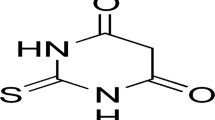
Abstract
The corrosion behavior of Alloy 22 (UNS N06022) according to the hydrogen sulfide, chloride, and pH in an anaerobic environment was investigated through electrochemical tests, surface analyses, and response surface methodology (RSM). Complex corrosion behavior model according to the HS− ion and Cl− ion concentrations and pH was obtained through RSM. The Cl− ion and pH had minimal effects on the corrosion behavior of Alloy 22. Meanwhile, the corrosion behavior of Alloy 22 changed significantly with increased HS− ion concentration. As the HS− ion concentration increases, sulfide ions are adsorbed on the metal surface, increasing the anodic dissolution of metal and suppressing the formation of Cr2O3 passive film, thereby deteriorating the passive properties of Alloy 22. Simultaneously, Mo in Alloy 22 forms MoS2, which acts as a pseudo-passive film to alleviate the corrosion resistance deterioration caused by HS− ions.
Graphical Abstract














Similar content being viewed by others
References
D. Shoesmith, Corrosion 62, 703–722 (2006). https://doi.org/10.5006/1.3278296
D.S. Hall, P.G. Keech, Corros. Eng. 52, 2–5 (2017). https://doi.org/10.1080/1478422X.2016.1275419
D. Féron, D. Crusset, J.-M. Gras, J. Nucl. Mater. 379, 16–23 (2008). https://doi.org/10.1016/j.jnucmat.2008.06.023
F. Zhang, C. Örnek, M. Liu, T. Müller, U. Lienert, V. Ratia-Hanby, L. Carpén, E. Isotahdon, J. Pan, Corros. Sci. 184, 109390 (2021). https://doi.org/10.1016/j.corsci.2021.109390
F. Cattant, D. Crusset, D. Féron, Mater. Today 11, 32–37 (2008). https://doi.org/10.1016/S1369-7021(08)70205-0
F. King, C. Lilja, K. Pedersen, P. Pitkänen, M. Vähänen An update of the state-of-the-art report on the corrosion of copper under expected conditions in a deep geologic repository, Swedish Nuclear Fuel and Waste Management Co. (2010)
F. King, Corrosion 69, 986–1011 (2013). https://doi.org/10.5006/0894
J.H. Payer, S. Finsterle, J.A. Apps, R.A. Muller, Energies 12, 1491 (2019). https://doi.org/10.3390/en12081491
D.-C. Kong, C.-F. Dong, X. Kui, X.-G. Li, Trans. Nonferrous Met. Soc. China 27, 1431–1438 (2017). https://doi.org/10.1016/S1003-6326(17)60165-1
F. King, in Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste, 2nd edn., ed. by M.J. Apted, J. Ahn (Woodhead Publishing, Cambridge, 2017), pp. 365–408
F. King, C. Lilja, M. Vähänen, J. Nucl. Mater. 438, 228–237 (2013). https://doi.org/10.1016/j.jnucmat.2013.02.080
G.S. Frankel, J.D. Vienna, J. Lian, X. Guo, S. Gin, S.H. Kim, J. Du, J.V. Ryan, J. Wang, W. Windl, Chem. Rev. 121, 12327–12383 (2021). https://doi.org/10.1021/acs.chemrev.0c00990
R. Carranza, JOM 60, 58–65 (2008). https://doi.org/10.1007/s11837-008-0009-z
R.B. Rebak, J.C. Estill, MRS Online Proc. Libr. (OPL). (2002). https://doi.org/10.1557/PROC-757-II4.1
D. Dunn, Y.-M. Pan, K. Chiang, L. Yang, G. Cragnolino, X. He, JOM 57, 49–55 (2005). https://doi.org/10.1007/s11837-005-0064-7
R.B. Rebak, Corrosion 65, 252–271 (2009). https://doi.org/10.5006/1.3319132
G.A. Cragnolino, D.S. Dunn, Y.-M. Pan, MRS Online Proc. Libr. (OPL) (2002). https://doi.org/10.1557/PROC-713-JJ1.6
J. Gray, C. Orme, Electrochim. Acta 52, 2370–2375 (2007). https://doi.org/10.1016/j.electacta.2006.08.043
A. Mishra, S. Ramamurthy, M. Biesinger, D. Shoesmith, Electrochim. Acta 100, 118–124 (2013). https://doi.org/10.1016/j.electacta.2013.03.161
A. Mishra, D. Shoesmith, Electrochim. Acta 102, 328–335 (2013). https://doi.org/10.1016/j.electacta.2013.03.177
J. Gray, J. Hayes, G. Gdowski, B. Viani, C. Orme, J. Electrochem. Soc. 153, B61 (2006). https://doi.org/10.1149/1.2160433
M.A. Rodriguez, Corros. Rev. (2012). https://doi.org/10.1515/corrrev.2011.022
N.S. Zadorozne, C. Giordano, M.A. Rodríguez, R.M. Carranza, R.B. Rebak, Electrochim. Acta 76, 94–101 (2012). https://doi.org/10.1016/j.electacta.2012.04.157
P. Jakupi, F. Wang, J. Noël, D. Shoesmith, Corros. Sci. 53, 1670–1679 (2011). https://doi.org/10.1016/j.corsci.2011.01.028
M.A. Rodríguez, R.M. Carranza, R.B. Rebak, J. Electrochem. Soc. 157, C1 (2009). https://doi.org/10.1149/1.3246790
K. Pedersen, Microbial Processes in Radioactive Waste Disposal, Technical Report, TR-00-04 (Swedish Nuclear Fuel and Waste Management Co., Stockholm, 2000)
J.-S. Lee, Y. Kitagawa, T. Nakanishi, Y. Hasegawa, K. Fushimi, J. Electrochem. Soc. 162, C685 (2015). https://doi.org/10.1149/2.0861512jes
M. Ahmadi, K. Rahmani, A. Rahmani, H. Rahmani, Pol. J. Chem. Technol. 19, 104–112 (2017). https://doi.org/10.1515/pjct-2017-0015
M. Wang, Z. Zhou, Q. Wang, Y. Liu, Z. Wang, X. Zhang, Results Phys. 15, 102708 (2019). https://doi.org/10.1016/j.rinp.2019.102708
M. Aliofkhazraei, A.S. Rouhaghdam, A. Heydarzadeh, Mater. Charact. 60, 83–89 (2009). https://doi.org/10.1016/j.matchar.2008.07.003
N.T. Chung, S.-R. Choi, J.-G. Kim, J. Electrochem. Soc. 169, 051503 (2022). https://doi.org/10.1149/1945-7111/ac700d/meta
N.T. Chung, Y.-S. So, W.-C. Kim, J.-G. Kim, Materials 14, 6596 (2021). https://doi.org/10.3390/ma14216596
S. Sarkar, I. Koley, R.K. Baranwal, A. Mukherjee, S. Lamichaney, G. Majumdar, J. Phys. Conf. Ser. 1593, 012040 (2020). https://doi.org/10.1088/1742-6596/1593/1/012040
G. Di Leo, F. Sardanelli, Eur. Radiol. Exp. 4, 18 (2020). https://doi.org/10.1186/s41747-020-0145-y
K. Rajkumar, M. Muthukumar, Appl Water Sci 7, 637–652 (2017). https://doi.org/10.1007/s13201-015-0276-0
S. Esmailzadeh, M. Aliofkhazraei, H. Sarlak, Prot. Met. Phys. Chem. Surf. 54, 976–989 (2018). https://doi.org/10.1134/S207020511805026X
Y.-H. Lee, S.-R. Choi, S.-J. Ko, J.-G. Kim, J. Phys. Chem. Solids 176, 111226 (2023). https://doi.org/10.1016/j.jpcs.2023.111226
J. Yang, H. Yang, H. Yu, Z. Wang, X. Zeng, Metall. Mater. Trans. A 48, 3583–3593 (2017). https://doi.org/10.1007/s11661-017-4087-9
K.F. Quiambao, S.J. McDonnell, D.K. Schreiber, A.Y. Gerard, K.M. Freedy, P. Lu, J.E. Saal, G.S. Frankel, J.R. Scully, Acta Mater. 164, 362–376 (2019). https://doi.org/10.1016/j.actamat.2018.10.026
Y. Xiao, J. Tang, Y. Wang, B. Lin, Z. Nie, Y. Li, B. Normand, H. Wang, Corros. Sci. 200, 110240 (2022). https://doi.org/10.1016/j.corsci.2022.110240
A. Oladoye, J. Carton, K. Benyounis, J. Stokes, A. Olabi, Surf. Coat. Technol. 304, 384–392 (2016). https://doi.org/10.1016/j.surfcoat.2016.07.023
X. Luo, R. Bai, D. Zhen, Z. Yang, D. Huang, H. Mao, X. Li, H. Zou, Y. Xiang, K. Liu, Ind. Crops Prod. 129, 405–413 (2019). https://doi.org/10.1016/j.indcrop.2018.12.029
H.-Y. Kim, Restor. Dent. Endod. 43, e34 (2018). https://doi.org/10.5395/rde.2018.43.e34
D.A. Jones, Principles and Prevention of Corrosion, 2nd edn. (Prentice Hall, Upper Saddle River, 1996)
W. Sun, S. Nešić, D. Young, R.C. Woollam, Ind. Eng. Chem. Res. 47, 1738–1742 (2008). https://doi.org/10.1021/ie070750i
M. Wiener, B. Salas, M. Quintero-Nunez, R. Zlatev, Corros. Eng. Sci. Technol. 41, 221–227 (2006). https://doi.org/10.1179/174327806X132204
P. Marcus, Electrochim. Acta 43, 109–118 (1998). https://doi.org/10.1016/S0013-4686(97)00239-9
V. Gattu, W. Ebert, J. Indacochea, S. Frank, Corros. Sci. 184, 109358 (2021). https://doi.org/10.1016/j.corsci.2021.109358
J. Dai, H. Feng, H.-B. Li, Z.-H. Jiang, H. Li, S.-C. Zhang, P. Zhou, T. Zhang, Corros. Sci. 174, 108792 (2020). https://doi.org/10.1016/j.corsci.2020.108792
J. Chivot, L. Mendoza, C. Mansour, T. Pauporté, M. Cassir, Corros. Sci. 50, 62–69 (2008). https://doi.org/10.1016/j.corsci.2007.07.002
A. Tomio, M. Sagara, T. Doi, H. Amaya, N. Otsuka, T. Kudo, Corros. Sci. 98, 391–398 (2015). https://doi.org/10.1016/j.corsci.2015.05.053
M. Shah, M. Ayob, R. Rosdan, N. Yaakob, Z. Embong, N. Othman, Sci. World J. (2020). https://doi.org/10.1155/2020/3989563
S.J. Ko, Y.H. Lee, K.S. Nam, E.H. Park, J.G. Kim, Mater. Chem. Phys. 302, 127747 (2023). https://doi.org/10.1016/j.matchemphys.2023.127747
C. Della Rovere, J. Alano, R. Silva, P. Nascente, J. Otubo, S. Kuri, Corros. Sci. 57, 154–161 (2012). https://doi.org/10.1016/j.corsci.2011.12.022
Z. Cui, S. Chen, Y. Dou, S. Han, L. Wang, C. Man, X. Wang, S. Chen, Y.F. Cheng, X. Li, Corros. Sci. 150, 218–234 (2019). https://doi.org/10.1016/j.corsci.2019.02.002
Y.-S. Kim, J.-G. Kim, Mater. Trans. 58, 76–84 (2017). https://doi.org/10.2320/matertrans.M2016310
P. Mourya, P. Singh, A. Tewari, R. Rastogi, M. Singh, Corros. Sci. 95, 71–87 (2015). https://doi.org/10.1016/j.corsci.2015.02.034
Y.-H. Lee, M.-S. Hong, S.-J. Ko, J.-G. Kim, Materials 14, 2722 (2021). https://doi.org/10.3390/ma14112722
A. Bahramian, M. Eyraud, S. Maria, F. Vacandio, T. Djenizian, P. Knauth, Corros. Sci. 149, 75–86 (2019). https://doi.org/10.1016/j.corsci.2018.12.026
H. Huang, F. Bu, Corros. Sci. 165, 108413 (2020). https://doi.org/10.1016/j.corsci.2019.108413
J. Moreto, C. Marino, W. Bose Filho, L. Rocha, J. Fernandes, Corros. Sci. 84, 30–41 (2014). https://doi.org/10.1016/j.corsci.2014.03.001
T. Bellezze, G. Giuliani, G. Roventi, Corros. Sci. 130, 113–125 (2018). https://doi.org/10.1016/j.corsci.2017.10.012
J.N. Kuhn, N. Lakshminarayanan, U.S. Ozkan, J. Mol. Catal. A: Chem. 282, 9–21 (2008). https://doi.org/10.1016/j.molcata.2007.11.032
J.A. Leiro, E.E. Minni, Philos. Mag. B 49, L61–L64 (1984). https://doi.org/10.1080/13642818408227640
D. Shuttleworth, J. Phys. Chem. 84, 1629–1634 (1980). https://doi.org/10.1021/j100449a038
S. Mischler, H. Mathieu, D. Landolt, Surf. Interface Anal. 11, 182–188 (1988). https://doi.org/10.1002/sia.740110403
M. Monnot, R.P. Nogueira, V. Roche, G. Berthomé, E. Chauveau, R. Estevez, M. Mantel, Appl. Surf. Sci. 394, 132–141 (2017). https://doi.org/10.1016/j.apsusc.2016.10.072
J.-G. Choi, L. Thompson, Appl. Surf. Sci. 93, 143–149 (1996). https://doi.org/10.1016/0169-4332(95)00317-7
I. Alstrup, I. Chorkendorff, R. Candia, B.S. Clausen, H. Topsøe, J. Catal. 77, 397–409 (1982). https://doi.org/10.1016/0021-9517(82)90181-6
J. Vissers, C. Groot, E. Van Oers, D. Beer, R. Prins, Bull. Soc. Chim. Belg. 93, 813–822 (1984). https://doi.org/10.1002/bscb.19840930822
M. Mantel, J. Wightman, Surf. Interface Anal. 21, 595–605 (1994). https://doi.org/10.1002/sia.740210902
Acknowledgements
This research was supported by the SungKyunKwan University and the BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF), as well as the Nuclear Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Korea government Ministry of Science and ICT (MSIT) (NRF-2021M2E1A1085195).
Author information
Authors and Affiliations
Contributions
YHL: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization. JSY: Conceptualization, Methodology, Data Curation, Writing—Review and Editing. YWK: Methodology, Validation, Data Curation, Writing—Review and Editing. JGK: Conceptualization, Methodology, Writing—Review and Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, YH., Yoo, JS., Kim, YW. et al. Corrosion Behavior of Alloy 22 According to Hydrogen Sulfide, Chloride, and pH in an Anaerobic Environment. Met. Mater. Int. (2024). https://doi.org/10.1007/s12540-023-01624-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12540-023-01624-2