Abstract
The middle Miocene (upper Serravallian, lower Volhynian) deposits at Karpov Yar near Naslavcea, northern Moldova, are among the few settings in which fossil fish are preserved with otoliths in situ. Here, we describe the new gobiid †Moldavigobius helenae gen. et sp. nov. from this locality. The taxon is characterized by small size (up to 34.2 mm SL), a compact body (body depth 17–21% SL), a fan-shaped caudal fin, large ctenoid scales (< 30 scales in the longitudinal row) and nearly square otoliths (sagittae) with a slender, shoe sole-shaped sulcus. It has 27 vertebrae, six spines in the first dorsal fin, one spine and 11 soft rays in both the second dorsal and the anal fin, 15–17 pectoral-fin rays, and 17 (9/8) segmented caudal-fin rays. The meristic characters of †Moldavigobius gen. nov., together with its sagitta shape, suggest a relationship with Lesueurigobius Whitley, 1950, but its fan-shaped caudal fin and the unique sulcus contour of the otoliths preclude its attribution to that genus. In addition, we re-assign an otolith-based species previously described as Knipowitschia suavis Schwarzhans, 2014 as a second member of †Moldavigobius gen. nov. Accordingly, †Moldavigobius gen. nov. was represented by at least two species in the Serravallian of the Eastern Paratethys (†M. helenae gen. et sp. nov., †M. suavis nov. comb.). Moreover, †M. suavis is also known from the Serravallian ichthyofauna of the SE Mediterranean. †Moldavigobius gen. nov. thus demonstrates the key role of fossil skeletal material with otoliths in elucidating the ancient diversity of the Gobioidei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Like many other types of Cenozoic sediments, the marine sediments of the middle Miocene from the Central and Eastern Paratethys region have yielded numerous specimens of fossil fish otoliths, while fish skeletons are comparatively rare in these deposits. This is also the case for the fossil remains of gobioid fishes (‘gobies’); indeed, it is mainly their otolith-based record that reveals that gobies were an abundant component of the marine middle Miocene ichthyofauna in the Central and Eastern Paratethys (Bratishko et al. 2015; Reichenbacher et al. 2019; Schwarzhans et al. 2020b). Interestingly, there is little overlap between the solely otolith-based fossil goby species known from the Central and Eastern Paratethys and those that are attested by skeletal material with otoliths in situ (see Carnevale et al. 2006; Schwarzhans et al. 2017; Reichenbacher and Bannikov 2022). This may in part be attributable to ecological and taphonomic factors, but the relative rarity of fossil skeletons may also play a role. Consequently, the discovery of goby skeletons that have retained their otoliths can shed new light on the overall composition of the middle Miocene goby fauna. In some cases, such fossils may also help to clarify the taxonomic status of fossil goby species that have been recognized on the basis of otolith data alone (see Reichenbacher and Bannikov 2022).
The locality Karpov Yar, near the township of Naslavcea, northern Moldova, is one of the very few localities that have yielded well preserved fossil goby skeletons with otoliths in situ, thus providing complementary sources of information for taxonomic classification. However, until recently, the goby fossils recovered from Karpov Yar have been left unidentified or in open nomenclature (Ionko 1954; Bannikov 2009, 2010, 2017, 2018). The present study is the second contribution based on our ongoing research on the goby fossils from Karpov Yar. In the first one (Reichenbacher and Bannikov 2022), we described four new extinct genera of the family Gobiidae, represented by six species. All of these displayed skeletal characters and scales reminiscent of the extant gobiid genus Lesueurigobius Whitley, 1950. However, their otoliths were incompatible with such a classification, and we interpreted these fossils as a stem lineage of the European Aphia clade within the Gobiidae. In this study, we present another new gobiid genus and species from Karpov Yar—†Moldavigobius helenae gen. et sp. nov.—which may be a relative of Lesueurigobius. In addition, we show that isolated fossil otoliths previously described as Knipowitschia suavis Schwarzhans, 2014 from the Serravallian of the SE Mediterranean and Eastern Paratethys represent another species of †Moldavigobius gen. nov.
Geological setting
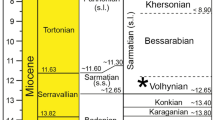
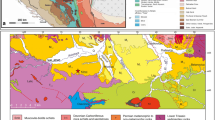
The sediments exposed in the Karpov Yar ravine in northern Moldova are lower Volhynian in age (Sarmatian sensu lato, c. 12 Ma; Reichenbacher and Bannikov 2022: fig. 1). The palaeogeographic location of Karpov Yar is situated in the western sector of the Eastern Paratethys, which was at that time a semi-closed inland sea with limited connections to the Central Paratethys and the Mediterranean Sea (Reichenbacher and Bannikov 2022: fig. 2). The fish-bearing sediments at Karpov Yar consist of diatomites and marls with some thin layers bearing mass occurrences of fossil fishes. For details of the geological profile at Karpov Yar, the fossil molluscs and previously described fish fauna from this locality, see Reichenbacher and Bannikov (2022).
Materials and methods
Fossil material
Articulated skeletons of six fossil specimens are included in this study. They are deposited in the Borissiak Palaeontological Institute of the Russian Academy of Sciences in Moscow, under the inventory numbers PIN 5274/49, PIN 5274/69, PIN 5274/73 [holotype], PIN 1306/68a, PIN 1306/68b, PIN 1306/68c. With the exception of PIN 1306/68b, all skeletons were preserved with otoliths in situ. Slabs revealing both the part (head to the right) and counterpart (head to the left) of the fossil skeleton were recovered for PIN 5274/73 and PIN 5274/49. The otoliths are kept in the Bavarian State Collection for Palaeontology and Geology (SNSB–BSPG) in Munich, Germany, under the inventory number SNSB–BSPG 2021 XI.
Methods
The fossil skeletons and otoliths were examined and photographed under a Leica M165 FC stereomicroscope equipped with a digital camera (Leica DC 200). Otoliths preserved in situ in the paratypes PIN 1306/68c (both sagittae) and PIN 1306/68a (right sagitta, left lapillus), and in the referred specimen PIN 5274/69 (right sagitta), were carefully extracted and stored separately; the right sagitta of each paratype and the lapillus were used for SEM imaging (HITACHI SU 5000 Schottky FE–SEM, Department of Earth and Environmental Sciences, LMU Munich). For the established terminologies of the gobiid sagitta and lapillus, see Reichenbacher and Bannikov (2022: fig. 4).
The morphometric parameters of the skeletons and otoliths correspond to those used in previous publications on extant goby species (e.g., Miller 2004; Liu et al. 2009; Iglésias et al. 2021) and in Reichenbacher and Bannikov (2022). All measurements were recorded to the nearest 0.1 mm using ImageJ (Schneider et al. 2012) and were normalized based on the standard length (SL) of the measured fish. Meristic characters comprised counts of abdominal and caudal vertebrae (including the terminal centrum), counts of fin rays (every discernible ray was counted), and, where possible, determination of the pterygiophore formula of the first dorsal fin (according to Birdsong et al. 1988) and the number of scales in the longitudinal row. Topographical terms refer to the natural anatomical location of the structure concerned, even when this is rotated or displaced in the fossil.
Calculation of the standard lengths of incomplete fish fossils
The paratypes PIN 1306/68a and PIN 1306/68b and the referred specimen PIN 5274/49 are incomplete, so their standard lengths could not be measured directly. In these cases, we used the length of the in-situ sagitta to calculate the corresponding SL for each specimen, based on the assumption that the standardized sagitta length should be the same (i.e., 4.65% SL) as that obtained from the (complete) holotype (PIN 5274/73) and the (complete) referred specimen (PIN 5274/69). This approach appears reasonable, because the ratio between the length of the sagitta and the SL is relatively stable within a gobiid species when specimens of the same size class are considered (Więcaszek et al. 2020), as is the case here. The only specimen that had no otoliths is the paratype PIN 1306/68b, which is an almost complete specimen but lacks the terminal centrum, hypural plates and caudal fin. We calculated the SL of PIN 1306/68b by assuming that it has the same size proportions as those measured in the holotype with respect to (i) the penultimate vertebra, terminal centrum and hypural plate, and (ii) head length and standard length. Both estimates resulted in a SL of 24.2 mm for PIN 1306/68b.
Abbreviations used in the text. α, inclination angle of sulcus; D1, first dorsal fin; D2, second dorsal fin; SL, standard length. A dagger (†) precedes the name of an extinct genus or species.
Institutional abbreviations. IRSNB, Royal Institute of Natural Sciences Belgium; NMP, National Museum Prague, Prague, Czech Republic; PIN, Borissiak Palaeontological Institute of the Russian Academy of Sciences, Moscow, Russia; ZM-CBSU, Zoological Museum Collection of the Biology Department at Shiraz University, Iran; ZSM, Bavarian State Collection of Zoology, Munich, Germany.
Systematic palaeontology
Infraclass Teleostei Müller, 1845 sensu Arratia (1999)
Order Gobiiformes Günther, 1880 sensu Betancur-R et al. (2017)
Suborder Gobioidei Jordan and Evermann, 1896 sensu Thacker et al. (2015)
Family Gobiidae Cuvier, 1816 sensu Nelson et al. (2016).
Genus †Moldavigobius gen. nov.
Type species. †Moldavigobius helenae gen. et sp. nov. (Figs. 1, 2, 3f–h).
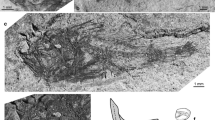
a–e †Moldavigobius helenae gen. et sp. nov. from Karpov Yar, near Naslavcea, northern Moldova. a1 holotype (counterpart), with right sagitta and both lapilli preserved in situ (PIN 5274/73b); a2 close-up of sagitta. b Skeleton of paratype PIN 1306/68b (no otoliths in situ). c1 Skeleton of paratype PIN 1306/68c (otoliths were extracted); c2, c3 close-ups of left (c2) and right (c3) extracted sagittae. d1 Incomplete skeleton of paratype PIN 1306/68a (otoliths were extracted); d2 close-up of extracted right sagitta; d3 close-up of extracted left lapillus (SEM image). e1 Skeleton of referred specimen PIN 5274/69 (otolith was extracted); e2 close-up of extracted right sagitta
Details of the holotype of †Moldavigobius helenae gen. et sp. nov. a Head of holotype (part, PIN 5274/73a). Note premaxilla with prominent postmaxillary process, and hyoid bar with five branchiostegal rays. b1–b4 Close-ups of holotype (counterpart, PIN 5274/73b). b1 Head with right sagitta and both lapilli preserved in situ, a well preserved T-shaped palatine (arrows indicate ethmoid and maxillary processes) and the articulation of branchiostegal rays 1–5 to the hyoid bar; b2 pectoral and pelvic girdles and fins (arrows indicate point of bifurcation of pelvic-fin rays); b3 scales on the flank (below insertion of D2) with well-preserved ctenii; b4 caudal skeleton exhibiting a single, large epural and 17 segmented caudal-fin rays. Abbreviations: bp, basipterygium; br, branchiostegal ray; ce, ceratohyal; cl, cleithrum; de, dentary; ect, ectopterygoid; eh, epihyal; ep, epural; hh, hypohyal; hyp, hypural plate; lap, lapillus; mx, maxilla; orb, orbit; pal, palatine; pectR, pectoral-fin rays; pelvR, pelvic-fin rays; pelvsp, pelvic-fin spine; pmx, premaxilla; pop, preopercle; ps, parasphenoid; q, quadrate; ra, radials of pectoral girdle; sag, sagitta; sy, symplectic; uh, urohyal; v, vomer
a–e Otoliths of †Moldavigobius suavis (Schwarzhans 2014) comb. nov. (shown in medial and dorsal views, dorsal view with lateral side down). a Holotype, right sagitta, Karaman Basin, SE Turkey (re-figured from Schwarzhans 2014: pl. 10, fig. 7). b Left sagitta (mirrored for better comparison), Karaman Basin, SE Turkey (re-figured from Schwarzhans et al. 2015: fig. 7.9). c–e Left sagittae (c, d, mirrored) and right sagitta (e) from Mangyshlak, Kazakhstan (re-figured from Bratishko et al. 2015: figs. 10.7, 10.8, 10.10). f–h †Moldavigobius helenae gen. et sp. nov.; f right sagitta preserved in situ in holotype PIN 5274-73a; g, h right sagittae extracted from paratypes PIN 1306/68a and PIN 1306/68c. i Knipowitschia thessala (Vinciguerra, 1827), right sagitta, Karla Lake, Greece (collection BR). Figures g–i are SEM images. All other figures are stereoscopic images
Other species. †Moldavigobius suavis (Schwarzhans, 2014) comb. nov. (Fig. 3a–e), which is an otolith-based species previously assigned to the extant oxudercid genus Knipowitschia Iljin, 1927 (see below).
Etymology. The name refers to where the taxon was first discovered (Moldavia) and to its general similarity to members of the Gobiidae. Gender masculine.
LSID ZooBank. This new genus is registered under urn:lsid:zoobank.org:pub:4C1FF532-2350-45A8-BF4D-C40A5A8F8810.
Stratigraphic range. Middle Miocene (upper Serravallian, lower Sarmatian, lower Volhynian).
Diagnosis. †Moldavigobius gen. nov. is a small gobiid fish; SL between 24.2 and 34.2 mm. Head moderately large (23.4–26.9% SL); body probably laterally compressed (as all specimens are preserved in lateral view); body depth 17.2–21.0% SL at origin of D1; anal fin inserted one vertebra behind D2; caudal peduncle relatively short (16.1–17.2% SL); caudal fin approximately as long as head (24.3% SL); length of abdominal part of vertebral column 63.9–66.4% of that of caudal part. Number of vertebrae 27 (10 + 17); D1 with six spines, last spine slightly further from preceding one (interspine distance is 3.8–4.4% SL); D1 pterygiophore formula not completely clear owing to some distortion of the neural spines, but it is probably 3-22110; D2 with relatively long spine (8.2–10.3% SL) and 11 segmented rays; anal fin with moderately long spine (4.1–6.2% SL) and 11 segmented rays. Pectoral fin with 15 to 17 rays; pelvic fin with relatively short spine (c. 3.8% SL) and five rays, rays terminating distant from anal-fin origin. Caudal fin fan-shaped, with 17 segmented rays (nine rays in the upper lobe) and a relatively large number of procurrent rays (9 dorsally, 8 ventrally). Relatively large ctenoid scales on body, number of scales in longitudinal row < 30.
Otoliths–Sagittae almost square, no marked posterodorsal projection (Fig. 3a–h). Sulcus moderately inclined (α < 10°–19.8°); shoe sole-shaped, with slender cauda and well developed dorsal ostial lobe; crista inferior with long, pronounced subcaudal iugum. Lapillus only known from type species, ovate with deep sulculus (Fig. 1d3).
Differential diagnosis. †Moldavigobius gen. nov. can be distinguished from the previously described ‘Lesueurigobius look-alikes’ from Karpov Yar (i.e., †Katyagobius Reichenbacher and Bannikov, 2022, †Pseudolesueurigobius Reichenbacher and Bannikov, 2022, †Sarmatigobius Reichenbacher and Bannikov, 2021, †Yarigobius Reichenbacher and Bannikov, 2022) by its fan-shaped caudal fin (vs. longish–lanceolate), fewer rays in D2 (11 vs. 14–16) and in anal fin (11 vs. 13–15), and a relatively longer abdominal portion of the vertebral column (64–66% of the length of the caudal part of the vertebral column, vs. 49–54%) (see Reichenbacher and Bannikov 2022: table 1). Furthermore, †Moldavigobius gen. nov. displays meristic counts, osteological traits and otolith characters that clearly set it apart from previously described Miocene marine and brackish fossil goby genera from the western Mediterranean and Paratethyan regions, i.e., †Eleogobius Gierl and Reichenbacher, 2015; †Hesperichthys Schwarzhans, Ahnelt, Carnevale and Japundžić, 2017; †Lepidocottus Sauvage, 1875; †Proneogobius Schwarzhans, Ahnelt, Carnevale and Japundžić, 2017; and †Protobenthophilus Schwarzhans, Ahnelt, Carnevale and Japundžić, 2017. In addition, the almost square shape of the otolith as well as the slender cauda and well-developed dorsal ostial lobe allow †Moldavigobius gen. nov. to be differentiated from the two middle Miocene otolith-based goby genera for which no skeletal remains are known, i.e., †Hoeseichthys Schwarzhans, Brzobohatý and Radwańska , 2020, and †Weilerigobius Schwarzhans, 2017. Finally, its otolith morphology (nearly square shape, slender cauda, well developed dorsal ostial lobe) and some body-related characters (e.g., fan-shaped fin, moderately deep body) distinguish †Moldavigobius gen. nov. from the extant genus Lesueurigobius Whitley, 1950 and other extant gobiid genera with the same or similar meristic traits (see “Discussion” section).
Remarks. An otolith-based extinct species has been reported as Knipowitschia suavis Schwarzhans, 2014 from the upper Serravallian marine and brackish deposits in the Karaman Basin, SE Turkey. Later, in Bratishko et al. (2015), the same species was also reported from the lower Serravallian (Konkian) shallow marine deposits at Mangyshlak, western Kazakhstan (Eastern Paratethys). The otoliths of this species resemble those of †Moldavigobius gen. nov. in their nearly square shape, absence of a marked posterodorsal angle, and presence of a moderately inclined, relatively slender sulcus adjoined by a well-developed subcaudal iugum (Fig. 3a–e). They differ from otoliths of extant Knipowitschia species insofar as ostium and cauda lie on nearly the same inclination axis, whereas in Knipowitschia the inclination angle of the cauda is much shallower (sometimes almost horizontal) than that of the ostium (Fig. 3i; see also Schwarzhans et al. 2015: figs. 7.6–8 for otoliths of K. longecaudata (Kessler, 1877), which is the type species of Knipowitschia). Moreover, the cauda is well developed in the fossil otoliths from the Karaman Basin and western Kazakhstan (Fig. 3a–e), and is not shortened or constricted like the otoliths of extant Knipowitschia species (Fig. 3i). Given the aforementioned similarities and differences, ‘Knipowitschia’ suavis can be assigned to †Moldavigobius gen. nov.
†Moldavigobius helenae gen. et sp. nov.
Figures 1, 2, 3f–h; Tables 1, 2
Type material. Holotype: PIN 5274/73; 34.2 mm SL; part (incomplete) and well-preserved counterpart with right sagitta and both lapilli preserved in situ (Fig. 1a). Three paratypes on a single slab: PIN 1306/68b is a well-preserved specimen but lacks the caudal skeleton and caudal fin (Fig. 1b), a mould of the sagitta is recognizable in its head; PIN 1306/68c is a posteriorly incomplete skeleton preserved in dorsolateral view, from which both sagittae were extracted (Fig. 1c); PIN 1306/68a is an incomplete skeleton from which a right sagitta and a left lapillus were extracted (Fig. 1d). This specimen is not included in Table 1 owing to its partial preservation.
Other material. The specimens PIN 5274/69 and PIN 5274/49 are tentatively assigned to †M. helenae gen. et sp. nov. on the basis of their otolith morphology. Specimen PIN 5274/69 is a complete, but poorly preserved skeleton with a right sagitta preserved in situ, which was extracted (Fig. 1e). PIN 5274/49 (part and counterpart) is a juvenile, largely incomplete specimen with both sagittae in situ. Its head is poorly preserved, while the abdominal vertebral column (comprising 10 vertebrae) and the first two caudal vertebrae can be discerned; this specimen is not included in Table 1 because of its poor preservation.
Type locality and age. Karpov Yar, Naslavcea, northern Moldova; lower Sarmatian (Volhynian).
Etymology. The species epithet honours Dr. Helen K. Larson (Museum and Art Gallery of the Northern Territory, and Museum of Tropical Queensland, Australia) for her meticulous work on the morphology, osteology and systematics of many extant goby genera and species.
LSID ZooBank. This new species is registered under urn:lsid:zoobank.org:act:8DDECDE1-BC3F-42EE-85C0-30719CAECB02.
Diagnosis. Morphological and meristic characters as for the genus.
Sagittae almost square, length–height index 0.97 ± 0.05 (Table 2), dorsal margin slightly lobed and weakly curved, no marked posterodorsal projection; sulcus shoe sole-shaped, moderately inclined (15.0°–18.9°), crista inferior with longish subcaudal iugum (Figs. 1a2, c2, c3, d2, e2, 3f–h). Lapillus ovate with well-developed sulculus (Fig. 1d3).
Differential diagnosis. The only other species of †Moldavigobius gen. nov. currently known is †M. suavis (Schwarzhans, 2014) comb. nov., of which only otoliths (sagittae) are known (see above and Fig. 3a–h). The otoliths of the two species differ in the curvature of the dorsal margin (faintly curved in †M. helenae gen. et sp. nov. vs. highly curved in †M. suavis), the configuration of the predorsal angle (sharp in †M. helenae gen. et sp. nov. vs. rounded), the position of the predorsal angle (same height as posterodorsal angle in †M. helenae gen. et sp. nov. vs. below posterodorsal angle), and the length of the subcaudal iugum (terminating before cauda end in †M. helenae gen. et sp. nov. vs. extending to cauda end and sometimes beyond). Further differential diagnosis as for the otoliths of the genus.
General description. Relatively small gobiid fish, with the largest (holotype) reaching 34.2 mm SL. Body moderately elongate and posteriorly slightly tapering, head of moderate size (23–27% SL), eyes 6–8% of SL, body depth at origin of D1 c. 17–21% SL, caudal peduncle slightly tapering towards caudal fin and relatively short (16–17% SL), caudal fin fan-shaped and moderately long (24.3% SL). For further body proportions and meristic counts, see Table 1.
Neurocranium. The neurocranium is preserved either in lateral (holotype, paratypes PIN 1306/68a, b) or dorsoventral view (paratype PIN 1306/68c). The frontal bones are long, narrow above the orbit and broad posteriorly. The parasphenoid is exposed at the lower margin of the orbit; it is straight, relatively narrow, with a broad posterior portion. The vomer has a broad, rounded anterior portion and a slender, pointed posterior process (Fig. 2a). Other details of the neurocranium are not recognizable. Scales are not present on the head.
Jaws. The mouth gape is moderately wide; the lower jaw articulation is situated more or less under the middle of the orbit (Fig. 2a). The dentary is narrow and long (9–10% SL); the angulo-articular is relatively massive and has a short, but relatively deep retroarticular process. The maxilla is slender and elongate, being slightly broader posteriorly and somewhat bent. The relatively long premaxilla is straight in lateral view; it has a thin and relatively long ascending process, a massive, rounded articular process and a well-developed, somewhat rounded postmaxillary process (Fig. 2a). Both the dentary and premaxilla bear large and small conical teeth, arranged in several rows; outer-row teeth are bigger than inner-row teeth. In specimen PIN 1306/68a, blunt teeth seem also to be present on the dentary. In the holotype, the bases of two enlarged teeth at the anterior portion of the premaxilla are recognizable.
Suspensorium, opercular apparatus and hyoid arch. The symplectic is a robust long rod; the metapterygoid is moderately long and slender (Fig. 2a). The quadrate is a roughly triangular bone with a deep and wide indentation in its posterior portion and a robust, thick and pointed posterior process (Fig. 2a). The palatine is T-shaped (indicated by arrows in Fig. 2b1); its proximal portion is not clearly detectable, but it seems to be short. The ectopterygoid is long, straight, somewhat broadened proximally and slender distally; it reaches the head of the palatine (Fig. 2b1). No entopterygoid is present. The preopercle, opercle and subopercle are poorly preserved, while the interopercle is not visible. The ceratohyal shows a relatively straight and slender anterior portion and a broadened posterior part; the epihyal is triangular (Fig. 2b1). The interhyal is not recognizable. Remains of the hypohyal and urohyal are preserved in the holotype (Fig. 2a); the latter is a thin, flat bone with a concave upper margin, a convex lower margin and an indented posterior margin. The number of branchiostegal rays is five; the first ray is slender, whereas rays 2–5 are relatively robust (Fig. 2a, b1). Branchiostegal ray 1 is attached slightly behind the middle of the slender part of the ceratohyal, rays 2–4 articulate with its broadened posterior part, and ray 5 inserts at the anteriormost edge of the epihyal (Fig. 2b1).
Branchial arches. Indistinct remains of gill arches are preserved in the holotype. The pharyngeal dentition includes both thick and slender conical teeth of multiple sizes.
Vertebral column. Total number of vertebrae is 27, of which 10 are abdominal. Vertebral centra are constricted in the middle; the centra of the abdominal vertebrae are relatively elongate. The length of the abdominal part of the vertebral column is 64–66% of the length of the caudal part (Table 1). The posteriormost abdominal vertebrae display relatively strong parapophyses. Thin ribs extend from vertebra 3 to vertebra 10; ribs of the last three pairs become successively shorter, with the last rib pair being much shorter than the preceding one. The haemal spines are more or less equally inclined posteriorly.
Pectoral girdle and fins. Only one of the anterior processes of the post-temporal is recognizable as a slender rod. The supracleithrum is a moderately elongate bone. The cleithrum is sturdy, long and slightly curved (Fig. 2a); scapula and coracoid are not preserved. Three radial bones are visible in the holotype, the fourth is obscured by the bones of the basipterygium (Fig. 2b2). The pectoral fin is slightly shorter than the pelvic fin and contains up to 17 rays (Fig. 2b2).
Pelvic girdle and fins. The pelvic fins are very close to each other and were probably united (Fig. 2b2); pelvic fin length is c. 15–19% SL. The pelvic spine is slender, its length is c. 3.8% SL. There are five pelvic-fin soft rays, which terminate distant from the anal-fin origin; each ray is bifurcated after 26–34% of its total length (Figs. 1a1, 2b2). Almost straight (very gently curved) elongate basipterygii are visible in the holotype (Fig. 2b2).
Dorsal fins. The first dorsal fin contains six long and slender spines terminating in short filaments (best seen in the paratype PIN 1306/68b; Fig. 1b). The second spine seems to be the longest (but the first spine is not completely preserved), spine V is clearly shorter, spine VI inserts after a small gap (Table 1). The first dorsal-fin pterygiophore inserts behind the neural spine of the third vertebra; the pterygiophore formula is most probably 3-22110 (based on holotype and paratype PIN 1306/68b), with the vacant interneural space (= interneural gap) located between the neural spines of vertebrae 7 and 8 (uncertainty in the pterygiophore formula is due to post-mortem distortion of the pterygiophores or neural spines).
The second dorsal fin originates above the last abdominal (holotype, paratype PIN 1306/68b) or first caudal vertebra (paratype PIN 1306/68a). It consists of a long, slender spine (8–10% SL) and 11 soft segmented rays. The rays display a comparatively long, undivided proximal portion before they begin to branch, and do not reach the dorsal procurrent caudal-fin rays (Fig. 1b). The first D2-pterygiophore inserts into the interneural space of vertebrae 8 and 9.
Anal fin. The anal fin comprises a moderately long spine (4–6% SL) and 11 soft rays. Rays are not completely preserved posteriorly, but appear to end distant from the beginning of the ventral procurrent caudal-fin rays. As observed in the D2 rays, the anal-fin rays also possess a comparatively long, undivided proximal portion before they begin to branch (Fig. 1a1). The point of insertion of the anal fin is one vertebra behind the origin of D2, i.e., below the first caudal vertebra (visible in holotype and paratype PIN 1306/68b) (Fig. 1a1, b). Two or three anal-fin pterygiophores insert anterior to the haemal spine of the first caudal vertebra.
Caudal endoskeleton and fin. The caudal fin is moderately long (24.3% of SL) and fan-shaped (Fig. 1a1). It is composed of 17 segmented principal rays; the number of principal rays in the upper lobe is 9 (Fig. 2b4). Ten procurrent rays are present dorsally and nine ventrally (visible in the holotype). The caudal skeleton is covered by large scales, which obscure its details. However, it is clear that the terminal centrum is fused with HYP3 + 4 and that a long slender hypural plate 5 is present (Fig. 2b4). A single large epural can clearly be seen, whose distal portion is roughly triangular (Fig. 2b4). The contour of the parhypural cannot be discerned. One or two vertical rows of relatively small ctenoid scales cover the proximal portion of the principal caudal-fin rays (Fig. 2b4).
Otoliths. The sagitta is squarish-to-rectangular in shape, length/height index 0.93–1.05 (Table 2). Dorsal margin weakly curved; no marked posterodorsal projection; anterior and posterior margins slightly incised in the middle (Figs. 1, 3f–h). Predorsal angle distinct, sharp, and positioned almost as high as posterodorsal angle, preventral and posteroventral angle pronounced. Sulcus shoe sole-shaped, relatively slender, moderately inclined (α = 15.0°–18.9°), cauda adjoined by crista inferior with well-developed subcaudal iugum terminating slightly before the end of the cauda.
Lapillus ovate, with rounded medial margin, relatively straight lateral margin and distinct sulculus (Fig. 1d3).
Scales. The body is densely covered with scales (Figs. 1a1, b, c1, 2b3), which are also present on the proximal part of the caudal fin (Fig. 2b4). Flank scales are ctenoid, with thickened posterior margin and about eight radii; the ctenii are sharp and relatively small and slender (Fig. 2b3). The number of longitudinal scales is about 30. Small cycloid belly scales and predorsal scales are also present.
Discussion
Assignment of †Moldavigobius gen. et sp. nov. at the family level
The presence of five branchiostegal rays, as seen in †Moldavigobius helenae gen. et sp. nov. (Fig. 2a), is regarded as a synapomorphy for the families Gobiidae and Oxudercidae (Wang et al. 2001; Gill and Mooi 2012). Further characteristic traits of the Gobiidae and Oxudercidae (although exceptions may occur) include a T-shaped palatine, absence of the endopterygoid, and presence of an interneural gap between the two dorsal fins (Akihito et al. 1984; Hoese 1984; Hoese and Gill 1993). Each of these traits can be clearly discerned in †M. helenae gen. et sp. nov. (see species description). Accordingly, †Moldavigobius gen. nov. can be classified as a member of the Gobiidae + Oxudercidae.
In deciding whether †Moldavigobius gen. nov. belongs to the Gobiidae or Oxudercidae, the configuration of the ‘palatopterygoquadrate complex’, the D1 pterygiophore formula and the number of epural bones are useful (Birdsong et al. 1988; Harrison 1989). The palatopterygoquadrate complex is made up of the palatine, ectopterygoid and quadrate bones, whose size and arrangement differ in certain respects between the two families (Harrison 1989; Thacker 2013; Reichenbacher et al. 2018). Characteristic for many Gobiidae is a short palatine (whose ventral tip ends with some distance from the quadrate) and a relatively long ectopterygoid (which extends almost to the head of the palatine) (Harrison 1989). This condition is found in †M. helenae gen. et sp. nov. (Fig. 2a, b1). Furthermore, Oxudercidae have a derived D1 pterygiophore formula sequence, which is 3-12210 (Harrison 1989). This configuration is not present in †Moldavigobius gen. nov.; its (probable) D1 pterygiophore formula sequence is 3-22110, which is a common D1 pterygiophore formula for the Gobiidae, albeit not a derived character (Birdsong et al. 1988). The number of epural bones is usually two in Oxudercidae (plesiomorphic condition) and one in Gobiidae (derived) (Birdsong et al. 1988; Harrison 1989). †Moldavigobius helenae gen. et sp. nov. shows a single epural (Fig. 2b4). In summary, †Moldavigobius gen. nov. can be assigned to the Gobiidae.
Possible assignment to an extant gobiid lineage and genus?
The Gobiidae can be divided into 14 lineages based on molecular data (Agorreta et al. 2013; Thacker 2015). However, little is known about the morphological synapomorphies that define each of these lineages and, as mentioned in Reichenbacher and Bannikov (2022), a phylogenetic matrix based on morphology that includes members of all 14 lineages is not available. Hence, the ‘best-fit’ approach sensu Penk et al. (2019) is used in the following to analyse whether †Moldavigobius gen. nov. can be assigned at the level of lineage.
We first chose the vertebral count seen in †Moldavigobius gen. nov. to explore possibly related extant lineages among the Gobiidae. The use of this specific trait is based on the available evidence, which indicates that vertebral counts are relatively constant within gobiid lineages (Birdsong et al. 1988). The choice is additionally justified because the 10 + 16 count is the plesiomorphic state; hence, the 10 + 17 count seen in †Moldavigobius gen. nov. is a derived condition (Reichenbacher and Bannikov 2022). Among the extant Gobiidae, a regular 10 + 17 vertebrae count occurs in Bathygobius, Glossogobius and Grallenia (all of which belong to the Glossogobius lineage), in both genera of the Aphia lineage (Aphia, Lesueurigobius), and in four genera of the highly diverse Gobius lineage (in Caffrogobius, Coryogalops, Hetereleotris, Sufflogobius) (Fig. 4).
Compilation of gobiid genera that share with Moldavigobius gen. nov. the count of 10 abdominal and 17 caudal vertebrae (vertebral counts compiled from Reichenbacher and Bannikov 2022: table 2). Numbers of anal- and second dorsal-fin rays and otolith morphology compiled from different literature sources (indicated with superscript numbers), otolith images for Glossogobius, Caffrogobius, Sufflogobius were refigured from Smale et al. (1995). Some additional morphological information is provided for Grallenia for which no otolith data were available. References: 1Miller and Stefanni (2001), Tornabene et al. (2010); 2Hoese and Allen (2009), Hoese et al. (2015); 3Shibukawa and Iwata (2007); 4Allen and Hammer (2018); 5Froese and Pauly (2022); 6Goren (1996); 7Kovačić et al. (2014); 8Hoese (1986a), Hoese and Larson (2005), Kovačić and Bogorodsky (2014); 9Hoese (1986b); 10Miller (1986). Scale bars for otoliths = 0.5 mm
In the second step, we used (i) the number of soft rays in the anal fin, and examined whether it contains one ray more or less than the second dorsal fin, and (ii) the otolith morphology. These characters have been shown to be taxonomically informative in previous publications (although exceptions occur). The number of soft rays in the anal fin is a diagnostic trait at the level of genus and species (e.g.,Miller 1986; Kovačić 2020), and the relationship between the numbers of rays in the anal fin and second dorsal fin is phylogenetically informative, with the presence of an extra ray in the anal fin (relative to the second dorsal fin) being the derived condition (Pezold 2004). The overall shape of a gobioid otolith, and particularly the shape and morphology of its sulcus (= ostium and cauda), are important diagnostic characters at the level of the genus, and are also of taxonomic value at the level of lineages (Gierl et al. 2018; Lombarte et al. 2018; Schwarzhans et al. 2020a, b).
Comparison with the Glossogobius lineage
Three genera of the Glossogobius lineage, i.e., Bathygobius, Glossogobius and Grallenia possess a regular 10 + 17 vertebrae count (Fig. 4).
Bathygobius and Glossogobius have lower ray counts in the anal fin than †Moldavigobius gen. nov. does (≤ 9 vs. 11), and one or two rays less in the anal fin than in the second dorsal fin (vs. equal counts). In addition, the sulcus shapes of their otoliths are clearly distinct from the sulcus of †Moldavigobius gen. nov. In Bathygobius, the cauda is almost horizontal (vs. inclined in †Moldavigobius gen. nov.); in Glossogobius, the lower sulcus margin is straight (vs. indented at the junction of ostium and cauda in †Moldavigobius gen. nov.). In addition, the subcaudal iugum is positioned at the transition between ostium and cauda in both Bathygobius and Glossogobius (vs. along the cauda) and is relatively weakly developed (vs. prominent) (Fig. 4). There is a superficial similarity between the overall shapes of the otoliths of Bathygobius, Glossogobius, and †Moldavigobius gen. nov., owing to the absence of a marked posterodorsal projection. However, this character state represents a plesiomorphic condition, since the otoliths of the Thalasseleotrididae, to which the (more derived) Gobiidae + Oxudercidae are sister (Gill and Mooi 2012; Thacker et al. 2015), also do not show a marked posterodorsal projection (see Schwarzhans 2019: figs. 99.9–11 for otoliths of Thalasseleotris iota; see also Gierl et al. 2022).
In Grallenia, up to 11 anal-fin rays can occur, and the number of soft rays in the anal fin can be the same as (or one less than) in the second dorsal fin (Fig. 4). As no otolith data are available for Grallenia, we investigated whether Grallenia and †Moldavigobius gen. nov. share other characteristic traits. This is obviously not the case as Grallenia is of very small size (< 23 mm SL vs. up to 34.2 mm in †Moldavigobius gen. nov.), always associated with coral reefs (vs. not), has a very slender body resembling that of a ‘sand goby’ (vs. compact body), and 15 segmented caudal-fin rays (vs. 17) (data for Grallenia from Allen and Hammer 2018). Taking all differences together, we do not consider †Moldavigobius gen. nov. to be closely related to the Glossogobius lineage (Fig. 4).
Comparison with the Gobius lineage
Four genera of the highly diverse Gobius lineage, namely, Caffrogobius, Coryogalops, Hetereleotris and Sufflogobius have a regular 10 + 17 vertebrae count (Fig. 4).
The monotypic genus Sufflogobius has 12–13 rays in both the second dorsal and the anal fin (vs. 11 in †Moldavigobius gen. nov.). The otoliths of Sufflogobius differ from those of †Moldavigobius gen. nov. in their irregular to trapezoid shape (vs. squarish) (Fig. 4). This is a rather unusual shape for a gobiid otolith, and may be related to the pelagic lifestyle of Sufflogobius.
Caffrogobius, Coryogalops and Hetereleotris share their anal-fin ray counts with †Moldavigobius gen. nov., but Caffrogobius and Coryogalops have at least one ray less in the anal fin than in the second dorsal fin (vs. equal in †Moldavigobius gen. nov.). The otoliths of Caffrogobius, Coryogalops and Hetereleotris are characterized by the presence of a marked posterodorsal projection (vs. absent in †Moldavigobius gen. nov.) (Fig. 4). It seems justified to assume that the presence of this projection is a derived character state for the Gobius lineage, because almost all its members have it, whereas it is lacking or at least much less developed in the other lineages of the Gobiidae (Gut et al. 2020; Schwarzhans et al. 2020a, b; unpublished data of BR). The otoliths of †Moldavigobius gen. nov. do not show this derived character state, and we, therefore, consider a close relationship of the new fossil taxon to the Gobius lineage to be unlikely.
Comparison to the Aphia lineage
A regular 10 + 17 vertebrae count occurs in both genera of the Aphia lineage (Aphia, Lesueurigobius) (Fig. 4). The number of the anal-fin rays in †Moldavigobius gen. nov. is at the lower limit of the corresponding counts in Aphia (11–15) and one ray less than the lower bound in Lesueurigobius (12–17) (Fig. 4). Both Aphia and Lesueurigobius can have one ray more in the anal fin than in the second dorsal fin, but the counts can also be the same (as in †Moldavigobius gen. nov.).
The otolith of Aphia is ovate and very different from the otolith of †Moldavigobius gen. nov. (Fig. 4). In contrast, the otoliths of Lesueurigobius resemble those of †Moldavigobius gen. nov. in their almost square shape and also in the length and inclination of the sulcus (Fig. 4). However, the cauda width is greater in Lesueurigobius than in †Moldavigobius gen. nov., while the dorsal lobe of the ostium is more pronounced in †Moldavigobius gen. nov. than in Lesueurigobius (Fig. 5). Further differences concern the overall otolith shape, which is characterized by the expanded, rounded posterodorsal region in both L. friesii and L. suerii, whereas the same region is angular and not expanded in †Moldavigobius gen. nov. Essentially, the otolith of †Moldavigobius gen. nov. gives the impression that it is ‘on the way’ to becoming a true Lesueurigobius otolith, i.e., is at a very early stage in such an evolutionary trajectory. Interestingly, Schwarzhans (2014) found that some otoliths of †M. suavis from the Karaman Basin are similar to juvenile otoliths of fossil Lesueurigobius from the same beds.
Otoliths of the extant gobiid Lesueurigobius Whitley (IRSNB, collection Chaine), and the extinct gobiid †Moldavigobius gen. nov. (PIN 1306/68a, PIN 1306/68c) with the contour of the sulcus highlighted. Otoliths of †Moldavigobius are mirrored for better comparison. All figures are SEM images and show the medial view
There are further similarities between †Moldavigobius gen. nov. and Lesueurigobius, e.g., in general body morphometry (see Supplementary Table in Reichenbacher and Bannikov 2022 for data on Lesueurigobius) and in the presence of large ctenoid scales. Nevertheless, †Moldavigobius gen. nov. is clearly not a member of the genus Lesueurigobius, because not only its otoliths, but also its fan-shaped caudal fin clearly differs from the lanceolate or longish caudal-fin shape of the extant species of Lesueurigobius (Maul 1971; Miller 1986). Taking all similarities and differences into account, †Moldavigobius gen. nov. may represent an ancient relative of Lesueurigobius and a possible member of the Aphia lineage.
Conclusions
The new gobioid genus and species †Moldavigobius helenae gen. et sp. nov. described in this paper represents a further contribution to the characterization of the unusually rich record of gobies recovered from the upper Serravalian deposits near Naslavcea in northern Moldova. Together with those reported in our previous study (Reichenbacher and Bannikov 2022), this new find is the fifth genus and the seventh species of gobioids from the locality, and their biodiversity at this site is far from being fully explored. †Moldavigobius helenae gen. et sp. nov. definitely belongs to the Gobiidae, and probably to the Aphia lineage.
Furthermore, the otoliths of †Moldavigobius gen. nov. revealed that an otolith-based species previously described as Knipowitschia suavis Schwarzhans, 2014 represents a second species of †Moldavigobius gen. nov. This is especially remarkable, because the extant genus Knipowitschia belongs to the family Oxudercidae (= Gobionellidae sensu Thacker 2013), whereas †Moldavigobius gen. nov. is an extinct genus of the Gobiidae. †Moldavigobius gen. nov. thus underlines the role of otoliths preserved in situ in elucidating the taxonomic diversity of ancient Gobioidei. Re-assignment of the ‘Knipowitschia’ suavis otoliths to the new genus also implies that the range of †Moldavigobius gen. nov. extended from the SE Mediterranean (presence of †M. suavis, see Schwarzhans 2014) to the western sector of the Eastern Paratethys (presence of †M. helenae gen. et sp. nov., this study) and the easternmost Eastern Paratethys (presence of †M. suavis, see Bratishko et al. 2015).
References
Agorreta, A., D. San Mauro, U. Schliewen, J.L. Van Tassell, M. Kovačić, R. Zardoya, and L. Rüber. 2013. Molecular phylogenetics of Gobioidei and phylogenetic placement of European gobies. Molecular Phylogenetics and Evolution 69 (3): 619–633. https://doi.org/10.1016/j.ympev.2013.07.017.
Akihito, M., T. Hayashi, K. Yoshino, T. Yamamoto. Shimada, and H. Senou. 1984. Suborder Gobioidei. In The fishes of the Japanese archipelago, ed. H. Masuda, K. Amaoka, C. Araga, T. Uyeno, and T. Yoshino, 236–289. Tokyo: Tokai University Press.
Allen, G.R., and M.P. Hammer. 2018. Grallenia larsonae, a new species of sandgoby (Pisces: Gobiidae) from northern Australia. Journal of the Ocean Science Foundation 31: 87–96.
Arratia, G. 1999. The monophyly of Teleostei and stem-group teleosts. Consensus and disagreements. In Mesozoic fishes 2 - Systematics and fossil record, ed. G. Arratia and H.-P. Schultze, 265–334. München: Verlag Dr. Friedrich Pfeil.
Bannikov, A.F. 2009. On early Sarmatian fishes from the Eastern Paratethys. Paleontological Journal 43 (5): 569–573. https://doi.org/10.1134/s003103010905013x.
Bannikov, A.F. 2010. Fossil vertebrates of Russia and adjacent countries. Fossil acanthopterygians fishes (Teleostei, Acanthopterygii). Moscow: GEOS.
Bannikov, A. F. 2017. New data on the distribution of the fossil gobiiform fishes in the Miocene of the Eastern Paratethys. In The 10th Indo-Pacific Fish Conference, Tahiti – 2–6 October 2017, Book of Abstracts, p. 138.
Bannikov, A. F. 2018. New data on the distribution of the gobiiform fishes (Gobioidei) in the Eastern Paratethys [Novye dannye o rasprostranenii bychkovidnykh ryb (Gobioidei) v Vostochnom Paratetise]. In Basic and Applied Paleontology. Materials of LXIV session of the Paleontological Society, 175–176. St. Petersburg: VSEGEI.
Betancur-R, R., E.O. Wiley, G. Arratia, A. Acero, N. Bailly, M. Miya, G. Lecointre, and G. Ortí. 2017. Phylogenetic classification of bony fishes. BMC Evolutionary Biology 17 (1): 162. https://doi.org/10.1186/s12862-017-0958-3.
Birdsong, R.S., E.O. Murdy, and F.L. Pezold. 1988. A study of the vertebral column and median fin osteology in gobioid fishes with comments on gobioid relationships. Bulletin of Marine Science 42 (2): 174–214.
Bratishko, A., W. Schwarzhans, B. Reichenbacher, Y. Vernyhorova, and S. Ćorić. 2015. Fish otoliths from the Konkian (Miocene, early Serravallian) of Mangyshlak (Kazakhstan): Testimony to an early endemic evolution in the Eastern Paratethys. Paläontologische Zeitschrift 89 (4): 839–889. https://doi.org/10.1007/s12542-015-0274-4.
Carnevale, G., A.F. Bannikov, W. Landini, and C. Sorbini. 2006. Volhynian (early Sarmatian sensu lato) fishes from Tsurevsky, North Caucasus, Russia. Journal of Paleontology 80 (4): 684–699. https://doi.org/10.1666/0022-3360(2006)80[684:vesslf]2.0.co;2.
Cuvier, G. 1816. Le Règne Animal distribué d'après son organisation pour servir de base à l'histoire naturelle des animaux et d'introduction à l'anatomie comparée. Tome II, Les reptiles, les poissons, les mollusques et les annélides. Paris: A. Belin.
Froese, R., and D. Pauly. 2022. FishBase. World Wide Web electronic publication. www.fishbase.org, version (12/2020).
Gierl, C., and B. Reichenbacher. 2015. A new fossil genus of Gobiiformes from the Miocene characterized by a mosaic set of characters. Copeia 103 (4): 792–805. https://doi.org/10.1643/ci-14-146.
Gierl, C., D. Liebl, R. Šanda, J. Vukić, H.R. Esmaeili, and B. Reichenbacher. 2018. What can goby otolith morphology tell us? Cybium 42 (4): 349–363. https://doi.org/10.26028/cybium/2018-424-006.
Gill, A.C., and R.D. Mooi. 2012. Thalasseleotrididae, new family of marine gobioid fishes from New Zealand and temperate Australia, with a revised definition of its sister taxon, the Gobiidae (Teleostei: Acanthomorpha). Zootaxa 3266 (1): 41–52. https://doi.org/10.11646/zootaxa.3266.1.3.
Gierl, C., M. Dohrmann, P. Keith, M. Humphreys, H.R. Esmaeili, J. Vukić, R. Šanda, and B. Reichenbacher. (2022). An integrative phylogenetic approach for inferring relationships of fossil gobioids (Teleostei: Gobiiformes). PLOS ONE 17(7), e0271121. https://doi.org/10.1371/journal.pone.0271121
Goren, M. 1996. A review of the southern African gobiid fish genus Caffrogobius Smitt, 1900. The J.L.B Smith Institute of Ichthyology Special Publication 57:1–28.
Günther, A. C. L. G. 1880. An introduction to the study of fishes. Edinburgh: Adam and Charles Black.
Gut, C., J. Vukić, R. Šanda, T. Moritz, and B. Reichenbacher. 2020. Identification of past and present gobies: Distinguishing Gobius and Pomatoschistus (Teleostei: Gobioidei) species using characters of otoliths, meristics and body morphometry. Contributions to Zoology 89: 282–323. https://doi.org/10.1163/18759866-bja10002.
Harrison, I.J. 1989. Specialization of the gobioid palatopterygoquadrate complex and its relevance to gobioid systematics. Journal of Natural History 23 (2): 325–353. https://doi.org/10.1080/00222938900770211.
Hoese, D.F. 1984. Gobioidei: relationships. In Ontogeny and systematics of fishes, ed. H.G. Moser, W.J. Richards, D.M. Cohen, M.P. Fahay, A.W. Kendall, and S.L. Richardson, 588–591. Gainesville, Florida: American Society of Ichthyologists and Herpetologists.
Hoese, D.F. 1986a. Descriptions of two new species of Hetereleotris (Pisces: Gobiidae) from the western Indian Ocean, with discussion of related species. The J.L.B. Smith Institute of Ichthyology Special Publication 41: 1–25.
Hoese, D.F., and G.R. Allen. 2009. Description of three new species of Glossogobius from Australia and New Guinea. Zootaxa 1981: 1–14.
Hoese, D.F., and A.C. Gill. 1993. Phylogenetic relationships of eleotridid fishes (Perciformes, Gobioidei). Bulletin of Marine Science 52 (1): 415–440.
Hoese, D.F., and H.K. Larson. 2005. Description of two new species of Hetereleotris (Gobiidae) from the south Pacific, with a revised key to species and synonymization of the genus Pascua with Hetereleotris. Zootaxa 1096: 1–16.
Hoese, D.F., R.K. Hadiaty, and F. Herder. 2015. Review of the dwarf Glossogobius lacking head pores from the Malili lakes, Sulawesi, with a discussion of the definition of the genus. Raffles Bulletin of Zoology 63: 14–26.
Hoese, D. F. 1986b. Family No. 240: Gobiidae. In Smiths' Sea Fishes, eds. M. M. Smith, and P. C. Heemstra, 774–807. Johannesburg: Macmillan South Africa.
Iglésias, S.P., J. Vukić, D.Y. Sellos, T. Soukupová, and R. Šanda. 2021. Gobius xoriguer, a new offshore Mediterranean goby (Gobiidae), and phylogenetic relationships within the genus Gobius. Ichthyological Research. https://doi.org/10.1007/s10228-020-00797-9.
Iljin, B.S. 1927. Keys to the gobies (Fam. Gobiidae) of the Sea of Azov and the Black Sea. Preliminary Communication. Trudy Azovsko-Černomorskoj Naučno-Promyslovoj Ékspedicii 2: 128–143.
Ionko, V.I. 1954. O nakhodke iskopayemykh ryb v nizhnesarmatskikh otlozheniyakh MSSR. Trudy Odesskogo Universiteta. Sbornik Geologo-Geograficheskogo Fakul’teta 2: 109–119.
Jordan, D.S., and B.W. Evermann. 1896. The fishes of North and Middle America: A descriptive catalogue of the species of fish-like vertebrates found in the waters of North America, North of the Isthmus of Panama Part I. Bulletin of the United States National Museum 47: 1–1240.
Kessler, K. F. 1877. The Aralo-Caspian Expedition. IV. Fishes of the Aralo-Caspio-Pontine ichthyological region. St. Petersburg.
Kovačić, M. 2020. Checklist of gobies (Teleostei: Gobiidae) of the Mediterranean Sea and a key for species identification. Zootaxa 4877 (1): 75–101. https://doi.org/10.11646/zootaxa.4877.1.3.
Kovačić, M., and S.V. Bogorodsky. 2014. A new species of Hetereleotris (Perciformes: Gobiidae) from the Red Sea. Zootaxa 3764 (4): 475–481. https://doi.org/10.11646/zootaxa.3764.4.7.
Kovačić, M., S.V. Bogorodsky, and A.O. Mal. 2014. Two new species of Coryogalops (Perciformes: Gobiidae) from the Red Sea. Zootaxa 3881 (6): 513–531. https://doi.org/10.11646/zootaxa.3881.6.2.
Liu, H.T.H., H. Ahnelt, G.A.C. Balma, and G.B. Delmastro. 2009. First record of Gobius roulei (Gobiidae) in the Ligurian Sea. Cybium 33 (3): 253–254.
Lombarte, A., M. Miletić, M. Kovačić, J.L. Otero-Ferrer, and V.M. Tuset. 2018. Identifying sagittal otoliths of Mediterranean Sea gobies: Variability among phylogenetic lineages. Journal of Fish Biology 92 (6): 1768–1787. https://doi.org/10.1111/jfb.13615.
Maul, G.E. 1971. On a new goby of the genus Lesueurigobius from off the Atlantic coast of Morocco and Madeira (Percomorphi, Gobioidea, Gobiidae). Bocagiana 29: 1–7.
Miller, P.J. 1986. Gobiidae. In Fishes of the north-eastern Atlantic and the Mediterranean (FNAM), ed. P.J.P. Whitehead, M.-L. Bauchot, J.-C. Hureau, J. Nielsen, and E. Tortonese, 1019–1085. Paris: UNESCO.
Miller, P.J., and S. Stefanni. 2001. The Eastern Pacific species of Bathygobius (Perciformes: Gobiidae). Revista De Biología Tropical 49: 141–156.
Miller, P. J. 2004. The freshwater fishes of Europe. Vol. 8/II. Gobiidae 2. Wiebelsheim: AULA-Verlag.
Müller, J. 1845. Über den Bau und die Grenzen der Ganoiden, und über das natürliche System der Fische. Physikalisch-Mathematische Abhandlungen Der Königlichen Akademie Der Wissenschaften Zu Berlin 1845: 117–216.
Nelson, J.S., T.C. Grande, and M.V.H. Wilson. 2016. Fishes of the World, 5th ed. Hoboken: Wiley.
Penk, S.B.R., M. Altner, A.F. Cerwenka, U.K. Schliewen, and B. Reichenbacher. 2019. New fossil cichlid from the middle Miocene of East Africa revealed as oldest known member of the Oreochromini. Scientific Reports 9 (10198): 1–24. https://doi.org/10.1038/s41598-019-46392-5.
Pezold, F. 2004. Phylogenetic analysis of the genus Gobionellus (Teleostei: Gobiidae). Copeia 2004 (2): 260–280. https://doi.org/10.1643/CI-02-218R3.
Reichenbacher, B., and A.F. Bannikov. 2022. Diversity of gobioid fishes in the late middle Miocene of northern Moldova, Eastern Paratethys – part I: An extinct clade of Lesueurigobius look-alikes. PalZ 96: 67–112. https://doi.org/10.1007/s12542-021-00573-8.
Reichenbacher, B., R. Gregorová, K. Holcová, R. Šanda, J. Vukić, and T. Přikryl. 2018. Discovery of the oldest Gobius (Teleostei, Gobiiformes) from a marine ecosystem of Early Miocene age. Journal of Systematic Palaeontology 16 (6): 493–513. https://doi.org/10.1080/14772019.2017.1313323.
Reichenbacher, B., S. Filipescu, and A. Miclea. 2019. A unique middle Miocene (Sarmatian) fish fauna from coastal deposits in the eastern Pannonian Basin (Romania). Palaeobiodiversity and Palaeoenvironments 99 (2): 177–194. https://doi.org/10.1007/s12549-018-0334-3.
Sauvage, M.H.E. 1875. Notes sur les poissons fossiles. Bulletin De La Société Géologique De France 3 (3): 631–642.
Schneider, C.A., W.S. Rasband, and K.W. Eliceiri. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9 (7): 671–675. https://doi.org/10.1038/nmeth.2089.
Schwarzhans, W. 2014. Otoliths from the middle Miocene (Serravallian) of the Karaman Basin, Turkey. Cainozoic Research 14 (1): 35–69.
Schwarzhans, W. 2017. A review of otoliths collected by W. Weiler from the Badenian of Romania and by B. Strashimirov from Badenian equivalents of Bulgaria. Cainozoic Research 17 (2): 167–191.
Schwarzhans, W., K. Bradić, and L. Rundić. 2015. Fish-otoliths from the marine-brackish water transition from the Middle Miocene of the Belgrade area, Serbia. Paläontologische Zeitschrift 89 (4): 815–837. https://doi.org/10.1007/s12542-015-0272-6.
Schwarzhans, W., H. Ahnelt, G. Carnevale, S. Japundžić, K. Bradić, and A. Bratishko. 2017. Otoliths in situ from Sarmatian (Middle Miocene) fishes of the Paratethys. Part III: tales from the cradle of the Ponto-Caspian gobies. Swiss Journal of Palaeontology 136 (1): 45–92. https://doi.org/10.1007/s13358-016-0120-7.
Schwarzhans, W., K. Agiadi, and G. Carnevale. 2020a. Late Miocene-Early Pliocene evolution of Mediterranean gobies and their environmental and biogeographic significance. Rivista Italiana Di Paleontologia e Stratigrafia 126 (3): 657–724.
Schwarzhans, W., R. Brzobohatý, and U. Radwańska. 2020b. Goby otoliths from the Badenian (middle Miocene) of the Central Paratethys from the Czech Republic, Slovakia and Poland: A baseline for the evolution of the European Gobiidae (Gobiiformes; Teleostei). Bollettino Della Società Paleontologica Italiana 59 (2): 125–173. https://doi.org/10.4435/bspi.2020.10.
Shibukawa, K., and A. Iwata. 2007. Grallenia, a new goby genus from the western Pacific, with descriptions of two new species (Perciformes: Gobiidae: Gobiinae). Bulletin of the National Museum of Nature and Science Series a, Supplement 1: 123–136.
Smale, M. J., G. Watson, and T. Hecht. 1995. Otolith atlas of southern African marine fishes. In Ichthyological Monographs. Grahamstown: J. L. B. Smith Institute of Ichthyology.
Thacker, C.E. 2013. Phylogenetic placement of the European sand gobies in Gobionellidae and characterization of gobionellid lineages (Gobiiformes: Gobioidei). Zootaxa 3619 (3): 369–382.
Thacker, C.E. 2015. Biogeography of goby lineages (Gobiiformes: Gobioidei): Origin, invasions and extinction throughout the Cenozoic. Journal of Biogeography 42 (9): 1615–1625. https://doi.org/10.1111/jbi.12545.
Thacker, C.E., T.P. Satoh, E. Katayama, R.C. Harrington, R.I. Eytan, and T.J. Near. 2015. Molecular phylogeny of Percomorpha resolves Trichonotus as the sister lineage to Gobioidei (Teleostei: Gobiiformes) and confirms the polyphyly of Trachinoidei. Molecular Phylogenetics and Evolution 93: 172–179. https://doi.org/10.1016/j.ympev.2015.08.001.
Tornabene, L., C.C. Baldwin, L.A. Weigt, and F.L. Pezold. 2010. Exploring the diversity of western Atlantic Bathygobius (Teleostei: Gobiidae) with cytochrome c oxidase-I, with descriptions of two new species. Aqua, International Journal of Ichthyology 16 (4): 141–170.
Wang, H.-Y., M.-P. Tsai, J. Dean, and S.-C. Lee. 2001. Molecular phylogeny of gobioid fishes (Perciformes: Gobioidei) based on mitochondrial 12S rRNA sequences. Molecular Phylogenetics and Evolution 20 (3): 390–408. https://doi.org/10.1006/mpev.2001.0957.
Whitley, G.P. 1950. New fish names. Proceedings of the Royal Society of New South Wales 1948–49: 44.
Więcaszek, B., A. Nowosielski, J. Dąbrowski, K. Górecka, S. Keszka, and A. Strzelczak. 2020. Fish size effect on sagittal otolith outer shape variability in round goby Neogobius melanostomus (Pallas 1814). Journal of Fish Biology 97 (5): 1520–1541. https://doi.org/10.1111/jfb.14521.
Acknowledgements
We are grateful to Ulrich Schliewen for constructive comments on character distribution in Gobiidae and Oxudercidae. We thank Dr. Helen Larson (Darwin, Australia) and Dr. Doug Hoese (Sydney, Australia) for providing helpful comments and sharing literature that was difficult to find. We also thank Radek Šanda (National Museum Praha, Czech Republic) and Jasna Vukić (Charles University, Praha, Czech Republic) for access to extant comparative material of the genus Knipowitschia. We are grateful to Hamid Reza Esmaeili and Reza Sadeghi (both Shiraz University) for providing the SEM image of Coryogalops tessellatus and we acknowledge Dirk Nolf and Kristiaan Hoedemakers (both Royal Institute of Natural Sciences Belgium, Brussels) for providing the SEM images of Lesueurigobius friesii and L. suerii otoliths from the collection in their care. The photographs of the skeletal-based material shown in Fig. 1 are courtesy of Mr. Sergey V. Bagirov (PIN, Moscow, Russia). Finally, we thank the two reviewers for their constructive comments and Dr. Paul Hardy (Düsseldorf, Germany) for critical reading of the manuscript. The authors have no conflict of interest to declare.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jürgen Kriwet.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reichenbacher, B., Bannikov, A.F. Diversity of gobioid fishes in the late middle Miocene of northern Moldova, Eastern Paratethys—Part II: description of †Moldavigobius helenae gen. et sp. nov.. PalZ 97, 365–381 (2023). https://doi.org/10.1007/s12542-022-00639-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-022-00639-1