Abstract
Hull fouling is considered to be the most significant vector of introduction of marine non-indigenous species (NIS) in the Madeira Archipelago (NE Atlantic) because these islands provide a vital passage route for many ships. The transfer of species between boat hulls and artificial substrates in marinas is known to be high. Bryozoans are among the most common groups of marine invertebrates growing on this type of substrate. In recent years, significant advances have been made in our knowledge about the biodiversity of bryozoans in the Madeira Archipelago. Nonetheless, the currently recognized numbers remain far from reflecting the actual bryozoan species richness. In this context, we examine bryozoan samples stemming from NIS monitoring surveys on artificial substrates along the southern coast of the Madeira Archipelago, in four recreational marinas and in two offshore aquaculture farms. This has yielded new information about ten bryozoan species. Two of them, Crisia noronhai sp. nov. and Amathia maderensis sp. nov., are described for the first time, although at least the first one was previously recorded from Madeira but misidentified. Bugula ingens, Cradoscrupocellaria insularis, Scruparia ambigua, and Celleporaria brunnea are recorded for the first time in Madeira. Moreover, the material of C. brunnea was compared with the type, and a biometric analysis was performed with material from the Atlantic and Mediterranean. All samples identified as C. brunnea in both regions are the same species, and the variations described in the literature apparently reflect high intracolonial variability. Finally, we provide new information for the descriptions of 4 additional bryozoans, namely, Crisia sp. aff. elongata, Cradoscrupocellaria bertholletii, Scrupocaberea maderensis, and Tricellaria inopinata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Madeira Archipelago is a group of Portuguese volcanic islands located in the NE Atlantic Ocean, 700 km off the Moroccan coast. Madeira is the largest of the two inhabited islands, with 144 km of coastline, whereas Porto Santo is located about 42 km northeast of Madeira island with about 33 km of coastline (Ramalhosa et al. 2019). Historically, the archipelago has provided a vital passage route for many ships between Europe, America and Africa because of its unique geographical position in the Atlantic Ocean, which offers an important port for re-fueling and rest stops (Castro et al. 2020).
Pioneer works on the bryozoan fauna from the Madeira Archipelago date back to the late nineteenth century (Busk 1858a, 1858b, 1859, 1860, 1861; Hincks 1880; Johnson 1897; Waters 1899; Norman 1909). Since then, several species have been added to this list, with most of the new records detected during the last two decades (d’Hondt 1985, Alves and Cocito 2002, Berning and Kuklinski 2008, Berning et al. 2008, Wirtz and Canning-Clode 2009, Berning 2012, Souto et al. 2014). In fact, Berning (2012) listed 140 species of bryozoans in Madeira and considered the island a “hotspot” of bryozoan diversity compared to other nearby regions. Furthermore, the author indicated that the known bryozoan species list for the archipelago was likely an underestimate (Berning 2012).
Accordingly, our knowledge of cheilostome bryozoan species from the Madeira Archipelago is far from complete, as several new records have been detected and inventoried in recent years due to comprehensive nonindigenous species (NIS) monitoring surveys, particularly in marinas along the southern coast (Canning-Clode et al. 2013a; Ramalhosa et al. 2017a, 2019). These recent monitoring surveys have increased sampling efforts and resulted in the discovery and description of three bryozoan species new to science (Souto et al. 2015, 2018). Nevertheless, the lack of a comprehensive checklist here makes the detection and dating of new anthropogenic introductions a complex challenge. Today, however, an estimated 150 species have been recorded. The increased detection in recent years also benefited from using Scanning Electron Microscopy (SEM) for identification instead of the optical microscopy used in past studies (Berning 2012; Souto et al. 2014; Ramalhosa et al. 2017a).
International shipping is one of the most significant vectors contributing to the spread and establishment of marine NIS, thus representing a significant threat to biodiversity in coastal marine ecosystems worldwide (Molnar et al. 2008; Carlton and Ruiz 2016). Furthermore, biological invasions in the sea have been increasing in recent years because maritime traffic facilitates the transport and arrival of NIS into new regions through the ballast water on ships and/or hull fouling (Kaluza et al. 2010, Clarke Murray et al. 2014). Indeed, hull fouling is the most significant vector of the introduction of marine NIS in the Madeira Archipelago coastal waters (Canning-Clode et al. 2013a; Ramalhosa et al. 2014, 2017a, 2017b, 2019; Ramalhosa and Canning-Clode 2015; Souto et al. 2018). Moreover, offshore aquaculture activities facilitate the local dispersion of NIS (Nunes et al. 2014; Campbell et al. 2017), posing a serious environmental threat to biodiversity and ecosystem function (Mack et al. 2000; Png-Gonzalez et al. 2021). Aquaculture artificial substrates may serve as stepping stones, offering novel niches for opportunistic colonizers, including NIS, favoring their dispersal (De Mesel et al. 2015) and supplying substrate to establish other NIS (Rius et al. 2011; Png-Gonzalez et al. 2021).
In this context, the current study examined samples of 10 bryozoan species stemming from NIS monitoring surveys conducted on artificial substrates along the south coast of the Madeira Archipelago in four recreational marinas and two offshore aquaculture farms. This research resulted in the description of two new bryozoan species and four new records for the archipelago.
Materials and methods
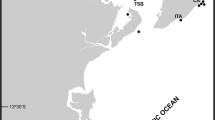
The bryozoan samples stem from several NIS monitoring surveys conducted along the south coast of the Madeira Archipelago (Fig. 1), particularly on artificial settlement panels deployed within four recreational marinas: Calheta (CA, 32°43′ N, 17°10′ W), Funchal (FX, 32°38′ N, 16°54′ W), Quinta do Lorde (QL, 32°44′ N, 16°42′ W), and Porto Santo Island (PS, 33°03′ N, 16°18′ W), between 2013 and 2019 (Fig. 1a–d). In addition, this includes samples collected during 2018 from two offshore aquaculture farms growing gilthead sea bream (Sparus aurata Linnaeus, 1758), Campanário (CAM, 32°39′ N, 17°3′ W) and Caniçal (CAN, 32°44′ N, 16°41′ W) (Fig. 1e, f). Following the design employed by Canning-Clode et al. (2011) and Ramalhosa et al. (2014), at each sampling site polyvinylchloride (PVC) experimental settling panels (14 × 14 × 0.3 cm) were deployed attached to a brick, They were positioned horizontally and facing downwards to favor the settlement of macro-invertebrates rather than macro-algae and hung at approximately 1 m depth from pontoons inside the four marinas and in the external float of seabream offshore cages at each aquaculture farm. Settlement plates were retrieved after three months to collect representative samples. Fouling communities from settlement plates were initially observed with a stereomicroscope (Leica S8 APO), and digital photographs of specimens were taken using an Olympus STYLUS TG-4 camera. Bryozoan specimens were first preserved in 95% ethanol and later examined with a stereomicroscope Leica MZ12. Colony fragments were selected and dried, and SEM photographs of uncoated material were taken using an FEI Inspect S50 SEM at the University of Vienna, Austria. Some samples were cleaned, and when necessary, fragments were bleached with sodium hypochlorite to remove organic material before SEM examination. The SEM was used with a back-scattered electron detector in low vacuum mode. Zooidal measurements were taken from the SEM images using the software ImageJ® (http://rsbweb.nih.gov/ij). In addition, historical specimens from the Norman Collection stored in the Museu Municipal de História Natural in Funchal (MMF), Madeira Island, Portugal, were also examined. Furthermore, type specimen of Celleporaria brunnea (Hincks, 1884) from the Natural History Museum of London (NHMUK) and specimens of this species from the Iberian Peninsula and Italy were studied for comparison. Finally, new specimens collected during the present study were deposited in MMF and the Natural History Museum of the University of Santiago de Compostela (MHNUSC) collections.
Madeira Archipelago with the location of the four marinas along the south coast of both Madeira and Porto Santo Islands shown by a red circle; a—Calheta (CA); b—Funchal (FX); c—Quinta do Lorde (QL), and; d—Porto Santo (PS); two aquaculture facilities shown by a white circle; e—Campanário (CAM) and; f—Caniçal (CAN)
Results
Phylum Bryozoa Ehrenberg, 1831
Class Stenolaemata Borg, 1941
Order Cyclostomatida Busk, 1852
Family Crisiidae Johnston, 1838
Genus Crisia Lamouroux, 1812
Crisia noronhai sp. nov. Souto, Ramalhosa & Canning-Clode
https://zoobank.org/23D69F5B-1684-4167-84C2-2D37C8985884
Crisia eburnea, Norman 1909: 277.
Material examined
Holotype– MMF48464: 13/01/2014, Funchal, two dry fragments from one specimen in a SEM stub; Paratypes– MMF48461, 44,786, 48,462: 11/10/2013, Quinta do Lorde; MMF48463: 15/10/2013, Calheta; MMF48465: 29/07/2014, Quinta do Lorde; MMF47068: 26/01/2015, Quinta do Lorde; MMF48466, 48,467: 30/10/2018, Caniçal; MMF48468: 48,469: 05/11/2018, Campanário. Other material–MMF44688 (Noronha 1907?, Funchal, Lido), MMF44775 (Noronha 1907?, Bay of Funchal).
Etymology
This species is dedicated to Adolfo César de Noronha (1873–1963), an enthusiastic naturalist and avid promoter of the Museu de História Natural do Funchal. For years, Noronha collected specimens of bryozoans from Madeira Archipelago that were included in Norman’s collection.
Description
Erect colonies forming dense tufts, between 1 and 2 cm in height, attached to the substrate by rhizoids formed from the basal branches of the colony. Branches, lightly incurving. Internodes formed by 7 and 16 zooids, with 9–11 zooids being the most common. Each internode has from 1 to 3 lateral branches; the first branch commonly forms at level of first zooid of an internode when two or three branches develop from the same internode, but from the third zooid when only one branch derive from one internode. Subsequent branches alternate on the opposite side from the parental internode, without a clear pattern in the sequence of appearance in the zooid position. Internodes articulate with joints ranging from colourless (in young portions of the colony) to light brown (old colony parts). Autozooids tubular, with a peristome around one-third of zooid length. Orifice facing forward, circular; in some cases, presenting a pointed process lateral to the orifice. The frontal surface is densely covered by pseudopores, without differences in their density between gonozooids and autozooids (average 1443.5 pseudopores/mm2). Several tubular rhizoids support the colony over the substrate, forming from the basal part of the first branches, never present in the younger portions of the colony. Gonozooids pyriform, rather prominent in profile, proportionally broad and reaching maximum width at the end of its length, with the distal part flat or slightly concave. Oeciostoma round, smaller than the autozooid’s aperture.
Remarks
The species described here presents morphological characteristics that differ from the known species belonging to the genus Crisia. The morphologically closest related species is Crisia eburnea (Linnaeus, 1758). Nevertheless, C. eburnea presents shorter internodes with fewer zooids (5–7 according to Kluge 1975 and Hayward and Ryland 1985), only one branch per internode, pyriform gonozooid, and a lower pore density on the zooidal wall, Crisia noronhai sp. nov., in turn, features longer internodes and gonozooids that are wider and whose distal part is concave. Crisia eburnea is widely distributed along the eastern Atlantic Ocean from Norway to the Mediterranean Sea. It was recorded by Norman (1909) from Madeira. Nevertheless, specimens collected by Noronha identified by Norman as C. eburnea and stored in the MMF, examined during the present study proved to be conspecific with C. norhonai..
d'Hondt (1988) described from Guipuzcoa (N Spain) C. eburnea subsp. Harmelini. This subspecies has a larger number of zooids per internode (8–18, more usually 13–14), closer to the observed zooid number in C. noronhai sp. nov. Nevertheless, a maximum of two branches are formed from one internode, and the morphology of the gonozooid is significantly different, being more elongated and narrower than in C. noronhai sp. nov.
Crisia noronhai sp. nov. is also similar to the Artic species, Crisia eburneodenticulata Smitt ms in Busk 1875, presenting a similar branching pattern. According to Kluge (1975), this species commonly has 11–13 zooids per internode and up to 20, but whose autozooids are almost entirely adnate, with a very short peristome and gonozooids that are pyriform but distally convex, attaining their maximum width at one-third of their lengths.
Finally, this study possibly confirms the identity of the previously unknown Crisia sp. samples collected since 2013 in all marinas in the Madeira Archipelago, as shown in Ramalhosa et al. (2019). Importantly, early records of this species date back to the 1900s until the present time.
Crisia sp. aff. elongata Milne-Edwards, 1838
?Crisia elongata Milne-Edwards, 1838: 203.
?Crisia elongata: Norman, 1909: 277; Harmer, 1915: 96, Pl. VII, figs. 1-8.
Material examined
MMF48470: 09/08/2013, Calheta; MMF48471: 14/10/2013, Funchal; MMF48472: 13/01/2014, Funchal; MMF45060: 04/08/2014, Calheta; MMF47045: 06/02/2015, Funchal.
Description
Colony erect, forming bushy tufts 1.5–2.5 cm high, comprising jointed biserial branches. Internodes very variable in size, including from 4 to 18 zooids that are generally longer in the distal parts and shorter in the basal part of the colony (and occasionally in medial parts of some colonies as well).. Generally, one branch is presented by internodes and two branches in the internodes with gonozooids. The joints are black. Autozooids are tubular, with a very short peristome and a circular orifice facing forward. Gonozooids pyriform with the broader portion distally, ooeciopore slit-like, without ooeciostomal rim. Autozooidal orifices adjacent to gonozooids are slightly narrower than when no gonozooids are present. The gonozooid frontal wall is densely porous, with an average of 1460 pseudopores/mm2 in autozooids and 3520 pseudopores/mm2 in gonozooids. Colonies attach to the substrate by tubular rhizoids forming from the basal part of the first branches.
Remarks
Unlike our material, Crisia elongata previously recorded in Madeira by Norman (1909) was collected in deeper waters, between 73 and 128 m deep (40–70 fathoms), and indicated as an uncommon species. Norman’s original specimen was not found, so we cannot be certain if it was conspecific with our material. In contrast, colonies from Ramalhosa et al. (2019) recorded as Crisia sp. from marinas of Calheta and Funchal, were partly examined and identified as Crisia sp. aff. elongata.
Crisia elongata was described from the Red Sea (Milne-Edwards 1838) and fossil and recent specimens were later recorded on several occasions. Currently, C. elongata is considered a widespread species in the Indian and Pacific Oceans (Harmer 1915; Lagaaij 1959; Winston 1986; Gordon 1989; Ziko et al. 2012; Chae et al. 2020), but also in the Mediterranean Sea (Rosso and Di Martino 2016). Madeiran specimens show some differences compared to specimens described from the Pacific, which have a fewer zooids per internode and proportionally longer gonozooids. Note, however, that differences also exist between the specimens figured by Gordon (1989) from Samoa, Winston (1986) from Panama, and those by Chae et al. (2020) from Korea; even twin gonozooids are described. These variations call for a re-description of C. elongata type material, and no formal identification is assumed for Madeiran specimens possibly representing a new species.
Class Gymnolaemata Allman, 1856
Order Ctenostomatida Busk, 1852
Family Vesiculariidae Hincks, 1880
Genus Amathia Lamouroux, 1812
Amathia madeirensis sp. nov. Souto, Ramalhosa & Ferrario.
https://zoobank.org/7BE3C5D7-F22F-450C-A664-B6B3A91E877A
Material examined
Holotype– MMF47097, 13/11/2017, Calheta.
Etymology
The name, madeirensis, alludes to the Madeira Archipelago, where the specimen was collected.
Description
Colony arborescent, branching, up to 5 cm high, forming dense light brown coloured tufts. Each branch is formed by cylindrical autozooid-bearing kenozooids separated by transverse septa. Branching is lateral, directly before the septa in all kenozooids. New stolons budded laterally at 45–60° to the stolonal axis. In-line stolons may be lightly deflected after ramification, giving the impression of a dichotomy. Kenozooids bear an extended group of 26–30 autozooids, occupying between half and two-thirds of the length of the kenozooids in their distal part. Autozooid groups are twisted, undergoing a 180–270° torsion around the kenozooid. The spiral direction is constant within a colony, but may differ between colonies, being either “clockwise” or “anticlockwise”.. The last zooid in a group and the first of the succeeding group are almost in the same plane.
Autozooids budded at the growing tips of each branch as small vesicles arranged in two series along the spiral. Autozooids cylindrical, but with subquadrangular apertures at the truncated distal ends. Autozooids closely packed, but not attached to eachother along their distal halves. Polypide with eight tentacles.
Remarks
Specimens from Madeira are morphologically closely related to Amathia pustulosa (Ellis & Solander, 1786). That species also has long zooids groups of up to 30 zooids per group. Nevertheless, the lateral walls of A. pustulosa zooids completely free, not joined to the adjacent zooids in the group (Prenant and Bobin 1956; Souto et al. 2010), whereas in A. madeirensis sp. nov. the autozooids are closely packed with their proximal halves joined to the adjacent zooids. Moreover, A. pustulosa presents many rhizooids that attach the colony to the substrate and generate new erect portions (Souto et al. 2010), which were not observed in the Madeiran specimens. Amathia pustulosa also has series of stolonar kenozooids missing lateral branches, whereas all kenozooids have lateral branches in A. madeirensis sp. nov.
Two other species, Amathia brasilensis Busk, 1886 and Amathia distans Busk, 1886, re-described by Fehlauer-Ale et al. (2011), have a similar colony morphology. Both species feature a very variable number of zooids per group, between 8 and 18 pairs in A. brasilensis, and 9–19 pairs in A. distans. Moreover, in both species the group of zooids form a complete spiral around the stolon and with the zooids mostly unjoined. Finally, both species have shorter and thinner stolons than A. madeirensis sp. nov., and A. distans has yellow spots in both stolons and autozooids.
Order Cheilostomatida Busk, 1852
Genus Scruparia Oken, 1815
Scruparia ambigua (d’Orbigny, 1841)
Eucratea ambigua d’Orbigny, 1841:pl. 3, figs. 13–17.
Scruparia ambigua: Hastings, 1941: 465; Hayward and Ryland, 1998,108, Fig. 19.
Material examined
MMF48474: 30/10/2018, Caniçal, on Cradoscrupocellaria bertholletii.
Description
Colony with encrusting and erect parts formed by uniserial chains of zooids. Encrusting uniserial portions with autoozoids budded from each zooid's distal part, and branches formed by lateral budding. Erect part arises from the frontal wall of the repent autozooids, forming uniserial chains of autozooids with branches always formed by frontal budding. Autozooids are slender and long, with their half distal parts being wider. Frontal oval opesia takes up about half of autozooid length and is located in the distal portion of the zooid. No brood chambers were observed in the Madeira specimens, but have benn described as bivalve brood chambers on dimorphic zooids (Zabala 1986; Hayward and Ryland 1998; Hayward and McKinney 2002; De Blauwe 2009).
Remarks
Scruparia ambigua is a widespread species recorded worldwide except for polar regions (Hayward and Ryland 1998). In general, this species is grows on bryozoans, algae, hydroids, stones and shells in shallow waters, not deeper than 50 m (Hayward and Ryland 1998), but also in artificial substrates (Ryland 1965; Beukhof et al. 2016; McCuller and Carlton 2018; Coolen et al. 2020). However, it was not previously recorded in the Madeira Archipelago. Here we present the first Madeiran record of this species, which was observed on artificial panels and on erect bryozoans and hydroids.
Family Bugulidae Gray, 1848
Genus Bugula Oken, 1815
Bugula ingensVieira et al., 2012
Bugula ingens Vieira et al., 2012: Figs. 7B, 10B, D and F.
Material examined
MMF48475: 05/11/2018, Campanário.
Description
Colony erect, with biserial branches of alternating and slightly rotated zooids simulating a uniserial pattern. Colony branches with 3 to 5 zooids between branches. Zooids are slightly calcified and elongated, with the frontal membrane occupying around one-third of the total zooid length. Distal corners of the zooids are slightly pointed but without spines. Avicularia pedunculate, present in all zooids, attached by a tubular cuspidate peduncle situated close to the proximal margin of the zooid and situated on the external wall of the zooid, in distal position to the previous zooid. Avicularia rounded with a hooked rostrum. Rostrum lateral edge irregular but not denticulate. Ovicell and ancestrula were not observed in the Madeiran specimens.
Remarks
Specimens found in Madeira are morphologically a member of the B. uniserialis-group (Vieira et al. 2012; Fehlauer-Ale et al. 2015). The specimens studied here agree with the description and biometrics of B. ingens without many differences. This species was described for only one locality, Meros, Ilha Grande, Angra dos Reis, Brazil (Vieira et al. 2012) and was not re-recorded after its original description. Bugula ingens is very similar to B. gnoma Vieira et al. 2012 and B. rochae Vieira et al. 2012, but as those authors pointed out, it is quite distinct from these two species by the conspicuously large avicularia.
Here we present the first record of this species on Madeira Island and outside its type locality in Brazil. The scarce of data about this species make difficult to know if this species can be considered as native or alien on the archipelago.
Family Candidae d’Orbigny, 1851
Genus Cradoscrupocellaria Vieira et al., 2013
Cradoscrupocellaria bertholletii (Audouin, 1826)
Acamarchis bertholletii Audouin, 1826: 241; Savigny, 1817: pl. 11, figs. 3.1–3.5.
Scrupocellaria bertholletii: Hincks, 1886: 258, pl. 9, figs. 1–2; Ramalho, 2006: 127, fig. 28; Cradoscrupocellaria bertholletii: Vieira et al. 2013: 7, figs. 2–3.
Material examined
MMF49208, 49,209: Calheta, 15/10/2013; MMF49210, 49,211, 49,212: Porto Santo, 23/10/2013; MMF48473: 30/10/2018, Caniçal; MMF48476: 05/11/2018, Campanário.
Remarks
Cradoscrupocellaria bertholletii was recently redescribed by Vieira et al. (2013), and new specimens collected during this study in Madeira correspond to the previous descriptions given for this species. Many records of this species exist worldwide, from shallow waters in New Zealand, Suez Canal, the Mediterranean and Brazil (Vieira et al. 2013). It was recorded previously in Madeira by Norman (1909). Vieira et al. (2013), in a mistake (Vieira, personal communication), write that Norman’s record could not be assigned to this species. However, its presence in Madeira was included based on a sample identified by Norman and labelled with the unpublished name Scrupocellaria bertholletii var. aperta, identified by Vieira et al. (2013) as C. bertholletii. Nevertheless, the description and figures presented by Norman (1909) agree with the characteristics of this species. In the absence of the original specimens described by Norman (1909), we consider his record as the first record of C. bertholletii for Madeira in the present work. More recently, the presence of this species in marinas around Madeira was recorded by Canning-Clode et al. (2013a) and Ramalhosa et al. (2019) as non-indigenous, but its status has recently been changed to cryptogenic (Castro et al. 2020). In the current study, its species is confirmed on the artificial substrate, and new data and SEM figures are provided.
Cradoscrupocellaria insularis Vieira et al., 2013
Cradoscrupocellaria insularis Vieira et al., 2013: Fig. 13.
Material examined
MMF48477: 30/10/2018, Caniçal.
Remarks
Cradoscrupocellaria insularis was recently described based on historical specimens collected in Cape Verde, besides specimens recorded in Madeira in 1857, identified initially as Scrupocellaria bertholletii and currently stored in the NHMUK (Vieira et al. 2013). New specimens collected on artificial panels during this work agree with the morphological description of C. insularis. This new record confirms the species' current presence in Madeira.
Genus Scrupocaberea Vieira et al., 2014
Scrupocaberea maderensis (Busk, 1860)
Scrupocellaria maderensis Busk, 1860: 280;
Scrupocellaria maderensis, Busk, 1861: 77, pl. 32, fig. 1; Norman 1909: 284. Scrupocaberea maderensis: Vieira et al. 2014: 17, figs. 15A–C; Souto et al. 2015: figs. 18–20.
Material examined
MMF47013: 24/08/2015, Quinta do Lorde; MMF47034: 17/12/2015, Quinta do Lorde; MF47046: 02/04/2016, Quinta do Lorde; MMF48478: 04/10/2018, Quinta do Lorde.
Remarks
Cradoscrupocellaria maderensis was described and figured from Madeira (Busk, 1860, 1861) as Scrupocellaria maderensis and recently transferred to the genus Scrupocaberea (Vieira et al. 2014). This species has been reported to be widespread in tropical and subtropical waters worldwide (Harmer 1926; Tilbrook 2006; Vieira et al. 2014), although the identity of these records should be revised (Vieira et al. 2014). In Madeira, this is the first record of C. maderensis on artificial substrates after the recent finding on rocky substrates at 11 m depth (Souto et al. 2015).
Genus Tricellaria Fleming, 1828
Tricellaria inopinata d'Hondt and Ambrogi, 1985
Tricellaria inopinata d'Hondt and Ambrogi, 1985: 35–46, figs. 2, 3.
Material examined
MMF48479, 48,480: 30/10/2018, Caniçal.
Remarks
Firstly described based on specimens collected in the lagoon of Venice (d'Hondt and Ambrogi 1985), T. inopinata is currently thought to have originated on the Pacific coast of North America (Dyrynda et al. 2000; De Blauwe and Faasse 2001). After its description, this species was recorded as NIS around the globe, from the north of Japan to Taiwan, Australia, New Zealand, and the Mediterranean Sea (Occhipinti Ambrogi and d'Hondt 1994). This species is also present in the North-East Atlantic Ocean, where it has shown a rapid expansion over the past few years (e.g. De Blauwe and Faasse 2001, Bishop et al. 2015, Porter et al. 2015, 2017; Reverter-Gil et al. 2019), even reaching the Azores (Micael et al. 2016). Nominal records of T. inopinata from Madeira were presented by Ramalhosa et al. (2019) as non-indigenous on artificial substrates from all marinas. In this study, the species' identity is confirmed for Madeira, and new data and SEM figures are provided.
Family Lepraliellidae Vigneaux, 1949
Genus Celleporaria Lamouroux, 1821
Celleporaria brunnea (Hincks, 1884)
(Figs. 11, 12, 13, and 14, Table 10)
Cellepora brunnea Hincks, 1884, p. 56; O'Donoghue and O'Donoghue, 1926, p. 21.
Holoporella brunnea: Hastings, 1929, p. 731, p. 16, fig. 7, 108–110; Osburn, 1952, p. 496, pl. 62, figs. 10–12; Soule, 1961, p. 33; Soule and Soule, 1965, p. 38, fig. 13, 14.
Celleporaria brunnea: Winston, 1986, p. 12, figs. 19–22; Soule et al. 1995, p. 267, fig. 101; Koçak, 2007, p. 192, fig. 2 A-D; Seo and Min 2009, p. 29, fig. 7; Canning-Clode et al. 2013b, fig. 2; Lodola et al. 2015, p. 265, figs.3–5; Lezzi et al. 2015, p. 1, fig. 2–3; McCuller and Carlton 2018, p. 153, fig. S18B.
Celleporaria sp. aff. brunnea: Harmelin, 2014, p. 316, fig. 6.
Material examined
MMF48482: 22/06/2018, Quinta do Lorde; MMF48481: 26/02/2019, Quinta do Lorde; MMF48483: 06/06/2019, Porto Santo; MHNUSC-698: Marina de Cascais, Portugal, 13/05/2016; MHNUSC-699: Ocean Revival, Algarve, Portugal, 12/07/2014.
Description
Colony encrusting, irregularly shaped, unilaminar to multilaminar depending on the development stage. Zooids white to grey, but with the operculum and mandible of avicularia dark brown and lophophore tentacles brown. Autozooids are rectangular, arranged in radiating linear series in the first layer, but mostly irregular in position and morphology in areas of frontal budding. Frontal shield granular, more so when the secondary calcification is extensive, with a marginal pore series. Primary orifice slightly wider than long, with the distal margin rounded and the proximal margin slightly bowed with a single rounded central sinus, very variable in size, and often pinched together at the top so that the sinus is almost closed, presenting considerable inter- and intra-colonial variability. Up to four widely spaced, distal oral spines are present in early ontogeny, with bases obscured by calcification, in most cases, only two are visible. The orifice rounded with a slight peristome, more raised proximally, with an umbo that supports an oval suboral-avicularium, perpendicular to the frontal plane and with a toothed distal rim. Vicarious avicularia large, spatulate, with dark brown spade-shaped columella and a parallel-sided rostrum. Ovicell noncleitral, widely open; covered by granular secondary calcification.
Remarks
Celleporaria brunnea was initially described in British Columbia by Hincks (1884), and the type specimen is preserved in the NHMUK (1886.3.6.33). It is a widespread species on the Pacific coast of North America, particularly in California (Soule 1961; Soule and Soule 1965; Soule et al. 1995; Winston 1986). Nevertheless, the native range is unclear, but the data suggest that the original distribution ranges from British Columbia to the Galapagos Islands, Ecuador (O'Donoghue and O'Donoghue 1926; Hastings 1929; Soule 1961; Soule and Soule 1965; Soule et al. 1995; Osman and Haugsness 1981; Keough and Downes 1982; Winston 1986). Recently, this species was recorded throughout the Mediterranean Sea with a swift expansion of its distribution (Koçak 2007; Çinar et al. 2008; Harmelin 2014 [as Celleporaria sp. aff. brunnea], Koçac and Aydin-Önen 2014; Lezzi et al. 2015; Lodola et al. 2015; Ferrario et al. 2016, 2018; Marić et al. 2017). It has also been recorded on the western Pacific coast from Korea (Seo and Min 2009; Lee et al. 2011) and more recently in Oahu, Hawai (McCuller and Carlton 2018). In 2013, C. brunnea was recorded for the first time in the Atlantic Ocean from a marina in Cascais, Portugal (Canning-Clode et al. 2013b), although this species has likely been present in the Atlantic at least since 2008 in the bay of Arcachon (André & Harmelin in DORIS 2021). Today, Celleporaria brunnea probably occurs in other areas of Portugal, particularly south of Lisbon because new records were detected in Arrabida and Algarve, mainly in harbours or on artificial substrates (see material examined section). Nevertheless, Vieira questioned the identity of some of these records (as personal communication in Harmelin 2014), noting certain differences between the material from Lebanon and specimens described from California. Harmelin (2014) described differences in the morphology of the small digitations from the sinus corners, and he considered the most evident differences to be in the sinus size and the size and frequency of interzooidal avicularia. Similar differences, such as in the sizes of the orifice and interzoidal avicularia or in sinus morphology, were also indicated by Lodola et al. (2015) and Lezzi et al. (2015) from Italian's specimens. Neither paperquestioned the identity of the species.
To solve this issue, the type specimen of C. brunnea, currently stored in the NHMUK (Fig. 12), was studied and compared with the Madeiran specimens. This comparison also included, data obtained from the figures in Winston (1986) and Soule et al. (1995) from Panama (Pacific coast) and Santa Barbara (California), respectively. Specimens from Lisbon and Algarve from the Atlantic coast as well as from Genova, Santa Margherita Ligure, La Spezia, Livorno, Olbia, Porto Torres, Porto Rotondo, Viareggio, Livorno and Caste Isardo, from the Mediterranean, were also included in the morphometric comparison. Furthermore, morphometric measurements of the orifice, length (including the sinus) and width, and size of the interzooidal avicularia were compared. A total of 384 measurements of orifices and 218 measurements of interzooidal avicularia were graphically compared (Fig. 13), and no significant differences were found between the colonies.
Here, we conclude that the morphological differences indicated by Harmelin (2014) apparently result from the high variability of certain characters. Thus, Harmelin indicated differences in the horizontal digitations in the sinus corners, which were observed to be very variable, as well as in the sinus size (Fig. 14). Other differences such as the frequency and size of the interzooidal avicularia, seem to be variable characters. The frequency was observed to be very variable from one part of the colony to another, and size seems to be related to the colony's ontogeny, being bigger in colonies with significant frontal development.
Hincks (1884) described only two lateral tall spines; nevertheless, in the type specimen studied, some zooids exhibit four oral spines, with the basal portion (the only preserved part) very variable in thickness (Fig. 12). This characteristic was also observed in specimens from Madeira with 4 or 2 oral spines on zooids of the colony margin to no spines in young zooids in other colony regions.
Considering the studied variability and the impossibility of sorting the specimens in different species based on morphological characters, the specimens from Madeira are considered to belong to C. brunnea. Records from Portugal by Canning-Clode et al. (2013b) also belong to this species. Moreover, the specimens studied, figured and described in the literature from the Mediterranean (Koçak 2007; Çinar et al. 2008; Koçac and Aydin-Önen 2014; Lezzi et al. 2015; Lodola et al. 2015, Ferrario et al. 2016, Marić et al. 2017), including the specimens recorded by Harmelin (2014, as Celleporaria sp. aff. brunnea), seem to belong to this species.
Discussion and conclusion
The ten bryozoan species included in the current work are associated with fouling communities collected in the Madeira Archipelago on artificial substrates. Two of these species, Crisia noronhai sp. nov. and Amathia madeirensis sp. nov., are described as new to science. Although C. noronhai sp. nov. here is described as a new species, its presence in Madeira was previously recorded by Norman (1909) and identified as C. eburnea. In addition, no data were found in the literature that indicates the previous presence of the new species Amathia madeirensis sp. nov. on the island. Finally, Crisia sp. aff. elongata may also be considered a new species, but at this stage, a specific determination is not possible. A re-description of this species is necessary and should be addressed in future studies.
Notably, B. ingens was known only from its original description in Brazil (Vieira et al. 2012). This species adds to the list of macroinvertebrates originating from Brazilian and Caribbean waters and recently detected in Madeira (Wirtz and Canning-Clode 2009; Canning-Clode et al. 2013a, b; Ramalhosa et al. 2014; Souto et al. 2015, 2016). This also includes the bryozoans Beania maxilladentata Ramalho et al., 2008 and Parasmitina alba Ramalho et al., 2011. These two species, previously known from the Brazilian coast (Ramalho et al. 2008, 2011; Vieira et al. 2010), were also recently recorded in Madeira (Souto et al. 2015, 2016).
Celleporaria brunnea is recorded in Madeira for the first time, and new data and SEM figures for T. inopinata are also provided. Both species are well known as NIS along European coasts, where their distribution is spreading very rapidly along the Atlantic and Mediterranean coasts. Morphological comparison of specimens from Madeira and other European localities with the type specimen confirmed the identity of the Atlantic and Mediterranean material as C. brunnea.
Scruparia ambigua is recorded here for the first time in Madeira. Since this species typically grows on artificial substrates and is commonly found in harbours and marinas around the world (Ryland 1965; Beukhof et al. 2016; McCuller and Carlton 2018; Coolen et al. 2020), its presence in Madeira suggests a recent introduction via shipping. As this species forms small, repent, and erect uniserial colonies, in most cases growing on other organisms such as other bryozoans or hydroids, colonies could also have gone unnoticed in previous works.
Furthermore, C. bertholleti, C. insularis, and S. maderensis, previously recorded in Madeira Island from natural substrates (Norman 1909; Vieira et al. 2013; and Busk 1860; 1861), are now also found growing on artificial substrates (Canning-Clode et al. 2013a, b; Souto et al. 2015; Ramalhosa et al. 2019; this study).
With regard to the distribution of the species between the localities studied in the current work (Table 11), only one species, Crisia noronhai sp. nov., was recorded in both marinas and aquaculture facilities (except for the Porto Santo sampling site). Five of the species studied were recorded in the aquaculture facilities of Caniçal, three of them, S. ambigua, C. insularis and T. inopinata, were recorded only from here, and C. bertholletii was shared in Campanário, Calheta, and Porto Santo. Bugula ingens was recorded only from the offshore aquaculture farm of Campanário, and S. maderensis was recorded only from the recreational marina of Quinta do Lorde. Crisia cf. elongata, Amathia madeirensis sp. nov., and Celleporaria brunnea were collected only in recreational marinas.
Bryozoans are an important component in fouling communities, and this study highlights the importance of monitoring programmes in ports for the early detection of NIS. Furthermore, the use of PVC panels has proven to be quite effective in collecting bryozoans, and further studies should be promoted to disentangle the identity and distribution of bryozoans, which would help broaden our knowledge on bryozoan richness in this hotspot area.
References
Allman GJ (1856) A monograph of the fresh-water polyzoa: including all the known species, both British and foreign. The Ray Society, London
Alves FMA, Cocito S (2002) A new bryozoan record (Bugula calathus minor) for the marine fauna of Madeira Island (NE Atlantic). Bocagiana 204:1–5
André F, Harmelin JG (2021) Celleporaria sp. aff. brunnea (Hincks, 1884). In: DORIS, 25/01/2021. https://doris.ffessm.fr/ref/specie/3880. Accessed 11 Mar 2021
Audouin JV (1826) Explication sommaire des planches de polypes de l’Egypte et de la Syrie, publiées par Jules-César Savigny. In: Audouin JV (ed.) Description de l'Egypte, ou recueil des observations et des recherches qui ont était faites en Egypte pendant l'expédition de l'armée française. Histoire Naturelle Tome 1, 4ème partie. Imprimerie Impériale, Paris
Berning B (2012) Taxonomic notes on some Cheilostomata (Bryozoa) from Madeira. Zootaxa 3236:36–54
Berning B, Kuklinski P (2008) North-east Atlantic and Mediterranean species of the genus Buffonellaria (Bryozoa, Cheilostomata): implications for biodiversity and biogeography. Zool J Linn Soc 152:537–566. https://doi.org/10.1111/j.1096-3642.2007.00379.x
Berning B, Tilbrook KJ, Rosso A (2008) Revision of the north-eastern Atlantic and Mediterranean species of the genera Herentia and Therenia (Bryozoa: Cheilostomata). J Nat Hist 42:1509–1547. https://doi.org/10.1080/00222930802109140
Beukhof ED, Coolen JWP, van der Weide BE, Cuperus J, de Blauwe H (2016) Records of five bryozoan species from offshore gas platforms rare for the Dutch North Sea. Mar Biodivers Rec 9(91):1–9. https://doi.org/10.1186/s41200-016-0086-6
Bishop JDD, Wood CA, Yunnie ALE, Griffiths CA (2015) Unheralded arrivals. Non-native sessile invertebrates in marinas on the English coast. Aquat Inv 10(3):249–264. https://doi.org/10.3391/ai.2015.10.3.01
Borg F (1941) On the structure and relationships of Crisina (Bryozoa, Stenolaemata). Arkiv För Zoologi 33A:1–44
Busk G (1852) An account of the Polyzoa, and sertularian zoophytes, collected in the Voyage of the Rattlesnake, on the coasts of Australia and the Louisiade Archipelago. In: MacGillivray J. (Ed.), Narrative of the Voyage of the H.M.S. Rattlesnake. Vol. 1. T. & W. Boone, London, pp. 343–402.
Busk G (1858) Zoophytology. On some Madeiran Polyzoa. Quart J Micros Sci 6:124–130
Busk G (1858) Zoophytology. On some Madeiran Polyzoa. Quart J Micros Sci 6:261–263
Busk G (1859) Zoophytology. On some Madeiran Polyzoa. Quart J Micros Sci 7:65–67
Busk G (1860) Zoophytology. Catalogue of the Polyzoa collected by J.Y. Johnson, Esq., at Madeira, in the years 1859 and 1860, with descriptions of new species. Quart J Micros Sci 8:213-214-280–285
Busk G (1861) Description of new Polyzoa collected by J.Y.Johnston Esq., at Madeira. Quart J Micros Sci New Ser 1:77–80
Busk G (1875) Catalogue of marine Polyzoa in the collection of the British Museum, III, Cyclostomata. Trustees of the Brithis Museum (natural History), London, 39 pp.
Busk G (1886) Report on the Polyzoa collected by H.M.S. Challenger during the years 1873–1876. Part 2. The Cyclostomata, Ctenostomata and Pedicellinea. Report on the Scientific Results of the Voyage of the H.M.S. “Challenger”, Zoology 17:1–47
Campbell ML, King S, Heppenstall LD, van Gool E, Martin R, Hewitt CL (2017) Aquaculture and urban marine structures facilitate native and non-indigenous species transfer through generation and accumulation of marine debris. Mar Pollut Bull 123(1–2):304–312
Canning-Clode J, Fofonoff P, Riedel GF, Torchin M, Ruiz GM (2011) The effects of copper pollution on fouling assemblage diversity: a tropical–temperate comparison. PLoS ONE 6:e18026. https://doi.org/10.1371/journal.pone.0018026
Canning-Clode J, Fofonoff P, McCann L, Carlton JT, Ruiz G (2013) Marine invasions on a subtropical island: fouling studies and new records in a recent marina on Madeira Island (Eastern Atlantic Ocean). Aquat Invasions 8:261–270. https://doi.org/10.3391/ai.2013.8.3.02
Canning-Clode J, Souto J, McCann L (2013) First record of Celleporaria brunnea (bryozoan: Lepraliellidae) in Portugal and in the East Atlantic. Mar Biodivers Rec 6:1–5
Carlton JT, Ruiz GM (2016) Anthropogenic vectors of marine and estuarine invasions: an overview framework, In: Canning-Clode (ed.). Biological invasions in changing ecosystems vectors, ecological impacts, management and predictions. Walter de Gruyter GmbH & Co KG. 24–36.
Castro N, Ramalhosa P, Jiménez J, Costa JL, Gestoso I, Canning-Clode J (2020) Exploring marine invasions connectivity in a NE Atlantic Island through the lens of historical maritime traffic patterns. Reg Stud Mar Sci 37:101333
Chae HS, Min BS, Zágorsek K, Yang HJ, Kil HJ, Seo JE (2020) Crisiidae (bryozoan: Cyclostomata) of Korea. J Sp Res 9(3):280–287
Çinar ME, Katağan T, Koçak F, Öztürk B, Ergen Z, Kocatas A, Önen M, Kirkim F, Bakir K, Kurt G, Dağli E, Açik S, Doğan A, Özcan T (2008) Faunal assemblages of the mussel Mytilus galloprovincialis in and around Alsancak Harbour (Izmir Bay, eastern Mediterranean) with special emphasis on alien species. J Mar Syst 71:1–17
Clarke Murray C, Gartner H, Gregr EJ, Chan K, Pakhomov E, Therriault TW (2014) Spatial distribution of marine invasive species: environmental, demographic and vector drivers. Divers Distri 20:824–836
Coolen JWP, van der Weide BE, Cuperus J, Blomberg M, Van Moorsel GWNM, Faasse MA, Bos OG, Degraer S, Lindeboom HJ (2020) Benthic biodiversity on old platforms, young wind farms, and rocky reefs. Ices J Mar Sci 77(3):1250–1265
d’Hondt JL (1985) Brachiopodes et Bryozoaires (Musorstom II). Mém Mus Natl Hist Nat 133:519–525
d’Hondt JL (1988) Bryozoaires marins de Guipúzcoa. Cah Biol Mar 29:513–529
d’Hondt JL, Ambrogi O (1985) Tricellaria inopinata, n.sp., un nouveau Bryozoaire Cheilostome de la faune Méditerrannée. Pubbl Della Stn Zool Napoli (Marine Ecology) 6:35–46
d’Orbigny (1841) Voyage dans l’Amérique méridionale, 5, Pt.4 Zoophytes
d’Orbigny A (1851) Paléontologie française, Terrains Crétacés, V, Bryozoaires. Victor Masson, Paris
De Blauwe H (2009) Mosdiertjes van de Zuidelijke bocht van de Noordzee: Determinatiewerk voor België en Nederland. Vlaams Instituut voor de Zee (VLIZ), Oostende, Belgium
De Blauwe H, Faasse MA (2001) Extension of the ranges of the bryozoans Tricellaria inopinata and Bugula simplex in the North-East Atlantic Ocean (Bryozoa: Cheilostomatida). Nederlandse Faunistische Mededelingen 14:103–112
De Mesel I, Kerckhof F, Norro A, Rumes B, Degraer S (2015) Succession and seasonal dynamics of the epifauna community on offshore wind farm foundations and their role as stepping stones for non-indigenous species. Hydrobiologia 756(1):37–50
Dyrynda PEJ, Fairall VR, Occhipinti Ambrogi A, d’Hondt JL (2000) The distribution, origins and taxonomy of Tricellaria inopinata d’Hondt and Occhipinti Ambrogi, 1985, an invasive bryozoan new to the Atlantic. J Nat Hist 34:1193–2006
Ehrenberg HG (1831) Symbolae physicae, seu icones et descriptiones corporum naturalium novorum aut minus cognitorum, quae ex itineribus per Libyam, Aegyptum, Nubiam, Dongalam, Syriam, Arabiam et Habessiniam à P. C. Hemprich et C. G. Ehrenberg à studio annis 1820–25 redierunt. Pars Zoologica 2, Animalia evertebrata exclusis Insectis. Vol. 4. Officina Academica. Berolini.
Ellis J, Solander DC (1786) The natural history of many curious and uncommon zoophytes, collected from various parts of the globe. White & Elmsly, London
Fehlauer-Ale KH, Vieira LM, Winston JE (2011) Molecular and morphological characterization of Amathia distans Busk and Amathia brasiliensis Busk (Bryozoa: Ctenostomata) from the tropical and subtropical Western Atlantic. Zootaxa 2962:49–62
Fehlauer-Ale KH, Winston JE, Tilbrook KJ, Nascimento KB, Vieira LM (2015) Identifying monophyletic groups within Bugula sensu lato (Bryozoa, Buguloidea). Zool Scripta 44(3):334–347
Ferrario J, Ulman A, Marchini A, Saracino F, Occipinti Ambrogi A (2016) Non-indigenous fouling species in the marina of Rome. Biol Mar Mediterr 23(1):224–225
Ferrario J, Rosso A, Marchini A, Occhipinti-Ambrogi A (2018) Mediterranean non-indigenous bryozoans: an update and knowledge gaps. Biodivers Conserv 27(11):2783–2794
Fleming J (1828) A history of British animals, exhibiting their descriptive characters and systematic arrangement of the genera and species of quadrupeds, birds, reptiles, fishes, Mollusca, and Radiata of the United Kingdom. Bell & Bradfute, Edinburgh
Gordon DP (1989) The marine fauna of New Zealand: Bryozoa: Gymnolaemata (Cheilostomida Ascophorina) from the western south Island continental shelf and slope. NZ Oceanogr Inst Mem 97:1–158
Gray JE (1848) List of the specimens of British Animals in the Collection of the British Museum. Part 1. Centroniae or radiated Animals. Trustees of the British Museum (Natural History), London
Harmelin JG (2014) Alien bryozoans in the eastern Mediterranean Seanew records from the coast of Lebanon. Zootaxa 3893:301–308
Harmer SF (1915) The Polyzoa of the Siboga Expedition. Part 1. Entoprocta, Ctenostomata and Cyclostomata. Siboga Expedition Rep 28A:1–180
Harmer SF (1926) The Polyzoa of the Siboga Expedition, 2. Cheilostomata Anasca. Siboga Expedition Rep 28b:183–501
Hastings AB (1929) Cheilostomatous Polyzoa from the vicinity of the Panama Canal collected by Dr C. Grassland on the Cruise of the S.Y. ‘St George.’ Proc Zool Soc 99:697–740
Hastings AB (1941) The British species of Scruparia (Polyzoa). Ann Mag Nat Hist 7(11):465–472
Hayward PJ, Ryland JS (1985) Cyclostome Bryozoans. Synopses of the British Fauna 34. E.J.Brill for the Linnaean Society, London
Hayward PJ, McKinney FK (2002) Northern Adriatic Bryozoa from the vicinity of Rovinj, Croatia. Bull Am Mus Nat Hist 270:1–139. https://doi.org/10.1206/0003-0090(2002)270%3c0001:nabftv%3e2.0.co;2
Hayward PJ, Ryland JS (1998) Cheilostomatous Bryozoa. Part 1. Aeteoidea – Cribrilinoidea. Synopses of the Br Fauna ns (2nd Edition) 10:1–366
Hincks T (1880) Contributions towards a general history of the marine Polyzoa. Part I. Madeiran Polyzoa. Ann Mag Nat Hist New Series 5 6:69–80
Hincks T (1884) Report on the Polyzoa of the Queen Charlotte Islands, 3. Ann Mag Nat Hist 13:49–58
Hincks T (1886) The polyzoa of the adriatic, I: a supplement to Prof. Heller’s ‘Die Bryozoen des adriatischen Meeres,’ 1867. Ann Mag Nat Hist 17:254–271
Johnson JY (1897) New cyclostomatous Bryozoa found at Madeira. Ann Mag Nat Hist Ser 6 20:60–65
Johnston G (1838) A History of British Zoophytes. W.H. Lizars, Edinburgh, London & Dublin
Kaluza P, Ko¨lzsch A, Gastner MT, Blasius B (2010) The complex network of global cargo ship movements. J Roy Soci Interface 7:1093–1103
Keough M, Downes B (1982) Recruitment of marine invertebrates: the role of active larval choices and early mortality. Oecologia 54:348–352
Kluge GA (1975) Bryozoa of the Northern Seas of the U.S.S.R. Smithosonian Institution and The National Science Foundation, Washington D.C
Koçak F (2007) A new alien bryozoan Celleporaria brunnea (Hincks, 1884) in the Aegean Sea (eastern Mediterranean). Sci Mar 71:191–195. https://doi.org/10.3989/scimar.2007.71n1191
Koçac F, Aydin-Önen S (2014) Epiphytic bryozoan community of Posidonia oceanica (L.) Delile leaves in two different meadows at disturbed and control locations. Mediterranean Ma Sci 15(2):390–397
Lagaaij R (1959) Some species of Bryozoa new to the Bowden Beds, Jamaica B.W.I. Micropaleontology 5(4):482–486
Lamouroux JVF (1812) Extrait d’un mémoire sur la classification des Polypiers coralligènes non entièrement pierreux. Nouv Bull Sci Soc Philos 3:181–188
Lamouroux JVF (1821) Exposition méthodique des genres de l’ordre des polypiers, avec leur description et celles des principales espèces figurées dans 84 planches; les 63 premiers appartenant a l’histoire naturelle des zoophytes d’Ellis et Solander. V.Agasse, Paris
Lee HJ, Kwan YS, Kong SR, Min BS, Seo JE, Won YJ (2011) DNA Barcode Examination of Bryozoa (class: Gymnolaemata) in Korean Seawater. Korean J Syst Zool 27(2):159–163
Lezzi M, Pierri C, Cardone F (2015) Presence of Celleporaria brunnea (Bryozoa: Lepraliellidae) in the Central Mediterranean: first occurrence in the Gulf of Taranto. Mar Biodivers Rec 8:1–8
Lodola A, Ferrario J, Occhipinti-Ambrogi A (2015) Further Mediterranean expansion of the non-indigenous bryozoan Celleporaria brunnea: multiple records along the Italian coasts. Sci Mar 79:263–274
Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10(3):689–710
Marić M, Ferrario J, Marchini A, Occhipinti-Ambrogi A, Minchin D (2017) Rapid assessment of marine non-indigenous species on mooring lines of leisure craft: new records in Croatia (eastern Adriatic Sea). Mar Biodivers 47:949–956
McCuller MI, Carlton JT (2018) Transoceanic rafting of Bryozoa (Cyclostomata, Cheilostomata, and Ctenostomata) across the North Pacific Ocean on Japanese tsunami marine debris. Aquat Invasions 13(1):137–162. https://doi.org/10.3391/ai.2018.13.1.11
Micael J, Jardim N, Núñez C, Occhipinti-Ambrogi A, Costa AC (2016) Some Bryozoa species recently introduced into the Azores: reproductive strategies as a proxy for further spread. Helgol Mar Res 70:7
Milne-Edwards H (1838) Mémoire sur les Crisies, les Hornéres et plusieurs autres Polypes vivants ou fossiles dont l’organisation est analogue à celle des Tubulipores. Ann Sci Nat Zool Biol 2(9):193–238
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6(9):485–492
Norman A (1909) The Polyzoa of Madeira and neighbouring islands. J Linn Soc Zool 30:275–314
Nunes AL, Katsanevakis S, Zenetos A, Cardoso AC (2014) Gateways to alien invasions in the European seas. Aquat Invasions 9(2):133–144
Occhipinti Ambrogi A, d'Hondt JL (1994) The invasion ecology of Tricellaria inopinata into the lagoon of Venice: morphological notes on larva and ancestrula. In: Hayward PJ, Ryland JS, Taylor PD (eds) Biology and Palaeobiology of Bryozoans. Olsen & Olsen, Fredensborg, pp 139–144
O’Donoghue CH, O’Donoghue E (1926) A second list of Bryozoa from the Vancouver Island region. Contrib Can Biol Fish New Ser 3:47–131
Oken L (1815) Lehrbuch der naturgeschichte, III, Zoologie Vol. Abteilung 1. Fleischlose Thiere, Leipzig
Osburn RC (1952) Bryozoa of the Pacific coast of America, part 2, Cheilostomata–Ascophora. Report of the Allan Hancock Pacific Expeditions 14:271–611
Osman RW, Haugsness JA (1981) Mutualism among sessile invertebrates: a mediator of competition and predation. Science 211:846–848
Png-Gonzalez L, Ramalhosa P, Gestoso I, Álvarez S, Nogueira N (2021) Non-indigenous species on artificial coastal environments: experimental comparison between aquaculture farms and recreational marinas. J Mar Sci Eng 9(10):1121
Porter JS, Spencer Jones M, Kukliński P, Rouse S (2015) First records of marine invasive non-native Bryozoa in Norwegian coastal waters from Bergen to Trondheim. Bioinv Rec 4(3):157–169. https://doi.org/10.3391/bir.2015.4.3.02
Porter JS, Nunn JD, Ryland JS, Minchin D, Spencer Jones M (2017) The status of non-native bryozoans on the north coast of Ireland. Bioinv Rec 6(4):321–320. https://doi.org/10.3391/bir.2017.6.4.04
Prenant M, Bobin G (1956) Bryozoaires, premiere partie, Entoproctes, Phylactolèmes, Ctenostomes. Fédération Française des sociétés de sciences naturelles, Paris
Ramalho LV (2006) Taxonomia, distribuição e introdução de espécies de briozoários marinhos (Ordens Cheilostomatida e Cyclostomata) do Estado do Rio de Janeiro. Thesis, UFRJ, Museu Nacional, Rio de Janeiro
Ramalho LV, Muricy G, Taylor PD (2008) Taxonomy of Beania Johnston, 184 0 (Bryozoa, Flustrina) from Arraial do Cabo, Río de Janeiro State, Brazil. Arq Museu Nac 66:499–508
Ramalho LV, Muricy G, Taylor PD (2011) Taxonomic revision of some lepraliomorph cheilostome bryozoans (Bryozoa: Lepraliomorpha) from Rio de Janeiro State, Brazil. J Nat Hist 45:767–798
Ramalhosa P, Canning-Clode J (2015) The invasive caprellid Caprella scaura Templeton, 1836 (Crustacea: Amphipoda: Caprellidae) arrives on Madeira Island, Portugal. BioInvasions Rec 4:97–102
Ramalhosa P, Camacho-Cruz K, Bastida-Zavala R, Canning-Clode J (2014) First record of Branchiomma bairdi McIntosh, 1885 (Annelida: Sabellidae) from Madeira Island, Portugal (northeastern Atlantic Ocean). BioInvasions Rec 3:235–239. https://doi.org/10.3391/bir.2014.3.4.04
Ramalhosa P, Souto J, Canning-Clode J (2017) Diversity of Bugulidae (Bryozoa, Cheilostomata) colonizing artificial substrates in the Madeira Archipelago (NE Atlantic Ocean). Helgol Mar Res 71:1–20
Ramalhosa P, Nebra A, Gestoso I, Canning-Clode J (2017) First record of the nonindigenous isopods Paracerceis sculpta (Holmes, 1904) and Sphaeroma walkeri Stebbing, 1905 (Isopoda, Sphaeromatidae) for Madeira Island. Crustaceana 90:1747–1764
Ramalhosa P, Gestoso I, Duarte B, Caçador I, Canning-Clode J (2019) Metal pollution affects both native and non-indigenous biofouling recruitment in a subtropical island system. Mar Pollut Bull 141:373–386
Reverter-Gil O, Souto J, Trigo JE (2019) New species and new records of bryozoans from Galicia (NW Spain). J Nat Hist 53(3–4):221–251
Rius M, Heasman KG, McQuaid CD (2011) Long-term coexistence of non-indigenous species in aquaculture facilities. Mar Pollut Bull 62(11):2395–2403
Rosso A, Di Martino E (2016) Bryozoan diversity in the Mediterranean Sea: an update. Mediterr Mar Sci 17(2):567–607. https://doi.org/10.12681/mms.1706
Ryland JS (1965) Catalogue of the main marine fouling organisms (found on ships coming into European waters), vol 2: Polyzoa. Organization for Economic Co-operation and Development, Paris
Savigny JC (1817) Description de l’Égypte, ou receuil des observations et des recherches qui ont été faites en Égypte pendant l’Expedition de ‘Armée française, Histoire naturelle, vol 2. Imprimerie Royale, Paris
Seo JE, Min BS (2009) A faunistic study on cheilostomatous bryozoans from the shoreline of South Korea, with two new species. Korean J Syst Zool 25:19–40
Soule JD (1961) Results of the Puritan-American Museum of Natural History expedition to western Mexico. 13. Ascophoran Cheilostomata (Bryozoa) of the Gulf of California. Am Mus Novit 2053:1–66
Soule DF, Soule JD (1965) The Ectoprocta (bryozoa) of Scammon’s Lagoon, Baja California, Mexico. Am Mus Nat Hist Novit 2199:1–56
Soule DF, Soule JD, Chaney HW (1995) Taxonomic Atlas of the benthic fauna of the Santa Maria Basin and western Santa Barbara Channel. The Bryozoa. Irene McCulloch Foundation Monograph Series, Number 2. Hancock Insitute of Marine Studies, University of Southern California, Los Angeles.
Souto J, Fernández-Pulpeiro E, Reverter-Gil O (2010) The genus Amathia Lamouroux (Bryozoa: Ctenostomata) in Iberian waters. Cah Biol Mar 51:181–195
Souto J, Reverter-Gil O, Ostrovsky AN (2014) New species of Bryozoa from Madeira associated with rhodoliths. Zootaxa 3795(2):135–151
Souto J, Kaufmann M, Canning-Clode J (2015) New species and new records of bryozoans from shallow waters of Madeira Island. Zootaxa 3925:581–593
Souto J, Ramalhosa P, Canning-Clode J (2018) Three non-indigenous species from Madeira harbors, including a new species of Parasmittina (Bryozoa). Mar Biodivers 48(2):977–986. https://doi.org/10.1007/s12526-016-0592-0
Tilbrook KJ (2006) Cheilostomatous Bryozoa from the Salomon Islands. Santa Barbara Museum of Natural History Monographs, 4 (Studies in Biodiversity Number 3)
Vieira LM, Migotto AE, Winston JE (2010) Shallow-water species of Beania Johnston, 1840 (Bryozoa, Cheilostomata) from the tropical and subtropical Western Atlantic. Zootaxa 2550:1–20
Vieira LM, Winston JE, Fehlauer-Ale KH (2012) Nine New Species of Bugula Oken (Bryozoa: Cheilostomata) in Brazilian Shallow Waters. PLoS ONE 7(7):e40492. https://doi.org/10.1371/journal.pone.0040492
Vieira LM, Spencer Jones M, Winston JE (2013) Cradoscrupocellaria, a new bryozoan genus for Scrupocellaria bertholletii (Audouin) and related species (Cheilostomata, Candidae): taxonomy, biodiversity and distribution. Zootaxa 3707(1):1–63
Vieira LM, Spencer Jones ME, Winston JE, Migotto AE, Marques AC (2014) Evidence for Polyphyly of the Genus Scrupocellaria (Bryozoa: Candidae) Based on a Phylogenetic Analysis of Morphological Characters. PLOS ONE 9(4):e95296
Vigneaux M (1949) Révision des Bryozoaires néogènes du Bassin d’Aquitaine et essai de classification. Mem Soc Geolog France 28:1–153
Waters AW (1879) On the Bryozoa (Polyzoa) of the Bay of Naples. Ann Mag Nat Hist 5(3):114–126
Waters AW (1899) Bryozoa from Madeira. J R Microsc Soc 1899:6–16. https://doi.org/10.1111/j.1365-2818.1899.tb00139.x
Winston JE (1986) An annotated check-list of coral-associated bryozoans. Am Mus Novit 2859:1–39
Wirtz P, Canning-Clode J (2009) The invasive bryozoan Zoobotryon verticillatum has arrived at Madeira Island. Aquat Invasions 4:669–670
Zabala M (1986) Fauna dels Briozous dels Països Catalans. Institut d’Estudis Catalans. Arxius De La Secció De Ciències 84:1–836
Ziko A, El Safori YA, SorogyA El, Abd El-Wahab M, El Dera N, Sheata W (2012) Bryozoa from northern Red Sea, Egypt: 1 Crisia (Cyclostomata). Hist Biol 24(2):113–119
Acknowledgements
We thank Leandro Vieira for the comments, Mary Spencer Jones form the NHM of London for the photography of the type specimen of C. brunnea, and João Silva from the Natural History Museum of Funchal (MMF) Madeira for processing all voucher specimens. We thank Porto Santo Line for their travel aid to the Island of Porto Santo and the administration of the four marinas used for this study (Porto Recreio da Calheta, Marina do Funchal, Marina da Quinta do Lorde (Caniçal), and Marina do Porto Santo) for allowing us to survey NIS diversity along the south coast of Madeira. Special thanks are due to Aquailha Aquacultura Lda., in particular, to Elvio Pontes and Nuno Malho, for their contribution to the operations and logistics of access to the aquaculture facilities (Campanário and Caniçal). We are grateful to Gonçalo Calado, João Encarnação and Subnauta diving center for the possibility to study the specimens collected during the colonization experiments carried out in the Ocean Revival underwater park (Algarve).
Funding
Open access funding provided by Austrian Science Fund (FWF). JS was supported by the Austrian Science Fund (FWF, projects numbers P33733-B and AP28594-B29). PR is funded by the project (UIDB/04292/2020) granted to MARE UI&I and partially funded by the Project Observatório Oceânico da Madeira-OOM (M1420-01–0145-FEDER-000001), co-financed by the Madeira Regional Operational Programme (Madeira 14–20), under the Portugal 2020 strategy, through the European Regional Development Fund (ERDF). SA is funded by Regional Agency for the Development of Research, Technology and Innovation (ARDITI) in the framework of MIMAR + project (MAC2/4.6d/249) and OCEANLIT project (MAC2/4.6d/302) funded by INTERREG MAC 2014–2020 programme. IGG is supported financially by a Maria Zambrano contract UCA under the grants call for the requalification of the Spanish university system 2021–2023, funded by the European Union–NextGenerationEU. JCC was funded by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P., under the Scientific Employment Stimulus Institutional Call (CEECINST/00098/2018). Finally, part of this study had the support of FCT through the strategic project UIDB/04292/2020 awarded to MARE and through project LA/P/0069/2020 granted to the Associate Laboratory ARNET. This is contribution 110 from the Smithsonian's MarineGEO and Tennenbaum Marine Observatories Network.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements.
Data availability
All data generated or analyzed during this study are included in this published article.
Author contribution
JS prepared the specimens for the taxonomical work, identified the samples, and wrote the first draft of the manuscript. PR and JCC designed and conducted the field experiments and contributed to the first draft. PR, JF, LPG, SA, IG, and NN contributed to fieldwork. All authors read and approved the manuscript.
Additional information
Communicated by A. Wehrmann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under https://zoobank.org/E9381D58-68F6-499D-918F-8A432F65E762
This article is a contribution to the Topical Collection Seamounts and oceanic archipelagos and their role for the biodiversity, biogeography, and dispersal of marine organisms
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Souto, J., Ramalhosa, P., Ferrario, J. et al. New species and new records of bryozoan species from fouling communities in the Madeira Archipelago (NE Atlantic). Mar. Biodivers. 53, 49 (2023). https://doi.org/10.1007/s12526-023-01355-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-023-01355-y