Abstract
The Listriodontinae were a common and widespread group of Suidae (pigs) that lived in an area extending from Portugal to China and to southern Africa. Here, we describe the new species Listriodon dukkar from Pasuda (Gujarat, India). It shares features with Li. pentapotamiae, evolved from it, and is the last representative of this lineage. The Listriodontinae flourished for about 10 million years, reached their maximum diversity and geographic extension during the Mid-Miocene Climatic Optimum (about 17–13.6 Ma), and their last records are close in age and date to ~ 9.8 Ma in the Indian Subcontinent, 9.78 Ma in Europe, and ~ 10 Ma in Africa. We review the environments in which the last listriodont lineages lived and went extinct. Their extinctions occurred against a background of increasing seasonality, vegetation change, a rise in bovid diversity and abundance, and local events, such as the European Vallesian Crisis and a dramatic drop in tragulid abundance in the Siwaliks. However, changes in the atmospheric pCO2 may have contributed to their decline and extinction in all their geographic distribution. Decreasing pCO2 is expected to have decreased sugar content and increased protein content of leaves and fruit. Hindgut fermenting Suoidea have higher protein requirements, while foregut fermenting Suoidea are more efficient in digesting sugars. Listriodontinae were probably foregut fermenters and were less well adapted in a low pCO2 world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Listriodontinae (Suidae, Suoidea) were a group of pigs that was common and widespread during the Early and Middle Miocene of the Old World (Van der Made 1996a; Pickford and Morales 2003; Orliac 2007, 2009) and that formed an important part of the ecosystems of their time. Within this group, various lineages evolved towards lophodonty (teeth with transverse crests), a convergence with the tapirs, with which initially they were confused (Nicolet 1844; von Meyer 1846; Lydekker 1876; Rusconi 1937). Tapirs are folivores and frugivores (Wilson and Mittermeier 2011) and a folivorous diet has been inferred for these pigs, as well (Leinders 1977a, b; Hunter and Fortelius 1994).
Here, we describe a new species of Listriodon from Pasuda (Kutch, India), a suoid species in which not only the molars were perfectly lophodont, but also the premolars. It is more derived than the Indian Li. pentapotamiae, with which it shares features and from which it evolved. This suggests that it is the last listriodont species from the Indian Subcontinent, which is in line with earlier interpretation of the age of Pasuda as early Late Miocene (Bhandari et al. 2015).
The Listriodontinae were widely distributed in Europe, the Indian Subcontinent, Asia, and Africa (Fig. 1) covering latitudes from 0 to about 50º. They reached their highest diversity during the Middle Miocene Climatic Optimum, after which they started to decline everywhere. This contrasts with the latitudinal gradient in extinction seen in most Suoidea, like the Tetraconodontinae (Van der Made 1992, 1999) and Hyotheriinae (Van der Made 2010). This synchronous decline of the Listriodontinae living in areas from the tropics to temperate climates suggests a global cause. Their decline started with the end of the Mid-Miocene Climatic Optimum at 13.6 Ma and their extinction coincided with an event in Europe that was baptised as the mid-Vallesian Crisis, or shorter Vallesian Crisis, that also marked the local extinction of the primitive Hominoidea (Agustí and Moyà Solà 1990; Moyà Solà and Agustí 1990).
Geographic distribution of Listriodon dukkar, Li. pentapotamiae, Listriodon sp. (either Li. dukkar or Li. pentapotamiae), Li. splendens, and Lopholistriodon (mainly after Van der Made, 1996a, b). In some cases, one dot is a cluster of localities: Gaj River indicates 14 HGSP localities and Chinji for 18 localities (IVAU and BSP collections)
This paper aims to describe the new species of Listriodon, to interpret the environment in which the Indian Listriodon lineage lived, and to discuss the possible reasons for decline and extinction of the Listriodontinae.
Geological background and antecedents on Pasuda
The lithostratigraphic sequence of Kutch basin is known to have lithological exposures with fossil fauna spanning from the Mesozoic to Pleistocene (Biswas 1992) (Fig. 2a, b). The Neogene deposits of the Kutch basins are classified into three formations as Khari Nadi, Chhasra, and Sandhan formations (Biswas 1992). The Oligocene Maniyara Fort Formation is overlain by the Khari Nadi Formation with a weak erosional unconformity due to a transgressive overlap (Catuneanu and Dave 2017). The Khari Nadi Formation comprises siltstone, sandstone, and clay, and is dated as early Miocene (Aquitanian: 23.03–20.43 ± 0.05 Ma; Hilgen et al., 2012). The uppermost part of this formation, with numerous bioturbation structures and pseudoconglomeratic beds on top, yields certain mammalian remains (Patnaik et al. 2014). The contact with the Chhasra Formation is conformable and gradational. This formation consists of grey to khaki green, laminated shale, siltstone, and argillaceous limestone at the lower part, and predominant siltstone and laminated silty shale at the upper part (Biswas 1992). The Chhasra Formation, dated as early Miocene (Burdigalian), has a disconformable contact with the younger Sandhan Formation (Biswas 1992). The Sandhan Formation is characterised by the presence of medium-to-coarse grain micaceous sandstone, quartzose sandstone, clay, siltstone, sandstone, and conglomerate. The coarse-grained sandstone consists of pebbles and concretionary nodules, and the conglomeratic beds have yielded both marine and terrestrial vertebrate remains (Bhandari et al. 2015; Singh et al. 2019; Sharma et al. 2021).
Geographic and stratigraphic position of the find. a Map showing the position of the study area within Kutch, Gujarat, India. b Geological map of the Kutch area with the fossil locality indicated by star mark. c Stratigraphic column of the Pasuda locality. d The site where the fossil of Listriodon dukkar sp. nov. was recovered
The present Listriodon specimen was recovered from the fossiliferous deposits exposed at Pasuda section (N23°14′27.81′′ and E70°11′00.59′′) situated about 1 km west of the Pasuda main road. Pasuda is a small village, situated approximately 65 km east of district Headquarter, Bhuj, of Kutch District of Gujarat, India. The lithostratigraphic section of the Pasuda locality comprises massive sand, silt, clay, and conglomerate beds (Fig. 2c, d). The latter contain calcareous nodules, agate pebbles, very coarse sand, and mudclasts, and has occasionally a coarsening upward sequence, suggesting a crevasse splay deposit (Bhandari et al. 2015). The conglomerate layer consisting of mud pebbles, nodules, and calcareous concretions yielded a rich assemblage of micro- and mega vertebrate fossils. Lithologically, these beds are different from Khari Nadi and Chhasra as the beds show abundant coarse-grained sandstone with pebbly clasts, calcareous nodules, agate pebbles, mud clast, and conglomerate, and show close similarity to the Sandhan Formation (Biswas 1992; Catuneanu and Dave 2017). The Tapar section, exposed nearly 2 km west of Pasuda, has a similar lithological and faunal composition and notably yielded the hominoid, Sivapithecus. Based on the presence and First Appearance Datum (FAD) of Hipparion, as well as on the associated fauna, Bhandari et al. (2015) proposed a Late Miocene (11–10 Ma) age for fossiliferous sites of Pasuda and Tapar. The Pasuda site is also known for mammalian fauna including murid rodents, Giraffokeryx punjabiensis, Giraffa priscilla (Bhandari et al. 2015) (see Table 1).
Materials and methods
The fossil from Pasuda described here (PUKP-1) is kept in the Centre of Advanced study in Geology, Panjab University, Chandigarh. This fossil has been compared with other fossils indicated in Table 2 and those described and figured by Van der Made (1996a), as well as many other specimens of Li. pentapotamiae housed at the Indian Museum, Kolkata.
The methods of study and tooth nomenclature follow Van der Made (1996a). This nomenclature is applicable to different groups of Artiodactyla, including Suoidea, Anthracotheriidae, and ruminants (which is better than having different nomenclatures for each of these groups). Molar cusps tend to have lobes or crests, usually a maximum of four, though not all are present in all taxonomic groups. These are called pre-, endo-, post-, and ectocrista or—cristid, depending on whether they originate on the anterior, interior, posterior, or exterior side of the tooth. Secondary cusplets or styles may be formed as part of these crests. The names are constructed in a logical way from general to particular: first comes the name of the cusp, then the position of the crest, secondary cusplet, or style. For example: protoprecrista, hypopreconulid. The premolars are simpler, but the nomenclature is the same (Fig. 3).
The age of many localities is estimated using magnetostratigraphy and interpolation between the boundaries of the chrons. Dating of the Geomagnetic Polarity Time Scale (GPTS) has improved and the changes are particularly notable in the time period that is of interest here. For instance, the upper boundary of the long chron C5n.2n has varied: 8.87, 8.92, 9.92, 9.987, and 9.984 Ma (La Breque et al. 1977; Berggren et al. 1985; Cande and Kent 1995; Lourens et al. 2004; Hilgen et al. 2012). The Siwalik sequence is a classic example of a long sedimentary sequence dated by magnetostratigraphy (Opdyke et al. 1979; Johnson et al. 1982; Tauxe and Opdyke 1982). The ages given for faunal events in the Siwaliks (e.g., Barry et al. 1982, 1995, 2002; Barry and Flynn 1990) and elsewhere have been recalculated and are given, save in the cases where the differences are minimal. Here, we use the MN units (Mein 1975; De Bruijn et al. 1992) and a local Spanish biozonation (Daams and Freudenthal 1981; Van Dam et al. 1997) for European biostratigraphy. Comparisons of magnetostratigraphically dated micromammal localities (Kälin and Kempf 2009; Van der Meulen et al. 2012) and Suoidea (Van der Made 2020) show the MN units to be diachronous. Recent dates for the MN units are provided by Hilgen et al. (2012), but see also Van der Made (2020).
Systematic palaeontology
The Listriodontinae are a group of Suidae which evolved wide and low incisors and which had a tendency to evolve from bunodont to sublophodont (e.g., species of Bunolistriodon and the African Lopholistriodon) to lophodont (Listriodon and species of Lopholistriodon). In Listriodon, there are two lineages: Li. pentapotamiae in the Indian Subcontinent and Li. splendens in an area that extended from Europe to Anatolia and China. Falconer (1868) named Li. pentapotamiae, thinking it was a tapir. Lydekker (1878) named Li. theobaldi, but Pickford (1988) considered it to be synonym of Li. pentapotamiae. The type specimen of Li. theobaldi comes from Hasnot. Barry et al. (1982) indicated the temporal range of Listriodon in the magnetostratigraphic Hasnot section (about 11.5–10.5 Ma), the last occurrence coinciding with the first of Hipparion in locality Y454. Van der Made (1996a) applied the name Li. pentapotamiae theobaldi to the more evolved and younger fossils and L. p. pentapotamiae to the older and more primitive ones. For the classification of the Listriodontinae, Van der Made (1996a, b, 2020) is followed here, though alternative classifications exist (Pickford and Morales 2003; Orliac 2007, 2009).
Some lineages of the Palaeochoeridae (or “Old World peccaries), a primitive group of Suoidea, also evolved towards lophodonty, such as Yunnanochoerus and a genus that was called Schizochoerus. The latter name is a junior homonym of a tapeworm and now is called Schizoporcus (Van der Made 2010). Schizochoerus has been reported from the Siwaliks, but the material more likely represents Yunnanochoerus (Van der Made 1997a; Pickford 2017). For the Palaeochoeridae, the classification by Van der Made (2010) is followed here. Lately, a different classification has been proposed (Pickford 2017), but see discussion by Van der Made (2020).
Institutional abbreviations. CASGPU—Centre of Advanced Study in Geology, Panjab University, Chandigarh, India; CJFV—Collection J. F. de Villalta, Barcelona, Spain; FISF—Forschungs-Institut Senckenberg, Frankfurt, Germany; FMNH—Finnish Museum of Natural History, Helsinki, Finland; GML—Geological Museum, Lisbon, Portugal; GSP—Geological Survey of Pakistan, Islamabad, Pakistan; HGSP—Howard University—Geological Survey of Pakistan; IM—Indian Museum, Kolkata, India; IPS—Institut Català de Paleontologia Miquel Crusafont, Sabadell; IVAU—Instituut Voor Aardwetenschappen Utrecht, The Netherlands; IVPP—Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; KNM—Kenya National Museums, Nairobi, Kenya; MHNCF—Musée d'Histoire Naturelle, La Chaux-de-Fonds, Switzerland; MLGSB—Museu i Laboratori de Geologia del Seminari, Barcelona, Spain; MNCN—Museo Nacional de Ciencias Naturale, Madrid, Spain; MNHN—Muséum national d’Histoire naturelle, Paris, France; MTA—Maden Tetkik ve Arama, Ankara, Turkey; NMB—Naturhistorisches Museum, Basel; Switzerland; NWW—Naturhistorisches Museum Wien, Vienna; Austria; PDTCFAU—Paleoantropoloji, Dil ve Tarih Cografya Facultesi, Ankara Universitesi, Ankara, Turkey; PIMUZ—Paläontologisches Institut und Museum der Universität, Zürich, Switzerland; SMNS—Staatliches Museum für Naturkunde, Stuttgart, Germany; SNSB-BSPG—Bayerische Staatssammlung für Paläontologie und Geologie, München, Germany; UPM—Laboratoire de sédimentologie et paléontologie, Université de Provence, Marseille, France.
Anatomical abbreviations. In addition to the common ones: Cm and Cf for the upper canines of males and females.
Superfamily Suoidea Gray, 1821
Family Suidae Gray, 1821
Subfamily Listriodontinae Gervais, 1859
Tribe Listriodontini Gervais, 1859
Listriodon von Meyer, 1846
Type species. Listriodon splendens von Meyer, 1846.
Diagnosis. Lophodont Listriodontinae with high crowned Cm that curve outwards and upwards (after Van der Made, 1996a, b).
Included species. L. splendens and L. pentapotamiae.
Listriodon dukkar sp. nov.
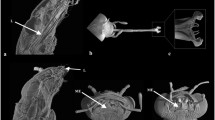
Holotype. PUKP-1—left maxilla with P3 till M3 (Fig. 4).
Type locality. Pasuda, Gujarat, India.
Age of the type locality. early Late Miocene (probably a little less than 10 Ma).
Zoobank LSID. urn:lsid:zoobank.org:act:03E63D53-F626-4A75-B671-3E3CFA0B43D9.
Diagnosis. species of Listriodon with wide P3−4 with perfect lophs, formed by the protocone and paracone, and with the posterior part of the crowns shortened as a result of the reduction of the metacone (in the P4), parapostcrista and protopostcrista.
Differential diagnosis. Listriodon dukkar differs from L. pentapotamiae in the P3 having a large protocone and well-developed protolophs and in the P3−4 having shorter metapostcristae and in being shorter and relatively wide. It differs from L. splendens in the P3 having a large protocone and well-developed protoloph and shorter metapostcrista and in the P4 lacking completely a metacone, having a more reduced protopostcrista, in the protocone being placed more anteriorly, forming a more perfect protoloph, and in being smaller. It differs from Lopholistriodon in having less elongate M1−2, less-developed cingula in the P3−4, in the P3 having a larger protocone and better developed protoloph, and in the P3−4 having more reduced parapostcristae and in being wider. It differs from Lo. kidogosana in being larger. Listriodon dukkar differs from Schizoporcus in having Mx with a smooth metaloph, without a tetrapreconule protruding from it or a furrow in the middle of its distal side, in the M1−2 being less elongate, in the P3−4 in having a well-developed protoloph and being wider, and in having a palatine groove that does not extend as far foreward as the premolars. It differs from S. sinapensis in being larger.
Etymology. The species name “dukkar” is from the common Gujarati name for pig or boar.
Description and comparison
Upper molars. The upper molars (Fig. 4) have two transverse crests; they are lophodont. Lophodont molars evolved in different mammals, such as Deinotherium (Proboscidea) and Pyrotherium (Pyrotheria), which were the size of elephants, and Tapiridae (Perissodactyla) and Suoidea (Artiodactyla), with sizes comparable to the fossils from Pasuda. The anterior cingulum of the upper molars of a tapir forms a cusp at the buccal side and there are small, but clear, crests on the anterior and posterior sides of the buccal cusps. These features are not present on the molars from Pasuda. Suoidea include fully lophodont species of the genera Listriodon and Lopholistriodon, sublophodont species of Bunolistriodon and Lopholistriodon spp. (all Suidae) and the lophodont Schizoporcus and Yunnanochoerus (Palaeochoeridae).
Fortelius et al. (1996) defined sublophodont as the state when the proximal pair of cusps of a molar forms a loph, but the distal cusps remain entirely or partially separate. If these cusps remain separate, there tends to be a sharp furrow marking the place where the two cusps meet and the wear facets and the dentine islands on the tips remain separate until wear is much advanced. Sublophodont Suoidea may retain a central cusp (tetrapreconule) placed behind the middle of the transverse valley (Bunolistriodon), which may have the posterior loph not straight, but with an angle in the middle, where the tetrapreconule protrudes forwards (Schizoporcus, Yunnanochoerus). The molars from the specimen from Pasuda are much worn, but it can be seen that the anterior sides of the metalophs are smooth and that there is no indication of a cusplet or crest (tetrapreconule) as in Schizoporcus and there is no furrow at the posterior side of the metaloph of the M2. The molars are fully lophodont as in Listriodon and some species of Lopholistriodon.
The molars of Schizoporcus are known to be elongate with wide transverse valleys. To some extent, this is obscured by the fact that molars that are more worn also tend to be shorter because of interproximal wear, which affects their proportions. Nevertheless, it is evident that the molars from Pasuda and of Listriodon are less elongate than those of Schizoporcus and also Lopholistriodon. The lines in Fig. 5 indicate the average length–width proportions of the different samples. The M3 of Yunnanochoerus has a very narrow second loph, which is not the case in Pasuda. The molars from Pasuda are comparable in size to Li. pentapotamiae, but tend to be smaller than those of Li. splendens, while they are larger than those of Yunnanochoerus lufengensis, S. sinapensis, and Lo. kidogosana (Fig. 5).
Bivariate diagrams the DAP (antero-posterior diameter), DT (transverse diameter), and DTa (DT of the anterior lobe) of the upper cheek teeth of selected Suoidea. Provenance of data as indicated in Table 1
Palate. What is left of the palate (Fig. 4) shows that there is no deep palatine groove next to the premolars and molars, which is present in Schizoporcus. This groove is less marked and extends far less anteriorly in the Suidae.
Fourth upper premolar. The P4 (Fig. 6b) has one buccal cusp, the paracone, which is connected by a loph with the protocone. Both cusps are about equally high and placed equally far forwards. The parapostcrista is reduced to a small ridge on the back of the paracone and the metacone is completely reduced. The protopostcrista is reduced to a low crest on the back of the protocone. In fact, the tooth consists of a protoloph and little more.
The premolars of selected Suoidea compared: a KNM BN992—right P3−4 of Lopholistriodon kidogosana from locality 2/10 in member 10 of the Ngorora Formation: (1) buccal and (2) occlusal views. b PUKP-1—Listriodon dukkar sp. nov. from Pasuda: (1) occlusal view of P3−4, (2) buccal view of P3−4, (3) anterior view of P3, (4) posterior view of P3, and (5) posterior view of P4. c BSP 1956II119—left P3 of Li. pentapotamiae: (1) lingual, (2) occlusal, (3) buccal, and (4) posterior views. d MNHN 3337—S. vallesiensis from the Middle Sinap Formation: (1) occlusal view of left P3−4 and (2) lingual view of P3. e IPS no number—left P2−4 of Li. splendens from Castell de Barberà: (1) lingual, (2) occlusal, and (3) buccal views, 4) anterior view of the P3, and (5) posterior view of the P4. f KNM FT3319—right P4 of Lo. akatidogus from Fort Ternan: (1) buccal and (2) occlusal views. g KNM FT3320—right P4 of Lo. akatidogus from Fort Ternan: (1) occlusal and (2) buccal views. h BSP-1956II115—left P4 of Li. pentapotamiae from Kanatti Chak 5, Chinji Formation: (1) occlusal, (2) buccal, and (3) anterior views. i MTA 1953—left P3 of Schizoporcus sinapensis from the Lower Sinap Formation: occlusal view. j IM B698—left P4 of Li. pentapotamiae: (1) lingual, (2) occlusal, (3) buccal, and (4) anterior views. k MNHN no number—left P3 of Li. splendens from the Lower Sinap Formation: lingual view
In Li. splendens, the metacone is placed more backwards at a level between the protocone and metacone, the protoprecrista curves towards the paracone, and the large protopostcrista curves towards the middle of the posterior cingulum (Fig. 6e). These crests, together with the paracone and metacone, form a longitudinal valley, very unlike in the specimen from Pasuda. There is a distinct metacone, separated on the lingual side from the paracone by a clear furrow. There is no evolutionary tendency to reduce the metacone; instead, it may become more separate from the paracone (Fig. 6e3), which seems to be a tendency towards a molarized P4 with two lophs, but this morphology was never reached and the longitudinal valley remains open posteriorly (Fig. 6e5). This tendency in the P4 is related to the trend in the P4 that the hypoconid becomes higher and placed more buccally and with a better developed hypoendocristid, which is a step in the direction of the formation of a hypolophid. The beginning hypolophid occludes with the cleft between the parapostcrista and metaprecrista of the P4. The P4 from Pasuda became reduced to one loph, suggesting that the hypoconid on the P4 became reduced.
The earliest Li. pentapotamiae have a morphology, which is still close to that of Li. splendens (Fig. 6h), but in the course of evolution, the metacone became reduced, and the protocone moved forwards and formed a transverse protoloph with the paracone (Fig. 6j). The protopostcrista also became reduced. This morphology is consistent with the P4 of this species having less well-developed hypoendocristids than Li. splendens. The P4 in Li. pentapotamiae shows an evolution towards the morphology seen in the P4 from Pasuda.
The P4 of Schizoporcus has the protoprecrista directed anteriorly, ending low near the middle of the anterior cingulum (Fig. 6d1). There is no connection to the paracone and no protoloph is formed. The longitudinal valley is well developed and wide open at the anterior side.
The P4 of Lo. akatidogus may have a well-developed distinct metacone (the tip of protocone in Fig. 6g is damaged) or no metacone at all (Fig. 6f). The latter morphology is close to that of Pasuda, but the protoloph is less well formed and the protopostcrista is strongly developed.
In Lo. kidogosana, the P4 has a well-developed protoloph and much reduced metacone (Fig. 6a). This morphology is close to that of the specimen from Pasuda, but the anterior cingulum is much more developed.
The P4 from Pasuda has a longitudinal fracture in the middle, which is about 1 mm wide at the base and which becomes narrower till the tip. This fracture increased the width (measured at the base), probably by around 1 mm. In Fig. 5, both measured and corrected widths are indicated. The P4 is among the widest (relative to the length) compared to Listriodon and is clearly much wider than in Schizoporcus and Lopholistriodon. This great width is probably related to the extreme development of the loph (increasing the width) and the complete reduction of the metacone (decreasing the length). The specimen from Pasuda is a little smaller than those of Li. pentapotamiae and clearly smaller than those of Li. splendens.
Third upper premolar. The P3 from Pasuda (Fig. 6b) has a morphology, which is essentially identical to that of the P4.
In Li. splendens (Fig. 6e), the P2−4 form a morphological cline and the protocone is smaller in the anterior premolars. However, there is an evolutionary tendency of this cusp to become larger and placed more forwards, so that the P3 becomes more similar to the P4. There is also a tendency for the metacone to become better developed in the P3, and there is even a metaendocrista, which would be a step in the direction of a posterior loph. However, primitive morphologies continue to occur (compare Fig. 6k with no distinct metacone and Fig. 6e). The development of an individual metacone has its counterpart in the development of a hypoconid with a morphology as in the P4 (Van der Made 1996a, Pl. 42, fig. 2). Even in the most evolved P3, the longitudinal valley still opens anteriorly (Fig. 6e4). The different characters of the P3 of Li. splendens are at a more primitive state than in the P4, but evolve in the same way, which is away from the morphology seen in the specimen from Pasuda.
In Li. pentapotamiae (Fig. 6c), the P3 is very similar to that of the primitive and earlier Li. splendens. The evolutionary change of the protocone in Li. pentapotamiae has not been documented in detail, but it seems that in the later Li. pentapotamiae, the protoprecrista is somewhat more developed and more directed towards the paracone, which would be a first step in the direction of the formation of a protoloph as seen in Pasuda. Li. pentapotamiae does not have a tendency to develop a separate metacone in the P3 nor to develop a large hypoconid in the P3.
In Lo. kidogosana (Fig. 6a), the paracone seems to have shifted towards the middle of the tooth with its protoprecrista and protopostcrista forming a crest that rotated from an antero-posterior to an oblique orientation. Also, the protocone shifted to a more anterior position and rotated its axis. These are steps in the direction of a transverse loph, but they differ in an essential way from the configuration in Pasuda, where the paracone is at the extreme buccal side. Another difference is in the extremely wide cingulum surrounding the whole tooth.
The P3 from Pasuda is relatively wide (compared to its length) and is one of the widest in Fig. 5. Only a few of the other Listriodon specimens are still wider, while the ones of Schizoporcus and Lopholistriodon are narrower. This great relative width is the result of the increased size of the protocone and its shift to a more anterior position contributing to the formation of the transverse loph, as well as of the reduction of the parapostcrista. The specimen from Pasuda is a little smaller than the P3 of Li. pentapotamiae and clearly smaller than those of Li. splendens.
Taxonomic discussion
The molars of the specimen from Pasuda have perfect lophs as in Listriodon and some of the species of Lopholistriodon. Lopholistriodon has several, mostly small species, with premolars that are narrower and with less-developed lophs than in Pasuda. Lopholistriodon akatidogus (= Lo. bartulensis) approaches Pasuda in size, but has much narrower premolars. The early Late Miocene Lo. kidogosana is much smaller than the species from Pasuda and has much more elongate molars. The molars from Pasuda are most similar to those of Listriodon. They are smaller than those of Li. splendens, but most of them are within the ranges of Li. pentapotamiae.
The well-formed lophs of the P3−4 are different from both species of Listriodon, though they are most similar to the morphology in Li. pentapotamiae. Listriodon splendens evolved from early Li. pentapotamiae. The protocone on its P4 retained a more posterior position and a well-developed protopostcrista, which together with the metacone, that evolved to be more separate from the paracone, could evolve into a metaloph. By contrast, in the evolution of Li. pentapotamiae, the metacone of the P4 became reduced, while the protocone moved forwards to form a loph with the paracone. The P3 shows the same tendencies, with a beginning individualisation of the metacone in Li. splendens, but not in Li. pentapotamiae. On the corresponding lower premolars, the increased hypoconid and hypoendocristid are a step towards a hypolophid in Li. splendens, while if there is any change in Li. pentapotamiae, it is towards a reduction of this structure. Both species were on divergent evolutionary pathways. With a totally reduced metacone of the P4, the specimen from Pasuda is closer to Li. pentapotamiae, but differs in a better developed loph of the P4 and in a P3 which has a perfect loph. In addition, both teeth differ from all other Listriodontinae in the reduction of the area behind the paracone or protoloph.
The morphology of the P3 and P4 from Pasuda is different from all other Listriodontinae, but also points to close affinities with Li. pentapotamiae, which we consider to be its ancestor. Though we assume that the change occurred by local evolution, at present no specimens of intermediate morphology have been published and the separation from Li. pentapotamiae is very clear. For these reasons, we name the new species Listriodon dukkar.
Discussion
Early late Miocene extinction of the Listriodontinae
Very different opinions are to be found in the literature on the temporal distribution of Li. pentapotamiae. According to Pilgrim (1926), this species occurred in the Chinji, Nagri, and Dhok Pathan Formations, but all the material he described or figured came from the Chinji Zone. Matthew (1929) cast doubt on the presence of Listriodon in the Middle Siwaliks. However, Colbert (1935) indicated the same distribution as Pilgrim and listed seven items from the lower part of the Middle Siwaliks at Nathor and Phadial. Hussain et al. (1979) and Barry et al. (1982) mentioned the co-occurrence of Hipparion and Listriodon in Daud Khel and locality Y454 in the Tatrot-Andar Kas section. Pickford (1988, Fig. 6) indicated in his range chart the occurrence of Listriodon in the very lowermost part of the Nagri Formation, overlapping with Hipparion. Raza et al. (2002) estimated the temporal overlap of Listriodon and Hipparion to be less than 0.5 My.
Various papers mention more recent and dated records of Listriodon. Flynn et al. (1995) and Barry et al. (2002) indicated the last record of Li. pentapotamiae as 9.6 Ma and 10.3 Ma, recalculated as 10.379 Ma and 10.478 Ma, respectively. The date 10.3 Ma has been repeated by various later authors, including Antoine et al. (2013), who also mentioned Listriodontini or Listriodon sp. with a last record in the approximate interval from 11 to 10 and perhaps even 8.4 Ma. Barry et al. (2002) mentioned a Schizochoerus gandakasensis with a time range of 10.1–8.7 Ma. In reality, this is a species of Yunnanochoerus, a sublophodont palaeochoerid which survived long after Listriodon (Van der Made 2010; Pickford 2017). The molars of all these genera are lophodont or sublophodont and very similar if worn. The Listriodon sp. of Antoine et al. (2013) has not been described, so we cannot discuss it, but given the coincidence in temporal distribution, we do not rule out the possibility that it might represent a Yunnanochoerus. Alternatively, it could represent Li. dukkar. Khan et al. (2012) described fossils from Sethi Nagri and assigned them to Li. pentapotamiae. This locality corresponds to loc. Y311 (Barry et al. 1982, 2002) with an approximate age of 10.72 Ma (range 10.108–10.035 Ma). In the Indian Siwaliks, the last record of Listriodon pentapotamiae is from Nurpur and dates to ~ 9.8 Ma (Patnaik 2013). This is based on an upper canine described by Vasihat et al. (1983) from Nurpur. The curvature of the canine forms a large part of the circumference of a circle as is common in Li. pentapotamiae and possibly Li. dukkar, while in the other Suidae of this time, the curvature forms a lesser part of a circle. This fossil comes from a section that has been magnetostratigraphically dated by Rao (1993) and that was reinterpreted by Sangode and Kumar (2003). This is the youngest dated fossil of the Listriodontinae in the Indian Subcontinent.
Even though we have the date of − 9.8 Ma for the last appearance of Listriodon in the Indian Subcontinent, we do not know the morphologically relevant features of the youngest samples. Nearly, all material described comes from the Chinji Formation or age equivalent strata (Pilgrim 1926; Colbert 1935; Pickford 1988; Van der Made 1996a). An exception is the paper by Khan et al. (2012), which describes Late Miocene material from Sethi Nagri, the type locality of the Nagri Formation, and assigned it to Li. pentapotamiae. The P4 figured by Khan et al. (2012) has a morphology that is normal for Li. pentapotamiae, with a hypoconid near the axis of the tooth and a very modest hypoendocristid. Although there are no upper premolars, nor P2-3 among the specimens, it seems that this material does not represent Li. dukkar, for which we expect a short talonid and reduced hypoconid in the P4.
The age of Pasuda is early Late Miocene and was roughly estimated to be between 11 and 10 Ma (Bhandari et al. 2015). Because Li. dukkar evolved from Li. pentapotamiae and because it is unlikely that two nearly identical species lived in a relatively small area, it is the last documented listriodont species known from the Indian Subcontinent, and given the differences in morphology, it should be clearly younger than Sethi Nagri (10.72 Ma). The locality Pasuda and the extinction of the Indian Listriodon lineage should also be younger and could be close to the last record of the genus at 9.8 Ma as mentioned by Patnaik (2013). Whereas the fossil record in the northern part of the Indian Subcontinent is particularly rich, in the southern part, it is poor. Listriodon dukkar could have retreated to the South and have survived there longer, but we do not have any fossil record, which could confirm or deny this.
In Europe, the last listriodont was Listriodon splendens. It was a very common species and is known from over 100 localities of the units MN6 to MN9. In MN9, it is known from Aveiras de Baixo, Azambujeira (both Portugal), Santiga, Can Ponsic I, Can Llobateres 1 (Spain), Doué-la-Fontaine (France) (Van der Made 1996a), and in addition, it is cited from the Vallesian of Can Flaqué, Relea, subsuelo de Sabadell, Creu del Batlle, Can Amat, and St. Miquel (Golple-Posse 1972), but we did not study this material. Its last dated occurrence is at Can Llobateres 1 (Van der Made 1990a, b, 1996a), a locality dated to 9.78 Ma based on magnetostratigraphy and close to the end of MN9, which is estimated as 9.7 Ma (Casanovas Vilar et al. 2014). No locality with Li. splendens has been assigned to a younger MN unit or has been dated to less than 9.78 Ma.
Asia is a huge continent. In western Asia, like in Europe, the last occurrence of Listriodon splendens is in MN9. It is known from Esme-Akçaköy and Çorak Yerler (Becker-Platen et al. 1975a, b), as well as from the Lower Sinap Formation (Ozansoy 1965). The fossils from Çorak Yerler come from two horizons, but have been studied as a whole, and based on different taxa, different correlations have been proposed, to the Kayadibi and Garkin Faunengruppen (Becker-Platen et al. 1975b). Sen et al. (1998) stated “In summary, there are obviously two different faunas at Çorak Yerler, one possibly of late Astaracian or early Vallesian and the other of late Vallesian or early Turolian.” Geraads (2013) suggested mixing of the collections. Kaya et al. (2016) mentioned two fossiliferous levels and, based on a magnetostratigraphic section with two reversals, favored an age between 8.13 and 7.55 Ma for the upper level, but did not obtain results for the lower level and did not mention Listriodon. The faunal list includes Listriodon (Becker-Platen 1975b), but Hippopotamodon major has also been reported from the locality (Fortelius et al. 1996). If we accept the presence of two fossiliferous levels of different ages (as suggested by Sen et al. 1998), it is possible that the lower level, with Listriodon, correlates to MN9 and the upper one, with H. major, to MN10 or 11.
The age of the Listriodon record from the Lower Sinap Formation can be estimated. In Ozansoy’s (1965) scheme, the Upper, Middle, and Lower Sinap Formation consist of beds 1–19, 20–43, and 44–47, respectively. A small fauna with Listriodon comes from bed 46, about 58 m below bed 25, the lowermost fossiliferous level of the Middle Sinap Formation. This fauna is generally considered to be late Middle Miocene or MN8 (e.g., Steininger et al. 1996). More recent work led to the recognition of a dense sequence of over 30 fossil localities in, or correlated to the Sinap Tepe (= Hill) composite magnetostratigraphic section (Kappelman et al. 2003). The entry of the hipparions is in locality 4 at a height of about 45–50 m and localities 88, 64, 104, and 65 are at heights of about 25–45 m in the profile. These four localities are correlated to chron C5n.2n–4n, which implies ages between 11.056 and − 10.7 Ma (Kappelman et al. 2003). This means that they are early Late Miocene (MN9) in age and not Middle Miocene (MN8). These localities do not have Hipparion. However, like bed 46, they have short faunal lists and probably lack Hipparion for that reason. The lowermost fossiliferous locality is loc. 65 with an interpolated age of 10.899 Ma (recalculated as 11.004 Ma). Probably, Ozansoy’s (1965) bed 46 was not much older than locality 65. Ozansoy’s Middle Sinap fossiliferous levels (beds 20, 23, 25) were identified as OZ01, OZ02, and S01 and are at a height of 125–130 m of Kappelman’s et al.’s (2003) section. Ozansoy’s bed 46 would have been 58 m lower than bed 25, which is at a height of about 67 m at the level of locality 93 in Kappelman’s et al.’s (2003) section, with an interpolated age of 10.488 Ma (recalculated as 10.576 Ma). It seems reasonable to assume an age for the Listriodon from Sinap between 11.004 and 10.576 Ma.
On the other side of Asia, various species of Listriodon were named on the basis of Chinese fossils, but these are indistinguishable from L. splendens (Van der Made 1996a). Deng et al. (2013) indicated the presence of Listriodon mongoliensis and two species of Hipparion in the lowermost Upper Miocene of the Guonigou Formation in the Linxia Basin.
The last African listriodont is Lopholistriodon kidogosana from Member D of the Ngorora Formation (Pickford and Wilkinson 1975). This member was reported to date to 9.82–9.68 Ma (Bishop and Pickford 1975), but more recent work suggested older ages for the Ngorora Fm (Tauxe et al. 1985; Deino et al. 1990) and Pickford (2001) situated locality 2/11, with the last Lopholistriodon kidogosana, between 11.84 and 11.54 Ma. Tsubamoto et al. (2017) described a tooth fragment of a listriodont with an estimated age of 10 Ma and assumed that Li. splendens or Li. pentapotamiae dispersed to Africa. This is only a small fragment with a morphology and size that also fit Lo. akatidogus (= Lo. bartulensis) and such an identification would not need to be explained by an intercontinental dispersal. In contrast to the two species of Listriodon, these two species of Lopholistriodon are known from fewer localities. In addition, it should be noted that the African upper Middle and lower Upper Miocene fossil record is less abundant than that from the preceding and following times and as a consequence, the time of extinction is less precisely known.
The last appearances of the Listriodontinae are in the early Late Miocene: Listriodon splendens in Europe and western Asia after 9.78 Ma, Listriodon dukkar in the Indian Subcontinent at or shortly after 9.8 Ma, and a possible Lopholistriodon akatidobus in Africa around 10 Ma. The ages of the decrease in abundance leading to extinction, the death of the very last individual, and the youngest recorded fossil of a species are not the same, but the difference is only relevant if we know it to be large compared to the precision we can obtain in dating. Given the temporal range of about 10 My of the Listriodontinae, the extinction of the last three lineages adapted to different climates and environments is remarkably close in time.
Listriodont ecology
Suoidea are specialized in rooting and during seasons when their favourite food is not available; they have access to a source of food for which they do not compete with other large mammals. They may also eat soil that is rich in organic material. Suoidea complement their diet with food of animal origin: animals they catch (e.g., rodents, reptiles, and birds), carrion, animals living in the soil (e.g., earthworms, larvae), and on occasions, this may make up 88% of their diet (Wilson and Mittermeier 2011). The specialized dentition of the Listriodontinae suggests that they did not ingest important quantities of food of animal origin. Skull morphology suggests that the Listriodontinae were never particularly strongly adapted to rooting and incisor morphology and wear indicate that they progressively abandoned the rooting habit (Leinders 1977a; Van der Made 1996a). This has several implications. One is that their diet was not complemented by animals living in the soil and they depended on leaves and fruit for their proteins. The other is that they are expected to have been limited to environments where nutritious leaves (and fruit) were available year-round.
Many Suoidea are very selective when feeding. The snout of Babyrousa is narrow and it is reported to select precisely different plant parts and in the case of captive individuals even to shell peanuts and peel bananas (Leus 1994). By contrast, the Listriodontinae have very wide snouts and were probably not very adept in precisely selecting the best plant parts. This is primarily due to their very wide incisors, which have been interpreted as an adaptation to bulk feeding on forbs but not on grasses (Van der Made 1996a, 2003b).
Because of the dental morphology and macrowear, Li. splendens has been interpreted to be a specialized folivore (Leinders 1977a, b) and this is confirmed by microwear analysis (Hunter and Fortelius 1994). Stable isotopes are compatible with folivorous diet, different from that of other Suidae (Quade et al. 1995; Domingo et al. 2012). Except for Phacochoerus and Hylochoerus, most species of wild pigs and peccaries have a preference for fruit and shift to roots and tubers when fruit is not available and domestic pigs have been reported to have a preference for sweet fruits (Leus 1994). Fruits tend to be rich in carbohydrates (sugar and starch), but tend to have less than 0.5% crude protein of the wet weight. Tapirs have lophodont cheek teeth, like Listriodon, and their diets consist of 63–86.5% of leaves, 8.1–37% of fruit, and the rest of twigs, bark, etc. (Wilson and Mittermeier 2011). We are not aware of anyone having addressed the possibility that Listriodontinae ingested important amounts of fruit.
Listriodon splendens lived in environments with abundant fruit. Fuss et al. (2018) documented caries in the hominid Dryopithecus from the locality St. Stefan in Austria (about 12.5 Ma), caused by the ingestion of fruit. The environment was forest, dominated by Quercus, Fagus, Castanea, Carya, Pterocarya, Engelhardia, and Juglans. Nine fossil plant species, mostly from the understory of the forest produced ripe sugar-rich fruits during the months June–December: Prunus sp. 1 and 2, Vitis sp., Elaeagnus sp., Morus cf. nigra, Arbutus sp., Castanea sp., Carya sp., and Toddalia sp. Listriodon splendens is one of the most abundant species from this locality and may have fed on the same fruits.
Suoidea may ingest large quantities of protein-rich nuts and acorns. Acorns allow Sus scrofa to gain weight to get through the winter. Their bunodont dentition is well suited for such a diet. Whether Listriodontinae ingested important quantities of nuts is unknown, but their lophodont dentition seems less suited for such a diet. The dental adaptations of Li. pentapotamiae and the last Lopholistriodon species are similar to those of Li. splendens and we assume that this was also the case with their diets.
Herbivores depend on a symbiosis with microorganisms which digest cellulose for them and thus also make cell contents available. Cellulose fermentation takes place in fermentation chambers either in the foregut or hindgut, and the position has further implications (Janis, 1976). Ruminants are the most specialized foregut fermenters. They are more efficient in digesting cellulose than hindgut fermenters, and take more time to digest food, and their digestive tract and contents comprise a larger proportion of the total bodyweight. Whereas food intake in ruminants is limited by rumen fill, hindgut fermenters may increase intake, decrease digestion time and efficiency, and, as a result, increase total nutrients obtained. Sugar is fermented along with cellulose in the rumen, and as a result, ruminants have lower blood sugar levels, while hindgut fermenters absorb the sugar before it reaches the place where fermentation takes place. The way nitrogen becomes available to the herbivore is different and seems to be more efficient in foregut fermenters. Ruminants have the most complex forestomachs, but also camels, hippopotamuses, peccaries, and the babirusas are foregut fermenters, while Potamochoerus, Phacochoerus, and Sus are hindgut fermenters (Langer 1986; Leus et al. 1999; Clauss et al. 2008). Peccaries and babirusas benefit from the formation of microbial proteins by fermentation in the forestomach. Those microbial proteins are subsequently digested in the stomach, resulting in higher protein digestibility and lower protein requirements. The hindgut fermenting Suidae have a more efficient access to sugars in fruit and leaves. Either foregut fermentation evolved several times in the Artiodactyla or it is the ancestral state and some Suidae became hindgut fermenters. Listriodontinae, which split off early from the other Suidae, may have been foregut fermenters, like the babirusas and peccaries. This may have had consequences for the protein requirements of the Listriodontinae.
The energy and protein requirements of several species of ungulates have been studied in detail. Borges et al. (2017) and Nogueira-Filho et al. (2014) gave the minimum daily protein intakes in relation to body weight: Pecari tayacu 514 mg N/kg0.75, Tayassu pecari 336.5 mg N/kg 0.75, Cervus elaphus 680 mg N/kg0.75, Odocoileus virginianus 710 mg N/kg0.75, and Philantomba monticola 643 mg N/kg0.75. The requirements are also expressed as a minimum % of crude protein of the diet (dry matter), which is 5.4% for Pecari tayacu, 4.5% for Tayassu pecari, 4–7.1% for Babyrousa babyrussa, 6.98% for Boselaphus, and 8.27% for Antilope cervicapra, but 12% for domestic pigs and even 15% for lactating sows (Das et al., 2012; Wilson and Mittermeier 2011; Nogueira-Filho et al. 2014; Borges et al. 2017). Though we cannot measure the protein requirements of the Listriodontinae, these may have been more similar to those of the peccaries and babirusas and even ruminants than of Sus scrofa. While the Listriodontinae were probably not omnivorous, did not root, nor eat nuts, their diet may have consisted of protein-rich leaves and possibly protein-poor, but sweet, fruit. Fruit has relatively low protein contents and fruit bats (Pteropodiade) overingest energy to meet their protein requirements (Thomas 1984). Obesity is a problem in overfed zoo babirusas to an extent that it may interfere with reproduction (Leus 1994), so overfeeding on low protein food may not have been an option for the Suoidea.
The extremely large male canines of Li. splendens, Bunolistriodon latidens, and B. meidamon have been interpreted to be used in display in inter-male interactions, suggesting a preference for habitats with good visibility at a distance and thus relatively open landscapes (Van der Made 1996a, 2003b). These canines reached heights (“lengths”) of about 30 cm, and had large diameters and large radii of curvature. While the height of these teeth can be measured only in relatively few complete specimens, the diameters and radii of curvature can be measured in many broken specimens. Although cheek teeth of Li. pentapotamiae are only a little smaller than those of Li. splendens, the canines of the males are much smaller (Fig. 7). Most Listriodontinae, including Lopholistriodon and Li pentapotamiae, retained modest canines, suggesting that these species may have lived in closed habitats, feeding on herbaceous vegetation in the under story or fallen fruit, while Li. splendens may have lived near the interface of closed and open environments or in a mosaic landscape.
Bivariate diagrams of the DAP, DT, and Ri (inner radius of curvature) of the male upper canine (Cm) of selected Listriodontinae. Provenance of data as indicated in Table 1
Body weight is an important parameter in ecology: it affects strength, speed, the relationship with predators, energy and protein requirements, food selection, and through intestine length and the passage time through the intestines, and it also affects digestion. Various ways to estimate body weight have been proposed, based on the length x width of the first lower molar (Legendre 1986), the length of the second upper molar (Fortelius et al. 1996), or the length x width of the astragalus (Martinez and Sudre 1995). The body weights of Li. dukkar, Lo. akatidogus, and Lo. akatikubas are estimated on the basis of the M2 as: 92 (n = 1), 77 (n = 1), and 23, 31, and 35 kg (minimum, mean, maximum; n = 4). The body weight (minimum, mean, maximum) of Li. pentapotamiae is estimated as 82, 122, and 182 kg (n = 11; M1), 61, 87, and 124 kg (n = 41; M2), and 32, 57, and 73 kg (n = 4; astragalus). For Li. splendens this is: 102, 148, and 281 kg (n = 58; M1), 70, 108, and 177 (n = 92; M2), and 88, 111, and 131 kg (n = 19; astragalus).
Legendre (1986) used cenograms to study ecology. As he used them, these are diagrams with the natural logarithm of the average body weight of a species on the vertical axis and the species ordered from large to small on the horizontal axis. He noted that there is a gap between the large and small mammals and that intermediate sizes are absent. This gap is very large in deserts, smaller in savannah and woodland, and absent in rain forest. This is logical in view of the anti predator behaviour: sprint and hide in small mammals living in or near closed environments, outrun the predators in the intermediate sized mammals living in more open landscapes, and defence in the large mammals. The short-legged Suidae are not the type of animals that outrun a predator, and especially small species like Lo. akatikubas would not venture far in an open environment.
Listriodont extinction in the European context
The context of the extinction of the last Listriodon in Europe is better known than for other regions of the world. Its last record is in Can Llobateres 1 (9.78 Ma) and coincides there with the last records of other species of Suoidea. An important turnover in the Suoidea in Spain and elsewhere in Europe, resulting in a major decrease in species richness, was documented (Van der Made 1988, 1990a, b, 1991, 1997c). This appeared to be part of a faunal event, which also involved the extinction of Hominoidea and was called the mid-Vallesian Crisis (Agustí and Moyà Solà 1990; Moyà Solà and Agustí 1990), later shortened to Vallesian Crisis. This event was also noted in the Bovidae: existing lineages went extinct and new arrivals included Tragoportax and Protoryx (Moyà Solà 1983; Moyà Solà and Agustí, 1990; Alcalá and Montoya, 1990).
The causes and extent of the Vallesian Crisis have been much debated in the literature. The Vallesian Crisis was believed to have been caused by a change towards more open and dry landscapes or to increased seasonality, resulting in an increase of deciduous vegetation and seasonal variations in the availability of fruit (Van Dam 1997; Suc et al. 1999; Agustí et al. 2003). It has also been correlated to Mi7, an oxygen isotope event around 9.7 Ma, reflecting decreased global temperatures (Agustí et al. 2013). The Vallesian Crisis has been considered to be continent-wide (e.g., Fortelius et al. 1996a) or only local (e.g., Madern et al. 2018). Hominoidea went extinct in western and central Europe, but are known from younger localities in Greece, Bulgaria, Turkey, and Georgia (Koufos and De Bonis 2006; Spassov et al. 2012; Kaya et al. 2016; Agusti et al. 2020), which has its parallel in longer survival of palaeotropical plants in SE Europe and the Caucasus (Kovar-Eder et al. 1996). The existence of the Vallesian Crisis has been doubted, based on the micromammal record (Casanovas Vilar et al. 2014).
The fossil record of the Suoidea supports the existence of the Vallesian Crisis (Fig. 8). Listriodon splendens, Propotamochoerus palaeochoerus, and Parachleuastochoerus steinheimensis were common species with temporal ranges lasting 5–2 My and their last record is at Can Llobateres. None of these species has been identified from any locality younger than MN9 (or 9.7 Ma) or dated younger than Can Llobateres 1 (9.78 Ma). Parachleuastochoerus crusafonti and Schizoporcus are less common, but both have their last record at La Tarumba (Van der Made 1990a, b, 1997c). La Tarumba is placed in MN10, is dated to 9.56 Ma (Casanovas Vilar et al. 2016), and is only very little younger than Can Llobateres 1. Conohyus is not very abundant and Albanohyus is rare, but both have their last record in MN9 (11.2–9.7 Ma), though no precise ages are available for these localities (Van der Made 1996b; Van der Made and Morales 2003). Nowhere in Europe or Anatolia, these Suoidea survived, nor do any of them co-occurr with H. major, a very common species from MN10 onwards. Within the genus Hippopotamodon (= Microstonyx), H. antiquus was replaced by H. major (Van der Made 1990a, b), which happened either late in MN9 or early in MN10. With up to eight contemporaneous species in Europe and up to five species in one locality before and one or two contemporaneous species after the Vallesian Crisis, European suoid species richness never recovered. While variations in sampling density may affect local sequences, this pattern is observed all over Europe. None of the mentioned species survived anywhere in Europe after MN9. Everywhere in Europe species richness decreased.
Temporal distribution of the European Listriodontinae and of the European and Anatolian Suoidea in relation to the Vallesian Crisis. The localities and squares indicate the first or last dated records of the Suoidea involved in this crisis, preferentially in dated sites. Ages of the dated sites after Casanovas-Vilar et al. (2014), Kappelman et al. (2003) and Van der Made (2003a). To the left reconstructed Northern Hemisphere air temperatures (relative to the pesent) and pCO2 estunated from δ18O (data from Van de Wal et al. 2011) and pCO2 based on the alkenone proxy (Super et al. 2018)
Agustí et al. (2003) described floral change coincident with the Vallesian Crisis. Previous to the crisis and still in Can Llobateres 1, there was a humid subtropical flora, while flora from Terrassa—Talud Sud Autopista (9.02 and 9.23 Ma) consists of 36 taxa, of which about 45% are deciduous trees, 15% Mediterranean elements, 33% evergreen trees, and 7% warm evergreen elements, suggesting about 1000 mm mean annual precipitation, mean annual temperatures between 16 and 19ºC, and seasonal availability of fruit. Listriodon may have lived in other habitats in Europe, and, for instance, is known from localities in the interior of Spain, which today and probably also in the Miocene were drier. However, its last records are in Can Llobateres and other localities in the same area, or with an expected similar environment, and the documented increase in seasonality, restricting the staple food of Listriodon, may have been decisive.
As far as the Suoidea are concerned, the Vallesian Crisis is a reality and Listriodon splendens became extinct as part of this crisis. If the study of other taxonomical groups cannot confirm the existence of the crisis or of environmental change, this can be because these groups were not affected in the same way. Though generally the age of the crisis is considered 9.7 Ma, the extinction of Listriodon and other Suoidea occurred after 9.78 Ma, that of still other Suoidea after 9.56 Ma, and the first dates for the replacing suid and new vegetation date to 9.4 Ma. Therefore, if there were two events, they occurred in the period from 9.78 to 9.4 Ma, and if it was only one, it was between 9.56 and 9.4 Ma. From the foregoing it appears that environmental change and increase in seasonality restricted the availability of the food of L. splendens, which may well have been the cause of its extinction in Europe.
Listriodont extinction in the West Asian context
The Anatolian fossil record is less complete than that of the whole of Europe and relevant material of the Suoidea has not yet been published, but the available data fit perfectly the pattern observed in Europe. Figure 9 shows the various species of Suoidea from Anatolia, which in Europe were involved in the Vallesian Crisis and shows a selection of relevant sites with their dates or with an approximate position according to biostratigraphy. The “Faunengruppen” (faunal associations), indicated in the figure, were defined by Becker-Platen et al. (1975a) on the basis of Turkish localities. These Faunengruppen were never widely used because of the MN units which were introduced at the same time (Mein 1975). As argued above, the locality Çorak Yerler has two different levels, one with Listriodon and another one with Hippopotamodon major. The Alçitepe Mb of the Eceabat Formation on the Gallipoli (Gelibolu) peninsula has several units: the Nebisuyu, Sigindere, and Degirmendere units, correlated to the Aragonian, Vallesian, and Turolian, respectively (Van der Made and Tuna 1999). The locality Nuri Yamut is in the Sigindere unit.
Temporal distribution of the Anatolian Suoidea in relation to the Faunengruppen and Pollenbilder, defined on Turkish sites (Becker-Platen et al. 1975a, b). The localities and squares indicate the first or last dated records of the Suoidea, preferentially in dated sites (see text). Ages of the dated sites after Becker-Platen et al. (1975b, 1977), Steininger et al. (1996) and Kappelman et al. (2003), updated after Hilgen et al. (2012)
Several of the sites in Fig. 9 overlie or underlie dated tuffs (Becker-Platen et al. 1975a, 1975b, 1977). A composite faunal list of the two localities Yeni Eskihisar 1 and 2 was used to define the Faunengruppe of this name. However, Yeni Eskihisar 2, mainly a micromammal locality, is overlain by two tuffs, dated 13.2 ± 0.35 Ma and 11.1 ± 0.2 Ma, which again are overlain by Yeni Eskihisar 1, which is mainly a large mammal locality. The latter locality has Hippopotamodon antiquus (Fortelius et al. 1996) and together with the date, this points to the locality being MN9 and Vallesian, while the other locality is MN7 + 8 and Aragonian. The localities Bayirköy and Eski Bayirköy overlie a tuff dated to 10.2 ± 0.15 Ma and apparently also one dated 9.25 ± 0.2 Ma (Becker-Platen et al. 1977, Fig. 9), but are correlated to the Kayadibi and Kinik Faunengruppe, respectively (Becker-Platen et al. 1975b). Ozansoy’s Middle Sinap fossiliferous beds 20, 23, and 25 correspond to OZ01, OZ02, and S01 of Kappelman et al. (2003), and have interpolated ages of 9.683–9.590 Ma (recalculated as 9.766–9.673 Ma).
As can be seen in Fig. 9, the Suoidea show the same pattern as in Europe. Hippopotamodon antiquus occurs in MN9, while localities of MN10 and younger have H. major, like in Europe. Even though H. major is known from many sites (e.g., Pickford and Ertürk 1979; Fortelius et al. 1996; Van der Made et al. 2013), none of these sites is dated and known to be older than Igbek, nor any is placed in an MN unit older than MN10. Bayirköy and Eski Bayirköy have Hippopotamodon, but we did not study the one from Bayirköy. Five of the eight species that went extinct or were replaced during the Vallesian Crisis in Europe did the same here, including Li. splendens. This happened between Sinap “bed 20” (9.673 Ma) and Igbek (9.118 Ma). Beyond Anatolia, the western Asian fossil record is not very well known, but the Hippopotamodon from Marageh Kopran I (Iran; about 9 Ma; Fortelius et al. 1996; Bernor et al. 1996), as well as in Udabno (Georgia; MN10) and Eldar (Azerbaidjan; MN10/11) are compatible with the event having occurred in a larger part of western Asia. Even though dating is less precise, the turnover may have happened at the same time. In Europe, the Vallesian Crisis occurred between 9.78/9.56 and 9.369 Ma and the faunal turnover in Anatolia occurred at about the same time between 9.659 and 9.118 Ma.
This faunal event in Anatolia seems to coincide with a change in the flora. In Turkey, a series of “Pollenbilder” or sporomorph associations (“zones”) have been defined and are correlated to the Faunengruppen and MN units (Benda et al. 1975; Benda and Meulenkamp 1990). These provide information on the environment in which Listriodon splendens lived and went extinct. The Eskihisar Pollenbild includes many tropical and few subtropical elements and the following Yeni-Eskihisar association is transitional. Listriodon splendens is present in localities correlated to those Pollenbilder, but not in sites correlated to the next one. The following Kizilhisar association has but few subtropical elements, but more conifers, species related to oaks, and sedges and grasses, indicative of steppe like to semi-arid conditions (Benda and Meulenkamp 1990). The Yeni Eskihisar Pollenbild is defined on a sample from the level of the Yeni Eskihisar 1 micromammal locality, but is said to be typical of the upper part of the Sekköy Formation (which also includes Yeni Eskihisar 2, < 11.1 Ma) (Becker-Platen et al. 1977). The Yeni Eskihisar Pollenbild is known only from a few sites with fossil mammals, but has been correlated to mammal sites. The transition to the Kizilhisar floral associations occurs between the Yeni Eskihisar 1 and Kayadibi mammal associations, and possibly within the Esme Akçaköy Faunengruppe (Benda et al. 1975). The locality Küçük Çekmece is of the Kayadibi faunal association, which has H. major and the Kizilhisar pollen association. Benda and Meulenkamp (1990) indicated that Kastellios Hill 1 in Crete has the Kizilhisar pollen association. This locality is placed in MN10 and correlated to chron C4Ar.2r (Steininger et al. 1996). This places the transition between the two pollen zones, which marks a decrease in temperature and a change to a landscape with more grasses between 11.1 ± 0.2 and 9.426–9.647 Ma. The whole Esme Akçaköy faunal association is between these two dates and precedes the faunal change, which also marks the MN9–10 transition. Benda’s Pollenbilder are not used anymore, and their use in correlation for the Lower and Middle Miocene has been criticised. However, these Pollenbilder have been found in or lithostratigraphically correlated to sites with fossil mammals of which the age is known and the environmental interpretation for these sites or times should remain valid. More recent work confirms in a broad sense a transition of more humid environments that included subtropical forests to steppe (Yavuz-Isik and Toprak 2010; Bouchal et al. 2017; Steinthorsdottir et al. 2021).
The available data show or suggest that: (1) the extinction of Listriodon in western Asia formed part of a turnover event, which involved the same species of Suoidea as the European Vallesian Crisis; (2) the events in both areas are coincident; and (3) also in western Asia, these faunal events may have coincided with a major floral change.
Listriodont extinction in the context of the Indian Subcontinent
Whereas suoid species richness in Europe declined during the Vallesian Crisis, it remained high in the Indian Subcontinent till well into the Pliocene (Van der Made 1991). Barry et al. (2002) found a generally low level of faunal turnover in the Siwaliks in the interval of 10.7–5.7 Ma, with peaks at 10.3, 7.8, and 7.3–7.0 Ma. The event at 10.3 Ma involved also non-suid taxa, but coincides with the last appearance of Li. pentapotamiae and Conohyus sindiensis. The last appearance of ?Hippopotamodon “Y450 unnamed species” and the first appearance of Hippopotamodon sivalense and Propotamochoerus hysudricus was indicated to be at 10.2 Ma. The temporal ranges of these and other Suoidea as given in the literature are indicated with thick lines in Fig. 9.
As we have seen already, there are records of Listriodon in the Indian Subcontinent, which are considerably younger than the 10.3 Ma, indicated by Barry et al. (2002), but this is not the only one. Conohyus sindiense (= Retroporcus) was cited from a magnetostratigraphic section at Tinau Khola (Munthe et al. 1983). This record is from a long interval with normal polarisation, correlated to C5n.2n, and, based on Hilgen et al. (2012), its interpolated age is 9.984 Ma.
Von Meyer (1866) described fossils from three different fossiliferous levels at Kushalgar and named Sanitherium schlagintweiti on the basis of material from a level with Hipparion. Later authors stated that the specimen came from Chinji-equivalent strata (Pilgrim 1926; Colbert 1935). The description of new material from Tapar from a level with Hipparion shows that the species did indeed survive till after the arrival of that equid (Bhandari et al. 2015).
Hippopotamodon major is present in Sethi Nagri (10.072 Ma), where it existed along with H. sivalense (Van der Made and Hussain 1989). This H. major may correspond to Dicoryphochoerus robustus and D. titanoides of Pilgrim (1926) and to ?Hippopotamodon “Y450 unnamed species” of Barry et al. (2002), because it is the only other Hippopotamodon mentioned by these authors. If this is correct, the curious situation exists that in the Indian Subcontinent, around 10.2 Ma, H. major is replaced by the larger H. sivalense, whereas in Europe and Anatolia, between 9.78 and 9.4 Ma, H. major replaced the large H. antiquus, which is very close to H. sivalense.
In Pickford’s (1988) scheme, Hyotherium pilgrimi occurred in the Chinji Formation and the very base of the Nagri Formation, and was replaced by Propotamochoerus hysudricus in the Nagri, Dhok Pathan, and Soan formations. Pickford (1988) named H. pilgrimi and listed in its synonymy the following species named by Pilgrim (1926): Propotamochoerus salinus, P. uliginosus, Dicoryphochoerus chisholmi, ?D. haydeni, and D. instabilis. All these species would have priority over H. pilgrimi, but this was not sufficiently discussed. The species P. salinus was described from Tapar as a new genus Kachchchoerus (Bhandari et al. 2015). Pickford’s (1988) generic diagnosis of Hyotherium states that the P4 is without sagittal cusplets. According to this criterion, the holotype of H. pilgrimi does not fit Hyotherium and it is more likely that Pilgrim (1926) was right and that it belongs to Propotamochoerus. Whatever the names applied, the primitive species of Propotamochoerus was replaced by P. hysudricus and this may have happened around 11 Ma (more conform to Pickford 1988) or around 10.3 Ma (more in line with Barry et al. 2002) or at a still other date, but this remains to be documented.
Lophochoerus nagrii was not mentioned by Barry et al. (2002), but according to Pickford (1988), it made a short appearance in Nagri times, but went extinct afterwards. Barry et al. (2002) gave the temporal distribution of Tetraconodon magnus as 10.0–9.3 Ma. In fact, there is a size increase in the lineage T. minor—T. intermedius—T. magnus (Van der Made 1999), but we lack precise data on the temporal distribution of these species. According to Barry et al. (2002), Schizochoerus gandakasensis appeared between 10.1 and 8.7 Ma. This genus is a junior homonym and its replacement name is Schizoporcus (Van der Made 2010), but this species belongs to Yunnanochoerus (Van der Made 1997a; Pickford 2017).
It appears, thus, that there is some evidence for a turnover in the Suoidea around 10.3–10.2 Ma according to the data of Barry et al. (2002), but that data from India and Nepal indicate that Listriodon and R. sindiense survived till around 9.8 Ma. The species involved are closely related to those in Europe and Anatolia, but not identical.
Barry et al. (2002) also gave data concerning a faunal turnover in the Tragulidae, with two species last occurring at 10.5–10.4 Ma and three species appearing at 10.4–10.3 Ma. It is not quite clear how this compares with the data presented by Barry (2014), showing that Tragulidae made up 30– > 60% of Tragulidae and Bovidae combined until 10 Ma, around 50% at 10 Ma and a decrease from about 20–5% from 9 to 6 Ma. This spectacular drop occurs broadly coincidently with the final extinction of Listriodon. Living Tragulidae are primarily frugivores, but may feed on leaves and occasionally eat carrion or may catch animals (Wilson and Mittermeier 2011). Many of the fossil tragulid species reached much larger sizes than the living species and may have been folivorous. Bovidae range from browsing folivores to grazing bulk feeders and their rise in abundance was probably related to a shift in vegetation.
Stable isotopes from soil carbonates and from teeth provide information on the environment and diet. However, this information is mainly restricted to differentiating C3 and C4 vegetation. After 7.3 Ma, C3 vegetation (probably trees and shrubs) is replaced by C4 vegetation (grasslands) in the Siwaliks of Pakistan (Cerling et al. 1993; Quade and Cerling 1995). Sanyal et al. (2004) studied soil carbonates near Haripur (India) and found a similar change around 6 Ma. Stable isotopes from tooth enamel of fossils from the Indian part of Siwaliks show Late Miocene dietary shifts among various mammallian taxa including primates (Patnaik 2015; Patnaik et al. 2014, 2019). Also it has been hypothesised that this shift in vegetation could have led to extinction of the hominoids in the Siwaliks (Patnaik et al. 2005). Stable isotope data suggest that Li. pentapotamiae lived in environments with C3 vegetation, which is in line with its dental morphology, and that it went extinct long before C4 grasses started to dominate the landscapes.
The Siwaliks of northern India and Nepal have an abundant record in leaf and fossil wood floras (Prasad 1971; Awasthi 1992; Prasad and Awasthi 1996; Prasad and Pandey 2008), but most of these fossil plant sites are situated in India (Awasthi 1992) and further to the east than the sites with Li. pentapotamiae. The fossil plants indicate a luxuriant humid forest environment, which in the Upper Siwaliks became drier, more open and with grasses. Of particular interest is a sedimentary sequence containing 52 horizons with plant macrofossils and 584 pollen samples at Surai Khola, tied to a magnetostratigraphic section, described by Corvinus and Rimal (2001). They interpreted tropical broad leaved evergreen rainforest and swampy environments from 13 Ma onwards, which is compatible with the sedimentology of the Chinji Formation in Pakistan (Zaleha 1997; Willis and Behrensmeyer 1995), a gradual shift towards moist semi-evergreen forest between some 10 Ma and 9 Ma, after about 7.5 Ma a shift to dry deciduous forest, and still later a shift to grasslands. They noted that the shift towards grasses in their section is much later than in Pakistan. The evergreen rain forest included trees like Dipterocarpus, which may be up to 50 m tall. Srivastava et al. (2018) used the plant fossils in the same section to estimate the principal climatic parameters for the period between 13 and 11 Ma as 1748–2869 mm mean annual precipitation and a mean annual temperature of 21.1–25.4 °C. For the period from 9.5 to 6.8 Ma, they estimated 2592–3151 mm mean annual precipitation (which is an increase) and a mean annual temperature of 26–-27 °C and estimated that seasonality in precipitation increased. This increase in precipitation contrasts with the smaller river channels in the Dhok Pathan Formation in Pakistan. Perhaps, one of the two is a local phenomenon. It should be taken into account that the Himalaya was already rising and that, at present, there is a narrow area of high precipitation along the mountain front, so fossil plant sites may not be representative of the environment more to the South and West, where most of the fossil mammals are found. Quade et al. (1995) analysed stable isotopes from soil carbonates at Surai Khola and found a similar pattern as in Pakistan.
The available geochemical, paleontological, and sedimentological information suggests that Li. pentapotamiae may have lived in humid environments with a luxuriant C3 vegetation, probably forest and that it may have fed on leaves of the plants of the understorey and perhaps also fruit. It survived into times with increasing seasonality. Its last records, around 9.8 Ma, date from a time when there was an increase in deciduous vegetation and an opening of the landscape, and coincided with the frugivorous Tragulidae becoming less abundant and Bovidae becoming more abundant. Listriodon went extinct well before landscapes became dominated by C4 grasses.
Listriodont extinction in the African context
In Europe and the Indian Subcontinent, suoid diversity was high with Cainochoerinae, Tetraconodontinae, Suinae, and Taucanaminae living along with the Listriodontinae, as testified by the co-occurrence of several of these in many localities (Van der Made, 1990a, b, 1991). By contrast, in Africa, diversity of the Listriodontinae was higher, while that of other Suoidea was much less (Van der Made, 1996a, b). A species of Lopholistriodon survived till about 10 Ma (Fig. 10).
Within the 10 My duration of the Listriodontinae in Africa, there were three major peaks in faunal turnover, around 17, 13.7, and 9.7 Ma (Van der Made 2014). The first two coincide with turnover in the Listriodontinae and sandwich the Mid-Miocene Climatic Optimum, which is when listriodonts reached their maximum geographic extension and diversity. The event around 9.7 Ma is close to the extinction of the Listriodontinae at ~ 10 Ma. It is noteworthy that the extinction of the last Listriodontinae and a major peak in faunal turnover in Africa are so close to the European Vallesian Crisis.
Initially, Bovidae formed a small proportion of the large mammals in Africa, but their diversity started to increase leading to a spectacular diversity of the Quaternary (Van der Made 2014, fig. 6). Other Suidae may have escaped direct competition, because of their omnivorous diet and rooting habits, but dental morphology suggests that Lopholistriodon was folivorous and more prone to suffering from the bovid radiation.
The eastern African palaeobotanic record is not very dense, but the best sequence of sites is from the Ngorora and Mpesida Formations in the Tugen Hills (Kenya; Jacobs et al. 2010), which also records the latest African Listriodontinae. The flora from Kabarsero (12.6 Ma) has 11 out of 25 taxa that grow today in wet forests or rain forests, including herbaceous plants and grasses that are found in the understory of wet forests (Jacobs and Kabuye 1987; Jacobs and Winkler 1992). Accordingly, the environment was interpreted as moist to wet forest. The flora from Waril (about 10 Ma) is dominated by a species of legume growing in seasonally dry environments (Jacobs et al. 1999). The last Lopholistriodon species must have lived in this environment and will have fed on this legume. The paleoflora from Kapturo (7.2–6.7 Ma) was interpreted as deciduous woodland with seasonality in the moisture (Jacobs and Deino 1996). The silicified wood from Rurmoch (> 6.37 Ma) with stem diameters of > 90 cm suggests trees over 50 m tall, and the association was interpreted as indicative of wet lowland rainforest, while stable isotopes from paleosols and tooth enamel indicate the presence of locally open habitats (Kingston et al. 2002).
These data suggest that Lopholistriodon lived in wet forest, may have fed on leguminous plants, and went extinct when the vegetation was on its way to become more seasonal and drier and when bovid diversity increased.
Possible reasons for the extinction of the Listriodontinae
The last record of the Listriodontinae in Europe is at 9.78 Ma, which is before the Vallesian Crisis, (9.78/9.56–9.396 Ma), in the Indian Subcontinent at ~ 9.8 Ma or somewhat later, and in Africa around 10 Ma. The extinctions in these areas seem to have occurred within a remarkably short period, suggesting a common cause. The three different lineages that went extinct lived at different latitudes in Europe and Anatolia (about 38–50° N), the Indian Subcontinent (about 21–34° N), and Africa (about 0°) and consequently in different climates and environments. If there is a common cause for these three extinctions in different continents, it has to be a global event or process, but most of the changes which coincided with the extinction of the Listriodontinae are local or gradual.
It has been suggested that the Vallesian Crisis was related to the Mi7 event (Agustí et al. 2013). Such events are global. Miller et al. (1991) named the Mi events for rapid increases in the values of the record of δ18O from the skeletons of benthic foraminifera in the Miocene. These events reflect sudden decreases in temperature and eustatic sea level and increases in global glacial ice volume. The timing of these events seems to be related to 400 ky, 100 ky, and 180 ky cyclic variations in precession, obliquity, and asymmetry of the obliquity cycle. Pagani et al. (1999b) situated the Mi6 event around 9.6 Ma and coincident with a peak value of pCO2, reaching the highest value (about 310 ppmv) in a period of over 15 My. The Mi7 event could date to 9.56, 9.40, 9.31, 9.22, 9.14, 9.04, or be still younger (Westerhold et al. 2005). It is possible that a temperature decrease combined with pronounced seasonality may have caused seasonal limitation of nutritive plant parts, causing the extinction of Suoidea and early European hominids, but it is more difficult to see how this may have affected the Listriodontinae living at the Equator.
Variations in atmospheric CO2 concentration are global and have a great impact on the vegetation and on herbivores, as well as humans. The projected increase in CO2 levels by 2050 is considered to put at risk 148.4 million people, who depend mainly on rice, wheat, barley, and potato for their protein intake (Medek et al. 2017). The protein content of these foods is expected to drop by 6–14%. Two billion people suffer from dietary iron and zinc deficiencies, which cause millions of death, and the problem is expected to increase with rising pCO2 (Myers et al. 2014). A rise in pCO2 will enhance productivity and will increase carbohydrate and sugar content in leaves, grains, and fruit (e.g., Högy and Fangmeier 2008; Dong et al. 2018; Yang et al. 2020), and it also leads to a decrease in zinc and iron, and protein content (Ehleringer et al., 2020). Low protein, zinc and iron intake affects growth and may lead to a series of pathologies. There is evidence that elevated pCO2 may lead to reduced growth rates in cattle (Ehleringer et al., 2020). Meeting the protein requirements of captive ruminants is a concern (e.g., Priebe and Brown 1987; Crissey 2005). There is reason to believe that changes in pCO2 may have affected the nutrition of Miocene ungulates.
There are various ways to reconstruct the pCO2 of the past (e.g., Pagani et al. 1999a; Pearson and Palmer 2000; Demicco et al. 2003; Sosdian et al. 2018) and the results may differ according to the method applied. During the past 200 My, atmospheric CO2 concentrations of over 1000 were common and peaks of over 2000 ppmv (parts per million volume) occurred (Pearson and Palmer 2000), but the Miocene pCO2 was much lower. Most methods give relatively low values for the Miocene (below 600 ppmv), but higher values would fit better existing climate models (Steinthorsdottir et al., 2021). In addition, some of the records lack detail; others are of short periods or have long hiatuses. Van de Wal et al. (2011) reconstructed detailed records of pCO2 and temperature for the past 20 My. Temperatures and pCO2 were high between about 17 and 13.6 Ma, a period that has been called the Mid-Miocene Climate Optimum (Figs. 8, 10). Around 13.6 Ma, there was a rapid decline in temperatures and pCO2 and then a more gradual decline until about 8 Ma, punctuated by several moments of acceleration. Notably, at 9.539 Ma, a record low value of pCO2 was reached. Super et al. (2018) used the alkenone proxy and the resulting curve (Fig. 8) differs from that of Van de Wal et al. (2011) in generally lower values, but both curves resemble each other in general shape with high values for the Mid-Miocene Climatic Optimum and lower values later. There is a peak low value at 9.4 Ma, but the record is not very dense with the next older data point at 9.7 Ma. Sosdian et al. (2018) used boron isotopes to reconstruct pCO2, gave various variants based on different assumptions and methods, but all resulted in higher estimated values. The general shapes of these pCO2 curves are similar to the curve by Van de Wal et al. (2011) in a marked decrease in the pCO2 after the Mid-Miocene Climatic Optimum and in having a peak low value at 9.874 Ma, though the density of the record is low, with the next data points at 10.604 and 9.244 Ma. The parts with denser data show important fluctuations, but the lesser densely sampled times cannot show such fluctuations.
The onset of the Middle Miocene Climatic Optimum coincided with faunal turnover events in Africa and Europe (Van der Made, 2014). It also marks the onset of the radiation of the Listriodontinae in Africa and Europe (Figs. 8, 10). Later during this period, Lopholistriodon in Africa and Listriodon in the Indian Subcontinent evolved perfect lophodonty, became specialized folivores, and Listriodon acquired a Eurasian distribution from Portugal to China. The end of the Mid-Miocene Climatic Optimum, around 13.6 Ma, coincides with the major peak in faunal turnover in Africa between 16 and 10 Ma (Van der Made, 2014) and coincides with the end of several listriodont lineages (Figs. 8, 10). Apparently Listriodontinae did well during this period of high temperatures and high pCO2 as they reached their greatest geographic expansion and diversity (Fig. 10). The decrease in pCO2 from 13.6 Ma onwards is mirrored by the rise in Bovidae in Africa (Van der Made 2014, fig. 6) and elsewhere, and in the decrease in browsers noted by Janis et al. (2000, 2004). The final extinction of the Listriodontinae around 9.8–9.4 Ma and a spectacular drop in the abundance of the Tragulidae in the Indian Subcontinent (Barry 2014), where they were most diverse, coincide with one of the moments when the decrease in pCO2 accelerated (Figs. 8, 10). The pCO2 according to Van de Wal et al. (2011) shows several peaks with each time lower values from about 9.75 Ma onward and a sustained decrease in values from about 9.62 onward, reaching a record low at about 9.58 and continued to decrease till 9.539 Ma and peaked several times about 100, 200, and 300 ka later. The temporal duration of these events covers or overlaps the last records of the last listriodont lineages. The curves by Super et al. (2018) and Sosdian et al. (2018) show peak low values at 9.7 and 9.874, which are close, given the lesser temporal precision due to their less dense records.
The increase in sugar and reduction of protein, Zn, and Fe contents of fruit and vegetables is well studied in relation to the possible rise in pCO2 in the near future, starting from the present levels around 400 ppm. Little or nothing is known about the effect of the drop in pCO2, from over 450 ppm during the Middle Miocene Climatic Optimum till about 350 ppm around 9.8–9.4 Ma, on sugar, starch, protein, Zn, and Fe contents of leaves and fruit. The fact that during this period, the high diversity of browsers, including Tragulidae, declined markedly, suggests that a decrease of sugar and starch in fruit and leaves could have been prejudicial to them and that this was not compensated by the higher content in other nutrients.
Janis (1989) classified living and fossil Artiodactyla in three categories, those with: (1) little or no fermentation (Suidae, Tayassuidae, Entelodontidae and Palaeogene families); (2) some foregut fermentation (for example Tragulidae, Anthracotheriidae); and (3) full foregut fermentation plus rumination (including ruminants and camels). Category 2 is somewhat adapted to a fibrous diet, but category 3 consists of “cell wall specialists”, still better adapted to the digestion of cellulose. She noted that, during the Late Eocene and Oligocene, primitive foregut fermenters (type 2) diversified at the cost of hindgut fermenters and that from the Middle Miocene onward, ruminating artiodactyls (type 3) replaced less specialized foregut fermenters and the Listriodontinae. At present, Tayassuidae and Babyroussa are considered to be foregut fermenters, while Phacochoerus and Sus (both Suinae) and Potamochoerus are considered to be hindgut fermenters (Langer 1986; Leus et al. 1999; Clauss et al. 2008).
The living Suinae are hindgut fermenters, and the available data suggest that they have higher protein requirements than the peccaries and babirusas, which are foregut fermenters. Propotamochoerus from the Chinji Formation is the first representative of this subfamily and was probably a hindgut fermenter. This subfamily radiated and progressively replaced other Suoidea, most of which are likely to have been foregut fermenters (Palaeochoeridae, Listriodontinae, Cainochoerinae, perhaps Hyotheriinae). At the time when pCO2 decrease suggests an increase in protein content, Suinae, which were less efficient, replaced other Suoidea, which were more efficient in the digestion of proteins. At the same time, there was a decrease in sugar content and the hindgut fermenting Suinae handle sugars more efficiently. Either access to protein may have been a limiting factor or more efficient sugar digestion may have been a plus, or both may have been decisive in the spread of hindgut fermenting Suoidea. Listriodontinae may have been more competitive in a high pCO2 world, because they were more protein efficient, while they could afford to be less sugar efficient.
Though many data are still lacking, it seems possible that the decreasing pCO2 led to changes in the nutrient composition of the vegetation, favoring some herbivores and omnivores over others. The extinction of the Listriodontinae in the different continents may have occurred when a critical pCO2 threshold was met.
Conclusions
We studied a new fossil from Pasuda and placed it in a wider context. Our work led to the following conclusions:
-
- The specimen from Pasuda belongs to the new species Listriodon dukkar.
-
- The new species has P3-4 with perfect lophs and evolved more towards perfect lophodonty than any other Suoidea, reflecting the highest degree of adaptation to folivory.
-
- Listriodon dukkar shares features with Li. pentapotamiae and evolved from it.
-
- The Listriodon pentapotamiae–Li. dukkar lineage was folivorous and lived in humid environments with a low degree of seasonality, probably including tropical evergreen forest.
-
- The last records of the three distinct lineages of Listriodontinae in the Indian Subcontinent (~ 9.8 Ma), Europe (9.78 Ma), and Africa (~ 10 Ma) are close in age and suggest a cause that was likely of global nature, acting in the different environments of these continents.
-
- These extinctions coincided with a variety of local events, including increasing seasonality, changes in vegetation, turnover in the mammals, and the European Vallesian Crisis. They also occurred against a background of the decline of the browsers and the rise of the Bovidae, but these trends occurred at different rates in the different continents. Though all these events may have contributed to the demise of the Listriodontinae, none explains the extinction of the Listridontinae in all their geographic distribution and apparently in a short period.
-
- The Listriodontinae radiated and did well when atmospheric pCO2 was relatively high, but went extinct during a period when pCO2 decreased. Changes in atmospheric pCO2 are independent of latitude and affect nutrient contents of plants and, thus, the competition between herbivores with different types of digestion and protein requirements. These observations suggest a causal relationship that may have acted at the same time throughout the area of distribution of the Listriodontinae
Availability of data and materials
The fossil from Pasuda described here (PUKP-1) is kept in the Centre of Advanced study in Geology, Panjab University, Chandigarh. The data are either published or given in Table 2.
Code availability
Not applicable.
References
Agustí, J., and S. Moyà-Solà. 1990. Mammal extinctions in the Vallesian (upper Miocene). In Extinction events in earth history, ed. E.G. Kauffman and O.H. Walliser, 425–432. Berlin, Heidelberg: Springer.
Agustí, J., A. Sanz de Siria, and M. Garcés. 2003. Explaining the end of the hominoid experiment in Europe. Journal of Human Evolution 45: 145–153.
Agustí, J., L. Cabrera, and M. Garcés. 2013. The vallesian mammal turnover: a late miocene record of decoupled land-ocean evolution. Geobios 46: 151–157.
Agustí, J., P. Piñero, M. Furió, H.A. Blain, A. Blanco-Lapaz, O. Oms, G. Chochisvili, and D. Lordkipanidze. 2020. Small vertebrates from the upper Miocene hominoid-bearing site of Udabno Georgia. Journal of Vertebrate Paleontology 39 (5): e1716776. https://doi.org/10.1080/02724634.2019.1716776.
Alcalá, L., and P. Montoya. 1990. Las faunas de macromamíferos del Turoliense inferior español. Paleontologia i Evolució 23: 111–119.
Antoine, P.O., G. Métais, M.J. Orliac, J.Y. Crochet, L.J. Flynn, L. Marivaux, A.R. Rajpar, G. Roohi, and J.L. Welcomme. 2013. Mammalian Neogene biostratigraphy of the Sulaiman Province, Pakistan. In Fossil mammals of Asia: neogene biostratigraphy and chronology, ed. X. Wang, L.J. Flynn, and M. Fortelius, 400–422. New York: Columbia University Press.
Awasthi, N. 1992. Changing patterns of vegetation through Siwalik succession. Palaeobotanist 40: 312–327.
Barry, J.C. 2014. Fossil tragulids of the Siwalik formation of Southern Asia. Zitteliana B 32: 53–61.
Barry, J.C., and L.J. Flynn. 1990. Key biostratigraphic events in the Siwalik sequence. In European neogene mammal chronology, ed. E.H. Lindsay, V. Fahlbusch, and P. Mein, 557–571. New York and London: Plenum Press.
Barry, J.C., E.H. Lindsay, and L.L. Jacobs. 1982. A biostratigraphic zonation of the middle and upper Siwaliks of the Potwar Plateau of northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 37: 95–130.
Barry, J.C., M.E. Morgan, L.J. Flynn, D. Pilbeam, L.L. Jacobs, E.H. Lindsay, S.M. Raza, and N. Solounias. 1995. Patterns of faunal turnover and diversity in the Neogene Siwaliks of Northern Pakistan. Palaeogeography, Palaeoclimatology Palaeoecology 115 (209): 220.
Barry, J.C., M.E. Morgan, L.J. Flynn, D. Pilbeam, A.K. Behrensmeyer, S.M. Raza, I.A. Khan, C. Badgley, J. Hicks, and J. Kelley. 2002. Faunal and environmental change in the late Miocene Siwaliks of northern Pakistan. Paleobiology 28 (2 Suppl. Mem. 3): 1–71.
Becker-Platen, J.D., O. Sickenberg, and H. Tobien. 1975a. Die Gliederung de känozoischen Sedimente der Türkei nach Vertebraten-Faunengruppen. Geologisches Jahrbuch, Reihe B 15: 19–46.
Becker-Platen, J.D., O. Sickenberg, and H. Tobien. 1975b. Vertebraten-Lokalfaunen der Türkei und ihre Altersstellung. Geologisches Jahrbuch, Reihe B 15: 47–100.
Becker-Platen, J.D., L. Benda, and P. Steffens. 1977. Litho- und biostratigraphische Deutung radiometrische Altersbestimmungen aus dem Jungtertiär der Türkei (Känozonikum und Braunkohlen der Türkei, 19). Geologisches Jahrbuch, Reihe B 25: 139–167.
Benda, L., and J. Meulenkamp. 1990. Biostratigraphic correlations in the Eastern Medterranean Neogene. 9. Sporomorph associations and event stratigraphy of the Eastern Mediterranean Neogene. Newsletters on Stratigraphy 23 (1): 1–10.
Benda, L., K. Heissig, and P. Steffens. 1975. Die Stellung der Vertebraten-Faunengruppen der Türkei innerhalb der chronostratigraphischen Systeme von Tethys und Paratethys. Geologisches Jahrbuch, Reihe B 15: 109–116.
Berggren, W.A., D.V. Kent, J.J. Flynn, and J.A. van Couvering. 1985. Cenozoic geochronology. Geological Society of America Bulletin 96: 1407–1418.
Bernor, R.L., N. Solounias, C.C. Swisher, and J.A. Van Couvering. 1996. The correlation of three classical “Pikermian” mammal faunas -Maragheh, Samos, and Pikermi- with the European MN unit system. In The evolution of western eurasian neogene mammal faunas, ed. R. Bernor, V. Fahlbusch, and W. Mittmann, 137–154. New York: Columbia University Press.
Bhandari, A., M. Pickford, and B.N. Tiwari. 2015. Basal Late Miocene mammal fauna from Tapar and Paduda, Kachchh. Münchener Geowissenschaftliche Abhandlungen, Reihe A Geologie Und Paläontologie 43: 1–4.
Bishop, W., and M.H.L. Pickford. 1975. Geology, fauna and palaeoenvironments of the Ngorora Formation, Kenya Rift Valley. Nature 254: 185–192.
Biswas, S.K. 1992. Tertiary Stratigraphy of Kutch. Journal of the Palaeontological Society of India 37: 1–29.
Borges, R.M., A. Mendes, S.S.C. Nogueira, J. Bindelle, and S.L.G. Nogueira-Filho. 2017. Protein requirements of collared peccary (Pecari tajacu). Tropical Animal Health and Production. https://doi.org/10.1007/s11250-017-1333-5.
Bouchal, J.M., S. Mayda, R. Zetter, F. Grímsson, F. Akgün, and T. Denk. 2017. Miocene palynofloras of the Tinaz lignite mine, Mugla, southwest Anatolia: taxonomy, palaeoecology and local vegetation change. Review of Palaeobotany and Palynology 243: 1–36.
Cande, S.C., and D. Kent. 1995. Revised calibration of the geomagnetic polarity timescale for the Late Cretaceous and Cenozoic. Journal of Geophysical Research: Solid Earth 100 (B4): 6093–6095.
Casanovas-Vilar, I., L.W. van den Hoek Ostende, M. Furió, and P.A. Madern. 2014. The range and extent of the Vallesian Crisis (Late Miocene): new prospects based on the micromammal record from the Vallès-Penedès basin (Catalonia, Spain). Journal of Iberian Geology 40 (1): 29–48.
Casanovas-Vilar, I., A. Madern, D.M. Alba, L. Cabrera, I. Garcia-Paredes, L.W. van den Hoek Ostende, D. DeMiguel, J.M. Robles, M. Furio, J. van Dam, M. Garcés, C. Angelone, and S. Moyà-Solà. 2016. The Miocene mammal record of the Valles-Penedes Basin (Catalonia). Comptes Rendus Palevol 15: 791–812.
Catuneanu, O., and A. Dave. 2017. Cenozoic sequence stratigraphy of the Kachchh Basin, India. Marine and Petroleum Geology 86: 1106–1132.
Cerling, T.E., Y. Wang, and J. Quade. 1993. Expansion of C4 ecosystems as an indicator of global ecologcial change in the late Miocene. Nature 361: 344–345.
Clauss, M., J. Nijboer, J.H.M. Loermans, T. Roth, J. Van der Kuilen, and A.C. Beynen Comparative digestion studies in wild suids at Rotterdam Zoo. Zoo Biology 27: 305–319.
Colbert, E.H. 1935. Siwalik Mammals in the American Museum of Natural History. Transactions of the American Philosophical Society 26: 407.
Corvinus, G., and L.N. Rimal. 2001. Biostratigraphy and geology of the Neogene Siwalik Group of the Surai Khola and Rato Khola areas in Nepal. Palaeogeography, Palaeoclimatology, Palaeoecology 165: 251–279.
Crissey, S. 2005. The complexity of formulating diets for zoo animals: A matrix. International Zoo Yearbook 39: 36–43.
Daams, R., and M. Freudenthal. 1981. Aragonian: The Stage concept versus Neogene Mammal zones. Scripta Geologica 62: 1–16.
Das, A., S. Katole, A. Kumar, S.P. Gupta, M. Saini, and D. Swarup. 2012. Feed consumption, nutrient utilization and serum metabolite profile of captive blackbucks (Antelope cervicapra) fed diets varying in crude protein content. Journal of Animal Physiology and Animal Nutrition 96: 442–449.
de Bruijn, H., R. Daams, G. Daxner-Höck, V. Fahlbusch, L. Ginsburg, P. Mein, J. Morales, E.P.J. Heizmann, D.F. Mayhew, A.J. van der Meulen, N. Schmidt-Kittler, and M. Telles Antunes. 1992. Report of the RCMNS working group on fossilmammals, Reisensburg 1990. Newsletters on Stratigraphy 26 (2/3): 65–118.
Deino, A., L. Tauxe, M. Monaghan, and R. Drake. 1990. 40Ar/39Ar age calibration of the litho- and palaeomagnetic stratigraphies of the Ngorora Formation, Kenya. Journal of Geology 98: 567–587.
Demicco, R.V., T.K. Lowenstein, and L.A. Hardie. 2003. Atmospheric pCO2 since 60 Ma from records of seawater pH, calcium, and primary carbonate mineralogy. Geology 31 (9): 793–796.
Deng T., Q. Zhan-Xiang, W. B.-Y. Wang Xiao-Ming, and H. Su-Kuan. 2013. Late cenozoic biostratigraphy of the Linxia Basin, Northweistern China. In Fossil Mammals of Asia: Neogene biostratigraphy and chronology, eds. Wang Xiao-ming, L.J. Flynn, and M. Fortelius, 243–273. New York: Columbia University Press.
Domingo, L.P.L., S.T. Koch, J. Morales. Grimes, and N. López-Martínez. 2012. Isotopic paleoecology of mammals and the Middle Miocene Cooling event in the Madrid Basin (Spain). Palaeogeography, Palaeoclimatology, Palaeoecology 339–341: 98–113.
Dong Jin-long, N., S..K.. L.. Gruda, Li. Xun, and D.. Zeng-qiang. 2018. Effects of elevated CO2 on nutritional quality of vegetables: a review. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00924.
Ehleringer, J.R., T.E. Cerling, and M.D. Dearing. 2020. Atmospheric CO2 as a global change driver influencing plant-animal interactions. Integrative and Comparative Biology 42: 424–430.
Falconer, H. 1868. XXV Notes on fossil remains found in the valley of the Indus below Attock and at Jubbulpoor. In Palaeontological memoirs and notes of the late Hugh Falconer, A.M., D.M. Volume 1: Fauna Antiqua Sivalensis, ed. C. Murchinson, 414–417. London: Robert Hardwicke.
Flynn, L.J., J.C. Barry, M.E. Morgan, D. Pilbeam, L.L. Jacobs, and E.H. Lindsay. 1995. Neogene Siwalik mammalian lineages: Species longevities, rates of change, and modes of speciation. Palaeogeography, Palaeoclimatology, Palaeoecology 115: 149–264.
Fortelius, M., J. van der Made, and R.L. Bernor. 1996. Middle and Late Miocene Suoidea of Central Europe and the Eastern Mediterranean: Evolution, Biogeography and Paleoecology. In The evolution of Western Eurasian Neogene Mammal Faunas, ed. R.L. Bernor, V. Fahlbusch, and H.W. Mittmann, 344–377. New York: Columbia University Press.
Fuss, J., G. Uhlig, and M. Böhme. 2018. Earliest evidence of caries lesion in hominids reveal sugar-rich diet for a Middle Miocene dryopithecine from Europe. PLoS One 13 (8): e0203307. https://doi.org/10.1371/journal.pone.0203307.
Geraads, D. 2013. Large Mammals from the Late Miocene of Çorakyerler, Çankiri Turkey. Acta Zoologica Bulgarica 65 (3): 381–390.
Gervais, P. 1859. Zoologie et Paléontologie françaises, 2nd ed. Paris: Bertrand.
Golpe-Posse, J.M. 1972. Suiformes del Terciario Español y sus yacimientos. Paleontolgía y Evolución 2: 1–197.
Gray, J.E. 1821. On the natural arrangement of vertebrose animals. London Medical Repository 15 (1): 296–310.
Hilgen, F.J., L.J. Lourens, and J.A. van Dam. 2012. The neogene period. In The geologic time scale 2012, eds. F.M. Gradstein, J.G. Ogg, M.B. Schmitz, and G.M. Ogg, 923–978. Elsevier.
Högy, P., and A. Fangmeier. 2008. Effects of elevated atmospheric CO2 on grain quality of wheat. Journal of Cereal Science 48: 580–591.
Hunter, J.P., and M. Fortelius. 1994. Comparative dental occlusal morphology, facet development, and microwear in two sympatric species Listriodon (Mammalia, Suidae) from the Middle Miocene of western Anatolia (Turkey). Journal of Vertebrate Paleontology 14 (1): 105–126.
Hussain, S.T., J. Munthe, S.M.I. Shah, R.M. West, and J.R. Lukacs. 1979. Neogene stratigraphy and fossil vertebrates of the Daud Khel Area, Mianwali District, Pakistan. Memoirs of the Geological Survey of Pakistan 13: 1–14.
Jacobs, B.F., and A.L. Deino. 1996. Test of climate-leaf physiognomy regression models, their application to two Miocene floras from Kenya, and 40Ar/39Ar dating of the Late Miocene Kapturo site. Palaeogeography, Palaeoclimatology, Palaeoecology 123 (1–4): 259–271.
Jacobs, B.F., and C.H.S. Kabuye. 1987. A middle Miocene (12.2 my old) forest in the East African Rift Valley, Kenya. Journal of Human Evolution 16: 147–155.
Jacobs, B.F., and D.A. Winkler. 1992. Taphonomy of a middle Miocene autochthonous forest assemblage, Ngorora Formation, central Kenya. Palaeogeography, Palaeoclimatology, Palaeoecology 99 (1–2): 31–40.
Jacobs, B.F., J.D. Kingston, and L.L. Jacobs. 1999. The origin of grass-dominated ecosystems. Annals of the Missouri Botanical Garden 86 (2): 590–643.
Jacobs, B.F., A.D. Pan, and C.R. Scotese. 2010. A Review of the Cenozoic Vegetation History of Africa. In Cenozoic Mammals of Africa, eds. L. Werdelin and W.J. Sanders, 57–72. Berkeley, Los Angeles, and London: University of California Press.
Janis, C. 1976. The evolutionary strategy of the Equidae and the origin of rumen and cecal digestion. Evolution 30: 757–774.
Janis, C.M. 1989. A climatic explanation for patterns of evolutionary diversity in ungulate mammals. Palaeontology 32: 463–481.
Janis, C.M., J. Damuth, and J.M. Theodor. 2000. Miocene ungulates and terrestrial primary productivity: Where have all the browsers gone? Proceedings of the National Aacademy of Sciences 97 (14): 7899–7904.
Janis, C.M., J. Damuth, and J.M. Theodor. 2004. The species richness of Miocene browsers, and implications for habitat type and primary productivity in the North American grassland biome. Palaeogeography, Palaeoclimatology, Palaeoecology 207: 371–398.
Johnson, N.M., N.D. Opdyke, G.D. Johnson, E.H. Lindsay, and R.A.K. Tahirkheli. 1982. Magnetic polarity stratigraphy and ages of Siwalik Group rocks of the Potwar Plaetau, Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 37: 17–42.
Kälin, D., and O. Kempf. 2009. High-resolution stratigraphy from the continental record of the Middle Miocene Northern Alpine Foreland Basin of Switzerland. Neues Jahrbuch Für Geolologie Und Paläontologie, Abhandlungen 254 (1–2): 177–235.
Kappelman, J., A. Duncan, M. Feseha, Juha Pekka Lunkka, D. Ekart, F. McDowell, T.M. Ryan, and C.C. Swisher III. 2003. Chronology. In Geology and paleontology of the Miocene Sinap formation, Turkey, ed. M. Fortelius, J. Kappelman, S. Sen, and R.L. Bernor, 41–66. New York: University of Columbia Press.
Kaya, F., N. Kaymakçi, F. Bibi, J.T. Eronen, C. Pehlevan, A.C. Erkman, C.G. Langereis, and M. Fortelius. 2016. Magnetostratigraphy and paleoecology of the hominid-bearing locality Çorakyerler, Tuglu Formation (Çankiri Basin, Central Anatolia). Journal of Vertebrate Paleontology. https://doi.org/10.1080/02724634.2015.1071710.
Khan, M.A., M. Akhtar, and T. Ikram. 2012. True ungulates from the Nagri type locality (Late Miocene), Northern Pakistan. The Journal of Animal and Plant Sciences, Supplementary Series 22: 1–59.
Kingston, J.D., B.F. Jacobs, A. Hill, and A. Deino. 2002. Stratigraphy, age and environments of the late Miocene Mpesida Beds, Tugen Hills, Kenya. Journal of Human Evolution 42 (1–2): 95–116.
Koufos, G.D., and L. de Bonis. 2006. New material of Ouranopithecus macedoniensis from late Miocene of Macedonia (Greece) and study of its dental attrition. Geobios 39: 223–243.
Kovar-Eder, J., Z. Kvacek, E. Zastawniak, R. Givulescu, L. Hably, D. Hihailjovic, J. Teslenko, and H. Walther. 1996. Floristic trends in the vegetation of the Paratethys surrounding areas during Neogene time. In The evolution of Western Eurasian Neogene Mammal Faunas, ed. R.L. Bernor, V. Fahlbusch, and S. Rietschel, 395–423. New York: Columbia University Press.
La Brecque, J.L., D.V. Kent, and S.C. Cande. 1977. Revised magnetic polarity time scale for Late Cretaceous and Cenozoic time. Geology 5: 330–335.
Langer, P. 1986. Large mammalian herbivores in tropical forests with either hindgut- or forestomach-fermentation. Zeitschrift Für Säugetierkunde 51: 173–187.
Legendre, S. 1986. Analysis of mammalian communities from the Late Eocene and Oligocene of southern France. Palaeovertebrata 16 (4): 191–212.
Leinders, J.J.M. 1977a. A functional interpretation of the dental morphology of Listriodon (Suina). Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen, Series B 80: 61–69.
Leinders, J.J.M. 1977b. The configuration of the wear facets on the premolars of Listriodon (Suidae, Artiodactyla) and its implications. Proceedings of the Koninklijke Nederlandse Akademie Der Wetenschappen, Series B 80: 360–366.
Leus, K.Y.G. 1994. Foraging behaviour, food selection and diet digestion of Babyrousa babyrussa (Suidae, Mammalia). Unpublished PhD thesis, 1–361. The University of Edinburgh.
Leus, K., G.P. Goodall, and A.A. Macdonald. 1999. Anatomy and histology of the babirusa (Babyrousa babyrussa) stomach. Comptes Rendus De L’académie Des Sciences Paris 322: 1081–1092.
Lourens, L., F. Hilgen, N.J. Shackleton, J. Laskar, and J. Wilson. 2004. Orbital tuning calibrations and conversions for the Neogene Period. In A Geologic Time Scale 2004, ed. F.M. Gradstein, J.G. Ogg, and A.G. Smith, 469–471. Cambridge: Cambridge University Press.
Lydekker, R. 1876. Wallace’s “geographical distribution of animals.” Nature 14 (364): 544.
Lydekker, R. 1878. Notices of Siwalik mammals. Records of the Geological Survey of India 11: 64–104.
Madern, P.A., J.M.M.S. van de Put, I. Casanovas-Vilar, and L.W. van den Hoek Ostende. 2018. Iberian micromammals show local extent of Vallesian Crisis. Palaeogeography, Palaeoclimatology, Palaeoecology 496: 18–31.
Martinez, J.N., and J. Sudre. 1995. The astragalus of Paleogene artiodactyls: Comparative morphology, variability and prediction of body mass. Lethaia 28 (3): 197–209.
Matthew, W.D. 1929. Critical observations upon Siwalik mammals (exclusive of Proboscidea). Bulletin American Museum of Natural History 56 (7): 437–560.
Medek, D.E., J. Schwartz, and S.S. Myers. 2017. Estimated effects of future atmospheric CO2 concentrations on protein intake and the risk of protein deficiency by country and region. Environmental Health Perspectives 125 (8): 087002.
Mein, P. 1975. Résultat du groupe de travail des vertébrés. In Report on activity of the R.C.M.N.S. working groups (1971–1975), ed. J. Senes, 78–81. Bratislava: Regional Committee on Mediterranean Neogene Stratigraphy.
Miller, K.G., J.D. Wright, and R.G. Fairbanks. 1991. Unlocking the ice house Oligocene–Miocene oxygen isotopes eustasy and margin erosion. Journal of Geophysical Research 96 (B4): 6829–6848.
Moyà-Solà, S. 1983. Los Boselaphini (Bovidae, Mammalia) del Neógeno de la Peniínsula Ibeérica. Universidad Autonoma de Barcelona. Publicaciones De Geología 18: 1–236.
Moyà-Solà, S., and J. Agustí. 1990. Bioevents and mammal successions in the Spanish Miocene. In European neogene mammal chronology, ed. E.H. Lindsay, V. Fahlbusch, and P. Mein, 357–373. New York and London: Plenum Press.
Munthe, J., B. Dongol, J.H. Hutchison, W.F. Kean, K. Munthe, and R.M. West. 1983. New fossil discoveries from the Miocene of Nepal include a hominoid. Nature 303: 331–333.
Myers, S.S., A. Zanobetti, I. Kloog, P. Huybers, A.D.B. Leakey, A.J. Bloom, E. Carlisle, L.H. Dietterich, G. Fitzgerald, T. Hasegawa, N.M. Holbrook, R.L. Nelson, M.J. Ottman, V. Raboy, H. Sakai, K.A. Sartor, J. Schwartz, S. Seneweera, M. Tausz, and Y. Usui. 2014. Increasing CO2 threatens human nutrition. Nature 510 (7503): 139–142.
Nicolet, C. 1844. Sur une dent fossile de Lophiodon. Bulletin De La Société Des Sciences Naturelles De Neuchâtel 1843–1844: 34.
Nogueira-Filho, S.L.G., R.M. Borges, A. Mendes, and C.T.S. Dias. 2014. Nitrogen requirements of white-lipped peccary (Mammalia, Tayassuidae). Zoo Biology 33: 320–326.
Opdyke, N.D., E. Lindsay, G.D. Johnson, N. Johnson, R.A.K. Tahirkheli, and M.A. Mirza. 1979. Magnetic polarity stratigraphy and vertebrate paleontology of the Upper Siwalik Subgroup of northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 27: 1–34.
Orliac, M. 2007. Le rôle des Listriodontinae dans la différenciation des Suidae (Mammalia): paléoanatomie, systématique, phylogénie. Unpublished PhD thesis, 1–705. Paris 6.
Orliac, M.J. 2009. The differentiation of bunodont Listriodontinae (Mammalia, Suidae) of Africa: New data from Kalodirr and Moruorot, Kenya. Zoological Journal of the Linnean Society 157: 653–678.
Ozansoy, F. 1965. Études des gisements continentaux et de Mammifères du Cénozoïque du Turquie. Mémoires De La Societé Géologique De France, Nouvelle Série 44: 1–92.
Pagani, M., K.H. Freeman, and M.A. Arthur. 1999a. Late Miocene atmospheric CO2 concentrations and the expansion of C4 grasses. Science 285: 876–879.
Pagani, M., K.H. Freeman, and M.A. Arthur. 1999b. Miocene evolution of atmospheric carbon dioxide. Paleoceanography 14 (3): 273–292.
Patnaik, R. 2013. Indian Neogene Siwalik mammalian biostratigraphy: An overview. In Fossil Mammals of Asia: Neogene biostratigraphy and chronology, ed. X. Wang, L.J. Flynn, and M. Fortelius, 423–444. New York: Columbia University Press.
Patnaik, R. 2015. Diet and habitat changes among Siwalik herbivorous mammals in response to Neogene and Quaternary climate changes: An appraisal in the light of new data. Quaternary International 371: 232–243.
Patnaik, R., D. Cameron, J.C. Sharma, and J. Hogarth. 2005. Evolution and extinction of Siwalik fossil apes: A review based on new fossil ape, palaeoecological and palaeoclimatological data. Anthropological Science 113 (1): 65–72.
Patnaik, R., T.E. Cerling, K.T. Uno, and J.G. Fleagle. 2014. Diet and habitat of Siwalik primates, Indopithecus, Sivaladapis and Theropithecus. Annales Zoologici Fennici 15 (21): 123–142.
Patnaik, R., N.P. Singh, D. Paul, and R. Sukumar. 2019. Dietary and habitat shifts in relation to climate of Neogene-Quaternary proboscideans and associated mammals of the Indian subcontinent. Quaternary Science Reviews 224: 105968.
Pearson, P.N., and M.R. Palmer. 2000. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406: 695–699.
Pickford, M. 1988. Revision of the Miocene suidae of the Indian subcontinent. Münchener Geowissenschaftliche Abhandlungen, Reihe a, Geologie Und Paläontologie 12: 1–91.
Pickford, M. 2001. Equidae in the Ngorora Formation, Kenya, and the first appearance of the family in East Africa. Revista Española De Paleontología 16 (2): 339–345.
Pickford, M. 2017. Revison of “peccary-like” Suoidea (Artiodactyla: Mammalia) from the Neogene of the Old World. Münchener Geowissenschaftliche Abhandlungen, Series A 46: 1–44.
Pickford, M., and C. Ertürk. 1979. Suidae and Tayassuidae from Turkey. Bulletin of the Geological Society of Turkey 22: 141–154.
Pickford, M., and J. Morales. 2003. New Listriodontinae (Suidae, Mammalia) from Europe and a review of listriodont evolution, biostratigraphy and biogeography. Geodiversitas 25: 1–58.
Pickford, M., and A.F. Wilkinson. 1975. Stratigraphic and phylogenetic implications of new Listriodontiae from Kenya. Netherlands Journal of Zoology 25: 128–137.
Pilgrim, G.E. 1926. The Fossil Suidae of India. Memoirs of the Geological Survey of India, New Series 8 (4): 1–65.
Prasad, K.N. 1971. Ecology of the fossil Hominoidea from the Siwaliks of India. Nature 232: 413–414.
Prasad, M., and N. Awasthi. 1996. Contribution to the Siwalik flora from Surai Khola sequence, western Nepal and its palaeoecological and phytogeographical implications. Palaeobotanist 43 (3): 1–42.
Prasad, M., and S.M. Pandey. 2008. Plant diversity and climate during Siwalik (Miocene-Pliocene) in the Himalayan foot hills of western Nepal. Palaeontographica Abteilung B 278 (1–3): 13–70.
Priebe, J.C., and R.D. Brown. 1987. Protein requirements of subadult nilgai antelope. Comparative Biochemistry and Physiology 88A (3): 495–501.
Quade, J., and T.E. Cerling. 1995. Expansion of C4 grasses in the late Miocene of northern Pakistan: Evidence from stable isotopes in paleosols. Palaeogeography, Palaeoclimatology, Palaeoecology 115 (1–4): 91–116.
Quade, J., T.E. Cerling, P. Andrews, and B. Alpagut. 1995. Paleodietary reconstruction of Miocene faunas from Pasalar, Turkey using stable carbon and oxygen isotopes of fossil tooth enamel. Journal of Human Evolution 28 (4): 373–384.
Rao, A.R. 1993. Magnetic-polarity stratigraphy of Upper Siwalik of north-western Himalayan foothills. Current Science 64 (11–12): 863–873.
Raza, S.M., I.U. Cheema, W.R. Downs, A.R. Rajpar, and S.C. Ward. 2002. Miocene stratigraphy and mammal fauna from the Sulaiman Range, Southwestern Himalayas, Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 186: 185–197.
Rusconi, C. 1937. “Listriodon Dupuyi” y sus relaciones con los tapires. Anales De La Sociedad Científica Argentina 123: 87.
Sangode, S.J., and R. Kumar. 2003. Magnetostratigraphic correlation of the Late Cenozoic fluvial sequences from NW Himalaya, India. Current Science 84 (8): 1014–1024.
Sanyal, P., S.K. Bhattacharya, R. Kumar, S.K. Ghosh, and S.J. Sangode. 2004. Mio-Pliocene monsoonal record from Himalayan foreland basin (Indian Siwalik) and its relation to vegetational change. Palaeogeography, Palaeoclimatology, Palaeoecology 205: 23–41.
Sen, S., G. Seyitoglu, L. Karadenizu, N. Kazanci, B. Varol, and H. Araz. 1998. Mammalian biochronology of Neogene deposits and its correlation with the lithostratigraphy in the Çankiri-Çorum Basin, central Anatolia, Turkey. Eclogae Geologicae Helvetiae 91: 307–320.
Sharma, K.M., N.A. Singh, R. Patnaik, R.P. Tiwari, N.P. Singh, Y.P. Singh, D. Choudhary, and S.K. Lalotra. 2021. Sharks and rays (chondrichthyes, elasmobranchii) from the Miocene sediments of Kutch, Gujarat, India: Paleoenvironmental and paleobiogeographic implications. Historical Biology. https://doi.org/10.1080/08912963.2021.1893712.
Singh, N.P., K.M. Sharma, R. Patnaik, N.A. Singh, and Y.P. Singh. 2019. Teleostei fish remains from the Miocene of Kutch, Gujarat, India: Palaeoenvironmental implications. Indian Journal of Geosciences 73 (2): 79–88.
Sosdian, S.M., R. Greenop, M.P. Hain, G.L. Foster, P.N. Pearson, and C.H. Lear. 2018. Constraining the evolution of Neogene ocean carbonate chemistry using the boron isotope pH proxy. Earth and Planetary Science Letters 498: 362–376. https://doi.org/10.1016/j.epsl.2018.06.017.
Spassov, N., D. Geraads, L. Hristova, G.N. Markov, G. Merceron, T. Tzankov, K. Stoyanov, M. Böhme, and A. Dimitrova. 2012. A hominid tooth from Bulgaria: The last pre-human hominid of continental Europe. Journal of Human Evolution 62: 138–145.
Srivastava, G., K.N. Paudayal, T. Utescher, and R.C. Mehrotra. 2018. Miocene vegetation shift and climate change: evidence from the Siwalik of Nepal. Global and Planetary Change 161: 108–120.
Steininger, F.F., W.A. Berggren, D.V. Kent, R.L. Bernor, S. Sen, and J. Agustí. 1996. Cirucum-Mediterranean Neogene (Miocene and Pliocene) marine-continental chronologic correlations of European Mammal Units. In The evolution of Western Eurasian Neogene Mammal Faunas, ed. R.L. Bernor, V. Fahlbusch, and S. Rietschel, 7–46. New York: Columbia University Press.
Steinthorsdottir, M., H.K. Coxall, A.M. de Boer, M. Huber, N. Barbolini, C.D. Bradshaw, N.J. Burls, S.J. Feakins, E. Gasson, J. Henderiks, A.E. Holbourn, S. Kiel, M.J. Kohn, G. Knorr, W.M. Kürschner, C. H. Lear, D. Liebrand, D.J. Lunt, T. Mörs, P.N. Pearson, M.J. Pound, H. Stoll, and C.A.E. Strömberg. 2021. The Miocene: The future of the past. Paleoceanography and Paleoclimatology 36: e2020PA004037. https://doi.org/10.1029/2020PA004037
Suc, J.P., S. Fauquette, M. Bessedik, A. Bertini, Zhuo Zheng, G. Clauzon, D. Suballyova, F. Diniz, P. Quézel, N. Feddi, M. Clet, E. Bessais, N. Bachiri Taoufiq, H. Meon, and N. Comboourieu-Nebout. 1999. Neogene vegetation changes in West European and West circum-Mediterranean areas. In Hominoid evolutiona and climatic change in Europe. Vol 1. The evolution of Neogene Terrestrial Ecostystems in Europe, eds. J. Agustí, L. Rook, and P. Andrews, 378–388. Cambridge: Cambridge University Press.
Super, J.R., E. Thomas, M. Pagani, M. Huber, C. O’Brien, and P.M. Hull. 2018. North Atlantic temperature and pCO2 coupling in the early-middle Miocene. Geology 46 (6): 519–522. https://doi.org/10.1130/G40228.1.
Tauxe, L., and N.D. Opdyke. 1982. A time framework based on magnetostratigraphy for the Siwalik sediments of the Khaur area, northern Pakistan. Palaeogeography, Palaeoclimatology, Palaeoecology 37 (1): 43–61.
Tauxe, L., M. Monaghan, R. Drake, G. Curtis, and H. Staudigel. 1985. Paleomagnetism of Miocene East African rift sediments and the calibration of the geomagnetic reversal time scale. Journal of Geophysical Research 90: 4639–4646.
Thomas, D.W. 1984. Fruit intake and energy budgets of frugivorous bats. Physiological Zoology 57 (4): 457–467.
Tsubamoto, T., Y. Kunimatsu, T. Sakai, M. Saneyoshi, D. Shimizu, N. Morimoto, H. Nakaya, and M. Nakatsukasa. 2017. Listriodontine suid and tragulid artiodactyls (Mammalia) from the upper Miocene Nakali Formation, Kenya. Paleontological Research 21 (4): 347–357.
van Dam, J.A. 1997. The small mammals from the Upper Miocene of the Teruel-Alfambra region (Spain): Paleobiology and pleoclimatic reconstructions. Geologica Ultraiectina 156: 1–204.
van de Wal, R.S.W., B. de Boer, L.J. Lourens, P. Köhler, and R. Bintanja. 2011. Reconstruction of a continuous high-resolution CO2 record over the past 20 million years. Climate of the Past 7: 1459–1469.
van der Made, J. 1988. Iberian Suoidea (pigs and peccaries). In Coloquio homenaje a Rafael Adrover 'Bioeventos and Sucesiones faunísticas en el Terciario Continental Ibérico', Sabadell, 14 - 16/12/1988, ed. Anonymous, 20–21. Sabadell: IPS.
van der Made, J. 1990a. Iberian Suoidea. Paleontologia i Evolució 23: 83–97.
van der Made, J. 1990b. A range chart for European Suidae and Tayassuidae. Paleontologia i Evolució 23: 99–104.
van der Made, J. 1991. Climatical changes and species diversity in Suoidea. In Abstracts, International Union for Quaternary Research, XIII International Congress, August 2–9, 1991. Beijing, ed. Anonymous, 365. Beijing.
van der Made, J. 1992. Migrations and climate. Courier Forschungsinstitut Senckenberg 153: 27–39.
van der Made, J. 1996a. Listriodontinae (Suidae, Mammalia), their evolution, systematics and distribution in time and space. Contributions to Tertiary and Quaternary Geology 33 (1–4): 3–254.
van der Made, J. 1996b. Albanohyus, a small Miocene pig. Acta Zoologica Cracoviense 38 (1): 293–303.
van der Made, J. 1997a. Systematics and stratigraphy of the genera Taucanamo and Schizochoerus and a classification of the Palaeochoeridae (Suoidea, Mammalia). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 100 (1–2): 127–139.
van der Made, J. 1997b. On Bunolistriodon (=Eurolistriodon) and kubanochoeres. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 100 (1–2): 141–160.
van der Made, J. 1997c. Los Suoidea de la Península Ibérica. In Avances en el conocimiento del Terciario Ibérico, eds. J.P. Calvo and J. Morales, 109–112. Cuenca.
van der Made, J. 1999. Biometrical trends in the Tetraconodontinae, a subfamily of pigs. Transactions of the Royal Society of Edinburgh, Earth Sciences 89: 199–225.
van der Made, J. 2003a. Fossil Suoidea of the Miocene Sinap Formation, Turkey. In Geology and paleontology of the Miocene Sinap formation, Turkey, eds. M. Fortelius, J. Kappelman, S. Sen, and R.L. Bernor, 308–327. New York: University of Columbia Press.
van der Made, J. 2003b. Suoidea (pigs) from the hominoid locality of Çandir in Turkey. Courier Forschungs-Institut Senckenberg 240: 149–179.
van der Made, J. 2010. The pigs and “Old World peccaries” (Suidae and Palaeochoeridae, Suoidea, Artiodactyla) from the Miocene of Sandelzhausen (southern Germany): Phylogeny and an updated classification of the Hyotheriinae and Palaeochoeridae. Paläontologische Zeitschrift 84: 43–121.
van der Made, J. 2014. Late Pleistocene European and Late Miocene African acceleration of faunal change in relation to climate and as a background to human evolution. Quaternary International 326–327: 431–447.
van der Made, J. 2020. The Suoidea from the Middle Miocene of Gracanica (Bugojno Basin, Bosnia and Herzegovina) - evolution, taxononomy, and biostratigraphy. Palaeobiodiversity and Palaeoenvironments 100 (2): 321–349.
van der Made, J., and D. Han. 1994. Suoidea from the hominoid locality Lufeng (Yunnan, China). Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen 97 (1): 27–82.
van der Made, J., and S.T. Hussain. 1989. “Microstonyx” major (Suidae, Artiodactyla) from Nagri. Estudios Geológicos 45: 409–416.
van der Made, J., and J. Morales. 2003. The pig Conohyus simorrensis from the Upper Aragonian of Alhambra, Madrid, and a review of the distribution of European Conohyus. Estudios Geológicos 59 (5–6): 303–312.
van der Made, J., and V. Tuna. 1999. A tetraconodontine pig from the Vallesian of Turkey. Transactions of the Royal Society of Edinburgh, Earth Sciences 89: 227–230.
van der Made, J., E. Güleç, and C.A. Erkman. 2013. Microstonyx (Suidae, Artiodactyla) from the Upper Miocene of Hayranli-Haliminhani, Turkey. Turkish Journal of Zoology 37: 106–122.
van der Meulen, A.J., I. García-Paredes, M.A. Álvarez-Sierra, L.W. van den Hoek Ostende, K. Hordijk, A. Oliver, and P. Peláez Campomanes. 2012. Updated Aragonian biostratigraphy: Small Mammal distribution and its implications for the Miocene European Chronology. Geologica Acta 10 (2): 159–179.
Vasishat, R.N., I.J. Suneja, R. Gaur, and S.R.K. Chopra. 1983. Neogene mammals (equids, rhinocerotids, suids and anthracotherids) from the Siwalik beds of Kangra District, Himachal Pradesh, India. Publications of the Centre of Advanced Study in Geology 13: 207–215.
von Meyer, H. 1846. Mitteilungen an Prof. Bronn gerichtet (Brief). Neues Jahrbuch Für Mineralogie, Geologie Und Paläontologie 1846: 462–476.
von Meyer, H. 1866. Ueber die Fossilen von Wirbeltieren welche die Herren von Schlagintweit von Ihren Reisen Indien und Hochasien mittgebracht haben. Palaeontographica 15: 1–40.
Westerhold, T., T. Bickert, and U. Röhl. 2005. Middle to late Miocene oxygen isotope stratigraphy of ODP site 1085 (SE Atlantic): new constrains on Miocene climate variability and sea-level fluctuations. Palaeogeography, Palaeoclimatology, Palaeoecology 217 (3–4): 205–222.
Willis, B.J., and A.K. Behrensmeyer. 1995. Fluvial systems in the Siwalik Miocene and Wyoming paleogene. Palaeogeography, Palaeoclimatology, Palaeoecology 115 (1–4): 13–35.
Wilson, D.E., and R.A. Mittermeier (eds). 2011. Handbook of the Mammals of the World. 2 Hoofed mammals. Lynx Edicions - Barcelona: 1–886.
Yang, X., P. Zhang, W. Zhen-Hua, L. Liu, H. Xiao-Tao, and L. Fu-Lai. 2020. Effects of CO2 fertilization on tomato fruit quality under reduced irrigation. Agricultural Water Management 230: 105985.
Yavuz-Isik, N., and V. Toprak. 2010. Palynostratigraphy and vegetation characteristics of Neogene continental deposits interbedded with the Cappadocia ignimbrites Central Anatolia, Turkey. International Journal of Earth Sciences 99: 1887–1897.
Zaleha, M.J. 1997. Fluvial and lacustrine palaeoenvironments of the Miocene Siwalik Group, Khaur area, northern Pakistan. Sedimentology 44: 349–368.
Acknowledgements
We thank V. González Gascón for preparing the map in Fig. 1 and the reviewers and editors, Drs. A. Souron, M. A. Khan, T. Mörs, and M. Reich, for their comments that improved the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. JvdM participates in projects PGC2018-095489-B-I00 and PGC2018-093925-B-C31 funded by the Spanish Ministerio de Ciencia, Inovación y Universidades (MCIU), Agencia Estatal de Investigación (AEI), and the European Regional Development Fund (ERDF). Rajeev Patnaik is supported by the Science and Engineering Research Board, Government of India, Grant number SERB-HRR/2018/000063.
Author information
Authors and Affiliations
Contributions
JvdM, DC, and RP wrote the paper, and field work and collecting were done by DC, NPS, KMS, NAS, and RP. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We do not have conflicts of interest.
Additional information
Handling Editor: Thomas Mörs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Made, J., Choudhary, D., Singh, N.P. et al. Listriodon dukkar sp. nov. (Suidae, Artiodactyla, Mammalia) from the late Miocene of Pasuda (Gujarat, India): the decline and extinction of the Listriodontinae. PalZ 96, 355–383 (2022). https://doi.org/10.1007/s12542-022-00606-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-022-00606-w