Abstract
In contrast to other kinds of biological interactions, symbiosis is a scarcely investigated aspect of the fossil record. This is largely due to taphonomic biases that often frustrate any attempt to make a strong case that two organisms shared an intimate association in life. Among extant marine vertebrates, the sea turtles (Cheloniidae and Dermochelyidae) bear a broad and diverse spectrum of epibiotic symbionts, including specialists such as the turtle barnacles (Chelonibiidae and Platyleapadidae). Here, we reappraise an early Oligocene (Rupelian) fossil cheloniid skeleton, featuring the remains of cirripedes on the exterior of its entoplastron, from the Rauenberg fossil-lagerstätte, southwestern Germany. The barnacle specimens are assigned to Protochelonibia melleni, an extinct protochelonibiine species and the geologically oldest known member of Chelonibiidae. In the light of taphonomic and palaeoenvironmental considerations, and given that the extant chelonibiids are mostly known as epizoic symbionts of sea turtles, we conclude that this unique fossil association resulted from the epizoic growth of the barnacles on the external surface of the plastron of the turtle during its lifetime. This remarkable fossil association provides evidence that chelonibiids, including the extinct protochelonibiines, have been chelonophilic epizoans for more than 30 Myr. A survey of the trace and body fossil records shows that platylepadids are also likely as old as the Rupelian as is their symbiotic association with cheloniid hosts. This early emergence of the modern-looking, turtle-dwelling barnacle lineages corresponds to a climate-driven phase of major radiation and taxonomic turnover among sea turtles at the Eocene–Oligocene transition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epibiosis corresponds to a particular type of symbiosis defined as a biological interaction in which a single host (also known as “basibiont”) supports one or more colonizers (or “epibionts”) on its skin or outer coating (Wahl and Mark 1999; Sagan and Margulis 2001). Epibiotic relationships may be either mutualistic (whereby both the organisms have a net benefit from the symbiotic relationship), commensalistic (whereby one organism benefits and the other is not significantly harmed or helped) or parasitic (whereby the parasite benefits while the host is harmed). Among extant marine vertebrates, the sea turtles (Testudines: Cheloniidae and Dermochelyidae) serve as basibionts to a broad and diverse spectrum of epibiotic symbionts (Frick and Pfaller 2013, and references therein). This is not surprising since in the marine environment any exposed, undefended surface is prone to colonization by marine propagules (Wahl 1989). Whereas most of the epibionts of marine turtles are opportunistic organisms that are normally found associated with inanimate substrates, several epizoan organisms exist that are found almost exclusively on sea turtles, most noticeably barnacles (Cirripedia) (Frick and Pfaller 2013). These “turtle barnacles” belong to the coronuloid families Chelonibiidae and Platylepadidae (Pilsbry 1916; Ross and Newman 1967; Ross and Frick 2007, 2011; Hayashi 2013; Hayashi et al. 2013; Zardus 2021) and play a key role as pioneer species, facilitating the colonization by subsequent epibionts (Frick et al. 2002). Furthermore, they provide refugia for other obligate symbionts (Frick and Pfaller 2013) and may eventually serve as target food items for some cleaner fish (e.g. the saddle wrasse Thalassoma duperrey; Losey et al. 1994). Though the symbiotic relationships between the filter-feeding coronuloids and their chelonian basibionts are often regarded as forms of commensalism, with the former profiting from a continuous flow of seawater carrying nutrient particles combined with a low risk of predation without significantly harming or benefiting the host, exceptions have been reported in case of severe infestation (e.g. Seigel 1983), and turtle barnacles are sometimes referred to as parasites (e.g. Zonneveld et al. 2022). As a matter of fact, there is some indication that some sea turtles may actively remove their epibiota by scrapping the shell against bottom rocks (Frick and McFall 2007).
In contrast to other kinds of biological interactions such as predator–prey relationships (Klompmaker et al. 2019), symbiotic associations (including mutualistic epibiosis) are a scarcely investigated aspect of the fossil record. This is largely due to taphonomic biases that often frustrate any attempt to provide unequivocal evidence that two organisms shared an intimate association in life (Tapanila 2008). Consequently, although symbiosis has long been recognized as a major ecological and evolutionary driving force (Tapanila and Ekdale 2007), case studies devoted to assessing the existence of symbiotic relationships—whether mutualistic, commensalistic, or parasitic—between fossil forms are relatively rare in the palaeontological literature. This has started to change during the last few years, especially in the light of new research approaches that promote the integration between trace and body fossil analyses to identify and qualify ancient symbiotic associations, often based on the detection of skeletal intergrowths and bioclaustration structures (e.g. Yuan et al. 2005; Tapanila 2008; Tapanila and Ekdale 2007; Stanley and Schootbrugge 2009; De Baets et al. 2015; Vinn and Wilson 2015, 2016; Vinn et al. 2015, 2016, 2018, 2019, 2021; Vinn 2016, 2017a, b; Robin et al. 2016, Robin 2021; Zonneveld et al. 2022). Only a few studies, however, have explored the deep past of sea turtle epibiosis based on palaeontological lines of evidence (e.g. Harzhauser et al. 2011).
Here, we reappraise a lower Oligocene sea turtle skeleton from the Rauenberg fossil-lagerstätte (Baden-Württemberg, southwestern Germany), featuring several barnacle shells on the external surface of one plastral bone (Alexander and Frey 2010; Maxwell et al. 2016). The chelonibiid affinities of the cirripedes are ascertained, and their epizoic relationship with the turtle is established on the basis of taphonomy and visual observations. Crucially, this unique association dates back to the very origin of the modern families of turtle barnacles, thus stimulating a broader discussion on the early evolution of this successful group of epizoic barnacles and its long-lasting symbiotic relationship with sea turtles.
Material and methods
Locality, geology and depositional environment
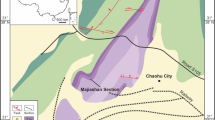
The fossil materials studied in the present paper originate from the abandoned Unterfeld clay pit near Rauenberg (formerly also known as the Frauenweiler–Wiesloch clay pit) (Alexander and Frey 2010) (Fig. 1A). The sediments outcropping therein were deposited during the early Oligocene (i.e. Rupelian) in the Western Paratethys and are part of the syn-rift sedimentary succession of the Upper Rhine Valley (Maxwell et al. 2016) (Fig. 1B). These deposits belong to the Hochberg Member of the Bodenheim Formation, and have been referred to the Calcareous Nannoplankton Zone NP23 and European Mammal Zone MP22–23, corresponding to an age of ca. 33 to 30 Ma, i.e. to the middle part of the Rupelian (Grimm et al. 2002; see also the summary in Maxwell et al. 2016).
(Palaeo)geographic framework. A Geographic setting of the Unterfeld abandoned clay pit near Rauenberg, southwestern Germany. Geographic coordinates: 49°16′14ʺ N; 8°40′22ʺ E. B Early Oligocene (Rupelian) palaeogeography of Europe, showing the location of Rauenberg along the Rhine Graben (modified after Maxwell et al. 2006; based on Spiegel et al. 2007)
The palaeontological content of the Unterfeld clay pit was recently reviewed by Maxwell et al. (2016). According to these authors, Rauenberg is one of the most significant early Oligocene fossil assemblages of Europe containing both marine and terrestrial elements of fauna and flora. Preservation is often superb and comprises complete and articulated skeletons that occasionally display soft tissue preservation. More than 300 different fossil taxa have been detected in the sediments of the Unterfeld clay pit. Of these, more than two-thirds are marine. Maxwell et al. (2016) interpreted the Rauenberg palaeoenvironment as marine, moderately shallow, low energy, and tropical or subtropical. The terrestrial vegetation consisted of mostly mixed broadleaf evergreen forests characterized by pine- and palm-rich coastal fringes. The marine fossil assemblage displays Paratethyan and boreal affinities.
Specimens, repository and anatomical terminology
The present paper deals with a peculiar fossil association involving a sea turtle skeleton and some acorn barnacle shells. The fossil material is housed in the palaeontological collections of the Staatliches Museum für Naturkunde Karlsruhe (SMNK). The chelonian specimen, consisting of an almost complete and largely articulated skeleton (SMNK-PAL 6608) assigned to Cheloniidae indet., was described in detail by Alexander and Frey (2010) and subsequently mentioned by Maxwell et al. (2016). Some elements of the turtle specimen (Fig. 2A) are encrusted with a dark, organic coating that may have originated from either the epidermis or bacterial mats that replaced the soft tissues (Alexander and Frey 2010) (Fig. 2B). Numerous globular, concretionary objects (likely derivatives of the gut contents) were found within the body cavity (Alexander and Frey 2010) (Fig. 2B). The occurrence of cirripede shells associated with the plastron of SMNK-PAL 6608 was noticed and briefly discussed by Alexander and Frey (2010) and Maxwell et al. (2016).
SMNK-PAL 6608, skeleton of Cheloniidae indet. from the lower Oligocene of the Unterfeld clay pit, A photograph and B corresponding explanatory line drawing. 1, cornu branchiale I; 2, cornu branchiale II; basihyoid; 4, right forelimb; 5, proximal part of right humerus; 6, left forelimb; 7, cervical vertebra; 8, caudal vertebra?; 9, dorsal vertebra; 10 right coracoid; 11, acromial process?; 12, pelvic girdle; 13–18, left peripherals; 19–23 right peripherals; 24, right hyoplastron; 25, right hypoplastron; 26, left hyoplastron; 27 left hypoplastron; 28, right epiplastron; 29 left epiplastron; 30, entoplastron; 31–32, right pleurals; 33–36, left pleurals; 37, probable gut contents; ?, unidentified skeletal elements. The occurrence of a dark organic coating is indicated by grey-shaded areas. Areas of barnacle attachment on the entoplastron are indicated by green-shaded areas as well as by arrows
The mural plate nomenclature embraced herein for the acorn barnacle shells follows that proposed by Yamaguchi and Newman (1990) based on possible homologies with pedunculate barnacle plates. Peculiar structures of the studied shells are hereby referred to mostly by following the nomenclatural proposals by Harzhauser et al. (2011) and Collareta et al. (2022c: Fig. 2).
Results
Two areas of attachment of barnacle shells occur on the external (i.e. ventral) surface of the entoplastron of SMNK-PAL 6608 (Fig. 2, 3A). Two heavily damaged, clustered shells are preserved on the right part of the shaft-like anterolateral expansion of this central, unpaired plastral bone (Fig. 2B, upper green-shaded area; Fig. 3B, top half). Their wall plates are characterized by trapezoidal parietes. Where abraded, the latter reveal the fabric of closely appressed, columnar blocks of carbonate that is typical of chelonibiid shells (Davis 1972; Collareta et al. 2022a). Radii are visible, although poorly preserved, and what remains of the orifice hints at a somewhat elliptical outline defined by a small number of compartments. There is no calcareous basal plate.
Shells of Protochelonibia melleni attached to the external surface of the entoplastron of SMNK-PAL 6608. A General view of the barnacle-bearing bone. B Details of the areas of barnacle attachment (arrows). C Close-up of the best preserved barnacle shell under different light conditions (the arrow indicates the dense pattern of internal longitudinal parietal septa). rd radius; R rostrum sensu stricto; RL rostrolatus
The second barnacle attachment area occurs along the right lateral margin of the entoplastron midway along the bone margin (Figs. 2B, lower green-shaded area; Fig. 3B, bottom half; Fig. 3C). It includes a fairly well-preserved shell as well as shreds of shelly material that likely belonged to an adjoining barnacle individual. The former specimen (Fig. 3B, arrow in square) comprises the well-preserved, taxonomically diagnostic tripartite compound rostrum (in which the rostrum sensu stricto and two rostrolatera are distinguishable: RL–R–RL; Fig. 3C). The opposite, carinal portion of the shell, which was originally situated across the lateral margin of the entoplastron, has collapsed and displays a slight displacement of the wall plates. As is typical of Protochelonibia (Harzhauser et al. 2011; Collareta and Newman 2020; Collareta et al. 2022a), the wide rostral complex is unfused, comprising three distinct compartments (i.e. R and the adjacent RLs, the right one being partly disarticulated from R) that are triangular in outline, similar in size, and contact each other through clearly visible suture lines (Fig. 3C). Similar to the two shells described above, the outer wall consists of closely appressed, pillar-like blocks of shelly material. Furthermore, the broken compartment apices reveal a dense pattern of internal longitudinal parietal septa (Fig. 3C). As observed in other specimens of Protochelonibia (Zullo 1982; Collareta et al. 2022a), the radii of both the RLs are sunken and finely striated (Fig. 3C). Moreover, as seen in some specimens of Protochelonibia melleni (e.g. Zullo 1982: figs. 4, 5), the outer wall of some compartments, primarily the right RL, is gently ribbed. In this specimen, too, no calcareous basal plate was observed.
Extant examples of sea turtles (Chelonia mydas) hosting turtle barnacles (Chelonibia testudinaria) on the external surface of the anterior plastron. A Photograph by Doug Perrine. Note the C. testudinaria shell (arrowhead) in the approximate position of the anterior margin of the entoplastron: the Rupelian cheloniid individual SMNK-PAL 6608 also hosted chelonibiid barnacles in the same region of the turtle shell during lifetime. B Black and white photograph by Mark Flint (see also Flint et al. 2009). Note that all the C. testudinaria shells have their rostral end facing anteriorly, as also observed in the fossil Protochelonibia melleni shell depicted in Fig. 3C: this may be somewhat significant as living C. testudinaria individuals are believed to be able to reorient themselves and even move on turtle shells to achieve dispositions that maximize the nutrient flow (Moriarty et al. 2008; Chan et al. 2021)
Synoptic overview of the evolutionary radiation of the better-known cheloniid sea turtles from the Late Cretaceous to the present, as recovered by the phylogenetic analysis by Weems and Brown (2017), compared to the chronostratigraphic distribution of the main groups of turtle barnacles. The chronostratigraphic distribution of Pacifichelys has been modified to account to the revised Tortonian age of Pacifichelys urbinai (Collareta et al. 2021). The chronostratigraphic distribution of the turtle barnacles is according to Collareta et al. (2022a, b, c and references therein). Question marks associated with the fossil record of the platylepadids indicate presumptive occurrences as witnessed by time-constrained trace fossils (Weems et al. 2014; Collareta et al. 2022b). The blue rectangle outlines the Rupelian cheloniid radiation phase and the corresponding episodes in the fossil history of turtle barnacles
In the light of the above considerations, we assign these barnacle shells to the extinct chelonibiid species Protochelonibia melleni, a Rupelian form that has been recently reported from the Rauenberg fossil-lagerstätte based on two clustered shells (Collareta et al. 2022a). Protochelonibia melleni is the earliest member of the monotypic subfamily Protochelonibiinae, which is currently known from fossils ranging chronostratigraphically from the lower Oligocene to the ?upper Pliocene (Harzhauser et al. 2011; Collareta et al. 2022a; Perreault et al. 2022).
Discussion
Interpretation of the Cheloniidae–Protochelonibia association
The most likely interpretation of the so far unique fossil association involving the indeterminate cheloniid skeleton SMNK-PAL 6608 and shells belonging to the extinct chelonibiid cirripede Protochelonibia melleni is that the barnacles dwelt on the external surface of the plastron of the living turtle. This hypothesis is supported by the fact that extant chelonibiids are common epibionts of modern sea turtles, infesting the surface of both skin and shell, although they also occur on sirenians and hard-shelled invertebrates like crustaceans and xiphosurans (e.g. Darwin 1854; Gruvel 1903; Pilsbry 1916; Ross and Newman 1967; Monroe and Limpus 1979; Monroe 1981; Newman 1996; Chan et al. 2009; Zardus 2021). In particular, the living species Chelonibia testudinaria is ubiquitous on sea turtles and has been reported from all the extant species of Cheloniidae (Epibiont Research Cooperative 2007; Hayashi 2013, and references therein), hence its vernacular name, “common turtle barnacle” (Balazs 1985; Zardus 2021). As observed elsewhere, the low-conical, somehow streamlined shell morphology of P. melleni is particularly reminiscent of that of members of C. testudinaria that attach onto actively swimming basibionts such as sea turtles (Collareta et al. 2022a). Corroborating this morphological inference, surface imprints that recall the sculpture of cheloniid carapacial scutes have been observed in a lower Miocene specimen of Protochelonibia submersa (Harzhauser et al. 2011). Usually, C. testudinaria superficially adheres onto the keratinous epidermal scutes of its hosts, sometimes even displaying limited movement capabilities (Moriarty et al. 2008; Chan et al. 2021). A similar attachment style may be reconstructed for P. melleni. However, this is by no means at odds with the preservation of the shells of P. melleni on the entoplastron of SMNK-PAL 6608. Indeed, the widespread occurrence of a dark, organic coating across the ventral aspect of this skeleton (Fig. 2B) suggests a delayed and/or incomplete degradation of the epidermis of the turtle (Alexander and Frey 2010). Furthermore, extant specimens of C. testudinaria occasionally penetrate the external, shedding keratinous layer of the turtle shell (Davis 1972).
Chelonibia testudinaria very rarely occurs as an encruster of bare mammalian bones (Monroe 1981; Collareta and Bianucci 2021), which may suggest a post-mortem encrustation of the entoplastron of SMNK-PAL 6608. However, the following arguments contradict this hypothesis: firstly, when C. testudinaria colonizes bare bones, its shells conform to the high-conical “patula” morph (Cheang et al. 2013; Zardus et al. 2014), which is otherwise typical of individuals from slow-moving hosts (e.g. crabs). The subpeltate morphology seen in SMNK-PAL 6608 is in turn typical of turtle-dwelling individuals (Monroe 1981; Collareta and Bianucci 2021). Secondly, the seafloor where SMNK-PAL 6608 settled was oxygen-depleted (Maxwell et al. 2016), and thus a hostile environment for sessile organisms such as acorn barnacles (Alexander and Frey 2010). Thirdly, even if the sea bottom was suitable as a habitat for acorn barnacles, the high degree of articulation of SMNK-PAL 6608 as well as the preservation of remains of the non-mineralized epidermis and probable gut contents suggest a rapid burial (Alexander and Frey 2010). This observation does not primarily preclude an infestation of the shell of the dead turtle by barnacle larvae, but a growth to the preserved shell size would not have been possible (see e.g. the growth rates reported for C. testudinaria by Sloan et al. 2014 and Ewers-Saucedo et al. 2015, and consider also that oxygen-depleted conditions are believed to inhibit barnacle growth; Nishizaki and Carrington 2015). Finally, SMNK-PAL 6608 was found dorsal side-up. The reason for this is that sea turtle carcasses may float for a fairly long amount of time with the carapace dome facing upwards as a consequence of the lungs being placed in a dorsal position within the thoracic cage (Santos et al. 2018). During decay, decomposition gases are trapped in the dome of the carapace, while the head, neck and flippers hang down, thus stabilizing the dorsal side-up floating configuration. This disposition is usually retained when the carcass sinks with the plastron contacting the sediment at the seafloor first, forming a stable resting plate. Referring to SMNK-PAL 6608, the presence of the foreflippers and some bony elements of the hyoid apparatus suggests that the turtle sank quite quickly, well before the completion of soft tissue decomposition, because the head and limbs tend to detach from floating tutle carcasses at an early stage (Brand et al. 2003). If SMNK-PAL 6608 sank back-first onto the seafloor, as needed for allowing the barnacles to settle and grow onto the plastron postmortem, the carcass must have subsequently rolled to be eventually buried dorsal side-up. However, the sediments exposed in the Unterfeld clay pit do not preserve any evidence of currents strong enough to tip over a turtle carcass as large as SMNK-PAL 6608 (Maxwell et al. 2016).
In the light of the above discussion, and in agreement with previous comments by Alexander and Frey (2010) and Maxwell et al. (2016), we conclude that the P. melleni shells preserved on the entoplastron of SMNK-PAL 6608 demonstrate the in vivo infestation of the external surface of the anterior plastron of the cheloniid turtle by epizoic chelonibiid barnacles—a condition that is frequently observed today (Fig. 4). Thus, the Cheloniidae–Protochelonibia fossil association described herein adds significantly to the sparse amount of palaeontological evidence for the co-occurence of coronuloid barnacles and their respective basibiont (Stewart et al. 2011; Collareta et al. 2016; Collareta 2020). In particular, SMNK-PAL 6608 and the co-occurring P. melleni shells represent the first such association in which representatives of an extinct coronuloid taxon are preserved in direct contact with the skeletal remains of their host.
Broader palaeobiological outcome
Extant coronuloid turtle barnacles are at present referred to two families: Chelonibiidae and Platylepadidae. Whereas the former currently consist of the sole genus Chelonibia, the latter are a diverse family composed of at least six genera and five subfamilies (Ross and Frick 2011; but see Chan et al. 2021 for a different assessment of the platylepadids). Molecular phylogenetic analyses suggest that the coronuloid barnacles evolved during the early Cenozoic. The platylepadids likely separated from the chelonibiids during the Oligocene or the early–middle Miocene (Hayashi et al. 2013). The fossil record, scattered and fragmentary as it is, only bears partial support to this molecular reconstruction. The oldest putative coronuloid, Emersonius cybosyrinx, comes from the upper Eocene (Priabonian) of Florida, USA (Ross and Newman 1967). It consists of a fused compound rostrum that features a serial repetition of box-like parietal chambers. Because this kind of organization appears to be unique to this barnacle species, E. cybosyrinx is at present assigned to its own family Emersoniidae (Newman 1996; Collareta et al. 2022c). The assignment of Emersoniidae to Coronuloidea remains tentative, because E. cybosyrinx would represent a highly idiosyncratic and surprisingly derived coronuloid (Collareta et al. 2022a). Furthermore, nothing is known about the possible basibionts of E. cybosyrinx (Ross and Newman 1967). The earliest chelonibiid, Protochelonibia melleni, comes from the lower Oligocene (Rupelian) of Mississippi, USA and southern Germany (Zullo 1982; Collareta and Newman 2020; Collareta et al. 2022a; this work). Like the early Miocene species Protochelonibia submersa, which likely lived on cheloniid sea turtles (Harzhauser et al. 2011), P. melleni is a member of the extinct subfamily Protochelonibiinae, which coexisted with members of the extant genus Chelonibia (the only known genus of Chelonibiinae) throughout most of the Neogene (Collareta et al. 2022a, c, and references therein; Perreault et al. 2022). The present paper unambiguously demonstrates that chelonibiids have been turtle-dwelling organisms for more than 30 million years, as well as that the extinct protochelonibiines have been chelonophilic epizoic organisms since at least Rupelian times.
Compared to the fossil record of chelonibiids, that of platylepadids is extremely fragmentary and limited to a handful of occurrences of the extant genus Platylepas from Pleistocene deposits (Ross 1963; Mimoto 1991; Collareta et al. 2019; Karasawa and Kobayashi 2022). According to Hayashi et al. (2013), this sparse fossil record is likely due to the fragility and small size of the shell of Platylepas and allied genera. Therefore, the trace fossil record may prove crucial for complementing the incomplete picture of the evolutionary history of platylepadids based on the body fossils alone (Hayashi et al. 2013; Collareta et al. 2022b). Indeed, a few probable platylepadid attachment scars incising carapacial elements of Cheloniidae have been recently reported from the Oligocene and Miocene (Hayashi et al. 2013; Weems and Sanders 2014; Collareta et al. 2022b), suggesting that platylepadids have a longer evolutionary history as epibionts of sea turtles than suggested by the body fossils alone.
In the light of the results presented herein, and with reference to the literature, we sum up the following conclusions regarding the early evolution of turtle barnacles:
-
1.
Coronuloids were likely already present as early as in late Eocene times, albeit with extinct morphotypes, whose host preferences are hiterto unknown.
-
2.
The oldest unequivocal chelonibiid, Protochelonibia melleni, occurs in lower Oligocene deposits from both sides of the Atlantic. Evidence for P. melleni being an epibiotic symbiont of cheloniid turtles is preserved in the fossil record, and the same holds true for the early Miocene Protochelonibia species P. submersa.
-
3.
Platylepas-like, shallowly burrowing turtle barnacles were possibly present during the early Oligocene, living on cheloniid basibionts.
All things considered, as early as in the early Oligocene, members of both Chelonibiidae and Platylepadidae had likely already developed growth strategies, shell morphologies and features of the membranous basis suitable for the attachment onto moulting keratinous substrates (Fig. 5). Interestingly, the rise of turtle barnacles coincides with a Rupelian phase of major radiation among Cheloniidae—one that followed the cooling event related to the onset of widespread Antarctic glaciation at the Eocene–Oligocene transition (Weems and Brown 2017) (Fig. 5). This radiation aligns with the extinction of several cheloniid turtle taxa (e.g. Euclastes and Puppigerus) along with many other groups of marine tetrapods, including the palaeophid sea snakes (Weems and Brown 2017). Thus, the early Oligocene emergence of the chelonophilic chelonibiids and platylepadids may reflect a major, climate-driven event of renewal and diversification of the global cheloniid biota (Fig. 5). A few late Eocene ancestors of the Oligocene cheloniids may already have been colonized by coronuloid (possibly basal chelonibiid) barnacles that could attach onto smooth, moulting, keratinous substrates. In order to confirm this scenario, additional finds of Palaeogene coronuloids as well as of well-preserved platylepadids are needed.
Conclusions
The external surface of the entoplastron of an indeterminate cheloniid skeleton from the lower Oligocene Unterfeld clay pit fossil-lagerstätte at Rauenberg (Baden-Württemberg, southwestern Germany) bears reasonably well-preserved acorn barnacle shells. These cirripede remains conform to the extinct protochelonibiine chelonibiid species Protochelonibia melleni. Because the extant chelonibiids are mostly known as epibionts of sea turtles, and based on taphonomic and palaeoenvironmental considerations, we conclude that the barnacle shells described herein provide evidence for an in vivo attachment of epizoic chelonibiids onto the plastron of the cheloniid turtle. This unequivocally demonstrates that chelonibiids have been turtle-dwelling barnacles for more than 30 million years. It also shows that the extinct protochelonibiines comprised chelonophilic epizoic organisms. Both the extant families of turtle barnacles apparently date back to at least the Rupelian, a time when members of Chelonibiidae (and possibly Platylepadidae) had already developed growth strategies and shell morphologies suitable for efficiently attaching onto the smooth, moulting, keratinous epidermal scutes of sea turtles. The early Oligocene emergence of the modern chelonophilic turtle barnacles apparently coincide with a major event of taxonomic turnover among the cheloniids, which in turn aligns with remarkable climate changes at the Eocene–Oligocene transition.
Availability of data and material
The fossil specimens described herein are stored in a publicly accessible museum collection (see details below).
References
Alexander, S., and E. Frey. 2010. Zwei Meeresschildkröten (Cheloniidae) aus der Tongrube Unterfeld bei Rauenberg (Unteroligozän, Rupelium). Kaupia 17: 73–105.
Balazs, G.H. 1985. Status and ecology of marine turtles at Johnston Atoll. Atoll Research Bulletin 285: 1–46.
Brand, L.R., M. Hussey, and J. Taylor. 2003. Taphonomy of freshwater turtles: Decay and disarticulation in controlled experiments. Journal of Taphonomy 1: 233–245.
Chan, B.K.K., N. Dreyer, A.S. Gale, H. Glenner, C. Ewers-Saucedo, M. Pérez-Losada, G.A. Kolbasov, K.A. Krandall, and J.T. Høeg. 2021. The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zoological Journal of the Linnean Society 193: 789–846. https://doi.org/10.1093/zoolinnean/zlaa160.
Chan, B.K.K., R.E. Prabowo, and K.S. Lee. 2009. Crustacean Fauna of Taiwan: Barnacles, Volume 1 – Cirripedia: Thoracica excluding the Pyrgomatidae and Acastinae. Keelung: National Taiwan Ocean University.
Cheang, C.C., L.M. Tsang, K.H. Chu, I.J. Cheng, and B.K.K. Chan. 2013. Host-specific phenotypic plasticity of the turtle barnacle Chelonibia testudinaria: A widespread generalist rather than a specialist. PLoS ONE 8: e57592. https://doi.org/10.1371/journal.pone.0057592.
Collareta, A. 2020. Discovery of complemental males in a Pliocene accumulation of Chelonibia testudinaria (Linnaeus, 1758), with some notes on the evolution of androdioecy in turtle barnacles. Neues Jahrbuch Für Geologie Und Paläontologie Abhandlungen 297: 193–203. https://doi.org/10.1127/njgpa/2020/0920.
Collareta, A., and G. Bianucci. 2021. The occurrence of the coronuloid barnacle Chelonibia Leach, 1817 as an encruster on mammalian bone in the central Mediterranean Sea. Acta Adriatica 62: 83–92.
Collareta, A., M. Bosselaers, and G. Bianucci. 2016. Jumping from turtles to whales: a Pliocene fossil record depicts an ancient dispersal of Chelonibia on mysticetes. Rivista Italiana Di Paleontologia e Stratigrafia 122: 35–44.
Collareta, A., M. Harzhauser, and M.W. Rasser. 2022a. New and overlooked occurrences of the rarely reported protochelonibiine “turtle” barnacles from the Oligocene and Miocene of Europe. PalZ 96: 197–206. https://doi.org/10.1007/s12542-021-00576-5.
Collareta, A., M. Merella, M. Bosselaers, S. Casati, A. Di Cencio, and G. Bianucci. 2022b. A Karethraichnus boring on a turtle shell bone from the Miocene of Italy is assessed as the attachment scar of a platylepadid symbiont. Neues Jahrbuch Für Geologie Und Paläontologie Abhandlungen 303: 327–337. https://doi.org/10.1127/njgpa/2022/1052.
Collareta, A., and W.A. Newman. 2020. Protochelonibia melleni (Zullo, 1982) comb. nov., an archaic barnacle from the lower Oligocene of Mississippi (USA), and its impact on the stratigraphic and geographic distribution of the early coronuloids of Western Tethys. Bollettino Della Società Paleontologica Italiana 59: 179–181.
Collareta, A., A. Reitano, A. Rosso, R. Sanfilippo, M. Bosselaers, G. Bianucci, and G. Insacco. 2019. The oldest platylepadid turtle barnacle (Cirripedia, Coronuloidea): A new species of Platylepas from the Lower Pleistocene of Italy. European Journal of Taxonomy 516: 1–17. https://doi.org/10.5852/ejt.2019.516.
Collareta, A., O. Lambert, F.G. Marx, C. de Muizon, R. Varas-Malca, W. Landini, G. Bosio, E. Malinverno, K. Gariboldi, A. Gioncada, M. Urbina, and G. Bianucci. 2021. Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): glimpses into the ancient humboldt Current ecosystem. Journal of Marine Science and Engineering 9: 1188. https://doi.org/10.3390/jmse9111188.
Collareta, A., W.A. Newman, G. Bosio, and G. Coletti. 2022c. A new chelonibiid from the Miocene of Zanzibar (Eastern Africa) sheds light on the evolution of shell architecture in turtle and whale barnacles (Cirripedia: Coronuloidea). Integrative Zoology 17: 24–43. https://doi.org/10.1111/1749-4877.12554.
Darwin, C. 1854. A monograph of the sub-class Cirripedia, with figures of all the species. The Balanidae, The Verrucidae, etc. London: Ray Society.
Davis, C.W. 1972. Studies on the barnacles epizoic on marine vertebrates (Unpublished MA thesis). San Francisco: California State University. https://scholarworks.calstate.edu/downloads/vh53x140n?locale=es. Accessed 30 April 2021.
De Baets, K., H. Keupp, and C. Klug. 2015. Parasites of ammonoids. In Ammonoid paleobiology: from anatomy to ecology, ed. C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes, 845–884. Berlin-Heidelberg: Springer.
Epibiont Research Cooperative. 2007. A synopsis of the literature on the turtle barnacles (Cirripedia: Balanomorpha: Coronuloidea) 1758–2007. ERC Special Publication 1: 1–62.
Ewers-Saucedo, C., M.D. Arendt, J.P. Wares, and D. Rittschof. 2015. Growth, mortality, and mating group size of an androdioecious barnacle: Implications for the evolution of dwarf males. Journal of Crustacean Biology 35: 166–176. https://doi.org/10.1163/1937240X-00002318.
Flint, M., J.C. Patterson-Kane, C.J. Limpus, T.M. Work, D. Blair, and P.C. Mills. 2009. Postmortem diagnostic investigation of disease in free-ranging marine turtle populations: A review of common pathologic findings and protocols. Journal of Veterinary Diagnostic Investigation 21: 733–759. https://doi.org/10.1177/104063870902100601.
Frick, M.G., and G. McFall. 2007. Self-grooming by loggerhead turtles in Georgia, USA. Marine Turtle Newsletter 118: 15.
Frick, M.G., and J.B. Pfaller. 2013. Sea turtle epibiosis. In The biology of sea turtles, vol. 3, ed. J. Wyneken, K.J. Lohmann, and J.A. Musick, 399–426. Boca Raton: CRC Press.
Frick, M.G., K.L. Williams, D. Veljacic, J.A. Jackson, and S.E. Knight. 2002. Epibiont community succession on nesting loggerhead sea turtles, Caretta caretta, from Georgia, USA In: A. Mosier, A. Foley, and B. Brost (eds) of the 20th Annual Symposium on Sea Turtle Biology and Conservation, Miami NOAA, Pp. 280–282
Grimm, K.I., M.C. Grimm, A. Köthe, and T. Schindler. 2002. Der “Rupelton” (Rupelium, Oligozän) der Tongrube Bott-Eder bei Rauenberg (Oberrheingraben, Deutschland). Courier Forschungsinstitut Senckenberg 237: 229–253.
Gruvel, A. 1903. Révision des Cirrhipèdes appartenant à la collection du Muséum d’Histoire Naturelle. Operculés. I. Partie Systematique. Nouvelles Archives du Muséum d’Histoire Naturelle 4: 95–170.
Harzhauser, M., W.A. Newman, and P. Grunert. 2011. A new Early Miocene barnacle lineage and the roots of sea-turtle fouling Chelonibiidae (Cirripedia, Balanomorpha). Journal of Systematic Palaeontology 9: 473–480. https://doi.org/10.1080/14772019.2010.528053.
Hayashi, R. 2013. A checklist of turtle and whale barnacles (Cirripedia: Thoracica: Coronuloidea). Journal of the Marine Biological Association of the United Kingdom 93: 143–182. https://doi.org/10.1017/S0025315412000847.
Hayashi, R., B.K.K. Chan, N. Simon-Blecher, H. Watanabe, T. Guy-Haim, T. Yonezawa, Y. Levy, T. Shuto, and Y. Achituv. 2013. Phylogenetic position and evolutionary history of the turtle and whale barnacles (Cirripedia: Balanomorpha: Coronuloidea). Molecular Phylogenetics and Evolution 67: 9–14. https://doi.org/10.1016/j.ympev.2012.12.018.
Karasawa, H., and N. Kobayashi. 2022. Cirripedes from the middle Pleistocene Atsumi Group, Japan, with a reevaluation of the genus Adna Sowerby, 1823 (Balanoidea: Pyrgomatidae). Bulletin of the Mizunami Fossil Museum 49: 67–93. https://doi.org/10.50897/bmfm.49.0_67.
Klompmaker, A.A., P.H. Kelley, D. Chattopadhyay, J.C. Clements, J.W. Huntley, and M. Kowalewski. 2019. Predation in the marine fossil record: Studies, data, recognition, environmental factors, and behavior. Earth-Science Reviews 194: 472–520. https://doi.org/10.1016/j.earscirev.2019.02.020.
Losey, G., G.H. Balazs, and L.A. Privitera. 1994. Cleaning symbiosis between the wrasse, Thalassoma duperrey, and the green turtle, Chelonia mydas. Copeia 1994: 684–690. https://doi.org/10.2307/1447184.
Maxwell, E.E., S. Alexander, G. Bechly, K. Eck, E. Frey, K. Grimm, J. Kovar-Eder, G. Mayr, N. Micklich, M. Rasser, A. Roth-Nebelsick, R.B. Salvador, P.R. Schoch, G. Schweigert, W. Stinnesbeck, K. Wolf-Schwenninger, and R. Ziegler. 2016. The Rauenberg fossil Lagerstätte (Baden-Württemberg, Germany): A window into early Oligocene marine and coastal ecosystems of Central Europe. Palaeogeography, Palaeoclimatology, Palaeoecology 463: 238–260. https://doi.org/10.1016/j.palaeo.2016.10.002.
Mimoto, K. 1991. Cirripedian fossils from the Pleistocene deposits of the southwestern part of Kochi Prefecture Shikoku. Fossils 51: 15–23. https://doi.org/10.14825/kaseki.51.0_15.
Monroe, R. 1981. Studies in the Coronulidae (Cirripedia) shell morphology, growth, and function, and their bearing on subfamily classification. Memoirs of the Queensland Museum 20: 237–251.
Monroe, R., and C.J. Limpus. 1979. Barnacles on turtles in Queensland waters with descriptions of three new species. Memoirs of the Queensland Museum 19: 197–223.
Moriarty, J.E., J.A. Sachs, and K. Jones. 2008. Directional locomotion in a turtle barnacle, Chelonibia testudinaria, on green turtles, Chelonia mydas. Marine Turtle Newsletter 119: 1–4.
Newman, W.A. 1996. Cirripedia; Suborders Thoracica and Acrothoracica. In Traité de Zoologie, Tome VII, Crustacés, Fascicule 2, ed. J. Forest, 453–540. Paris: Masson.
Nishizaki, M.T., and E. Carrington. 2015. The effect of water temperature and velocity on barnacle growth: Quantifying the impact of multiple environmental stressors. Journal of Thermal Biology 54: 37–46. https://doi.org/10.1016/j.jtherbio.2015.02.002.
Perreault, R.T., A. Collareta and J.S. Buckeridge. 2022. A new species of the archaic “turtle barnacle” genus Protochelonibia (Coronuloidea, Chelonibiidae) from the upper Rupelian Chickasawhay Formation of Mississippi (U.S.A.). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 305: 225–235. https://doi.org/10.1127/njgpa/2022/1087.
Pilsbry, H.A. 1916. The sessile barnacles (Cirripedia) contained in the collections of the U.S. National Museum; including a monograph of the American species. Bulletin of the United States National Museum 93: 1–366.
Robin, N. 2021. Importance of data on fossil symbioses for parasite–host evolution. In The evolution and the fossil record of parasitism: coevolution and paleoparasitological techniques, ed. K. De Baets and J.W. Huntley, 51–73. Cham: Springer.
Robin, N., O. Béthoux, E. Sidorchuk, Y. Cui, Y. Li, D. Germain, A. King, F. Berenguer, and D. Ren. 2016. A Carboniferous mite on an insect reveals the antiquity of an inconspicuous interaction. Current Biology 26: 1376–1382. https://doi.org/10.1016/j.cub.2016.03.068.
Ross, A. 1963. Chelonibia in the Neogene of Florida. Quarterly Journal of the Florida Academy of Science 26: 221–233.
Ross, A., and M.G. Frick. 2007. From Hendrickson (1958) to Monroe, Limpus (1979) and beyond: An evaluation of the turtle barnacle Tubicinella cheloniae. Marine Turtle Newsletter 118: 2–5.
Ross, A., and M.G. Frick. 2011. Nomenclatural emendations of the family-group names Cylindrolepadinae, Stomatolepadinae, Chelolepadinae, Cryptolepadinae, and Tubicinellinae of Ross, Frick, 2007 – including current definitions of family-groups within the Coronuloidea (Cirripedia: Balanomorpha). Zootaxa 3106: 60–66.
Ross, A., and W.A. Newman. 1967. Eocene Balanidae of Florida, including a new genus and species with a unique plan of “turtle-barnacle” organization. American Museum Novitates 2288: 1–21.
Sagan, D., and L. Margulis. 2001. Eukaryotes, Origin of. In Encyclopedia of Biodiversity, ed. S.A. Levin, 623–633. San Diego: Academic Press.
Santos, B.S., D.M. Kaplan, M.A. Friedrichs, S.G. Barco, K.L. Mansfield, and J.P. Manning. 2018. Consequences of drift and carcass decomposition for estimating sea turtle mortality hotspots. Ecological Indicators 84: 319–336. https://doi.org/10.1016/j.ecolind.2017.08.064.
Seigel, R.A. 1983. Occurrence and effects of barnacle infestations on diamondback terrapins (Malaclemys terrapin). American Midland Naturalist 109: 34–39. https://doi.org/10.2307/2425512.
Sloan, K., J.D. Zardus, and M.L. Jones. 2014. Substratum fidelity and early growth in Chelonibia testudinaria, a turtle barnacle especially common on debilitated loggerhead (Caretta caretta) sea turtles. Bulletin of Marine Science 90: 581–597. https://doi.org/10.5343/bms.2013.1033.
Spiegel, C., J. Kuhlemann, and W. Frisch. 2007. Tracing sediment pathways by zircon fission track analysis: Oligocene marine connections in Central Europe. International Journal of Earth Sciences 96: 363–374. https://doi.org/10.1007/s00531-006-0097-3.
Stanley, G.D., and B. Schootbrugge. 2009. The evolution of the coral–algal symbiosis. In Coral Bleaching: Patterns, Processes, Causes and Consequences, ed. M.J.H. Hoppen and J.M. Laugh, 7–19. Berlin-Heidelberg: Springer.
Stewart, J.R., S. Aspinall, M. Beech, P. Fenberg, P. Hellyer, N. Larkin, S.W. Lokier, F.G. Marx, M. Meyer, R. Miller, P.S. Rainbow, J.D. Taylor, J.E. Whittaker, K. Al-Mehsin, and C.J. Strohmenger. 2011. Biotically constrained palaeoenvironmental conditions of a mid-Holocene intertidal lagoon on the southern shore of the Arabian Gulf: Evidence associated with a whale skeleton at Musaffah, Abu Dhabi, UAE. Quaternary Science Reviews 30: 3675–3690. https://doi.org/10.1016/j.quascirev.2011.09.004.
Tapanila, L. 2008. Direct evidence of ancient symbiosis using trace fossils. Paleontological Society Papers 14: 271–287. https://doi.org/10.1017/S1089332600001728.
Tapanila, L., and A.A. Ekdale. 2007. Early history of symbiosis in living substrates trace-fossil evidence from the marine record. In Leif Tapanila, ed. A.A. Ekdale, 339–349. Trace Fossils Concepts Problems Prospects: Elsevier.
Vinn, O. 2016. Symbiotic endobionts in Paleozoic stromatoporoids. Palaeogeography, Palaeoclimatology, Palaeoecology 453: 146–153. https://doi.org/10.1016/j.palaeo.2016.04.027.
Vinn, O. 2017a. Symbiosis in Late Devonian-Mississippian corals: A review. Palaeobiodiversity and Palaeoenvironments 97: 723–729. https://doi.org/10.1007/s12549-017-0284-1.
Vinn, O. 2017b. Symbiosis between Devonian corals and other invertebrates. Palaios 32: 382–387. https://doi.org/10.2110/palo.2017.005.
Vinn, O., and M.A. Wilson. 2015. Symbiotic interactions in the Ordovician of Baltica. Palaeogeography, Palaeoclimatology, Palaeoecology 436: 58–63. https://doi.org/10.1016/j.palaeo.2015.06.044.
Vinn, O., and M.A. Wilson. 2016. Symbiotic interactions in the Silurian of Baltica. Lethaia 49: 413–420. https://doi.org/10.1080/08912963.2016.1161032.
Vinn, O., A. Ernst, and U. Toom. 2016. Earliest symbiotic rugosans in cystoporate bryozoan Ceramopora intercellata Bassler, 1911 from Late Ordovician of Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology 461: 140–144. https://doi.org/10.1016/j.palaeo.2016.08.016.
Vinn, O., A. Ernst, and U. Toom. 2018. Symbiosis of cornulitids and bryozoans in the Late Ordovician of Estonia (Baltica). Palaios 33: 290–295. https://doi.org/10.2110/palo.2018.018.
Vinn, O., A. Ernst, M.A. Wilson, and U. Toom. 2019. Symbiosis of conulariids with trepostome bryozoans in the Upper Ordovician of Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology 518: 89–96. https://doi.org/10.1016/j.palaeo.2019.01.018.
Vinn, O., A. Ernst, M.A. Wilson, and U. Toom. 2021. Symbiosis of cornulitids with the cystoporate bryozoan Fistulipora in the Pridoli of Saaremaa, Estonia. Lethaia 54: 90–95. https://doi.org/10.1111/let.12385.
Vinn, O., M.A. Wilson, U. Toom, and M.A. Mõtus. 2015. Earliest known rugosan-stromatoporoid symbiosis from the Llandovery of Estonia (Baltica). Palaeogeogr Palaeoclim 431: 1–5. https://doi.org/10.1016/j.palaeo.2015.04.023.
Wahl, M. 1989. Marine epibiosis. I. fouling and antifouling: some basic aspects. Marine Ecology Progress Series 58: 175–189.
Wahl, M., and O. Mark. 1999. The predominately facultative nature of epibiosis: Experimental and observational evidence. Marine Ecology Progress Series 187: 59–66. https://doi.org/10.3354/meps187059.
Weems, R.E., and K.M. Brown. 2017. More-complete remains of Procolpochelys charlestonensis (Oligocene, South Carolina), an occurrence of Euclastes (upper Eocene, South Carolina), and their bearing on Cenozoic pancheloniid sea turtle distribution and phylogeny. Journal of Paleontology 91: 1228–1243. https://doi.org/10.1017/jpa.2017.64.
Weems, R.E., and A.E. Sanders. 2014. Oligocene pancheloniid sea turtles from the vicinity of Charleston, South Carolina, USA. Journal of Vertebrate Paleontology 34: 80–99. https://doi.org/10.1080/02724634.2013.792826.
Yamaguchi, T., and W.A. Newman. 1990. A new and primitive barnacle (Cirripedia: Balanomorpha) from the North Fiji Basin abyssal hydrothermal feld, and its evolutionary implications. Pacific Science 44: 135–155.
Yuan, X., S. Xiao, and T.N. Taylor. 2005. Lichen-like symbiosis 600 million years ago. Science 308: 1017–1020. https://doi.org/10.1126/science.1111347.
Zardus, J.D. 2021. A global synthesis of the correspondence between epizoic barnacles and their sea turtle hosts. Integrative Organismal Biology. https://doi.org/10.1093/iob/obab018.
Zardus, J.D., D.T. Lake, M.G. Frick, and P.D. Rawson. 2014. Deconstructing an assemblage of “turtle” barnacles: Species assignments and fickle fidelity in Chelonibia. Marine Biology 161: 45–59. https://doi.org/10.1007/s00227-013-2312-7.
Zonneveld, J.P., M.K. AbdelGawad, and E.R. Miller. 2022. Ectoparasite borings, mesoparasite borings, and scavenging traces in early Miocene turtle and tortoise shell: Moghra Formation, Wadi Moghra. Egypt. Journal of Paleontology 96: 304–322. https://doi.org/10.1017/jpa.2021.92.
Zullo, V.A. 1982. A new species of the turtle barnacle Chelonibia Leach, 1817, (Cirripedia, Thoracica) from the Oligocene Mint Spring and Byram Formations of Mississippi. Mississippi Geology 2: 1–6.
Acknowledgements
We are grateful to Doug Perrine and Mark Flint for generously sharing their own photographs of extant chelonibiids and cheloniids. Constructive reviews by John-Paul Zonneveld, Olev Vinn and John S. Buckeridge significantly helped to improve an earlier version of the present paper.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. The research of AC is supported by a LinnéSys: Systematics Research Fund grant (funded by the Linnean Society of London and the Systematics Association).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest/competing interests to declare.
Additional information
Handling Editor: Hans Suess.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collareta, A., Rasser, M.W., Frey, E. et al. Turtle barnacles have been turtle riders for more than 30 million years. PalZ 97, 353–363 (2023). https://doi.org/10.1007/s12542-022-00641-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-022-00641-7