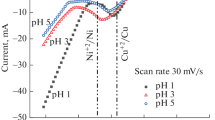
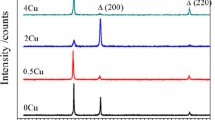
Abstract
In this work we report attempts to inhibit corrosion of Cu substrates in 0.6 M NaCl solution by coating with 100 nm Ni film and post-annealing with oxygen at different temperatures, in order to convert the nickel to nickel oxide. Electrochemical impedance spectroscopy (EIS) and polarization measurement analyses were used to obtain electrochemical data. The correctness of the EIS results was confirmed by Kramers–Kronig transformation, while fitting of the data (Nyquist and Bode diagrams) to suitable equivalent electrical circuits showed that the highest corrosion enhancement is achieved for the Ni/Cu sample annealed at 473 K, resulting in a 98% corrosion inhibition enhancement factor (η%). Polarization measurements also showed that this sample has the lowest corrosion current density, lowest corrosion rate and highest corrosion potential with a 97% corrosion inhibition efficiency factor (PE%). Consistent results are achieved for EIS and polarization measurements which are then correlated with the nanostructure of the films using X-ray diffraction and atomic force microscope analyses.
Graphic Abstract







Similar content being viewed by others
References
K. Rahmouni, M. Keddam, A. Srhiri, H. Takenouti, Corros. Sci. 47, 3249–3266 (2005)
K.M. Ismail, A.M. Fathi, W.A. Badawy, Corrosion 60, 795–803 (2004)
A. Varea, E. Pellicer, S. Pané, Bradley J. Nelson, S. Suriñach, M. Dolors Baró, J. Sort, Int. J. Electrochem. Sci. 7, 1288–1302 (2012)
C.-H. Chang, T.-C. Huang, C.-W. Peng, T.-C. Yeh, H.-I. Lu, W.-I. Hung, C.-J. Weng, T.-I. Yang, J.-M. Yeh, Carbon 50, 5044–5051 (2012)
G. Kear, B.D. Barker, F.C. Walsh, Corros. Sci. 46, 109–135 (2004)
P.A. Selembo, M.D. Merrill, B.E. Logan, J. Power Sour. 190, 271–278 (2009)
G. Gece, S. Bilgiç, Corros. Sci. 52, 3435–3443 (2010)
K.-H. Ahn, K.-G. Song, H.-Y. Cha, I.-T. Yeom, Desalination 122, 77–84 (1999)
J. McGeough, M. Leu, K. Rajurkar, A. De Silva, Q. Liu, CIRP Ann. 50, 499–514 (2001)
S. Rossi, F. Deflorian, F. Venturini, J. Mater. Process. Technol. 148, 301–309 (2004)
D. Landolt, Corrosion and Surface Chemistry of Metals (EPFL Press, Lausanne, 2007)
G. Yu, L. Zeng, F. Zhu, C. Chai, W. Lai, J. Appl. Phys. 90, 4039–4043 (2001)
S. Nandy, U. Maiti, C. Ghosh, K. Chattopadhyay, J. Phys.: Condens. Matter 21, 115804 (2009)
B. Subramanian, M.M. Ibrahim, V. Senthilkumar, K. Murali, V. Vidhya, C. Sanjeeviraja, M. Jayachandran, Physica B 403, 4104–4110 (2008)
T. Manago, T. Ono, H. Miyajima, I. Yamaguchi, K. Kawaguchi, M. Sohma, Thin Solid Films 374, 21–26 (2000)
K.X. Steirer, J.P. Chesin, N.E. Widjonarko, J.J. Berry, A. Miedaner, D.S. Ginley, D.C. Olson, Org. Electron. 11, 1414–1418 (2010)
G.H. Guai, M.Y. Leiw, C.M. Ng, C.M. Li, Adv. Energy Mater. 2, 334–338 (2012)
J.-K. Kang, S.-W. Rhee, Thin Solid Films 391, 57–61 (2001)
H. Lu, G. Scarel, M. Alia, M. Fanciulli, S.-J. Ding, D.W. Zhang, Appl. Phys. Lett. 92, 222907 (2008)
I. Porqueras, E. Bertran, Thin Solid Films 398, 41–44 (2001)
D. Jiang, J. Qin, X. Wang, S. Gao, Q. Liang, J. Zhao, Vacuum 86, 1083–1086 (2012)
Z. Jiao, M. Wu, Z. Qin, H. Xu, Nanotechnology 14, 458 (2003)
J. Singh, D.E. Wolfe, J. Mater. Sci. 40, 1–26 (2005)
M. Schönleber, D. Klotz, E. Ivers-Tiffée, Electrochim. Acta 131, 20–27 (2014)
B.A. Boukamp, Solid State Ion. 62, 131–141 (1993)
B. Díaz, J. Światowska, V. Maurice, A. Seyeux, B. Normand, E. Härkönen, M. Ritala, P. Marcus, Electrochim. Acta 56, 10516–10523 (2011)
Y. Zhang, D. Seghete, A. Abdulagatov, Z. Gibbs, A. Cavanagh, R. Yang, S. George, Y.-C. Lee, Surf. Coat. Technol. 205, 3334–3339 (2011)
H.S. Bahari, H. Savaloni, Mater. Res. Express 6, 086570 (2019)
E.E. Stansbury, R.A. Buchanan, Fundamentals of Electrochemical Corrosion (ASM International, Russell, 2000)
G. Honjo, J. Phys. Soc. Jpn. 4, 330–333 (1949)
T. Barr, J. Vac. Sci. Technol. 14, 660–665 (1977)
J. Iijima, J.-W. Lim, S.-H. Hong, S. Suzuki, K. Mimura, M. Isshiki, Appl. Surf. Sci. 253, 2825–2829 (2006)
I. Platzman, R. Brener, H. Haick, R. Tannenbaum, J. Phys. Chem. C 112, 1101–1108 (2008)
P. Keil, D. Lützenkirchen-Hecht, R. Frahm, AIP Conf. Proc. 882, 490–492 (2007)
D. Santos-Cruz, S. Mayén-Hernández, F. de Moure-Flores, J. Campos-Álvarez, M. Pal, J. Santos-Cruz, Results Phys. 7, 4140–4144 (2017)
S.-K. Lee, H.-C. Hsu, W.-H. Tuan, Mater. Res. Express 19, 51–56 (2016)
K. Khojier, H. Savaloni, E. Amani, Appl. Surf. Sci. 289, 564–570 (2014)
K. Khojier, H. Savaloni, Vacuum 84, 770–777 (2010)
H.Y. Ma, C. Yang, S.H. Chen, Y.L. Jiao, S.X. Huang, D.G. Li, J.L. Luo, Electrochim. Acta 48, 4277–4289 (2003)
O.E. Barcia, O.R. Mattos, Electrochim. Acta 35, 1601–1608 (1990)
O.E. Barcia, O.R. Mattos, N. Pebere, B. Tribollet, J. Electrochem. Soc. 140, 2825–2832 (1993)
H. Ma, S. Chen, L. Niu, S. Shang, S. Li, S. Zhao, Z. Quan, J. Electrochem. Soc. 148, B208–B216 (2001)
S.L. Li, Y.G. Wang, S.H. Chen, R. Yu, S.B. Lei, H.Y. Ma, D.X. Liu, Corros. Sci. 41, 1769–1782 (1999)
M.-S. Hong, I.-J. Park, J.-G. Kim, Met. Mater. Int. 23, 708–714 (2017)
C. Liu, Q. Bi, A. Leyland, A. Matthews, Corros. Sci. 45, 1257–1273 (2003)
Y.-S. Kim, S.-H. Kim, J.-G. Kim, Met. Mater. Int. 21, 1013–1022 (2015)
D.A. Jones, Principles and Prevention of Corrosion, 2nd edn. (Prentice Hall, Upper Saddle River, 1996), pp. 109–113
H.W. Bode, Network Analysis and Feedback Amplifier Design (D. Van Nostrand Company), pp. 303–335 (1945)
H. Saifi, M. Bernard, S. Joiret, K. Rahmouni, H. Takenouti, B. Talhi, Mater. Chem. Phys. 120, 661–669 (2010)
A. Dermaj, N. Hajjaji, S. Joiret, K. Rahmouni, A. Srhiri, H. Takenouti, V. Vivier, Electrochim. Acta 52, 4654–4662 (2007)
V.K.W. Grips, V. Ezhil Selvi, H.C. Barshilia, K.S. Rajam, Electrochim. Acta 51, 3461–3468 (2006)
Z. Chai, J. Li, X. Lu, D. He, RSC Adv. 4, 39365–39371 (2014)
S.S. Mirhashemihaghighi, J. Maurice, V. Seyeux, A. Klein, L.H. Harkonen, E. Ritala, M.P. Marcus, J. Electrochem. Soc. 162, C377–C384 (2015)
S. Mirhashemihaghighi, J. Światowska, V. Maurice, A. Seyeux, S. Zanna, E. Salmi, M. Ritala, P. Marcus, Corros. Sci. 106, 16–24 (2016)
S.-H. Lee, J.-G. Kim, J.-Y. Koo, Eng. Fail. Anal. 17, 1424–1435 (2010)
Y.-W. Jang, J.-H. Hong, J.-G. Kim, Met. Mater. Int. 15, 623–629 (2009)
Acknowledgements
This work was carried out with the support of the University of Tehran. We wish to thank professor F. Placido for his comments and the reviewing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Fig. 7.
Rights and permissions
About this article
Cite this article
Bahari, H.S., Savaloni, H. Corrosion Inhibition of Cu Coated with Ni and Annealed with Flow of Oxygen in NaCl Solution as a Function of Annealing Temperature. Met. Mater. Int. 26, 1621–1633 (2020). https://doi.org/10.1007/s12540-019-00422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-019-00422-z