Abstract
Toarciconiopteryx dipterosimilis gen. et sp. nov. is described from the Lower Toarcian of Grimmen (Western Pomerania, Germany) based on a hind wing. This enigmatic wing superficially resembles a dipteran forewing, but analysis in detail establishes that it belongs to the Neuroptera. We assign it to the Coniopterygidae with great confidence by its great concordance with the hind wings of that family, but a small possibility remains that it might belong to the Dipteromantispidae, although the very derived haltere-like hind wings of all its known members are entirely unlike it. We, therefore, consider it to be the oldest record of Coniopterygidae. We create the new subfamily Toarciconiopteryginae subfam. nov. for it, which is distinguished from other Coniopterygidae by its hind wings possessing two branches of RP and a proximal forking of M. These conditions are also known in some Sialidae (Megaloptera), supporting the hypothesis that Coniopterygidae is the sister group of all other Neuroptera, as Megaloptera is considered by most authors to be sister to Neuroptera. New interpretations of some aspects of the Coniopterygidae venation are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Coniopterygidae is composed of the smallest neuropterans, with reduced wing venation, and bodies and wings with a waxy covering (Meinander 1972). Today, ca. 570 species of the family are widely distributed across the globe (Oswald 2022). Four subfamilies are recognized: Aleuropteryginae, Coniopteryginae, Brucheiserinae, and Cretaconiopteryginae (Sziráki 2007; Liu and Lu 2017). The vast majority of modern species belong to the first two subfamilies, and only five to the latter two.
Fossil Coniopterygidae are numerous, occurring almost exclusively in amber. They are known from Barremian Lebanese amber (Whalley 1980; Azar et al. 2000); Albian El Soplao amber, Spain (Pérez-de la Fuente et al. 2019); upper Albian Charentese amber, France (Nel et al. 2005); mid-Cretaceous Burmese amber, Myanmar (Engel 2004, 2016; Engel and Grimaldi 2008; Sziráki 2016, 2017; Liu and Lu 2017; Li et al. 2019a, b; Ružičková et al. 2019); Turonian New Jersey amber, USA (Grimaldi 2000; Engel 2002); Cenomanian/Turonian Vendean amber, France (Perrichot et al. 2014); upper Cenomanian Nizhnyaya Agapa amber, northern Siberia (Makarkin and Perkovsky 2019); Santonian Yantardakh amber, northern Siberia (Meinander 1975; Makarkin and Perkovky 2017); earliest Eocene Oise amber, France (Nel et al. 2005) and Cambay amber, India (Grimaldi et al. 2013); upper Eocene Baltic (Pictet-Baraban and Hagen 1856; Enderlein 1910, 1930; Meinander 1975; Dobosz and Krzemiński 2000; Engel 2010; Sziráki and Gröhn 2015) and Rovno ambers (Ukraine) (Kupryjanowicz and Makarkin 2008); Miocene Dominican amber (Meinander 1998; Engel and Grimaldi 2007; Grimaldi et al. 2013) and Zhangpu amber, China (Chen et al. 2022a, b).
Only two species of the family are known as compression fossils, one from the Oligocene of France (Nel 1991) and the other from the Jurassic, i.e., Juraconiopteryx zherichini Meinander 1975 from the Upper Jurassic of Karatau (Kazakhstan). The latter is represented by a single specimen with poorly preserved characters not informative to determine its systematic position within the family. Meinander (1975), however, assigned Juraconiopteryx to Aleuropteryginae “in view of the structure of the head and the antennae” (p. 54).
The Jurassic monotypic genus Archiconiopteryx Enderlein 1909 from the lower Toarcian of Dobbertin (Germany) was for a long time considered a member of the Coniopterygidae and the oldest record of the family (Enderlein 1909, 1910, 1911, 1930; Tjeder 1957; Meinander 1972, 1975, 1979, 1990; Nel 1991; Carpenter 1992; Ohm 1995; Weitschat and Wichard 1998; Dobosz and Krzemiński 2000). However, its single species (Archipsylla liasina Handlirsch 1906) was initially described as belonging to Archipsyllidae in Homoptera (Handlirsch 1906–1908). Archipsyllidae, with the type species Archipsylla primitiva Handlirsch 1906 is now ascribed to the order Permopsocida (see Huang et al. 2016; = Tetrastigmoptera: Kluge 2019). Ansorge (1996) examined additional specimens of the species from contemporaneous localities at Grimmen and Dobbertin, and has allocated it to another family, the Archiconiopterygidae in Sternorrhyncha (Hemiptera), which has been accepted by most authors (e.g., Grimaldi and Engel 2005; Szwedo 2018). In any case, Archiconiopterygidae do not belong to Neuroptera.
In this paper, we describe a second Jurassic species of Coniopterygidae, based on an enigmatic, rather small wing with reduced venation. It is the oldest known member of the family, from the Lower Jurassic (lower Toarcian, ca. 182 MA).
Materials and methods
The specimen is in a carbonate concretion of the Lower Toarcian (exaratum subzone) marine “Green series” clay from the former clay pit Klein Lehmhagen near Grimmen in Western Pomerania, Northeast Germany (Fig. 1). This locality is well known for its diverse, numerous, and well-preserved insect fossils (Ansorge 1996, 2003; Kirejtshuk and Ansorge 2022).
a Paleogeographic map of Middle and Western Europe showing Lower Toarcian insect localities. CEB Central European Basin, NSB North Sea Basin, PB Paris Basin, 1 Grimmen, 2 Dobbertin, 3 Ahrensburg, 4 Braunschweig area, 5 Upper Franconia, 6 Kerkhofen, 7 Irlbach, 8 Holzmaden, 9 Aselfingen, 10 Hemmiken, 11 Bascharage, 12 Withby, 13 Gloucestershire (modified after Ansorge 2003). b Section of the Lower Jurassic of Grimmen showing the occurrence of the holotype of Toarciconiopteryx dipterosimilis gen. et sp. nov. (modified after Ansorge 2007), U.P. Upper Pliensbachian
Neuroptera is not well known from the exaratum subzone, about 3% of all insects: 2.7% of 1200 specimens (Ansorge 1996), 3.3% of 2042 specimens (Ansorge 2003), and currently 2.8% of 3300 specimens (JA, pers. data). From the slightly older elegantulum concretions (elegantulum subzone), only one neuropteran is known, the holotype of Mesosmylina falciferum Ansorge 1996. The Lower Toarcian neuropteran assemblage of Grimmen is dominated by rather small specimens of Prohemerobiidae, broad-winged Neuroptera that were treated as Brongniartiellidae (their actual family affinity is unclear), and Epigambriinae (Ithonidae s. l.), with a wing size smaller than 10–15 mm (Ansorge 1996; Makarkin et al. 2021). Larger taxa are extremely rare or absent at Grimmen, but are better known from the Lower Toarcian of Lower Saxony [Fig. 1a (4)] and Bavaria [Fig. 1a (6)], e.g., Kalligrammatidae, Tetanoptilon Bode 1953, Panfilovia Makarkin, 1990 and Paractinophlebia Handlirsch 1906 (Bode 1953; Ponomarenko 1996; Ansorge and Makarkin 2021).
We mostly follow the venational terminology of Breitkreuz et al. (2017), but that of venation details (e.g., spaces, veinlets) follows Oswald (1993). Crossveins are designated after the longitudinal veins which they connect and are numbered in sequence from the wing base, e.g., 1ra-rp, 2ra-rp, crossveins connecting RA and RP. Contrary character states of compared taxa are provided in brackets.
Systematic palaeontology
Order Neuroptera Linnaeus 1758
Family Coniopterygidae Burmeister 1839
Subfamily Toarciconiopteryginae subfam. nov.
Diagnosis. In hind wing, the anterior trace of RP with two pectinate branches [one branch in other subfamilies]; M forked in its proximal half [in distal half in other subfamilies].
Genus Toarciconiopteryx gen. nov.
Type and only species. Toarciconiopteryx dipterosimilis sp. nov.
Etymology. From the Toarcian and Coniopteryx, the type genus of Coniopterygidae, referring to the age and family affinity of this genus. Gender feminine.
Diagnosis. As subfamily.
Toarciconiopteryx dipterosimilis sp. nov.
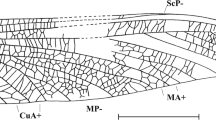
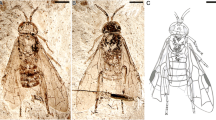
Toarciconiopteryx dipterosimilis gen. et sp. nov. (holotype, LGA 2752) from the Lower Toarcian of Grimmen, Germany. a part, b counterpart, and c hind wing venation. Scale bar 1 mm (all to same scale). A1 first anal vein, Cu (cu) cubitus, CuA anterior cubitus, CuP posterior cubitus, ff flexion fold, M (m) media, MA anterior media, MP posterior media, R(r) radius, RA (ra) anterior radius, RP (rp) posterior radius, RP1, proximal-most branch of RP, RP2, branch of RP distad RP, Sc subcosta
Etymology. From Diptera and the Latin similis [-e], similar, resemble, referring to the superficial similarity of this only known wing with a dipteran (nematoceran) wing.
Type material. Holotype LGA 2752, deposited in Museum für Naturkunde (Berlin, Germany), J. Ansorge Grimmen collection. A nearly complete, well-preserved hind wing.
Type locality and horizon. Northern Germany: Grimmen. Lower Jurassic: lower Toarcian, “Green Series” clay of exaratum subzone.
Diagnosis. As for genus.
Description. Hind wing—ca. 4.50–4.60 mm long, 1.55 mm wide (Fig. 2). Costal space in general narrow, dilated proximally and distally, very constricted medially. Sc long, distal part poorly discernible (Fig. 3); two poorly discernible veinlets in proximal dilated part; no veinlets discernible in distal dilated part. Subcostal space broad, basal crossvein located proximad ra-rp1; no distal crossveins discernible. Subcostal flexion fold between Sc and RA well developed, convex. RA rather stout, entering margin far before wing apex; no veinlets detected. RA space broad, with two crossveins: ra-rp1 short, straight; ra-rp2 long, oblique, slightly sinuous. RP originating close to wing base (poorly discernible); its anterior trace entering margin slightly before wing apex, with two long single branches. RP1 originating in proximal part of RP (at about half wing length), proximad ra-rp2; RP2 originating in distal part, approximately at mid-way between origin of RP1 and termination of anterior trace of RP. Basal crossvein between RP and M (1r-m) poorly discernible, relatively long, continuing anterior trace of RP. Stem of M very thin, basally very poorly discernible, forked in proximal half of wing. MA and MP divergent, simple; MA thin proximad intramedian crossvein. Long setae on M not detected. One intramedian crossvein (ma-mp) long, located distad crossvein distal m-cu and proximad distal crossvein 2r-m. Distal crossvein m-cu (connecting MP and CuA) long. Cu very stout, continuing in concave, very stout, simple CuA. CuP originating far from wing base, much thinner that CuA, simple. A1 rather long, simple. A2, A3 not preserved. Wing membrane slightly fuscous, single-colored. Veins mainly fuscous.
Remarks. We consider this wing to be a hind wing because of its general venation and its convex RA and concave CuA. In all Neuropterida in which CuA is well developed, it is concave in the hind wing and convex in the forewing (Adams 1960; Makarkin et al. 2009). The venation of Coniopterygidae differs in the fore and hind wings (like in other Neuroptera) in different extent. In particular, RP originates proximally in the hind wing (except advanced Coniopteryginae) as in Toarciconiopteryx dipterosimilis sp. nov., but clearly distally in the forewing of all Coniopterygidae.
The venation in the basal part of the wing is poorly discernible, especially crossveins (these veins are very thin and probably the same color as the membrane).
Discussion
Order and family affinity of Toarciconiopteryx gen. nov.
The hind wing of Toarciconiopteryx gen. nov. is relatively short, with strongly reduced venation. It certainly belongs to Neuroptera, and nothing in the venation contradicts this. Its initial resemblance to the forewing venation of Diptera is only superficial. It clearly differs by the structure of CuP, which in Diptera is mostly reduced or weak and incomplete in form of a pseudovein running subparallel to CuA, but is well developed here as in most Neuroptera. Other character states of Toarciconiopteryx gen. nov. may be present in Diptera.
The venation of some minute Mecoptera is also reduced. However, their M and CuA in the hind wing are always proximately fused (see e.g., Novokshonov 1997: figs. 8, 22d, 38), but these veins are widely spaced in the new genus for entire length.
The hind wings of the minute Permian Nanosialidae (Megaloptera?), whose venation is reduced, possess two character states absent in the new genus: the costal space is not constricted medially, and a dark pterostigma is present (Shcherbakov 2013: Fig. 24). However, Toarciconiopteryx sp. nov. may theoretically represent a new family of Megaloptera, as its hind wing venation is similar to that of some Sialidae in some aspects. However, this similarity may result from a closer phylogenetic relationship of Coniopterygidae with Megaloptera than other neuropteran families (see below). Also, the costal space of the megalopteran hind wing is never constricted medially.
Among Neuropterta, the wing resembles the forewings of the Cretaceous Dipteromantispidae, but the hind wing in that family is modified to a haltere-like structure (Makarkin et al. 2013: Fig. 1). The venation of Dipteromantispidae differs in various ways from that of T. dipterosimilis sp. nov., in particular by the short forewing Sc (see Liu et al. 2016, 2017b). However, complete (i.e., not haltere-like) hind wings of this family are not yet known, and Toarciconiopteryx might belong to that family.
Other genera with the relatively reduced hind wing venation occur in extant Hemerobiidae (e.g., Psectra Hagen, Zachobiella Banks: Makarkin 1994: Figs. 2, 5, 7), Sisyridae (e.g., Climacia McLachlan: Flint 1998: Figs. 34, 35), extant Dilaridae (e.g., Neonallachius Nakahara: Liu et al. 2017a: figs. 10D, E), and many Cretaceous to Recent Berothidae (e.g., Aspöck 1989: Fig. 1; Archibald and Makarkin 2004: Fig. 6; Makarkin 2018: Fig. 3). However, all of these genera bear numerous, usually closely spaced subcostal veinlets and trichosors, and have most longitudinal veins forked, often more than once. The venation of all small Triassic and Jurassic Neuroptera is also dense and have at least some of these character states.
Sialidopsis similis Martynov 1937 (Permithonidae) probably has the most reduced hind wing venation of Permian Neuroptera, but it differs from that of Toarciconiopteryx gen. nov. by a terminal forking of the longitudinal veins, four branches of RP, and the costal space not being constricted (Martynov 1937). In almost all Mantispidae, the hind wing costal space is strongly constricted medially (like in T. dipterosimilis sp. nov.), but their venation (even in species with most reduced venation) is otherwise very different (e.g., by the presence of a pterostigma and the reduced CuP in most species).
Therefore, the family Coniopterygidae is the only real candidate for Toarciconiopteryx gen. nov. within the Neuroptera. The size and wing venation of this genus fit rather well with Coniopterygidae, differing only in details. Its wing is concordant with that of other coniopterygids by similar wing shape, a narrow and medially constricted costal space, the broad subcostal and RA spaces, scarce crossveins, and all longitudinal veins not being forked distally.
Systematic position of Toarciconiopteryx gen. nov. within the Coniopterygidae
The genus Toarciconiopteryx gen. nov. does not belong to any of the four known subfamilies of Coniopterygidae with any certainty. Its hind wing is distinguished from that of other coniopterygids by two character states: the presence of two branches of RP and the proximal forking of M. Therefore, we assign the genus to its own subfamily Toarciconiopteryginae subfam. nov. Unfortunately, there are no clear venational apomorphies; these two diagnostic character conditions are probably plesiomorphic. Below, we compare the hind wing venation of all coniopterygid subfamilies in more detail.
The mid-Cretaceous subfamily Cretaconiopteryginae with two genera from Burmese amber (i.e., Cretaconiopteryx Liu and Lu 2017 and Archaeconis Chen et al. 2022a, b) is thought to represent a putative basal-most lineage of the family (Liu and Lu 2017). According to Liu and Lu (2017), it possesses a number of plesiomorphic conditions: segmented galea; the presence of plicatures; denser crossvenation; and the proximal origin of RP in both the fore and hind wings. Putative autapomorphies of this subfamily are: the forewing RA and RP terminally fused forming a loop, and the zig-zagged forewing CuP. Of these plesiomorphic characters, one is detected in Toarciconiopteryx dipterosimilis sp. nov., i.e., RP originates near the wing base (Fig. 4a). We found that the longitudinal orientation of 1r-m (not crossvein-like), the presence of 1ra-rp, and the presumable A3 are three other plesiomorphic conditions of Cretaconiopteryginae; the first two of these are shared by this subfamily and Toarciconiopteryx gen. nov. Therefore, three plesiomorphic character states of Cretaconiopteryginae are also found in Toarciconiopteryginae, and these have no synapomorphies.
Hind wing venation of Coniopterygidae subfamilies. a Cretaconiopteryx grandis, Cretaconiopteryginae (based mainly on the photographs in Liu and Lu 2017: figs. 1A, 2A, with addition of some details from other photographs and drawings in Liu and Lu 2017 and Li et al. 2019b), b Flintoconis gozmanyi Sziráki 2007, Brucheiserinae (based on the photograph in Sziráki 2007: Fig. 15), c Aleuropteryx juniperi Ohm, 1968, Aleuropteryginae (redrawn from Meinander 1972: Fig. 11F), d Neosemidalis nervalis Meinander 1972, Coniopteryginae (redrawn from Meinander 1972: Fig. 105G). The vein labeling is changed. All not to scale. A1–A3 first-to-third anal veins, Cu cubitus, CuA anterior cubitus, CuP posterior cubitus, J jugal vein. M (m) media, MA anterior media, MP posterior media, R(r) radius, RA (ra) anterior radius, RP (rp) posterior radius, Sc subcosta
The South American Brucheiserinae contain two genera and four species (Navás 1927; Riek 1975; Sziráki 2007). The position of this group within the Neuropterida was for a long time unclear, as the two known species of the genus Brucheiser Navás, 1927 have a strongly abnormal wing venation. Only the discovery of the larva (Sziráki and Flint 2007) and imagoes of the genus Flintoconis Sziráki 2007 with ‘normal’ venation allowed a resolution of the problem.
The hind wing venation of Flintoconis shares with Cretaconiopteryginae at least four character states: dense crossvenation, a proximal origin of RP, the presence of the presumable A3, and the distal connecting crossvein between of Sc and RA (the first three are plesiomorphic). Also, the crossvein 1r-m is almost longitudinally oriented (Fig. 4b). The simple M is the only venational apomorphy of the subfamily, but it occurs also in some Aleuropteryginae, e.g., Coniocompsa Enderlein, 1905. Two plesiomorphic conditions are shared by Brucheiserinae and Toarciconiopteryginae, i.e., the proximal origin of RP and the almost longitudinally oriented 1r-m.
The subfamilies Aleuropteryginae and Coniopteryginae have similar hind wing venation, e.g., the crossvein 1r-m in both is more or less perpendicular to M (not longitudinally oriented), an apomorphic state (Fig. 4c, d). The venation of Coniopteryginae is more advanced, distinguished from Aleuropteryginae mainly by two apomorphic states: the distal location of the RP origin, and the proximal fusion of M and R in many species. The widely spaced Sc and RA in these two subfamilies not connecting by a distal crossvein is similar to a condition seen in Toarciconiopteryginae (this area is, however, poorly discernible in the new species). The proximal origin of RP is the only plesiomorphic state shared by Toarciconiopteryginae and Aleuropteryginae.
Therefore, the hind wing venation of Toarciconiopteryginae is one of the most plesiomorphic in the family along with that of Cretaconiopteryginae. Nevertheless, Toarciconiopteryginae appear to be more primitive by their plesiomorphic RP and M.
Phylogenetic position of Coniopterygidae
The Coniopterygidae is an unique family in the Neuroptera by several character states which differentiate it from all others of the order: in the larva: by six Malpighian tubules [more than six in all other Neuroptera] and two-segmented antennae [at least three in all other Neuroptera]; in the imago: by the presence of the gula (a median plate on the ventral part of the head closing the occipital foramen) [absent in all other Neuroptera], the hypodermal wax glands and the abdominal plicatures [absent in all other Neuroptera], and flattened fourth tarsomeres [not flattened in other Neuroptera (except Babinskaiidae, see below)] (Sziráki 1996; Zimmermann et al. 2009; Aspöck et al. 2012; Randolf and Zimmermann 2019). Also, the Coniopterygidae possess several autapomorphies in their sperm structure (Zizzari et al. 2008). Furthermore, several characters in the ovariole structure, the transcriptional activity of the oocyte nucleus, and dynamics of previtellogenic growth of Coniopterygidae significantly differentiate this family from those of other families of Neuroptera (Kubrakiewicz et al. 1998).
Based on the above morphological evidence and molecular analyses, Coniopterygidae are considered by most authors to be the sister group to all other Neuroptera (e.g., Yang et al. 2012; Wang et al. 2017; Engel et al. 2018; Winterton et al. 2018; Vasilikopoulos et al. 2020). However, there are alternative hypotheses: Coniopterygidae as the sister-group to Sisyridae (Aspöck et al. 2001), Dilaridae (Haring and Aspöck 2004), or to the dilarid clade (Dilaridae and berothoids) (e.g., Aspöck and Aspöck 2008; Zimmermann et al. 2009; Randolf et al. 2014, 2017).
At least two imaginal character conditions of Coniopterygidae are found in Megaloptera and are absent in all other Neuroptera: (1) the presence of the gula, and (2) fourth tarsomeres are dilated and flattened (Sziráki 1996; Randolf and Zimmermann 2019). In addition, Sziráki (1996) found that the male genitalia of Brucheiserinae are similar to those of the megalopteran Corydalinae (Corydalidae) in some details.
The gula is well developed in all Megaloptera (see Kelsey 1954: Fig. 2, labeled ‘gulasternum’; Yang and Liu 2010: Figs. 4, 5). The head of Megaloptera is prognathous. Headrick and Gordh (2009) believed that “the gula is a derived condition that is found in some but not all prognathous heads” formed “in the membranous neck region between the lateral extinctions of the postocciput” (p. 15). However, the head of Coniopterygidae is orthognathous, and so, it is impossible to associate the presence of the gula here with head prognathism.
Claws of Coniopterygidae (a), Sialidae (b) and Chrysopidae (c). a Semidalis aleyrodiformis (Stephens, 1836) (image courtesy of D. Zimmermann), b Sialis sp. (image courtesy of T. Vshivkova), c Ceraeochrysa cincta (Schneider, 1851) (image courtesy of C. Martins). Scale bars 0.01 mm (a), 0.1 mm (b), and 0.05 mm (c). bd basal dilatation, bp basal protuberance
The dilated and flattened fourth tarsomeres are present in Sialidae (Yang and Liu 2010: Figs. 17), and its structure is very similar to that of Coniopterygidae (Tjeder 1957: Fig. 4). The fourth tarsomeres are strongly bilobed in megalopteran Corydasialidae (Wichard et al. 2005: Figs. 5, 6; Liu et al. 2017c: Fig. 3; VM. pers. obs.). However, the bilobed fourth tarsomeres of Corydasialidae might have evolved independently from those of Coniopterygidae and Sialidae as their structure is different. All tarsomeres in the Cretaceous myrmeleontoid family Babinskaiidae have broad lateral wing-like dilatations, especially well developed in Gigantobabinskaia godunkoi Makarkin and Staniczek (Makarkin and Staniczek 2019: Fig. 2c).
We found other characters which are present in Coniopterygidae and Megaloptera, but are absent in other Neuroptera.
Both Coniopterygidae (see Tjeder 1957: Fig. 4), and Megaloptera (Sialidae and at least some Corydalidae) (VM, pers. obs.) bear the basal protuberance on claws of legs (Fig. 5a, b). Claws of other Neuroptera do not possess such a protuberance; the basal dilation of claws in some Chrysopidae differs in structure; it does not look like a protuberance (Fig. 5c).
The partially desclerotized forewing MA is considered a synapomorphy of Sialidae by Ansorge (2001). The very thin M and the proximal part of MA in the hind wing of Toarciconiopteryx dipterosimilis sp. nov. might also be interpreted as desclerotization. The structure and general configuration of M, and its relationships with the proximal part of RP in this species are very similar to those of some Sialidae: M is thin and forked proximally; MA and MP are sometimes simple; RP is originating near the wing base; the basal part of RP (before 1r-m) is very short; and the crossvein 1r-m is rather short, oriented longitudinally (e.g., Adams 1960: Fig. 2; Wichard 1997: Fig. 4; Price et al. 2012: Fig. 9; Liu et al. 2014: Fig. 4).
The presence of these shared character states in Coniopterygidae and Megaloptera supports the hypothesis that this family is basal in Neuroptera as Megaloptera are now widely accepted as a sister group of Neuroptera (e.g., Haring and Aspöck 2004; Zhao et al. 2014; Wang et al. 2017; Winterton et al. 2018; Vasilikopoulos et al. 2020). Coniopterygidae are estimated by molecular analysis to have originated ca. 294 Ma (upper Carboniferous to lower Permian) (Winterton et al. 2018) to ca. 281 Ma (middle Permian) (Vasilikopoulos et al. 2020). The finding of Toarciconiopteryx dipterosimilis sp. nov. in the Lower Jurassic, the venation of which has changed little since that time, supports the assumption that this is a very ancient family.
It is interesting that the oldest genus of Sialidae is also known from a nearby contemporaneous locality (Dobbertin, locality 2 in Fig. 1a), represented by an incomplete wing of Dobbertinia reticulata Handlirsch 1920 (Ansorge 2001). The scarcity of both Coniopterygidae and Sialidae in these lower Toarcian marine sediments can be explained by the assumption that their habitats could be located far from the coast, and they did not fly far.
Some remarks on the venation of Coniopterygidae
The relationship of the distal Sc and RA in Cretaconiopteryginae and Brucheiserinae is important for the interpretation of their relationship in Aleuropteryginae and Coniopteryginae. In the latter two subfamilies, only one distal subcostal crossvein is present. Currently, it is interpreted as Sc2 (a second, posterior branch of Sc) which is then the apparent RA, while actual RA “terminates or partially overlaps the base of the apical Sc2 abscissa” (Breitkreuz et al. 2017: p.19, Fig. 7); Sc1 is interpreted as a first, anterior branch of Sc and continues Sc. This interpretation is based on the pupal tracheation of Conwentzia psociformis Curtis (Withycombe 1922). The new data allow to reinterpret this relationship.
Cretaconiopteryginae and Brucheiserinae bear two distal crossveins. The more proximal one is obviously a true crossvein, 2sc-ra. However, the more distal one may be interpreted both as a crossvein (3sc-ra) or is part of Sc sharply turned toward RA. The 3sc-ra of Cretaconiopteryginae is located at the end of Sc, and either interpretations might be valid, while 3sc-ra of Brucheiserinae is located relatively distant from the end of both veins, and its identity as a crossvein is more evident than in Cretaconiopteryginae. Therefore, we interpret it as the crossvein 3sc-ra. It is hard to know if the distal crossvein of Aleuropteryginae and Coniopteryginae is homologous with 2sc-ra or 3sc-ra. If it is homologous with 2sc-ra, then a part of Sc distad 2sc-ra is Sc; if it is homologous with 3sc-ra, then a part of Sc distad 3sc-ra is single veinlet of Sc. Both interpretations differ from the currently generally accepted hypothesis.
Both Cretaconiopteryginae and Brucheiserinae bear a vein which we interpret as the presumptive A3. The structure and number of anal veins in Coniopterygidae have not been previously analyzed. A1 is simple and A2 is bifurcated in all members of the family, including probably Toarciconiopteryginae (A2 is not preserved in the latter). A vein between the stem of A2 and the posterior margin in Cretaconiopteryginae and Brucheiserinae is interpreted as a crossvein (Liu and Lu 2017; Li et al. 2019b) or a jugal vein (Sziráki and Flint 2007: Fig. 16). However, it may be treated as A3 which is basally fused with A2 for a considerable distance. This vein is long and oblique in Flintoconis and does not resemble a crossvein.
Data availability
Not applicable.
References
Adams, P.A. 1960. The relationship of the Protoperlaria and the Entopterygota. Psyche 65 (for 1958): 115–127.
Ansorge, J. 1996. Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläontologische Abhandlungen 2: 1–132.
Ansorge, J. 2001. Dobbertinia reticulata Handlirsch 1920 from the Lower Jurassic of Dobbertin (Mecklenburg/ Germany)—the oldest representative of Sialidae (Megaloptera). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 9: 553–564. https://doi.org/10.1127/njgpm/2001/2001/553
Ansorge, J., and V.N. Makarkin. 2021. The oldest giant lacewings (Neuroptera: Kalligrammatidae) from the Lower Jurassic of Germany. Palaeoworld 30: 296–310. https://doi.org/10.1016/j.palwor.2020.07.001
Ansorge, J. 2003. Insects from the Lower Toarcian of Middle Europe and England. Acta Zoologica Cracoviensia 46 (suppl.): 291–310.
Ansorge, J. 2007. Lower Jurassic clay pit of Klein Lehmhagen near Grimmen. In Geo-Pomerania Excursion guide, eds. R.-O. Niedermeyer, R. Dobracki, and K. Schütze. Biuletyn Państwowego Instytutu Geologicznego 424: 37–41.
Archibald, S.B., and V.N. Makarkin. 2004. A new genus of minute Berothidae (Neuroptera) from Early Eocene amber of British Columbia, Canada. Canadian Entomologist 136: 59–74. https://doi.org/10.4039/N03-043
Aspöck, U. 1989. Nyrma kervillea Navás—eine Berothide! (Neuropteroidea: Planipennia). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 41: 19–24.
Aspöck, U., and H. Aspöck. 2008. Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola). Systematic Entomology 33: 97–127. https://doi.org/10.1111/j.1365-3113.2007.00396.x
Aspöck, U., J.D. Plant, and H.L. Nemeschkal. 2001. Cladistic analysis of Neuroptera and their systematic position within the Neuropterida (Insecta: Holometabola: Neuropterida: Neuroptera). Systematic Entomology 26: 73–86.
Aspöck, U., E. Haring, and H. Aspöck. 2012. The phylogeny of the Neuropterida: Long lasting and current controversies and challenges (Insecta: Endopterygota). Arthropod Systematics & Phylogeny 70 (2): 119–129. https://doi.org/10.3897/asp.70.e31758
Azar, D., A. Nel, and M. Solignac. 2000. A new Coniopterygidae from the Lebanese amber. Acta Geologica Hispanica 35: 31–36.
Bode, A. 1953. Die Insektenfauna des Ostniedersächsischen Oberen Lias. Palaeontographica (Abteilung A) 103: 1–375.
Breitkreuz, L.C.V., S.L. Winterton, and M.S. Engel. 2017. Wing tracheation in Chrysopidae and other Neuropterida (Insecta): a resolution of the confusion about vein fusion. American Museum Novitates 3890: 1–44. https://doi.org/10.1206/3890.1
Burmeister, H.C.C. 1839. Handbuch der Entomologie. Zweiter Band. Besondere Entomologie. Zweite Abtheilung. Kaukerfe. Gymnognatha. (Zweite Hälfte; vulgo Neuroptera), i–xii + 757–1050. Berlin: Theod. Chr. Friedr. Enslin.
Carpenter, F.M. 1992. Superclass Hexapoda. In Treatise on Invertebrate Paleontology. Part R. Arthropoda 4, ed. Kaesler, R.L., vols 3 and 4, pp. 1–655. Boulder, Colorado: The Geological Society of America & Lawrence, Kansas: University of Kansas.
Chen, Z.L., C.C. Li, and X.Y. Liu. 2022a. New dustywings (Insecta: Neuroptera: Coniopterygidae) from the Miocene Zhangpu amber. Palaeoworld https://doi.org/10.1016/j.palwor.2022.01.003
Chen, Z.L., Z.L. Zhang, D. Zhuo, and X.Y. Liu. 2022b. New dustywings of the extinct subfamily Cretaconiopteryginae (Neuroptera: Coniopterygidae) from mid-Cretaceous amber of northern Myanmar. Cretaceous Research 105381. https://doi.org/10.1016/j.cretres.2022.105381
Dobosz, R., and W. Krzemiński. 2000. A new species of the Coniopterygidae (Neuroptera) from Baltic amber. Polskie Pismo Entomologiczne 69: 219–224.
Enderlein, G. 1909. Zur Kenntnis frühjurassischer Copeognathen und Coniopterygiden und über das Schicksal der Archipsylliden. Zoologischer Anzeiger 34: 770–776.
Enderlein, G. 1910. Über die Beziehungen der fossilen Coniopterygiden zu den recenten und über Archiconiocompsa prisca nov. gen. nov. spec. Zoologischer Anzeiger 35(22): 673–677.
Enderlein, G. 1911. Die fossilen Copeognaten und ihre Phylogenie. Palaeontographica 58: 279–360.
Enderlein, G. 1930. Die Klassifikation der Coniopterygiden auf Grund der recenten und fossilen Gattungen. Archiv für klassifikatorische und phylogenetische Entomologie 1 (2): 98–114.
Engel, M.S. 2002. A new dustywing (Neuroptera: Coniopterygidae) in Turonian amber from New Jersey, with a reassessment of Glaesoconis in Neoconian amber from Lebanon. Journal of the Kansas Entomological Society 75: 38–42.
Engel, M.S. 2004. The dustywings in Cretaceous Burmese amber (Insecta: Neuroptera: Coniopterygidae). Journal of Systematic Palaeontology 2: 133–136. https://doi.org/10.1017/S1477201904001191
Engel, M.S. 2010. A new genus of dustywings allied to Archiconiocompsa in Baltic amber (Neuroptera: Coniopterygidae). Transactions of the Kansas Academy of Sciences 113 (3/4): 145–150. https://doi.org/10.1206/0003-0082(2007)3587[1:TNFODA]2.0.CO;2
Engel, M.S. 2016. Two new genera of Cretaceous dustywings in amber from northern Myanmar (Neuroptera: Coniopterygidae). Novitates Paleoentomologicae 17: 1–16. https://doi.org/10.17161/np.v0i17.6465
Engel, M.S., and D.A. Grimaldi. 2007. The Neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates 3587: 1–58. https://doi.org/10.1206/0003-0082(2007)3587[1:TNFODA]2.0.CO;2
Engel, M.S., and D.A. Grimaldi. 2008. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nova Supplementa Entomologica 20: 1–86.
Engel, M.S., S.L. Winterton, and L.C.V. Breitkreuz. 2018. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us? Annual Review of Entomology 63: 531–551. https://doi.org/10.1146/annurev-ento-020117-043127
Flint, O.S., Jr. 1998. New species and records of Climacia from the neotropics (Neuroptera, Sisyridae). Acta Zoologica Fennica 209: 107–117.
Grimaldi, D.A. 2000. A diverse fauna of Neuropterodea in amber from the Cretaceous of New Jersey. In Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey, ed. D.A. Grimaldi, 259–303. Leiden: Backhuys Publishers.
Grimaldi, D.A., and M.S. Engel. 2005. Evolution of the insects, i–xv + 1–755. Cambridge, UK: Cambridge University Press.
Grimaldi, D.A., M.S. Engel, P.C. Nascimbene, and H. Singh. 2013. Coniopterygidae (Neuroptera: Aleuropteryginae) in amber from the Eocene of India and the Miocene of Hispaniola. American Museum Novitates 3770: 1–20. https://doi.org/10.1206/3770.2
Handlirsch, A., 1906–1908. Die fossilen Insekten und die Phylo-genie der rezenten Formen. Ein Handbuch für Palaeontologen und Zoologen, i–ix + 1–1430. Leipzig: Wilhelm Engelmann. [Issued in 1906 (pp. 1–640); 1907 (pp. 641–1140); 1908 (pp. 1120–1430)].
Haring, E., and U. Aspöck. 2004. Phylogeny of the Neuropterida: A first molecular approach. Systematic Entomology 29: 415–430. https://doi.org/10.1111/j.0307-6970.2004.00263.x
Headrick, D.H., and G. Gordh. 2009. Anatomy: head, thorax, abdomen, and genitalia. In Encyclopedia of insects. 2nd ed., eds. V.H. Resh and T.C. Ring, 1–20. Amsterdam etc.: Elsevier.
Huang, D.-Y., G. Bechly, P. Nel, M.S. Engel, J. Prokop, D. Azar, C.Y. Cai, T. van de Kamp, A.H. Staniczek, R. Garrouste, and L. Krogmann. 2016. New fossil insect order Permopsocida elucidates major radiation and evolution of suction feeding in hemimetabolous insects (Hexapoda: Acercaria). Scientific Reports 6(1): 23004. https://doi.org/10.1038/srep23004
Kelsey, L.P. 1954. The skeleto-motor mechanism of the dobson fly, Corydalus cornutus. Part I. Head and prothorax. Cornell University Agricultural Experiment Station Memoir 334: 1–51.
Kirejtshuk, A.G. and J. Ansorge. 2022. An extraordinarily preserved new genus and species of Trachypachidae (Coleoptera, Adephaga) from the early Jurassic of Germany and a review of fossil trachypachid genera. Historical Biology Historical 35(8):1-18. https://doi.org/10.1080/08912963.2022.2071709
Kluge, N.J. 2019. Cladoendesis of Parametabola and systematic position of the extinct taxon Tetrastigmoptera taxon nov. Entomological Review 99 (3): 362–383. https://doi.org/10.1134/S0013873819030084
Kubrakiewicz, J., I. Jedrzejowska, and S.M. Bilinski. 1998. Neuropteroidea – different ovary structure in related groups. Folia Histochemica et Cytobiologica 36: 179–187.
Kupryjanowicz, J., and V.N. Makarkin. 2008. Archiconiocompsa prisca Enderlein (Neuroptera: Coniopterygidae): The first neuropteran fossil in Rovno amber (Ukraine). Entomologica Fennica 19: 25–31. https://doi.org/10.33338/ef.84410
Li, H.Y., X.M. Bai, W.W. Zhang, B. Wang, and X.Y. Liu. 2019a. Taxonomic notes on dustywings of Aleuropteryginae (Insecta, Neuroptera, Coniopterygidae) from the mid-Cretaceous Burmese amber. Cretaceous Research 98: 122–135. https://doi.org/10.1016/j.cretres.2019.02.008
Li, H.Y., B. Wang, and X.Y. Liu. 2019b. First description of the male of Cretaconiopteryx grandis Liu & Lu, 2017 (Neuroptera: Coniopterygidae) from the Cretaceous Burmese amber. Zootaxa 4674 (4): 482–490. https://doi.org/10.11646/zootaxa.4674.4.7
Linnaeus, C. 1758. Systema naturae per regna tria naturae secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 10th ed. Vol. 1, 1–824. Holmiae, Salvii.
Liu, X.Y., and X.M. Lu. 2017. A remarkable new genus of Cretaceous dustywings (Neuroptera: Coniopterygidae) in amber from northern Myanmar. Zoological Systematics 42 (3): 380–389. https://doi.org/10.11865/zs.201716
Liu, X.Y., B.W. Price, F. Hayashi, F. de Moor, and D. Yang. 2014. Revision of the Megaloptera (Insecta: Neuropterida) of Madagascar. Zootaxa 3796: 320–336. https://doi.org/10.11646/zootaxa.3796.2.5
Liu, X.Y., H. Aspöck, S.L. Winterton, W.W. Zhang, and U. Aspöck. 2017a. Phylogeny of pleasing lacewings (Neuroptera: Dilaridae) with a revised generic classification and description of a new subfamily. Systematic Entomology 42: 448–471. https://doi.org/10.1111/syen.12225
Liu, X.Y., X.M. Lu, and W.W. Zhang. 2017b. New genera and species of the family Dipteromantispidae (Insecta: Neuroptera) in amber from the Cretaceous of Myanmar and New Jersey. Cretaceous Research 72: 18–25. https://doi.org/10.1016/j.cretres.2016.12.007
Liu, X.Y., X.M. Lu, and W.W. Zhang. 2017c. Phylogenetic position of Corydasialidae (Insecta: Neuropterida) revisited based on a significant new fossil in Cretaceous amber of Myanmar. Journal of Systematic Palaeontology 15: 571–581. https://doi.org/10.1080/14772019.2016.1200148
Liu, X.Y., X.M. Lu, and W.W. Zhang. 2016. Halteriomantispa grimaldii gen. et sp. nov.: A new genus and species of the family Dipteromantispidae (Insecta: Neuroptera) from the mid-Cretaceous amber of Myanmar. Zoological Systematics 41(2): 165–172. https://doi.org/10.11865/zs.201615
Makarkin, V.N. 1994. Oriental Hemerobiidae (Neuroptera) described by Waro Nakahara. Raffles Bulletin of Zoology 42: 917–926.
Makarkin, V.N. 2018. A new species of Haploberotha (Neuroptera: Berothidae) from mid-Cretaceous Burmese amber. Cretaceous Research 90: 375–381. https://doi.org/10.1016/j.cretres.2018.06.011
Makarkin, V.N., and E.E. Perkovsky. 2017. A new species of Glaesoconis Meinander (Neuroptera: Coniopterygidae) from the Santonian Taimyr amber. Cretaceous Research 75: 120–124. https://doi.org/10.1016/j.cretres.2017.03.019
Makarkin, V.N., and E.E. Perkovsky. 2019. New Coniopterygidae (Neuroptera) from the upper Cenomanian Nizhnyaya Agapa amber, northern Siberia. Cretaceous Research 93: 107–113. https://doi.org/10.1016/j.cretres.2018.09.006
Makarkin, V.N., and A.H. Staniczek. 2019. A new large-sized genus of Babinskaiidae (Neuroptera) from mid-Cretaceous Burmese amber. Cretaceous Research 104 (104196): 1–6. https://doi.org/10.1016/j.cretres.2019.104196
Makarkin, V.N., D. Ren, and Q. Yang. 2009. Two new species of Kalligrammatidae (Neuroptera) from the Jurassic of China, with comments on venational homologies. Annals of the Entomological Society of America 102 (6): 964–969. https://doi.org/10.1603/008.102.0606
Makarkin, V.N., Q. Yang, and D. Ren. 2013. A new Cretaceous family of enigmatic two-winged lacewings (Neuroptera). Fossil Record 16: 67-75. https://doi.org/10.1002/mmng.201300002
Makarkin, V.N., J. Ansorge, and A.V. Khramov. 2021 Revision of Epigambriinae Handlirsch, stat. nov., a subfamily of Early Jurassic Ithonidae s. l. (Neuroptera). Palaeoentomology 4(6): 516–531. https://doi.org/10.11646/palaeoentomology.4.6.1
Martynov, A.V. 1937. Permian fossil insects of Kargala and their relationships. Trudy Paleontologicheskogo Instituta 7 (2): 1–92 [In Russian].
Meinander, M. 1972. A revision of the family Coniopterygidae (Planipennia). Acta Zoologica Fennica 136: 1–357.
Meinander, M. 1975. Fossil Coniopterygidae (Neuroptera). Notulae Entomologicae 55: 53–57.
Meinander, M. 1979. The phylogeny and geographical distribution of the Aleuropteryginae (Neuroptera, Coniopterygidae). Annales Entomologici Fennici 45: 16–23.
Meinander, M. 1990. The Coniopterygidae (Neuroptera, Planipennia). A check-list of the species of the world, descriptions of new species and other new data. Acta Zoologica Fennica 189: 1–95.
Meinander, M. 1998. Coniopterygidae (Neuroptera) in amber from the Dominican Republic. Journal of Neuropterology 1: 33–36.
Navás, L. 1927. Veinticinco formas nuevas de insectos. Boletín de la Sociedad Ibérica de Ciencias Naturales 26: 48–75.
Nel, A., V. Perrichot, and D. Azar. 2005. New and poorly known fossil Coniopterygidae in Cretaceous and Cenozoic ambers (Insecta: Neuroptera). Annales Zoologici 55: 1–7. https://doi.org/10.3161/0003454053642103
Nel, A. 1991. Nouveaux insectes neuropteroïdes fossiles de l'Oligocene de France (Neuroptera et Megaloptera). Bulletin du Muséum National d'Histoire Naturelle (4e sér., C) 12 [for 1990]: 327–349.
Novokshonov, V.G. 1997. Early evolution of scorpionflies (Insecta: Panorpida), 140 pp., 10 pls. Moscow: Nauka [in Russian].
Ohm, P. 1995. Coniopterygidae in Bernstein-Einschlüssen. Eine vorläufige Übersicht. In 3. Treffen deutschsprachiger Neuropterologen (Schloß Schwanberg, D 97348 Rödelsee, 7.–9. April 1995). Galathea: Berichte des Kreises Nürnberger Entomologen e.V. Supplement 2: 19–20.
Oswald, J.D. 1993. Revision and cladistic analysis of the world genera of the family Hemerobiidae (Insecta: Neuroptera). Journal of New York Entomological Society 101: 143–299.
Oswald, J.D. 2022. Bibliography of the Neuropterida. Lacewing Digital Library, Research Publication No. 2. http://lacewing.tamu.edu/Biblio/Main. Accessed 27 Feb 2022
Pérez-de la Fuente, R., X. Delclòs, E. Peñalver, and M.S. Engel. 2019. A new dustywing (Neuroptera: Coniopterygidae) from the Early Cretaceous amber of Spain. Palaeoentomology 2: 279–288. https://doi.org/10.11646/PALAEOENTOMOLOGY.2.3.13
Perrichot, V., R. Garrouste, S. Azar, and A. Nel. 2014. A new genus of dustywings (Neuroptera: Coniopterygidae) in Late Cretaceous Vendean amber. In Fossil arthropods in Late Cretaceous Vendean amber (northwestern France), ed. V. Perrichot. Paleontological Contributions 10F: 10–28.
Pictet-Baraban, F.J., and H.A. Hagen. 1856. Die im Bernstein befindlichen Neuropteren der Vorwelt. In Die im Bernstein befindlichen organischen Reste der Vorwelt gesammelt, in Verbindung mit Mehreren bearbeitet und herausgegeben von Dr. Georg Carl Berendt. Bd 2, Abt. 2, 41–125. Berlin: Nicholaische Buchhandlung.
Ponomarenko, A.G. 1996. Upper Liassic neuropterans (Insecta) from Lower Saxony, Germany. Russian Entomological Journal 4(1/4) [for 1995]: 73–89.
Price, B.W., X.Y. Liu, F.C. de Moor, and M.H. Villet. 2012. A review of the alderfly genus Leptosialis Esben-Petersen (Megaloptera, Sialidae) with description of a new species from South Africa. Zookeys 201: 27–41. https://doi.org/10.3897/zookeys.201.2623
Randolf, S., and D. Zimmermann. 2019. Small, but oh my! Head morphology of adult Aleuropteryx spp. and effects of miniaturization (Insecta: Neuroptera: Coniopterygidae). Arthropod Structure & Development 50: 1–14. https://doi.org/10.1016/j.asd.2019.02.001
Randolf, S., D. Zimmermann, and U. Aspöck. 2014. Head anatomy of adult Nevrorthus apatelios and basal splitting events in Neuroptera (Neuroptera: Nevrorthidae). Arthropod Structure & Development 42: 111–136. https://doi.org/10.3897/asp.72.e31890
Randolf, S., D. Zimmermann, and U. Aspöck. 2017. Head anatomy of adult Coniopteryx pygmaea Enderlein, 1906: Effects of miniaturization and the systematic position of Coniopterygidae (Insecta: Neuroptera). Arthropod Structure & Development 46: 304–322. https://doi.org/10.1016/j.asd.2016.12.004
Riek, E.F. 1975. On the phylogenetic position of Brucheiser argentinus Navás, 1927 and description of a second species from Chile (Insecta: Neuroptera). Studies on the Neotropical Fauna 10: 117–126.
Ružičková, D., A. Nel, and J. Prokop. 2019. New dustywings from mid-Cretaceous of Myanmar amber reveal unexpected diversity (Neuroptera: Coniopterygidae). ZooKeys 827: 139–152. https://doi.org/10.3897/zookeys.827.31961
Shcherbakov, D.E. 2013. Permian ancestors of Hymenoptera and Raphidioptera. Zookeys 358: 45–67. https://doi.org/10.3897/zookeys.358.6289
Sziráki, G. 1996. Female internal genitalia of Megalithone tillyardi Riek, 1974 with comments on the systematic position of the neuropterous families (Neuroptera: Ithonidae). Folia Entomologica Hungarica 57: 277–284.
Sziráki, G. 2007. Studies on Brucheiserinae (Neuroptera: Coniopterygidae), with description of the second genus of the subfamily. Acta Zoologica Academiae Scientiarum Hungaricae 53 (Suppl. 1): 231–254.
Sziráki, G. 2016. A new dusty lacewing genus and species (Neuroptera: Coniopterygidae) from Cretaceous Burmese amber. Folia Historico-Naturalia Musei Matraensis 40: 89–93.
Sziráki, G. 2017. Taxonomic position of Paranimboa groehni Sziráki, 2016, with remarks on the Cretaceous genus Paranimboa Engel, 2016 (Neuroptera, Coniopterygidae). Folia Historico-Naturalia Musei Matraensis 41: 181–182.
Sziráki, G., and C. Gröhn. 2015. Presence of two extant genera of dusty lacewings (Neuroptera: Coniopterygidae) in Baltic amber, with remarks on some earlier described fossil taxa. Folia Historico-Naturalia Musei Matraensis 39: 63–71.
Sziráki, G., and O.S., Jr. Flint. 2007. Larva of Brucheiser penai Riek, 1975 (Neuroptera Coniopterygidae). Annali del Museo Civico di Storia Naturale di Ferrara 8 [for 2005]: 45–48.
Szwedo, J. 2018. The unity, diversity and conformity of bugs (Hemiptera) through time. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107 (2/3): 109–128. https://doi.org/10.1017/S175569101700038X
Tjeder, B. 1957. Neuroptera-Planipennia. The Lace-wings of Southern Africa. 1. Introduction and families Coniopterygidae, Sisyridae, and Osmylidae. In: South African Animal Life. Results of the Lund University Expedition in 1950–1951. Vol. 4, eds. B. Hanström, P. Brinck, and G. Rudebec, G., 95–188. Uppsala: Almqvist & Wiksells Boktryckri AB.
Vasilikopoulos, A., B. Misof, K. Meusemann, D. Lieberz, T. Flouri, R.G. Beutel, O. Niehuis, T. Wappler, J. Rust, R.S. Peters, A. Donath, L. Podsiadlowski, C. Mayer, D. Bartel, A. Böhm, S.L. Liu, P. Kapli, C. Greve, J.E. Jepson, X.Y. Liu, X. Zhou, H. Aspöck, and U. Aspöck. 2020. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evolutionary Biology 20: 64. https://doi.org/10.1186/s12862-020-01631-6.
Wang, Y.Y., X.Y. Liu, I.J. Garzón-Orduña, S.L. Winterton, Y. Yan, U. Aspöck, H. Aspöck, and D. Yang. 2017. Mitochondrial phylogenomics illuminates the evolutionary history of Neuropterida. Cladistics 33: 617–636. https://doi.org/10.1111/cla.12186
Wang, Y.Y., X.F. Zhou, L.M. Wang, X.Y. Liu, D. Yang, and A. Rokas. 2019. Gene selection and evolutionary modeling affect phylogenomic inference of Neuropterida based on transcriptome data. International Journal of Molecular Sciences 20 (5): 1072. https://doi.org/10.3390/ijms20051072.
Weitschat, W., and W. Wichard. 1998. Atlas der Pflanzen und Tiere im Baltischen Bernstein, 256 pp., 92 pls. München: Dr. Friedrich Pfeil Verlag.
Whalley, P.E.S. 1980. Neuroptera (Insecta) in amber from the Lower Cretaceous of Lebanon. Bulletin of the British Museum of Natural History (Geology) 33: 157–164.
Wichard, W., C. Chatterton, and A. Ross. 2005. Corydasialidae fam. n. (Megaloptera) from Baltic amber. Insect Systematics and Evolution 36: 279–283. https://doi.org/10.1163/187631205788838410
Wichard, W. 1997. Schlammfliegen aus Baltischem Bernstein (Megaloptera, Sialidae). Mitteilungen aus dem Geologisch-Paläontologischen Institut der Universität Hamburg 80: 197–211.
Winterton, S.L., A.R. Lemmon, J.P. Gillung, I.J. Garzon, D. Babano, D.K. Bakkes, L.C.V. Breitkreuz, M.S. Engel, E. Moriarty Lemmon, X.Y. Liu, R.J.P. Machado, J.H. Skevington, J.H., and J.D. Oswald. 2018. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Systematic Entomology 43: 330–354. https://doi.org/10.1111/syen.12278
Withycombe, C.L. 1922. The wing venation of the Coniopterygidae. Entomologist 55: 224–225.
Yang, Q., V.N. Makarkin, S.L. Winterton, A.V. Khramov, and D. Ren. 2012. A remarkable new family of Jurassic insects (Neuroptera) with primitive wing venation and its phylogenetic position in Neuropterida. PLoS ONE 7 (9): e44762. https://doi.org/10.1371/journal.pone.0044762
Yang, D., and X.K. Liu. 2010. Fauna Sinica. Vol. 51 (Insecta, Megaloptera), viii + 457 pp. Beijing: Science Press.
Zhao, C.J., X.Y. Liu, and Yang, D. 2014. Wing base structural data support the sister relationship of Megaloptera and Neuroptera (Insecta: Neuropterida). PLoS One 9: e114695. https://doi.org/10.1371/journal.pone.0114695
Zimmermann, D., W. Klepal, and U. Aspöck. 2009. The first holistic SEM study of Coniopterygidae (Neuroptera)—structural evidence and phylogenetic implications. European Journal of Entomology 106: 651–662. https://doi.org/10.14411/eje.2009.081
Zizzari, Z., P. Lupetti, C. Mencarelli, and R. Dallai. 2008. Sperm ultrastructure and spermiogenesis of Coniopterygidae (Neuroptera, Insecta). Arthropod Structure & Development 37: 410–417. https://doi.org/10.1016/j.asd.2008.03.001
Acknowledgements
The authors would like to thank Elena Lukashevich (Palaeontological Institute of RAS, Moscow) for her helpful comments on an originally supposed dipteran affinity of the fossil; Caleb C. Martins (Universidade Estadual do Maranhão, Caxias, Brazil), Dominique Zimmermann (Natural History Museum Vienna, Vienna, Austria), and Tatiana Vshivkova (Federal Scientific Center of the East Asia Terrestrial Biodiversity, Vladivostok, Russia) for providing SEM microphotographs; and Bruce Archibald (Department of Earth, Ocean, and Atmospheric Sciences, University of British Columbia, Canada) for editing of the English. Finally, we thank two anonymous reviewers and the Associate Editor Joachim T. Haug for their valuable suggestions to improve this manuscript. The research of VNM were carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 121031000151-3).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joachim T. Haug.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makarkin, V.N., Ansorge, J. The oldest dustywing (Neuroptera: Coniopterygidae) from the Lower Jurassic of Germany. PalZ 98, 105–116 (2024). https://doi.org/10.1007/s12542-023-00662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-023-00662-w