Abstract
The first Mesozoic scorpion from Italy, Protobuthus ziliolii sp. nov., is here described and named thanks to a single specimen. This new species comes from the Besano Formation (Middle Triassic) of Monte San Giorgio, a UNESCO World Heritage Locality (WHL). Taphonomical analysis allows interpretation of the specimen as a full-body fossil, rather than an exuvia. Different analytical techniques, such as optical, UV, and SEM microscopy, reveal different characters, not visible together with a single method. The new species is assigned to the family Protobuthidae. Protobuthus ziliolii is the first arachnid to be reported from the Besano Formation and the Mesozoic of Italy, the second from the Monte San Giorgio WHL, and the second species of the genus Protobuthus in the world. This discovery corroborates the previously hypothesized nearshore deposition for the genesis of the upper portion of the Besano Formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial arthropods are among the rarest fossils in the paleontological record (Wills 2001), and arachnids represent just a small part of this record. The phylogeny of Arachnida is a highly debated topic, the monophyly of Arachnida itself being challenged, and many different relationships between orders proposed (e.g., Shultz 2007; Dunlop 2010; Dunlop et al. 2014; Giribet 2018; Howard et al. 2019; Ballesteros and Sharma 2019; Lozano-Fernandez et al. 2019; Noah et al. 2020). Although controversial, we follow the latest phylogenetic analyses that place Xiphosura and Eurypterida within Arachnida (Ballesteros and Sharma 2019; Noah et al. 2020). Therefore, according to Dunlop et al. (2020), 2519 valid species are recognized among extinct taxa.
The fossil record of scorpions consisted of 145 valid species (Dunlop et al. 2020) until very recently when another scorpion species was described from the Meride Limestone (Magnani et al. 2022), doubling with this contribution the number of species unearthed from the Monte San Giorgio WHL. This number—contrary to the fossil record of all other extant arachnid orders—is skewed and higher toward the Paleozoic, making scorpions the most diverse arachnids of that Era, with a total of 81 valid species. Scorpions are also the only arachnid order with a well-defined stem- and crown-group in the Paleozoic (Stockwell 1989; Jeram 1994, 1998). Compared to this great diversity, Mesozoic and Cenozoic scorpions are quite rare, counting 39 valid species in the Mesozoic and 27 in the Cenozoic. Until the discovery of large amber deposits in Myanmar and the study of their inclusions (e.g., Lourenço 2002; Santiago-Blay et al. 2004; Lourenço and Beigel 2011; Lourenço 2012 and subsequent papers—see Selden and Ren 2017 for a full list of references), the Triassic period was the richest of the Mesozoic, with 9 (now 11) valid species described (Dunlop et al. 2020).
Here, we describe in detail a new Triassic scorpion, previously only noticed (Viaretti et al. 2020), which represents the first from the Mesozoic of Italy. This fossil comes from the “Sasso Caldo” site (Besano Formation, Middle Triassic, Monte San Giorgio UNESCO WHL), located on the NW slope of Monte Pravello, between Besano and Porto Ceresio (Varese, N Italy). This contribution also represents the first report ever of an arachnid from the Besano Formation and a step forward in the knowledge of Triassic arachnid paleobiodiversity.
Geological framework
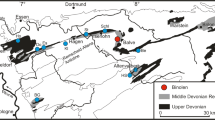
The Besano Formation crops out on Monte San Giorgio (Lombardy, Italy; and Canton Ticino, Switzerland; Fig. 1) and is one of the richest and well-known sites of Middle Triassic paleobiodiversity (e.g., Rieppel 2019) on par with more recently discovered Triassic sites in China (e.g., Benton et al. 2013). The Middle Triassic carbonate succession of Monte San Giorgio consists of four different formations deposited on a carbonate platform of the western margin of the Neo-Tethys (Bernasconi 1994; Furrer 1995; Röhl et al. 2001; Etter 2002; Stockar et al. 2012): these are the San Salvatore Dolomite, the Besano Formation, the San Giorgio Dolomite, and the Meride Limestone (Fig. 1). After the deposition of the Anisian Salvatore Dolomite, the development of a 30–130 m deep and approximately 20 km wide basin resulted in the deposition of the Besano Formation (e.g., Bernasconi 1991, 1994; Bernasconi and Riva 1993; Furrer 1995), from which a great part of the fossil fauna of Monte San Giorgio has been recovered (e.g., Bürgin et al. 1989; Furrer 2003). The Besano Formation is a 5 to 16 m thick alternation of black shales and variably laminated, organic-rich dolomitic banks; subordinate cineritic tuffs are dated as late Anisian-early Ladinian (Brack and Rieber 1986, 1993; Mundil et al. 1996; Brack et al. 2005; Wotzlaw et al. 2017).
The formation is divided into three portions (Rohl et al. 2001): the upper and the lower portions of the Besano Fm. represent a restricted, shallow, inter-to-subtidal carbonate platform rich in nearshore vertebrates, whereas the middle part is a slightly deeper intraplatform basin, from which a great number of ichthyosaurian remains and other pelagic vertebrates come (e.g., Dal Sasso and Pinna 1996; Brinkmann 1997; Maish and Matzke 1998; Renesto et al. 2020; Bindellini et al. 2021; Bindellini and Dal Sasso 2022). Recent biozonation of the Sasso Caldo site (Bindellini et al. 2019; Bindellini 2022), based on index-fossil invertebrates (ammonoids and the bivalve Daonella), allowed confident correlation of this stratigraphic section with the stratigraphy and biozonation of the coeval Swiss localities (Rieber 1973; Brack and Rieber 1993; Brack et al. 2005). This means that, at Sasso Caldo, only the middle and the upper portion of the Besano Formation crop out. The scorpion comes from the upper part of the outcrop (G. Teruzzi, pers. com. 2019), which is dated back to the earliest Ladinian (Bindellini et al. 2019).
Material and methods
Material
BES SC 1973, an almost complete fossil scorpion from the Besano Formation (Monte San Giorgio, UNESCO WHL). The specimen, 44.7 mm long, is deposited in the fossil collection of the Museo di Storia Naturale di Milano (MSNM), and it is preserved as part and counterpart in two little slabs of a finely laminated, dolomitic matrix enriched in organic matter (Fig. 2).
Specimen BES SC 1973, Protobuthus ziliolii sp. nov. a Visible light photo. b UV light photo. c Collage of SEM images taken in BSE (Back-Scattered Electrons) mode. d DEM (Digital Elevation Model) obtained through photogrammetric scan of the specimen. Scale bar equals 5 mm. For further details, see Fig. 8
Methods
To observe the anatomical features in this small-sized specimen, we used a Leica MZ9-5 stereomicroscope equipped with a plan 1.0 × lens, 10/20 × oculars, and a 0.63 to 6.0 zoom. A Wild Heerbrugg TYP 308,700 camera lucida was mounted on top for detailed drawings. Fluorescence induced by ultraviolet light (365 nm) was used to better see organic remains and highlight their chemical compositional differences, following previous studies (e.g. Tischlinger 2002; Hone et al. 2010; Dal Sasso and Maganuco 2011; Crippa and Masini 2022). Scanning electron microscopy (SEM) images and elemental peaks were obtained by analyzing microsamples, taken from carefully selected areas of the specimen, with a Jeol JSM 5610 LV (IXRF Systems Inc.) equipped with an EDS 500 spectrometer. Lastly, a macro-photogrammetric scan of the surface of the main slab, where most of the specimen is preserved, was performed; this allowed the output of a DEM (Digital Elevation Model), which helped to distinguish the surface morphology of the fossil (Fig. 2).
We followed the anatomical nomenclature of Vachon to define the carapace and mesosoma carinae (Vachon 1952) and the trichobothrial pattern (Vachon 1973).
Taphonomical remarks
The scorpion from Besano is exposed in dorsal view in the main slab (Fig. 2). Contrary to the first report (Viaretti et al. 2020), in-depth anatomical examination shows that the preservation of this specimen is sub-optimal. Nonetheless, diagnostic features are still present and distinctive. Prosoma, mesosoma, and metasoma are preserved in their entirety, whereas the limbs are quite damaged: the preserved legs are folded and stacked on the back of the scorpion; only isolated parts of the pedipalps are still present.
To evaluate whether the specimen is an exuvia (molt) or a full-body fossil, we carried out a taphonomical and morphological analysis, according to the criteria established by McCoy and Brandt (2009), which used the relative position of selected anatomical elements of the scorpion body. These criteria included the integrity and posture of the body line, the relative position of the legs and the pedipalps, as well as the telescoping of segments. Another criterion was the relative position of the chelicerae, which was the only one impossible to evaluate because in BES SC 1973 the remains of the mouth parts are only putative (Fig. 3, see below).
Prosoma of BES SC 1973. a Visible light photo of the counterpart. b Interpretation of the whole prosoma; c SEM (BSE) image of the median eyes on the counterpart. d Prosoma region preserved on the main slab. Scale bar equals 1 mm. Anatomical: cl central lateral carina; ol ocular lateral carina; pl posterior lateral carina; pm posterior median carina; ps posterior sub-median carina
The body line is straight, with the only exception of the metasoma (i.e., the tail), which is curved. The remains of the pedipalps are retained alongside the body and there is no trace of telescoping on the segments, neither the mesosomal nor the metasomal one. The walking legs are the most damaged part of the fossil. They are visible only on the right side of the scorpion, where only one of them still shows most of the segment jointed, whereas the others consist of single or coupled segments, all lumped together on the right side of the body and overlapping the back of the scorpion.
Based on these observations, we regard specimen BES SC 1973 to be a full-body fossil (Table 1). In fact, according to the experimental data provided by McCoy and Brandt (2009), in the totality of the examined cases, the carcasses show a straight body line and no telescoped segments; the walking legs are folded against the body in most cases (77%), and the pedipalps are pulled in toward the prosoma in more than half of the cases (54%). McCoy and Brandt (2009) also showed that appendages and metasomal segments are the first parts of a scorpion carcass to disarticulate. In sum, the scorpion described in this paper, albeit not fully matching the conditions reported, can be interpreted as the remains of a carcass that was left exposed for a sufficient period of time required to degrade the appendages, which could have been affected by different biostratinomic processes like scavenging, post-mortem contraction, and/or displacement due to light bottom currents.
Systematic paleontology
This publication is registered under Zoobank LSID urn:lsid:zoobank.org:pub:1E17AB1F-ACEB-452E-95EFF68F60246769.
Class Arachnida Lamarck, 1801
Order Scorpiones C. L. Koch 1837
Suborder Neoscorpiones Thorell and Lindstrӧm, 1885.
Infraorder Orthosterni Pocock, 1911
Superfamily Buthoidea C. L. Koch, 1837 (sensu Lourenço, 2000).
Family Protobuthidae Lourenço and Gall, 2004
Remarks. The definition of high-level taxonomic categories is a particularly difficult task when dealing with scorpions. Several authors have tried to solve the issue by using different techniques and combinations of characters, but not without controversies (e.g., Prendini 2000; Soleglad and Fet 2003; Fet et al. 2005; Prendini and Wheeler 2005; Santibáñez-López et al. 2019). The proposed diagnosis of the families involves various characters, such as carinae, median eyes, and overall shape of some anatomical parts, but the synapomorphies mainly used in the definition of extant families are found in the trichobothrial pattern. In fossil specimens, the use of trichobothria is strongly limited by the often poor preservation; it is therefore preferred to use morphological features more commonly preserved in the scorpion fossil record (Jeram 1994). Lourenço and Gall (2004) diagnosed the family Protobuthidae using all the characters present in the description of the genus Protobuthus, including a “very incomplete” trichobothrial pattern. Moreover, in their character list, they included a “probable” composition of the dentate margins of fixed fingers and “not observable” tibial spurs on the legs. This diagnosis fits the species Protobuthus elegans Lourenço and Gall 2004, as well as the specimen BES SC 1973; however, such vague descriptions of some characters, along with the lack of clear diagnostic characters, raise problems in the use of this taxonomic category. The specimen here described does not improve much the family-level diagnosis, as a clear and well-defined trichobothrial pattern is still missing to define the Protobuthidae.
Genus Protobuthus Lourenço and Gall 2004
Type species. Protobuthus elegans Lourenço and Gall 2004
Species Protobuthus ziliolii sp. nov.
Etymology. The species is dedicated to Michele Zilioli (MSNM), for the skillful and painstaking support he has given to paleontological research, in particular the fundamental contribution to exceptional discoveries about soft-tissue preservation in fossils (e.g., Dal Sasso and Maganuco 2011), and to this work itself.
Type specimen. Almost complete and articulated fossil specimen, labeled as BES SC 1973 in the catalog of the Museo di Storia Naturale di Milano (BES SC is an acronym for Besano Sasso Caldo), and coded as 20.S288-2.5 in the Inventario Patrimoniale dello Stato (State Heritage Database).
Type locality. Sasso Caldo site, Besano, Monte San Giorgio, Varese Province, NW Lombardy, N. Italy. Geographical coordinates: 45°54′03.7"N 8°55′10.6"E, elev. 650 m.
Type horizon and distribution. Upper Besano Formation (sensu Röhl et al. 2001), uppermost Anisian/lowermost Ladinian (uppermost N. secedensis Zone/lowermost E. curioni Zone sensu Brack et al. 2005), Middle Triassic.
LSID ZooBank. This new species is registered under urn:lsid:zoobank.org:act:735361AC-1C9F-4118-A190-1B446DEA4A89.
Diagnosis. Protobuthus with a strongly carinate carapace. Three main carinae: one strong posterior median carina and two lyra-shaped carinae. Anterior margin of the carapace convex, forming a small lobe. Median ocular tubercle more anteriorly located than in Protobuthus elegans Lourenço and Gall 2004. Mesosoma strongly tricarinate from segment II to segment V.
P. ziliolii differs from P. elegans because of the carinate carapace, which in P. elegans has a smooth tegument and lacks carinae or clearly visible furrows (Fig. 3). The carapace outline is also more triangular in P. ziliolii. Additionally, in P. ziliolii the shaft of the fixed finger of the chela is straight, whereas in P. elegans it is slightly curved medially (Fig. 4). P. elegans also differs by having a shorter metasoma with more-slender segments, particularly segments IV and V, where the length/depth ratio is double that in P. ziliolii (Table 2). The vesicle/aculeus ratio and the number of metasomal carinae are also different in the two species.
Description
Medium- to small-sized scorpion (overall length, 44.7 mm) with a very slender body (Table 2). Mesosoma with 7 segments; metasoma with 5 segments. Telson slender without subaculear tooth. Appendages badly preserved, consisting in right femur, left chela of the pedipalps, and various leg segments.
Prosoma. Prosomal shield (carapace) trapezoidal with a convex, almost lobed, anterior margin. Three strong carinae visible on the shield (Fig. 3): one posterior median carina (pm), located behind the median eyes and reaching the posterior margin of the carapace. The other two strong carinae are lyra-shaped (Hjelle 1990) and result from the fusion of three different carinae: ocular lateral carinae (ol), central lateral carinae (cl), and posterior sub-median carinae (ps). Additionally, two small and finer posterior lateral carinae (pl) are present. Median ocular tubercle located halfway between the anterior margin and the center of the carapace; median eyes are separated by a half ocular diameter.
Mesosoma. Tergite I bears seven carinae, of which five in continuity with the prosomal carinae. The median three are in continuity with the posterior median and sub-median carinae, and converge at the end of the tergite to form one strong axial carina. The remaining carinae are located on tergite I, two on each side: the ones on the external sides are in continuity with the posterior lateral carinae on the prosoma, whereas the others start from tergite I and become stronger from tergite II. Tergites II-VII are tricarinate, with three strong median carinae, one axial and two sub-median, and bear one pair of secondary carinae on each side, not always visible. The surface between carinae is rather smooth.
Metasoma. Segments from I to IV squared, particularly I and IV where the length/depth ratio is around one (Table 2). Segment V smaller and more slender. Segments II and IV with the most complete ornamentation pattern, where 6 carinae can be observed: dorsal, lateral-dorsal, intermedian, lateral-ventral, and two ventral. Telson weakly granular, in particular the aculeus. Vesicle with an oval shape and two weak lateral carinae; aculeus slender and longer than vesicle; subaculear tooth absent (Fig. 5).
Pedipalps. Badly preserved. The one on the right is still partly articulated, with coxa and femur recognizable thanks to morphology and relative position with the carapace and to each other; the patella is possibly present, but poor preservation prevents reliable identification. Another element preserved on the right pedipalp is the chela’s fixed finger, characterized by a rounded, inflated base and a straight shaft (Fig. 4).
The left pedipalp is mostly missing, but an isolated fragment near the left antero-lateral margin of the carapace is likely an entire article, partially covered distally by rock matrix. The exposed part of the fragment consists of a joint surface, three sides separated by carinae and a trichobothrial pattern. The trichobothria near the joint surface suggest that the fragment is the proximal end. Moreover, due to the lack of a clear concavity, which usually characterizes the tibial proximal end of scorpions (e.g., Vachon 1952; Sissom 1990; Fet and Kovařík 2020), and thanks to the presence of a right-angled small indentation, similar to the femoral proximal end of scorpions, we interpret the article fragment as the left femur of BES SC 1973. Seven trichobothria are visible on the segment: three on the internal surface (interpreted as i1?, i2, and i3?) and four on the dorsal surface (interpreted as d1, d2?, d3?, d4?, and d5) (Fig. 6), which, under the stereomicroscope, all appear slightly protruding from the article’s surface. Their presence and distribution are consistent with our diagnosis of left femur.
Legs. Badly preserved, only one leg easily recognizable albeit lying unnaturally above the mesosoma, with femur, patella, and tibia still articulated (Fig. 7). Each segment bears at least three carinae and, in addition, the femur shows at least two weak carinae with a transverse disposition.
Articulated leg of BES SC 1973 in visible light (a) and a SEM (BSE) image (b). Scale bar equals 1 mm. For anatomical abbreviations, see Fig. 8
Interpretative drawing of BES SC 1973 (a) and the specimen as preserved on the main slab (b). Scale bar equals 5 mm. Anatomical: ch chelicerae; co coxa; fe femur; ff fixed finger; me median eyes; ms mesosomal segment; mt metasomal segment; pa patella; pr prosoma; ta tarsus; te telson; ti tibia. Question marks indicate putative structures
Discussion
Seven Triassic scorpion families need to be evaluated to properly assess the taxonomic attribution of specimen BES SC 1973. These families are as follows: Mesophonidae Wills 1910; Willsiscorpionidae Kjellesvig-Waering 1986; Gallioscorpionidae Lourenço and Gall 2004; Stenoscorpionidae Kjellesvig-Waering 1986; Spongiophonidae Kjellesvig-Waering 1986; Isobuthidae Petrunkevitch 1913; and Protobuthidae Lourenço and Gall 2004. Comparisons with BES SC 1973 are made below.
Mesophonidae, Willsiscorpionidae, and Gallioscorpionidae are characterized by an anterior median protrusion on the carapace which bears the median eyes or, when this structure is absent, by the median eyes located in line with the anterior margin of the carapace, as in Willsiscorpio bromsgroviensis (Wills 1910). BES SC 1973 completely lacks any kind of anterior protrusion, the anterior margin of the carapace is slightly rounded, and the median eyes are located halfway between the anterior margin and carapace center. On the other hand, these characters are diagnostic of the family Protobuthidae (as stated above, see Systematic paleontology).
Any affinity of our specimen with the family Stenoscorpionidae can also be excluded since the latter is characterized by a prosomal shield divided in two halves by a longitudinal groove.
The families Spongiophonidae and Isobuthidae are both difficult to compare with the specimen here described due to the lack of diagnostic features preserved in BES SC 1973 and the fossil specimens assigned to the two families. The family Spongiophonidae is represented only by isolated tergites and a sternum articulated to four coxae, characterized by a peculiar ornamentation made of polygonal pustules (Wills 1947). Such a character is absent in BES SC 1973, so attribution to this family can be excluded. Finally, the family Isobuthidae includes only one Triassic scorpion, Bromsgroviscorpio willsi Kjellesvig-Waering 1986, erected upon a single half abdominal plate, which is impossible to compare with BES SC 1973 because not preserved in the latter. The remaining species belonging to Isobuthidae are Paleozoic and the specimen BES SC 1973 does not bear any diagnostic character of the family, a condition preventing comparisons.
In sum, we assign BES SC 1973 to Protobuthidae on the basis of its small-to-medium size, slender body, median ocular tubercle located anteriorly to the center of the carapace, eyes separated by less than an ocular diameter, lack of subaculear tooth on the telson, and aculeus longer than the vesicle. These characters are also diagnostic for the genus Protobuthus and the species P. elegans (Lourenço and Gall 2004). In contrast, the carinae on the metasomal segments and the trichobothrial pattern are inconsistent with such a diagnosis. However, the incomplete state of these characters, along with the sub-optimal preservation of this single specimen, is too weak to justify the erection of a new genus; therefore, we assign BES SC 1973 to the genus Protobuthus. Nevertheless, based on the description above, specimen BES SC 1973 represents the holotype of a new species: Protobuthus ziliolii sp. nov., characterized by the presence of three main carinae on the prosomal shield, a more anteriorly located median ocular tubercle, the straight shaft of the fixed finger of the chela, a longer and thicker metasoma, and a longer aculeus. The description of a new species from the family Protobuthidae increases the relevance of this scorpionid taxon during the Triassic faunal recovery.
Lourenço and Gall (2004) included the family Protobuthidae in the superfamily Buthoidea, but such an assignment has been questioned (Baptista et al. 2006). The phylogenetic position of these Triassic scorpions is crucial because they bridge the Carboniferous Palaeopisthacanthidae Kjellesvig-Waering 1986, and the Cretaceous Archaeobuthidae Lourenço, 2001, filling the absence of orthostern scorpions in the early phases of the Mesozoic (Baptista et al. 2006). The genus Protobuthus was eventually included in an unnamed group of closely related Mesozoic scorpions representing basal lineages without extant relatives, alongside Archaeobuthus Lourenço, 2001 and Palaeoburmesebuthus Lourenço, 2002 (Baptista et al. 2006). As a consequence, P. ziliolii should also be included in this group of basal scorpions. However, although incomplete, the trichobothrial pattern of P. ziliolii (Fig. 6) seems to reflect an intermediate condition between two of the patterns described by Soleglad and Fet (2001). On the dorsal surface of the femur, we have located trichobothria that can be interpreted as d1, d2?, d3?, d4? and d5 (“?” indicating putative detections). The arrangement of these trichobothria recalls the modern pattern β of the Type A, a typical buthid pattern (Vachon 1973, 1975; Soleglad and Fet 2001). More recently, patterns α and β have been subdivided into three subpatterns by Soleglad and Fet (2003) and their evaluation here is useful to interpret the trichobothrial pattern of P. ziliolii. These subpatterns are the alignment of d1–d3, the alignment of d3-d4, and the placement of d2; for each of them, a primitive (basal), an α, and a β arrangement were defined by the authors.
The dorsal trichobothrial pattern of the femur in P. ziliolii is intermediate between the primitive and the β arrangement: trichobothria d1–d3 are parallel to the dorsointernal carina, and d2 lies on the dorsal surface (primitive arrangement); on the other hand, trichobothria d3–d4 point away from the dorsoexternal carina (β arrangement). In fact, the trichobothrial pattern of the dorsal surface is intermediate between the basal pattern, found in Archaeobuthus and Pseudochactas Gromov 1998, and the modern β Type A pattern, found in buthid scorpions. The disposition of the putative trichobothria detected on the internal surface of P. ziliolii led us to the interpretation of i1?, i3? and i4?. These putative trichobothria are arranged similarly to those on the internal surface of the femur of Pseudochactas, where these trichobothria are somewhat in line and parallel to the dorsointernal carina. However, bad preservation of the pedipalps in BES SC 1973 prevents confident detection of femoral trichobothria; d1 and d5 are an exception, since their position is well-defined in our specimen. Still, as unfortunately common when dealing with fossils, this is an interpretation of an incomplete structure, nonetheless useful to define and describe the position of the trichobothria. Given the different taxonomic positions that may result from different trichobothrial interpretations, we follow the classification of Protobuthidae by Lourenço and Gall (2004). More and better-preserved specimens are needed to clarify the taxonomic position of the family Protobuthidae.
BES SC 1973 was recovered in the upper portion of the Besano Fm. (G. Teruzzi, pers. com., 2019). According to Rohl et al. (2001), its layers were deposited in a shallow inter-to-subtidal carbonate platform and are rich in nearshore species, including small sauropterygians, marine gastropods, terrestrial protorosaurs, and plants. The finding of a scorpion in the portion of the formation at the Sasso Caldo site corroborates the hypothesis of a near shoreline in that depositional phase which was closer to the shore than the older central portion, where a strong pelagic influence is documented (see “Geological framework”).
Conclusions
BES SC 1973 is a small-sized fossil scorpion recovered from the upper portion of the Besano Formation (Early Ladinian), at the Sasso Caldo site (Monte San Giorgio, UNESCO WHL). The specimen is almost complete, with sub-optimal preservation, and consists of a main slab and its counterpart. After careful morphological analysis carried out through optical microscopy, UV, and SEM, BES SC 1973 can be assigned to the taxon Protobuthus ziliolii sp. nov., included in the family Protobuthidae. This finding is the first arachnid recorded from the Besano Fm., the second from Monte San Giorgio WHL, and the second species attributed to the genus Protobuthus. The specimen also represents the first Mesozoic scorpion from Italy. The new species is diagnosed by the presence of a strongly carinate carapace, with one posterior median carina, two symmetrical lyra-shaped carinae (each resulting from the fusion of a posterior sub-median carina, a central lateral carina, and an ocular lateral carina) and two posterior lateral carinae. Other diagnostic characters are the median ocular tubercle located halfway between the anterior margin and the center of the carapace, the straight shaft of the fixed finger, the longer and thicker metasoma, and longer aculeus. With the description of this new species, the diversity of Triassic scorpions increases, as well as the interest toward Lower and Middle Triassic successions, which could likely bear a more diversified arthropod fauna. Moreover, the presence of a terrestrial arthropod corroborates the hypothesis of a near shoreline environment during the deposition of the upper Besano Formation.
The discovery of new specimens could clarify the incomplete, putative, or unknown characters of the new species, i.e. the trichobothrial pattern and the ventral side, and shed light on the recovery and evolution of these arachnids after the end-Permian extinction.
Data availability
BES SC 1973 is housed in the paleontological collection of Museo di Storia Naturale di Milano. All data are provided in the text and figures.
References
Ballesteros, J.A., and P.P. Sharma. 2019. A critical appraisal of the placement of Xiphosura (Chelicerata) with account of known sources of phylogenetic error. Systematic Biology 68: 896–917. https://doi.org/10.1093/sysbio/syz011.
Baptista, C., J.A. Santiago-Blay, M.E. Soleglad, and V. Fet. 2006. The Cretaceous scorpion genus, Archaeobuthus, revisited (Scorpiones: Archaeobuthidae). Euscorpius 35: 1–40.
Benton, M.J., Q. Zhang, S. Hu, Z.Q. Chen, W. Wen, J. Liu, J. Huang, C. Zhou, T. Xie, J. Tong, and B. Choo. 2013. Exceptional vertebrate biotas from the Triassic of China, and the expansion of marine ecosystems after the Permo-Triassic mass extinction. Earth Sciences Review 125: 199–243. https://doi.org/10.1016/j.earscirev.2013.05.014.
Bernasconi, S.M. 1991. Geochemical and microbial controls on dolomite formation and organic matter production/preservation in anoxic environments a case study from the Middle Triassic Grenzbitumenzone, Southern Alps (Ticino, Switzerland). Swiss Federal Institute of Technology Zürich, Switzerland, PhD thesis.
Bernasconi, S.M. 1994. Geochemical and microbial controls on dolomite formation in anoxic environments: a case study from the Middle Triassic (Ticino, Switzerland). Contributions to Sedimentology. 19: 1–109.
Bernasconi, S.M., and A. Riva. 1993. Organic geochemistry and depositional environment of a hydrocarbon source rock: the Middle Triassic Grenzbitumenzone Formation, Southern Alps, Italy/Switzerland. In: Generation, Accumulation and Production of Europe’s Hydrocarbons (vol.3). ed. A.M. Spencer, 179–190. Springer, Berlin, Heidelberg.
Bindellini, G. 2022. Study of the paleontological record of the Besano Formation (Middle Triassic) at “Sasso Caldo”, Varese, UNESCO WHL Monte San Giorgio. Università degli Studi di Milano, Italy, PhD thesis.
Bindellini, G., M. Balini, G. Teruzzi, and C. Dal Sasso. 2019. Ammonoid and Daonella zonation of the Sasso Caldo quarry (Besano Formation, Middle Triassic). In: Strati 2019, 3rd International Congress on Stratigraphy. ST2.4 Ammonoids in stratigraphy: Abstract book. ed. F.M. Petti, G. Innamorati, B. Carmina, and D. Germani, 87.
Bindellini, G., and C. Dal Sasso. 2022. First skeletal remains of Helveticosaurus from the Middle Triassic Italian outcrops of the Southern Alps, with remarks on an isolated tooth. Rivista Italiana Di Paleontologia E Stratigrafia 128: 625–641.
Bindellini, G., A.S. Wolniewicz, F. Miedema, T.M. Scheyer, and C. Dal Sasso. 2021. Cranial anatomy of Besanosaurus leptorhynchus Dal Sasso and Pinna, 1996 (Reptilia: Ichthyosauria) from the Middle Triassic Besano Formation of Monte San Giorgio, Italy/Switzerland: taxonomic and palaeobiological implications. PeerJ 9: e11179. https://doi.org/10.7717/peerj.11179.
Brack, P., and H. Rieber. 1986. Stratigraphy and ammonoids of the lower Buchenstein beds of the Brescian Prealps and Giudicarie and their significance for the Anisian/Ladinian Boundary. Eclogae Geologicae Helvetiae 79: 181–225.
Brack, P., and H. Rieber. 1993. Towards a better definition of the Anisian/Ladinian boundary: new biostratigraphic data and correlations of boundary sections from the Southern Alps. Eclogae Geologicae Helvetiae 86: 415–527.
Brack, P., H. Rieber, A. Nicora, and R. Mundil. 2005. The Global boundary Stratotype section and point (GSSP) of the Ladinian stage (Middle Triassic) at Bagolino (Southern Alps, Northern Italy) and its implications for the Triassic time scale. Episodes 28: 233–244.
Brinkmann, W. 1997. Die Ichthyosaurier (Reptilia) aus der Mitteltrias des Monte San Giorgio (Tessin, Schweiz) und von Besano (Lombardei, Italien) - der aktuelle Forschungsstand. Vierteljarsschrift Der Naturforschenden Gesellschaft in Zürich 142: 69–78.
Bürgin, T., O. Rieppel, P.M. Sander, and K. Tschanz. 1989. The fossils of Monte San Giorgio. Scientific American 260: 74–81.
Crippa, G., and S. Masini. 2022. Photography in the ultraviolet and visible violet spectra: unravelling methods and applications in palaeontology. Acta Palaeontologica Polonica 67 (3): 685–702. https://doi.org/10.4202/app.00948.2021.
Dal Sasso, C., and G. Pinna. 1996. Besanosaurus leptorhynchus n. gen. n. sp., a new shastasaurid ichthyosaur from the Middle Triassic of Besano (Lombardy, N. Italy). Paleontologia Lombarda, Nuova Serie 4: 3–23.
Dal Sasso, C., and S. Maganuco. 2011. Scipionyx samniticus (Theropoda: compsognathidae) from the lower Cretaceous of Italy. Osteology, ontogenetic assessment, phylogeny, soft tissue anatomy, taphonomy and paleobiology. Memorie Della Società Italiana Di Scienze Naturali e Del Museo Di Storia Naturale Di Milano 37: 1–282.
Dunlop, J.A. 2010. Geological history and phylogeny of Chelicerata. Arthropod Structure & Development 39 (2–3): 124–142. https://doi.org/10.1016/j.asd.2010.01.003.
Dunlop, J.A., J. Borner, and T. Burmester. 2014. Phylogeny of the Chelicerates: Morphological and Molecular Evidence. In Deep Metazoan Phylogeny: the backbone of the tree of life: new insights from analyses of molecules, morphology and theory of data analysis, ed. J.W. Waegele and T. Bartolomaeus, 395–408. Berlin: De Gruyter.
Dunlop, J.A., D. Penney, and D. Jekel. 2020. A summary list of fossil spiders and their relatives.World Spider Catalog. Natural History Museum Bern, online at http://wsc.nmbe.ch , version 20.5. Accessed 17 May 2021.
Etter, W. 2002. Monte San Giorgio: Remarkable Triassic marine vertebrates. In: Exceptional fossil preservation. A unique view on the evolution of marine life. ed. D.J. Bottjer, W., Etter, J.W. Hagadorn, and C.M. Tang, 220–242. New York: Columbia University Press.
Fet, V., and F. Kovařík. 2020. New scorpion taxa (Arachnida: Scorpiones) described in the journal “Euscorpius” in 2002–2020. Euscorpius. 2020 (300): 1–31.
Fet, V., S.E. Michael, and G. Lowe. 2005. A new trichobothrial character for the high-level systematics of Buthoidea (Scorpiones: Buthida). Euscorpius 2005 (23): 1–40.
Furrer, H. 1995. The Kalkschieferzone (Upper Meride Limestone; Ladinian) near Meride (Canton Ticino, Southern Switzerland) and the evolution of a Middle Triassic intraplatform basin. Eclogae Geologicae Helvetiae. 88: 827–852.
Furrer, H. 2003. Der Monte San Giorgio im Südtessin-vom Berg der Saurier zur Fossil-Lagerstätte internationaler Bedeutung. Neujahrsblatt Der Naturforschenden Gesellschaft in Zürich. 206: 1–64.
Giribet, G. 2018. Current views on chelicerate phylogeny—a tribute to Peter Weygoldt. Zoologischer Anzeiger. 273: 7–13. https://doi.org/10.1016/j.jcz.2018.01.004.
Gromov, A.V. 1998. A new family, genus and species of scorpions (Arachnida, Scorpiones) from southern central Asia. Zoologichesky Zhurnal 77 (9): 1003–1008.
Hjelle, J.T. 1990. Anatomy and morphology. In The biology of scorpions, ed. G.A. Polis, 9–63. Stanford, California: Stanford University Press.
Hone, D.W.E., H. Tischlinger, X. Xu, and F. Zhang. 2010. The extent of the preserved feathers on the four-winged dinosaur Microraptor gui under ultraviolet light. PLoS ONE 5 (2): e9223. https://doi.org/10.1371/journal.pone.0009223.
Howard, R.J., G.D. Edgecombe, D.A. Legg, D. Pisani, and J. Lozano-Fernandez. 2019. Exploring the evolution and terrestrialization of scorpions (Arachnida: Scorpiones) with rocks and clocks. Organisms Diversity & Evolution 19 (1): 71–86. https://doi.org/10.1007/s13127-019-00390-7.
Jeram, A.J. 1994. Carboniferous Orthosterni and their relationship to living scorpions. Palaeontology 37: 513–550.
Jeram, A.J. 1998. Phylogeny and classification of Palaeozoic scorpions. In Proceedings of the 17th European Colloquium of Arachnology, Edinburgh, 1997. ed. P.A. Selden, 17–31. The British Arachnological Society, Burnham Beeches.
Kjellesvig-Waering, E.N. 1986. A restudy of the fossil Scorpionida of the world. Palaeontographica Americana 55: 1–287.
Koch, C.L. 1837. Scorpionen. Übersicht des Arachnidensystems. Nürnberg CH. Zeh’sche Buchhandlung 1: 36–39.
Lamarck, J.B.P.A. 1801. Systême des animaux sans vertèbres. Paris: Lamarck and Deterville.
Lourenço, W.R. 2000. Panbiogéographie, les familles des scorpions et leur répartition géographique. Biogeographica 76: 21–39.
Lourenço, W.R. 2001. A remarkable scorpion fossil from the amber of Lebanon. Implications for the phylogeny of Buthoidea. Comptes Rendus De L’académie Des Sciences Paris, Sciences De La Terre Et Des Planètes 332: 641–646. https://doi.org/10.1016/S1251-8050(01)01583-X.
Lourenço, W.R. 2002. The first scorpion fossil from the Cretaceous amber of Myanmar (Burma). New implications for the phylogeny of Buthoidea. Comptes Rendus Palevol 1: 97–101. https://doi.org/10.1016/S1631-0683(02)00017-9.
Lourenço, W.R. 2012. About the scorpion fossils from the Cretaceous amber of Myanmar (Burma) with the descriptions of a new family, genus and species. Acta Biologica Paranaense 41: 75–87.
Lourenço, W.R., and A. Beigel. 2011. A new scorpion fossil from the Cretaceous amber of Myanmar (Burma). New Phylogenetic Implications. Comptes Rendus Palevol 10 (8): 635–639. https://doi.org/10.1016/j.crpv.2011.08.001.
Lourenço, W.R., and J.C. Gall. 2004. Fossil scorpions from the Buntsandstein (Early Triassic) of France. Comptes Rendus Palevol. 3: 369–378. https://doi.org/10.1016/j.crpv.2004.06.006.
Lozano-Fernandez, J., A.R. Tanner, M. Giacomelli, R. Carton, J. Vinther, G.D. Edgecombe, and D. Pisani. 2019. Increasing species sampling in chelicerate genomic-scale datasets provides support for monophyly of Acari and Arachnida. Nature Communications 10 (1): 1–8. https://doi.org/10.1038/s41467-019-10244-7.
Magnani, F., R. Stockar, and W.R. Lourenço. 2022. A new family, genus and species of fossil scorpion from the Meride Limestone (Middle Triassic) of Monte San Giorgio (Switzerland). Faunitaxys 10 (24): 1–7.
Maisch, M.W., and A.T. Matzke. 1998. Observations on Triassic ichthyosaurs. Part II: a new ichthyosaur with palatal teeth from Monte San Giorgio. Neues Jahrbuch Fur Geologie Und Palaontologie 1: 26–41. https://doi.org/10.1127/njgpm/1998/1998/26.
McCoy, V.E., and D.S. Brandt. 2009. Scorpion taphonomy: criteria for distinguishing fossil scorpion molts and carcasses. The Journal of Arachnology 37: 312–320. https://doi.org/10.1636/SH09-07.1.
Mundil, R., P. Brack, M. Meier, H. Rieber, and F. Oberli. 1996. High resolution U-Pb dating of Middle Triassic volcaniclastics: time-scale calibration and verification of tuning parameters for carbonate sedimentation. Earth and Planetary Science Letters 141: 137–151. https://doi.org/10.1016/0012-821X(96)00057-X.
Noah, K.E., J. Hao, L. Li, X. Sun, B. Foley, Q. Yang, and X. Xia. 2020. Major revisions in arthropod phylogeny through improved supermatrix, with support for two possible waves of land invasion by chelicerates. Evolutionary Bioinformatics 16: 1176934320903735.
Petrunkevitch, A. 1913. A monograph of the terrestrial Palaeozoic Arachnida of North America. Transactions of the Connecticut Academy of Arts and Sciences 18: 1–137.
Pocock, R.I. 1911. A monograph of the terrestrial Carboniferous Arachnida of Great Britain. Monographs of the Palaeontographical Society 64: 1–84.
Prendini, L. 2000. Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): An exemplar approach. Cladistics 16 (1): 1–78. https://doi.org/10.1111/j.1096-0031.2000.tb00348.x.
Prendini, L., and W.C. Wheeler. 2005. Scorpion higher phylogeny and classification, taxonomic anarchy, and standards for peer review in online publishing. Cladistics 21 (5): 446–494. https://doi.org/10.1111/j.1096-0031.2005.00073.x.
Renesto, S., C. Dal Sasso, F. Fogliazza, and C. Ragni. 2020. New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin. Acta Paleontologica Polonica 65: 511–522. https://doi.org/10.4202/app.00731.2020.
Rieber, H. 1973. Die Triasfauna der Tessiner Kalkalpen. Cephalopoden aus der Grenzbitumenzone (Mittlere Trias) des Monte San Giorgio (Kt. Tessin, Switzerland). Schweizerische Palaeontologische Abhandlungen 93: 1–96.
Rieppel, O. 2019. Mesozoic sea dragons: Triassic marine life from the ancient tropical lagoon of Monte San Giorgio. Bloomington: Indiana University Press.
Röhl, H.J., A. Schmid-Röhl, H. Furrer, A. Frimmel, W. Oschmann, and L. Schwark. 2001. Microfacies, geochemistry and palaeoecology of the Middle Triassic Grenzbitumenzone from Monte San Giorgio (Canton Ticino, Switzerland). Geologia Insubrica 6: 1–13.
Santiago-Blay, J.A., V. Fet, M.E. Soleglad, and S.R. Anderson. 2004. A new genus and subfamily of scorpions from Lower Cretaceous Burmese amber (Scorpiones: Chaerilidae). Revista Iberica De Aracnología 9: 3–1.
Santibáñez-López, C.E., E. González-Santillán, L. Monod, and P.P. Sharma. 2019. Phylogenomics facilitates stable scorpion systematics: reassessing the relationships of Vaejovidae and a new higher-level classification of Scorpiones (Arachnida). Molecular Phylogenetics and Evolution 135: 22–30. https://doi.org/10.1016/j.ympev.2019.02.021.
Selden, P.A., and D. Ren. 2017. A review of Burmese amber arachnids. The Journal of Arachnology 45 (3): 324–343. https://doi.org/10.1636/JoA-S-17-029.
Shultz, J.W. 2007. A phylogenetic analysis of the arachnid orders based on morphological characters. Zoological Journal of the Linnean Society 150 (2): 221–265. https://doi.org/10.1111/j.1096-3642.2007.00284.x.
Sissom, W.D. 1990. Systematics, Biogeography and Paleontology. In The Biology of Scorpions, ed. G.A. Polis, 31–80. Stanford, California: Stanford University Press.
Soleglad, M.E., and V. Fet. 2001. Evolution of scorpion orthobothriotaxy—a cladistic approach. Euscorpius 1: 1–38.
Soleglad, M.E., and V. Fet. 2003. High-level systematic and phylogeny of the extant scorpions (Scorpiones: orthosterni). Euscorpius 11: 1–175.
Stockar, R., P.O. Baumgartner, and D. Condon. 2012. Integrated Ladinian bio-chronostratigraphy and geochronology of Monte San Giorgio (Southern Alps, Switzerland). Swiss Journal of Geosciences 105: 85–108. https://doi.org/10.1007/s00015-012-0093-5.
Stockwell, S.A. 1989. Revision of the phylogeny and higher classification of scorpions (Chelicerata). University of California, Berkeley, PhD thesis.
Thorell, T., and G. Lindström. 1885. On a Silurian scorpion from Gotland. Konglige Svenska Vetenskaps-Akademiens Handlingar 21: 1–33.
Tischlinger, H. 2002. Der Eichstätter Archaeopteryx im langwelligen UV-Licht. [The Eichstätt specimen of Archaeopteryx under longwave ultraviolet light]. Archaeopteryx 20: 21–38.
Vachon, M. 1952. Études sur les scorpions. Institut Pasteur d’Algérie.
Vachon, M. 1973. [1974]. Étude des caractères utilisés pour classer les familles et les genres de scorpions (Arachnides). 1. La trichobothriotaxie en arachnologie. Sigles trichobothriaux et types de trichobothriotaxie chez les scorpions. Bulletin Du Muséum National D’histoire Naturelle 3: 857–957.
Vachon, M. 1975. Sur l’utilisation de la trichobothriotaxie du bras des pédipalpes des Scorpions (Arachnides) dans le classement des genres de la famille de Buthidae Simon. Comptes Rendus Des Séances De L’académie Des Science 281: 1597–1599.
Viaretti, M., G. Bindellini, and C. Dal Sasso. 2020. An exceptionally well-preserved scorpion from the Besano Formation (Monte San Giorgio, Middle Triassic, Southern Alps): Preliminary study. In Fossilia. Volume 2020. ed. S. Bartolini Lucenti, O. Cirilli, and L. Pandolfi, 53–55. https://doi.org/10.32774/FosRepPal.2020.0614
Wills, L.J. 1910. On the fossiliferous Lower Keuper Rocks of Worcestershire: with descriptions of some of the plants and animals discovered therein. Proceedings of the Geologists’ Association 21: 249–331.
Wills, L.J. 1947. A Monograph of British Triassic Scorpions. Part I Pages i–iv, 1–74; Plates I–VI. Monographs of the Palaeontographical Society. 100: i–74.
Wills, M.A. 2001. How good is the fossil record of arthropods? An assessment using the stratigraphic congruence of cladograms. Geological Journal 36: 187–210. https://doi.org/10.1002/gj.882.
Wotzlaw, J.F., P. Brack, and J.C. Storck. 2017. High-resolution stratigraphy and zircon U-Pb geochronology of the Middle Triassic Buchenstein Formation (Dolomites, northern Italy): precession-forcing of hemipelagic carbonate sedimentation and calibration of the Anisian-Ladinian boundary interval. Journal of the Geological Society 175: 71–85. https://doi.org/10.1144/jgs2017-052.
Acknowledgements
We thank Giorgio Teruzzi (MSNM), who directed the Sasso Caldo excavations for several years, and the volunteers of the former “Gruppo paleontologico di Besano”, who unearthed the specimens studied here, and many other exceptional fossils. We also thank Michele Zilioli (MSNM) for SEM analysis and macrophotography, the Soprintendenza Archeologia, Belle Arti e Paesaggio of the provinces of Como, Lecco, Sondrio, Monza-Brianza, Pavia, and Varese for permissions. We warmly thank Mike Latronico for improving the English writing. This paper is part of a Ph.D. project (G. Bindellini) focusing on the Besano Formation fauna, led by the Università Statale di Milano (M. Balini) in agreement with the Museo di Storia Naturale di Milano (C. Dal Sasso). Finally, we thank the editor Michael Rasser and the anonymous reviewers for their constructive comments which improved our manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Joachim T. Haug.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viaretti, M., Bindellini, G. & Dal Sasso, C. A new Mesozoic scorpion from the Besano Formation (Middle Triassic, Monte San Giorgio UNESCO WHL), Italy. PalZ 97, 505–517 (2023). https://doi.org/10.1007/s12542-023-00659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-023-00659-5