Abstract
Aethozoid ctenostome bryozoans are an unusual, small group of solitary ctenostome bryozoans, occurring almost exclusively in deep-sea habitats. Currently, there are only five species belonging to four, still insufficiently known genera, which have been reported from the Atlantic and Pacific Oceans. Recent examination of sediment core samples from an active volcanic area near Mayotte revealed a high abundance of aethozoids, recorded for the first time in the Indian Ocean. A comparative approach identified the specimens as belonging to a new species, Aethozoon flavum sp. nov. There are particular characters diagnostic of this new species, such as basally oriented duplicature bands, a highly denticulate proximal vestibular wall, and a highly elongated anal tube terminating in an almost vestibular anus. This species is the first ctenostome observed at depths of over 3.000 m in the Indian Ocean. Morphological characters are compared among all aethozoids, but still require more detailed analyses in most species. Aethozoids appear to be globally distributed and often occur in high numbers, which indicates that additional efforts will increase their distribution and species range. Ultimately, additional studies will be able to show the ecological importance of these bryozoans and molecular studies should reveal more about their diversity and phylogenetic affinities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bryozoa is a moderately large phylum of lophotrochozoans comprising mostly colonial, suspension feeders (Ryland 1970, 2005). Colonies consist of iterated modules or individuals termed zooids. Each regular or feeding zooid (known as an autozooid) carries a ciliated tentacle crown or lophophore, which is used for feeding among other functions, and an associated U-shaped digestive tract (Mukai et al. 1997). Along with associated neuro-muscular tissue, these structures are traditionally termed the ‘polypide’ in bryozoans, whereas the body wall is termed as cystid. The latter can be unmineralized or mineralized in form of calcium carbonate, depending on the taxonomic clade. Besides coloniality, the second characteristic of all bryozoans is the retractability of the polypide inside the body wall (Schwaha et al. 2020).
Two large clades are distinguished among bryozoans, Phylactolaemata and Myolaemata (Schwaha et al. 2020). Whereas the former comprises a small group of freshwater bryozoans, the latter represents the bulk of over 6.000 extant, mostly marine, species (Bock and Gordon 2013). Myolaemata is divided into Stenolaemata (Cyclostomata) and Gymnolaemata, with the latter comprising ctenostomes and cheilostomes. Ctenostome bryozoans are an assemblage of unmineralized gymnolaemates which are paraphyletic and gave rise to the monophyletic Cheilostomata, which have mineralized skeletons (Todd 2000; Waeschenbach et al. 2012; Taylor and Waeschenbach 2015).
Bryozoans occur globally in all aquatic systems from freshwater lakes or rivers, intertidal and subtidal areas and even deep-sea habitats. The term 'deep sea' is generally used to describe environments that extend beyond the continental shelf, typically from a depth of approximately 200 m downwards. This begins with the bathyal zone, followed by the abyssal and hadal zones, with the latter representing depths exceeding the abyssal plain at ~ 6000 m (Tyler 2003). Research on deep-sea bryozoans mostly resulted from numerous voyages or cruises starting from the late nineteenth century until recent times (see Grischenko et al. 2019 for a summary). Initially, ctenostome and cyclostome bryozoans were not reported from areas beyond 3000 m (Schopf 1969), but later studies revealed a cryptic fauna of ctenostome bryozoans (d’Hondt 1976) that required careful sorting or specific collection techniques (Hayward 1981).
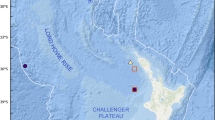
A recent series of surveys off eastern Mayotte (North Mozambique Channel, Indian Ocean) revealed the presence of a new very active volcanic edifice named Fani Maoré at 3500 m depth (Feuillet et al. 2021). The first visual survey of Fani Maoré and surrounding sediments did not reveal observable macro- or megafauna (Zeppilli and Pernet 2021). However, a rich meiofaunal community was recovered from sediments around this edifice, including the presence of numerous solitary bryozoans, which were preliminarily identified as aethozoids. Aethozoidae is a little-known family of ctenostome bryozoans with five species in four genera (Schwaha 2020a). Most records are from deep-sea habitats, but shallower specimens have also been encountered (Schwaha et al. 2019). In this study we provide a detailed analysis of the Indian Ocean specimens, and describe a new species of aethozoid bryozoans, which is also the first report of this group for the Indian Ocean.
Material and methods
Material and sampling location
Samples were collected near the Fani Maoré volcanic edifice in the east insular slope of Mayotte (Comoros Archipelago, southwestern Indian Ocean) during the oceanographic campaign ‘GeoFLAMME’ on board the R/V Pourquoi pas? on 14–26 May 2021. Fani Maoré was discovered in May 2018 on the distal part of the volcanic ridge that runs off the eastern flank of Mayotte. This pyramidal, volcanic edifice is 820-m tall and has numerous radial ridges of coalesced pillow-lava mounds intercalated with flatter areas of channelized lava or sheet flows emplaced at high effusion rates (Clague et al. 2018, Chadwick et al. 2019).
Samples of accumulated sediment were taken by an Oktopus multi-corer at five different sites across the newly formed volcanic substrate (Table 1). On each deployment, three half-cores of 34.6 cm2 surface were dedicated to meiofaunal taxonomic studies. Each sample was subsequently sliced in horizontal layers as follows: first layer at 0–1 cm, second layer at 1–3 cm, third layer at 3–5 cm, fourth layer at 5–10 cm, fifth layer at 10–15 cm. All sediment layers were fixed in 4% neutralised formalin.
Methods
Each sediment layer was profusely washed with 32 µm of filtered tap water to remove formalin remnants prior to meiofauna extraction. For this process, the sediment was passed through a 1-mm sieve (for possible separation of possible macrofauna and large particles) and a 32-µm sieve (for meiofauna isolation). Meiofauna was extracted with LUDOX® colloidal silica centrifugation (Burgess 2001), then preserved in 4% neutralised formalin and dyed with Phloxine B (80 mg/l in formalin). Meiofaunal samples were examined with a stereomicroscope at the Centre National de Tri d’Océanographie Biologique (CENTOB, Ifremer, France). Specimens were classified per metazoan phylum and counted, including the studied Bryozoa.
Fixed bryozoan specimens were documented and analysed with a Nikon SMZ25 stereomicroscope equipped with a DsRi2 microscope camera (Nikon, Tokyo, Japan). Zooidal dimensions were measured with the software FIJI (Schindelin et al. 2012). Selected zooids were transferred into glycerol and mounted on standard microscope slides and sealed afterwards with nail polish. Mounted samples were documented and analysed with a Nikon NiU compound microscope equipped with the same camera mentioned earlier.
For histological analysis, zooids were dehydrated in a graded ethanol series, followed by infiltration and embedding into Agar Low Viscosity Resin (Agar Scientific, Stansted, UK). Cured resin blocks were serially sectioned with a Leica® UC6 ultramicrotome (Leica® Microsystems, Wetzlar, Germany) and a Diatome HistoJumbo diamond knife (Diatome, Switzerland) at a thickness of 1 µm. Section series were stained with toluidine blue and sealed in resin. Afterwards, series were documented with the aforementioned NiU microscope. Image stacks of zooids were processed with FIJI prior to their import into the reconstruction software Amira (2022.2) (ThermoFisher). Stacks were first aligned in Amira, segmented and afterwards visualized. Snapshots were taken with the Amira software.
Results
Taxonomic account
Class Gymnolaemata Allman, 1856
Clade *Ctenostomata* Busk, 1852
Family Aethozoidae d’Hondt, 1983 (sensu Reverter-Gil et al. 2016)
Genus Aethozoon Hayward, 1978
Aethozoon flavum sp. nov.
https://zoobank.org/03152A98-44F2-4484-99A9-31B220F2486A
Material examined: 'MTB02' (deflation zone): 12°49′23.725''S, 45°44′30.217''E, 3562 m depth, 'MTB03' (NE volcano): 12°52′28.466''S, 45°45′44.719''E, 3511 m depth, 'MTB04' (NE volcano): 12°54′32.35''S, 45°48′4.37''E, 3530 m depth. Types deposited in Muséum National d’Histoire Naturelle, Paris (France) under accession codes: holotype MNHN-IB-2017-723; paratypes MNHN-IB-2017-724 to MNHN-IB-2017-727.
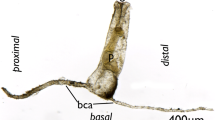
Description: Solitary or pseudo-solitary bryozoans with elongated zooids. Zooid mainly consist of an elongated peristome (Figs. 1, 2, 3), measuring 1787–2996 µm in length (mean 2281 µm, n = 8), width 135–229 µm (mean 179 µm, n = 8), basal area slightly wider 295–392 µm (mean 257 µm, n = 8), containing gonads, digestive tract, retractor muscles and funicular system (Figs. 4, 5, 6). Kenozooidal cystid appendage (kca) on proximo-basal side of zooid and non-kenozooidal one (nca) opposite on distal side, attaching slightly more frontally (Figs. 2, 3, 4). Kca may be missing, sometimes two present (Fig. 2b). Both appendage types of variable length, nca 384–2119 µm (mean 896 µm, n = 8), kca 473–1244 µm (mean 821 µm, n = 8); when longer with terminal expansion carrying developing polypide bud (Fig. 2c). Kenozooidal appendage separated by pore-plate with approximately four special cells (Fig. 7). Frontal side of vestibular wall and often cystid appendages with distinct, strong yellow coloration, sometimes also observed in other areas including the gut (Figs. 1, 2). Intertentacular organ may be present at adneural tentacles (Fig. 7a). Zooids simultaneous hermaphrodites with spermatogenic tissue and ovaries with several small oligolecithal oocytes (Figs. 6b–c; 7b). Polypide with 14 or 15 tentacles (Fig. 7a) occupying middle third of retracted zooid. Foregut short, cardia highly elongated and thin tube expanding on a voluminous sac-shaped caecum directed basally (Figs. 3, 8). Intestine elongated with very long anal tube terminating almost in vestibular area (Figs. 3c, d; 8d, e). Vestibular wall highly elongated occupying top third of retracted zooid (Figs. 1–3, 9). Distal vestibular area quadrangular (Fig. 9b) and usually wide with distinct yellow coloration (Figs. 1, 2, 9a), more proximally with strongly denticulate, dense cuticle (Fig. 9c). Short, vestigial diaphragmatic collar (Fig. 9d). Diaphragm inconspicuous with indistinct sphincter (Fig. 9e). Four sets of parieto-vestibular muscles, bilaterally arranged (Figs. 3, 4a, b). Four duplicature bands – two projecting frontally, two basally (Figs. 3, 4b, c, e, f). Funicular system single or branched, from caecal tip to pore-plate and basal body wall (Figs. 3, 4, 5).
Details of single zooids of Aethozoon flavum sp. nov. a Holotype (MNHN-IB-2017-723) showing long, non-kenozooidal cystid appendages; b Paratype (MNHN-IB-724) showing shorter cystid appendages including two shorter kenozooidal ones; c Paratype (MNHN-IB-725) showing zooid devoid of polypide and kenzooidal cystid appendage with distal bud. Abbreviations: b = bud; kca = kenozooidal cystid appendage; nca = non-kenozooidal cystid appendage; ply = polypide
Schematic drawing of Aethozoon flavum sp. nov. Abbreviations: alt = anal tube; am = apertural muscles; bdb = basal duplicature band; ca = cardia; cae = caecum; db = duplicature band; fg = foregut; fuc = funicular cords; kca = kenozooidal cystid appendage; int = intestine; nca = non-kenozooidal cystid appendage; py = pylorus; o = orifice; rm = retractor muscles; v = vestibulum; vw = vestibular wall
3D-reconstructions based on serial histological sections of Aethozoon flavum sp. nov.. a–d Reconstruction of one zooid; a Lateral view showing general structure; b Detail of frontal area; c Detail of basal duplicature bands; d Detail of the lophophore and digestive tract; e, f Another zooid. Digestive tract and duplicature bands in two opposing lateral views. Abbreviations: a = anus; alt = anal tube; am = apertural muscles; bdb = basal duplicature band; ca = cardia; cae = caecum; db = duplicature band; fg = foregut; fux = funicular cords; kca = kenozooidal cystid appendage; int = intestine; nca = non-kenozooidal cystid appendage; rm = retractor muscles; v = vestibulum
Reproductive organs, whole mounts of Aethozoon flavum sp. nov. a Basal area of zooid with developing gonads; b Basal area with ripe gonads showing particular association of the spermatogenic tissue with funicular cords; c General view of basal area; d Detail of c showing developing oocytes and spermatogenic tissue. Abbreviations: cae = caecum; dg = developing gonad; fuc = funicular cord; kca = kenozooidal cystid appendage; nca = non-kenozooidal cystid appendage; ov = ovary; pm = parietal muscles; rm = retractor muscles; spt = spermatogenic tissue
Cystid appendages of Aethozoon flavum sp. nov. a Non-kenozooidal cystid appendage, whole mount; b Kenozooidal cystid appendage showing pore-plate; c, d Sections showing details of pore-plate and separation of kenozooidal cystid appendages with specialized cells. Abbreviations: ci = cincture cell; fuc = funicular cord; kca = kenozooidal cystid appendage; li = limiting cell; nca = non-kenozooidal cystid appendage; ov = ovary; pm = parietal muscles; pop = pore plate; rm = retractor muscles; spe = special cell
Digestive tract of Aethozoon flavum sp. nov. a–c Same specimen; a Overview; b Detail of the foregut and parts of the midgut; c Detail of the caecum contents; d, e Same specimen; d Detail of the vestibular anus; e Digestive tract showing highly elongated anal tube from the intestine to the anus. Abbreviations: a = anus; alt = anal tube; ca = cardia; cae = caecum; cw = cystid wall; fg = foregut; fuc = funicular cord; int = intestine; l = lophophore; py = pylorus; rm = retractor muscles; ts = tentacle sheath
Vestibular area of Aethozoon flavum sp. nov. a Overview of a zooid showing general proportions and large size of the vestibular area. Transparent section planes indicate approximate section area in; b–e; b Section of the distal vestibular area; c Section of the proximal vestibular area; d Section of the collar area of the vestibulum; e Section of the diaphragm. Abbreviations: am = apertural muscles; c = collar; cy = cystid appendage; dis = diaphragmatic sphincter; dt = digestive tract; l = lophophore; o = orifice; v = vestibulum; vw = vestibular wall
Etymology: The specific Latin epithet of the new species, flavum, means yellow and alludes to the distinct yellow coloration of the specimens.
Type locality: Western Indian Ocean, Fani Maoré volcanic edifice.
Discussion
Morphological characters and diversity of aethozoids
The general morphology of Aethozoon flavum sp. nov. conforms well to other aethozoids, as it bears elongated zooids and various appendages (Reverter-Gil et al. 2016; Schwaha et al. 2019; Schwaha 2020a; Schwaha and Ott 2020). Its size range accords more with those of Franzenella limicola (Franzén, 1960) (length 1–2.5 mm) and Aethozooides uraniae (Schwaha et al. 2019) (length 1–3 mm), but cystid appendages and gut morphology are most similar to those of Aethozoon pellucidum Hayward, 1978.
Franzenella limicola occurs in shallow habitats and has a high number of occasionally branching cystid appendages, supposedly supported by musculature (Franzén 1960; Berge et al. 1985). Aethozooides uraniae has two basal cystid appendages, more or less opposite to each other, plus mid-peristomial to rare frontal appendages (Schwaha et al. 2019). Like A. pellucidum, A. flavum sp. nov. has up to two distal, kenozooidal appendages, but below a mid-peristomial position. The lack of the characteristical pair of basal appendages supports the assignment of A. flavum to the genus Aethozoon.
The tentacle number of 14 in A. flavum sp. nov. differs from the 10 in A. uraniae (Schwaha et al. 2019) and is more similar to the 12–14 of F. limicola (Franzén 1960). The tentacle number in A. pellucidum is elusive and has previously been guessed at 30 (Hayward 1978) or could not be estimated in later studies (Hayward and Erseus 1980). Although A. pellucidum represents the largest of all aethozoids (~ 6–9 mm), tentacle numbers rarely exceed over 20 in ctenostomes (Schwaha 2020a).
The gut morphology is best known in Aethozooides uraniae, which is rather short and straight compared to A. flavum sp. nov. (Schwaha et al. 2019). The latter corresponds better to the situation in A. pellucidum (Hayward 1978; Hayward and Erséus 1980), but also in F. limicola (Franzén 1960) with elongated cardiac portions and a more frontal position of the caecum in retracted zooids. A highly elongated anal tube has also been indicated in A. pellucidum and F. limicola and is also weakly present in A. uraniae. In the latter three species, however, it usually terminates via a lophophoral anus, whereas A. flavum sp. nov. shows the most elongated anal tube observed and has a more vestibular anus (see Schwaha 2020b for differentiation of ctenostome anuses).
The apertural area of A. flavum sp. nov. has two remarkable features. First, duplicature bands that project basally (in comparison, proximally to other bryozoans) have never been observed in any other bryozoan (Schwaha et al. 2011, 2020; Schwaha 2020c). Four duplicature bands are common, but usually all of them project in the same direction and not in opposite directions. Aethozooides uraniae has four, short duplicature bands extended frontally from the tentacle sheath (Schwaha et al. 2019). Second, the proximal vestibular wall shows a thick, denticulate cuticle obstructing the vestibulum. Thick vestibular walls are common among ctenostome bryozoans (Decker et al. 2021; Schwaha 2021), but a denticulate structure is unusual and probably only previously reported in Harmeriella terebrans Borg, 1940, which supposedly uses these structures as a boring apparatus into other bryozoans (Borg 1940), a function not feasible for solitary bryozoans such as aethozoids. At least in A. uraniae such a denticulate structure is not present, however, none of the other species or genera of aethozoids have been analysed in this respect with comparable histological methods.
A collar, as an acellular defensive structure projecting from the diaphragm into the vestibulum, is present in the apertural area of most ctenostome bryozoans (Schwaha 2020a). Among aethozoids, it is short in F. limicola, A. uraniae (Schwaha et al. 2019) and A. flavum sp. nov. (this study), whereas it is highly elongated in A. pellucidum (Hayward 1978; Grischenko et al. 2019). The highly denticulate vestibular wall of A. flavum sp. nov. probably renders a long collar superfluous as it obstructs the passage of the vestibulum, which is otherwise achieved via the collar.
The funicular cord or system is a thin peritoneal tissue strand with musculature that generally projects from the proximal end of the caecum to the body wall (Schwaha 2020c). In several ctenostomes, it forms a colonial system of integration by creating a network of tissue strands in contact with interzooidal pore-plates (Schwaha et al. 2020). In aethozoids, it either is present as a simple cord not associated with the interzooidal pore-plate in A. uraniae (Schwaha et al. 2019) or projects basally from the caecum to branch into several strands, also associated with pore-plates, as found in F. limicola (Franzén 1960), and A. flavum sp. nov. of the present study. No data is available for A. pellucidum. Because studied specimens of F. limicola and A. flavum sp. nov. showed sexual reproduction, the extent of the funicular branching could correlate to gonad development.
Diversity and distribution of aethozoids
Aethozoon flavum sp. nov. is only the fifth species of Aethozoidae and the second of the genus Aethozoon (Schwaha et al. 2019). The type species, A. pellucidum, was originally found in the Norwegian Sea, Atlantic Ocean (Hayward 1978; Hayward and Erseus 1980) and later reported from other areas in the Atlantic (see Reverter-Gil et al. 2016 for a summary). Aethozooides uraniae was recently described from the deep-sea hypersaline anoxic basins (DHAB) haloclines of the Mediterranean (Bernhard et al. 2015; Schwaha et al. 2019). Aethozoon pellucidum was recently found in the Pacific Ocean (Grischenko et al. 2019), from the Kuril-Kamchatka Trench at > 7000 m depth, comprising the deepest record of an aethozoid and of ctenostomes. Previously it was only recorded from New Caledonia in the Pacific Ocean (d’Hondt and Gordon 1996). The present study documents the first report of an aethozoid bryozoan from the Indian Ocean and supplements the general picture of a global distribution of the family both geographically and bathymetrically.
The high abundance of aethozoids in the analysed core samples is of particular interest. Although distributed in patches, a mean of 252 individuals per/cm2 was observed in samples of Aethozooides uraniae (Bernhard et al. 2015). Specimens of the current core samples of A. flavum sp. nov. also showed a high abundance of specimens in samples (mean 22 individuals per 34,6 cm2 core diameter), being in some cases the second most abundant taxon in the sample, only after nematodes. In addition to deep-sea aethozoids, studies on the shallow-water inhabiting F. limicola showed abundant specimens (mean 27.5 individuals per 6 cm core diameter) in 6 out of 24 cores, with a total number of 662 individuals in seven months (Berge et al. 1985). Apparently, aethozoids can have an important role in soft-sediment ecosystems, both in shallow and deeper environments. We have little knowledge on the ecological and trophic networks in such ecosystems, but we found probable retarian tests in the gut of Aethozoon flavum sp. nov. of the current study (Fig. 8), which gives a first indication of what aethozoids might feed on.
So far, sequence data for any aethozoid are still lacking. As previously indicated, it is currently unknown whether specimens identified as A. pellucidum, which show a wide geographical and bathymetric distribution, actually belong to the same species (Hayward and Erséus 1980; Grischenko et al. 2019). As advocated in previous ctenostome studies (Decker et al. 2021; Schwaha et al. 2022) and also shown for aethozoids (Schwaha et al. 2019), detailed soft-body morphological analyses are essential for species identification and distinction. For most species, however, detailed histological analyses are still missing. Likewise, the finding of A. pellucidum in the Pacific Ocean only concerned a single zooid (Grischenko et al. 2019), which restricted detailed morphological analyses. We conclude that a holistic methodology, combining both morphological and prospective molecular approach will yield insights into the species diversity of this unexplored group of bryozoans.
Reproductive aspects
Aethozoids are one of few examples of genuinely solitary bryozoans (Schwaha 2020a). While the presence of kenozooids, as true polymorphs, may provoke questions about the solitary nature of this clade, we argue that, on one hand, there is never more than one feeding autozooid present in such animals. On the other hand, there is strong indication that kenozooidal cystid appendages are actually asexual budding stages that will ultimately separate from the parent specimen. This was recently also indicated in Aethozooides uraniae, where evidently some of the kenozooidal appendages were buds, or at least part of them were buds (Schwaha et al. 2019). In the current study, we found similar evidence in Aethozoon flavum sp. nov. where the terminal end of one of the kenozooidal appendages showed an advanced budding stage (Fig. 2c). Consequently, the kenozooidal appendage of the mother zooid may represent the non-kenozooidal, caudate, cystid appendage of the bud. Elongated, proximal and tubular cystid parts are typical for other ctenostomes such as some nolellids or arachnidiids (Schwaha 2020a). Ultimately, we think that all kenozooidal appendages in A. flavum sp. nov. are various budding stages that ultimately will separate from the mother animal. Given the abundance encountered and the lack of anything showing more than two functional feeding zooids or often even traces of a polypide, we consider the entire budding and separation processes from the mother zooid as very fast. This is also supported by the few budding stages observed in samples.
Concerning sexual reproduction, we detected an inter-tentacular organ in A. flavum sp. nov., a character found in zygote-spawning species associated with numerous small oligolecithal eggs and planktotrophic larval development (Ostrovsky and Porter 2011; Ostrovsky 2020). As recently summarized, Aethozooides uraniae and Franzenella limicola have an intertentacular organ (Schwaha et al. 2019), whereas information on A. pellucidum and all species of Solella Schwaha et al. 2019 is still lacking. Suffice it to say, the observations on A. flavum sp. nov. confirm that aethozoids generally appear to have a reproductive mode with oligolecithal eggs and planktotrophic larvae. Although larvae have, not surprisingly, never been observed for aethozoids, it should favour a wider distribution of the species owing to the long-lived nature of planktotrophic larvae (Reed 1991; Gruhl 2020). Other, true colonial deep-sea ctenostome bryozoans have lecithotrophic larvae including brooding (Schwaha pers. observation), and also the other solitary form of bryozoan, the Monobryozoidae, probably show lecithotrophic development too (Ott 1972; Schwaha 2020a; Schwaha and Ott 2020). The consequence of these different sexual reproductive strategies remains unknown and requires additional studies.
References
Allmann GJ (1856) A monograph of the Freshwater Polyzoa, including all the known species, both British and Foreign. The Ray Society, London
Berge JA, Leinass HP, Sandiy K (1985) The solitary bryozoan, Monobryozoon limicola, Franzén (Ctenostomata), a comparison of mesocosm and field samples from Oslofjorden, Norway. Sarsia 70:91–94
Bernhard JM, Morrison CR, Pape E, Beaudoin DJ, Todario MA, Pachiadaki MG, Kormas KA, Edgcomb VP (2015) Metazoans of redoxcline sediments in Mediterranean deep-sea hypersaline anoxic basins. BMC Biol 13:105
Bock P, Gordon DP (2013) Phylum Bryozoa Ehrenberg, 1831. Zootaxa 3703:67–74
Borg F (1940) On the genus Tubiporella and a new boring Bryozoan. Zool Bidr Uppsala 18:415–437
Burgess R (2001) An improved protocol for separating meiofauna from sediments using colloidal silica sols. Mar Ecol Prog Ser 214:161–165. https://doi.org/10.3354/meps214161
Busk G (1852) Catalogue of marine Polyzoa in the collection of the British Museum. I. Cheilostomata. Trustees of the British Museum, London
Chadwick WW Jr, Rubin KH, Merle SG, Bobbitt AM, Kwasnitschka T, Embley RW (2019) Recent eruptions between 2012 and 2018 discovered at West Mata submarine volcano (NE Lau Basin, SW Pacific) and characterized by new ship, AUV, and ROV data. Front Mar Sci 20:495. https://doi.org/10.3389/fmars.2019.00495
Clague DA, Paduan JB, Caress DW, Chadwick WW Jr, Le Saout M, Dreyer BM, Portner RA (2018) High-resolution AUV mapping and targeted ROV observations of three historical lava flows at Axial Seamount. Oceanography 30:82–99. https://doi.org/10.5670/oceanog.2017.426
d’Hondt JL (1976) Bryozoaires cténostomes bathyaux et abyssaux de l’Atlantique Nord. In: Pouyet S (ed) Bryozoa 1974. Université Claude Bernard, Lyon, pp 311–333
d’Hondt JL (1983) Tabular keys for identification of the recent Ctenostomatous Bryozoa. Mém Inst Océanogr Monaco 14:1–134
d’Hondt JL, Gordon DP (1996) Bryozoa: Cténostomes et Cheilostomes Cellularines, Scrupariines et Malacostèges) des campagnes MUSORSTOM autour de la Nouvelle-Caledonie. Res Camp Musorstom 15:55–123
Decker S, Gordon DP, Spencer Jones ME, Schwaha T (2021) A revision of the ctenostome bryozoan family Pherusellidae, with description of two new species. J Zool Syst Evol Res 59:963–980
Feuillet N, Jorry S, Crawford WC, Deplus C, Thinon I, Jacques E, Saurel JM, Lemoine A, Paquet F, Satriano C, Aiken C, Foix O, Kowalski P, Laurent A, Rinnert E, Cathalot C, Donval J-P, Guyader V, Gaillot A, Scalabrin C, Moreira M, Peltier A, Beauducel F, Grandin R, Ballu V, Daniel R, Pelleau P, Gomez J, Besançon S, Geli L, Bernard P, Bachelery P, Fouquet Y, Bertil D, Lemarchand A, Van der Woerd J (2021) Birth of a large volcanic edifice offshore Mayotte via lithosphere-scale dyke intrusion. Nat Geosc 14:787–795
Franzén A (1960) Monobryozoon limicola n.sp., a ctenostomatous Bryozoan from the detritus layer on soft sediment. Zool Bidr Uppsala 33:135–148
Grischenko AV, Hirose M, Schwaha T, Chernyshev AV (2019) First record of an abyssal and hadal bryozoan fauna from the Kuril-Kamchatka Trench. Prog Oceanogr 176:102130. https://doi.org/10.1016/j.pocean.2019.102130
Gruhl A (2020) Larval structure and metamorphosis. In: Schwaha T (ed) Handbook of Zoology, Bryozoa. De Gruyter, Berlin, pp 123–14
Hayward PJ (1978) Two species of Ctenostomata (Bryozoa) from the Norwegian Sea. Sarsia 63:159–162
Hayward PJ (1981) The Cheilostomata (Bryozoa) of the deep sea. Galathea Rep 15:21–68
Hayward PJ, Erseus C (1980) Morphology and distribution of Aethozoon pellucida (Bryozoa Ctenostomata). Sarsia 65:243–248
Mukai H, Terakado K, Reed CG (1997) Bryozoa. In: Harrison FW, Woollacott RM (eds) Microscopic anatomy of invertebrates. Wiley-Liss, New York, Chichester, pp 45–206
Ostrovsky AN, Porter JS (2011) Pattern of occurrence of supraneural coelomopores and intertentacular organs in Gymnolaemata (Bryozoa) and its evolutionary implications. Zoomorphology 130:1–15
Ostrovsky AN (2020) Sexual reproduction in Bryozoa. In: Schwaha T (ed) Handbook of Zoology. Bryozoa. De Gruyter, Berlin pp 101–122
Ott JA (1972) Monobryozoon bulbosum n. sp., a new solitary interstitial bryozoan from the west Atlantic coast. Cah Biol Mar 13:421–428
Reed CG (1991) Bryozoa. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine Invertebrates. VI. Echinoderms and Lophophorates. The Boxwood Press, Pacific Grove, California, pp 85–245
Reverter-Gil O, Souto J, Fernández Pulpeiro E (2016) Fauna Iberica. Vol 43. Bryozoa 1. Ctenostomata. Museo Nacional de Ciencias Naturales, Madrid
Ryland JS (1970) Bryozoans. Hutchinson University Library, London
Ryland JS (2005) Bryozoa: an introductory overview. Denisia 19:9–20
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Meth 9:676–682
Schopf TJM (1969) Geographic and depth distribution of the Phylum Ectoprocta from 200 to 6,000 meters. Proc Am Phil Soc 113:464–474
Schwaha T (2020a) Ctenostomata. In: Schwaha T (ed) Handbook of zoology. Bryozoa. De Gruyter, Berlin, pp 269–316
Schwaha T (2020b) O anus, where art thou? An investigation of ctenostome bryozoans. J Morphol 281:914–922. https://doi.org/10.1002/jmor.21146
Schwaha T (2020c) Morphology of bryozoans. In: Schwaha T (ed) Handbook of Zoology: Bryozoa. De Gruyter, Berlin pp 57–100
Schwaha T (2021) Morphology of ctenostome bryozoans. 3. Elzerina, Flustrellidra, Bockiella. J Morphol 282: 633–651
Schwaha T, Ott JA (2020) 17 Bryozoa. In: Schmidt-Rhaesa A (ed) Guide to the Identification of Marine Meiofauna. Pfeil Verlag, Munich, pp 321–326
Schwaha T, Wood TS, Wanninger A (2011) Myoanatomy and serotonergic nervous system of the ctenostome Hislopia malayensis: evolutionary trends in bodyplan patterning of Ectoprocta. Front Zool 8:11
Schwaha T, Edgcomb VP, Bernhard JM, Todaro MA (2019) Aethozooides uraniae, a new deep sea genus and species of solitary bryozoan from the Mediterranean with a revision of the Aethozoidae. Mar Biodiv 48:1843–1856
Schwaha T, Ostrovsky AN, Wanninger A (2020) Key novelties in the evolution of aquatic colonial phylum Bryozoa: Evidence from soft body morphology. Biol Rev 95:696–729
Schwaha T, Winston JE, Gordon DP (2022) Morphology of ctenostome bryozoans: 5. Sundanella, with description of a new species from the Western Atlantic and the Multiporata concept. J Morphol 283:1139–1162
Taylor PD, Waeschenbach A (2015) Phylogeny and diversification of bryozoans. Palaeontology 58:585–599. https://doi.org/10.1111/pala.12170
Todd JA (2000) The central role of ctenostomes in bryozoan phylogeny. In: Herrera Cubilla A, Jackson JBC (eds) Proceedings of the 11th International Bryozoology Association Conference. Smithsonian Tropical Research Institute, Balboa, pp 104–135
Tyler PA (2003) Ecosystems of the deep oceans. Elsevier, Amsterdam, Boston, London
Waeschenbach A, Taylor PD, Littlewood DTJ (2012) A molecular phylogeny of bryozoans. Mol Phyl Evol 62:718–735. https://doi.org/10.1016/j.ympev.2011.11.011
Zeppilli D, Pernet EJ (2021) GEOFLAMME Cruise leg3 Pourquoi pas ?/Victor 6000 14–26 May 2021, Mayotte. Cruise report (biology and ecology) https://campagnes.flotteoceanographique.fr/campagnes/18001297/fr/
Acknowledgements
We thank the chief scientists, the scientific party and the crew of R/V Pourquoi pas? during the cruise GEOFLAMME cruise, RV Pourquoi pas ?, https://doi.org/https://doi.org/10.17600/18001297. Authors thank Stephane Jorry and Eve-Julie Pernet for helping in sediment sampling. Thanks to two reviewers for their comments and corrections of the manuscript.
Funding
Open access funding provided by University of Vienna. This work was supported by the BLUE REVOLUTION project (Biodiversity underestimation in our bLUe planEt: artificial intelligence REVOLUTION in benthic taxonomy) funded by the Interdisciplinary Graduate School for the blue planet (ANR-17-EURE-0015) and Ifremer (Institut français de recherche pour l'exploitation de la mer).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No animal testing was performed during this study.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
Data is available on reasonable request.
Author contribution
DZ and DC designed the study. AG and DC sorted all material. TS performed all morphological analyses and wrote the manuscript draft, TS, AG, and DC analysed the data. All authors contributed to the writing of the manuscript and approved the final version of the manuscript.
Additional information
Communicated by B. W. Hoeksema
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under https://zoobank.org/References/1CF34EB4-5566-44E5-8262-38EA151AD01A
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwaha, T., Zeppilli, D., González-Casarrubios, A. et al. The first deep-sea ctenostome bryozoan from the Indian Ocean: Aethozoon flavum sp. nov.. Mar. Biodivers. 54, 19 (2024). https://doi.org/10.1007/s12526-024-01409-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-024-01409-9