Abstract
Seamounts and remote oceanic islands serve as valuable natural laboratories in which to study patterns and processes in marine biodiversity. A central hypothesis arising from studies of these systems is the ecological function of seamounts as stepping-stones for dispersal and population connectivity. Evidence of this mechanism exists for a range of taxa, including coral reef fishes, but is still lacking from many tropical seamounts in remote regions. In this study, we used remotely operated vehicles and baited remote underwater video systems to survey fish and benthic communities between 1 and 100 m on seamounts in the Coral Sea Marine Park (CSMP), Australia. We found evidence to support the stepping-stone model of ecological connectivity from new observations of 16 coral reef fishes which have previously not been recorded by quantitative surveys in the region. The widespread distribution of many of these species throughout the full latitudinal extent of the CSMP suggests that there is greater connectivity between mesophotic habitats in the Coral Sea and surrounding biogeographic regions than previously known. We also found a wide variety of mesophotic habitats and recorded significant depth range extensions for 78 fishes in these habitats. This further highlights the potential role of increased habitat area and heterogeneity in a stepping-stone effect throughout the region. Four of the fish occurrence records represent significant range extensions into the Coral Sea from adjacent biogeographic regions, and 13 fishes recorded by this study in the CSMP are not known from the neighbouring Great Barrier Reef, despite its close proximity. Although the Coral Sea remains relatively understudied, these findings suggest that larger-scale models of marine biogeography are relevant to communities in the region, particularly at mesophotic depths. Given the extent and the spatial arrangement of seamounts in the Coral Sea, our findings emphasise that the region is an important link between the centre of marine biodiversity in the Coral Triangle and the Southwest Pacific. Greater mesophotic sampling effort and genetic studies are necessary to understand the nature of connectivity and to establish the role of regional seamount chains, like the Coral Sea reefs, in broader marine biogeographic processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Coral Sea, in the southwest Pacific Ocean, is the second largest tropical marginal sea on earth and is characterised by a complex bathymetry and diverse seascape of distinct marine habitats (Ceccarelli et al. 2013; McKinnon et al. 2014). These habitats include the deep sea, submerged banks, canyons, island chains and large oceanic coral reef systems atop of seamounts rising from deep waters (up to 3000 m) (Davies et al. 1989; Bridge et al. 2019). Ecologically, seamounts have been variously considered as either stepping-stones for marine dispersal that can promote regional connectivity via chains of suitable habitat across deep open oceans (Hubbs 1959; Rowden et al. 2010; Mazzei et al. 2021) and conversely, as isolated islands that can give rise to unique ecological communities (Richer de Forges et al. 2000; Hobbs et al. 2008, 2012; McClain et al. 2009). The general application of these contrasting hypotheses remains unclear and it is broadly agreed that the ecological function of seamounts in marine connectivity depends on multiple factors including geomorphology, depth, spatial isolation, oceanographic processes and taxa-specific dispersal capabilities (Mcclain 2007; Clark and Bowden 2015; Miller and Gunasekera 2017; Pinheiro et al. 2017).
The basis of the function of seamounts as stepping-stones for the dispersal of marine organisms depends on the availability of suitable habitat at a given seamount, the distance between seamounts and the nature and direction of ocean currents and localised seamount-generated flows (Rowden et al. 2010). The typical arrangement of seamounts in linear chains is a particularly important feature of this hypothesis. In combination with large-scale ocean currents, this spatial arrangement can facilitate the successive transport of marine larvae across oceanic basins or extending from mainland coastlines (Mazzie et al. 2021; Simon et al. 2022). Moreover, localised seamount-generated hydrodynamics are a unique feature of these habitats and the stepping-stone model of connectivity. These mechanisms may include closed recirculating currents that can trap and retain larvae over seamount summits, subsequently enhancing recruitment and settlement along seamount chains (Mulineaux and Mills 1997; Sponaugle et al. 2002). Despite wide recognition as highly productive biodiversity hotspots and this important function for connectivity in both tropical and temperate oceans, seamounts remain one of the least explored and studied marine biomes on earth (Clark et al. 2010b; Wagner et al. 2020; Yesson et al. 2021). Consequently, many regional seamount chains require considerably greater sampling effort to establish patterns of biodiversity and the mechanisms driving ecological connectivity with wider biogeographic regions (Rogers 2018).
Most seamount reef systems in the Coral Sea occur in Australia’s Exclusive Economic Zone (EEZ) and are managed as the Coral Sea Marine Park (CSMP). Together with the French Natural Park of the Coral Sea (Le Parc Naturel de la Mer de Corail), the Coral Sea possesses the largest combined protected area in the world (Director of National Parks 2018). In Australia’s CSMP, over 30 individual reef systems are spread across 22° of latitude on the Queensland and Marion Plateaus and constitute ~24,000 km2 of shallow-water (< 30 m) emergent coral reef habitat (Bridge et al. 2019). The CSMP is bordered by major global marine biodiversity and productivity hotspots; Australia’s Great Barrier Reef (GBR) to the west, the Coral Triangle (specifically, Papua New Guinea and the Solomon Islands) to the north, Vanuatu and New Caledonia to the east and the Tasman Sea to the south (Fig. 1). It is therefore not surprising that the CSMP supports a relatively high diversity of reef fish (~1200 species) and high abundance and biomass of sharks and other large predatory fishes (Randall et al. 1997; Ceccarelli et al. 2013; Stuart-Smith et al. 2013; Hoey et al. 2022). Shallow-water reef habitats in the CSMP have also been shown to support unique coral and reef fish communities that are distinct from those of the adjacent GBR to the west and share more similarities with those found in New Caledonia to the east (Ceccarelli et al. 2013; Hoey et al. 2020). Oceanographic processes and historic environmental conditions explain a significant proportion of the evolutionary processes driving genetic connectivity and biodiversity patterns within Coral Sea populations and between surrounding regions (Ceccarelli et al. 2013; Kessler and Cravatte 2013; Payet et al. 2022). However, the extent and spatial arrangement of coral reef habitat on seamounts in the Coral Sea is also a significant component of ecological connectivity in this region and within the wider Central Pacific Ocean.
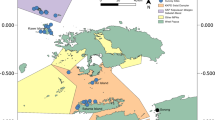
a Location of the Coral Sea relative to major marine biodiversity regions adapted from dissimilarity analysis between shallow species occurrence data by Kulbicki et al. (2013) and Spalding et al. (2007) for nested ecoregions within bioregions; b Bathymetry of the Coral Sea from the same region bounded in red as in a (data from Beaman 2012). Most seamounts in the Coral Sea Marine Park can be seen in a chain on the Queensland plateau, offshore to the east from the Great Barrier Reef. The Southwest Pacific region in the Coral Sea extends east to New Caledonia and north to the border of the Coral Triangle and Papua New Guinea. The northern seamounts of the Coral Sea extend to the border with the Central Indo-Pacific region, west through the Torres Strait. Arrows indicate conceptual borders and connectivity with adjacent biogeographical regions
Although recent large-scale monitoring efforts (Hoey et al. 2020, 2022) and some baseline surveys (Ayling and Ayling 1984; Oxley et al. 2004) have established quantitative ecological data for shallow-water coral reefs in the CSMP, the remote nature of reefs in the Coral Sea mean, they remain poorly documented compared to those in the surrounding GBR or the Coral Triangle (Ceccarelli 2010). Additionally, there is a paucity of research conducted on coral reefs below 30 m compared to shallow reefs, particularly in Australia (Pyle and Copus 2019, Eyal et al. 2021). Mesophotic coral ecosystems (MCEs) are defined as light-dependent coral reef communities in depths of 30–150 m (Loya et al. 2016). The exceptionally clear oligotrophic waters of the Coral Sea allow light penetration to considerable depths,and several exploratory studies have confirmed the presence of MCEs in the region to depths of up to 125m (Sarano and Pichon 1988; Bongaerts et al. 2011; Muir et al. 2015; Englebert et al. 2015, 2017). At these depths, the complex bathymetries of the Coral Sea seamounts are also highly variable, both among and within individual reef systems (Harris et al. 2003; Beaman 2012). Some rise as vertical walls to the surface (e.g. Bougainville and Osprey reefs), while others have less abrupt slopes and many possess near-horizontal areas along flanks and submerged shelves (e.g. Holmes Reefs and East Diamond Islet). On more moderate slopes, where hard substrate is present and light availability is optimal, mesophotic depths can support high percentage cover of photosynthetic habitat-forming taxa (Pérez-Rosales et al. 2022). Hard and soft corals, macroalgae, sponges and large benthic foraminifera are all important constituents of MCEs and in turn provide habitats for other marine organisms (Slattery and Lesser 2012; Lesser et al. 2018). This said, the extent of MCEs or other important benthic habitats between 0 and 150 m have not been quantified or confirmed at most Coral Sea reefs. Further, the few exploratory studies of MCEs in the CSMP are restricted to a select number of reefs and have all focused on benthic organisms, specifically scleractinian corals. To date, there have been no quantitative surveys of fish communities in the CSMP, or the Coral Sea more broadly, at depths below 20 m. The ecology and biodiversity of mesophotic coral reef fish communities in the Coral Sea are therefore scarcely known.
Two major global marine biogeographical regions meet in the Coral Sea; the Southwest Pacific and the Central Indo-Pacific, the latter of which includes the Coral Triangle (Kulbicki et al. 2013) (Fig. 1b). There has been increasing recognition that “peripheral habitats” (typically isolated archipelagos, marginal seas and seamounts) in regions surrounding major biodiversity centres can also export biodiversity and connect biogeographical regions, rather than function only as isolated population sinks (Bowen et al. 2013; Simon et al. 2022). Over the past 10–20 years, updated species checklists from locations in the Southwest Pacific have listed occurrence records for several Indo-Pacific fishes not previously reported from these areas (Randall et al. 2003; Fricke et al. 2011b, a). Additionally, aquarist collections from one location in the central Coral Sea Marine Park (Holmes Reefs) have also reported deep-water fishes typically known only from either the Central Indo-Pacific or Central Pacific regions (Fenton Walsh, pers. com). Large-scale connectivity patterns are not well established in the Coral Sea, but these observations suggest greater connectivity between the Central Indo-Pacific and Western Pacific region through the Coral Sea than may currently be known. This may be particularly true for mesophotic species that are not recorded in shallow reef community surveys but utilise deeper reef habitats.
In this study, we used Remotely Operated Vehicles (ROVs) and Baited Remote Underwater Video systems (BRUVs) to survey fish and benthic communities in the Coral Sea Marine Park at depths between 1 and 100 m. Here, we present the first occurrence records of 16 species from quantitative ecological surveys of coral reef fishes from seamounts in the Coral Sea. We also compare fish species richness between shallow-water monitoring surveys and ROV/BRUV surveys and identify a range of mesophotic habitats found in the CSMP during this study. We discuss these observations in the context of regional connectivity, biogeography and the importance of seamounts in tropical coral reef seascapes.
Material and methods
Four separate voyages in the Coral Sea Marine Park were undertaken in 2021, 2022 and 2023. ROVs and BRUVs were used to survey fish communities and benthic habitats at 17 reefs and between 1 and 100 m (Fig. 2). Single-camera BRUVs were deployed for 1 h following the standard operating procedures outlined in Langlois et al. (2020). ROVs (BlueRobotics BlueROV2) were fitted with a forward-facing stereo-video system (SVS) to enable length estimates to be made. SVS cameras (Paralenz or GoPro Hero 8 systems) were calibrated prior to surveys using the software CAL and the associated calibration method (SeaGis Pty, Australia). ROV transects, each 30 × 5 m, were conducted parallel to the reef contour using a timed swim method (ROV speed 0.2 m/s for 2 min 30 s) at a constant depth (+/− 2 m). For each ROV deployment, two transects were conducted within each 10-m depth band, starting with the deepest transects and working upwards to the shallows. Sufficient horizontal and vertical separation was attained between transects and between depth bands by the known speed and time of the ROV. Three GoPro Hero8 cameras inside deep-rated T-housings were mounted facing outwards left and right and downwards on the ROV. These cameras were set to the timelapse photo function (1 photo every 10 s), capturing an image of the benthos every ~2 m (15 photos per transect). For fish community data, videos were interrogated in EventMeasure (SeaGIS, Pty Australia), and each individual fish entering the frame (BRUV) or transect field-of-view (ROV) was identified to the lowest possible taxonomic resolution. For ROV stereo-video footage, length estimates were also made of individual fishes in each transect (fork length) using the software EventMeasure Stereo (SeaGIS, Pty Australia). All major habitats at each reef were surveyed (outer reef, lagoon, back-reef and reef passes between the lagoons and leeward outer reef), with BRUVs mostly conducted in lagoons and inner reef areas due to the steep sides of many Coral Sea seamount reefs.
Map of the Coral Sea, East Coast Australia and survey sites from this study. All sites in the CSMP surveyed by BRUV, ROV and shallow diver surveys are labelled by text. Locations with yellow stars indicate reefs with new locality observations for coral reef fishes. The Holmes Reefs, where several of these species have been previously noted by aquarium fish collectors, are marked by a red circle. Reefs marked with a black circle indicate locations where no new observations of reef fishes were recorded.
We compared species records from shallow underwater visual census monitoring surveys conducted by divers on SCUBA (1–10 m depth) at the same reefs during the same voyages (Hoey et al. 2021, 2022; Galbraith et al. 2022), as well as fish species records from previous surveys of the same reefs conducted by the Reef Life Survey Foundation (Edgar and Stuart-Smith 2014). Occurrence records and locations for species only recorded by BRUV and ROV surveys in this study were obtained from the Ocean Biodiversity Information System (OBIS 2023a) and Global Biodiversity Information Facility (GBIF.org 2023a) and were cross-referenced with other online databases; Eschmeyer’s Catalogue of Fishes (Fricke et al. 2023), Atlas of Living Australia (ALA 2023), Fishes of Australia (Bray and Gomon 2023), Reef Life Survey (RLS 2023), FishBase (Froese and Pauly 2023), CSIRO Codes for Australian Aquatic Biota (Rees et al. 2023) and the Australian Faunal Directory (ABRS 2020) as well as taxonomic experts. Known depth records for all fishes recorded were also extracted from FishBase using the rfishbase package (Boettiger et al. 2012) and compared to depths at which they were observed by ROV and BRUV surveys. To illustrate notable range extensions for three species, previous extant range extents were calculated as Extent of Occurrence (EOO) based on records obtained from the aforementioned databases and plotted in R (R Core Development Team 2023) using the packages ggmaps (Kahle and Wickham 2013) and maps (Becker et al. 2022).
Results
A total of 274 ROV transects and 108 BRUV drops were analysed and cumulatively recorded 361 species of fishes from 41 families from depths between 1 and 100 m. Of these 361 species, 73 were recorded exclusively by BRUVs, 105 exclusively by ROV and the remaining 183 were recorded by both methods (Online Resource 1). Of the total 361 fish species, 128 (36%) were observed at depths below their reported maximum known depth as listed on the FishBase database (Online Resource 2). Thirty-four of these depth record extensions are for species observed at depths greater than double their previously reported maximum depth.
Compared to available data from shallow (< 10 m) underwater visual census surveys in the Coral Sea (Ceccarelli et al. 2013; Stuart-Smith et al. 2013; Hoey et al. 2020, 2021, 2022), this study recorded 50 additional species. Prior to this study, thirteen of these 50 species were previously only known from a single location in the central Coral Sea through observations and/or collections by aquarium fish collectors at the Holmes Reefs (F.Walsh pers. Com). Outside of these collections, these are the first observations of these thirteen species from quantitative fish community surveys in the Coral Sea Marine Park, and broader Coral Sea region (Table 1).
A further three species have single records from Boot (Anampses melanurus, Atlas of Living Australia, 2023a), Frederick (Mulloidichthys pfluegeri, Atlas of Living Australia, 2023b) and Osprey (Valenciennea helsdingenii, Australian Faunal Directory 2023) reefs respectively, but were recorded in our surveys at 11 other reefs (Table 1). These records increase the range extent of these three species in the region by between 4 and 12° of latitude.
Four species recorded by ROV in the CSMP represent notable range extensions based on previous global occurrence records. Hoplolatilus randalli (Allen, Erdman & Hamilton, 2010), a relatively newly described species of tile fish (family Malacanthidae), is currently known only from Indonesia, the Philippines, Palau, Yap and the Solomon Islands (Froese and Pauly 2023). A total of eight individuals were recorded at reefs spanning the northern and central CSMP (Ashmore and Lihou Reefs and East Diamond Islet), all at depths below 70 m (Table 1, Fig. 3a). We mostly observed H. randalli in pairs beside large mounds of rubble, apparently built by the fish over their burrows. The observations from this study are the southernmost occurrence records for the species and expand the known extent of occurrence for H. randalli by almost 10° of latitude. Cephalopholis polleni (Bleeker, 1868) was previously only known in Australian waters from the Cocos (Keeling) and Christmas Islands in the Indian Ocean. Elsewhere, C. polleni, (family Serranidae), occurs at scattered localities on oceanic islands across the Indian Ocean and wider Indo-Pacific (Bray 2023). In the Coral Sea, this study recorded one individual C. polleni at 97 m under a ledge at Osprey Reef by ROV survey (Fig. 3b), and it has also been collected at Holmes Reefs (Fenton Walsh pers. com). These Coral Sea records extend the southern range of C. polleni in the Southwest Pacific by 6° of latitude. Pseudanthias flavicauda (Randall & Pyle, 2001) was recorded by ROV survey at Osprey and Bougainville reefs in the northern Coral Sea. P. flavicauda (family Serranidae) is known from the Central and Southwest Pacific (Bray 2022; Froese and Pauly 2023), and recently from Tonga (Fricke et al 2011b) and New Caledonia (Fricke and Kulbicki 2007). We observed abundant schools of P. flavicauda between 80 and 100 m at Osprey and Bougainville Reefs, and although also collected from the Holmes Reefs (Fenton Walsh pers. com), the observations from this study are the most western records for this species and the most northern extent in the Coral Sea (Fig. 3c). Bodianus paraleucosticticus (Gomon, 2006) was found in ROV surveys at Lihou and Osprey Reefs at depths between 70 and 90 m. Together with collections from Holmes Reefs, these new observations of B. paraleucosticticus (family Labridae) extend the previously reported distribution west from New Caledonia and south from Papua New Guinea into the Coral Sea (Fig. 3d).
Current extent of occurrence plotted as coloured hulls for a Hoplolatilus randalli; b Cephalopholis polleni; c Pseudanthias flavicauda; d Bodianus paraleucosticticus. Occurrence data were obtained from OBIS (2023b,c,d,e) and GBIF (2023b,c,d,e). New observations of each species from the Coral Sea by this study are represented by yellow stars
The presence of mesophotic coral ecosystems was confirmed at all 17 of the reefs surveyed by ROV and BRUV (Fig. 4). Several sites possessed remarkable hard coral cover (preliminary estimates ~70–85%) at depths between 50 and 100 m (Fig. 5a and d). Multiple other non-coral dominated mesophotic habitats were also found including Cycloclypeus fields (large benthic foraminifera) (Fig. 5b), octocoral dominated walls (Fig. 5c), seagrass (Fig. 5e) and extensive Halimeda meadows (Fig. 5f).
Twelve species of coral reef fish recorded by ROV and BRUV surveys in the Coral Sea Marine Park (CSMP) between depths of 50 and 100 m. All are previously known from one reef location in the CSMP but are reported here from multiple other reefs spanning the full latitudinal extent of the CSMP. a Pogonoperca punctata; b Pycnochromis leucura; c Valenciennea helsdingenii; d Abalistes filamentosus; e Liopropoma sp. “yellow tail”; f Xanthichthys auromarginatus; g Mulloidichthys pfluegeri; h Anampses melanurus; i Cirrhilabrus roseafascia; j Genicanthus bellus; k Hoplolatilus marcosi; l Pyronotanthias aurulentus. C. roseafascia, M. pfluegeri and H. marcosi have been recorded on the outer GBR shelf break (Sih et al. 2017) but were previously not confirmed to be widely distributed throughout the Coral Sea
Varied mesophotic coral ecosystems and other mesophotic habitats in the Coral Sea a areas of high coral cover, 77 m, Lihou Reef; b Oxychellinus orientalis and Cirrhilabrus bathyphilus on a dense slope of Cycloclypeus at 82 m, East Diamond Islet; c Oxycheilinus orientalis on a steep wall with soft corals and gorgonians, 94 m, Osprey Reef; d high abundance of fishes with high and complex coral cover, 57 m Bougainville Reef; e seagrass (Halophila gradients) at 43 m East Diamond Islet; f dense Halimeda meadows at 67 m, Lihou Reef
Discussion
The new occurrence records presented here span a considerable latitudinal gradient and provide evidence of more widespread distributions for multiple fishes at mesophotic depths in the region than previously known. These observations are consistent with the role of seamounts as stepping-stones for mesophotic fishes within the Coral Sea and between other neighbouring biogeographic regions. The provision and amount of suitable habitats at mesophotic depths throughout the seamount chain, together with the spatial arrangement of seamounts across the Coral Sea basin, are likely key mechanisms supporting a stepping-stone model of ecological connectivity in the region.
Total habitat area and the arrangement of a variety of habitat types are fundamental components of species-area-isolation relationships that drive biodiversity patterns (MacArthur and Wilson 1967; Connor and McCoy 1979; Fahrig 2013; Hanski 2015). The presence of multiple deep-water habitats at individual Coral Sea reefs highlights that there is considerably greater habitat area and habitat heterogeneity within the Coral Sea than known from shallow reefs alone. This is significant given that most metrics of ecological isolation comprise some measure of patch size combined with distance from nearest neighbouring habitat and the properties of the surrounding matrix (Moilanen and Nieminen 2002; Prugh et al. 2008). Compared to continental scales (thousands of kilometres), seamounts in the Coral Sea are separated by relatively small distances (< 450 km maximum distance). Isolation can both positively and negatively affect biodiversity, either via demographic effects (Hanski et al. 2013; Fahrig 2013; Jones et al. 2020) or distance from anthropogenic influences (Demartini et al. 2008; Williams et al. 2011; Bennett et al. 2018). In the context of this study, the relatively small distance between many reefs throughout the seamount chain, in combination with increased habitat area and heterogeneity at mesophotic depths, may represent an optimal level of isolation between populations. This in turn would facilitate dispersal and enhanced connectivity for some taxa through the stepping-stone model (Baum et al. 2004; Saura et al. 2014).
The relationships between increased habitat area and reduced isolation, together with levels of habitat heterogeneity and quality, also drive biodiversity through other ecological dynamics (Gratwicke and Speight 2005; Szangolies et al. 2022). For example, Halimeda spp. meadows, seagrass and other macroalgae habitats are known to provide valuable nursery habitats for reef fish settlement and recruitment (Sambrook et al. 2019; Tang et al. 2020; Sievers et al. 2020). Although this function has not been extensively tested in MCEs, Halimeda meadows are known to support diverse mesophotic fish communities (Langston and Spalding 2017; Spalding et al. 2019), and we found these habitats on deep outer reef slopes and in lagoons of all the Coral Sea reefs surveyed. Given their isolation from other coastal nursery habitats, these habitats may be particularly important for the early life stages of fishes and invertebrates, and thereby the replenishment and maintenance of Coral Sea populations. Similarly, areas of high coral cover at mesophotic depths increase total habitat area and resource availability for coral-associated and dependent fishes. Although resources at range margins, including depth, can be of lower quality and affect the physiological condition of some reef fishes (Munday 2001; Srinivasan 2003; Hoey et al. 2007), others including highly specialised obligate coral-feeding butterflyfishes have been shown to access equal or greater resources from deeper reefs without impacting their fitness (MacDonald et al. 2018; MacDonald et al. 2019). Further, other studies of energetic trade-offs associated with marginal coral reef habitats have found that deep reefs support robust subpopulations through demographic and reproductive plasticity (Goldstein et al. 2016). Biological traits including habitat preference, diet and dispersal ability therefore also strongly influence connectivity differentially between species and ontogenetic stages (Hixon and Jones 2005; Goldstein et al. 2017). In the context of this study, this is evident from the function of seamounts as stepping-stones for deep-sea invertebrates, where mismatches between expected and observed dispersal patterns can be explained by a combination of environmental parameters and biological traits (Miller and Gunasekera 2017).
The occurrence of several fishes in the Coral Sea which were previously only known from either the Indo-Pacific region or South and Western Central Pacific also aligns with other ecological hypotheses explaining larger-scale patterns in marine biodiversity. “The biodiversity feedback” theory proposes that peripheral regions actively contribute to the export of taxa and lineages back to biodiversity hotspots, rather than acting only as sink populations (Bowen et al. 2013). Range expansions into the Coral Sea from both the Central Indo-Pacific (e.g. H. randalli) and the Western Central Pacific (e.g. P. flavicauda) suggest multiple directions of connectivity between these biogeographical regions through the Coral Sea. The proximity of the Coral Sea to the global centre of marine biodiversity, the Coral Triangle in the Indo-Pacific, mean reef habitats in the Coral Sea may represent a particularly important link in larger-scale patterns of reef fish biodiversity, rather than isolated populations with limited distribution (Hobbs et al. 2009; Budd and Pandolfi 2010). Though often speculated, prior to this study, there has been scant empirical evidence to support the role of the Coral Sea seamounts as stepping-stones for reef fish populations (but see Van Herwerden et al. 2009), or in contributing to biodiversity feedback between regions.
The stepping-stone model of dispersal has been shown to contribute to the biodiversity feedback process for reef fish assemblages in other regional seamount chains (Pinheiro et al. 2015, 2018; Mazzei et al. 2021). From studies in the Southwest Atlantic, coastal populations closer to the Brazilian continental shelf represent areas of higher biodiversity, and genetic connectivity exists in both directions between these populations and the most offshore seamounts (Simon et al. 2022). Interestingly, of the 16 new occurrence records found in this study from the Coral Sea Marine Park, 13 are not known from the neighbouring Great Barrier Reef. At least nine of these species are deep-water specialists, typically only known from depths greater than 30 m and up to 120 m. We include H. marcosi in these deep-water specialists which, prior to these multiple new Coral Sea observations, is only reported in Australia from a single individual at 100 m at the GBR shelf break (Sih et al. 2017) and has not been recorded from the reefs of the GBR itself. Although a lack of observations on the GBR for these 13 species may reflect low sampling from mesophotic depths, the shallow geomorphology of the GBR shelf (30–50 m, Hopley 2006) likely restricts the establishment of populations of the deep-water specialist, or mesophotic, species reported here. Indeed, the occurrence of these mesophotic species throughout the full latitudinal extent of the CSMP, but not on the GBR, suggests that connectivity for these species is greater latitudinally along the seamount chain, where deep-water habitat is available between the Coral Triangle and island chains of the Southwest Pacific.
Connectivity patterns in the Coral Sea remain unclear but for shallow-water studies, both genetic analyses (Planes et al. 2001; Payet et al. 2022) and dispersal-driven connectivity models (Treml et al. 2008) suggest that connectivity between the Coral Sea and GBR is generally weak. The barriers to connectivity between these regions have not been fully established, but the spatial separation of these two regions by deep open water and the lack of mesophotic habitat on the GBR would certainly contribute to a dispersal barrier for mesophotic species from the Coral Sea seamounts to the shallow GBR shelf. Further studies utilising genetic sampling of mesophotic fishes with extended ranges throughout the CSMP and from neighbouring regions are required to test these aspects of the biodiversity feedback hypothesis. Regional and localised oceanographic processes will also determine the nature and direction of population connectivity and barriers to dispersal throughout deep reefs of the region. For example, dispersal via large-scale ocean currents may be the main mode of seamount colonisation for some taxa (Leal and Bouchet 1991), but localised seamount-generated flows may be more important for explaining the distribution of others (Richer de Forges et al. 2000). Finally, although there is limited connectivity between shallow and mesophotic ecological assemblages in many regions for some taxa (Morais and Santos 2018; Bongaerts and Smith 2019; Stefanoudis et al. 2019), the depth extensions for 158 species in the Coral Sea reported by this study suggest that boundaries between shallow and deep assemblages may be shifted deeper here than community breaks known from other regions (Lesser et al. 2019). Although MCEs clearly warrant conservation actions and scientific investigation independent from shallow-water reefs (Bridge et al. 2013; Rocha et al. 2018), it is recognised that the degree of MCE species overlap with shallow-water assemblages can vary considerably by taxon and location (Laverick et al. 2018). This necessitates further ecological and environmental sampling from MCEs in remote geographic regions, like the Coral Sea, and in understudied habitats, like seamounts.
A second mechanism, particularly relevant to patterns of mesophotic fish diversity, is the “Habitat Persistence Hypothesis” (HPH) (Copus et al. 2022). This theory posits that during periods of lower relative sea level, deeper marine habitats can persist, particularly on complex bathymetries where there are horizontal and low aspect areas, while shallow-water habitats are dried out and communities here are lost. Multiple biogeographical hypotheses of global patterns and processes in reef fish diversity are supported to varying degrees by often overlapping empirical evidence (Mora et al. 2003; Gaither and Rocha 2013; Bowen et al. 2013; Cowman et al. 2017). Yet, most of this evidence is derived from shallow-water coral reefs (< 30 m) which are estimated to represent only 20% of global coral reef habitat (Pyle and Copus 2019). These data therefore must be considered incomplete regarding both species inventories and the extent of available habitat. Indeed, unlike shallow-water reef fishes, diversity for mesophotic fishes does not appear to attenuate with distance from the Coral Triangle (Pyle 2000, 2005; Pyle and Copus 2019), and this mismatch suggests that there are further mechanisms shaping reef fish diversity than have currently been considered (Pinheiro et al. 2023). Again, although the lack of mesophotic sampling effort in the region must be acknowledged, the absence of fishes which are reported in this study from the adjacent GBR aligns with several mechanisms proposed by the HPH. During periods of lower relative sea level, species richness may have been retained in deeper habitats of the Coral Sea, and in these persisting habitats, evolutionary processes would continue among populations, potentially driving higher rates of speciation. Certainly, taxonomic revisions and redefined species complexes within some reef fish genera demonstrate high levels of speciation across broad distributions which span island chains, oceanic islands and seamounts in the Western and Indo-Pacific. These include the Pomacentrus philippinus group, which in the Coral Sea is represented by P. imitator but in the adjacent GBR is P. magniseptus (Allen et al. 2017), the goby genus Nemateleotris (Tea and Larson 2023) and multiple Pseudanthias species (Anderson 2018, 2022; Gill 2022) many of which are deep-water specialists. The compilation and comparison of updated species inventories is required to test the applicability of the HPH in the Coral Sea, as well as genetic sampling to establish patterns of connectivity between mesophotic populations in adjacent regions. The complex bathymetries of the Coral Sea seamounts do, however, constitute a significant system that aligns with many of the mechanisms proposed by the HPH and is a promising region in which to test these concepts further.
Increased mesophotic surveys will likely continue to increase diversity records for the region and comprehensive fish species checklists for the Coral Sea Marine Park, and Coral Sea region more broadly, will undoubtedly continue to expand on the observations presented by this study. Recent large-scale survey efforts in the region have collected the most detailed bathymetry data for these reefs to date (Carroll et al. 2021; Beaman et al. 2022; Brooke and Schmitt Ocean Institute 2022) and have substantially expanded our understanding of deep-sea habitats of the Coral Sea. Unfortunately, despite the evident value of deep-sea exploration, technical constraints on large ship-based ROVs mean that such work often only focuses on mesophotic habitats for short periods of time. Our findings highlight the utility of small, affordable ROVs as an effective tool for conducting mesophotic surveys in remote regions where technical diving is often not feasible. Although beyond the scope of this study, trait-based analysis of the fish community data collected by ROV surveys would be an informative line of further investigation to establish how ecological characteristics (e.g. body size, depth range, habitat preference, dispersal ability) contribute to habitat use and geographical ranges throughout the Coral Sea. Further, despite significant advances in understanding connectivity and recruitment patterns in tropical reef fishes (Jones et al. 1999; Mora et al. 2003; Mora 2004; Planes et al. 2009; Jones 2015; Almany et al. 2017), ecological studies from seamounts are typically focused on cold-water, true deep-sea taxa (Rowden et al. 2005, 2010; Pitcher et al. 2007; Clark et al. 2010a; Rogers 2018). Coral reef communities from tropical seamounts, including those inhabiting MCEs, are therefore underrepresented in an already understudied global marine habitat but are known to support abundant and diverse reef fish communities (Letessier et al. 2019; Galbraith et al. 2021; Leitner et al. 2021). As biodiversity hotspots and important patch habitats for connectivity, seamounts should more widely be considered global conservation priorities in coral reef seascapes (McCook et al. 2009; Riva and Fahrig 2022; Thompson et al. 2023). Increased mesophotic community surveys in the Coral Sea region and in other tropical seamount chains will contribute to baseline knowledge of reef fish community structure and species distributions in these habitats. Further observational studies examining species turnover between seamounts should also be supported by genetic sampling to establish the nature and direction of population connectivity throughout tropical seamount chains and within adjacent biogeographical regions.
The findings from this study support the stepping-stone model of dispersal for seamounts in a tropical seascape. The new records of 16 fishes recorded throughout the Coral Sea Marine Park confirm that the geographic range of many tropical reef fishes is more widespread than currently reported. Sampling deficiencies in the region and at mesophotic depths are clearly a significant reason behind new species observations. Nevertheless, these new records from the Coral Sea contribute to evidence that tropical seamounts are important habitats for reef fish dispersal and connectivity between the Indo-Pacific, Coral Triangle and Western Pacific region. The discovery of high coral-cover mesophotic reefs, combined with other diverse deep-water benthic communities, demonstrates the potential of mesophotic habitats of the Coral Sea to provide valuable corridors for the dispersal of coral reef fishes. The spatial arrangement of the Coral Sea seamounts along the boundary of major biogeographical regions also suggests that these tropical seamounts function in processes driving large-scale marine biodiversity patterns. These include but are likely not limited to biodiversity feedback between peripheral regions and centres of marine biodiversity and differences in the distribution and diversity of mesophotic fishes driven by habitat persistence through periods of sea-level change. These paradigms may operate across other regional tropical seamount chains and suggests these habitats have an important role in the maintenance and regulation of global biodiversity patterns for coral reef taxa.
References
ALA (2023) Atlas of Living Australia website at http://www.ala.org.au. Accessed 4 March 2023
ABRS 2020 Australian Faunal Directory. Australian Biological Resources Study, Canberra., https://biodiversity.org.au/afd/home, Accessed 21 March 2023
Allen G, Museum WA, Erdmann MV et al (2017) Descriptions of four new species of damselfishes ( Pomacentridae ) in the Pomacentrus philippinus complex from the tropical western Pacific Ocean. Ocean Sci 25:47–76. https://doi.org/10.5281/zenodo.317395
Allen GR, Erdmann M V, Hamilton AM (2010) Hoplolatilus randalli, a new species of sand tilefish (Pisces: Malacanthidae) from the tropical western Pacific with comments on the validity of H. luteus. Aqua: International Journal of Ichthyology,16:171–186
Almany GR, Planes S, Thorrold SR et al (2017) Larval fish dispersal in a coral-reef seascape. Nat Ecol Evol 1:148. https://doi.org/10.1038/s41559-017-0148
Anderson WD (2022) Additions and emendations to the annotated checklist of anthiadine fishes (Percoidei: Serranidae). Zootaxa 5195(6):567–578. https://doi.org/10.11646/zootaxa.5195.6.5
Anderson WD (2018) Annotated checklist of anthiadine fishes (Percoidei: Serranidae). Zootaxa 4475:1–62. https://doi.org/10.11646/zootaxa.4475.1.1
Atlas of Living Australia (2023a) Anampses melanurus occurrence records https://doi.org/10.26197/ala.6aff80e8-d157-4549-857b-6950c4949ca5. Accessed 21/03/2023
Atlas of Living Australia (2023b) Mulloidichthys pfluegeri occurrence records https://doi.org/10.26197/ala.0e0763bf-259f-45e7-84d1-ec8ae391355b. Accessed 21/03/2023
Australian Faunal Directory (2023) Valenciennea_helsdingenii occurrence records https://biodiversity.org.au/afd/taxa/Valenciennea_helsdingenii. Accessed 21/03/2023
Ayling AM, Ayling AL (1984) Coral Sea National Nature Reserves. Report on a preliminary survey of the Lihou Reef and Herald/Coringa National Nature Reserves. Unpublished report for ANPWS
Baum KA, Haynes KJ, Dillemuth FP, Cronin JT (2004) The matrix enhances the effectiveness of corridors and stepping stones. Ecology 85:2671–2676. https://doi.org/10.1890/04-0500
Beaman RJ (2012) Project 3DGBR: Great Barrier Reef and Coral Sea geomorphic features (MTSRF 2.5i.1, JCU). eAtlas.dataset. https://eatlas.org.au/data/uuid/25685ba5-6583-494f-974d-cce2f3429b78. Accessed Mar 21 2023
Beaman RJ, Picard K, Miller A (2022) RV Falkor surveys in Australia 2020-2021. In: Maschke J (ed) Hydrospatial 2021 Conference, 16-18 Feb 2022. Australasian Hydrographic Society,. Cairns, Australia
Becker R., Wilks R., Brownrigg R, et al (2022) maps: Draw Geographical Maps https://cran.r-project.org/package=maps
Bennett E (1832) Observations on a collection of fishes from Mauritius, presented by Mr Telfair, with characters of new genera and species, remaining portion. Proc Comm Sci Corresp Zool Soc London 1:165–169
Bennett S, Halford AR, Choat JH et al (2018) Geography and island geomorphology shape fish assemblage structure on isolated coral reef systems. Ecol Evol 8:6242–6252. https://doi.org/10.1002/ece3.4136
Bleeker P (1868) Description de deux espèces inédites d’Epinephelus rapportées de l’île de la Réunion par M.M. Pollen et van Dam. Versl en Meded der K Akad van Wet (Afdeeling Natuurjunde) 2:336–341
Bleeker P (1858) Bijdrage tot de kennis der vischfauna van den Goram-Archipel. Natuurwetenschappelijk Tijdschr voor Ned Indië 15:97–218
Bleeker P (1857) Achtste bijdrage tot de kennis der vischfauna van Amboina. Acta Soc Regiae Sci Indo-Neêrlandicae 2:1–102
Bray DJ and Gomon MF (2023) Fishes of Australia. Museums Victoria and OzFishNet, http://fishesofaustralia.net.au/. Accessed 21 March 2023
Boettiger C, Lang DT, Wainwright PC (2012) Rfishbase: exploring, manipulating and visualizing FishBase data from R. J Fish Biol 81:2030–2039. https://doi.org/10.1111/j.1095-8649.2012.03464.x
Bongaerts P, Bridge TCL, Kline DI et al (2011) Mesophotic coral ecosystems on the walls of Coral Sea atolls. Coral Reefs 30:335–335. https://doi.org/10.1007/s00338-011-0725-7
Bongaerts P, Smith TB (2019) Beyond the “deep reef refuge” hypothesis: a conceptual framework to characterize persistence at depth. In: Coral Reefs of the World Vol 12. Springer,Cham, pp 881–895, https://doi.org/10.1007/978-3-319-92735-0_45
Bowen BW, Rocha LA, Toonen RJ, Karl SA (2013) The origins of tropical marine biodiversity. Trends Ecol Evol 28:359–366. https://doi.org/10.1016/J.TREE.2013.01.018
Bray DJ (2023) Cephalopholis polleni in Fishes of Australia, https://fishesofaustralia.net.au/home/species/5007. Accessed 25 Mar 2023
Bray DJ (2022) Pseudanthias flavicauda in Fishes of Australia. https://fishesofaustralia.net.au/home/species/4998. Accessed 27 Mar 2023
Bridge TC, Hughes TP, Guinotte JM, Bongaerts P (2013) Call to protect all coral reefs. Nat Clim Chang 3:528–530. https://doi.org/10.1038/nclimate1879
Bridge TCL, Beaman RJ, Bongaerts P, et al (2019) The Great Barrier Reef and Coral Sea. In: Loya Y, Puglise KA, Bridge TCL (eds) Mesophotic coral ecosystems. In: Coral reefs of the world vol 12. Springer,Cham, pp 351–367. https://doi.org/10.1007/978-3-319-92735-0_20
Brooke B, Schmitt Ocean Institute (2022) Schmidt Ocean Institute expedition report: seamounts, canyons and reefs of the Coral Sea. https://doi.org/10.5281/zenodo.7308219
Budd AF, Pandolfi JM (2010) Evolutionary novelty is concentrated at the edge of coral species distributions. Science 328:1558–1561. https://doi.org/10.1126/science.1188947
Burgess WE (1978) Two new species of tilefishes (family Branchiostegidae) from the western Pacific. Trop Fish Hobbyist 26:43–47
Carroll A, Brooke B, Nichol S, et al (2021) Seamounts, reefs and canyons of Australia’s Coral Sea and Great Barrier Reef marine parks: a comprehensive deep sea study. In: ICRS 2021 - 14th International Coral Reef Symposium. Abstract no.: ICRS2021-2115.
Ceccarelli DM (2010) Research and monitoring in Australia’s Coral Sea: a review. Report for DSEWPaC available at https://parksaustralia.gov.au/marine/pub/scientific-publications/Ceccarelli-Coral-Sea-Research-Review-Final-2011.pdf
Ceccarelli DM, David McKinnon A, Andréfouët S et al (2013) The Coral Sea: physical environment, ecosystem status and biodiversity assets. In: Adv Marine Bio 66(213-90):213–290. https://doi.org/10.1016/B978-0-12-408096-6.00004-3
Clark M, Bowden D (2015) Seamount biodiversity: high variability both within and between seamounts in the Ross Sea region of Antarctica. Hydrobiologia 761:161–180. https://doi.org/10.1007/s10750-015-2327-9
Clark MR, Rowden AA, Schlacher T et al (2010a) The ecology of seamounts: structure, function, and human impacts. Annu Rev Mar Sci 2:253–278. https://doi.org/10.1146/annurev-marine-120308-081109
Clark MR, Schlacher TA, Rowden AA et al (2010b) Science priorities for seamounts: research links to conservation and management. PLoS One 7:e29232. https://doi.org/10.1371/journal.pone.0029232
Connor EF, McCoy ED (1979) The statistics and biology of the species-area relationship. Am Nat 113:791–833
Copus JM, Pyle RL, Bowen BW et al (2022) The habitat persistence hypothesis: a new perspective on the distribution of coral-reef organisms. Front Biogeograp 14(4). https://doi.org/10.21425/F5FBG57427
Cowman PF, Parravicini V, Kulbicki M, Floeter SR (2017) The biogeography of tropical reef fishes: endemism and provinciality through time. Biol Rev 92:2112–2130. https://doi.org/10.1111/brv.12323
Davies P, Symonds P, Feary D, Pigram C (1989) The evolution of carbonate platforms of northeast Australia. In: Crevello P, Wilson J, Sarg J, Read J (eds) Controls on carbonate platform and basin development. SEPM Special Publications, Tulsa, pp 233–258
Demartini EE, Friedlander AM, Sandin SA, Sala E (2008) Differences in fish-assemblage structure between fished and unfished atolls in the northern Line Islands, central Pacific. Mar Ecol Prog Ser 365:199–215. https://doi.org/10.3354/meps07501
Director of National Parks (2018) Coral Sea Marine Park management plan. Canberra. Available at https://parksaustralia.gov.au/marine/pub/plans/coral-sea-management-plan-2018.pdf. Accessed 24 Mar 2023
Edgar G, Stuart-Smith R (2014) Reef Life Survey (RLS): Global reef fish dataset 10.15468/qjgwba accessed via GBIF.org. Accessed 27 March 2023
Englebert N, Bongaerts P, Muir P et al (2015) Deepest zooxanthellate corals of the Great Barrier Reef and Coral Sea. Mar Biodivers 45:1–2. https://doi.org/10.1007/s12526-014-0221-8
Englebert N, Bongaerts P, Muir PR et al (2017) Lower mesophotic coral communities ( 60- 125 m depth ) of the Northern Great Barrier Reef and Coral Sea. PLoS One:1–16. https://doi.org/10.1371/journal.pone.0170336
Eyal G, Laverick JH, Bongaerts P, et al. (2021) Mesophotic coral ecosystems of the Great Barrier Reef are understudied and underexplored. Front Mar Sci 8:. https://doi.org/10.3389/fmars.2021.622856
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663. https://doi.org/10.1111/jbi.12130
Fricke R, Earle JL, Pyle RL, Séret B (2011a) Checklist of the fishes. In: Bouchet P, Le Guyader H, Pascal O (eds) The natural history of Santo - Patrimoines naturels. Paris (MNHN, PNI), Marseille (PNI), pp 343–409
Fricke R, Kulbicki M (2007) Checklist of the shore fishes of New Caledonia. In: Ayri C., Richer de Forges B (eds) Compendium of marine species from New Caledonia. 2nd edition, 2nd Editio. pp 357–401, pls. 15/1 and 15/2
Fricke R, Kulbicki M, Wantiez L (2011b) Checklist of the fishes of New Caledonia, and their distribution in the Southwest Pacific Ocean (Pisces). Stuttgarter Beitrage Zur Naturkunde, Ser A:341–463
Fricke R, Eschmeyer WN, R. van der Laan (eds) (2023) Eschmeyer’s Catalog of Fishes: Genera, species, references electronic version accessed 18 Oct 2023. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Froese R, Pauly D (2023) FishBase. In: World wide web electron. Publ. www.fishbase.org
Gaither MR, Rocha LA (2013) Origins of species richness in the Indo-Malay-Philippine biodiversity hotspot: evidence for the centre of overlap hypothesis. J Biogeogr 40:1638–1648. https://doi.org/10.1111/jbi.12126
Galbraith G, McClure E, Barnett A, et al (2022) Diving into the deep: the unique deep habitats of the Coral Sea Marine Park. Report prepared for Parks Australia. https://doi.org/10.13140/RG.2.2.10516.68487/1
Galbraith GF, Cresswell BJ, McCormick MI et al (2021) High diversity, abundance and distinct fish assemblages on submerged coral reef pinnacles compared to shallow emergent reefs. Coral Reefs 40:335–354. https://doi.org/10.1007/s00338-020-02044-z
GBIF.org (2023a), GBIF Home Page. Available from: https://www.gbif.org. Accessed 1 Mar 2023
GBIF.org (2023b) GBIF Occurrence Download https://doi.org/10.15468/dl.uyvhj9 [Accessed 1 March 2023]
GBIF.org (2023c) GBIF Occurrence Download https://doi.org/10.15468/dl.f7ak26 [Accessed 1 March 2023]
GBIF.org (2023d) GBIF Occurrence Download https://doi.org/10.15468/dl.qspxgx [Accessed 1 March 2023]
GBIF.org (2023e) GBIF Occurrence Download https://doi.org/10.15468/dl.zjpsfj [Accessed 1 March 2023]
Gilbert C (1905) II. The deep-sea fishes. In: Jordan D., Evermann B. (eds) The aquatic resources of the Hawaiian Islands. Bulletin of the United States Fish Commission : 23(2). pp 575–713 figs 230–276 pls 66–101
Gill AC (2022) Revised definitions of the anthiadine fish genera Mirolabrichthys Herre and Nemanthias Smith, with description of a new genus (Teleostei: Serranidae). Zootaxa 5092:41–66. https://doi.org/10.11646/zootaxa.5092.1.2
Goldstein ED, D’Alessandro EK, Sponaugle S (2017) Fitness consequences of habitat variability, trophic position, and energy allocation across the depth distribution of a coral-reef fish. Coral Reefs 36:957–968. https://doi.org/10.1007/s00338-017-1587-4
Goldstein ED, D’alessandro EK, Sponaugle S (2016) Demographic and reproductive plasticity across the depth distribution of a coral reef fish. Nat Publ Gr. https://doi.org/10.1038/srep34077
Gomon MF (2006) A revision of the labrid fish genus Bodianus with descriptions of eight new species. Rec Aust Museum 58:1–133. https://doi.org/10.3853/j.0812-7387.30.2006.1460
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667. https://doi.org/10.1111/j.0022-1112.2005.00629.x
Hanski I (2015) Habitat fragmentation and species richness. J Biogeogr 42:989–993. https://doi.org/10.1111/jbi.12478
Hanski I, Zurita GA, Bellocq MI, Rybicki J (2013) Species-fragmented area relationship. Proc Natl Acad Sci U S A 110:12715–12720. https://doi.org/10.1073/PNAS.1311491110/SUPPL_FILE/PNAS.201311491SI.PDF
Harris P, Heap A, Passlow V, et al. (2003) Geomorphic features of the continental margin of Australia, Geoscience Australia, Record 2003/30, 142pp
Hixon MA, Jones GP (2005) Competition, predation, and density-dependent mortality in demersal marine fishes. Ecology 86:2847–2859. https://doi.org/10.1890/04-1455
Hobbs J-PA, Jones GP, Munday PL et al (2012) Biogeography and the structure of coral reef fish communities on isolated islands. J Biogeogr 39:130–139. https://doi.org/10.1111/j.1365-2699.2011.02576.x
Hobbs JPA, Choat JH, Robbins WD et al (2008) Unique fish assemblages at world’s southernmost oceanic coral reefs, Elizabeth and Middleton Reefs, Tasman Sea, Australia. Coral Reefs 27:15. https://doi.org/10.1007/S00338-007-0301-3/FIGURES/1
Hobbs JPA, Frisch AJ, Allen GR, Van Herwerden L (2009) Marine hybrid hotspot at Indo-Pacific biogeographic border. Biol Lett 5:258–261. https://doi.org/10.1098/rsbl.2008.0561
Hoey A, Harrison H, McClure E, et al (2021) Coral Sea Marine Park Coral Reef Health Survey 2021. Report prepared for Parks Australia, https://doi.org/10.13140/RG.2.2.30008.06405/1
Hoey A, McClure E, Burn D, et al (2022) Coral Sea Marine Park Coral Reef Health Survey 2022. Report prepared for Parks Australia. https://doi.org/10.13140/RG.2.2.28552.60161/1
Hoey AS, Harrison H., Pratchett M. (2020) Coral Reef Health in the Coral Sea Marine Park – Surveys 2018-2020, Report prepared for Parks Australia. [Accessed 1 March 2023] Available at https://parksaustralia.gov.au/marine/pub/scientific-publications/Coral-Sea-Coral-Reef-Health-Report-2020.pdf
Hoey J, McCormick MI, Hoey AS (2007) Influence of depth on sex-specific energy allocation patterns in a tropical reef fish. Coral Reefs 26:603–613. https://doi.org/10.1007/s00338-007-0246-6
Hopley D (2006) Coral reef growth on the shelf margin of the Great Barrier Reef with special reference to the Pompey complex. J Coast Res 22. https://doi.org/10.2112/05A-0012.1
Hubbs CL (1959) Initial discoveries of fish faunas on seamounts and offshore banks in the Eastern Pacific. Pac Sci 13(4):311–316
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804. https://doi.org/10.1038/45538
Jones GP (2015) Mission impossible: unlocking the secrets of coral reef fish dispersal. In: Mora C (ed) Ecology of fishes on coral reefs. Cambridge University Press, Cambridge, pp 16–27
Jones GP, Barone G, Sambrook K, Bonin MC (2020) Isolation promotes abundance and species richness of fishes recruiting to coral reef patches. Mar Biol 167:167. https://doi.org/10.1007/s00227-020-03772-0
Kahle D, Wickham H (2013) ggmap: spatial visualization with ggplot2. R Journal 5:144–161. https://doi.org/10.32614/RJ-2013-014
Kessler WS, Cravatte S (2013) Mean circulation of the Coral Sea. J Geophys Res Ocean 118:6385–6410. https://doi.org/10.1002/2013JC009117
Kulbicki M, Parravicini V, Bellwood DR et al (2013) Global biogeography of reef fishes: a hierarchical quantitative delineation of regions. PLoS One 8:81847. https://doi.org/10.1371/journal.pone.0081847
Langlois T, Goetze J, Bond T et al (2020) A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods Ecol Evol 11:1401–1409. https://doi.org/10.1111/2041-210X.13470
Langston RC, Spalding HL (2017) A survey of fishes associated with Hawaiian deep-water Halimeda kanaloana (Bryopsidales: Halimedaceae) and Avrainvillea sp. (Bryopsidales: Udoteaceae) meadows. PeerJ 2017. https://doi.org/10.7717/peerj.3307
Laverick JH, Piango S, Andradi-Brown DA et al (2018) To what extent do mesophotic coral ecosystems and shallow reefs share species of conservation interest? A systematic review. Environ Evid 7:1–13. https://doi.org/10.1186/s13750-018-0127-1
Leitner A, Friedrich T, Kelley C et al (2021) Biogeophysical influence of large-scale bathymetric habitat types on mesophotic and upper bathyal demersal fish assemblages: a Hawaiian case study. Mar Ecol Prog Ser 659:219–236. https://doi.org/10.3354/meps13581
Lesser MP, Slattery M, Laverick JH et al (2019) Global community breaks at 60 m on mesophotic coral reefs. Glob Ecol Biogeogr 00:1–14. https://doi.org/10.1111/geb.12940
Lesser MP, Slattery M, Mobley CD (2018) Biodiversity and functional ecology of mesophotic coral reefs. Annu Rev Ecol Evol Syst 49:49–71. https://doi.org/10.1146/annurev-ecolsys-110617
Letessier TB, Mouillot D, Bouchet PJ et al (2019) Remote reefs and seamounts are the last refuges for marine predators across the Indo-Pacific. PLoS Biol 17:e3000366. https://doi.org/10.1371/journal.pbio.3000366
Loya Y, Eyal G, Treibitz T et al (2016) Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Coral Reefs 35:1–9. https://doi.org/10.1007/s00338-016-1410-7
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, New Jersey
MacDonald C, Bridge TCL, McMahon KW, Jones GP (2019) Alternative functional strategies and altered carbon pathways facilitate broad depth ranges in coral-obligate reef fishes. Funct Ecol 33:1962–1972. https://doi.org/10.1111/1365-2435.13400
MacDonald C, Jones GP, Bridge TCL (2018) Marginal sinks or potential refuges? Costs and benefits for coral-obligate reef fishes at deep range margins. Proc R Soc B Biol Sci 285:20181545. https://doi.org/10.1098/rspb.2018.1545
Mazzei EF, Pinheiro HT, Simon T et al (2021) Mechanisms of dispersal and establishment drive a stepping stone community assembly on seamounts and oceanic islands. Mar Biol 168:1–11. https://doi.org/10.1007/S00227-021-03919-7/FIGURES/4
Mcclain CR (2007) Seamounts: identity crisis or split personality? J Biogeogr (J 34:2001–2008. https://doi.org/10.1111/j.1365-2699.2007.01783.x
McClain CR, Lundsten L, Ream M et al (2009) Endemicity, biogeography, composition, and community structure on a Northeast Pacific Seamount. PLoS One 4:e4141. https://doi.org/10.1371/journal.pone.0004141
McCook LJ, Almany GR, Berumen ML et al (2009) Management under uncertainty: guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 28:353–366. https://doi.org/10.1007/s00338-008-0463-7
McKinnon AD, Williams A, Young J et al (2014) Tropical marginal seas: priority regions for managing marine biodiversity and ecosystem function. Annu Rev Mar Sci 6:415–437. https://doi.org/10.1146/annurev-marine-010213-135042
Miller KJ, Gunasekera RM (2017) A comparison of genetic connectivity in two deep sea corals to examine whether seamounts are isolated islands or stepping stones for dispersal. Sci Rep 7:46103. https://doi.org/10.1038/srep46103
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83:1131–1145. https://doi.org/10.1890/0012-9658(2002)083[1131:SCMISE]2.0.CO;2
Mora C (2004) Importance of dispersal in coral reef fishes. Electronic Theses and Dissertations. 2400. https://scholar.uwindsor.ca/etd/2400. Accessed 1 Mar 2023
Mora C, Chittaro PM, Sale PF et al (2003) Patterns and processes in reef fish diversity. Nature 421:933–936. https://doi.org/10.1038/nature01393
Morais J, Santos BA (2018) Limited potential of deep reefs to serve as refuges for tropical Southwestern Atlantic corals. Ecosphere 9. https://doi.org/10.1002/ecs2.2281
Muir P, Wallace C, Bridge TCL, Bongaerts P (2015) Diverse staghorn coral fauna on the mesophotic reefs of North-East Australia. PLoS One 10:e0117933. https://doi.org/10.1371/journal.pone.0117933
Mullineaux LS, Mills SW (1997) A test of the larval retention hypothesis in seamount-generated flows. Deep Sea Res I 44(5):745–770
Munday PL (2001) Fitness consequences of habitat use and competition among coral-dwelling fishes. Oecologia 128:585–593. https://doi.org/10.1007/s004420100690
OBIS (2023a) Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.obis.org. Accessed 24 Mar 2023
OBIS (2023b) Distribution records of Hoplolatilus randalli (Allen, Erdmann & Hamilton, 2010) [Dataset] (Available: Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.obis.org. Accessed: 21 March 2023)
OBIS (2023c) Distribution records of Cephalopholis polleni (Bleeker 1868) [Dataset] (Available: Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.obis.org. Accessed: 21 March 2023)
OBIS (2023d) Distribution records of Pseudanthias flavicauda (Randall and Pyle 2001) [Dataset] (Available: Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.obis.org. Accessed: 21 March 2023)
OBIS (2023e) Distribution records of Bodianus paraleucosticticus (Gomon 2006) [Dataset] (Available: Ocean Biodiversity Information System. Intergovernmental Oceanographic Commission of UNESCO. www.obis.org. Accessed: 21 March 2023)
Oxley W, Ayling A, Cheal A, Osborne K (2004) Marine surveys undertaken in the Elizabeth and Middleton Reefs Marine National Nature Reserve, December 2003, Townsville, Qld. Australian Institute of Marine Science
Payet SD, Pratchett MS, Saenz-Agudelo P et al (2022) Demographic histories shape population genomics of the common coral grouper (Plectropomus leopardus). Evol Appl 15:1221–1235. https://doi.org/10.1111/EVA.13450
Pérez-Rosales G, Hernández-Agreda A, Bongaerts P et al (2022) Mesophotic depths hide high coral cover communities in French Polynesia. Sci Total Environ 844:157049. https://doi.org/10.1016/J.SCITOTENV.2022.157049
Pinheiro HT, Bernardi G, Simon T et al (2017) Island biogeography of marine organisms. Nature 549:82–85. https://doi.org/10.1038/nature23680
Pinheiro HT, MacDonald C, Quimbayo JP et al (2023) Assembly rules of coral reef fish communities along the depth gradient. Curr Biol:1–10. https://doi.org/10.1016/j.cub.2023.02.040
Pinheiro HT, Mazzei E, Moura RL et al (2015) Fish biodiversity of the Vitória-Trindade Seamount Chain, Southwestern Atlantic : an updated database. PLoS One 10:e0118180. https://doi.org/10.1371/journal.pone.0118180
Pinheiro HT, Rocha LA, Macieira RM et al (2018) South-western Atlantic reef fishes: zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers Distrib 24:951–965. https://doi.org/10.1111/ddi.12729
Pitcher TJ, Morato T, Hart PJB, et al (2007) Seamounts: ecology, fisheries & conservation. Blackwell Publishing Ltd.
Planes S, Doherty PJ, Bernardi G (2001) Strong genetic divergence among populations of a marine fish with limited dispersal, Acanthochromis polyacanthus, within the Great Barrier Reef and the Coral Sea. Evolution (NY) 55:2263–2273. https://doi.org/10.1111/j.0014-3820.2001.tb00741.x
Planes S, Jones GP, Thorrold SR (2009) Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci 106:5693–5697. https://doi.org/10.1073/pnas.0808007106
Prugh LR, Hodges KE, Sinclair ARE, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. Proc Natl Acad Sci U S A 105:20770–20775. https://doi.org/10.1073/pnas.0806080105
Pyle LR (2000) Assessing undiscovered fish biodiversity on deep coral reefs.pdf. Mar Technol Soc J 34:82–91. https://doi.org/10.4031/MTSJ.34.4.11
Pyle RL (2005) Recent discoveries of new fishes inhabiting deep Pacific coral reefs, with biogeographic implications. In: 7th Indo-Pacific Fish Conference (IPFC7). Taipei, Taiwan
Pyle RL, Copus JM (2019) Mesophotic coral ecosystems: introduction and overview. In: Loya Y, Puglise K, Bridge T (eds) Mesophotic coral ecosystems. Coral Reefs of the World, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-92735-0_1
R Core Development Team (2023) R: a language and environment for statistical computing
Randall JE, Allen GR and Steene RC (1997) The complete divers’ and fisherman’s guide to fishes of the Great Barrier Reef and Coral Sea, second edition, Crawford House Publishing
Randall JE, Williams J, Smith D, et al (2003) Checklist of the shore and epipegagic fishes of Tonga. Washington DC
Randall JE (1975) A revision of the Indo-Pacific angelfish genus Genicanthus, with descriptions of three new species. Bull Mar Sci 25:393–421
Randall JE, Lubbock R (1982) Three new labrid fishes of the genus Cirrhilabrus from the southwestern Pacific. Occas Pap Bernice P Bish Museum 25:12pp
Randall JE, McCosker JE (1982) Two new serranid fishes of the genus Anthias from the central Pacific. J Aquaric 2:59–69
Randall JE, Pyle RL (2001) Four new serranid fishes of the anthiine genus Pseudanthias from the South Pacific. Raffles Bull Zool 49:19–34
Rees, AJJ, Yearsley, GK, Gowlett-Holmes K and Pogonoski J (2023) Codes for Australian aquatic biota (on-line version). CSIRO National Collections and Marine Infrastructure (formerly CSIRO Marine and Atmospheric Research), World Wide Web electronic publication, 1999 onwards. Available at: https://www.marine.csiro.au/data/caab/. Accessed 4 March 2023
RLS (2023) Reef Life Survey website https://reeflifesurvey.com/survey-data. Accessed 25 March 2023
Richer de Forges B, Koslow JA, Poore GCB (2000) Diversity and endemism of the benthic seamount megafauna in the southwest Pacific. Nature 405:944–947. https://doi.org/10.1038/35016066
Riva F, Fahrig L (2022) The disproportionately high value of small patches for biodiversity conservation. Conserv Lett 15:1–7. https://doi.org/10.1111/conl.12881
Rocha LA, Pinheiro HT, Shepherd B et al (2018) Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361:281–284. https://doi.org/10.1126/science.aaq1614
Rogers AD (2018) The biology of seamounts: 25 years on. Adv Mar Biol 79:137–224. https://doi.org/10.1016/BS.AMB.2018.06.001
Rowden AA, Clark MR, Wright IC (2005) Physical characterisation and a biologically focused classification of " seamounts " in the New Zealand region. New Zeal J Mar Freshw Res 39. https://doi.org/10.1080/00288330.2005.9517374
Rowden AA, Dower JF, Schlacher TA et al (2010) Paradigms in seamount ecology: fact, fiction and future. Mar Ecol 31:226–241. https://doi.org/10.1111/j.1439-0485.2010.00400.x
Sambrook K, Hoey AS, Andréfouët S et al (2019) Beyond the reef: the widespread use of non-reef habitats by coral reef fishes. Fish Fish 20:903–920. https://doi.org/10.1111/faf.12383
Sarano F, Pichon M (1988) Morphology and ecology of the deep fore reef slope at Osprey Reef (Coral Sea). In: Choat J. (ed) Proceedings of the 6th International Coral Reef Symposium. Townsville, Australia
Saura S, Bodin Ö, Fortin MJ (2014) Stepping stones are crucial for species’ long-distance dispersal and range expansion through habitat networks. J Appl Ecol 51:171–182. https://doi.org/10.1111/1365-2664.12179
SeaGis Pty Australia CAL EventMeasure. TransectMeasure https://www.seagis.com.au/
Sievers KT, McClure EC, Abesamis RA, Russ GR (2020) Non-reef habitats in a tropical seascape affect density and biomass of fishes on coral reefs. Ecol Evol 10:13673. https://doi.org/10.1002/ECE3.6940
Sih TL, Cappo M, Kingsford M (2017) Deep-reef fish assemblages of the Great Barrier Reef shelf-break (Australia). Sci Rep 7:10886. https://doi.org/10.1038/s41598-017-11452-1
Simon T, Pinheiro HT, Santos S et al (2022) Comparative phylogeography of reef fishes indicates seamounts as stepping stones for dispersal and diversification. Coral Reefs 41:551–561. https://doi.org/10.1007/S00338-021-02178-8/FIGURES/2
Slattery M, Lesser MP (2012) Mesophotic coral reefs: a global model of community structure and function. Proc 12th Coral Reef Symp 1:9−13
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX, ... Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57(7):573–583
Spalding HL, Amado-Filho GM, Bahia RG, et al. (2019) Macroalgae. In: Loya, Y., Puglise, K., Bridge, T. (eds) Mesophotic coral ecosystems. Coral Reefs of the World, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-92735-0_29
Sponaugle S, Cowen RK, Shanks A et al (2002) Predicting self-recruitment in marine populations biophysical correlates and mechanisms. Bull Mar Sci 70(1):341–375
Srinivasan M (2003) Depth distributions of coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 137:76–84. https://doi.org/10.1007/s00442-003-1320-6
Stefanoudis PV, Rivers M, Smith SR et al (2019) Low connectivity between shallow, mesophotic and rariphotic zone benthos. R Soc Open Sci 6:190958. https://doi.org/10.1098/rsos.190958
Steindachner F (1900) Fische aus dem Stillen Ocean. Ergebnisse einer Reise nach dem Pacific (Schauinsland, 1896-1897). nzeiger der Kais Akad der Wissenschaften Math Klasse. Wien 37:174–178
Stuart-Smith R, Crawford T, Cooper A, et al (2013) Coral Sea marine biodiversity. University Of Tasmania Report. https://hdl.handle.net/102.100.100/502860
Szangolies L, Rohwäder MS, Jeltsch F (2022) Single large and several small habitat patches: a community perspective on their importance for biodiversity. Basic Appl Ecol 65:16–27. https://doi.org/10.1016/j.baae.2022.09.004
Tang S, Graba-Landry A, Hoey AS (2020) Density and height of Sargassum influence rabbitfish (f. Siganidae) settlement on inshore reef flats of the Great Barrier Reef. Coral Reefs 39:467–473. https://doi.org/10.1007/s00338-020-01908-8
Tea YK, Larson HK (2023) Synopsis of the ptereleotrine goby genus Nemateleotris, with description of a new species from the western and central Pacific Ocean (Teleostei: Gobiidae). Raffles Bull Zool 71:248–266. https://doi.org/10.26107/RBZ-2023-0019
Thompson CDH, Meeuwig JJ, Friedlander AM, Sala E (2023) Remote seamounts are key conservation priorities for pelagic wildlife. Conserv Lett 00:e12993. https://doi.org/10.1111/conl.12993
Valenciennes A (1830) in Cuvier G & Valenciennes, A Histoire naturelle des poissons. Tome Sixième. Livre sixième. Partie I. Des Sparoïdes; Partie II. Des Ménides. v. 6: i-xxiv + 6 pp. + 1-559, Pls. 141-169. [Valenciennes is author of pp. 1-425, 493-559; Cuvier 426-491. i-x]
Van Herwerden L, Howard Choat JH, Newman SJ et al (2009) Complex patterns of population structure and recruitment of Plectropomus leopardus (Pisces: Epinephelidae) in the Indo-West Pacific: Implications for fisheries management. Mar Biol 156:1595–1607. https://doi.org/10.1007/s00227-009-1195-0
Wagner D, Friedlander AM, Pyle RL et al (2020) Coral reefs of the high seas: hidden biodiversity hotspots in need of protection. Front Mar Sci 7:2296–7745. https://doi.org/10.3389/fmars.2020.567428
Williams ID, Richards BL, Sandin SA et al (2011) Differences in reef fish assemblages between populated and remote reefs spanning multiple archipelagos across the Central and Western Pacific. J Mar Biol 2011:1–14. https://doi.org/10.1155/2011/826234
Yesson Y, Letessier TB, Nimmo-Smith A et al. (2021) Improved bathymetry leads to >4000 new seamount predictions in the global ocean – but beware of phantom seamounts! UCL Open Eviron. Vol. 4. https://doi.org/10.14324/111.444/ucloe.000030
Acknowledgements
We are grateful to JH Choat, GP Jones and C MacDonald for thoughtful discussions and comments on this manuscript. Thanks also to D Bray, A Hay, M McNeil, J Pogonoski, YK Tea and F Walsh for assistance with cross referencing species distributions and identification. ROV and BRUV surveys were logistically supported by the skippers of the MV Iron Joy, Rob Benn, and MV Argo, Jeff Farnham, and their crew. Photo of B. paraleucosticticus in Fig. 3d courtesy of Richard Bajol/Alain Daoulas. We also thank three anonymous reviewers for their engagement with and constructive feedback on our manuscript. Field studies in the Coral Sea Marine Park were carried out with permission from the Director of National Parks (permit number PA2020-00092-3).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Funding for all surveys was provided by the Australian Government’s Our Marine Parks Grants (Rounds 2 and 3) to ASH, GFG and ECM (grant numbers 4-FISKTNX and 4-HAY3RAP respectively) and the ARC Centre of Excellence for Coral Reef Studies (ASH). Additional ROV surveys in July 2021 were facilitated by Parks Australia with operational support from Queensland Department of Environment and Science as part of the Coral Sea Island Health voyage.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This research was conducted under James Cook University Animal Ethics permit A2721.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
All species recorded by ROV and BRUV in this study are provided as Online Resource 1. A list of depth extensions for fishes compared to FishBase records is provided in Online Resource 1. Occurrence data used to plot Fig. 3 were downloaded from the OBIS database (OBIS 2023a,b,c,d,e), GBIF database (GBIF 2023a,b,c,d,e) and are provided as a csv file at 10.5281/zenodo.7844209.
Author contribution
All authors contributed to the study conception and design. Material preparation and data collection were performed GFG and BJC. Analysis was performed by GFG, BJC and ECM. The first draft of the manuscript was written by GFG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Additional information
Communicated by K. H. George
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the Topical Collection Seamounts and oceanic archipelagos and their role for the biodiversity, biogeography, and dispersal of marine organisms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galbraith, G.F., Cresswell, B.J., McClure, E.C. et al. Tropical seamounts as stepping-stones for coral reef fishes: range extensions and new regional distributions from mesophotic ecosystems in the Coral Sea, Australia. Mar. Biodivers. 54, 17 (2024). https://doi.org/10.1007/s12526-024-01404-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-024-01404-0