Zealand, South Australia, and Cabinda (Angola) in the Southern Hemisphere (data were downloaded from the Paleobiology Database on 15 February, 2022, using the following parameters: taxon = Cetacea, time interval = Rupelian; Uhen et al. 2022). Marx and Fordyce (2015) argued that lower Oligocene deposits had been poorly sampled so far which finally reflects a globally deficient record.
Rothausen (1967) suspected climatic reasons for the lack of cetaceans in the area of question in form of a warm-water barrier that prevented the further northern advance of cetaceans, in this special case, he wrote about the Squalodontoidea, the dominant cetacean group of Oligocene time. In a later study, Walliser et al. (2015) could reconstruct a water surface temperature in the Mainz Basin ranging between 12.3° and 22.0 °C, based on δ18O isotopes of Glycymeris shells and on δ18OPO4 isotopes of Kaupitherium teeth. Still, the only occurrences of cetaceans from the Rupelian to date from the North Sea are those of a ?squalodontid (Ravn 1926).
Therefore, the assumed low diversity and dispersal in the European waters can surely be considered to be a reason why no cetacean remain occurred before in the Mainz Basin/Upper Rhine Graben. A deficient sampling can be excluded because of the long tradition of collecting in that area (see above). A warm-water barrier as a reason for the lack of cetaceans in the record so far in the Mainz Basin or Upper Rhine Graben on the basis of water temperature is not convincing and the North Sea, with its direct connection to the Upper Rhine Graben, obviously had a very low abundance of cetaceans in the Rupelian.
Bianucci et al. (2011) postulate migration routes of southern hemisphere representatives (mammalodontids, waipatiids) to the Parathetys and Mediterranean realms (“Mediterranean-Indopacific Seaway”) during the late Oligocene because of related finds in deposits of the Maltese Islands. Assuming that the Eckelsheim specimen belongs to or is a descendant of Llanocetus, which is essentially known from the South Atlantic, we must assume that a migration route through the entire Atlantic realm must have been possible during the early Oligocene, independent of changes in oceanic gateways in context with global tectonics and climatic alterations in that epoch.
Life behaviour of the Eckelsheim cetacean, for example, regarding nutrition is speculative at this stage. As mentioned above, there are no attrition traces definable on the Eckelsheim tooth and a raptorial feeding uncertain. The principal cusp has a broken apex, probably a result of damage during sedimentation processes.
Conclusions
The here presented find is the first report of a cetacean in the Mainz Basin. This single tooth of the later Rupelian shows greatest similarities with mandibular cheek teeth of the early toothed mysticete Llanocetus denticrenatus which is known from the latest Eocene of the Antarctic region and which is the second-oldest described mysticete so far (Fordyce and Marx 2018).
Cetaceans seem to be extremely rare in the Mainz Basin resp. Upper Rhine Graben despite centuries of intense fossil hunting. One strong reason for this may have been the generally low diversity and dispersal in the North Atlantic and its adjacent oceanic basins during the Rupelian (see Uhen et al. 2022). Another reason might lie in the preservation biases. The global cooling after the Eocene–Oligocene transition resulted in a marine regression. This could have either limited the deposition of cetaceans or destroyed already deposited specimens as erosion expanded.
Considering the rich faunal content of the Mainz basin, here of the Eckelsheim site (as presented in the chapter “Faunal assemblage of the Alzey Formation”), a lack of food resources would not be explicable even for a whale, although finally we do not know how many of the species described from the Alzey Formation could plausibly serve as whale food and that we have to date no idea about the diet of the species to which the tooth belonged.
Data availability
All data are provided in the text and figures. The described specimen is stored in a publicly accessible collection.
References
Albright III, L.B., A.E. Sanders, and J.H. Geisler. 2018. An unexpectedly derived odontocete from the Ashley Formation (upper Rupelian) of South Carolina, U.S.A. Journal of Vertebrate Paleontology 38 (4): e1482555.
Bahlo, E., and H. Tobien. 1982. Bestandsaufnahme der Säugetiere im „prä-aquitanen“ Tertiär des Mainzer Beckens. Mainzer geowissenschaftliche Mitteilungen 10: 131–157.
Barnes, L.G., M. Kimura, H. Furusawa, and H. Sawamura. 1994. Classification and distribution of Oligocene Aetiocetidae (Mammalia; Cetacea; Mysticeti) from western North America and Japan. The Island Arc 3 (4): 392–431.
Barucco, D. 2012. Neosqualodon, il mistero dei cetacei preistorici degli Iblei. Agorà 40: 52–56.
Bearzi, G., S. Bonizzoni, N.L. Santostasi, N.B. Furey, L. Eddy, V.D. Valavanis, and O. Gimenez. 2016. Dolphins in a scaled-down Mediterranean: The Gulf of Corinth’s odontocetes. In Advances in Marine Biology, Volume 75: Mediterranean Marine Mammal Ecology and Conservation, ed. Notarbartolo di Sciara, G., M. Podestà, and B.E. Curry, 297–331. Amsterdam: Academic Press.
Berg, D.E. 1984. Amphibien und Reptilien im “prä-aquitanen” Tertiär des Mainzer Beckens. Mainzer geowissenschaftliche Mitteilungen 13: 115.
Berger, J.-P., B. Reichenbacher, D. Becker, M. Grimm, K. Grimm, L. Picot, A. Storni, C. Pirkenseer, C. Derer, and A. Schaefer. 2005. Paleogeography of the Upper Rhine Graben (URG) and the Swiss Molasse Basin (SMB) from Eocene to Pliocene. International Journal of Earth Sciences 94 (4): 697–710.
Berta, A. 1991. New Enaliarctos* (Pinnipedimorpha) from the Oligocene and Miocene of Oregon and the role of “enaliarctids“ in pinniped phylogeny. Smithsonian Contributions to Paleobiology 69: 1–33.
Bianucci, G., M. Gatt, R. Catanzariti, S. Sorbi, C.G. Bonavia, R. Curmi, and A. Varola. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios 44 (6): 549–585.
Boessenecker, R.W., and S.J. Boessenecker. 2019. Paleontology of the “Ashley Phosphate Beds” of Charleston: Insights from Northbridge Park, Charleston, South Carolina. In Field excursions in the Carolinas: Guides for the 2019 GSA Southeastern Section Meeting, ed. Chadwick, J., and S.C. Jaume. Geological Society of America Field Guide 53: 1–8.
Boessenecker, R.W., D. Fraser, M. Churchill, and J.H. Geisler. 2017a. A toothless dwarf dolphin (Odontoceti: Xenorophidae) points to explosive feeding diversification of modern whales (Neoceti). Proceedings of the Royal Society B 284: 20170531.
Boessenecker, R.W., E. Ahmed, and J.H. Geisler. 2017b. New records of the dolphin Albertocetus meffordorum (Odontoceti: Xenorophidae) from the lower Oligocene of South Carolina: Encephalization, sensory anatomy, postcranial morphology, and ontogeny of early odontocetes. PLoS ONE 12 (11): e0186476.
Boessenecker, R.W., M. Churchill, E.A. Buchholtz, B.L. Beatty, and J.H. Geisler. 2020. Convergent evolution of swimming adaptations in modern whales revealed by a large macrophagous dolphin from the Oligocene of South Carolina. Current Biology 30 (16): 3267–3273.
Boessenecker, R.W., B.L. Beatty, and J.H. Geisler. 2023. New specimens and species of the Oligocene toothed baleen whale Coronodon from South Carolina and the origin of Neoceti. PeerJ 11: e14795.
Boy, J.A. 1975. Eine neue Selachier-Faunula aus dem mitteloligozänen Meeressand des Mainzer Beckens. Notizblatt des Hessischen Landesamtes für Bodenforschung 103: 71–101.
Braun, A. 1851a. Die fossile Fauna und Flora des Mainzer Beckens. Wirbellose Thiere. In Handbuch der Geognosie zum Gebrauche bei seinen Vorlesungen und zum Selbstudium mit besonderer Berücksichtigung der geognostischen Verhältnisse des Grossherzogthums Baden. Zweite, verbesserte und vermehrte Auflage, ed. Walchner, F.A., 1112–1141. Karlsruhe: Christian Theodor Groos.
Braun, A. 1851b. Wirbelthiere. In Handbuch der Geognosie zum Gebrauche bei seinen Vorlesungen und zum Selbstudium mit besonderer Berücksichtigung der geognostischen Verhältnisse des Grossherzogthums Baden. Zweite, verbesserte und vermehrte Auflage, ed. Walchner, F.A., 1142–1144. Karlsruhe: Christian Theodor Groos.
Brisson, A.D. 1762. Regnum animale in Classes IX. distributum, sive Synopsis methodica sistens generalem Animalium distributionem in Classes IX, and duarum primarum Classicum, Quadrupedum scilicet and Cetaceorum, particularem divisionem in Ordines, Sectiones, Genera and Species. Leiden: Haak.
Corrie, J.E., and R.E. Fordyce. 2022. A redescription and re-evaluation of Kekenodon onamata (Mammalia: Cetacea), a late-surviving archaeocete from the late Oligocene of New Zealand. Zoological Journal of the Linnean Society 196: 1637–1670.
Costa, M., M. Fumagalli, and A. Cesario. 2019. Review of cetaceans in the Red Sea. In Oceanographic and Biological Aspects of the Red Sea, ed. Rasul, N.M.A., and I.C.F. Stewart, 281–303. Cham: Springer.
de Muizon, C. 1987. The affinities of Notocetus vanbenedeni, an Early Miocene platanistoid (Cetacea, Mammalia) from Patagonia, Southern Argentina. American Museum Notitates 2904: 1–27.
de Muizon, C., G. Bianucci, M. Martínez-Cáceres, and O. Lambert. 2019. Mystacodon selenensis, the earliest known toothed mysticete (Cetacea, Mammalia) from the late Eocene of Peru: anatomy, phylogeny, and feeding adaptations. Geodiversitas 41: 401–499.
Deméré, T.A., and A. Berta. 2008. Skull anatomy of the Oligocene toothed mysticete Aetiocetus weltoni (Mammalia; Cetacea): Implications for mysticete evolution and functional anatomy. Zoological Journal of the Linnean Society 154: 308–352.
Deutsche Stratigraphische Kommission. 2002. Stratigraphische Tabelle von Deutschland 2002, ed. Menning M. and A. Hendrich, Chart 96x130 cm or Folded Chart A4. Potsdam: GeoForschungsZentrum and Frankfurt a.M.: Forsch.-Inst. Senckenberg.
Deutsche Stratigraphische Kommission. 2016. Stratigraphische Tabelle von Deutschland 2016, ed. Menning M. and A. Hendrich, Chart B0-Format 100x141 cm or Folded Chart A4. Potsdam: Deutsches GeoForschungsZentrum.
Doebl, F., and V. Sonne. 1973. Mikrofauna und -flora des Unteren Meeressandes (Rupel). 1. Sandgrube am Steigerberg bei Wendelsheim (Mainzer Becken) a. Aufschluß und Fossilinhalt. Mainzer geowissenschaftliche Mitteilungen 2: 27–33.
Dooley, A.C., Jr. 2003. A review of the eastern North American Squalodontidae (Mammalia: Cetacea). Jeffersoniana 11: 1–26.
Emlong, D. 1966. A new archaic cetacean from the Oligocene of Northwest Oregon. Bulletin of the Museum of Natural History, University of Oregon 3: 1–51.
Fitzgerald, E.M.G. 2006. A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales. Proceedings of the Royal Society B 273: 2955–2963.
Fitzgerald, E.M.G. 2010. The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zoological Journal of the Linnean Society 158: 367–476.
Fitzgerald, E.M.G. 2016. A late Oligocene waipatiid dolphin (Odontoceti: Waipatiidae) from Victoria, Australia. Memoirs of Museum Victoria 74: 117–136.
Fordyce, R.E. 1977. The development of the Circum-Antartic current and the evolution of the Mysticeti (Mammalia: Cetacea). Palaeogeography, Palaeoclimatology, Palaeoecology 21: 265–271.
Fordyce, R.E. 1981. Systematics of the odontocete whale Agorophius pygmaeus and the family Agorophiidae (Mammalia: Cetacea). Journal of Paleontology 55 (5): 1028–1045.
Fordyce, R.E. 1994. Waipatia maerewhenua, new genus and new species (Waipatiidae, new family), an archaic late Oligocene dolphin (Cetacea: Odontoceti: Platanistoidea) from New Zealand. In Contributions in Marine Mammal Paleontology Honoring Frank C. Whitmore, Jr., ed. Berta, A. and T.A. Deméré, Proceedings of the San Diego Society of Natural History 29: 147–176.
Fordyce, R.E. 2002. Simocetus rayi (Odontoceti: Simocetidae, new family): A bizarre new archaic Oligocene dolphin from the eastern North Pacific. Smithsonian Contributions to Paleobiology 93: 185–222.
Fordyce, R.E. 2003. Cetacean evolution and Eocene-Oligocene oceans revisited. In From Greenhouse to Icehouse: The Marine Eocene-Oligocene Transition, ed. Prothero, D.R., Ivany, L.C. and E. Nesbitt, 154–170. New York: Columbia University Press.
Fordyce, R.E. 2018. Cetacean evolution. In Encyclopedia of marine mammals, 3rd Edition, ed. Würsig, B., Thewissen, J.G.M. and K.M. Kovacs, 180–185. Amsterdam: Academic Press.
Fordyce, R.E. and C. de Muizon. 2001. Evolutionary history of cetaceans: a review. In Secondary adaptation of tetrapods to life in water, ed. Mazin, J.-M. and V. de Buffrénil, 169–233. Munich: Pfeil.
Fordyce, R.E., and F.G. Marx. 2018. Gigantism precedes filter feeding in baleen whale evolution. Current Biology 28 (10): 1670–1676.
Gaetán, C.M., M.R. Buono, and L.C. Gaetano. 2019. Prosqualodon australis (Cetacea: Odontoceti) from the early Miocene of Patagonia, Argentina: Redescription and phylogenetic analysis. Ameghiniana 56 (1): 1–27.
Geisler, J.H., R.W. Boessenecker, M. Brown, and B.L. Beatty. 2017. The origin of filter feeding in whales. Current Biology 27 (13): 2036–2042.
Glaessner, M.F. 1955. Pelagic fossile (Aturia, penguins, whales) from the Tertiary of South Australia. Records of the South Australian Museum 11 (4): 353–372.
Godfrey, S., M.D. Uhen, J.E. Osborne, and L.E. Edwards. 2016. A new specimen of Agorophius pygmaeus (Agorophiidae, Odontoceti, Cetacea) from the early Oligocene Ashley Formation of South Carolina, USA. Journal of Paleontology 90 (1): 154–169.
Gray, J.E. 1864. On the Cetacea which have been observed in the seas surrounding the British Islands. Proceedings of the Scientific Meetings of the Zoological Society of London 1864: 195–248.
Grimm, M.C. 2005. Beiträge zur Lithostratigraphie des Paläogens und Neogens im Oberrheingebiet (Oberrheingraben, Mainzer Becken, Hanauer Becken). Geologisches Jahrbuch Hessen 132: 79–112.
Grimm, K.I. 2006. Meeresverbindungen im Rupelium Mitteleuropas—Paläobiogeographische Untersuchungen anhand von Foraminiferen. Geologisches Jahrbuch Hessen 133: 19–27.
Grimm, K.I., and M.C. Grimm. 2003. Geologischer Führer durch das Mainzer Tertiärbecken. In Die fossilen Wirbellosen des Mainzer Tertiärbeckens. Teil 1–1, ed. Grimm, K.I., M.C. Grimm, F.O. Neuffer, and H. Lutz, Mainzer Naturwissenschaftliches Archiv Beiheft 26: 1–158.
Grimm, K.I., and M.C. Grimm. 2005. Die Alzey-Formation (Rupelium, Mainzer Becken) am Steigerberg bei Eckelsheim: Sedimentologische, sequenzstratigraphische und biostratigraphische Untersuchungen eines transgressiven Küstensystems. Geologica et Palaeontologica 39: 79–108.
Grimm, K.I., M.C. Grimm, and T. Schindler. 2000. Lithostratigraphische Gliederung im Rupelium/Chattium des Mainzer Beckens, Deutschland. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 218: 343–397.
Grimm, K.I., M. Grimm, G. Radtke, D. Kadolsky, P. Schäfer, J.L. Franzen, T. Schindler, and M. Hottenrott. 2011. Mainzer Becken. In Stratigraphie von Deutschland IX. Tertiär, Teil 1, ed. Deutsche Stratigraphische Kommission, Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften 75: 133–209.
Gürs, K. 1995. Revision der marinen Molluskenfaunen des Unteren Meeressandes (Oligozän, Rupelium) des Mainzer Beckens (unpubl. Ph.D. thesis). Johannes Gutenberg-Universität Mainz.
Hampe, O., C. Hartkopf-Fröder, and F. von der Hocht. 2019. Neue Walüberreste—Squalodontidae, ?Eomysticetidae—aus dem Oberoligozän des Rheinlandes. Archäologie im Rheinland 2018: 60–62.
Hardenbol, J., J. Thierry, M.B. Farley, T. Jacquin, P.-C. de Graciansky, and P.R. Vail. 1998. Mesozoic and Cenozoic sequence chronostratigraphy framework of European basins. In Mesozoic and Cenozoic sequence stratigraphy of European basins, ed. Graciansky, P.-C. de, J. Hardenbol, T. Jacquin, and P.R. Vail, SEPM Special Publication 60: 3–13.
Hartkopf, C., and K.R.G. Stapf. 1984. Sedimentologie des Unteren Meeressandes (Rupelium, Tertiär) an Inselstränden im W-Teil des Mainzer Beckens (SW-Deutschland). Mitteilungen der POLLICHIA 71: 5–106.
Hoyt, E. 2005. Marine protected areas for whales, dolphins and porpoises: A world handbook for cetacean habitat conservation. London: Earthscan.
Ivany, L.C., W.P. Patterson, and K.C. Lohmann. 2000. Cooler winters as a possible cause of mass extinctions at the Eocene/Oligocene boundary. Nature 407: 887–890.
Jicha, B.R., D.W. Scholl, and D.K. Rea. 2009. Circum-Pacific arc flare-ups and global cooling near the Eocene-Oligocene boundary. Geology 37 (4): 303–306.
Karl, H.-V., and G. Tichy. 1999. Zur Taxonomie eines neuen Tribus von Seeschildkröten aus dem Oligozän von Deutschland (Testudines: Chelonioidea). Joannea Geologie und Paläontologie 1: 61–77.
Katz, M.E., B.S. Cramer, J.R. Toggweiler, G. Esmay, C. Liu, K.G. Miller, Y. Rosenthal, B.S. Wade, and J.D. Wright. 2011. Impact of Antarctic Circumpolar Current development on late Paleogene ocean structure. Science 332: 1076–1079.
Kellogg, R. 1923. Description of two squalodonts recently discovered in the Calvert Cliffs, Maryland; and notes on the shark-toothed cetaceans. Proceedings of the United States National Museum 62 (6): 1–69.
Kellogg, R. 1936. A review of the Archaeoceti. Carnegie Institution of Washington Publication 482: 1–366.
Kennett, J.P. 1977. Cenozoic evolution of Antarctic glaciation, the Circum-Antarctic Ocean, and their impact on global paleoceanography. Journal of Geophysical Research 82 (27): 3843–3860.
Kuhn, W. 2008. Das Brandungskliff von Eckelsheim. Die Rekonstruktion eines 30 Millionen Jahre alten Küstenstreifens. Alzeyer Geschichtsblätter 37: 3–24.
Kuhn, W. 2012. Küstenverläufe: Veränderungen und Wandel in der Geologie des Mainzer Beckens. Heimatjahrbuch des Landkreises Alzey-Worms 47: 153–157.
Lambert, O. 2005. Phylogenetic affinities of the long-snouted dolphin Eurhinodelphis (Cetacea, Odontoceti) from the Miocene od Antwerp. Belgium. Palaeontology 48 (3): 653–679.
Lambert, O., M. Martínez-Cáceres, G. Bianucci, C. Di Celma, R. Salas-Gismondi, E. Steurbaut, M. Utbina, and C. de Muizon. 2017. Earliest mysticete from the Late Eocene of Peru sheds new light on the origin of baleen whales. Current Biology 27: 1535–1541.
Lepsius, G.R. 1882. Halitherium schinzi die fossile Sirene des Mainzer Beckens. Abhandlungen des Mittelrheinischen Geologischen Vereins 1: 1–200.
Lepsius, G.R. 1883. Das Mainzer Becken geologisch beschrieben. Darmstadt: Bergsträsser.
Linnè, C. 1758. Systema naturae per Regna tria naturae, secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio Decima, Reformata. Stockholm: Laurentii Salvii.
Martínez-Cáceres, M., O. Lambert, and C. de Muizon. 2017. The anatomy and phylogenetic affinities of Cynthiacetus peruvianus, a large Dorudon-like basilosaurid (Cetacea, Mammalia) from the late Eocene of Peru. Geodiversitas 39 (1): 7–163.
Marx, F.G., and R.E. Fordyce. 2015. Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity. Royal Society Open Science 2 (4): 140434.
Marx, F.G., C.-H. Tsai, and R.E. Fordyce. 2015. A new early Oligocene toothed ‘baleen’ whale (Mysticeti: Aetiocetidae) from western North America: one of the oldest and the smallest. Royal Society Open Science 2: 150476.
Marx, F.G., O. Lambert, and M.D. Uhen. 2016. Cetacean paleobiology. Chichester: Wiley Blackwell.
Mayr, G. 2006. An osprey (Aves: Accipitridae: Pandioninae) from the early Oligocene of Germany. Senckenberiana lethaea 86 (1): 93–96.
Mchedlidze, G.A. 1970. Nekotorye obshchie cherty istorii kitoobraziych. Chast’ 1. Tbilisi: Metsnierba.
Mchedlidze, G.A. 1984. General features of the paleobiological evolution of Cetacea. New Dehli: Oxonian Press.
Mitchell, E.D. 1989. A new cetacean from the Late Eocene La Meseta Formation, Seymour Island, Antarctic Peninsula. Canadian Journal of Fisheries and Aquatic Sciences 46: 2219–2235.
Morlo, M. 1996. Carnivoren aus dem Unter-Miozän des Mainzer Beckens. Senckenbergiana lethaea 76: 193–249.
Neuffer, F.O., K. Rothausen, and V. Sonne. 1978. Fossilführende Rinnenfüllung im Unteren Meeressand an einer Insel-Steilküste des Mitteloligozän-Meeres (Steigerberg bei Eckelsheim, Mainzer Becken)—1. Aufschluß. Makro- und Mikrofauna. Mainzer geowissenschaftliche Mitteilungen 6: 99–120.
Nungesser, K. 2021. Das Tertiär des Mainzer Beckens. Erdgeschichte und Fossilien in Rheinhessen und Umgebung. Bielefeld: Steinkern.
Okazaki, Y. 1982. A Lower Miocene squalodontid from the Ashiya Group, Kyushu, Japan. Bulletin of the Kitakyushu Museum of Natural History 4: 107–112.
Okazaki, Y. 1988. Oligocene squalodont (Cetacea: Mammalia) from the Ashiya Group, Japan. Bulletin of the Kitakyushu Museum of Natural History 8: 75–80.
Okazaki, Y. 2012. A new mysticete from the upper Oligocene Ashiya Group, Kyushu, Japan and its significance to mysticete evolution. Bulletin of the Kitakyushu Museum of Natural History and Human History Series A 10: 129–152.
Pardo, M.A., N. Silverberg, D. Gendron, E. Beier, and D.M. Palacios. 2013. Role of environmental seasonality in the turnover of a cetacean community in the southwestern Gulf of California. Marine Ecology Progress Series 487: 245–260.
Peigné, S., and E.P.J. Heizmann. 2003. The Amphicyonidae (Mammalia: Carnivora) from Ulm-Westtangente (MN 2, Early Miocene), Baden-Württemberg, Germany—Systematics and ecomorphology. Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie) 343: 1–133.
Peredo, C.M., and N.D. Pyenson. 2018. Salishicetus meadi, a new aetiocetid from the late Oligocene of Washington State and implications for feeding transitions in early mysticete evolution. Royal Society Open Science 5 (4): 172336.
Peredo, C.M., N.D. Pyenson, and A.T. Boersma. 2017. Decoupling tooth loss from the evolution of baleen in whales. Frontiers in Marine Science 4 (67): 1–11.
Pilleri, G. 1985. The Miocene Cetacea of the Belluno Sandstones (eastern southern Alps). Memorie di Scienze Geologiche 37: 1–250.
Pirkenseer, C., J.P. Berger, and B. Reichenbacher. 2013. The position of the Rupelian/Chattian boundary in the southern Upper Rhine Graben based on new records of microfossils. Swiss Journal of Geosciences 106 (2): 291–301.
Pledge, N.S. 2005. A new species of early Oligocene cetacean from Port Willunga, South Australia. Memoirs of the Queensland Museum 51 (1): 123–133.
Poust, A.W., and R.W. Boessenecker. 2018. Expanding the geographic and geochronologic range of early pinnipeds: New specimens of Enaliarctos from Northern California and Oregon. Acta Palaeontologica Polonica 63 (1): 25–40.
Pross, J., A. Bruch, and Z. Kvacek. 1998. Paläoklima-Rekonstruktionen für den Mittleren Rupelton (Unter-Oligozän) des Mainzer Beckens auf der Basis mikro-und makrobotanischer Befunde. Mainzer geowissenschafliche Mitteilungen 27: 79–92.
Ravn, J.P.J. 1926. On a cetacean, Squalodon (Microzeuglodon?) Wingei n.sp., from the Oligocene of Jutland. Meddelelser fra Dansk Geologisk Forening 7: 45–54.
Reinecke, T., F. von der Hocht, and L. Dufraing. 2015. Fossil basking sharks of the genus Keasius (Lamniformes, Cetorhinidae) from the boreal North Sea Basin and Upper Rhine Graben: evolution of dental characteristics from the Oligocene to late Middle Miocene and description of two new species. Palaeontos 28: 39–98.
Rice, D.W. 1998. Marine mammals of the world. Systematics and distribution. Society for Marine Mammalogy Special Publication 4: 1–231.
Rothausen, K. 1967. Die Klimabindung der Squalodontidae (Odontoceti, Mamm.) und anderer mariner Vertebrata. Sonderveröffentlichungen des Geologischen Instituts der Universität Köln 13: 157–166.
Rothausen, K. 1968a. Die systematische Stellung der europäischen Squalodontidae (Odontoceti, Mamm.). Paläontologische Zeitschrift 42 (1/2): 83–104.
Rothausen, K. 1968b. Die Squalodontidae (Odontoceti, Mamm.) im Oligozän und Miozän Italiens. Memorie degli Istituti di Geologia e Mineralogia dell’ Università di Padova 26: 1–19.
Rothausen, K. 1988. Carnivoren im Kalktertiär (Oberoligozän – Untermiozän) des Mainzer Beckens (1. Amphicyonidae). Geologisches Jahrbuch A110: 241–260.
Rothausen, K. and V. Sonne. 1984. Mainzer Becken. Sammlung Geologischer Führer 79. Berlin: Bornträger.
Rousse, S., P. Duringer, and K.R.G. Stapf. 2012. An exceptional rocky shore preserved during Oligocene (Late Rupelian) transgression in the Upper Rhine Graben (Mainz Basin, Germany). Geological Journal 47 (4): 388–408.
Sanders, A.E., and L.G. Barnes. 2002. Paleontology of the late Oligocene Ashley and Chandler Bridge Formations of South Carolina, 2: Micromysticetus rothauseni, a primitive cetotheriid mysticete (Mammalia: Cetacea). Smithsonian Contributions to Paleobiology 93: 271–293.
Sanders, A.E. and J.H. Geisler. 2015. A new basal odontocete from the upper Rupelian of South Carolina, U.S.A., with contributions to the systematics of Xenorophus and Mirocetus (Mammalia, Cetacea). Journal of Vertebrate Paleontology 35 (1): e890107.
Schaarschmidt, F. 1982. Bestandsaufnahme der Makroflora im „prä-aquitanen“ Tertiär des Mainzer Beckens. Mainzer geowissenschaftliche Mitteilungen 10: 19–28.
Schäfer, P. 2012. Mainzer Becken. Stratigraphie – Paläontologie – Exkursionen. Sammlung Geologischer Führer 79, 2nd ed. Stuttgart: Bornträger.
Schindler, T. 2011. Die zeitliche Entwicklung der Chondrichthyer-Faunen der Selztal-Gruppe (Rupelium, U. Oligozän) des Mainzer Beckens (SW-Deutschland) Ursachen und Potenziale. Mainzer Naturwissenschaftliches Archiv 48: 155–170.
Schindler, T. 2013a. Biostratonomie der Wirbeltiere in der Wonsheim-Wendelsheim-Bucht (Alzey-Formation, Oligozän; Mainzer Becken, SW-Deutschland). Mainzer Naturwissenschaftliches Archiv 50: 67–80.
Schindler, T. 2013b. Litho- und Biofazies der Alzey-Formation (Selztal-Gruppe, Oligozän) am Südwestrand der Steigerberg-Insel (Mainzer Becken, SW-Deutschland). Mainzer Naturwissenschaftliches Archiv 50: 49–66.
Schindler, T. 2013c. Vogelreste aus der Alzey- und der Bodenheim-Formation (Unteroligozän) des Mainzer Beckens (Südwestdeutschland). Mainzer Naturwissenschaftliches Archiv 50: 147–153.
Schindler, T., and G. Mayr. 2004. Der erste Vogelrest der Alzey-Formation (Rupelium, Oligozän) des Mainzer Beckens (SW-Deutschland) – ein Greifvogel (Accipitridae). Mainzer Naturwissenschaftliches Archiv 42: 21–26.
Schindler, T. and M. Poschmann. 2001. Palmen und Brachiopoden. Neue Funde an der oligozänen Küste von Eckelsheim. Fossilien 18 (5): 274–278.
Schindler, T., and A. Schneider. 2020. Ein Nashornzahn aus der Sandgrube Eckelsheim. Fossilien 37 (3): 63–64.
Schindler, T., K. Nungesser, A. Müller, and K.I. Grimm. 2009. Die Alzey-Formation der klassischen Lokalität Welschberg bei Waldböckelheim (Rupelium, Oligozän, Mainzer Becken) – Ergebnisse neuer Grabungen. Jahresberichte und Mitteilungen des Oberrheinischen Geologischen Vereins, Neue Folge 91: 37–87.
Schindler, T., K.I. Grimm, M.C. Grimm, and K. Nungesser. 2021. Lithostratigrafische Neudefinition und Untergliederung der Sulzheim-Formation (Oligozän; Mainz-Becken, SW-Deutschland). Mainzer Naturwissenschaftliches Archiv 58: 5–16.
Schopp, H. 1888. Der Meeressand zwischen Alzey und Kreuznach. Abhandlungen der Grossherzoglich Hessischen Geologischen Landesanstalt zu Darmstadt 1 (3): 343–392.
Shackleton, N.J. 1986. Paleogene stable isotope events. Palaeogeography, Palaeoclimatology, Palaeoecology 57 (1): 91–102.
Syring, J.C. 2015. Die otolithenbasierten Teleostei-Faunen aus dem Rupelium s.str. des Mainzer Beckens (Unteroligozän, Rheinland-Pfalz, Deutschland) – Systematik, Paläoökologie, Paläobiogeographie und Erstellung einer vorläufigen, otolithenbasierten Biostratigraphie (unpubl. Ph.D. thesis). Humboldt-Universität zu Berlin.
Tanaka, Y., and R.E. Fordyce. 2015. Historically significant late Oligocene dolphin Microcetus hectori Benham 1935: a new species of Waipatia (Platanistoidea). Journal of the Royal Society of New Zealand 45 (3): 135–150.
Tate-Jones, M.K., C.M. Peredo, C.D. Marshall, and S.S.B. Hopkins. 2020. The dawn of Desmatophocidae: A new species of basal desmatophocid seal (Mammalia, Carnivora) from the Miocene of Oregon, U.S.A. Journal of Vertebrate Paleontology 40 (4): e1789867.
Tomiya, S., and Z.J. Tseng. 2016. Whence the beardogs? Reappraisal of the middle to late Eocene ‘Miacis’ from Texas, USA, and the origin of Amphicyonidae (Mammalia, Carnivora). Royal Society Open Science 3 (10): 160518.
Uhen, M.D. 2004. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): An archaeocete from the middle to late Eocene of Egypt. University of Michigan Papers on Paleontology 34: 1–222.
Uhen, M.D. 2007. Evolution of marine mammals: Back to the sea after 300 million years. The Anatomical Record 290 (6): 514–522.
Uhen, M.D. 2008. A new Xenorophus-like odontocete cetacean form the Oligocene of North Carolina and a discussion of the basal odontocete radiation. Journal of Systematic Palaeontology 6 (4): 433–452.
Uhen, M.D. 2010. The origin(s) of whales. Annual Review of Earth and Planetary Sciences 38: 189–219.
Uhen, M.D., and N.D. Pyenson. 2007. Diversity estimates, biases, and historiographic effects: Resolving cetacean diversity in the Tertiary. Palaeontologia Electronica 10 (2): 1–22.
Uhen, M.D., Clapham, M.E. and C.R. Marshall. 2022. The Paleobiology Database. https://paleobiodb.org/#/
Vávra, N. 1983. Bryozoen aus dem Unteren Meeressand (Mitteloligozän) von Eckelsheim (Mainzer Becken, Bundesrepublik Deutschland). Mainzer Naturwissenschaftliches Archiv 21: 67–123.
von der Hocht, F. 1978. Bestandsaufnahme der Chondrichthyes-Fauna des Unteren Meeressandes (Oligozän, Rupelium) im Mainzer Becken. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie 15 (3): 77–83.
Voss, M., and O. Hampe. 2017. Evidence for two sympatric sirenian species (Mammalia, Tethytheria) in the early Oligocene of Central Europe. Journal of Paleontology 91 (2): 337–367.
Walchner, F.A. 1850. Handbuch der Geognosie zum Gebrauche bei seinen Vorlesungen und zum Selbstudium mit besonderer Berücksichtigung der geognostischen Verhältnisse des Grossherzogthums Baden. Zweite, verbesserte und vermehrte Auflage. 7. Lieferung. Karlsruhe: Christian Theodor Groos.
Walliser, E.O., B.R. Schöne, T. Tütken, J. Zirkel, K.I. Grimm, and J. Pross. 2015. The bivalve Glycymeris planicostalis as a high-resolution paleoclimate archive for the Rupelian (early Oligocene) of central Europe. Climate of the past 11 (4): 653–668.
Wang, X., R.J. Emry, C.A. Boyd, J.J. Person, S.C. White, and R.H. Tedford. 2022. An exquisitely preserved skeleton of Eoarctos vorax (nov. gen. et sp.) from Fitterer Ranch, North Dakota (early Oligocene) and systematics and phylogeny of North American early arctoids (Carnivora, Caniformia). Society of Vertebrate Paleontology Memoir 22: 1–123.
Whitmore, F.C. jr. and A.E. Sanders. 1976. Review of the Oligocene Cetacea. Systematic Zoology 25 (4): 304–320
Zachos, J.C., T.M. Quinn, and K.A. Salamy. 1996. High-resolution (104 years) deep-sea foraminiferal stable isotope records of the Eocene-Oligocene climate transition. Paleoceanography 11 (3): 251–266.
Ziegler, P.A. 1990. Geological Atlas of Western and Central Europe. Shell Internationale Petroleum Maatschappij B.V. Mijdrecht: Verweij B.V.
Acknowledgements
We are deeply grateful to Kirsten and Matthias Grimm, Mainz, for profound discussions on local geology and palaeogeography in connection with the discovery. We thank Thomas Schindler, Koblenz/Mainz, for information about the current situation of the Eckelsheim outcrop and Dirk Gille, Kassel, for facts about the discovery circumstances of the presented tooth. Many thanks also to Wolf-Dieter Heinrich, Berlin, for the insightful conversation on the variation of carnivore teeth. We are indebted to Michele Kaiser, Berlin/Bochum, for preparation and stabilizing the tooth and to Bernhard Schurian, Berlin, for providing high resolution images of the specimen utilizing stacking method. Finally, we thank an anonymous reviewer for the constructive comments and helpful suggestions for improvement.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Handling Editor: Thomas Mörs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hampe, O., von der Hocht, F. The first cetacean from the early Oligocene of the SW German Mainz Basin: a probable cheek tooth of a mysticete (Mammalia: Cetacea). PalZ 98, 161–174 (2024). https://doi.org/10.1007/s12542-023-00676-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-023-00676-4