Abstract
The pterosaur assemblage of the mid-Cretaceous Kem Kem Group of Morocco is reviewed. This analysis examines their taxonomy, palaeoecology and palaeobiology with comments on taphonomy. New material permits the rediagnosis of the azhdarchoids Alanqa saharica and Afrotapejara zouhrii. Several specimens are reported that do not fit within the paradigms of previously named taxa. They represent three distinct jaw morphotypes, but are not assigned to new taxa here. The assemblage is highly diverse, including four tooth-bearing taxa assigned to Ornithocheiridae and five named taxa and three additional morphotypes assigned to Azhdarchoidea. The Kem Kem Group assemblage is the most diverse for any pterosaur-bearing fluvial deposit and one of the most diverse of any pterosaur assemblage. The assemblage is heavily biased in terms of preservation with an as yet unexplained high abundance of jaw fragments. We highlight the importance of fragmentary material in pterosaur studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pterosaurs were a diverse and important part of Mesozoic faunas, ranging from the Triassic to the end of the Cretaceous and occurring worldwide. Their fossil record, however, is rather scant. Until recently, relatively little was known about pterosaurs from Africa, or from the middle Cretaceous. In just over two decades however, a large number of pterosaur fossils have been collected from the Cretaceous Kem Kem Group in Morocco (Mader and Kellner 1999; Ibrahim et al. 2010, 2020; Martill et al. 2018, 2020a; Jacobs et al. 2019, 2020; McPhee et al. 2020; Smith et al. 2020a). This has transformed the Kem Kem Group into an important horizon for understanding the diversity and evolution of pterosaurs in the Cretaceous, not only in Africa but beyond. Here, we provide a detailed review of the Kem Kem Group pterosaurs. We evaluate the pterosaurs in terms of their taxonomy, palaeoecology, palaeobiology, taphonomy and evolution, both in a spatial context that includes Africa and elsewhere in the world and in a temporal context: the Cretaceous.
Historical narrative
The pterosaur material from Africa has previously been reviewed by Kellner et al. (2007), Barrett et al. (2008), and Ibrahim et al. (2020). The fossil record of pterosaurs in Africa is sparse, comprising just 18 fossil localities/horizons in 12 countries (see Fig. 1, Table S1), compared to at least 55 in North America, for example (Barrett et al. 2008). This includes body and trace fossils, ranging from the Lower Jurassic (Hettangian) to the Upper Cretaceous (end Maastrichtian) (see Fig. 2, Table S1), an interval of over 100 million years. This patchy distribution reflects both the overall record of pterosaurs, and the fact that, historically, Africa has seen less study than much of the rest of the World.
Chronostratigraphic chart showing the temporal distribution of pterosaur bearing deposits of continental Africa. See Fig. 1 for localities corresponding to the numbers and SI Table S1 for detailed locality information and references. Red star indicates the Kem Kem Group. Note that the Mibladen, Morocco and DR Congo pterosaur localities are not included because their precise ages are not known
The first pterosaur from Africa, reported by Reck (1931), comes from the Upper Jurassic (Kimmeridgian-Tithonian) Tendaguru Beds of Tanzania. The first possibly Cretaceous African pterosaur was described by Swinton (1948) from the Democratic Republic of Congo. It comprises a partial pterodactyloid metacarpal IV, probably from an ornithocheirid (Fig. S1). Later, pterosaur material was described from Algeria, Angola, Cameroon, Egypt, Madagascar, Morocco, Niger, Senegal, South Africa and Tunisia (see Figs. 1, 2, Table S1 for references). Morocco has the most pterosaur-bearing deposits in Africa with six localities/horizons (three trace fossil and three body fossil) (see Figs. 1, 2, Table S1).
French explorer and palaeontologist René Lavocat was the first to recover pterosaur remains, from beds now termed the Kem Kem Group in the 1940s and 1950s. These early discoveries consisted of isolated ornithocheirid teeth, now in the collection of Muséum National d'Histoire Naturelle, Paris (MNHN) (Fig. 3) (Ibrahim et al. 2020). The first pterosaur bone reported from the Kem Kem Group was an isolated azhdarchid cervical vertebra (LINHM 014) mentioned in an abstract by Kellner and Mader (1996) and later figured by Rodrigues et al. (2011, fig. 4). Also in an abstract, Mader and Kellner (1997) described an ornithocheirid jaw fragment (LINHM 016) which became the holotype of Siroccopteryx moroccensis Mader and Kellner (1999, figs. 2, 3). This represented the first pterosaur to be named from the Kem Kem Group.
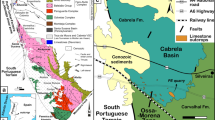
Map of south-east Morocco showing the Kem Kem Group exposures. Kem Kem Group exposures outline adapted from Sereno et al. (1996)
The first indication of edentulous pterosaurs in the Kem Kem Group assemblage was published by Wellnhofer and Buffetaut (1999) who described three toothless jaw fragments (BSP 1993 IV 338; BSP 1997 I 67; BSP 1996 I 36) identified as a pteranodontid, tapejarid and azhdarchid, respectively. These would later be referred to the ?chaoyangopterid Apatorhamphus gyrostega McPhee et al. 2020; tapejarid Afrotapejara zouhrii Martill et al., 2020a and the azhdarchid Alanqa saharica Ibrahim et al. 2010. However, the first edentulous pterosaur from the Kem Kem to be named was Alanqa saharica (Ibrahim et al. 2010).
Subsequently, four more edentulous taxa, Xericeps curvirostris Martill et al. 2018, Apatorhamphus gyrostega McPhee et al. 2020, Afrotapejara zouhrii Martill et al. 2020a, and Leptostomia begaaensis Smith et al. 2020a were described. In addition, a second toothed taxon, Coloborhynchus fluviferox Jacobs et al. 2019 and material attributable to the genera Anhanguera and Ornithocheirus were described (Jacobs et al. 2019, 2020). For a detailed history of exploration of the Kem Kem Group see Ibrahim et al. (2020). See Table S2 for all pterosaur material described and figured from the Kem Kem Group.
Locality
The Kem Kem Group is a widespread stratigraphic unit exposed mainly in the northwestern Sahara Desert of Morocco and along the southern flank of the Atlas Mountain fold belt (Ibrahim et al. 2020; Fig. 4). Lateral equivalents occur extensively in neighbouring Algeria (Alloul et al. 2018; Ibrahim et al. 2020).
Most fossils from the Kem Kem Group made available by local collectors come from outcrops in the Tafilalt region, where a nearly continuous outcrop flanks the valleys of the Oued Ziz and Oued Gheris and rims the immense plain of the Tafilalt and the sand dunes of Erg Chebbi.
By far the most prolific source of fossils is found along a stretch of the outcrop between Gara Sbaa and Taouz, between Takmout and Douira, the area around Zrigat and between Tarda and Goulmima (Fig. 4). Some fossils are also mined near Tadighoust from a folded outcrop at the foot of the Atlas Mountains. Extensive areas of outcrop to the east and west remain unexplored for fossils (e.g., in the Anoual and Ouarzazate basins).
The most fossiliferous outcrops appear to be those along a ~ 250 km escarpment of the Guir and Kem Kem hamadas along the Moroccan–Algerian border (Ibrahim et al. 2010, 2020). The northern extent of the escarpment is located ~ 30 km south of Errachidia, extending southeast towards Taouz along the western edge of the Hamada du Guir. It then extends southwest following the western edge of the Hamada du Kem Kem (Ibrahim et al. 2020) (see Fig. 4). Pterosaur remains have been collected from most of the extent of the Kem Kem Group from Takmout in the north to Gara Tabroumit in the south.
Geological and stratigraphical context
The Kem Kem Group is represented by an approximately 200 m thick sequence of mainly siliciclastic strata and is divided into two distinct formations, a lower sandy unit, the Ifezouane Formation, and an upper mudstone unit, the Aoufous Formation following the nomenclature of Ettachfini and Andreu (2004). A revised stratigraphic nomenclatural scheme was proposed by Ibrahim et al. (2020) for geographically more restricted outcrops in the southern Tafilalt, but we do not employ it here, finding that the scheme of Ettachfini and Andreu (2004) can be extended easily into this region.
In the Tafilalt region, the Kem Kem Group lies with angular and gently topographic unconformity on folded marine Palaeozoic strata (Ibrahim et al. 2010; Martill et al. 2018), with the base of the Ifezouane Formation usually being composed of a thin breccia or conglomerate (Cavin et al. 2010). Elsewhere, the Kem Kem Group appears to lie on Middle to Upper Jurassic strata with only minor unconformity, as at Tadighoust and in the Anoual Basin. Almost everywhere the Kem Kem Group is overlain by a thick succession of Cenomanian–Turonian carbonates of the Akrabou Formation (Ibrahim et al. 2010; Martill et al. 2018).
The Kem Kem Group’s two formations, the Ifezouane Formation and overlying Aoufous Formation, differ in their lithology. The Ifezouane Formation is characterised by reddish fluvial detritic sandstones, with high angled, metre-thick cross-bedding representing channel fills, with intercalations of pedogenic overbank mudstones (Belvedere et al. 2013), pinkish sand, and thin conglomerates with quartz pebbles (Cavin et al. 2010). The overlying Aoufous Formation is characterised by variegated mudstones and marls, with calcitic palaeosols, thin intercalations of evaporites, detrital sandstone and micro-conglomerates, and carbonate cemented layers (Cavin et al. 2010; Belvedere et al. 2013). The vast majority of vertebrate material comes from intraformational, mud-flake conglomerates in the upper Ifezouane Formation (Martill et al. 2018).
Age
The age of the Kem Kem Group is problematic due to the rarity of biostratigraphically useful fossils. Correlations with the Bahariya Formation of Egypt based on similar vertebrate assemblages have been used to infer an early Cenomanian age, particularly due to the shared presence of the dinosaur Spinosaurus and the sawfish Onchopristis (Sereno et al. 1996). The overlying limestones of the Akrabou Formation contain the ammonite Neolobites vibrayeanus (d'Orbigny 1840), of late Cenomanian age (Cavin et al. 2010). Therefore, the Kem Kem Group may be late Albian to early Cenomanian (Ibrahim et al. 2020) or older. Further work is needed to refine the age of the beds.
Palaeoenvironment
The Kem Kem Group was deposited in a continental environment dominated by a fluvial setting, with some lacustrine horizons. Rare, short-lived shallow marine environments are recorded in some northern localities (Adardor et al. 2021; Beevor et al. 2021). Overall, the lithologies record a transgressive sequence terminating in a thick succession of fully marine carbonates represented by the Cenomanian Akrabou Formation (Ettachfini and Andreu 2004; Ibrahim et al. 2010; Belvedere et al. 2013). The Ifezouane Formation has been interpreted as a braided river system (Belvedere et al. 2013). These deposits are detritic in nature and are strictly freshwater in origin as demonstrated by the presence of lungfishes, sirenid urodeles and frogs (Cavin et al. 2010); the high diversity of turtles (Ibrahim et al. 2020) is also consistent with a freshwater environment. The Ifezouane Formation fines upwards into the low-energy deltaic, estuarine and playa lake deposits of the Aoufous Formation (Belvedere et al. 2013; Martill et al. 2018) where fossils include freshwater gastropods and rare unionid bivalves (DMM, NI, RES pers. obs.). Assuming extant vertebrates associated with recent river systems can be used as analogues, then it seems likely that the Kem Kem Group palaeo-river system incorporated a wide range of possible niches. Environments likely included river channels, river banks, sandbars, oxbows, marshes, estuaries, and tidal flats.
Materials and methods
Kem Kem Group pterosaur material was examined in the following collections: BSPG, CMN, FSAC, MNHM, MSNM, NHMUK and UCRC. A variety of techniques were used to examine the material, including light microscopy, scanning electron microscopy (SEM) and X-ray computed tomography (XCT). Several specimens were thin-sectioned using standard techniques. The specimens were imaged using a Nikon D5600 DSLR camera and images processed using Combine ZP and Corel Draw Graphics Suite X8. Specimens were topographically scanned using an Einscan Pro + 3D scanner and scans processed using Geomagic Design X. Joint fieldwork by multiple universities (University of Portsmouth, University College Dublin, University of Detroit Mercy, University of Casablanca, University of Bath) was conducted in the Tafilalt region of Morocco over the past 12 years to collect fossil material and record taphonomic and sedimentological data. Our comparisons and criteria for taxonomic assignment of postcranial material are limited to the clades present within the Kem Kem Group: Azhdarchoidea and Ornithocheiridae.
Definitions
The following terminology describing pterosaur jaw elements is used throughout: lateral angle—angle between the dorsal/ventral margin and the occlusal surface as seen in lateral view; dorsal/ventral angle—the angle between the occlusal margins as seen in dorsal/ventral view; rostrum—the fused left and right premaxillae and maxillae; mandible—the lower jaw.
Rationale and problems
The isolated and fragmentary nature of Kem Kem Group pterosaur remains renders taxonomic distinction and determination of pterosaur diversity challenging. The high abundance, in the Kem Kem Group of pterosaur jaw fragments (see taphonomy section), especially those attributable to Azhdarchoidea has resulted in the erection of seven pterosaur species founded solely on fragmentary jaw material. In all cases the holotype is little more than a jaw tip, and all are anterior of the nasoantorbital fenestra or the divergence of the mandibular rami.
The similarity between upper and lower jaws, as seen in more complete fossils elsewhere (e.g., Chaoyangopteridae of the Yixian Formation of China) sometimes renders distinction of rostra and mandibles difficult. It is possible that species currently erected on upper jaws may become synonyms of species erected on lower jaws and vice versa. However, this similarity can also be useful; the similar profiles and cross-sections of the rostrum and mandibles, for Alanqa and Leptostomia, for example, make it possible to refer these fossils to the same species even in the absence of association.
Niche partitioning, different feeding strategies and diets can result in dramatic modifications to the skull and feeding apparatus (i.e., the jaws and teeth), as seen in birds (e.g., Abzhanov et al. 2004). In pterosaurs, profound differences are seen in jaw morphology across higher clades, presumably reflecting diverse feeding strategies (Witton and Naish 2008; Navarro et al. 2018; Pêgas et al. 2021a). Thus, subtle differences in jaw morphology seen in Kem Kem Group pterosaurs may provide clues for evaluating species diversity. However, small differences (or sometimes even large differences) may also reflect ontogenetic variation, interspecific variation, and/or sexual dimorphism (Bennett 1992; Manzig et al. 2014; Wang et al. 2014; Pinheiro and Rodrigues 2017).
The impact of ontogenetic change on the morphology of the pterosaur skeleton has been discussed in detail by several authors for Jurassic pterosaurs (Bennett 1995, 2007; Hone et al. 2021) and the Late Cretaceous Pteranodon (Bennett 1993). However, the consequences of ontogenetic variation on overall jaw morphology for Kem Kem Group pterosaurs is far from understood (Smith et al. 2021).
It does appear that immature individuals of some Kem Kem Group edentulous pterosaurs (e.g., Alanqa saharica and Apatorhamphus gyrostega) have a similar morphology (cross-sectional outline, jaw profile, and foramina distribution) to their adult counterparts (Smith et al. 2021). Therefore, although many of the morphological differences exhibited by Kem Kem Group jaws are likely not the result of ontogenetic variation, a cautious approach to naming taxa must still be taken.
Remarkably, and for reasons not as yet fully understood, not a single Kem Kem Group pterosaur jaw fragment exhibits either the mandibular symphysis divergence or the anterior border of the nasoantorbital fenestra, which are diagnostic characters for distinguishing lower and upper jaws (respectively). This is especially surprising as some 200 pterosaur jaw fragments have now been collected from the Kem Kem Group. In addition, no associated material has been discovered where the association is convincingly genuine.
Consequently, the approach of naming taxa based on sometimes subtle morphological differences (i.e., jaw cross-sectional outline) is likely less reliable than any analysis involving more complete skulls, but these appear to be valuable diagnostic characters, which have been used in other pterosaurs. Nonetheless, the Kem Kem Group pterosaur material is frequently preserved in three-dimensions and parameters such as rostral angle, bone-wall thickness and cross-sectional shape (see Fig. 5) can be reliably obtained from the material, which often cannot be obtained from more two-dimensionally preserved specimens such as those from the Solnhofen Limestone Formation, Niobrara Chalk Formation, or Jehol Group. When combined with data derived from techniques such as XCT scanning and thin-section palaeohistology, internal structure can also be incorporated into any analysis (e.g., Martill et al. 2020a), although we note this is not a technique available to all.
Diagram showing the cross-section change across the jaw in edentulous pterosaurs from the Kem Kem Group. A Alanqa saharica holotype mandibular symphysis (FSAC-KK 26); B Afrotapejara zouhrii holotype rostrum (FSAC-KK 5004); C Xericeps curvirostris holotype mandible (FSAC-KK 10700); D Apatorhamphus gyrostega holotype rostrum (FSAC-KK 5010); E, Leptostomia begaaensis holotype rostrum (FSAC-KK 5075); F Leptostomia begaaensis paratype mandibular symphysis (FSAC-KK 5076); G jaw ‘morphotype B’, ?mandibular symphysis (FSAC-KK 5085). Cross-sections and jaws not to scale
The advantage of studying isolated remains is their abundance; since most fossils are incomplete, focusing on skulls and skeletons ignores the vast majority of the pterosaur fossil record. The material here provides a remarkable insight into pterosaur evolution for a time and place that is otherwise unknown.
Distinguishing between rostrum and mandible
Distinguishing between fragmentary and isolated rostra and mandibles in edentulous pterosaurs is challenging, especially when specimens lack key landmarks (i.e., anterior margin of the NAOF or the divergence of the mandibular rami). In taxa where both the upper and lower jaws are preserved the overall morphology (i.e., cross-sectional outline, profile) is often very similar (Smith et al. 2020b), as for example in Albadraco Solomon et al. 2020. Generally, mandibles have a smaller lateral angle than rostra, but exceptions are known where the lateral angle of the mandible is somewhat larger than that of the upper jaws (e.g., Bakonydraco galaczi and Pteranodon longiceps: see McPhee et al. 2020).
For Kem Kem Group edentulous pterosaurs, we use a combination of similar overall morphology and lateral angle to tentatively identify upper jaw and lower jaw pairs. In particular we rely on the overall cross-sectional outline and the degree of smoothness/sharpness of the dorsal/ventral border, but other parameters are used too, including presence or absence of median tuberosities on the occlusal surface, topography of the occlusal surface and distribution of neural foramina, and cortical bone thickness.
For toothed pterosaurs, upper jaws are distinguished from lower jaws by the presence of a median ridge on the palatal surface, opposed to a median groove present on the occlusal surface of lower jaws. These features are widespread in ornithocheirans (Rodrigues and Kellner 2013) and are seen in the rare Kem Kem Group examples where the relevant element is preserved (Jacobs et al. 2019, 2020).
Other identifications of upper and lower jaws of isolated jaw fragments in other deposits (e.g., Aerotitan and Mistralazhdarcho) may also require revaluation.
Institutional abbreviations
FSAC Faculté de Sciences Aïn Chock, Laboratoire de Géosciences, Université Hassan II, Casablanca, Morocco; BSPG Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany (formerly BSP); CMN Canadian Museum of Nature, Ottawa, Canada (formerly NMC); LINHM Long Island Natural History Museum, Long Island, USA (there is no current record of this institution); MN Museu Nacional/Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (collection likely destroyed by the catastrophic fire of 2018); MNHN Muséum national d’Histoire naturelle, Paris, France; MSNM Museo Civico di Storia Naturale, Milan, Italy; NHMUK Natural History Museum, London, United Kingdom (formerly BMNH); RGP (RMCA) Royal Museum for Central Africa—Registre Général Paléontologie, Tervuren, Belgium; SMNK Staatliches Museum für Naturkunde Karlsruhe, Germany; UCRC University of Chicago Research Collection, Chicago, USA; UOP University of Portsmouth, School of the Environment, Geography and Geosciences collection, UK.
Results
The following account is based on material described in the literature and newly collected material described herein. Data on size, morphology, and identification of specimens is provided in Tables 1, 2 and 3, with additional details supplied in the supplementary information (Table S2).
Taphonomy
The Kem Kem Group pterosaur material occurs as isolated but three-dimensionally preserved elements, which are often broken (Ibrahim et al. 2020). Although fragmentary the internal structures at macroscopic and microscopic levels are often extremely well preserved (Williams et al. 2021). The deposit is unusual compared to other pterosaur-bearing deposits, in being dominated by edentulous jaw fragments (Martill et al. 2018). This bias towards the preservation of jaw fragments, is as yet unexplained but could be a consequence of robustness of triangular shaped bones, hydrodynamic sorting, pterosaur autecology, selective predation/scavenging, collecting bias or any combination of these. The Kem Kem Group has a high abundance of ornithocheirid teeth but ornithocheirid skeletal material is comparatively rare. Presently, there are no convincing explanations for this disparity. Other significant anomalies include the complete lack of syncarpals, elements that are both robust and common in other pterosaur bone concentrations, such as the Cambridge Greensand Member of England (Unwin, 2001).
Systematic palaeontology
Pterosauria Kaup, 1834
Pterodactyloidea Plieninger, 1901
Azhdarchoidea Nesov, 1984 sensu Unwin, 2003
Tapejaridae Kellner, 1989
Afrotapejara Martill, Smith, Unwin, Kao, McPhee, Ibrahim, 2020
Type and only species. Afrotapejara zouhrii Martill, Smith, Unwin, Kao, McPhee, Ibrahim, 2020
Diagnosis. As for type species A. zouhrii.
Genus Zoobank reference number. urn:lsid:zoobank.org:act:4D288F04-8FEF-493E-8767-A3B448CCF2C2
Species Zoobank reference number. urn:lsid:zoobank.org:act:4CFBB0A7-6865-4DE6-ABEE-E1698E7B2334
Afrotapejara zouhrii Martill, Smith, Unwin, Kao, McPhee, Ibrahim, 2020
Synonymy. All mentions where the citation refers to specimens here identified as A. zouhrii are included, as are all mentions of A. zouhrii.
- 1999:
-
‘Tapejaridae… fragment of anterior mandibular symphysis’—Wellnhofer and Buffetaut, p. 137, fig. 5 (specimen BSP 1997 I 67)
- 2007:
-
‘Pteranodontidae… mandibular symphysis’—Kellner et al., p. 262, figs. 1–2 (specimen MN [UFRJ] 7054-V)
- 2008 :
-
Azhdarchidae indet. ‘posterior fragment of premaxilla’—Averianov et al., p. 641 (specimen BSP 1997 I 67 [spec. number not reported])
- 2010 :
-
‘Tapejaridae… anterior portion of mandibular symphysis’—Ibrahim et al., p. 1, table 1 (specimen BSP 1997 I 67)
- 2011 :
-
‘Tapejaridae… partial lower jaw’—Rodrigues et al., p. 156 (specimen BSP 1997 I 67)
- 2014 :
-
Alanqa saharica IBRAHIM et al.—Averianov, p. 6, fig. 1 (specimens BSP 1997 I 67, MN [UFRJ] 7054-V)
- *2020a:
-
Afrotapejara zouhrii MARTILL et al.—Martill et al., p. 1, figs. 4–15, tables 1–3
- 2020:
-
Afrotapejara zouhrii MARTILL et al.—Ibrahim et al., p. 69, figs. 97, 100
- 2020 :
-
Apatorhamphus gyrostega MCPHEE et al.—Ibrahim et al., p. 121–122, fig. 100 (specimens FSAC-KK 29, FSAC-KK 32, UCRC PV 161)
- 2020b :
-
Afrotapejara zouhrii MARTILL et al.—Martill et al., p. 10, tables 2–3
- 2020b :
-
Afrotapejara zouhrii MARTILL et al.—Smith et al., p. 122, fig. 14
- 2021 :
-
Afrotapejara zouhrii MARTILL et al.—Martill et al., p. 5
- 2021 :
-
Afrotapejara zouhrii MARTILL et al.— Smith et al., p. 2, table S1
Holotype. FSAC-KK 5004 (Fig. 6A–E), partial rostrum anterior to the nasoantorbital fenestra, missing the anterior terminus.
Holotype rostrum FSAC-KK 5004 (A–E) and tentatively referred mandibular symphysis BSP 1997 I 67 (F–I) of Afrotapejara zouhrii. A, F in right lateral view; B, G in occlusal view; C in dorsal view; H in ventral view, D in anterior view, E in posterior view and I cross-sectional outline. Scale bars represent 10 mm. Cross-sectional outline redrawn from Wellnhofer and Buffetaut (1999)
Type locality and horizon. Takmout, Oued Ziz valley, Errachidia Province, south-east Morocco; ?Albian-Cenomanian, Ifezouane Formation, Kem Kem Group.
Referred material. Seven jaw fragments. Rostral fragments: FSAC-KK 5006, FSAC-KK 5007 (referred by Martill et al. 2020a) and MN (UFRJ) 7054 V (referred by Ibrahim et al. (2020); ?mandibular symphysis: BSP 1997 I 67 (Fig. 6F–I, referred by Martill et al. 2020a); indeterminate jaw fragments FSAC-KK 29, FSAC-KK 32 and UCRC PV 161 (referred here).
Original diagnosis. (from Martill et al. 2020a). Tapejarid pterosaur with the down-turned tip typical of the family. Distinguished from other tapejarids by the following characters: presence of a row of small foramina on the lateral margins of the rostrum located close to the occlusal margin of the jaw, and a small, boss-like protuberance located posteriorly on the occlusal surface.
Revised diagnosis. A reanalysis of the holotype requires a slightly revised diagnosis. Tapejarid pterosaur with the down-turned tip typical of the family. Distinguished from other tapejarids by the following characters: presence of two rows of small foramina on the lateral margins of the rostrum, one located close to the occlusal margin of the jaw and the other towards the dorsal margin, and a small, boss-like protuberance located posteriorly on the occlusal surface.
Remarks. An occlusal protuberance has been reported in members of Azhdarchoidea (e.g., Alanqa saharica), and occlusal surface modifications have been reported in some tapejarids (e.g., Caupedactylus ybaka -a pair of grooves that diverge posteriorly, and Caiuajara dobruskii—two elongate boss-like ridges extending posteriorly). Occlusal modifications are widespread within Azhdarchoidea (see discussion) and differ in morphology and location. However, at present we have insufficient information to use the presence, morphology and position of these modifications for taxonomic assignment.
Distinguishing between rostrum and mandible. Both jaws of Afrotapejara zouhrii were identified by Martill et al. (2020a). The holotype upper jaw (FSAC-KK 5004, Fig. 6A–E) of A. zouhrii has a downturn as seen in all other tapejarids where the upper jaw is preserved. The average lateral angle of the holotype is approximately 20°. The upper jaw also has the start of a posteriorly located median boss on the occlusal surface, and the start of a sagittal crest. This combination of features makes it easily identifiable as an upper jaw. A lower jaw originally described by Wellnhofer and Buffetaut (1999) (BSP 1997 I 67, Fig. 6F–I) was tentatively referred to A. zouhrii (Martill et al., 2020a) as it has a comparable cross-section to the holotype and referred material of A. zouhrii (see Figs. 5, 6). It has a straight occlusal margin and a concave ventral margin. Its overall morphology is comparable to the lower jaws of other tapejarids (e.g., Tapejara).
Azhdarchidae Nesov, 1984
Alanqa Ibrahim, Unwin, Martill, Baidder, Zouhri, 2010
Type and only species. Alanqa saharica Ibrahim, Unwin, Martill, Baidder, Zouhri, 2010
Diagnosis. As for type and only known species A. saharica.
Genus Zoobank reference number. urn:lsid:zoobank.org:act:B6244462-2CDC-409A-91F7-5A5A1F1A99BC
Species Zoobank reference number. urn:lsid:zoobank.org:act:19995B00-FFB3-4747-8A37-17EBFCA8B2B2
Alanqa saharica Ibrahim, Unwin, Martill, Baidder, Zouhri, 2010
Synonymy. All mentions where the citation refers to specimens here identified as A. saharica are included, as are all mentions of A. saharica. Annotations follow Matthews (1973). Where multiple publications have the same year, they are listed in author alphabetical order.
- 1999:
-
‘?Azhdarchidae… anterior fragment of premaxilla’—Wellnhofer and Buffetaut, p. 136, Fig. 4 (specimen BSP 1996 I 36)
- 2008 :
-
‘anterior end of the premaxilla of Azhdarchidae(?)’—Averianov, et al., p. 641 (specimen BSP 1996 I 36)
- *2010:
-
Alanqa saharica IBRAHIM et al.—Ibrahim, et al., p. 1, Table 1–2, Figs. 2–4
- 2010 (non):
-
Alanqa saharica IBRAHIM et al.—Ibrahim et al., p. 3, Fig. 4, Tables 1, 2 (specimens BSP 1993 IX 338, FSAC-KK 27)
- 2010 :
-
Alanqa saharica IBRAHIM et al.—Vremir, p. 652
- 2011:
-
Alanqa saharica IBRAHIM et al.—Rodrigues et al., p. 150
- 2012 :
-
Alanqa saharica IBRAHIM et al.—Averianov, p. 43
- 2012 :
-
Alanqa saharica IBRAHIM et al.—Novas et al., p. 1448
- 2013 :
-
Alanqa saharica IBRAHIM et al.—Witton, p. 248
- 2014:
-
Alanqa saharica IBRAHIM et al.—Averianov, p. 1, Fig. 1
- 2014 (non):
-
Alanqa saharica IBRAHIM et al.—Averianov, p. 6, Fig. 1 (specimens BSP 1993 IX 338, BSP 1997 I 67, CMN 50,859, MN [UFRJ] 7054-V)
- 2015:
-
Alanqa saharica IBRAHIM et al.—Martill and Ibrahim, p. 59, Table 1, Figs. 3–5
- 2016 :
-
Alanqa saharica IBRAHIM et al.—Pêgas et al., p. 13
- 2017 :
-
Alanqa saharica IBRAHIM et al.—Masrour, et al., p. 774
- 2018 :
-
Alanqa saharica IBRAHIM et al.—Longrich, et al., p. 23
- 2018 :
-
Alanqa saharica IBRAHIM et al.—Martill et al., p. 1, Fig. 6
- 2018 :
-
Alanqa saharica IBRAHIM et al.—Pêgas et al., p. 6
- 2018 :
-
Alanqa saharica IBRAHIM et al.—Vremir et al., p. 7
- 2019 :
-
Alanqa saharica IBRAHIM et al.—Jacobs et al., p. 77
- 2020:
-
Alanqa saharica IBRAHIM et al.—Ibrahim et al., p. 10, Fig. 98
- 2020 (non):
-
Alanqa saharica IBRAHIM et al.—Ibrahim et al., p. 123 (specimens CMN 50,859 and FSAC-KK 28)
- 2020 :
-
Alanqa saharica IBRAHIM et al.—Jacobs et al., p. 2
- 2020a :
-
Alanqa saharica IBRAHIM et al.—Martill et al., p. 1
- 2020 :
-
Alanqa saharica IBRAHIM et al.—McPhee et al., p. 1
- 2020 :
-
Alanqa saharica IBRAHIM et al.—Solomon et al., p. 6
- 2020a :
-
Alanqa saharica IBRAHIM et al.—Smith et al., p. 4
- 2020b :
-
Alanqa saharica IBRAHIM et al.—Smith et al., p. 123, Fig. 14
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Andres, p. 203, Fig. 1
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Andres and Langston, p. 96
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Campos, p. 5
- 2021a :
-
Alanqa saharica IBRAHIM et al.—Pêgas et al., p. 630
- 2021b:
-
Alanqa saharica IBRAHIM et al.—Pêgas et al., p. 2, Figs. 9–11
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Martill et al., p.5, Fig. 5
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Smith et al., p. 2, Figs. 2–3, 6, Tables 2, S1
- 2021 :
-
Alanqa saharica IBRAHIM et al.—Williams et al., supplemental information
- 2021 (non):
-
Alanqa sp. IBRAHIM et al.—Williams et al., p.1, Figs. 1–2 (specimen FSAC-KK 5077)
Holotype. FSAC-KK 26 (Fig. 7A–E), mandibular symphysis, anterior of the divergence of the mandibular rami.
Mandibular symphyses of Alanqa saharica. A–E holotype FSAC-KK 26 and F–H referred specimen in private collection, 3D prints UOP-PAL KK 0006 and FSAC-KK 5213 (digital images). A, F In left lateral view; B, G in occlusal view; C, H in ventral view; D-E, cross-section images (D anterior and E posterior). Scale bars represent 10 mm, cross-sections not to scale
Type locality and horizon Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ? Albian—Cenomanian, Ifezouane Formation, Kem Kem Group.
Referred material. Nine jaw fragments. Rostra: FSAC-KK 5204 and FSAC-KK 5205 (referred here; Fig. 8); mandibular symphyses: BSP 1996 I 36 (referred by Ibrahim et al. 2010), FSAC-KK 4000 and a specimen in a private collection (Fig. 7F–H), 3D prints of which are numbers UOP-PAL-KK 0006 and FSAC-KK 5213 (both specimens referred by Martill and Ibrahim 2015); indeterminate jaw fragments: FSAC-KK 4002 (referred by Martill et al. 2021); FSAC-KK 5078, FSAC-KK 5079, FSAC-KK 5080 (immature individuals referred by Smith et al. 2021).
The holotype FSAC-KK 26 was originally described as a mandibular symphysis by Ibrahim et al. (2010) based on its low lateral angle. The specimen was later reinterpreted as a premaxilla by Ibrahim et al. (2020). Here we agree with the original description as a mandibular symphysis (see discussion below). Specimen BSP 1996 I 36, described originally as an anterior premaxilla of an indeterminate azhdarchid (Wellnhofer and Buffetaut 1999), was reinterpreted as a mandible by Ibrahim et al. (2010) and referred to A. saharica. Here we tentatively agree with the interpretation by Ibrahim et al. (2010) of BSP 1996 I 36 as a partial mandibular symphysis of A. saharica. FSAC-KK 4000 is a fragment of occlusal surface posteriorly located in the jaw and bearing bony protuberances, identified as a fragment of rostrum by Martill and Ibrahim (2015). Here we interpret it as a fragment of mandibular symphysis because of the similarity of its bony protuberances to those of the holotype.
Original diagnosis. (from Ibrahim et al. 2010). Elongate mandibular symphysis (length/maximum depth ratio 0.10) with remarkably straight dorsal and ventral profile in lateral view and a well-developed ‘‘V’’-shaped midline ridge that bounds the posterior end of the occlusal surface of the mandibular symphysis, bifurcates posteriorly and projects well above the occlusal margin of the symphysis.
Revised diagnosis. Reanalysis of the holotype enables a revision of the diagnosis thus: elongate mandibular symphysis with a straight dorsal and ventral profile in lateral view, a cross-sectional outline which is triangular anteriorly and becomes ‘Y’ shaped posteriorly with concave lateral margins (Fig. 7), and bifurcating bony protuberances on the posterior occlusal surface, which project above the occlusal margin but do not meet anteriorly (Fig. 7).
Two specimens are considered premaxillae of A. saharica (FSAC-KK 5204 and FSAC-KK 5205). We can supplement the original diagnosis with the following characters: rostrum with a similar overall morphology to the mandibular symphysis (cross-section and profile) but with a single median boss located posteriorly on the occlusal surface (Fig. 8).
Remarks. Several other fragmentary jaws have been referred to A. saharica including BSP 1993 IX 338, FSAC-KK 27 (referred by Ibrahim et al. 2010), BSP 1997 I 67, CMN 50859, MN (UFRJ) 7074-V (referred by Averianov 2014) and FSAC-KK 28 (referred by Ibrahim et al. 2020). All were subsequently referred to other taxa. Specimens BSP 1993 IX 338 and CMN 50859 were re-evaluated and referred to Apatorhamphus gyrostega by McPhee et al. (2020). Jaw fragment FSAC-KK 27 was also reinterpreted and referred to A. gyrostega by Ibrahim et al. (2020) on account of its rounded dorsum. Specimens BSP 1997 I 67 and MN (UFRJ) 7074-V were also reinterpreted and referred to Afrotapejara zouhrii by Martill et al. (2020a) and Ibrahim et al. (2020) respectively. We identify FSAC-KK 28 as Azhdarchoidea indet. jaw ‘morphotype A’ (see below).
In addition to jaw fragments, some authors referred postcranial elements to Alanqa saharica because at the time A. saharica was the only named taxon of azhdarchoid pterosaur recorded from the Kem Kem Group. Cervical vertebrae CMN 50801, LINHM 014 and FSAC-KK 5077 were referred by Averianov, (2014) and Williams et al. (2021) to this taxon. In the latter case Williams et al. (2021) identified the vertebra as Alanqa sp. A right humerus, CMN 50814, was also referred to A. saharica by Averianov (2014). We consider postcranial azhdarchoid material from the Kem Kem Group to be non-diagnostic at genus or species level, and, until associated material is found, postcranial material cannot be referred with certainty to any jaw-based taxon. Therefore, we identify the vertebrae CMN 50801, FSAC-KK 5077 and LINHM 014 as Azhdarchidae indet. and the humerus CMN 50814 as Azhdarchoidea indet. (see below).
Distinguishing between rostrum and mandible. The holotype of Alanqa saharica (FSAC-KK 26, Fig. 7A–E) was originally described as a lower jaw (Ibrahim et al. 2010), and subsequently several authors followed this interpretation (Rodrigues et al. 2011; Averianov 2014; Martill and Ibrahim 2015; Martill et al. 2018; Vullo et al. 2018; McPhee et al. 2020) whereas more recently, it has been suggested it is an upper jaw (Ibrahim et al. 2020). Several specimens were referred to A. saharica as upper jaws including BSP 1993 IX 338 (Ibrahim et al. 2010) and FSAC-KK 4000 (Martill and Ibrahim 2015). Specimen BSP 1993 IX 338 was subsequently referred to Apatorhamphus gyrostega (Ibrahim et al. 2020). The occlusal modification on the holotype of A. saharica described by Ibrahim et al. (2010) was misinterpreted and is the same as that seen on FSAC-KK 4000. Two specimens referred here to A. saharica, with a comparable morphology (cross-sectional outline, foramina distribution) but with a single median boss on the occlusal surface (FSAC-KK 5204 and FSAC-KK 5205, Fig. 8), represent the rostrum of this taxon. The holotype has a lateral angle of ~ 4°, as does a similar specimen in a private collection figured by Martill and Ibrahim (2015) (3D-print UOP-PAL KK 0006 and FSAC-KK 5213, Fig. 7F–H). Specimen FSAC-KK 5205 has a lateral angle of ~ 6°. FSAC-KK 5204 is missing its dorsal margin, and therefore a lateral angle cannot be measured. The lateral angle is generally larger in the upper jaw compared to the lower, therefore we tentatively interpret FSAC-KK 26, UOP-PAL KK 0006/FSAC-KK 5213 and FSAC-KK 4000 as lower jaws and FSAC-KK 5204 and FSAC-KK 5205 as upper jaws.
Azhdarchidae indet.
Cervical vertebrae
Material. Mid-series cervical vertebrae: CMN 50801 and LINHM 014 (described by Rodrigues et al. 2011); FSAC-KK 34 (described by Ibrahim et al. 2010); FSAC-KK 3088 (described by Ibrahim et al. 2020); FSAC-KK 5077 (described by Williams et al. 2021); FSAC-KK 5083 (described by Smith et al. 2021); FSAC-KK 7251 (referred here, Fig. S2); FSAC-KK 5214 (referred here, Fig. S3); FSAC-KK 5215 (referred here, Fig. S4); FSAC-KK 5216 (referred here, Fig. S6). Posterior cervical vertebra: FSAC-KK-5218 (referred here Fig. S10).
Taxonomic assignment. Elongate mid cervicals (C3-C7) from the Kem Kem deposits that exhibit the following cervical morphotypes: 1–6, and 8 (see below) are assigned here to Azhdarchidae. These vertebrae are clearly distinguished from those of ornithocheiroids which are relatively short with a high ‘spine line’ neural spines and large foramina on the lateral surfaces of the centrum (Wellnhofer 1991; Kellner 1995). Among azhdarchoids, azhdarchid mid-series cervical vertebrae are distinguished by their remarkable elongation, reduced, vestigial, or even absent neural spine, absence of foramina on the lateral surfaces of the centrum, a neural arch that is confluent with the centrum forming a tubular vertebral body, a neural canal that is subsumed into the centrum and large dorsoventrally flattened zygapophyses (e.g., Howse 1986; Frey and Martill 1996; Averianov 2010). Several of these features are present on these Kem Kem mid-cervicals, supporting their assignment to Azhdarchidae.
The cervical vertebra FSAC-KK 5218 (cervical morphotype 9) has an almost quadrilateral outline in dorsal/ventral view, with vertebral length subequal to its width. This morphology compares closely to that of cervical number 8 of Azhdarcho (Averianov 2010) and cervical number 3 (Vremir, 2010) or cervical number 7 (Naish and Witton 2017) of Hatzegopteryx sp. The C8 described by Averianov (2010) bears foramina on the lateral surfaces of the centrum, similar to those on FSAC-KK 5218 (Fig S10), whereas lateral foramina do not appear to be present on the cervical referred to Hatzegopteryx sp. A comparable morphology is evident in mid-series cervicals of thalassodromeids which also have a squarish outline but differ in exhibiting a well-developed hatchet-like neural spine and multiple large foramina on the lateral surfaces (Vila Nova et al. 2015). A neural spine appears to be absent on the cervical referred to Hatzegopteryx sp. (Naish and Witton 2017) but may have been present on the cervical assigned to Azhdarcho. Overall, the morphology of FSAC-KK 5218 matches most closely that of the C8 of azhdarchids, therefore it is identified here as a posterior cervical vertebra (?C8) of an indeterminate species of Azhdarchidae.
Remarks. There is no current record of LINHM, therefore the location of LINHM 014 described by Rodrigues et al. (2011) is currently unknown.
Notarium
Material. FSAC-KK 5207, partial notarium fragment including anterior-most dorsal vertebra (Fig. 9A–E).
Pterosaur notaria from the Kem Kem Group. A–E azhdarchoid anterior notarium fragment (FSAC-KK 5207): A, anterior view; B posterior view; C right lateral view; D dorsal view and E ventral view. F–I ornithocheirid anterior notarium fragment (FSAC-KK 5208): F anterior view; G right lateral view; H dorsal view and I ventral view. Scale bars represent 10 mm
Taxonomic assignment. A well-preserved but incomplete dorsal vertebra (FSAC-KK 5207; Fig. 9A–E), lacking the neural spine but retaining the proximal portion of a rib which is fused to the right side of the vertebra, appears to be the anteriormost element of a partially fused notarium and thus from position 10 (= dorsal number 1) in the vertebral column. This specimen compares closely to the first dorsal in the notarium of several azhdarchoids including thalassodromines (Aires et al. 2013: fig. 4), Tupuxuara (Aires et al. 2020: fig. 7) and Quetzalcoatlus (Andres and Langston 2021: fig. 26). Typical azhdarchoid features include pneumatopores flanking the neural canal and short, robust, laterally directed parapophyses. Notably, in anterior view, the ventral margin of the centrum bears a distinctive, rounded, midline notch, a feature that, for those few species where the notarium is well preserved (Aires et al. 2020) appears to be restricted to Quetzalcoatlus. Furthermore, in terms of its general shape and proportions FSAC-KK 5207 compares closely to the first notarial vertebra of Quetzalcoatlus, consequently this specimen can be confidently assigned to Azhdarchidae.
Wing phalanx
Material. FSAC-KK 5212, right wing phalanx 1 missing distal end (Fig. 10, referred here).
Taxonomic assignment Wing phalanges are simple structures and show relatively little morphological variation across Pterodactyloidea, generally only differing in the extent to which individual phalanges contribute to the length of the wing-finger and in cross-sectional outline. In azhdarchoids, for example, the wing-phalanx one typically forms at least 40%, or more, of the wing-finger and as a consequence is relatively elongate, with in Zhejiangopterus and Quetzalcoatlus, length/width ratios of between 25 and 30. Azhdarchoid wing phalanges two and three are also distinguished by a ‘T-shaped’ cross-section resulting from the presence of an elongate ridge that extends along the ventral margin of the phalanx (Martill and Frey 1999). In other pterodactyloids wing phalanges are oval, sub-triangular, or D-shaped in cross-section. A wing-phalanx one (FSAC-KK 5212) from the Kem Kem deposits (Fig. 10) lacking its distal termination is likely to have exceeded a length/width ratio of 25, when complete. Moreover, the proximal articulation is unusual in that the profile of the dorsal articular facet, when seen in dorsal aspect, is asymmetrical with the portion of the arc that faces proximally of markedly greater length than the portion that faces posteriorly. This asymmetry is also evident in the wing-phalanx one of the azhdarchids Azhdarcho, Quetzalcoatlus and, seemingly, Zhejiangopterus, and is possibly restricted to azhdarchids because in other azhdarchoids such as Tupuxuara the articular facet appears to be much more symmetrical. In ornithocheirids, the dorsal articular facet is symmetrical, subtends a greater arc and occupies only about 50% of the total width of the proximal articular end of the phalanx, unlike FSAC-KK 5212, Quetzalcoatlus and Azhdarcho where the dorsal articular facet extends across more than 66% of the antero-posterior width of the proximal articulation. FSAC-KK 5212 can be confidently referred to Azhdarchoidea and likely represents Alanqa or one of the other putative Kem Kem azhdarchids.
?Azhdarchidae indet.
Femur
Material. FSAC-KK 7141 (Fig. 11A–D), complete right femur (referred here).
Hind-limb elements of Kem Kem Group pterosaurs. A-D ?azhdarchid complete right femur (FSAC-KK 7141): A posterior view; B anterior view; C proximal view and D distal view. E–H azhdarchoid left tibia with fused fibula missing distal end (FSAC-KK 7140): E medial view; F lateral view; G magnified lateral view showing partial fused fibula (arrows) and H proximal view. Scale bars represent 10 mm
Taxonomic assignment. A well-preserved near complete right femur (FSAC-KK 7141) lacking only part of the proximal head can be confidently assigned to Azhdarchoidea. It lacks typical features of ornithocheiroids including a femoral head directed at > 160°, a heavily reduced greater trochanter and a more or less straight shaft of uniform diameter throughout much of its length (e.g., Kellner and Tomida 2000: Fig. 51). By contrast, FSAC-KK 7141 exhibits a slender femoral neck, a well-developed and proximally directed greater trochanter, a prominent pneumatopore in the base of the posterior aspect of the femoral neck, a long slender slightly medially curved shaft that is narrowest immediately distal to the proximal articulation and gently increases in diameter posteriorly, and a relatively complex distal articulation in which the central sulcus (which separates the lateral and medial condyles) is flanked on either side by a shallow groove that extends anteroposteriorly and gently bisect each articular condyle. These accessory grooves are well developed, for example, in Azhdarcho (Averianov 2010: fig. 34 J) but present in only an incipient state here, though visible as slight arcuate excavations of the distal condyles in posterior aspect (Fig. 11A). These features, most notably the pneumatopore and complex distal articulatory surfaces, are apomorphies of Azhdarchoidea (e.g., Unwin 2003). Within this clade, FSAC-KK 7141 shows closest similarity to the femora of the thalassodromeid Tupuxuara (IMCF 1052) and the azhdarchid Azhdarcho (Averianov 2010) but is less robustly constructed than the femur of Quetzalcoatlus (Andres and Langston 2021). Similarly, it is more elongate and slender than the femora of tapejarids such as Sinopterus (e.g. IVPP V.12693, D2525) and Tupandactylus (Beccari et al. 2021) and of chaoyangopterids such as Chaoyangopterus (IVPP 13,397) and Jidapterus (Wu et al. 2017). Pending the discovery of more complete remains, FSAC-KK 7141 is identified as ?Azhdarchidae.
?Chaoyangopteridae Lü et al., 2008
Apatorhamphus McPhee, Ibrahim, Kao, Unwin, Smith, Martill, 2020
Type and only species. Apatorhamphus gyrostega McPhee, Ibrahim, Kao, Unwin, Smith, Martill, 2020
Diagnosis. As for type species A. gyrostega.
Genus Zoobank reference number urn:lsid:zoobank.org:act:BB7F9696-23CC-4A54-BCB3-6D1D7A31826A
Species Zoobank reference number urn:lsid:zoobank.org:act:86D6C1C7-9CD3-46AA-9CED-20E3B1BC2EF6
Apatorhamphus gyrostega McPhee, Ibrahim, Kao, Unwin, Smith, Martill, 2020
Synonymy. All mentions where the citation refers to specimens here identified as A. gyrostega are included, as are all mentions of A. gyrostega.
- 1999:
-
‘?Pteranodontidae… anterior part of premaxilla’—Wellnhofer and Buffetaut, p. 135, Figs. 2–3 (specimen BSP 1993 IX 338)
- 2006 ‘ :
-
Pteranodontidae or Azhdarchidae… edentulous rostral tip’—Rodrigues et al., p. 116A (specimen CMN 50,859)
- 2008 :
-
Azhdarchid ‘mandibular beak’—Averianov et al., p. 641 (specimen BSP 1993 IX 338 [spec. no not stated])
- 2010 :
-
Alanqa saharica IBRAHIM et al.—Ibrahim et al., p. 3, Fig. 4, Tables 1, 2 (specimens BSP 1993 IX 338, FSAC-KK 27)
- 2011 ‘ :
-
Pteranodontidae… lower jaw’—Rodrigues et al., p. 156 (specimen BSP 1993 IX 338)
- 2011 :
-
‘Dsungaripteroidea indet… ?lower jaw’—Rodrigues et al., p. 150, Fig. 1 (specimen CMN 50,859)
- 2014 :
-
Alanqa saharica IBRAHIM et al.—Averianov, p. 6, Fig. 1 (specimens BSP 1993 IX 338, CMN 50,859)
- *2020:
-
Apatorhamphus gyrostega MCPHEE et al.—McPhee et al., p. 1, Figs. 3–10, Table 1
- 2020:
-
Apatorhamphus gyrostega MCPHEE et al.—Ibrahim et al., p. 70, Fig. 96
- 2020 :
-
Alanqa saharica IBRAHIM et al.—Ibrahim et al., p. 123 (specimen CMN 50,859)
- 2020 (non):
-
Apatorhamphus gyrostega MCPHEE et al.—Ibrahim et al., p. 121–122, Fig. 100 (specimens FSAC-KK 29, FSAC-KK 32, UCRC PV 161)
- 2020 :
-
Apatorhamphus gyrostega MCPHEE et al.—Jacobs et al., p. 2
- 2020a :
-
Apatorhamphus gyrostega MCPHEE et al.—Martill et al., p. 1
- 2020b :
-
Apatorhamphus gyrostega MCPHEE et al.—Smith et al., p. 123, Fig. 14
- 2021 :
-
Apatorhamphus gyrostega MCPHEE et al.—Andres, p. 205, Fig. 1
- 2021 :
-
Apatorhamphus gyrostega MCPHEE et al.—Martill et al., p. 5
- 2021 :
-
Apatorhamphus gyrostega MCPHEE et al.—Smith et al., p. 2, Figs. 2–3, Tables 2, S1
Holotype. FSAC-KK 5010 (Fig. 12), a portion of rostrum anterior to the margin of the nasoantorbital fenestra, missing the anterior end (3D print UOP-PAL-KK 0001).
Type locality and horizon. Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ?Albian—Cenomanian, Ifezouane Formation, Kem Kem Group.
Referred material. 11 jaw fragments. ?rostra: FSAC-KK 27 (referred by Ibrahim et al. 2020), FSAC-KK 5011, FSAC-KK 5012, FSAC-KK 5014, BSP 1993 IX 338 (referred by McPhee et al. 2020) and FSAC-KK 5084 (referred by Smith et al. 2021); ?mandibular symphyses: FSAC-KK 5013, CMN 50859 (referred by McPhee et al. 2020) and two indeterminate jaw fragments of immature individuals FSAC-KK 5081 and FSAC-KK 5082 (referred by Smith et al. 2021).
Diagnosis (from McPhee et al. 2020). Apatorhamphus gyrostega can be diagnosed by a unique combination of characters: cross-sectional profile has an inverted U-shape anteriorly, posteriorly developing a more teardrop-like outline as the lateral margins become slightly convex (a possible autapomorphy) and rostrum long and edentulous, with a straight occlusal border and slightly concave anterior dorsal border in lateral view. The bone wall is massively thickened at the rostrum tip (autapomorphy). The occlusal surface is moderately concave with paired, slightly off-set foramina; foramina of the occlusal surface are slit-like anteriorly becoming circular posteriorly (possibly autapomorphic) and a single row of slit-like neurovascular foramina on the lateral margins is aligned parallel to the dorsal margin.
Remarks. Specimens FSAC-KK 29, FSAC-KK 32 and UCRC PV 161 referred by Ibrahim et al. (2020) to A. gyrostega are reinterpreted here as Afrotapejara zouhrii, based on the following characters: triangular cross-section outline with convex lateral surfaces; presence of parallel vertical septa and two rows of foramina on the lateral surfaces. Specimen CMN 50859 incorrectly cited as ‘CMN 50895’ (pg. 123 Ibrahim et al. 2020).
Distinguishing between rostrum and mandible. The rostrum and mandible of Apatorhamphus gyrostega were described by McPhee et al. (2020). Both have a similar overall morphology and were distinguished as upper and lower jaws by their lateral angle (upper jaws had a higher lateral angle compared to lower jaws). The holotype rostrum (FSAC-KK 5010, Fig. 12) has a lateral angle of approximately 12° and the referred possible mandible (FSAC-KK 5013) has a lateral angle of approximately 8°.
Azhdarchoidea Nesov 1984 (sensu Unwin 2003)
Family incertae sedis
Leptostomia Smith, Martill, Kao, Zouhri, Longrich, 2020
Type and only species. Leptostomia begaaensis Smith, Martill, Kao, Zouhri, Longrich, 2020
Diagnosis. As for type species L. begaaensis
Genus Zoobank reference number urn:lsid:zoobank.org:act:1CAC21B5-E226-47EA-9432-C50E09650D0D.
Species Zoobank reference number urn:lsid:zoobank.org:act:52,727,043-2A97-4FDA-B586-D97E27CC0595
Leptostomia begaaensis Smith, Martill, Kao, Zouhri, Longrich, 2020
Synonymy. All mentions of L. begaaensis are included.
- *2020a:
-
Leptostomia begaaensis SMITH et al.—Smith et al., p. 1, Figs. 2–10, Tables 1
- 2020a:
-
Leptostomia begaansis SMITH et al.—Smith et al., p. 12. Lapsus calami
- 2021 :
-
Leptostomia begaaensis SMITH et al.—Andres, p. 212, Fig. 1
- 2021 :
-
Leptostomia begaaensis SMITH et al.—Andres and Langston, p. 89
- 2021 :
-
Leptostomia begaaensis SMITH et al.—Smith et al., table S1
Holotype. FSAC-KK 5075 (Fig. 13A–E), a fragment of rostrum from anterior to the margin of the nasoantorbital fenestra, missing the terminus.
Paratype. FSAC-KK 5076 (Fig. 13F–H), a partial mandibular symphysis lacking any divergence of the mandibular rami, missing the anterior end.
Type locality and horizon. Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ?Albian—Cenomanian, upper Ifezouane Formation, Kem Kem Group.
Diagnosis. (from Smith et al. 2020a). Edentulous pterosaur, with a long and slender beak lacking dorsal or ventral crests. The following features are autapomorphic: lateral and dorsal rostral angles (sensu McPhee et al. 2020, tb. 1) less than or equal to an arc of three degrees; cross-sectional outline of anterior rostrum and mandibular symphysis semi-circular; cross-section of rostrum and mandibular symphysis with thick cortices and reduced central cavity.
Distinguishing between rostrum and mandible. The incomplete upper and lower jaws of Leptostomia begaaensis, a possible probe-feeding pterosaur, were described by Smith et al. (2020a). Both jaws have a similar shape with a semi-circular cross-sectional outline, but minor differences suggest one is a lower and the other is an upper jaw. There is no reason to believe they represent remains from a single individual. The holotype rostrum (FSAC-KK 5075, Fig. 13A–E) has a median ridge on the occlusal surface that extends for the length of the specimen, whereas the paratype mandible (FSAC-KK 5076, Fig. 13F–H) has a complimentary median occlusal groove that flattens anteriorly. They are identified as upper and lower jaw based upon their lateral angles, where the holotype has a slightly higher lateral angle compared to the paratype (~ 2.5° vs 2.0°) (Smith et al. 2020a), and the presence of a ridge on the rostrum and groove on the mandible.
Xericeps Martill, Unwin, Ibrahim, Longrich, 2018
Type and only species. Xericeps curvirostris Martill, Unwin, Ibrahim, Longrich, 2018.
Diagnosis. As for type species X. curvirostris.
Genus Zoobank reference number urn:lsid:zoobank.org:act:0E0893BC-AF20-4CF0-AFB5-0BE3A1090EF3.
Species Zoobank reference number urn:lsid:zoobank.org:act:46AEB1A1-D274-4D07-A1EA-B42315349A28.
Xericeps curvirostris Martill, Unwin, Ibrahim, Longrich, 2018
Synonymy. All mentions of X. curvirostris are included
- *2018 :
-
Xericeps curvirostris MARTILL et al.—Martill et al., p. 1, Figs. 3–6, Table 1
- 2018 :
-
‘Xericeps (holotype FSAC-KK 10,700)’ MARTILL et al.—Vullo et al., p. 4
- 2019 :
-
Xericeps curvirostris MARTILL et al.—Jacobs et al., p. 77
- 2020:
-
Xericeps curvirostris MARTILL et al.—Ibrahim et al., p. 69, Fig. 99
- 2020 :
-
Xericeps curvirostris MARTILL et al.—Jacobs et al., p. 2
- 2020 :
-
Xericeps curvirostris MARTILL et al.—McPhee et al., p. 1, Fig. 9, Table 1
- 2020 :
-
Xericeps curvirostris MARTILL et al.—Solomon et al., p. 6
- 2020b :
-
Xericeps curvirostris MARTILL et al.—Smith et al., p. 123, Fig. 14
- 2021 :
-
Xericeps curvirostris MARTILL et al.—Andres, p. 212, Fig. 1
- 2021 :
-
Xericeps curvirostris MARTILL et al.—Campos, p. 5
- 2021 :
-
Xericeps curvirostris MARTILL et al.—Martill et al., p. 5
- 2021b:
-
Xericeps curvirostris MARTILL et al.—Pêgas et al., p. 3, Figs. 10, 12, 14
- 2021 :
-
Xericeps curvirostris MARTILL et al.—Smith et al., p. 2, Table S1
Holotype FSAC-KK 10700 (Fig. 14A–E), partial mandibular symphysis.
Type locality and horizon. Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ?Albian—Cenomanian, Ifezouane Formation, Kem Kem Group.
Referred material. Partial mandible FSAC-KK 5203 (Fig. 14F–H) (referred here).
Diagnosis (from Martill et al. 2018). Lower jaw upcurved (dorsally recurved), with occluding surface exhibiting a concave profile. Ventral margin lacking keel, but with continuous longitudinal midline sulcus (autapomorphy); occlusal surface with paired ridges, confined to the posterior portion of the mandibular symphysis, that project slightly dorsal to the dentary’s lateral margin, thereby defining a broad midline groove. Deep sulcus on occluding surface of mandibular symphysis shallows anteriorly into jaw tip. Thick cortices of dentary anteriorly. Lateral surface of lower jaw tip distinctly convex, cross-section U-shaped.
Remarks. Due to the lack of autapomorphic features of Azhdarchidae on the holotype and referred material of X. curvirostris, we consider X. curvirostris an indeterminate non-tapejarid azhdarchoid, following Martill et al. (2018).
Distinguishing between rostrum and mandible Only the lower jaw of Xericeps curvirostris has so far been described. The holotype (FSAC-KK 10700, Fig. 14A–E) is dorsally curved with a deep sulcate occlusal surface that deepens posteriorly. A further specimen (FSAC-KK 5203, Fig. 14F–H) has been discovered with a comparable morphology. It appears that the jaws of X. curvirostris are less common than other Kem Kem Group pterosaurs. The corresponding upper jaw of Xericeps is unknown.
Azhdarchoidea indet.
Jaw morphotype A
Referred material Two edentulous fragments: FSAC-KK 28 (originally referred to A. saharica by Ibrahim et al. 2020) and FSAC-KK 5206 (referred here; Fig. 15).
Description. Both specimens have a triangular cross-section with acute dorsal and occlusal apices, and flat lateral surfaces. Specimens FSAC-KK 28 and FSAC-KK 5206 have a gently sulcate occlusal surface with paired foramina on the occlusal surface. The lateral surface has a single row of foramina located medially (see Table 1 for measurements).
Remarks. Of the edentulous pterosaur taxa from the Kem Kem Group, two have a triangular cross-sectional outline: Alanqa saharica and Afrotapejara zouhrii. The acute dorsal and occlusal apices of these jaws distinguish them from Alanqa saharica and Afrotapejara zouhrii, both of which have more rounded apices. Furthermore, the flat lateral surfaces of these specimens distinguish them from Alanqa saharica, which has a more concave lateral surface and Afrotapejara zouhrii, which has convex lateral surfaces anteriorly which become more concave posteriorly. Significantly, these specimens differ from all described Kem Kem Group edentulous pterosaurs, suggesting they represent an as yet unidentified taxon. We await better material before attempting to establish a new taxon.
Jaw morphotype B, aff. Apatorhamphus
Referred material. FSAC-KK 5085, a partial ?mandible missing the anterior tip and not extending posteriorly as far as the divergence of the mandibular rami (Fig. 16).
Description. The specimen is a fragment of jaw, likely a mandibular symphysis that lacks any trace of the diverging rami and lacks the anterior tip. It is free from matrix and has a maximum length of 57.3 mm, a maximum height of 7.3 mm, and a maximum width of 5.8 mm (Table 1). Much of the surface is pitted where sand grains of the original matrix have been pressed into the bone surface. Along its entire length the cross-sectional outline presents a U-shaped profile. The occlusal surface is gently sulcate, becoming slightly deeper posteriorly. The lateral angle is ~ 1.5° and the dorsal angle is ~ 1.7°. All surfaces have elongated neural foramina, those of the lateral margins being highly elongate and aligned in two medial rows parallel to the long axis of the jaw (Fig. 16A, B). On the occlusal surface neurovascular foramina are arranged in alternating pairs (Fig. 16D). In cross-section, the bone wall appears thickened, especially in the vertices where it has a maximum breadth of 2.1 mm (Fig. 16E–F). The bone wall is thinnest at the posterior termination where it is approximately 1.0 mm thick (Fig. 16F).
Remarks. This specimen exhibits a unique combination of characters. The cross-sectional outline is U-shaped, similar to that of Apatorhamphus gyrostega and its lateral and dorsal angles are low, suggesting an extremely elongate, slender, needle-shaped jaw comparable to that of Leptostomia (see below). The morphology hints at a distinct taxon and perhaps a different feeding ecology. More material of this pterosaur is needed to determine its validity as a new taxon and its potential phylogenetic affinities.
Jaw morphotype C
Referred material Partial jaw fragments FSAC-KK 5200, FSAC-KK 5201 and FSAC-KK 5202 (Fig. 17, referred here).
Jaw fragments of jaw ‘morphotype C’. A–D FSAC-KK 5201; E–I, FSAC-KK 5200; J–N FSAC-KK 5202. A, E, J in lateral view; B, F, K in dorsal/ventral view; C, G, L in occlusal view; D, H, N in posterior view; I in anterior view and M in lateral view but with some of the distortions caused by breakage and compaction digitally removed. Scale bars represent 10 mm
Descriptions. All three specimens show some signs of compaction and damage. This is most extensive on FSAC-KK 5201, which has most of its dorsal section and large fragments of the lateral surface missing. Compaction has caused the posterior lateral margins on specimen FSAC-KK 5200 to fold in medially. FSAC-KK 5202 (Fig. 17J–N) has a dorsally curved profile, which is likely the result of distortion (Fig. 17M). This is evident when looking at the occlusal surface, which appears ‘twisted’ (Fig. 17L).
All three specimens have a similar morphology with a U-shaped cross-sectional outline and a sulcate occlusal surface that deepens posteriorly. The lateral surfaces have a single row of small foramina, which are positioned slightly more towards the dorsal margin. The occlusal surface bears paired foramina. Specimens FSAC-KK 5200 and FSAC-KK 5201 are missing the anterior portion of the jaw, whereas FSAC-KK 5202 extends almost to the jaw tip. Specimen FSAC-KK 5200 has a lateral angle of approximately 7° whereas that of FSAC-KK 5202 is approximately 6°. As specimen FSAC-KK 5201 lacks the majority of the dorsal margin a lateral angle cannot be accurately measured.
All three specimens have a median boss-like eminence on the occlusal surface. FSAC-KK 5202 has only the anterior-most portion of the boss preserved. The boss is more extensively preserved in specimens FSAC-KK 5200 and FSAC-KK 5201, which shows that the boss widens and heightens posteriorly. Specimen FSAC-KK 5200 has the boss broken off at a point level with the occlusal margin, whereas in specimen FSAC-KK 5201 the boss projects approximately 19 mm above the occlusal margin. All three specimens have a slight downward curve to the occlusal margin posteriorly (see Fig. 17) (see Table 1 for measurements).
Remarks. The U-shaped cross-sectional outline of all three specimens suggests possible affinities with either Apatorhamphus or Xericeps. However, due to the fragmentary nature of the material, it is not possible to determine the affinities of the taxon they represent. We posit three possibilities: 1, they are the lower jaw of Apatorhamphus which may have borne a median boss; 2, they are the upper jaw of Xericeps, which may have been straight, unlike the curved lower jaw typical of this species; 3, they represent a new taxon.
Indeterminate jaw fragment
Material. FSAC-KK 31, a jaw fragment missing the dorsal surface, originally referred to A. saharica by Ibrahim et al. (2010), but not figured.
Remarks. Due to the specimen only comprising the occlusal surface, a complete cross-sectional outline cannot be confidently determined. Therefore, assignment of this specimen to a particular taxon is not currently possible because multiple azhdarchoids occur in the Kem Kem Group.
Cervical vertebrae
Material. Mid-series cervical vertebrae: FSAC-KK 5217, FSAC-KK 7177 (referred here, Fig. S8); cervical vertebra fragments FSAC-KK 34 (described by Ibrahim et al. 2010). Posterior cervical vertebrae (cervical IX/dorsal vertebra I [cervicalised dorsal vertebrae]): FSAC-KK 5219 and FSAC-KK 5220 (referred here, Fig. 18).
Taxonomic assignment. FSAC-KK 34 (indeterminate cervical morphotype), FSAC-KK 5217 and FSAC-KK 7177 (cervical morphotype 7), exhibit several features, including elongation, partial coalescence of the neural arch and centrum and the presence of pneumatic openings dorsal to and either side of the neural canal that support their identification as cervicals of one or more species of azhdarchoid pterosaurs. Their relative shortness and the presence of a well-developed, tall, blade-like neural spine are not consistent with the morphology of azhdarchid mid-series cervicals. These vertebrae are comparable to those of tapejarids and chaoyangopterids but our knowledge of vertebral anatomy in these pterosaurs is not sufficient to determine to which of these families they may belong.
A superbly preserved, almost complete single posterior cervical vertebrae (FSAC-KK 5219), likely the ninth, compares closely to the ninth cervical of azhdarchoids including Azhdarcho (Averianov 2010: fig. 16) and indeterminate thalassodromines (Aires et al. 2013; fig. 3; Vila Nova et al., 2015; fig. 7). Unlike the ninth cervical of ornithocheirids (e.g., Anhanguera; Wellnhofer, 1991: fig. 11) where the neural spine and the cotyle are relatively narrow and the lateral surfaces of the centrum are pierced by multiple pneumatopores, in azhdarchoids the neural spine is relatively broad, as is the cotyle, and pneumatopores are absent from the lateral surfaces of the centrum. Notably, the prominent pneumatopores flanking the neural canal of FSAC-KK 5218 are absent in Quetzalcoatlus, though deep blind pits are reported at this location (Andres and Langston 2021).
Morphology of cervical vertebrae. Nine azhdarchoid mid-series cervical vertebra (C3-C8) morphotypes were identified from the Kem Kem Group (Figs. 19, S2–S10, Table 2). The morphotypes vary in the presence/absence of anterior lateral and dorsal foramina; posterior lateral and dorsal foramina; foramina on the lateral surfaces of the centrum and foramina on the anterior end of the ventral surface beneath the cotyle, here referred to as sub-cotylar foramina (see Table 2). Most of the specimens had a relatively similar length to width ratio of ~ 2, apart from morphotype 9 (M9) which had a width subequal to its length. Morphotype 7 (M7) (Fig. S8) has a taller neural spine than all other Kem Kem Group cervical vertebrae, a character widespread within Azhdarchoidea, and is therefore referred to Azhdarchoidea indet. rather than Azhdarchidae indet. These cervical morphotypes likely represent a combination of different cervical positions within the neck of an individual, interspecific variation, and ontogenetic variation.
Reconstructed Kem Kem Group azhdarchoid mid-series cervical vertebra (C3-C8) morphotypes. M1 based on FSAC-KK 7251 (Fig. S2); M2 based on FSAC-KK 5214 (Fig. S3); M3 based on FSAC-KK 5215 (Fig. S4); M4 based on CMN 50801 (Fig. S5); M5 based on FSAC-KK 5216 (Fig. S6); M6 based on FSAC-KK 3088 (Fig. S7); M7 based on FSAC-KK 5217 and FSAC-KK 7177 (Fig. S8); M8 based on FSAC-KK 5083 (Fig. S9); M9 based on FSAC-KK 5218 (Fig. S10). Not drawn to scale
Scapulocoracoid
Material. FSAC-KK 5210 (Fig. 20A–C), right scapulocoracoid missing articular facets on the coracoid and scapula (referred here).
Azhdarchoid right scapulocoracoid (FSAC-KK 5210) and humerus (FSAC-KK 5211) from the Kem Kem Group. A–C right scapulocoracoid missing distal articulations on both scapula and coracoid: A posterior view; B lateral view, oriented to better show the glenoid; C anterior view. D–G right humerus with damaged proximal end, distal end and deltopectoral crest: D, dorsal view; E ventral; F lateral view and G medial view. Scale bars represent 10 mm
Taxonomic assignment. A near complete scapulocoracoid (FSAC-KK 5210) lacking only its distal terminations can be confidently identified as azhdarchoid. This specimen lacks any of the many diagnostic features of the scapulocoracoid of ornithocheiroids (e.g., Wellnhofer 1991; Veldmeijer 2003) including a relatively short scapula with a highly constricted shaft and strongly expanded proximal and distal terminations, a prominent procoracid tubercle, and small or no coracoid flange. By contrast, the general proportions of FSAC-KK 5210, the presence of a well-developed coracoid flange and a supraglenoid tubercle that is separated from the glenoid by a distinct gap are typical features of the azhdarchoid scapulocoracoid. Among azhdarchoids, FSAC-KK 5210 compares most closely to the scapulocoracoid of Tupuxuara (IMCF 1052) although the gap between the glenoid buttress and the supraglenoid tubercle is relatively much greater in the Kem Kem specimen. The latter feature is observed in Quetzalcoatlus (Andres and Langston 2021) but the scapulocoracoid of this azhdarchid differs in other respects, most notably the presence of a massive coracoid flange that rounds into the glenoid buttress and marked flexure medially of the scapula at approximately mid-length.
Humeri
Material. Humeri: CMN 50814, right humerus with proximal and distal ends but lacking a small section of the shaft (referred by Rodrigues et al. 2011); FSAC-KK 5211 (Fig. 20D–G), right humerus with damage to proximal and distal ends (referred here).
Taxonomic assignment Fragmentary remains of two pterosaur humeri, CMN 50814 (Rodrigues et al. 2011: figs. 7, 8) and FSAC-KK 5211, have been recovered from the Kem Kem deposits. Both are easily distinguished from the humeri of ornithocheiroids, which exhibit a suite of unique characters: a distinctive deltopectoral crest that has a long base and bears a terminal expansion that is twisted (warped) obliquely to the humeral shaft (Bennett, 1989); a prominent pneumatic opening on the anconal surface of the proximal end of the humerus; and the distal end of the humerus has a sub-triangular outline); neither of these features is present in FSAC-KK 5211 or CMN 50814. As noted by Rodrigues et al. (2011), CMN 50814 compares closely to azhdarchoid humeri. Where comparable, FSAC-KK 5211 is identical to CMN 50814, and the former also exhibits two additional features, a flange-like deltopectoral crest that extends perpendicular to the humeral long axis (Witton et al., 2009) and a highly constricted shaft, with markedly expanded proximal and distal terminations that, among pterosaurs, compare most closely to the humeri of azhdarchoids. The identity of the Kem Kem humeri can be further resolved because the distal termination of CMN 50814, when viewed in distal aspect, presents a complex structure that is almost identical to that of the humeri of Azhdarcho and Quetzalcoatlus. Notably, the profile appears thicker and more rounded than in other azhdarchoids such as Tupuxuara, where it has a more rectangular outline. In addition, there is a distinct rounded notch toward the anterior of the entepicondyle unlike that of Tupuxuara which lacks this feature.
Ulnae
Material. FSAC-KK 5209, left ulna missing the proximal end; FSAC-KK 7142, proximal end of ?right ulna (Fig. 21, referred here).
Ulnae fragments from the Kem Kem Group. A–C azhdarchoid proximal fragment of ?right ulna (FSAC-KK 7142): A anterior view; B posterior view and C, proximal view. D–G azhdarchoid distal left ulna (FSAC-KK 5209), shaft left unprepped to show mud-flake conglomerate: D anterior view; E posterior view; F distal view and G ventral view. Scale bars represent 10 mm
Taxonomic assignment. Seen in distal view, the ulnae of ornithocheiroids are principally distinguished from those of other pterodactyloids by the relatively narrow transverse width of the dorsal condyle (e.g., Wellnhofer 1985: fig. 37c). By contrast, in non-ornithocheiroids, the transverse width of the dorsal condyle is greater than that of the transverse width of the ventral half of the distal end of the ulna. This is especially pronounced in azhdarchoids such as Azhdarcho (Averianov 2010), Quetzalcoatlus (Andres and Langston 2021), and Tupuxuara (DMU pers. obs.). The presence of a strongly expanded dorsal condyle in FSAC-KK 5209 an incomplete left ulna, and its similarity in all respects to the ulnae of other azhdarchoids (Azhdarcho, Quetzalcoatlus and Tupuxuara) support the assignment of this specimen to Azhdarchoidea. Within this clade ulna morphology is not sufficiently well known as to permit the assignment of isolated ulnae to a particular group though we note here that FSAC-KK 5209 is remarkably similar to the ulnae of the neoazhdarchoids (Tupuxuara and Quetzalcoatlus).
The proximal articular portion of a right ulna (FSAC-KK 7142) is beautifully preserved and exhibits fine anatomical detail including a well-developed, partially fused olecranon on the posterior aspect and a prominent pneumatic opening on the anterior aspect. The ‘C-shaped’ profile, evident in proximal view, with a deeply excavated anterior margin, is typical of azhdarchoids (e.g., Andres and Langston 2021: fig. 31I, J) and quite unlike the more triangular profile, in proximal view, of the ulna of ornithocheiroids, the anterior margin of which is irregular with slightly convex projections (e.g., ‘Santanadactylus’; Wellnhofer 1991: fig. 27). FSAC-KK 7142 differs in minor respects from the corresponding elements of Quetzalcoatlus and Tupuxuara (IMCF 1052), notably in that the ventral articular facet is relatively large. In the absence of more complete specimens, and details of ulnar morphology in other azhdarchoids, FSAC-KK 7142 is assigned to Azhdarchoidea indet.
Metacarpal IV
Material. FSAC-KK 4001 (Fig. 22), left metacarpal IV missing proximal end (referred here).
Taxonomic assignment. The well-preserved distal portion of a wing-metacarpal (MC IV), FSAC-KK 4001, exhibits several features typical of azhdarchoids. The shaft is relatively elongate and slender. In azhdarchoids such as Tapejara (Martill et al. 2013) and Quetzalcoatlus (Andres and Langston 2021) the length: minimum width ratio of this element is approximately 1:20, whereas ornithocheiroids (e.g., Anhanguera piscator, Barbosania gracilirostris and Santanadactylus araripensis) have a relatively short, more robust wing-metacarpal with a slenderness ratio of 1:10. The shape of the distal condyle, when seen in anterior or posterior view, is asymmetrical whereas it is symmetrical in ornithocheiroids and also bears a median ridge (Kellner and Tomida 2000: fig. 42), which is absent in azhdarchoids and other pterosaurs. In addition, the dorsal profile of the wing-metacarpal is somewhat excavated immediately proximal to the distal condyle (e.g., Averianov 2010; Martill et al. 2013) to accommodate the wing-phalanx 1 when it is fully flexed, whereas this margin remains more or less level in ornithocheiroids (e.g., Kellner and Tomida 2000: fig. 42). The wing-metacarpal of azhdarchids exhibits a distinctive anterior flexure of the distal portion of the shaft + distal condyle in Quetzalcoatlus (Andres and Langston, 2021: fig. 38); Zhejiangopterus (DMU pers. obs.) and an azhdarchid from Dinosaur Park (Godfrey and Currie 2005: fig. 16.9). This is seemingly absent in FSAC-KK 4001, suggesting that it is azhdarchoid but not azhdarchid.
Tibiotarsus
Material. FSAC-KK 7140, left tibia missing distal end (Fig. 11E–H, referred here).
Taxonomic assignment. A single incomplete tibiotarsus (FSAC-KK 7140), lacks its distal end, but is otherwise well preserved (Fig. 11E–H). It consists of a slender, elongate, composite tubular structure dominated by the tibia, but bearing a small splint-like fibula which is fused proximally to the proximal end of the tibia and extends distally along its lateral margin to a point at approximately the mid-length of the tibia where it merges into the tibia shaft. The development of the fibula suggests that FSAC-KK 7140 does not represent an ornithocheiroid in that the fibula is reduced to a tiny splint (in Pteranodon) or entirely absent (ornithocheirids) in that clade (Unwin 2003). By contrast, FSAC-KK 7140 compares closely to tibiotarsi of Tupuxuara (IMCF 1052) and Quetzalcoatlus (Andres and Langston, 2021: fig. 46), most notably in terms of the morphology of the proximal articulation, which is inclined posteriorly and laterally, and bears a distinctive notch in the anterolateral margin. Further comparisons are severely constrained by the lack of uncompressed examples of the tibiotarsus in other azhdarchoids, and pterodactyloids, more generally. Consequently, until more complete, better-preserved remains are found we assign FSAC-KK 7140 to an indeterminate species within Azhdarchoidea.
Ornithocheiroidea Seeley, 1891
Ornithocheiridae sensu Unwin, 2003
Anhanguera Campos and Kellner, 1985
Anhanguera cf. A. piscator Kellner and Tomida, 2000
Referred specimen. FSAC-KK 5005 (Fig. 23), a partial mandibular symphysis.
Locality and horizon. Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ?Albian—Cenomanian, upper Ifezouane Formation, Kem Kem Group.
Remarks. This specimen is comparable to A. piscator on account of the shape of the mandibular crest in lateral view and the position of the teeth.
Coloborhynchus Owen, 1874
Genus Zoobank reference number urn:lsid:zoobank.org:act:26335C50-25FD-4BA1-8183-FFE40A0F51C0.
Type species. Coloborhynchus clavirostris Owen, 1874
Coloborhynchus fluviferox Jacobs, Martill, Ibrahim, Longrich, 2019.
Species Zoobank reference number urn:lsid:zoobank.org:act:41DB8186-0F23-4C59-AF2E-38C5FF3752AE.
Synonymy. All mentions of C. fluviferox are included.
- *2019 :
-
Coloborhynchus fluviferox JACOBS et al.—Jacobs et al., p. 77, Figs. 3–8, Tables 1–2
- 2019 :
-
Coloborhynchus fluviferox JACOBS et al.—Pêgas et al., p. 1278
- 2020:
-
Nicorhynchus fluviferox (JACOBS et al.)—Holgado and Pêgas, p. 743, Figs. 3, 10–11
- 2020 :
-
Coloborhynchus fluviferox JACOBS et al.—Ibrahim, et al., p. 69
- 2020 :
-
Coloborhynchus fluviferox JACOBS et al.—Jacobs et al., p. 1, Fig. 10
- 2020 :
-
Coloborhynchus fluviferox JACOBS et al.—McPhee, et al., p. 1
- 2021 :
-
Coloborhynchus fluviferox JACOBS et al.—Smith et al., Table S1
Holotype. FSAC-KK 10701 (Fig. 24A–E), anterior rostrum displaying the alveoli of the first, second, and partial third tooth pairs.
Coloborhynchus anterior rostrum fragments from the Kem Kem Group. A–E holotype of Coloborhynchus fluviferox, FSAC-KK 10701; F–J Coloborhynchus sp. in private collection, casts FSAC-KK 5024, SMNK-PAL 45833. A, F in anterior view; B, G in posterior view; C, H in left lateral view; D, I in ventral view; E in dorsal view and J in slightly oblique dorsal view. Scale bars represent 10 mm
Type locality and horizon. Southern Morocco, possibly Aferdou N'Chaft, Hassi el Begaa, Errachidia Province in south-eastern Morocco, ?Albian—Cenomanian, Ifezouane Formation, Kem Kem Group.
Diagnosis. Based on Jacobs et al. (2019). Species of Coloborhynchus in which the upturned palate (90-degree upturn) has a slightly depressed deltoid face. The deltoid face defines a high isosceles triangle with concave dorsolateral margins in anterior view. The deltoid face bears two shallow, sub-circular depressions located dorsal to tooth pair one. Dorsally, this becomes a shallow groove defined by low ridges that transition into a broad rugose anterodorsal margin of the premaxilla. Central point of alveoli for first tooth pair level with dorsal border of second tooth pair. Mediodorsal crest rises steeply from dorsally turned palatal margin at an angle of 60°.
Remarks. Coloborhynchus fluviferox was referred to a new genus, Nicorhynchus by Holgado and Pêgas (2020) for which Coloborhynchus capito Seeley, 1870 from the Cambridge Greensand was made the type species (N. capito). The distinguishing features used to separate Nicorhynchus from Coloborhynchus are subtle shape differences in the anterior deltoid facet of the rostrum tip. Here we consider such subtle differences to possibly be sufficient to diagnose species but are of little or no importance at generic level. Such minor differences may reflect ontogenetic changes, interspecific variation, or sexual dimorphism. We have studied the relevant material and note here that it is highly abraded and fragmentary. Thus, such small morphological differences are likely to be of little or no taxonomic significance. Accordingly, we consider Nicorhynchus to be a subjective junior synonym of Coloborhynchus. Holgado and Pêgas (2020) also name a new species Nicorhynchus (Coloborhynchus) smaugi, but this is not described or figured, with no specimen referral; thus, this taxon is a nomen nudum.
Coloborhynchus sp.
Referred material. An anterior fragment of rostrum in a private collection (Fig. 24F–J), casts specimen numbers FSAC-KK 5024, SMNK PAL 45833.
Remarks. Jacobs et al. (2020) described and figured a specimen of Coloborhynchus from a private collection that differed somewhat from C. fluviferox, notably in anterior cross-sectional shape (more closely resembling that of C. clavirostris) and a central groove in the premaxillary crest. Jacobs et al. (2020) suggested the specimen might be a new species, but refrained from naming it, referring to it as ‘Coloborhynchus sp. A’. Holgado and Pêgas (2020) referred this specimen to Nicorhynchus fluviferox. However, as discussed above, we synonymise Nicorhynchus with Coloborhynchus. It is possible that the differences between FSAC-KK 5024/SMNK PAL 45833 and the holotype of C. fluviferox are the result of ontogenetic changes and/or sexual dimorphism. More fossils and a better understanding of ontogeny and dimorphism in pterosaurs are needed to help resolve these issues.
Ornithocheirus Seeley, 1870.
Ornithocheirus cf. O. simus Owen (1861)
Age and distribution (from Jacobs et al., 2020). The holotype of O. simus (CAMSM B54.428) is from the Cambridge Greensand (Cenomanian, with derived fossils of Albian age), of Cambridgeshire, England, UK (Unwin, 2001). O. simus has not previously been recorded outside of southern England.
Referred specimen. Anterior fragment of premaxilla in a private collection (Fig. 25), casts specimen numbers FSAC-KK 5025, SMNK PAL 45831.
Locality and horizon. Aferdou N’Chaft, near Hassi el Begaa, Errachidia Province, south-east Morocco; ?Albian-Cenomanian, upper Ifezouane Formation, Kem Kem Group.
Remarks. This specimen is comparable to the holotype of O. simus on account of its tooth placement, tooth size, and jaw outline (Jacobs et al. 2020). Given the geographic and perhaps temporal separation between the two, they may represent distinct species. The Kem Kem material is too fragmentary for detailed comparisons and its relationships to O. simus must await the discovery of more complete remains.
Siroccopteryx Mader and Kellner, 1999
Type and only species. Siroccopteryx moroccensis Mader and Kellner, 1999
Diagnosis. As for type species S. moroccensis
Genus Zoobank reference number. urn:lsid:zoobank.org:act:7E4CA95A-78CE-41F5-950F-533FCB092ACD
Species Zoobank reference number. urn:lsid:zoobank.org:act:379D4E2F-140B-4759-ACE3-A8068D3FF857.
Siroccopteryx moroccensis Mader and Kellner, 1999
Synonymy. All mentions where the citation refers to specimen LINHM 016, now known as S. moroccensis are included, as are all mentions of S. moroccensis.
- 1997 :
-
Anhangueridae—Mader and Kellner, p. 62A
- *1999:
-
Siroccopteryx moroccensis MADER and KELLNER—Mader and Kellner, p. 2, Figs. 2–3
- 2001 :
-
Siroccopteryx moroccensis MADER and KELLNER—Dalla Vecchia et al., p. 223
- 2001:
-
Coloborhynchus moroccensis (MADER and KELLNER)—Unwin, p. 206
- 2003 :
-
Coloborhynchus moroccensis (MADER and KELLNER)—Frey et al., p. 60
- 2003 :
-
Siroccopteryx moroccoensis MADER and KELLNER—Pereda Suberbiola et al., p. 79. Lapsus calami
- 2003 :
-
Coloborhynchus moroccensis (MADER and KELLNER)—Unwin, p. 145, Table 1
- 2003 :
-
Siroccopteryx moroccensis MADER and KELLNER—Veldmeijer, p. 98
- 2006 :
-
Coloborhynchus moroccensis (MADER and KELLNER)—Unwin, p. 272
- 2006 :
-
Siroccopteryx moroccensis MADER and KELLNER—Veldmeijer, p. 41
- 2007 :
-
Siroccopteryx moroccensis MADER and KELLNER—Kellner et al., p. 259, Table 2
- 2008 :
-
Coloborhynchus moroccensis (= Siroccopteryx moroccensis) MADER and KELLNER—Barrett et al., p. 85
- 2008:
-
Siroccopteryx moroccensis MADER and KELLNER—Rodrigues and Kellner, p. 222
- 2009 :
-
Coloborhynchus (= “Siroccopteryx”) moroccensis MADER and KELLNER—Vullo and Neraudeau, p. 280
- 2010 :
-
Siroccopteryx moroccanus MADER and KELLNER—Cavin et al., p. 399. Lapsus calami
- 2010 :
-
Coloborhynchus moroccensis (MADER and KELLNER)—Ibrahim et al., p. 1, Table 1
- 2010 :
-
Siroccopteryx moroccensis MADER and KELLNER—Rodrigues and Kellner, p. 163
- 2011 :
-
Siroccopteryx moroccensis MADER and KELLNER—Rodrigues et al., p. 150
- 2012:
-
Coloborhynchus moroccensis (MADER and KELLNER)—Martill and Unwin, p. 4, Fig. 8
- 2013 :
-
Siroccopteryx moroccensis MADER and KELLNER—Andres and Myers, p. 13
- 2013 :
-
Siroccopteryx moroccensis MADER and KELLNER—Rodrigues and Kellner, p. 33
- 2014 :
-
Siroccopteryx moroccensis MADER and KELLNER—Bantim et al., p. 206
- 2015 :
-
Coloborhynchus moroccensis (MADER and KELLNER)—Martill, p. 377
- 2015 :
-
Coloborhynchus (= Siroccopteryx) moroccensis MADER and KELLNER—Myers, p. 7
- 2017 :
-
‘Coloborhynchus moroccensis or Siroccopteryx moroccensis’ MADER and KELLNER—Masrour et al., p. 774
- 2018 :
-
Siroccopteryx sp.—Dridi, p. 275
- 2019:
-
Siroccopteryx moroccensis MADER and KELLNER—Jacobs et al., p. 77, Figs. 6–8, Table 2
- 2019 :
-
Siroccopteryx moroccoensis MADER and KELLNER—McCurry et al., p. 249. Lapsus calami
- 2019 :
-
Siroccopteryx moroccensis MADER and KELLNER—Pêgas et al., p. 1278
- 2019 :
-
Siroccopteryx moroccensis MADER and KELLNER—Pentland et al., p. 2
- 2020:
-
Siroccopteryx moroccensis MADER and KELLNER—Holgado and Pêgas, p. 744, Figs. 8, 12
- 2020 :
-
Siroccopteryx moroccensis MADER and KELLNER—Ibrahim et al., p. 69, Fig. 95
- 2020 :
-
Siroccopteryx moroccensis MADER and KELLNER—Jacobs et al., p. 1, Fig. 10
- 2021 :
-
Siroccopteryx moroccensis MADER and KELLNER—Andres, p. 212, Fig. 1
- 2021 :
-
Siroccopteryx moroccensis MADER and KELLNER—Fernandes et al., p. 14
- 2021 :
-
Siroccopteryx moroccensis MADER and KELLNER—Richards et al., p. 2
- 2021 :
-
Siroccopteryx moroccensis MADER and KELLNER—Smith et al., Table S1
Holotype. LINHM 016 (Fig. 26), anterior section of rostrum.
Type locality and horizon.? Albian-Cenomanian Kem Kem Group, Morocco.
Diagnosis. (from Mader and Kellner, 1999). Anhanguerid pterosaur with the anterodorsal margin of the premaxillary sagittal crest almost straight and a comparatively massive and broad snout with the anterior tip straight and higher than in Anhanguera but lower than Tropeognathus and less laterally expanded than in ‘Coloborhynchus’ wadleighi.
Remarks. There is no current record of LINHM, therefore the location of LINHM 016 is not known.
Ornithocheiridae indet.
Jaw fragment
Material FSAC-KK 33, partial mandibular ramus referred by Ibrahim et al. (2020).
Remarks. Assignment of this ramus fragment to a specific taxon is not possible because multiple ornithocheirids are present within the Kem Kem Group.
Isolated teeth
Material. BSP 1993 IX 4, 590–596 (morphotype 1) BSP 1993 IX 314, 597–607 (morphotype 2) BSP 1993 IX 332, 608–617 (morphotype 3) BSP 1993 IX 618–621 (morphotype 4) (described by Wellnhofer and Buffetaut 1999), FSAC-KK 44, FSAC-KK 197, FSAC-KK 885–887, FSAC-KK 941 (described by Ibrahim et al. 2020), FSAC-KK 17001 (described by Martill et al. 2018), LINHM 007 (described by Mader and Kellner 1997), MNHN MRS 1108 (referred here, Fig. 3).
Remarks. Assignment of any of these teeth to a specific taxon is not currently possible because multiple taxa of ornithocheirids are present in the Kem Kem Group, and have similar (and highly variable) tooth morphologies. There is no current record of LINHM, therefore the location of LINHM 007 described by Mader and Kellner (1997) is currently unknown.
Notarium
Material. FSAC-KK 5208, anterior notarium fragment comprising the anterior most three and partial fourth dorsal vertebrae (Fig. 9, referred here).
Taxonomic assignment A notarium consisting of three co-ossified vertebral centra and possibly the anterior section of a fourth (FSAC-KK 5208) is uncrushed but badly damaged and lacks most surficial details. It differs in several important respects from FSAC-KK 5207, identified as azhdarchid: it bears massive ventrolaterally directed parapophyses that extend well beyond the lateral margins of the centrum; the cotyle is dorsoventrally pinched, resulting in a lemniscate profile; and blind pits, rather than pneumatopores, flanking the neural canal. All these features are present in ornithocheiroid notaria including, for example, Pteranodon (Bennett, 2001: figs. 45–47), ‘Santanadactylus’ (Wellnhofer et al., 1983: fig. 2), Ornithocheirus (Kellner et al., 2013), and Coloborhynchus (Veldmeijer, 2003: fig. 7). Thus, FSAC-KK 5208 is assigned here to an indeterminate species of Ornithocheiridae.
Discussion
Modification to the occlusal surface
Bony protuberances occur on the occlusal surfaces of the jaws of at least three Kem Kem Group azhdarchoids; Afrotapejara zouhrii (FSAC-KK 5004; Fig. 27J), Alanqa saharica (FSAC-KK 26, FSAC-KK 4000, UOP-PAL KK 0006/FSAC-KK 5213, FSAC-KK 5204–5205; Fig. 27A–E) and Xericeps curvirostris (FSAC-KK 10700; Fig. 27I) (Ibrahim et al. 2010; Martill and Ibrahim 2015; Martill et al. 2018, 2020a). They are also observed on three jaws referred to here as ‘morphotype C’ (FSAC-KK 5200–5202; Fig. 27F–H).
Photographs (left) and digital 3D model images generated from topographic scans (right) of bony protuberances on the occlusal surface of Kem Kem Group edentulous pterosaur jaw fragments in occlusal view. A Alanqa saharica mandibular symphysis FSAC-KK 4000; B Alanqa saharica mandibular symphysis UOP-PAL KK 0006 and FSAC-KK 5213; C Alanqa saharica holotype mandibular symphysis FSAC-KK 26 (topographic scan unavailable); D Alanqa saharica rostrum FSAC-KK 5205; E Alanqa saharica rostrum FSAC-KK 5204; F jaw ‘morphotype C’ FSAC-KK 5201; G jaw ‘morphotype C’ FSAC-KK 5200; H jaw ‘morphotype C’ FSAC-KK 5202; I Xericeps curvirostris holotype mandibular symphysis FSAC-KK 10700; J Afrotapejara zouhrii holotype rostrum FSAC-KK 5004. Arrow indicates bony protuberance on occlusal surface. Scale bars represent 10 mm
Alanqa saharica has complementary bony protuberances on the upper and lower jaws. The holotype mandibular symphysis (FSAC-KK 26) was originally described as having a ‘V’ shaped caudally bifurcating midline ridge on the occlusal surface (Ibrahim et al. 2010, Fig. 27C). Subsequently Martill and Ibrahim (2015) described a specimen (FSAC-KK 4000), which was attributed to a possible rostrum of cf. Alanqa saharica, with “paired, elongate tapered protuberances extending from a position exterior to the lateral margins of the jaw posteriorly and extend toward the median line of the occlusal surface of the jaw.” The structures did not meet in the middle but tapered to a point anteriorly and posteriorly (Fig. 27A). A second specimen with similar structures (held in a private collection) was also figured (Martill and Ibrahim, 2015 Fig. 5; 3D print UOP-PAL KK 0006/FSAC-KK 5213; Figs. 7F–H and 25B here). Martill and Ibrahim (2015) proposed that the structure seen on the holotype mandible was complimentary to the structures on FSAC-KK 4000 and UOP-PAL KK 0006/FSAC-KK 5213. Re-examination of the holotype has indicated that these structures are identical (see Fig. 27A–C) and that all three specimens are most likely mandibular symphyses of Alanqa saharica. Two specimens (FSAC-KK 5204 and FSAC-KK 5205) have been discovered with a cross-section and shape, that while similar to the mandibular symphyses of A. saharica, differs in the presence of a single median eminence (boss) on the occlusal surface (see Fig. 27D–E). This eminence extends medially for approximately 110 mm along the occlusal surface of FSAC-KK 5204, widening, rising and becoming more prominent posteriorly, attaining a maximum height of 8 mm and maximum width of 5 mm. In the case of FSAC-KK 5205 the eminence extends for at least 90 mm along the occlusal surface, and reaches a maximum height of 9 mm and width of 4 mm. These bones are interpreted as sections of rostra, composed of fused premaxillae-maxillae, with the single eminence complementing the paired structures seen on the occlusal surface of the lower jaws. This construction consisting of a midline ridge, keel, or boss of bone borne on the occlusal surface of the rostrum that matches a groove-like structure on the occlusal surface of the mandibular symphysis is a common but not universal feature of pterosaurs (e.g., Martill and Ibrahim 2015).
Bony protuberances similar to those found on the holotype of Alanqa saharica are also present on the rostrum of the holotype of Xericeps curvirostris (FSAC-KK 10700, Fig. 27I). However, these elongate, tapered protuberances are much narrower in Xericeps, measuring 2 mm in width and projecting 1.5 mm above the occlusal surface (see Fig. 27I). Posterior to these protuberances the occlusal surface becomes deeply sulcate (see Fig. 14 and cross-section Fig. 5), deepening posteriorly to a maximum depth of 9 mm. This deeply sulcate occlusal surface could accommodate a large median protuberance similar to that seen in the upper jaw of A. saharica. However, as rostra of X. curvirostris have yet to be recovered this hypothesis remains untested.
Three jaw fragments (FSAC-KK 5200-5202) here referred to as jaw ‘morphotype C’, exhibit a U-shaped cross-section and bear a large median eminence on the occlusal surface (see Fig. 17, 27F–H). These fragments could represent mandibular symphyses of Apatorhamphus gyrostega due to their similar cross-sectional outline and low lateral angle. However, this is not consistent with the standard configuration in pterosaurs where such structures are borne on the rostrum. Moreover, not one of the specimens referred to A. gyrostega by McPhee et al., (2020) displays a sulcus that would accommodate a boss although this may be because these specimens are not sufficiently complete as to preserve these features. Alternatively, these specimens could represent the rostrum of X. curvirostris, which is straight, with the large median boss fitting in the deeply sulcate occlusal surface of the mandibular symphysis. Such an animal would possess a gape perhaps comparable to the African Openbill (Anastomus lamelligerus). A third possibility is that these specimens represent an as yet unnamed species with affinities to either A. gyrostega or X. curvirostris.
The rostrum of the holotype of Afrotapejara zouhrii (FSAC-KK 5004) also bears a small posteriorly located ‘boss’ on its occlusal surface (Fig. 27J). Such a feature has not previously been reported for Tapejaridae. However, modifications of the occlusal surface are seen in the tapejarids Caupedactylus ybaka and Caiuajara dobruskii. Caupedactylus ybaka has a slightly raised area between two grooves located on the posterior part of the rostrum (Kellner, 2013: fig. 3c), referred to as ‘a low palatal boss’ by Pêgas et al. (2021b), whereas Caiuajara dobruskii has two elongate boss-like ridges, extending posteriorly and similar to those observed in A. saharica (Manzig et al., 2014: fig. 5a). In addition to a low median eminence anteriorly, the mandibular symphysis of the putative tapejarid Bakonydraco galaczi also exhibits a transverse eminence located at approximately the mid-point of the mandibular symphysis (Ősi et al., 2005: fig. 2; Pêgas et al., 2021b).
Several other azhdarchoid pterosaurs also exhibit modifications to the occlusal surface of the jaws. The ?mandibular symphysis of the azhdarchid Mistralazhdarcho maggii Vullo et al., 2018 has a large single median eminence that protrudes dorsally approximately 9 mm above the lateral margin of the occlusal surface (Vullo et al., 2018: fig. 1). A low but prominent median longitudinal eminence was also reported on the occlusal surface of the ?premaxilla/maxilla (considered a lower jaw by Pêgas et al., 2021b) of the holotype of the azhdarchid Aerotitan sudamericanus Novas et al., 2012 (Novas et al., 2012: fig. 2D, H; Pêgas et al., 2021b: fig. 4). This feature is, however, not clearly evident in their figures. Comparable structures are also present on the jaws of the thalassodromeids Tupuxuara leonardii Kellner and Campos, 1994, which has a large median eminence on the rostrum of the holotype (Kellner and Campos, 1994: figs. 3–6), and Thalassodromeus sethi which also has an occlusal median eminence on the rostrum and an interlocking sulcus on the mandibular symphysis (Pêgas et al. 2018, 2021a). The mandibular symphysis of Tupuxuara lacks a complimentary sulcus that could accommodate the large median eminence on the rostrum (Pêgas et al. 2021b). A median ridge is also present on the mandibular symphysis of the holotype of the tapejaromorph Keresdrakon vilsoni (Kellner et al. 2019: fig. 4), but the rostrum lacks a complementary sulcus (Kellner et al. 2019; Pêgas et al. 2021b). The mandibular symphysis of the holotype of Argentinadraco barrealensis Kellner and Calvo 2017 (MUCPv-1137) bears a groove bounded on either side by lateral ridges that converge but do not meet anteriorly (Kellner and Calvo 2017: figs. 2–3), a structure comparable to that present on mandibular symphyses of Alanqa saharica and Xericeps curvirostris. To summarise, modifications to the occlusal surfaces of the jaws are widespread within Azhdarchoidea, present on both the rostra and mandibular symphyses and often complement each other.
The function of the occlusal ridges and grooves in azhdarchoid jaws is most likely related to feeding. The robust nature of these structures, which have thickened bony cortices and extensive modification to the trabeculae directly beneath or above the structure (Martill and Ibrahim 2015), suggests that they were used for crushing or shearing hard prey items such as crustaceans, shelled molluscs or small vertebrates (e.g., turtles) (Martill and Ibrahim 2015; Pêgas et al. 2021a). But other functions cannot be ruled out. Martill and Ibrahim (2015) speculate, for example, that the protuberances could have acted as anchoring points for soft tissues that formed ‘cheeks’ or elaborate display structures.
Phylogeny
Of the eight major pterosaur clades present in the late Early Cretaceous—early Late Cretaceous (Ornithocheiridae, Ctenochasmatinae, Gnathosaurinae, Lonchodectidae, Tapejaridae, Chaoyangopteridae, Thalassodromidae and Azhdarchidae) (Fig. 28), at least three are present in the Kem Kem Group (Azhdarchidae, Ornithocheiridae and Tapejaridae) and possibly four if Apatorhamphus gyrostega is a chaoyangopterid. A. gyrostega was tentatively referred to ?Chaoyangopteridae by McPhee et al. (2020) based on its similar jaw profile to other chaoyangopterids including Jidapterus and Chaoyangopterus. In a recent phylogenetic analysis by Andres (2021), A. gyrostega was recovered as a chaoyangopterid. The presence of a chaoyangopterid in the Kem Kem Group would also extend the range of the family into the Albian and possibly the Cenomanian depending on the age of the Kem Kem Group. Alanqa saharica was originally referred to Azhdarchidae by Ibrahim et al. (2010), based on several features including its straight, elongate, slender and low angled jaw profile and its Y-shaped cross-section (Ibrahim et al. 2010; Pêgas et al. 2021b). However, Longrich et al. (2018) recovered Alanqa as a thalassodromid, but this was not well supported and was coded based on their interpretation of the holotype as an upper jaw (Longrich et al. 2018; Pêgas et al. 2021b).

Adapted from Lü et al., 2010
Time-calibrated phylogeny of the main pterodactyloid pterosaur clades showing their temporal ranges. Solid bar indicates the known ranges and coloured lines inferred ‘ghost’ ranges. Bars in red show the clades present in the Kem Kem Group; pink horizontal bar with * indicates potential age range of the Kem Kem Group. Chaoyangopteridae ‘broken’ bar indicates range of Chaoyangopteridae if Apatorhamphus gyrostega is a chaoyangopterid.
Pêgas et al. (2021b) recovered Alanqa saharica as a sister taxon to the tapejaromorph Keresdrakon vilsoni outside of Azhdarchidae in a clade they called Alanqidae. This was based on the presence of a ‘posterior V-shaped median ridge’ (apomorphy) on the occlusal surface. However, due to our reinterpretation of this structure on the holotype of A. saharica as a pair of ridges like that seen on FSAC-KK 4000 (see Fig. 27), this phylogenetic placement is likely incorrect.
Xericeps curvirostris was recovered as a sister taxon to Argentinadraco barrealensis by Pêgas et al. (2021b). This was based on the presence of ‘paired ridges bordered by paired sulci’ on the occlusal surface. In their study, Xericeps curvirostris and Argentinadraco barrealensis formed a sister group to a trichotomy within Chaoyangopteridae containing Chaoyangopterus, Jidapterus and Lacusovagus based upon an upturned lower jaw (Pêgas et al. 2021b). We disagree that Chaoyangopterus and Jidapterus, have upturned lower jaws like X. curvirostris; the jaws on these taxa may be ever so slightly upcurved but this is likely an artifact of compaction (see Martill et al. 2018). Regarding the features it shares with Argentinadraco (the pair of ridges), this character state is also seen on the holotype and referred specimens of A. saharica (FSAC-KK 26, Fig. 27A–C). Pêgas et al. (2021b) also suggested that X. curvirostris has a similar foramina distribution to some chaoyangopterids. Foramina distribution is highly variable within Azhdarchoidea and therefore should not be used as a feature for taxonomic assignment until better evaluated. Therefore, we do agree that X. curvirostris could be a chaoyangopterid, but we consider that, due to the fragmentary nature of the material and limited number of characters, it is not possible to regard X. curvirostris with any confidence as a chaoyangopterid at the present time.
In addition, in a recent analysis by Andres (2021), Alanqa saharica, Leptostomia begaaensis, and Xericeps curvirostris were recovered as thalassodromids within Thalassodrominae. We are sceptical of any assignment of these taxa with any certainty to a pterosaur clade due to the highly fragmentary nature of the material with limited characters. We therefore take a cautious approach and consider L. begaaensis and X. curvirostris indeterminate non-tapejarid azhdarchoids. However, we do agree with the original assignment of A. saharica to Azhdarchidae based on its straight and elongate jaw, which is very similar to that of Quetzalcoatlus. Any phylogenetic analysis based on such fragmentary material should always be treated cautiously.
Diversity and palaeoecology of Kem Kem Group pterosaurs
The Kem Kem Group pterosaurs are represented by two of the four main pterodactyloid clades, ornithocheiroids and azhdarchoids. Ornithocheiridae is the only one of the three principal ornithocheiroid clades to have been recorded in the Kem Kem Group so far. The other groups, are known to have existed at that time (Istiodactylidae) or, based on phylogenetic inference, are likely to have existed (Pteranodontia) but remain unrecorded.
The toothed pterosaurs Anhanguera, Coloborhynchus and Siroccopteryx have been interpreted as aerial piscivores (e.g., Veldmeijer et al. 2007; Amiot et al. 2010; Tütken and Hone 2010; Bestwick et al. 2020; Pêgas et al. 2021a). This is based upon tooth morphology and arrangement, theoretical modelling of fishing behaviour (Veldmeijer et al. 2007), microwear analyses (Bestwick et al. 2020), tooth carbon isotope ratios indicative of aquatic foraging (Amiot et al. 2010; Tütken and Hone 2010), and bite force analyses (Pêgas et al. 2021a). The similarities in jaw and dental morphology between Anhanguera and Coloborhynchus and the other Kem Kem Group ornithocheirids, Ornithocheirus and Siroccopteryx, suggest that they were also piscivores. However, the differing tooth arrangements (dental location, spacing, size and number) and jaw morphologies of the four Kem Kem Group ornithocheirids, might indicate that they occupied subtly different niches, foraging in different ways and/or specialising on different prey. It is also conceivable that these pterosaurs could have exploited other prey, such a small reptiles or dinosaurs or even other pterosaurs.
There is far less agreement regarding the palaeoecology of the edentulous azhdarchid pterosaurs, and several alternative lifestyles have been proposed. These include: carrion feeding (Lawson 1975); probe feeding (Langston 1981; Lehman and Langston 1996; Smith et al. 2020a); terrestrial foraging (Chatterjee and Templin 2004; Witton 2007; Witton and Naish 2008, 2013; Padian et al. 2021); a heron-like lifestyle (Bennett 2001; Padian et al. 2021) and skim/dip feeding (Nesov 1984; Kellner and Langston 1996; Martill 1997; Prieto 1998). These possible palaeoecological scenarios have been summarised and reviewed by Humphries et al. (2007) and Witton and Naish (2008).
Due to the range of sedimentary environments in which azhdarchid fossils occur ranging from shallow-marine (e.g., Frey and Martill, 1996; Pereda Suberbiola et al. 2003; Longrich et al. 2018) to fluvial and lacustrine settings (e.g., Lawson 1975; Averianov 2010; Ibrahim et al. 2020) it is unlikely that all azhdarchids had the same lifestyle. Similarly, due to the long temporal range (at least 47 my from the Aptian to Maastrichtian) of Azhdarchidae and their morphological disparity, it is unlikely that individual species occupied the same ecological niche through time. Nevertheless, it is unlikely that they were skim/dip feeders due to their skull and jaw morphology (Humphries et al. 2007). A role as terrestrial foragers argued for by Witton and Naish (2008, 2013) is plausible. In this case it is proposed that some azhdarchids were generalists feeding on larger invertebrates, small vertebrates, and perhaps carrion, with a lifestyle somewhat similar to that of storks. However, it is equally plausible that some azhdarchids were heron-like feeders patrolling the banks of rivers, lakes, and shorelines, feeding on a range of vertebrates and invertebrates, albeit without the heron’s ability to dart its neck at speed.
It is therefore possible that the Kem Kem Group azhdarchid Alanqa saharica had a lifestyle similar to that of a stork or heron, either foraging and fishing along the Kem Kem river system or foraging on its extensive flood plains. The presence of bony protuberances and corresponding groove in the jaws of A. saharica have been interpreted as a structure for crushing hard foods, such as shelled molluscs and smaller vertebrates such as (perhaps) turtles and juvenile crocodilians (Martill and Ibrahim 2015; Pêgas et al. 2021a).
The edentulous Leptostomia begaaensis has been interpreted as a probe feeder based on its long, narrow, dorsoventrally compressed beak with thickened bone walls. Such adaptations would decrease the resistance caused by inserting the beak into sediment, as well as strengthening it against compressional and bending stresses (Smith et al. 2020a).
Like other chaoyangopterids, which have been interpreted as piscivores and generalists (Beskwick et al., 2018), it is likely that the Kem Kem Group ?chaoyangopterid Apatorhamphus gyrostega was a piscivore/generalist, possibly with a similar lifestyle to Alanqa saharica.
Many diets have been suggested for tapejarids, including herbivory/frugivory/nucivory (Wellnhofer and Kellner 1991; Wang and Zhou 2006; Meijer et al. 2007; Vullo et al. 2012; Henderson 2017; Pêgas et al. 2021a), carnivory (Vullo et al. 2012), piscivory (Wang and Zhou 2006; Unwin and Martill 2007), insectivory (Wang et al. 2008); Vullo et al. 2012), and a generalist feeding ecology (Pêgas et al. 2021a). These suggestions are based upon comparative anatomy, functional morphology, and associations with certain environments (Bestwick et al. 2018) and have rarely been tested. Recently, reconstructions of the jaw musculature of the tapejarids Tapejara wellnhoferi and Caupedactylus ybaka by Pêgas et al. (2021a) inferred that they were a frugivore/nucivore and generalist, respectively. It is likely due to the morphological disparity, geographical range (see Martill et al. 2020b: fig. 7), and range of sedimentary environments that tapejarids are found in, from possible desert (Manzig et al. 2014) to fluvial and lacustrine (e.g., Martill et al. 2020a) to shallow-marine (Kellner 1989), that they had a wide range of diets. Morphological comparisons with extant birds can be problematic, not least because many birds exhibit a superficially similar jaw morphology to tapejarids but exhibit a wide range of diets, as seen, for example, in frugivorous parrots and the helmeted vanga (Euryceros prevostii), which has a generalist diet consisting of insects and small vertebrates such as lizards (Marca and Thorstrom 2000). It is therefore difficult to establish the diet of Afrotapejara zouhrii, and tapejarids in general, but this taxon may have been a generalist similar to Caupedactylus ybaka as proposed by Pêgas et al. (2021a).
Flaplings to giants
The pterosaur material of the Kem Kem Group is unusual compared to most of the other pterosaur-bearing deposits in that it contains remains from both immature individuals (Smith et al. 2021) and giant individuals. Material of immature individuals comprises several jaw fragments referred to Alanqa saharica and Apatorhamphus gyrostega and a possible azhdarchid cervical vertebra (Smith et al. 2021). This cervical vertebra (FSAC-KK 5083) hints at a pterosaur with a wingspan of approximately 1 m. The giant material comprises several very large teeth, jaw fragments, cervical vertebrae, ulnae, and femora of both ornithocheirid and azhdarchoid pterosaurs. These specimens have estimated wingspans of up to 6–7 m. Large jaw fragments suggest that Alanqa saharica and Apatorhamphus gyrostega likely reached large sizes. These immature and giant forms presumably occupied different ecological niches (ontogenetic niche partitioning), which would have expanded the ecological diversity of the Kem Kem Group pterosaur assemblage without extending its taxonomic diversity (Smith et al. 2021).
Diversity and palaeoecology of mid-Cretaceous pterosaurs
Excluding teeth, pterosaurs from the Kem Kem Group are now represented by more than 400 remains consisting primarily, but not only fragments of rostra and mandibular symphyses, but also including a range of postcranial remains, among them numerous vertebrae and incomplete limb bones (Tables 3, S2). Assuming these remains each represent a single individual, then the assemblage, as currently understood, is comparable in scale to that of other pterosaur concentration Lagerstätten (Dean et al. 2016). This, and the ongoing intensive study of this assemblage in terms of its taxonomic composition, summarised in this paper, permit comparisons with other important mid-Cretaceous pterosaur assemblages including the Crato and Santana formations of Brazil (e.g., Kellner and Tomida 2000; Unwin and Martill 2007), the Cambridge Greensand Member (Unwin 2001; Smith et al. 2021) and Lower Chalk of England (Bowerbank 1851; Owen 1851), and the Sannine Limestone Formation of Lebanon (Kellner et al. 2019).
At the most inclusive taxonomic levels, these assemblages are broadly comparable in terms of taxonomic diversity, with ornithocheirids, azhdarchoids and ctenochasmatoids (represented by lonchodectids) occurring in most assemblages, the exceptions being the Crato Formation and the Kem Kem Group where ctenochasmatoids are unrecorded and the Lower Chalk of England where azhdarchoids are absent (though it should be noted that this sequence has yielded considerably fewer specimens). In addition, and as noted previously by Dean et al. (2016), there is a strong correlation between numbers of specimens recovered and diversity at the species level. Relatively high levels of diversity, approaching or exceeding 10 species, are to be found in deposits such as the Crato and Santana formations, the Cambridge Greensand Member, and now the Kem Kem Group where the assemblages approach or exceed 100 specimens. Consequently, the relatively low diversity recorded in the Lower Chalk (two species) and Sannine Limestone (three species) likely reflect the small number of specimens collected to date.
The Kem Kem Group pterosaur assemblage is of particular interest in that this deposit has yielded the greatest number of species, representing four distinct clades (Ornithocheiridae, Azhdarchidae, Tapejaridae and ?Chaoyangopteridae) yet to be recorded from a fluvial system. Higher diversity has been reported for pterosaurs in Cretaceous lacustrine Lagerstätten such as the Yixian and Jiufotang formations of the Chinese Jehol Group (Unwin et al. 2000; Lü et al. 2006) and some lagoonal and quasi-marine horizons such as the Crato and Santana formations of Brazil (Unwin and Martill 2007). However, these strata have been more intensively sampled and studied compared to the Kem Kem Group.
There is some evidence to suggest a correlation between particular palaeoenvironments and the kinds of pterosaurs to be found in those environments. Thus, azhdarchoids are present and often common in ‘continental’ and ‘marginal continental’ sequences such as the Kem Kem Group and Crato and Santana formations, but rare or even absent from fully marine deposits such as the Cambridge Greensand, Lower Chalk, and the stratigraphically younger Niobrara Formation of the United States. By contrast, ornithocheirids, which seem to have been able to inhabit any environments in which water bodies occur, are practically ubiquitous in mid-Cretaceous deposits, including the Kem Kem Group.
Conclusions
The Kem Kem Group pterosaur assemblage is highly diverse with nine named taxa and at least three distinct and as yet unnamed jaw morphotypes, hinting at even higher diversity (Fig. 29). This suggests that the Kem Kem Group samples a period of high pterosaur diversity and that it represents a sedimentary environment with diverse habitats. Despite the fragmentary nature of the fossils, the pterosaur-yielding horizons in the upper part of the Ifezouane Formation can be considered as a concentration Lagerstätte and, because the preservation is excellent (fossils are 3-D and bone micro-histology is preserved with sub-cellular level detail), also as a conservation Lagerstätte too (albeit not in the usually accepted sense of the term). Thus, the Kem Kem Group pterosaurs provide valuable insights into pterosaur palaeobiology with excellent preservation of both micro and macro internal structures.
Simplified reconstruction of the pterosaur assemblage Kem Kem Group river system. 1. Leptostomia begaaensis; 2. Apatorhamphus gyrostega; 3. Alanqa saharica; 4. Xericeps curvirostris; 5. Afrotapejara zouhrii; 6. Ornithocheirus simus; 7. Coloborhynchus fluviferox; 8. Anhanguera piscator; 9. Siroccopteryx moroccensis; 10. Spinosaurus aegyptiacus; 11. rebbachisaurid sauropods; 12 a tree-like representation of Welwitschiophyllum. Artwork by Emily Pilavachi
The preservation of the pterosaur assemblage is heavily biased taphonomically, with a presently unexplained high abundance of jaw fragments anterior to the nasoantorbital fenestra or divergence of the mandibular rami. Skeletal fragments are dominated by edentulous taxa whereas teeth of ornithocheirids are extremely abundant, suggesting this group was not scarce in the Kem Kem Group environment. The deposit is also unusual in that some of the edentulous forms comprise both immature and giant individuals of the same species, suggesting that they may have lived alongside each other but occupied different ecological niches. The first pterosaur remains from the Kem Kem Group were reported a quarter of a century ago. There have been numerous publications on the pterosaur assemblage since then, and likely many more discoveries remain to be made. Despite its fragmentary nature, the material has lent itself to taxonomic, palaeoecological, biogeographic, and functional morphological studies and, though perhaps less convincingly, phylogenetic studies.
Presently, this pterosaur assemblage is the most diverse known assemblage from a fluvial setting, containing examples of taxa that otherwise have been reported from lacustrine and marine palaeoenvironments and is the most diverse assemblage in Africa.
References
Abzhanov, A., M. Protas, B.R. Grant, P.R. Grant, and C.J. Tabin. 2004. Bmp4 and morphological variation of beaks in Darwin’s finches. Science 305: 1462–1465.
Adardor, S., H. Haddoumi, A. Rachdi, E.M. Ettachfini, L. Baidder, R. Chennouf, and A. Charrière. 2021. Jurassic-Cretaceous red beds of the southern front of Moroccan Central High Atlas (Aghbalou N’Kerdouss-Tadighoust region): Sedimentological, lithostratigraphical and paleogeographical studies. Journal of African Earth Sciences 178: 104185.
Aires, A.S., A.W. Kellner, R.T. Müller, L.R. Da Silva, C.P. Pacheco, and S. Dias-Da-Silva. 2013. New postcranial elements of the Thalassodrominae (Pterodactyloidea, Tapejaridae) from the Romualdo Formation (Aptian–Albian), Santana Group, Araripe Basin, Brazil. Palaeontology 57: 343–355.
Aires, A.S., L.M. Reichert, R.T. Müller, F.L. Pinheiro, and M.B. Andrade. 2020. Development and evolution of the notarium in Pterosauria. Journal of Anatomy 238: 400–415.
Alloul, T., J.C. Rage, R. Hamdidouche, and N.E. Jalil. 2018. First report on Cretaceous vertebrates from the Algerian Kem Kem beds. A new procoelous salamander from the Cenomanian, with remarks on African Caudata. Cretaceous Research 84: 384–388.
Amiot, R., X. Wang, C. Lécuyer, E. Buffetaut, L. Boudad, L. Cavin, Z. Ding, F. Fluteau, A.W.A. Kellner, H. Tong, and F. Zhang. 2010. Oxygen and carbon isotope compositions of middle Cretaceous vertebrates from North Africa and Brazil: Ecological and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology 297: 439–451.
Andres, B. 2021. Phylogenetic systematics of Quetzalcoatlus Lawson 1975 (Pterodactyloidea: Azhdarchoidea). Journal of Vertebrate Paleontology 41: 203–217.
Andres, B., and W. Langston Jr. 2021. Morphology and taxonomy of Quetzalcoatlus Lawson 1975 (Pterodactyloidea: Azhdarchoidea). Journal of Vertebrate Paleontology 41: 46–202.
Andres, B., and T.S. Myers. 2013. Lone star pterosaurs. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 103: 383–398.
Averianov, A.O. 2010. The osteology of Azhdarcho lancicollis Nessov, 1984 (Pterosauria, Azhdarchidae) from the Late Cretaceous of Uzbekistan. Proceedings of the Zoological Institute RAS 314: 264–317.
Averianov, A.O. 2012. Ornithostoma sedgwicki–valid taxon of azhdarchoid pterosaurs. Proceedings of the Zoological Institute of the Russian Academy of Sciences 316: 40–49.
Averianov, A.O. 2014. Review of taxonomy, geographic distribution, and paleoenvironments of Azhdarchidae (Pterosauria). ZooKeys 432: 1–107.
Averianov, A.O., M.S. Arkhangelsky, and E.M. Pervushov. 2008. A new Late Cretaceous azhdarchid (Pterosauria, Azhdarchidae) from the Volga Region. Paleontological Journal 42: 634–642.
Bantim, R.A., A.A. Saraiva, G.R. Oliveira, and J.M. Sayao. 2014. A new toothed pterosaur (Pterodactyloidea: Anhangueridae) from the Early Cretaceous Romualdo Formation, NE Brazil. Zootaxa 3869: 201–223.
Barrett, P.M., R.J. Butler, N.P. Edwards, and A.R. Milner. 2008. Pterosaur distribution in time and space: An atlas. Zitteliana B28: 61–107.
Beccari, V., F.L. Pinheiro, I. Nunes, L.E. Anelli, O. Mateus, and F.R. Costa. 2021. Osteology of an exceptionally well-preserved tapejarid skeleton from Brazil: Revealing the anatomy of a curious pterodactyloid clade. PLoS ONE 16: e0254789.
Beevor, T., A. Quigley, R.E. Smith, R.S. Smyth, N. Ibrahim, S. Zouhri, and D.M. Martill. 2021. Taphonomic evidence supports an aquatic lifestyle for Spinosaurus. Cretaceous Research 117: 104627.
Belvedere, M., N.E. Jalil, A. Breda, G. Gattolin, H. Bourget, F. Khaldoune, and G.J. Dyke. 2013. Vertebrate footprints from the Kem Kem beds (Morocco): A novel ichnological approach to faunal reconstruction. Palaeogeography, Palaeoclimatology, Palaeoecology 383: 52–58.
Bennett, S.C. 1989. A pteranodontid pterosaur from the Early Cretaceous of Peru, with comments on the relationships of Cretaceous pterosaurs. Journal of Paleontology 63: 669–677.
Bennett, S.C. 1992. Sexual dimorphism of Pteranodon and other pterosaurs, with comments on cranial crests. Journal of Vertebrate Paleontology 12: 422–434.
Bennett, S.C. 1993. The ontogeny of Pteranodon and other pterosaurs. Paleobiology 19: 92–106.
Bennett, S.C. 1995. A statistical study of Rhamphorhynchus from the Solnhofen Limestone of Germany: Year-classes of a single large species. Journal of Paleontology 69: 569–580.
Bennett, S.C. 2001. The osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon Part II. Size and functional morphology. Palaeontographica Abteilung A 260: 113–153.
Bennett, S.C. 2007. A review of the pterosaur Ctenochasma: Taxonomy and ontogeny. Neues Jahrbuch Für Geologie Und Paläontologie, Abhandlungen 245: 23–31.
Bestwick, J., D.M. Unwin, R.J. Butler, D.M. Henderson, and M.A. Purnell. 2018. Pterosaur dietary hypotheses: A review of ideas and approaches. Biological Reviews 93: 2021–2048.
Bestwick, J., D.M. Unwin, R.J. Butler, and M.A. Purnell. 2020. Dietary diversity and evolution of the earliest flying vertebrates revealed by dental microwear texture analysis. Nature Communications 11: 1–9.
Bowerbank, J.S. 1851. On the pterodactyles of the Chalk Formation. Proceedings of the Zoological Society of London 19: 14–20.
Campos, H.B.N. 2021. A new azhdarchoid pterosaur from the Late Cretaceous Javelina Formation of Texas. Biologia 77: 1–9 (article retracted).
Cavin, L., H. Tong, L. Boudad, C. Meister, A. Piuz, J. Tabouelle, M. Aarab, R. Amiot, E. Buffetaut, G. Dyke, and S. Hua. 2010. Vertebrate assemblages from the early Late Cretaceous of southeastern Morocco: An overview. Journal of African Earth Sciences 57: 391–412.
Chatterjee, S., and R.J. Templin. 2004. Posture, locomotion, and paleoecology of pterosaurs. Geological Society of America, Special Publications 376: 1–64.
Campos, D.A., and A.W.A. Kellner. 1985. Un novo exemplar de Anhanguera blittersdorffi (Reptilia, Pterosauria) da formaçao Santana, Cretaceo Inferior do Nordeste do Brasil. In: Congresso Brasileiro de Paleontologia, Rio de Janeiro, Resumos: 13.
Dalla Vecchia, F.M., P. Arduini, and A.W.A. Kellner. 2001. The first pterosaur from the Cenomanian (Late Cretaceous) Lagerstätten of Lebanon. Cretaceous Research 22: 219–225.
Dean, C.D., P.D. Mannion, and R.J. Butler. 2016. Preservational bias controls the fossil record of pterosaurs. Palaeontology 59: 225–247.
d'Orbigny, A. 1840–1842. Paléontologie française: Terrains crétacés. 1. Cephalopodes Masson, Paris (1840–1842) pp. 1–120 (1840), 121–430 (1841), 431–662 (1842).
Dridi, J. 2018. New fossils of the giant pholidosaurid genus Sarcosuchus from the Early Cretaceous of Tunisia. Journal of African Earth Sciences 147: 268–280.
Ettachfini, E.M., and B. Andreu. 2004. Le Cénomanien et le Turonien de la plate-forme Préafricaine du Maroc. Cretaceous Research 25: 277–302.
Fernandes, D.L., I. Nunes, and F.R. Costa. 2021. A taxonomic approach on diagnostic characters used to define new pterosaur taxa and an estimation of pterosaur diversity. Anais Da Academia Brasileira De Ciências 93: e20201568.
Frey, E., and D.M. Martill. 1996. A reappraisal of Arambourgiania (Pterosauria, Pterodactyloidea): One of the world’s largest flying animals. Neues Jahrbuch Für Geologie Und Paläontologie, Abhandlungen 199: 221–247.
Frey, E., D.M. Martill, and M.C. Buchy. 2003. A new crested ornithocheirid from the Lower Cretaceous of northeastern Brazil and the unusual death of an unusual pterosaur. Geological Society, London, Special Publications 217: 55–63.
Godfrey, S.J., and P.J. Currie. 2005. Pterosaurs. In Dinosaur provincial park. A spectacular ancient ecosystem revealed, ed. P.J. Currie and E.B. Koppelhus, 292–311. Bloomington: Indiana University Press.
Henderson, D.M. 2017. Using three-dimensional, digital models of pterosaur skulls for the investigation of their relative bite forces and feeding styles. Geological Society, London, Special Publications 455: 25–44.
Holgado, B., and R.V. Pêgas. 2020. A taxonomic and phylogenetic review of the anhanguerid pterosaur group Coloborhynchinae and the new clade Tropeognathinae. Acta Palaeontologica Polonica 65: 743–761.
Hone, D.W., J.M. Ratcliffe, D.K. Riskin, J.W. Hermanson, and R.R. Reisz. 2021. Unique near isometric ontogeny in the pterosaur Rhamphorhynchus suggests hatchlings could fly. Lethaia 54: 106–112.
Howse, S.C.B. 1986. On the cervical vertebrae of the Pterodactyloidea (Reptilia: Archosauria). Zoological Journal of the Linnean Society 88: 307–328.
Humphries, S., R.H. Bonser, M.P. Witton, and D.M. Martill. 2007. Did pterosaurs feed by skimming? Physical modelling and anatomical evaluation of an unusual feeding method. PLoS Biology 5: e204.
Ibrahim, N., D.M. Unwin, D.M. Martill, L. Baidder, and S. Zouhri. 2010. A new pterosaur (Pterodactyloidea: Azhdarchidae) from the Upper Cretaceous of Morocco. PLoS ONE 5: e10875.
Ibrahim, N., P.C. Sereno, D.J. Varricchio, D.M. Martill, D.B. Dutheil, D.M. Unwin, L. Baidder, H.C. Larsson, S. Zouhri, and A. Kaoukaya. 2020. Geology and paleontology of the Upper Cretaceous Kem Kem Group of eastern Morocco. ZooKeys 928: 1–216.
Jacobs, M.L., D.M. Martill, N. Ibrahim, and N. Longrich. 2019. A new species of Coloborhynchus (Pterosauria, Ornithocheiridae) from the mid-Cretaceous of North Africa. Cretaceous Research 95: 77–88.
Jacobs, M.L., D.M. Martill, D.M. Unwin, N. Ibrahim, S. Zouhri, and N.R. Longrich. 2020. New toothed pterosaurs (Pterosauria: Ornithocheiridae) from the middle Cretaceous Kem Kem beds of Morocco and implications for pterosaur palaeobiogeography and diversity. Cretaceous Research 110: 104413.
Kaup, J.J. 1834. Versuch einer Eintheilung der Saugethiere in 6 Stämme und der Amphibien in 6 Ordnungen. Isis 3: 311–315.
Kellner, A.W.A. 1989. A new edentate pterosaur of the Lower Cretaceous from the Araripe Basin, Northeast Brazil. Anais Da Academia Brasileira De Ciências 61: 439–446.
Kellner, A.W.A. 1995. The relationships of the Tapejaridae (Pterodactyloidea) with comments on pterosaur phylogeny. In: Sixth Symposium on Mesozoic Terrestrial Ecosystems and Biota, Short Papers. China Ocean Press, Beijing, pp. 73–77.
Kellner, A.W.A. 2013. A new unusual tapejarid (Pterosauria, Pterodactyloidea) from the Early Cretaceous Romualdo Formation, Araripe Basin, Brazil. Earth and Environmental Science Transactions of the Royal Society of Edinburgh 103: 409–421.
Kellner, A.W.A., and J.O. Calvo. 2017. New azhdarchoid pterosaur (Pterosauria, Pterodactyloidea) with an unusual lower jaw from the Portezuelo Formation (Upper Cretaceous), Neuquén Group, Patagonia, Argentina. Anais Da Academia Brasileira De Ciências 89: 2003–2012.
Kellner, A.W.A., and D.A. Campos. 1994. A new species of Tupuxuara (Pterosauria, Tapejaridae) from the Early Cretaceous of Brazil. Anais Da Academia Brasileira De Ciências 66: 467–474.
Kellner, A.W.A., and W. Langston Jr. 1996. Cranial remains of Quetzalcoatlus (Pterosauria, Azhdarchidae) from Late Cretaceous sediments of Big Bend National Park, Texas. Journal of Vertebrate Paleontology 16: 222–231.
Kellner, A.W.A., and B.J. Mader. 1996. First report of Pterosauria (Pterodactyloidea, Azhdarchidae) from Cretaceous rocks of Morocco. Journal of Vertebrate Paleontology 16: 45A.
Kellner, A.W.A., and Y. Tomida. 2000. Description of a new species of Anhangueridae (Pterodactyloidea) with comments on the pterosaur fauna from the Santana Formation (Aptian-Albian), northeastern Brazil. National Science Museum Monographs 17: IX–137.
Kellner, A.W.A., A. Mello and T. Ford. 2007. A survey of pterosaurs from Africa with the description of a new specimen from Morocco. Paleontologia: Cenários de Vida 1: 257–267.
Kellner, A.W.A., D.A. Campos, J.M. Sayao, A.A. Saraiva, T. Rodrigues, G. Oliveira, L.A. Cruz, F.R. Costa, H.P. Silva, and J.S. Ferreira. 2013. The largest flying reptile from Gondwana: A new specimen of Tropeognathus cf. T. mesembrinus Wellnhofer, 1987 (Pterodactyloidea, Anhangueridae) and other large pterosaurs from the Romualdo Formation, Lower Cretaceous, Brazil. Anais Da Academia Brasileira De Ciências 85: 113–135.
Kellner, A.W.A., L.C. Weinschütz, B. Holgado, R.A. Bantim, and J.M. Sayao. 2019. A new toothless pterosaur (Pterodactyloidea) from Southern Brazil with insights into the paleoecology of a Cretaceous desert. Anais Da Academia Brasileira De Ciências 91: e20190768.
Langston, W. 1981. Pterosaurs. Scientific American 244: 122–137.
Lawson, D.A. 1975. Pterosaur from the latest Cretaceous of West Texas: Discovery of the largest flying creature. Science 187: 947–948.
Lehman, T.M., and W. Langston Jr. 1996. Habitat and behaviour of Quetzalcoatlus: Paleoenvironmental reconstruction of the Javelina Formation (Upper Cretaceous), Big Bend National Park, Texas. Journal of Vertebrate Paleontology 16: 48A.
Longrich, N.R., D.M. Martill, and B. Andres. 2018. Late Maastrichtian pterosaurs from North Africa and mass extinction of Pterosauria at the Cretaceous-Paleogene boundary. PLoS Biology 16: e2001663.
Lü, J., S. Ji, C. Yuan, and Q. Ji. 2006. Pterosaurs from China, 147. Beijing: Geological Publishing House.
Lü, J., D.M. Unwin, L. Xu, and X. Zhang. 2008. A new azhdarchoid pterosaur from the Lower Cretaceous of China and its implications for pterosaur phylogeny and evolution. Naturwissenschaften 95: 891–897.
Lü, J., D.M. Unwin, X. Jin, Y. Liu, and Q. Ji. 2010. Evidence for modular evolution in a long-tailed pterosaur with a pterodactyloid skull. Proceedings of the Royal Society b: Biological Sciences 277: 383–389.
Mader, B.J., and A.W.A. Kellner. 1997. First occurrence of Anhangueridae (Pterosauria, Pterodactyloidea) in Africa. Journal of Vertebrate Paleontology 17: 62A.
Mader, B., and A.W.A. Kellner. 1999. A new anhanguerid pterosaur from the Cretaceous of Morocco. Boletim Do Museu Nacional Serie Geologia 45: 1–11.
Manzig, P.C., A.W.A. Kellner, L.C. Weinschütz, C.E. Fragoso, C.S. Vega, G.B. Guimarães, L.C. Godoy, A. Liccardo, J.H. Ricetti, and C.C. de Moura. 2014. Discovery of a rare pterosaur bone bed in a Cretaceous desert with insights on ontogeny and behavior of flying reptiles. PLoS ONE 9: e100005.
Marca, G., and R. Thorstrom. 2000. Breeding biology, diet and vocalization of the Helmet Vanga, Euryceros prevostii, on the Masoala Peninsula, Madagascar. Ostrich 71: 400–403.
Martill, D.M. 1997. From hypothesis to fact in a flight of fantasy: the responsibility of the popular scientific media. Geology Today 13: 71–73.
Martill, D.M. 2015. First occurrence of the pterosaur Coloborhynchus (Pterosauria, Ornithocheiridae) from the Wessex Formation (Lower Cretaceous) of the Isle of Wight, England. Proceedings of the Geologists’ Association 126: 377–380.
Martill, D.M., and E. Frey. 1999. A possible azhdarchid pterosaur from the Crato Formation (Early Cretaceous, Aptian) of northeast Brazil. Geologie En Mijnbouw 78: 315–318.
Martill, D.M., and N. Ibrahim. 2015. An unusual modification of the jaws in cf. Alanqa, a mid-Cretaceous azhdarchid pterosaur from the Kem Kem beds of Morocco. Cretaceous Research 53: 59–67.
Martill, D.M., and D.M. Unwin. 2012. The world’s largest toothed pterosaur, NHMUK R481, an incomplete rostrum of Coloborhynchus capito (Seeley, 1870) from the Cambridge Greensand of England. Cretaceous Research 34: 1–9.
Martill, D.M., M. O’Sullivan, and C. Newman. 2013. A possible azhdarchid pterosaur (Pterosauria, Azhdarchidae) in the Durlston Formation (Early Cretaceous, Berriasian) of southern England. Cretaceous Research 43: 26–39.
Martill, D.M., D.M. Unwin, N. Ibrahim, and N. Longrich. 2018. A new edentulous pterosaur from the Cretaceous Kem Kem beds of south eastern Morocco. Cretaceous Research 84: 1–12.
Martill, D.M., M. Green, R.E. Smith, M. Jacobs, and J. Winch. 2020a. First tapejarid pterosaur from the Wessex Formation (Wealden Group: Lower Cretaceous, Barremian) of the United Kingdom. Cretaceous Research 113: 104487.
Martill, D.M., R.E. Smith, D.M. Unwin, A. Kao, J. McPhee, and N. Ibrahim. 2020b. A new tapejarid (Pterosauria, Azhdarchoidea) from the mid-Cretaceous Kem Kem beds of Takmout, southern Morocco. Cretaceous Research 112: 104424.
Martill, D.M., R.E. Smith, N. Longrich, and J. Brown. 2021. Evidence for tactile foraging in pterosaurs: A sensitive tip to the beak of Lonchodraco giganteus (Pterosauria, Lonchodectidae) from the Upper Cretaceous of southern England. Cretaceous Research 117: 104637.
Masrour, M., C. Pascual-Arribas, M. de Ducla, N. Hernández-Medrano, and F. Pérez-Lorente. 2017. Anza palaeoichnological site. Late Cretaceous. Morocco. Part I. The first African pterosaur trackway (manus only). Journal of African Earth Sciences 134: 766–775.
Matthews, S.C. 1973. Notes on open nomenclature and on synonymy lists. Palaeontology 16: 713–719.
McCurry, M.R., A.R. Evans, E.M. Fitzgerald, C.R. McHenry, J. Bevitt, and N.D. Pyenson. 2019. The repeated evolution of dental apicobasal ridges in aquatic-feeding mammals and reptiles. Biological Journal of the Linnean Society 127: 245–259.
McPhee, J., N. Ibrahim, A. Kao, D.M. Unwin, R.E. Smith, and D.M., Martill. 2020. A new ?chaoyangopterid (Pterosauria: Pterodactyloidea) from the Cretaceous Kem Kem beds of southern Morocco. Cretaceous Research 110: 104410.
Meijer, H.J.M., M.M.E. Van Der Meij, I. Van Waveren and A.J. Weldmeijer. 2007. Linking skull morphology to feeding in Tapejaridae: adaptations to frugivory in Tapejara wellnhoferi. In: 3rd International Meeting on Pterosaurs, Bavaria State Collection for Palaeontology, Munich pp. 25.
Myers, T.S. 2015. First North American occurrence of the toothed pteranodontoid pterosaur Cimoliopterus. Journal of Vertebrate Paleontology 35: e1014904.
Naish, D., and M.P. Witton. 2017. Neck biomechanics indicate that giant Transylvanian azhdarchid pterosaurs were short-necked arch predators. PeerJ 5: e2908.
Navarro, C.A., E. Martin-Silverstone, and T.L. Stubbs. 2018. Morphometric assessment of pterosaur jaw disparity. Royal Society Open Science 5: 172130.
Nesov, L.A. 1984. Upper Cretaceous pterosaurs and birds from Central Asia. Paleontological Journal 1: 47–57.
Novas, F.E., M. Kundrat, F.L. Agnolín, M.D. Ezcurra, P.E. Ahlberg, M.P. Isasi, A. Arriagada, and P. Chafrat. 2012. A new large pterosaur from the Late Cretaceous of Patagonia. Journal of Vertebrate Paleontology 32: 1447–1452.
Ősi, A., D.B. Weishampel, and C.M. Jianu. 2005. First evidence of azhdarchid pterosaurus from the Late Cretaceous of Hungary. Acta Palaeontologica Polonica 50: 777–787.
Owen, R. 1851. On a new species of pterodactyle (Pterodactylus compressirostris, Owen) from the Chalk; with some remarks on the nomenclature of the previously described species. Proceedings of the Zoological Society of London 19: 21–34.
Owen, R. 1861. Monograph on the fossil Reptilia of the Cretaceous Formations: Supplement No. III. Monographs of the Palaeontographical Society: 1–19.
Owen, R. 1874. Fossil Reptilia of the Mesozoic Formations, I Pterosauria. Monographs of the Palaeontographical Society 27: 1–14.
Padian, K., J.R. Cunningham Jr., W. Langston, and J. Conway. 2021. Functional morphology of Quetzalcoatlus Lawson 1975 (Pterodactyloidea: Azhdarchoidea). Journal of Vertebrate Paleontology 41: 218–251.
Pêgas, R.V., M.E.D.C. Leal, and A.W.A. Kellner. 2016. A basal tapejarine (Pterosauria; Pterodactyloidea; Tapejaridae) from the Crato Formation, Early Cretaceous of Brazil. PLoS ONE 11: e0162692.
Pêgas, R.V., F.R. Costa, and A.W.A. Kellner. 2018. New information on the osteology and a taxonomic revision of the genus Thalassodromeus (Pterodactyloidea, Tapejaridae, Thalassodrominae). Journal of Vertebrate Paleontology 38: e1443273.
Pêgas, R.V., B. Holgado, and M.E.C. Leal. 2019. On Targaryendraco wiedenrothi gen. nov. (Pterodactyloidea, Pteranodontoidea, Lanceodontia) and recognition of a new cosmopolitan lineage of Cretaceous toothed pterodactyloids. Historical Biology 33: 1–15.
Pêgas, R.V., F.R. Costa, and A.W.A. Kellner. 2021a. Reconstruction of the adductor chamber and predicted bite force in pterodactyloids (Pterosauria). Zoological Journal of the Linnean Society 193: 602–635.
Pêgas, R.V., B. Holgado, L.D.O. David, M.A. Baiano, and F.R. Costa. 2021b. On the pterosaur Aerotitan sudamericanus (Neuquén Basin, Upper Cretaceous of Argentina), with comments on azhdarchoid phylogeny and jaw anatomy. Cretaceous Research 129: 104998.
Pentland, A.H., S.F. Poropat, T.R. Tischler, T. Sloan, R.A. Elliott, H.A. Elliott, J.A. Elliott, and D.A. Elliott. 2019. Ferrodraco lentoni gen. et sp. nov., a new ornithocheirid pterosaur from the Winton Formation (Cenomanian–lower Turonian) of Queensland, Australia. Scientific Reports 9: 1–13.
Pereda-Suberbiola, X., N. Bardet, S. Jouve, M. Iarochène, B. Bouya, and M. Amaghzaz. 2003. A new azhdarchid pterosaur from the Late Cretaceous phosphates of Morocco. Geological Society, London, Special Publications 217: 79–90.
Pinheiro, F.L., and T. Rodrigues. 2017. Anhanguera taxonomy revisited: Is our understanding of Santana Group pterosaur diversity biased by poor biological and stratigraphic control? PeerJ 5: e3285.
Plieninger, F. 1901. Beitrӓge zur Kenntnis der Flugsaurier. Palaeontographica 48: 65–90.
Prieto, I.R. 1998. Morfología funcional y hábitos alimentarios de Quetzalcoatlus (Pterosauria). Coloquios De Paleontologia 49: 129–144.
Reck, H. 1931. Die deutschostafrikanischen Flugsaurier. Centralblatt Für Mineralogie, Geologie Und Paläontologie B 7: 321–336.
Richards, T.M., P.E. Stumkat, and S.W. Salisbury. 2021. A new species of crested pterosaur (Pterodactyloidea, Anhangueridae) from the Lower Cretaceous (upper Albian) of Richmond, North West Queensland, Australia. Journal of Vertebrate Paleontology 41: e1946068.
Rodrigues, T., and A.W.A. Kellner. 2008. Review of the pterodactyloid pterosaur Coloborhynchus. Zitteliana B28: 219–228.
Rodrigues, T., and A.W.A. Kellner. 2010. Note on the pterosaur material described by Woodward from the Recôncavo Basin, Lower Cretaceous, Brazil. Revista Brasileira De Paleontologia 13: 159–164.
Rodrigues, T., and A.W.A. Kellner. 2013. Taxonomic review of the Ornithocheirus complex (Pterosauria) from the Cretaceous of England. ZooKeys 308: 1–112.
Rodrigues, T., A.W.A. Kellner, B. Mader, and D. Russell. 2006. Brief report on new pterosaur (Pterosauria, Pterodactyloidea) specimens from the Cretaceous of Morocco. Journal of Vertebrate Paleontology 26: 116A.
Rodrigues, T., A.W.A. Kellner, B. Mader, and D. Russell. 2011. New pterosaur specimens from the Kem Kem beds (Upper Cretaceous, Cenomanian) of Morocco. Rivista Italiana Di Paleontologia e Stratigrafia 117: 149–160.
Seeley, H.G. 1870. The Ornithosauria: an elementary study of the bones of pterodactyls, made from fossil remains found in the Cambridge Upper Greensand, and arranged in the Woodwardian Museum of the University of Cambridge, Xii + 135. Deighton: Bell, and Co., Cambridge.
Seeley, H.G. 1891. On the shoulder girdle in Cretaceous Ornithosauria. Annals and Magazine of Natural History Series 6 (7): 438–445.
Sereno, P.C., D.B. Dutheil, M. Iarochene, H.C. Larsson, G.H. Lyon, P.M. Magwene, C.A. Sidor, D.J. Varricchio, and J.A. Wilson. 1996. Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science 272: 986–991.
Smith, R.E., D.M. Martill, A. Kao, S. Zouhri, and N. Longrich. 2020a. A long-billed, possible probe-feeding pterosaur (Pterodactyloidea:? Azhdarchoidea) from the mid-Cretaceous of Morocco, North Africa. Cretaceous Research 118: 104643.
Smith, R.E., D.M. Martill, D.M. Unwin, and L. Steel. 2020b. Edentulous pterosaurs from the Cambridge Greensand (Cretaceous) of eastern England with a review of Ornithostoma Seeley, 1871. Proceedings of the Geologists’ Association 132: 110–126.
Smith, R.E., A. Chinsamy, D.M. Unwin, N. Ibrahim, S. Zouhri, and D.M. Martill. 2021. Small, immature pterosaurs from the Cretaceous of Africa: Implications for taphonomic bias and palaeocommunity structure in flying reptiles. Cretaceous Research 130: 105061.
Solomon, A.A., V.A. Codrea, M. Venczel, and G. Grellet-Tinner. 2020. A new species of large-sized pterosaur from the Maastrichtian of Transylvania (Romania). Cretaceous Research 110: 104316.
Swinton, W.E. 1948. A Cretaceous pterosaur from the Belgian Congo. Bulletin De La Societé Belge De Géologie, Paléontologie, Et Hydrologie 47: 234–238.
Tütken, T., and D.W.E. Hone. 2010. The ecology of pterosaurs based on carbon and oxygen isotope analysis. Acta Geoscientica Sinica 31: 65–67.
Unwin, D.M. 2001. An overview of the pterosaur assemblage from the Cambridge Greensand (Cretaceous) of Eastern England. Mitteilungen Aus Dem Museum Für Naturkunde in Berlin, Geowissenschaftliche Reihe 4: 189–221.
Unwin, D.M. 2003. On the phylogeny and evolutionary history of pterosaurs, 217, 139–190. London: Special Publications, Geological Society.
Unwin, D.M. 2006. Pterosaurs from deep time, 347. New York: Pi Press.
Unwin, D.M., J. Lü, and N.N. Bakhurina. 2000. On the systematic and stratigraphic significance of pterosaurs from the Lower Cretaceous Yixian Formation (Jehol Group) of Liaoning, China. Fossil Record 3: 181–206.
Unwin, D.M., and D.M. Martill. 2007. Pterosaurs of the Crato Formation. In The Crato fossil beds of Brazil: Window into an ancient world. Cambridge University Press, Cambridge, 475–524
Veldmeijer, A.J. 2003. Description of Coloborhynchus spielbergi sp. nov. (Pterodactyloidea) from the Albian (Lower Cretaceous) of Brazil. Scripta Geologica 125: e139.
Veldmeijer, A.J. 2006. Toothed pterosaurs from the Santana Formation (Cretaceous; Aptian-Albian) of northeastern Brazil: a reappraisal on the basis of newly described material. Doctoral dissertation, Utrecht University, 269 pp.
Veldmeijer, A.J., M. Signore, and E. Bucci. 2007. Predator-prey interaction of Brazilian Cretaceous toothed pterosaurs: A case example. In Predation in organisms, ed. A.M.T. Elewa, 295–308. Springer Berlin, Heidelberg.
Vila Nova, B.C., J.M. Sayão, M.C. Langer, and A.W.A. Kellner. 2015. Comments on the cervical vertebrae of the Tapejaridae (Pterosauria, Pterodactyloidea) with description of new specimens. Historical Biology 27: 771–781.
Vremir, M. 2010. New faunal elements from the Late Cretaceous (Maastrichtian) continental deposits of Sebeş area (Transylvania). Acta Musei Sabesiensis 2: 635–684.
Vremir, M., G. Dyke, Z. Csiki-Sava, D. Grigorescu, and E. Buffetaut. 2018. Partial mandible of a giant pterosaur from the uppermost Cretaceous (Maastrichtian) of the Hațeg Basin, Romania. Lethaia 51: 493–503.
Vullo, R., and D. Neraudeau. 2009. Pterosaur remains from the Cenomanian (Late Cretaceous) paralic deposits of Charentes, western France. Journal of Vertebrate Paleontology 29: 277–282.
Vullo, R., J. Marugan-Lobon, A.W.A. Kellner, A.D. Buscalioni, B. Gomez, M. De La Fuente, and J.J. Moratalla. 2012. A new crested pterosaur from the Early Cretaceous of Spain: The first European tapejarid (Pterodactyloidea: Azhdarchoidea). PLoS ONE 7: e38900.
Vullo, R., G. Garcia, P. Godefroit, A. Cincotta, and X. Valentin. 2018. Mistralazhdarcho maggii, gen. et sp. nov., a new azhdarchid pterosaur from the Upper Cretaceous of southeastern France. Journal of Vertebrate Paleontology 38: 1–16.
Wang, X., and Z. Zhou. 2006. Pterosaur assemblages of the Jehol Biota and their implication for the Early Cretaceous pterosaur radiation. Geological Journal 41: 405–418.
Wang, X., A.W.A. Kellner, Z. Zhou, and D.A. Campos. 2008. Discovery of a rare arboreal forest-dwelling flying reptile (Pterosauria, Pterodactyloidea) from China. Proceedings of the National Academy of Sciences of the United States of America 105: 1983–1987.
Wang, X., A.W.A. Kellner, S. Jiang, Q. Wang, Y. Ma, Y. Paidoula, X. Cheng, T. Rodrigues, X. Meng, J. Zhang, and N. Li. 2014. Sexually dimorphic tridimensionally preserved pterosaurs and their eggs from China. Current Biology 24: 1323–1330.
Wellnhofer, P. 1985. Neue Pterosaurier aus der Santana-Formation (Apt) der Chapada do Araripe Brasilien. Palaeontographica. Abteilung A 187: 105–182.
Wellnhofer, P. 1991. Additional pterosaur remains from the Santana Formation (Aptian) of the Chapada do Ararripe, Brazil. Palaeontographica Abteilung A 215: 43–101.
Wellnhofer, P., and E. Buffetaut. 1999. Pterosaur remains from the Cretaceous of Morocco. Paläontologische Zeitschrift 73: 133–142.
Wellnhofer, P., and A.W.A. Kellner. 1991. The skull of Tapejara wellnhoferi Kellner (Reptilia, Pterosauria) from the Lower Cretaceous Santana Formation of the Araripe Basin, Northeastern Brazil. Mitteilungen Der Bayerischen Staatssammlung Für Paläontologie Und Historische Geologie 31: 89–106.
Wellnhofer, P., E. Buffetaut, and P. Gigase. 1983. A pterosaurian notarium from the Lower Cretaceous of Brazil. Paläontologische Zeitschrift 57: 147–157.
Williams, C.J., M. Pani, A. Bucchi, R.E. Smith, A. Kao, W. Keeble, N. Ibrahim, and D.M. Martill. 2021. Helically arranged cross struts in azhdarchid pterosaur cervical vertebrae and their biomechanical implications. iScience 24: 102338.
Witton, M.P. 2007. Titans of the skies: Azhdarchid pterosaurs. Geology Today 23: 33–38.
Witton, M.P. 2013. Pterosaurs: Natural history, evolution, anatomy, 291. Princeton University Press.
Witton, M.P., and D. Naish. 2008. A reappraisal of azhdarchid pterosaur functional morphology and paleoecology. PLoS ONE 3: e2271.
Witton, M.P., and D. Naish. 2013. Azhdarchid pterosaurs: Water-trawling pelican mimics or “terrestrial stalkers”? Acta Palaeontologica Polonica 60: 651–660.
Witton, M.P., D.M. Martill, and M. Green. 2009. On pterodactyloid diversity in the British Wealden (Lower Cretaceous) and a reappraisal of “Palaeornis” cliftii Mantell, 1844. Cretaceous Research 30: 676–686.
Wu, W.H., C.F. Zhou, and B. Andres. 2017. The toothless pterosaur Jidapterus edentus (Pterodactyloidea: Azhdarchoidea) from the Early Cretaceous Jehol Biota and its paleoecological implications. PLoS ONE 12: e0185486.
Acknowledgements
A great many people have helped us in our researches on Kem Kem Group pterosaurs over nearly two decades. We are especially grateful to Drs. Dino Frey and Anusuya Chinsamy-Turan. For technical support we thank Richard Hing, Geoff Long, Bob Loveridge, William Keeble, Ben Morrison and Dr. Alex Kao, all sometime based at the University of Portsmouth. For assistance with field work we thank Simon Penn, Rab Smyth, Michael Oates, James McPhee, and many undergraduate and MRes students of DMM and NI. For assistance with artwork we thank Lucy Smith, Emily Pilavachi and Julian Kiely. For assistance and access to specimens in collections we thank Drs. Mike Day (NHMUK), Ronan Allain (MNHN), Oliver Rauhut (BSPG), Florias Mees and Jonathan Brecko (RMCA) and Bryn Mader for supplying images. We especially thank the many people in Morocco who have assisted us in the field, collected significant fossil remains, and offered us incredible hospitality, including Mustapha Meharich and Mohammed Ben Sekkou. We also thank our veteran team members Matteo Fabbri, Simone Maganuco and Cristiano Dal Sasso for helping us collect large numbers of vertebrate fossils over the last few years. Mr. Ian Eaves is warmly thanked for making specimens in his collection available for study. We thank Dr. Alexander Averianov, an anonymous reviewer and the editors of Paläontologische Zeitschrift, Dr. Michael Rasser and Dr Hans-Dieter Sues for their very helpful comments, which greatly improved the manuscript.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Hans-Dieter Sues.
Supplementary Information
Below is the link to the electronic supplementary material.
12542_2022_642_MOESM1_ESM.jpg
Fig. S1. RGP 2091, left metacarpal IV from an indeterminate pterodactyloid, possibly an ornithocheirid from the ?Upper Jurassic-Lower Cretaceous ?Lualaba Series of the Democratic Republic of the Congo figured and described by Swinton (1948). A, anterior view; B, dorsal view; C, posterior view; D, distal view. The specimen was imaged by Jonathan Brecko (RMCA) in the framework of the DIGIT04 project funded by BELSPO. Scale bars represent 10 mm
12542_2022_642_MOESM2_ESM.jpg
Fig. S2. FSAC-KK 7251, indeterminate azhdarchid mid-series cervical vertebra morphotype 1 (M1) from the Kem Kem Group. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, in posterior view. Scale bars represent 50 mm
12542_2022_642_MOESM3_ESM.jpg
Fig. S3. Digital images of FSAC-KK 5214, a 3D print of an indeterminate azhdarchid mid-series cervical vertebra morphotype 2 (M2) from the Kem Kem Group, in a private collection. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, in posterior view. Scale bars represent 50 mm
12542_2022_642_MOESM4_ESM.jpg
Fig. S4. FSAC-KK 5215, indeterminate azhdarchid mid-series cervical vertebra morphotype 3 (M3) from the Kem Kem Group. A, in dorsal view; B, in ventral view; C, in right lateral view; D, in anterior view; E, in posterior view. Scale bars represent 10 mm
12542_2022_642_MOESM5_ESM.jpg
Fig. S5. CMN 50801, indeterminate azhdarchid mid-series cervical vertebra morphotype 4 (M4) from the Kem Kem Group. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, in posterior view. Scale bars represent 10 mm. Images from Rodrigues et al., 2011
12542_2022_642_MOESM6_ESM.jpg
Fig. S6. FSAC-KK 5216, indeterminate azhdarchid mid-series cervical vertebra morphotype 5 (M5) from the Kem Kem Group. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, image of 3D model of the anterior end generated from XCT scan; F, in posterior view. Scale bars represent 10 mm
12542_2022_642_MOESM7_ESM.jpg
Fig. S7. FSAC-KK 3088, indeterminate azhdarchid mid-series cervical vertebra morphotype 6 (M6) from the Kem Kem Group. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, in posterior view. Scale bars represent 10 mm
12542_2022_642_MOESM8_ESM.jpg
Fig. S8. Indeterminate azhdarchoid mid-series cervical vertebrae morphotype 7 (M7) from the Kem Kem Group. A-E, FSAC-KK 5217; F-I, FSAC-KK 7177. A, F, in dorsal view; B, G, in ventral view; C, in right lateral view; D, I, in anterior view; E, in posterior view; H, in left lateral view. Scale bars represent 10 mm
12542_2022_642_MOESM9_ESM.jpg
Fig. S9. FSAC-KK 5083, indeterminate azhdarchid mid-series cervical vertebra morphotype 8 (M8) from the Kem Kem Group, from Smith et al., 2021. A, in dorsal view; B, in ventral view; C, in right lateral view; D, in anterior view; E, in posterior view. Scale bars represent 10 mm
12542_2022_642_MOESM10_ESM.jpg
Fig. S10. Digital images of FSAC-KK 5218, a 3D print of an indeterminate azhdarchid cervical vertebra (?C8) morphotype 9 (M9) from the Kem Kem Group, in a private collection. A, in dorsal view; B, in ventral view; C, in left lateral view; D, in anterior view; E, in posterior view. Scale bars represent 20 mm.
12542_2022_642_MOESM13_ESM.stl
A 3D topographic scan of FSAC-KK 5024/SMNK PAL 45833, an anterior rostrum fragment of Coloborhynchus sp. from the Kem Kem Group.
12542_2022_642_MOESM14_ESM.stl
A 3D topographic scan of FSAC-KK 5025/SMNK PAL 45831, an anterior rostrum fragment of Ornithocheirus cf. simus from the Kem Kem Group.
12542_2022_642_MOESM16_ESM.stl
A 3D topographic scan of FSAC-KK 5214, an indeterminate azhdarchid mid-series cervical vertebra (morphotype 2) from the Kem Kem Group.
12542_2022_642_MOESM17_ESM.stl
3D scan of FSAC-KK 5218 A 3D topographic scan of FSAC-KK 5218, an indeterminate azhdarchid cervical vertebra (morphotype 9) from the Kem Kem Group.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, R.E., Ibrahim, N., Longrich, N. et al. The pterosaurs of the Cretaceous Kem Kem Group of Morocco. PalZ 97, 519–568 (2023). https://doi.org/10.1007/s12542-022-00642-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-022-00642-6