Abstract
Despite the large number of species in the group Coleoptera (beetles), it is usually relatively easy to identify an adult beetle as such due to certain common characteristics. Among beetle larvae, however, there is a larger variability of body organisation. In some lineages, specialised larval morphologies are carried on into the adult phase by heterochrony, more exactly paedomorphosis. Such evolutionary events resulted in larviform females, as they occur in some extant representatives of Lycidae (net-winged beetles) and Lampyridae (fireflies). However, such larviform individuals, larvae or paedomorphic females, have been very rarely described in the fossil record until now and were restricted to Cenozoic ambers. Here, we report fossil larviform representatives, resembling larvae of the groups Lampyridae and Lycidae in certain aspects, from 100-million-year-old Myanmar amber. We furthermore discuss the morphological similarities and differences of the three new specimens in relation to extant larviform representatives of the groups and possible relationships of the new fossils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The group Coleoptera is extremely species-rich, with more than 380,000 formally described species of beetles (e.g. McKenna et al. 2019). Still, most people are able to identify an adult representative of the group as a beetle. This is possible due to the rather stereotypic morphology of an adult beetle: when viewed from above (dorsal view), head, pronotum, and the two elytra (forewings) are apparent (with few exceptions such as rove beetles). Especially, the straight anterior–posterior line between the elytra is very different from the arrangement in other winged representatives of Insecta, as there is, for example, no apparent prominent part of the scutellum (triangular sclerite between the wing bases).
A larger variability of body organisation within Coleoptera is represented by the larval stages. A strong differentiation between larvae and adults in beetles is possible due to a certain evolutionary decoupling of these stages (e.g. Scholtz 2005). Selective pressures can, therefore, lead to highly specialised larval morphologies. In some lineages of Coleoptera, such specialised larval morphologies have been carried on into the adult life phase by heterochrony, more exactly paedomorphosis (Kundrata and Bocak 2011; McMahon and Hayward 2016; Rosa et al. 2020). This has led to larviform females, i.e. adult females that retain an overall larva-like morphology. Phylogenetic reconstructions clearly indicate that this phenomenon has evolved independently in several lineages.
A quite famous case is that of the “trilobite larva” (Mjöberg 1925). Females and larvae of the group Platerodrilus (in older literature also as Duliticola; Masek and Bocak 2014) have tiny heads with stout antennae, laterally drawn out thorax tergites, and processes on the abdomen segments. In fact, also within the closely related larger group Lycidae, highly specialised larvae and other paedomorphic females are known. Lycidae includes up to 4600 species (Masek et al. 2018 p. 2; Molino-Olmedo et al. 2020, p. 1), yet of only 2% the larvae are indeed known (Levkanicova and Bocak 2009 p. 210); still the morphological diversity (≈ disparity) of these is astonishing (Levkanicova and Bocak 2009, Figs. 2, 4, 5 p. 215).
Very similar larvae are also known in the group Lampyridae (most similar in Emeia pseudosauteri; Fu et al. 2012, Figs. 5, 6, p. 4). Also in many representatives of Lampyridae (fireflies), a group of about 2,200 species (Martin et al. 2019, p. 1), females are larviform. However, most firefly larvae differ from the trilobite-type larvae; they appear less heavily derived, at least on a first glance. Lycidae and Lampyridae are both ingroups of Elateroidea. The two groups have been suggested to be sister groups in some analyses (Kundrata and Bocak 2011), but not in others (e.g. Kundrata et al. 2014).
Representatives of Lycidae and Lampyridae have been found as fossils, especially often in different types of amber. So far, all fossil finds seem to be winged adults, no larviform individual, neither larva nor paedomorphic female, has been reported so far. Here, we report larviform specimens that share many characters with extant trilobite-type beetle larvae and fireflies (and their paedomorphic females), but also differ in certain aspects.
Material and methods
Material
In the centre of this study are three fossil specimens, BUB 3369, BUB 3845, and BUB 3989. The specimens originate from 100-million-year-old Cretaceous Kachin amber (99 million years old, hence rounded to about 100 million years old) from the Hukawng Valley, Myanmar (Cruickshank and Ko 2003; Shi et al. 2012; Yu et al. 2019). They come from the collection of one of the authors (PM). The specimens were legally exported from Myanmar before 2017 (see discussion in Haug et al. 2020).
Documentation methods
The specimens were documented on a Keyence VHX-6000 digital microscope. White and black background and different illumination settings were used (cross-polarised co-axial light and low-angle ring light; Haug et al. 2013a, 2018). Several images of varying focus and several adjacent image details as well as different exposure times (HDR, cf. Haug et al. 2013b) were combined, resulting in composite images (Haug et al. 2011). All images were further processed and colour-marked with Adobe Photoshop CS2.
Description of specimens
Specimen BUB 3369
General
Elongate, slightly dorso-ventrally compressed fusiform body, with slightly tapering anterior and posterior part. Total body length ~ 3.38 mm. Body differentiated into head and trunk (Fig. 1). Trunk differentiated into anterior trunk region (thorax) and posterior trunk region (abdomen). Head prognathous (mouthparts facing forward). Thorax with three segments (pro-, meso-, metathorax). Thorax with one pair of long locomotory appendages (legs) on each segment. Abdomen with nine discernible units. Trunk units with well-sclerotised tergites, laterally drawn out into dorso-lateral processes. In dorsal view, second row of similar processes discernible ventro-laterally, partially hidden by dorso-lateral ones. Body bears short setae, especially discernible on abdomen laterally.
Specimen BUB 3369, specimen resembling larviform fireflies: a habitus in dorsal view; b colour-marked version of a; c habitus in ventral view; d details of head in ventral view; e colour-marked version of d; f detail of spine-like process on terminal end, flipped forward; g colour-marked version of f. a2–a6, abdomen segment 2–6; at, antenna; da, dorso-lateral process on abdomen segment; fe, femur; hc, head capsule; li, labium; md, mandible; ms, mesothorax; mt, metathorax; mx, maxilla; pt, prothorax; ti, tibia (or tibio-tarsus?); va, ventro-lateral process on abdomen segment
Head
Head roughly pentagonal in dorsal view, wider than long, 1.6× (~ 0.26 mm long and ~ 0.43 mm wide), partially hidden by prothorax tergite dorsally (Fig. 1a, b). No stemmata discernible. Antenna (appendage of post-ocular segment 1) with three elements discernible (Fig. 1a–c). Antenna longer than head capsule, 1.3× (~ 0.34 mm long) and relatively wide (~ 0.07 mm wide). Most distal element the longest (~ 0.19 mm), significantly longer than other two elements, 1.25×. Labrum (derivative of ocular segment) continuous with head capsule (no sutures discernible), anterior edge bears multiple small setae (at least 16).
Mandibles (appendages of post-ocular segment 3), sickle-shaped (Fig. 1d, e), strongly sclerotised, with no setae discernible. Right mandible partially hidden by the counterpart. Left mandible ~ 0.25 mm long and ~ 0.06 mm wide at its widest point.
Maxillae (appendages of post-ocular segment 4), differentiated in proximal part (stipes?) and palp. Proximal part elongate, longer than wide, 6.4 × (~ 0.24 mm long), with median protrusion facing anteriorly (galea, lacinia, or mala). Latero-distally with palp (~ 0.09 mm long) with four elements discernible (Fig. 1d, e). No setae discernible.
Labium (conjoined appendages of post-ocular segment 5) with no clear sutures between elements discernible. Distally with pair of possible palps (Fig. 1d, e).
Trunk
Prothorax sub-hexagonal in dorsal view, with posterior part slightly drawn out posteriorly (Fig. 1a, b), wider than long, 1.6 × (max. length ~ 0.47 mm, max. width ~ 0.77 mm). Mesothorax sub-hexagonal in dorsal view, largest thorax segment, wider than other thorax segments, 1.1× (max. length ~ 0.48, max. width ~ 0.85 mm). Metathorax sub-hexagonal in dorsal view, wider than long, 1.9× (max. length ~ 0.41 mm, max. width ~ 0.8 mm).
Legs relatively long, longer than mesothorax, 1.8× (0.85 mm long), with five discernible elements (Fig. 1c): coxa, trochanter, femur, tibio-tarsus, and distal claw.
Abdomen segments 1–7 sub-similar, wider than long (~ 0.2 mm long, 0.73–0.91 mm wide), with abdomen segment 4 being widest (Fig. 1a–c). Abdomen segment 8 longer and narrower than previous segments (~ 0.27 mm long, ~ 0.57 mm wide). Last discernible unit, terminal end (possible compound of several segments), semicircular in dorsal view, with single dorsal process postero-medially. Process with triangular proximal and pin-like distal part (Fig. 1f, g).
Specimen BUB 3989
General
Elongate, slightly dorso-ventrally compressed fusiform body, with slightly tapering anterior and posterior part. Total body length ~ 2.31 mm. Body differentiated into head and trunk (Fig. 2). Trunk differentiated into anterior trunk region (thorax) and posterior trunk region (abdomen). Head prognathous (mouthparts facing forward). Thorax with three segments (pro-, meso-, metathorax). Thorax with one pair of long locomotory appendages (legs) on each segment. Abdomen with nine discernible units. Trunk units with well-sclerotised tergites, laterally drawn out into dorso-lateral processes. In dorsal view, second row of similar processes discernible ventro-laterally, partially hidden by dorso-lateral ones. Body bears short setae, especially discernible on abdomen laterally.
Specimen BUB 3989, specimen resembling larviform fireflies: a habitus in dorsal view; b colour-marked version of a. a4–a8, abdomen segment 4–8; at, antenna; da, dorso-lateral process on abdomen segment; hc, head capsule; ms, mesothorax; mt, metathorax; mx, maxilla; pt, prothorax; te, terminal end; va, ventro-lateral process on abdomen segment
Head
Head roughly pentagonal in dorsal view, slightly longer than wide, 1.1× (~ 0.3 mm long and ~ 0.28 mm wide) (Fig. 2a, b). No stemmata discernible. Antenna (appendage of post-ocular segment 1) club-shaped, with three elements discernible (Fig. 2a, b). Antenna shorter than head capsule, 1.3× (~ 0.22 mm long) and relatively wide (~ 0.05 mm wide). Most distal element the longest (~ 0.16 mm), significantly longer than other two elements, 2.65×, bears multiple setae.
Labrum (derivative of ocular segment) continuous with head capsule (no sutures discernible), anterior edge bears single discernible micro-seta (Fig. 2b).
Mandibles (appendages of post-ocular segment 3) with no setae discernible. Both mandibles partially hidden by labrum in dorsal view.
Maxillae (appendages of post-ocular segment 4) presumably differentiated in proximal part (not discernible) and palp. Palp (~ 0.12 mm long) with multiple elements distinguishable (Fig. 2b), exact number not discernible. Proximal part of palp bears a single seta (Fig. 2b).
Labium (conjoined appendages of post-ocular segment 5) not discernible.
Trunk
Prothorax sub-hexagonal in dorsal view (Fig. 2a, b), wider than long, 2× (max. length ~ 0.28 mm, max. width ~ 0.57 mm). Mesothorax sub-hexagonal in dorsal view, sub-similar to prothorax (max. length ~ 0.29, max. width ~ 0.57 mm). Metathorax trapezoidal in dorsal view, wider than long, 3 × (max. length ~ 0.21 mm, max. width ~ 0.64 mm), shortest and widest segment of thorax.
Legs relatively long, longer than mesothorax, 2.2× (~ 0.64 mm long), with five presumed elements: coxa, trochanter, femur, tibio-tarsus, and distal claw. Femur, tibio-tarsus and claw clearly discernible (Fig. 2a, b).
Abdomen segments 1–7 sub-similar, wider than long (~ 0.10–0.21 mm long, ~ 0.59–0.75 mm wide), with abdomen segment 4 being longest and abdomen segment 2 widest (Fig. 2a, b). Posterior rim of abdomen segment 8 medially convex in dorsal view. Last discernible unit, terminal end (possible compound of several segments), rhomboid in dorsal view, with single dorsal process postero-medially. Process with triangular proximal and pin-like distal part (Fig. 2b).
Specimen BUB 3845
General
Elongate body, with slightly tapering posterior part. Total body length ~ 3.92 mm. Body differentiated into head and trunk (Fig. 3). Trunk differentiated into anterior trunk region (thorax) and posterior trunk region (abdomen). Head prognathous (mouthparts facing forward). Thorax with three segments (pro-, meso-, metathorax). Thorax with one pair of long locomotory appendages (legs) on each segment. Abdomen with nine discernible units. Trunk units with well-sclerotised tergites, laterally drawn out into dorso-lateral processes. On abdomen dorso-lateral processes longer and thinner, rod-like, with distal parts facing posteriorly (Fig. 3a, b). In dorsal view on thorax, second row of similar processes discernible ventro-laterally on thorax, partially hidden by dorso-lateral ones. In ventral view on abdomen, second row of significantly shorter processes discernible ventro-laterally (Fig. 4a, b). Head, antennae, prothorax and legs bear short setae.
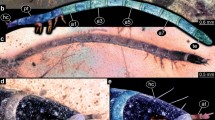
Specimen BUB 3845, trilobite-like beetle: a habitus in dorsal view; b colour-marked version of a; c details of antennae, arrows indicate four distinct structures on distal surface; d details of locomotory appendage (leg). a2–a6, abdomen segment 2–6; cl, claw (tarsungulum?); fe, femur; hc, head capsule; ms, mesothorax; mt, metathorax; pt, prothorax; ti, tibia (or tibio-tarsus?)
Specimen BUB 3845, trilobite-like beetle, continued. a habitus in ventral view; b detail of trunk region, including locomotory appendage (leg). cl, claw (tarsungulum?); cx, coxa; da, dorso-lateral process on abdomen segment; dt, dorso-lateral process on thorax segment; fe, femur; mp, maxillary palp; ti, tibia (or tibio-tarsus?); tr, trochanter; va, ventro-lateral process on abdomen segment; vt, ventro-lateral process on thorax segment
Head
Head semicircular in dorsal view, wider than long, 1.8× (~ 0.26 mm long and ~ 0.43 mm wide), partially hidden by prothorax tergite dorsally (Fig. 3a, b). No stemmata discernible. Antenna (appendage of post-ocular segment 1) club-shaped, with three elements discernible (Figs. 3a–c, 4a). Antenna longer than head capsule, 2.1× (~ 0.63 mm long) and relatively wide (~ 0.2 mm wide). Most distal element the longest (~ 0.41 mm), longer than other two elements together, 1.9×, bears multiple micro-setae. Distal surface of most distal antenna element with four distinct globular structures (Fig. 3c).
Labrum (derivative of ocular segment) continuous with head capsule (no sutures discernible), anterior edge bears multiple micro-setae.
Mandibles (appendages of post-ocular segment 3) not discernible.
Maxillae (appendages of post-ocular segment 4) presumably differentiated in proximal part (not discernible) and palp. Palp of maxilla (~ 0.13 mm long) with three elements distinguishable (Fig. 4a). No setae discernible.
Labium (conjoined appendages of post-ocular segment 5) not discernible.
Trunk
Prothorax trapezoidal in dorsal view (Fig. 3a, b), with posterior part drawn out posteriorly (ventro-lateral processes), wider than long, 1.8× (max. length ~ 0.74 mm, max. width ~ 1.37 mm). Prothorax tergite hexagonal, wider than long, 1.6× (max. width ~ 1.21 mm). Mesothorax trapezoidal in dorsal view, wider than long, 2.9× (max. length ~ 0.51, max. width ~ 1.49 mm), tergite trapezoidal, wider than long, 2.5× (max. width ~ 1.29 mm). Metathorax trapezoidal in dorsal view, wider than long, 3.3× (max. length ~ 0.42 mm, max. width ~ 1.39 mm), tergite trapezoidal, wider than long, 3.2× (max. width ~ 1.33 mm).
Legs relatively long, longer than whole thorax, 1.3× (~ 2.1 mm long), with five discernible elements (Figs. 3d, 4b): coxa, trochanter, femur, tibio-tarsus, and distal claw.
Abdomen segments 1–7 sub-similar, slightly tapering antero-posteriorly, due to dorso-lateral processes significantly wider than long (~ 0.15–0.26 mm long, ~ 1.23–1.58 mm wide), with abdomen segment 3 being widest (Fig. 3a, b). Abdomen segment 8 possibly longer and narrower than previous segments but partially inaccessible. Last unit, terminal end (possible compound of several segments), not discernible. Dorso-lateral abdomen processes significantly longer (max. 0.51 mm long) than ventro-lateral abdomen processes (max. 0.14 mm long) (Fig. 4b).
Discussion
Identity of the new fossil specimens: the coarse frame
All three fossils reported here share certain features. The overall arrangement of the body segments into head, thorax with three appendage-bearing segments, and abdomen with 11 or slightly less segments immediately identifies the specimens as representatives of Insecta (Hexapoda of many authors). Absence of wings in combination with a tibio-tarsus either indicates a position outside Pterygota or immature stages of a representative of Holometabola. The arrangement of the mouthparts, absence of genitalia and compound eyes support the latter interpretation. The overall campodeiform habitus is best compatible with an interpretation as a larva of a beetle (Coleoptera).
In the specimen with accessible mouthparts, the mandibles are sickle-shaped. In combination with the overall habitus, specimens BUB 3369 and BUB 3989 remind of larvae of the group Cantharidae (e.g. LeSage 1991 Fig. 34.465a p. 430). Yet, the trunk segments of the three specimens have short lateral processes, one row of more dorsal and one row of more ventral processes. Such processes seem to be absent in larvae of Cantharidae.
Processes as in the fossils are particularly known from certain representatives of the group Elateroidea, most prominently in the ingroups Lycidae (net-winged beetles), Lampyridae (fireflies) and Drilini (false firefly beetles) and especially in their larvae (but also in paedomorphic females; more on this aspect below). The here reported specimens are unlikely to be representatives of Drilini, as in larvae of this group prominent urogomphi are developed, which are absent in the new fossils. In addition, the abdomen processes become longer towards the posterior end in larvae of Drilini (Baalbergen et al. 2016 Fig. 3k p. 167), which is not the case in the fossils.
Also in some other beetle groups, the larvae have at least comparable processes (e.g. Silphidae, Erotylidae, Chrysomelidae, and Coccinellidae). Larvae of Silphidae lack the prominent sickle-shaped mandibles, have urogomphi not seen in any of the here described specimens, and lack the stout antenna present in the here presented specimens (e.g. Mahlerova et al. 2021). Larvae of Erotylidae and Chrysomelidae with processes differ from the here described specimens in possessing urogomphi and lacking similar mandibles (e.g. Skelley 2009; Świętojańska 2009). In larvae of Coccinellidae, the antennae are less prominent than in our specimens and do not show distal structures comparable to the ones seen here (e.g. Ślipiński 2007).
Comparison to larvae of Lampyridae and Lycidae
Specimens BUB 3369 and BUB 3989 appear sub-similar to each other and resemble modern larvae of Lampyridae (e.g. Riley et al. 2021). Many modern larvae of Lampyridae have two rows of processes on the trunk segments as in the fossils (example here: LaBella and Lloyd 1991 Fig. 34.463 p. 428). Other larvae of Lampyridae appear more onisciform having broad tergo-pleura-like protrusions (LaBella and Lloyd 1991 Fig. 34.462 p. 428), not displaying a prominent two-row arrangement. The two fossils are clearly more similar to the first type of larvae. The fossils have especially strong similarities to the larva of Luciola atra (Branham 2011 Fig. 4.15.2D p. 143).
BUB 3845 resembles modern larvae of Lycidae (“trilobite larvae”) in several aspects, but also differs in certain features. For example, the head is in relation to the body rather small in modern larvae of Lycidae (Mjöberg 1925 Fig. 1 pl. III; Lok 2008 Fig. 1 p. 176; Kusy et al. 2019 Fig. 2A p. 913), but is of a more “normal” proportion in the fossil. The shape of the head is indeed rather similar (Kazantsev and Zaitsev 2008 Fig. 13 p. 286). In addition, the short antennae of the fossils strongly resemble antennae of modern net-winged beetle larvae (McCabe and Johnson 1979 Fig. 1 p. 284; Miller 1997 Fig. 1 p. 7; Kazantsev and Nikitsky 2011 Fig. 1–22 pl. 2; Fanti et al. 2017 Fig. 2 p. 140). Yet, in modern larvae only two elements are apparent in the antenna (e.g. see supplementary information in Bocak et al. 2016), while in the fossil there are clearly three such elements. Other morphological features characteristic for modern larvae of Lycidae such as a circular pygopodium or non-opposable, longitudinally divided mandibles (e.g. Bocak and Matsuda 2003) are not accessible in the fossil larva and can, therefore, not be used for the present discussion.
The exact morphology of the trunk processes varies in the modern larvae of Lycidae:
(1) There are rod-like processes, comparable to those in the fossil (Mjöberg 1925 Figs. 1, 2, 7 pl. III; Brues 1941 pl. II p. 31, pl. III p. 32 upper; Levkanicova and Bocak 2009 Fig. 2 p. 215; Masek and Bocak 2014 Fig. 2, 3 p. 37, Figs. 32, 33 p. 47, Figs. 35, 36, 38, 39, 42 p. 49; Masek et al. 2014 Figs. 12, 15, 16 p. 138; Kusy et al. 2019 Fig. 2E p. 913).
(2) There are short processes (Mjöberg 1925 Figs. 2a, 3 pl. III; Lok 2008 Fig. 1, p. 176; Masek and Bocak 2014 Fig. 43, p. 49).
(3) There are very thin processes (Mjöberg 1925 Fig. 5 pl. III; Brues 1941 pl. III p. 32 lower; Levkanicova and Bocak 2009 Fig. 4 p. 215; Masek and Bocak 2014 Fig. 40 p. 49; Kusy et al. 2019 Fig. 3O p. 916).
(4) There are tergo-pleura-like processes (similar to those of some larvae of Lampyridae, see above; Masek and Bocak 2014 Fig. 37 p. 49; Masek et al. 2014 Figs. 13, 14 p. 138; Kusy et al. 2019 Fig. 2A p. 916).
(5) Finally, there are some more aberrant larvae with very strange processes (Levkanicova and Bocak 2009 Fig. 5 p. 215).
While the overall trilobite-larva-type morphology seems pretty unique, there are in fact some firefly larvae that appear very similar to trilobite beetle larvae (Fu et al. 2012 Fig. 5 p. 4, Fig. 30 p. 16). Hence BUB 3845 resembles, concerning the trunk processes, certain modern larvae of Lycidae (those with rod-like processes), but also certain larvae of Lampyridae.
The complicated phylogenetic frame of Elateroidea
As pointed out above, the specimens reported here resemble modern-day larvae of Lycidae and Lampyridae in certain aspects, in a more distant way also larvae of Cantharidae.
Cantharidae has been resolved as the sister group to Lampyridae + Lycidae (e.g. Kundrata and Bocak 2011 Fig. 2 p. 371), or to Lampyridae with Lycidae as an earlier branch within Elateroidea (Kundrata et al. 2014 Fig. 1; Rosa et al. 2020 Fig. 6), or to Lycidae with Cantharidae + Lycidae as sister group to a larger group including Lampyridae (Douglas et al. 2021 Fig. 2), or with the three groups not being directly related, but all as ingroups of Elateroidea (Zhang et al. 2018; Fig. 2; Cai et al. 2022; Fig. 1). In the first case, the similarities of the larvae of Lycidae, Lampyridae, and Cantharidae may characterise (as an apomorphy) a group including only these three ingroups. In the latter cases, one might suggest that the similarities may be interpreted as a result of convergent evolution. Alternatively, the specific larval morphology may characterise a rather basal node within Elateroidea, with more further derived lineages (e.g. Elateridae) having secondarily further modified larval morphology.
Most likely, a combination of both aspects, i.e. retained ancestral features and convergences, may be the best explanation. Convergent evolution of larval forms within this part of the tree of Elateroidea seems to be common, also explaining why some firefly larvae basically appear like trilobite beetle larvae (Fu et al. 2012 Fig. 5 p. 4, Fig. 30 p. 16).
This situation makes a more definite interpretation of the here reported fossils challenging: two of the specimens resemble modern larvae of Lampyridae and the other mostly larvae of Lycidae (but also certain larvae of Lampyridae). It seems unlikely that the latter specimen is a representative of Lycidae (“crown group”) given the rather large head and the three elements in the antennae; yet, these may represent plesiomorphic characters that are further derived in the modern representatives. The fossil may, therefore, be interpreted as an early offshoot of the lineage (“stem-lineage representative”) or simply as a possible sister species.
Yet, given the character distribution as discussed above, the interpretation of the three fossils is even more complicated. They might potentially represent larvae of offshoots of the evolutionary lineage towards Lampyridae + Lycidae, Lampyridae + Cantharidae, Cantharidae + (Lampyridae + Lycidae), (Lampyridae + Cantharidae) + (smaller ingroup of Elateroidea), or also Lycidae + ((Lampyridae + Cantharidae) + smaller ingroup of Elateroidea). In all these parts of the tree, we can at least expect a rather similar-appearing larval morphology. Combined with the quite common occurrence of convergent evolution in Elateroidea (e.g. Kundrata and Bocak 2011), we cannot easily exclude that the new specimens are representatives of these parts of the tree. Especially, the rather large head of specimen BUB 3845 may well represent a plesiomorphic feature, indicating that, despite its overall trilobite-larva-type appearance, the specimen might be a representative of a more ancestral lineage.
Are the new fossils larvae?
We have pointed out that the three new fossils resemble larvae of Lampyridae and Lycidae in certain aspects. Does that immediately identify them as larvae? In other lineages, this might already be sufficient (but see also discussion in Haug (2020) on this problem with larviform fossils in general). However, especially in Elateroidea, and even more so in Lycidae and Lampyridae, many females retain an overall larviform morphology and never reach the “typical adult-beetle-type” of morphology (Kundrata and Bocak 2011). This leaves the possibility that the specimens are larviform females.
Larviform females of Lampyridae often have an adult-like anterior body (especially head, prothorax) and only the posterior trunk resembles that of the larvae (e.g. Kundrata and Bocak 2011 Fig. 1 p. 370; Branham 2011 Fig. 4.15.2B p. 143; Dong et al. 2021 Fig. 101 p. 464). Larviform females of Lycidae strongly resemble their larvae in overall appearance (e.g. Kundrata and Bocak 2011 Fig. 1 p. 370; McMahon and Hayward 2016 Fig. 3 p. 510).
Based on this comparison, BUB 3369 and BUB 3989 are more likely larvae than paedomorphic females, given their head morphology. For BUB 3845 this aspect is less easy to be evaluated. The rather small size of all specimens is clearly more indicative for an interpretation of all three specimens as larvae, but it cannot be fully excluded that one of them is a very small-sized paedomorphic female.
The fossil record of Lycidae and Lampyridae
So far, 16 fossil species of Lycidae have been formally described (numbers combined from Molino-Olmedo et al. 2020 and Li et al. 2021), but 3 of these have been interpreted as representatives of Tenebrionoidea (Bocak et al. 2022), resulting in 13 accepted species. Most of these were based on specimens preserved in amber: 3 species are known from Miocene Dominican amber (Kazantsev 2012a; Ferreira and Ivie 2017), at least 4 species are known from Eocene Baltic amber (Kazantsev 2013), and apparently at least 3 species from Cretaceous Kachin amber from Myanmar (numbers combined from Bocak et al. 2019, 2022; Molino-Olmedo et al. 2020; Li et al. 2021).
Fossils of Lampyridae are even rarer. Also here, only few specimens are known from non-amber deposits (e.g. Wedmann et al. 2010). At least three species in Eocene Baltic amber (Kazantsev 2012b, c; Alekseev 2019) have been formally described, a single one from Cretaceous Kachin amber (Kazantsev 2015; Alekseev 2019). A larva of Lampyridae has recently been reported from Dominican amber (Oligocene–Miocene; Ferreira et al. 2022).
The fossil record of larviform individuals of Elateroidea
Klausnitzer (2003) reported, for Baltic amber, that beetle larvae are rarely preserved and often difficult to interpret. Due to the strong evolutionary independence between larvae and adults, fossils of larvae are important for holometabolans, including beetles, when attempting to reconstruct ecological aspects of fossil faunas (see discussion in Baranov et al. 2019), instead of inferring larval or other life stages based on adult males.
Many larvae of the group Elateroidea have rather peculiar morphologies and, therefore, should be recognisable when found as fossils, which has been suggested as a pre-requisite by Klausnitzer (2003). Still, few fossil larviform individuals of Elateroidea have so far been reported in addition to the larva of Lampyridae from Dominican amber mentioned above (Ferreira et al. 2022).
Larvae of Eucnemidae have been found preserved in sedimentary rocks (Chang et al. 2016) and in amber (Zippel et al. 2022 early view). There, legless vermiform to buprestiform appearance in combination with often jagged heads allowed an easy identification of the fossils.
Larvae of the very species-poor group Brachypsectridae have been found in various types of amber (Dominican, about 15 million years old, Baltic, about 35 million years, Kachin, about 100 million years old; recently summarised in Haug et al. 2021). These larvae have highly specialised processes on all trunk segments.
A possible fossil larva of the group Cantharidae was reported by Fowler (2019 Fig. 2 p. 141). More such larvae seem to be present in various collections, but have not been described in detail so far (pers. obs.)
In summary, the overall fossil record of larviform representatives of Elateroidea is still quite scarce. The here reported fossils are, therefore, possibly an important addition to this still rather limited fossil record.
Data availability
All data are provided in the text and the figures.
Code availability
Not applicable.
References
Alekseev, V.I. 2019. New extinct Eocene Coleoptera in Baltic amber of Friedhelm Eichmann’s collection (Germany). Baltic Journal of Coleopterology 19: 11–22.
Baalbergen, E., R. Schelfhorst, and M. Schilthuizen. 2016. Drilus larvae in the Netherlands (Coleoptera: Elateridae: Drilini). Entomologische Berichte 76: 165–173.
Baranov, V., C. Hoffeins, H.-W. Hoffeins, and J.T. Haug. 2019. More than dead males: Reconstructing the ontogenetic series of terrestrial non-biting midges from the Eocene amber forest. Bulletin of Geosciences 94: 187–199.
Bocak, L., and K. Matsuda. 2003. Review of the immature stages of the family Lycidae (Insecta: Coleoptera). Journal of Natural History 37: 1463–1507.
Bocak, L., R. Kundrata, C.A. Fernández, and A.P. Vogler. 2016. The discovery of Iberobaeniidae (Coleoptera: Elateroidea): A new family of beetles from Spain, with immatures detected by environmental DNA sequencing. Proceedings of the Royal Society B 283: 20152350.
Bocak, L., Y. Li, and S. Ellenberger. 2019. The discovery of Burmolycus compactus gen. et sp. nov. from the mid-Cretaceous of Myanmar provides the evidence for early diversification of net-winged beetles (Coleoptera, Lycidae). Cretaceous Research 99: 149–155.
Bocak, L., P. Müller, M. Motyka, and D. Kusy. 2022. Prototrichalus is transferred to the Tenebrionoidea: A comment on Molino-Olmedo et al., 2020,‘The description of Prototrichalus gen. nov. and three new species from Burmese amber supports a mid-Cretaceous origin of the Metriorrhynchini (Coleoptera, Lycidae)’. Cretaceous Research 133: 104837.
Branham, M.A. 2011. Lampyridae Latreille, 1817. In Handbook of Zoology, Coleoptera, Beetles, Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia Partim), vol. 2, ed. R.A.B. Leschen, R.G. Beutel, and J.F. Lawrence, 141–149. Berlin and New York: Walter de Gruyter.
Brues, C.T. 1941. The Sumatran “Trilobite Larva.” Psyche 48 (1): 24–33.
Cai, C., E. Tihelka, M. Giacomelli, J.F. Lawrence, A. Ślipiński, R. Kundrata, S. Yamamoto, M.K. Thayer, A.F. Newton, R.A.B. Leschen, M.L. Gimmel, L. Lü, M.S. Engel, P. Bouchard, D. Huang, D. Pisani, and P.C.J. Donoghue. 2022. Integrated phylogenomics and fossil data illuminate the evolution of beetles. Royal Society Open Science 9: 211771.
Chang, H., J. Muona, P. Hanyong, X. Li, W. Chen, M. Teräväinen, R. Dong, Y. Qiang, Z. Xingliao, and J. Songhai. 2016. Chinese Cretaceous larva exposes a southern Californian living fossil (Insecta, Coleoptera, Eucnemidae). Cladistics 32: 211–214.
Cruickshank, R.D., and K. Ko. 2003. Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences 21: 441–455.
Dong, Z., V. Yiu, G. Liu, J. He, R. Zhao, Y. Peng, and X. Li. 2021. Three new species of Lamprigera Motschulsky (Coleoptera, Lampyridae) from China, with notes on known species. Zootaxa 4950: 441–468.
Douglas, H.B., R. Kundrata, A.J. Brunke, H.E. Escalona, J.T. Chapados, J. Eyres, R. Richter, K. Savard, A. Ślipiński, D. McKenna, and J.R. Dettman. 2021. Anchored phylogenomics, evolution and systematics of Elateridae: Are all bioluminescent Elateroidea derived click beetles? Biology 10: 451.
Fanti, F., V. Di Taddeo, and M. Bocci. 2017. Description of the larva of Lygistopterus anorachilus Ragusa, 1883 (Coleoptera: Lycidae). Baltic Journal of Coleopterology 17 (2): 137–145.
Ferreira, V.S., and M.A. Ivie. 2017. The first fossil species of the extant genus Cessator Kazantsev (Coleoptera: Lycidae): A new Leptolycini from Dominican Amber. The Coleopterists Bulletin 71: 57–60.
Ferreira, V.S., A. Solodovnikov, M.A. Ivie, and R. Kundrata. 2022. Dominican amber net-winged beetles suggest stable paleoenvironment as a driver for conserved morphology in a paedomorphic lineage. Scientific Reports 12: 5820.
Fowler, M.J. 2019. Eocene world: Imaging fossil insects in Baltic amber. Bulletin of the Amateur Entomologists’ Society 78: 139–146.
Fu, X.H., L.A. Ballantyne, and C. Lambkin. 2012. Emeia gen. nov., a new genus of Luciolinae fireflies from China (Coleoptera: Lampyridae) with an unusual trilobite-like larva, and a redescription of the genus Curtos Motschulsky. Zootaxa 3403: 1–53.
Haug, J.T. 2020. Why the term “larva“ is ambiguous, or what makes a larva? Acta Zoologica 101: 167–188.
Haug, C., G. Mayer, V. Kutschera, D. Waloszek, A. Maas, and J.T. Haug. 2011. Imaging and documenting gammarideans. International Journal of Zoology 2011: 380829.
Haug, C., K.R. Shannon, T. Nyborg, and F.J. Vega. 2013a. Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Bolétin De La Sociedad Geológica Mexicana 65: 273–284.
Haug, J.T., C.H.G. Müller, and A. Sombke. 2013b. A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity and Evolution 13: 425–432.
Haug, J.T., P. Müller, and C. Haug. 2018. The ride of the parasite: A 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters 4: 31.
Haug, J.T., D. Azar, A. Ross, J. Szwedo, B. Wang, A. Arillo, V. Baranov, J. Bechteler, R. Beutel, V. Blagoderov, X. Delclòs, J. Dunlop, K. Feldberg, R. Feldmann, C. Foth, R.H.B. Fraaije, A. Gehler, D. Harms, L. Hedenäs, M. Hyžny, J.W.M. Jagt, E.A. Jagt-Yazykova, E. Jarzembowski, H. Kerp, P.K. Khine, A.G. Kirejtshuk, C. Klug, D.S. Kopylov, U. Kotthoff, J. Kriwet, R.C. McKellar, A. Nel, C. Neumann, A. Nützel, E. Peñalver, V. Perrichot, A. Pint, E. Ragazzi, L. Regalado, M. Reich, J. Rikkinen, E.-M. Sadowski, A.R. Schmidt, H. Schneider, F.R. Schram, G. Schweigert, P. Selden, L.J. Seyfullah, M.M. Solórzano-Kraemer, J.D. Stilwell, B.W.M. van Bakel, F.J. Vega, Y. Wang, L. Xing, and C. Haug. 2020. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated April 21, 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber. PalZ 94: 431–437.
Haug, J.T., A. Zippel, G.T. Haug, C. Hoffeins, H.-W. Hoffeins, J.U. Hammel, V. Baranov, and C. Haug. 2021. Texas beetle larvae (Brachypsectridae)—the last 100 million years reviewed. Palaeodiversity 14: 161–183.
Kazantsev, S.V. 2012a. A new lycid genus from the Dominican Amber (Insecta, Coleoptera, Lycidae, Leptolycinae, Leptolycini). Psyche 2012: 982141.
Kazantsev, S.V. 2012b. A new Luciolinae firefly (Coleoptera: Lampyridae) from the Baltic Amber Luciolinae (Coleoptera: Lampyridae). Russian Entomological Journal 21: 319–320.
Kazantsev, S.V. 2012c. New omethid and lampyrid taxa from the Baltic Amber (Insecta: Coleoptera). Zootaxa 3186: 59–63.
Kazantsev, S.V. 2013. A new fossil genus of net-winged beetles, with a brief review of amber Lycidae (Insecta: Coleoptera). Zootaxa 3608: 94–100.
Kazantsev, S.V. 2015. Protoluciola albertalleni gen. n., sp. n., a new Luciolinae firefly (Insecta: Coleoptera: Lampyridae) from Burmite amber. Russian Entomological Journal 24 (4): 281–283.
Kazantsev, S.V., and N.B. Nikitsky. 2011. Larvae of net-winged beetles (Lycidae: Coleoptera) of the European part of Russia and the Caucasus. Kaukasian Entomological Bulletin 7 (2): 129–134.
Kazantsev, S.V., and A.A. Zaitsev. 2008. Description of larval and imaginal stages of new species from the genera Pseudacroleptus Pic, 1911 and Ceratoprion Gorham, 1880 (Coleoptera: Lycidae: Leptolycinae). Russian Entomological Journal 17 (3): 283–292.
Klausnitzer, B. 2003. Käferlarven (Insecta: Coleoptera) in Baltischem Bernstein—Möglichkeiten und Grenzen der Bestimmung. Entomologische Abhandlungen 61: 103–108.
Kundrata, R., and L. Bocak. 2011. The phylogeny and limits of Elateridae (Insecta, Coleoptera): Is there a common tendency of click beetles to soft-bodiedness and neoteny? Zoologica Scripta 40: 364–378.
Kundrata, R., M. Bocakova, and L. Bocak. 2014. The comprehensive phylogeny of the superfamily Elateroidea (Coleoptera: Elateriformia). Molecular Phylogenetics and Evolution 76: 162–171.
Kusy, D., M. Motyka, M. Bocek, M. Masek, and L. Bocak. 2019. Phylogenomic analysis resolves the relationships among net-winged beetles (Coleoptera: Lycidae) and reveals the parallel evolution of morphological traits. Systematic Entomology 44: 911–925.
LaBella, D.M., and J.E. Lloyd. 1991. Lampyridae (Cantharoidea). In Immature insects, ed. F.W. Stehr, 427–428. Dubuque, Iowa: Kendall-Hunt Publishing Co.
LeSage, L. 1991. Cantharidae (Cantharoidea) (inclduding Chauliognathidae). In Immature insects, ed. F.W. Stehr, 429–431. Dubuque, Iowa: Kendall-Hunt Publishing Co.
Levkanicova, Z., and L. Bocak. 2009. Identification of net-winged beetle larvae (Coleoptera: Lycidae) using three mtDNA fragments: A comparison of their utility. Systematic Entomology 34 (2): 210–221.
Li, Y.D., E. Tihelka, and D. Huang. 2021. Murcybolus gen. nov., a new net-winged beetle genus from mid-Cretaceous Burmese amber (Coleoptera: Lycidae: Burmolycini). Zootaxa 4966: 77–83.
Lok, A.F.S.L. 2008. A Singapore trilobite larva, Duliticola species. Nature in Singapore 1: 175–178.
Mahlerová, K., P. Jakubec, M. Novák, and J. Růžička. 2021. Description of larval morphology and phylogenetic relationships of Heterotemna tenuicornis (Silphidae). Scientific Reports 11: 16973.
Martin, G.J., K.F. Stanger-Hall, M.A. Branham, L.F. Da Silveira, S.E. Lower, D.W. Hall, X.-Y. Li, A.R. Lemmon, E.M. Lemmon, and S.M. Bybee. 2019. Higher-level phylogeny and reclassification of Lampyridae (Coleoptera: Elateroidea). Insect Systematics and Diversity 3 (6): 11.
Masek, M., and L. Bocak. 2014. The taxonomy and diversity of Platerodrilus (Coleoptera, Lycidae) inferred from molecular data and morphology of adults and larvae. ZooKeys 426: 29–63.
Masek, M., M.A. Ivie, V. Palata, and L. Bocak. 2014. Molecular phylogeny and classification of Lyropaeini (Coleoptera: Lycidae) with description of larvae and new species of Lyropaeus. Raffles Bulletin of Zoology 62: 136–145.
Masek, M., M. Motyka, D. Kusy, M. Bocek, Y. Li, and L. Bocak. 2018. Molecular phylogeny, diversity and zoogeography of net-winged beetles (Coleoptera: Lycidae). Insects 9 (4): 154.
McCabe, T.L., and L.M. Johnson. 1979. Larva of Calopteron terminale (Say) with additional notes on adult behavior (Coleoptera: Lycidae). Journal of the New York Entomological Society 87: 283–288.
McKenna, D.D., S. Shin, D. Ahrens, M. Balke, C. Beza-Beza, D.J. Clarke, A. Donath, H.E. Escalona, F. Friedrich, H. Letsch, S. Liu, D. Maddison, C. Mayer, B. Misof, P.J. Murin, O. Niehuis, R.S. Peters, L. Podsiadlowski, H. Pohl, E.D. Scully, E.V. Yan, X. Zhou, A. Ślipiński, and R.G. Beutel. 2019. The evolution and genomic basis of beetle diversity. Proceedings of the National Academy of Sciences 116: 24729–24737.
McMahon, D.P., and A. Hayward. 2016. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecological Entomology 41: 505–515.
Miller, R.S. 1997. Immature stages of Plateros floralis (Melsheimer) and discussion of phylogenetic relationships (Coleoptera: Lycidae). The Coleopterists’ Bulletin 51: 1–12.
Mjöberg, E. 1925. The mystery of the so called “trilobite larvae” or “Perty’s larvae” definitely solved. Psyche 32 (3): 119–156.
Molino-Olmedo, F., V.S. Ferreira, M.A. Branham, and M.A. Ivie. 2020. The description of Prototrichalus gen. nov. and three new species from Burmese amber supports a mid-Cretaceous origin of the Metriorrhynchini (Coleoptera, Lycidae). Cretaceous Research 111: 104452.
Riley, W.B., S.P. Rosa, and L.F. Lima da Silveira. 2021. A comprehensive review and call for studies on firefly larvae. PeerJ 9: e12121.
Rosa, S.P., C. Costa, K. Kramp, and R. Kundrata. 2020. Hidden diversity in the Brazilian Atlantic rainforest: The discovery of Jurasaidae, a new beetle family (Coleoptera, Elateroidea) with neotenic females. Scientific Reports 10: 1544.
Scholtz, G. 2005. Homology and ontogeny: Pattern and process in comparative developmental biology. Theory in Biosciences 124: 121–143.
Shi, G., D.A. Grimaldi, G.E. Harlow, J. Wang, J. Wang, M. Yang, W. Lei, Q. Li, and X. Li. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research 37: 155–163.
Skelley, P.E. 2009. Pleasing fungus beetles of the West Indies (Coleoptera: Erotylidae: Erotylinae). Insecta Mundi 0082: 1–94.
Ślipiński, A. 2007. Australian Ladybird Beetles (Coleoptera: Coccinellidae): their biology and classification. Melbourne: CSIRO Publishing.
Świętojańska, J. 2009. The Immatures of Tortoise Beetles with Bibliographic Catalogue of All Taxa (Coleoptera: Chrysomelidae: Cassidinae), vol. 16. Wroclaw: Biologica Silesiae.
Wedmann, S., M. Poschmann, and T. Hörnschemeyer. 2010. Fossil insects from the Late Oligocene Enspel Lagerstätte and their palaeobiogeographic and palaeoclimatic significance. Palaeobiodiversity and Palaeoenvironments 90: 49–58.
Yu, T., R. Kelly, L. Mu, A. Ross, J. Kennedy, P. Broly, F. Xia, H. Zhang, B. Wang, and D. Dilcher. 2019. An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences 116: 11345–11350.
Zhang, S.Q., L.H. Che, Y. Li, D. Liang, H. Pang, A. Ślipiński, and P. Zhang. 2018. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nature Communications 9: 205.
Zippel, A., C. Haug, P. Müller, and J.T. Haug. 2022. The first fossil false click beetle larva preserved in amber. PalZ. https://doi.org/10.1007/s12542-022-00638-2.
Acknowledgements
Three anonymous reviewers kindly provided helpful comments for improving the manuscript. We thank J. Matthias Starck, Munich, for long-time support. We are grateful to all people providing low-cost, open-access or open-source software. This is LEON publication #36.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is kindly supported by the German Research Foundation (DFG Ha 6300/6-1) and by the Volkswagen Foundation with a Lichtenberg Professorship to JTH.
Author information
Authors and Affiliations
Contributions
Conceptualisation: CH, AZ, and JTH; methodology: CH, AZ, PM, and JTH; investigation: CH, AZ, PM, and JTH; writing—original draft preparation: CH, AZ, and JTH; writing—review and editing: CH, AZ, PM, and JTH; funding acquisition: JTH; resources: PM and JTH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest and no competing interests.
Additional information
Handling Editor: Christian Klug.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haug, C., Zippel, A., Müller, P. et al. Unusual larviform beetles in 100-million-year-old Kachin amber resemble immatures of trilobite beetles and fireflies. PalZ 97, 485–496 (2023). https://doi.org/10.1007/s12542-023-00648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-023-00648-8