Abstract
The deep-sea holothurian Chiridota heheva Pawson & Vance, 2004 was recently recognised as a cosmopolitan species which exploits the organic enrichment at three types of deep-sea reducing environments: hydrothermal vents, cold seeps, and organic falls. Here, we apply phylogenetic reconstruction and species delimitation approaches using new COI and 12S sequence data to show that C. heheva is genetically congruent with the only other hydrothermal vent holothurian, Chiridota hydrothermica Smirnov & Gebruk, 2000, with strong supporting morphological, ecological, and biogeographical parallels between the two. As such, we propose that C. heheva is a junior synonym of C. hydrothermica, and that subsequent chiridotid holothurians discovered at deep-sea reducing environments likely also belong to this single globally distributed species. As a species endemic to deep-sea reducing environments yet known at sites across the Atlantic, Indian, and Pacific Oceans, this unique holothurian provides an interesting case study for connectivity, biogeography, and speciation in the deep sea, which has important implications for deep-sea conservation planning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Holothurians are distinctive members of deep-sea benthic communities at abyssal plains (Gubili et al. 2017), seamounts (Lundsten et al. 2009), canyons (Fernández-Rodríguez et al. 2019), and hadal trenches (Liu et al. 2021). They are also among the most visible megafaunal members at the periphery of chemosynthetic habitats, with two species from the genus Chiridota Eschscholtz, 1829 recorded at deep-sea reducing environments (Smirnov et al. 2000; Pawson and Vance 2004; Thomas et al. 2020).
Chiridota hydrothermica Smirnov & Gebruk, 2000 is known only from hydrothermal vents, with records in the Southwest Pacific basins (type locality, Manus Basin) and along the Southeast Pacific Rise (Smirnov et al. 2000). In contrast, Chiridota heheva Pawson & Vance, 2004 has been reported at three types of deep-sea reducing environments: hydrothermal vents, cold seeps, and organic falls. Chiridota heheva was originally described from cold seeps and wood falls in the Northwest Atlantic, Gulf of Mexico, and Caribbean Sea (Pawson and Vance 2004), and was later discovered at cold seeps in the East Atlantic and South China Sea, as well as one hydrothermal vent location along the Southwest Indian Ridge (Thomas et al. 2020). In addition to their widespread geographical distributions, both C. hydrothermica and C. heheva are described as endemic to their respective reducing environments and share broad similarities in ecology and morphology, differing only in tentacle arrangement and digit structure (Pawson and Vance 2004). Conventionally, the new material from the Southwest Indian Ridge hydrothermal vents would have been identified as C. hydrothermica based on habitat differentiation (Copley et al. 2016); however, as the specimens could not be separated from C. heheva by either morphological or molecular evidence, Thomas et al. (2020) concluded that it must be C. heheva.
The strong morphological, ecological, and biogeographical similarities, as well as the discovery of C. heheva at hydrothermal vents, prompted Thomas et al. (2020) to propose that the two species are conspecific. It remains unclear whether they can be separated using molecular barcode sequences as there were no comparative sequence data for C. hydrothermica at the time. Recently, another study has sequenced Chiridota material from a vent habitat, at the type locality of C. hydrothermica, but species-level identification was not determined (Sun et al. 2021). Specimens from the type locality are crucial to resolving the systematics of these reducing environment chiridotids; therefore, new sequence data from Sun et al. (2021) of a Chiridota sp. from the location of the first discovered population of C. hydrothermica could be taken as definitive to answering this conundrum.
Here, we present new evidence for a single widespread Chiridota species across all deep-sea reducing environments, using novel sequence data from the type locality of C. hydrothermica (Sun et al. 2021). Based on genetic species delimitation, we propose that C. heheva is a junior synonym of C. hydrothermica.
Material and methods
Phylogenetic analysis
Cytochrome C oxidase I (COI) and 12S rRNA sequences were extracted from Sun et al.’s (2021) published mitogenomes of Chiridota heheva from the South China Sea (GenBank accession no. MW357261) and Chiridota sp. from the Manus Basin (MW357262). These sequences were trimmed and aligned with the 15 holothurian taxa included in the original phylogeny produced for C. heheva (Table 1; Thomas et al. 2020), using the default multiple alignment settings in Geneious (Version 11.0.5), and combined in Mesquite (Version 3.31). Monte Carlo Markov Chain (MCMC) Bayesian phylogenetic analyses were conducted in MrBayes (Version 3.2.6) as described in Thomas et al. (2020), using GTR+G and HKY+G combined evolutionary models for COI and 12S, respectively. Phylogenetic trees were edited using FigTree (Version 1.4.3) and InkScape (Version 1.0.1).
Species delimitation
An initial pairwise distance analysis was conducted for the COI gene, as described in Thomas et al. (2020), to determine the patterns of genetic variability within Chiridota from deep-sea reducing environments. The medium and maximum values were compared to the pairwise distances of other holothurian species to gauge the intra- and interspecific genetic variation (Thomas et al. 2020). This delineation was then verified using the Automatic Barcode Gap Discovery (ABGD) and Assemble Species by Automatic Partitioning (ASAP) methods (Puillandre et al. 2012; Puillandre et al. 2021), and the Bayesian Poisson Tree Processes model (bPTP) (Zhang et al. 2013), following default parameter settings. The trimmed COI Fasta alignment (532 bp) was used as input for ABGD and ASAP. The COI phylogenetic tree output from MrBayes was converted to Newick format in FigTree (Version 1.4.3) for input to bPTP.
Results
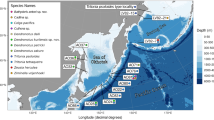
Phylogenetic reconstruction based on COI and 12S mitochondrial gene fragments recovered a single clade encompassing all Chiridota from deep-sea reducing environments, including the Chiridota sp. specimen from the type locality of C. hydrothermica (Bayesian inference posterior probability > 95; Fig. 1). The COI sequences of these chiridotid specimens have a median pairwise distance of 0.024 and a maximum distance of 0.043, separating a specimen from the Worm Hole cold seep in the East Atlantic and the Manus Basin hydrothermal vent specimen from the Southwest Pacific (Table 2). These intraspecific genetic distances are comparable to the genetic variability of other holothurian species (Thomas et al. 2020) and are consistent with previous echinoderm DNA barcoding studies (Hoareau and Boissin 2010; Layton et al. 2016; Boissin et al. 2017).
Phylogenetic tree obtained from combined Bayesian analysis of the cytochrome c oxidase I (COI) and 12S rRNA genes from 17 apodid taxa, showing the phylogenetic relationship between holothurian material attributed to Chiridota hydrothermica by this study (as indicated by the black line). New chiridotid sequences from Sun et al. 2021 are highlighted in bold. Asterisks (*) indicate Bayesian Inference posterior probability values > 95. Scale bar represents the quantity of genetic change, with 0.07 nucleotide substitutions per site. Chiridota specimens collected from hydrothermal vents are highlighted in red, cold seeps in blue, and wood falls in purple. Bars on right represent the top species delimitation outputs using the Bayesian Poisson Tree Processes model (bPTP), Automatic Barcode Gap Discovery (ABGD) and Assemble Species by Automatic Partitioning (ASAP) methods
Furthermore, three independent species delimitation approaches using COI barcode data also supported this hypothesis (Fig. 1). Both bPTP solutions, the maximum likelihood partition and the highest supported Bayesian partition found by simple heuristic search, supported a delimitation with all Chiridota from deep-sea reducing environments as a single species (species support = 0.710; Online Resource 1). The two top scoring ASAP outputs endorsed delimitation as a single species, with the barcode gap identified between 0.080 and 0.153, respectively (Online Resource 1). In contrast, the median interspecific genetic distance across the four Chiridota species included in the phylogenetic analysis was 0.224 (Online Resource 1). All ABGD primary partitions also delimited a single species encompassing all sequences from reducing environment Chiridota. Based on this evidence, and owing to the priority of C. hydrothermica (ICZN 2012 Art. 23.1), we propose that C. heheva is a junior synonym of C. hydrothermica and recommend that the taxonomy of the species is revised to reflect the new genetic data.
Systematics
Order Apodida (Brandt, 1835)
Family Chiridotidae Östergren, 1898
Genus Chiridota Eschscholtz, 1829
Chiridota hydrothermica Smirnov & Gebruk, 2000
Synonymy: Chiridota heheva Pawson & Vance, 2004
Chresonymy as presented in Table 3 of Thomas et al. (2020) and including more recent publications listed below:
Chiridota hydrothermica Smirnov & Gebruk, 2000 (p.322, figs.1, 3–5)
Chiridota heheva Pawson & Vance, 2004 (p.3, figs.2-3): Thomas et al. 2020; Cleland et al. 2021; Sun et al. 2021; Wang et al. 2022; Zhang et al. 2022
Chiridota sp.: Reid et al. 2020; Xu et al. 2020; Sun et al. 2021; Boulart et al. 2022
Chiridota cf. heheva: Dueñas et al. 2021
Type locality: Manus Basin, the ‘Red Star B’ site, 03°06.63′S 150°21.62′E, depth 2628 m
Chiridota hydrothermica holotype is stored at the P.P. Shirshov Institute of Oceanology, Moscow, Russia (Smirnov et al. 2000); synonymized C. heheva holotype is stored at the National Museum of Natural History, Smithsonian Institution, Washington DC, USA (Pawson and Vance 2004).
Amended diagnosis: Diagnosis revised from Smirnov et al. (2000), Pawson and Vance (2004), and Thomas et al. (2020). Slender, worm-like holothurians, up to 300 mm in length. Twelve circumoral peltato-digitate tentacles with approximately twenty (up to thirty) comparatively small finger-like digits arranged along the margin of a very large terminal disc. Calcareous ring radial pieces without perforations for the passage of radial nerve. Body wall ossicles are six-spoked wheels, 66 to 207 μm in diameter, with uniform denticles along inner edge of uppermost rim. Tentacle ossicles are rods, 100 to 280 μm in length, with variable distal branching and irregular protuberances across exterior. More than 20 slender Polian vesicles. Ciliated funnels absent or rare. Intestine looped.
Distribution: Known from hydrothermal vent fields in the Manus and North Fiji Basins in the West Pacific, and along the Southeast Pacific Rise and Southwest Indian Ridge; cold seeps in the South China Sea, on the Florida Escarpment in the eastern Gulf of Mexico, the Blake Ridge in the Northwest Atlantic, and pockmarks in the Central Eastern Atlantic; and wood falls in the Caribbean Sea and Northwest Atlantic. Depth range 1360 to 3998 m.
Remarks: Inhabitant of bathyal and abyssal reducing environments: hydrothermal vents, cold seeps, and organic falls. Tentacles are free and extended during feeding. Extended remarks on the morphology, ecology, and behaviour of C. hydrothermica can be found in Thomas et al. (2020).
Discussion
Unlike many studies that have uncovered cryptic diversity within widespread marine invertebrates (including echinoderms, e.g., Boissin et al. 2017; Gubili et al. 2017), Chiridota hydrothermica is evidently a single cosmopolitan deep-sea species. Though uncommon, this is not unheard of in deep-sea invertebrates, as discussed by Thomas et al. (2020), and serves as a cautionary example when inferring species based on minor morphological variation.
While species delimitation based on a single barcoding gene has previously been criticised (Dupuis et al. 2012; Dellicour and Flot 2018; Ahrens et al. 2021), several studies have confirmed that DNA barcoding can successfully distinguish different echinoderm species (Ward et al. 2008; Hoareau and Boissin 2010; Layton et al. 2016). Single-locus species delimitation does require testing using a variety of methods (Puillandre et al. 2021): this study uses a combination of results from phylogenetic analysis of two mitochondrial genes and three different species delineation methods to determine the synonymy of C. hydrothermica and C. heheva, as well as morphological characters. Interestingly, the top scoring ASAP and ABGD primary partitions also suggested the grouping of the two Euapta Östergren, 1898 species used as outgroups in this study, as well as Chiridota laevis (O. Fabricius, 1780) with Chiridota albatrossi Edwards, 1907 (Online Resource 1). This is likely a factor of apodid holothurians being often overlooked (Thomas et al. 2020), with a lack of genetic data in depositories such as NCBI’s GenBank and BOLD. However, these results demonstrate the potential shortfalls of these species delimitation methods and the uncertainty stemming from single-locus data, highlighting the importance of testing species partitions against other evidence within an integrative taxonomy approach (Puillandre et al. 2021), especially when studying non-model organisms (Pedraza-Marrón et al. 2019).
We emphasise that the proposed synonymy of C. hydrothermica and C. heheva is not based on genetic data alone. In addition, morphological similarities, as well as analogous ecological niches occupied by the species, including broadly overlapping ecology, habitat, and zonation are also considered (Thomas et al. 2020).
Connectivity among populations
Many deep-sea holothurian taxa have expansive ranges, with several species exhibiting trans-oceanic distributions. For example, Arctic Elpidiidae species Elpidia heckeri Baranova, 1989 and Kolga hyalina Danielssen & Koren, 1879 have wide circumpolar distributions (Rogacheva 2007), while Peniagone vitrea Théel, 1882 is known across the Pacific Ocean and even into the Southern Ocean (Kremenetskaia et al. 2021). Such widespread species make interesting case studies for connectivity and speciation in the deep sea (Gubili et al. 2017) and have important implications for deep-sea conservation planning (Hilário et al. 2015; Baco et al. 2016). As a globally distributed species, C. hydrothermica presents a particularly extreme example, suggestive of a longer pelagic larval duration and greater dispersal potential (Hilário et al. 2015), and merits further study. Furthermore, the occurrence of a species at all three major types of deep-sea reducing environments is relatively rare (e.g. Georgieva et al. 2015), so phylogenetic study of animals that exploit the margins of chemosynthetic environments, such as Chiridota, may reveal new insights to the complexities of biogeography and speciation at these insular ecosystems (Rogers et al. 2012; Thomas et al. 2020).
A holothurian species that is globally widespread but restricted to deep-sea reducing environments contrasts with distinct regional faunas and recognised biogeographical patterns of vent-endemic species (Van Dover et al. 2002; Rogers et al. 2012). Among available sequence data, there are however some groupings that correlate with location and habitat (Fig. 1). The two Southwest Indian Ridge hydrothermal vent chiridotids form the basal grade of the clade, while the three East Atlantic cold seep and single Caribbean Sea wood fall specimen, are clustered together with significant support (PP = 100). Sun et al.’s (2021) new South China Sea cold seep sequence is grouped with the other two Chiridota from the same region (PP = 80), whereas the Southwest Pacific hydrothermal vent Chiridota sp. stands alone within the clade encompassing all non-vent chiridotids (PP = 50). Based on these few specimens, there are four distinct biogeographic regions for C. hydrothermica that are generally congruent with the known biogeography for deep-sea reducing environments (Rogers et al. 2012): Atlantic Ocean (including Gulf of Mexico and Caribbean Sea), Indian Ocean, Northwest Pacific (including South China Sea), and Southwest Pacific. This, however, does not include the known C. hydrothermica populations recorded at the Southeast Pacific Rise hydrothermal vents (Smirnov et al. 2000), as they currently lack genetic data.
These phylogenetic results could reflect varied gene flow between regions, with a marked difference between intraregional pairwise distances (0.000–0.013) and interregional pairwise distances (0.017–0.043) (Table 2). Other widespread species at deep-sea reducing environments, such as the bipolar tubeworm Sclerolinum contortum Smirnov, 2000, present similar levels of genetic connectivity between populations (Georgieva et al. 2015). However, this pattern could also be an artefact of the small number of genetic sequences available which do not reflect the entire range (Audzijonyte and Vrijenhoek 2010). The addition of taxonomically unconfirmed records of Chiridota sp. potentially expand C. hydrothermica’s range to hydrothermal vents on the Central and Southeast Indian Ridges and within other Southwest Pacific basins (Lau and Woodlark), active-seepage asphalt mounds on the Angolan Margin and in the Gulf of Mexico, and wood falls in the South China Sea (Thomas et al. 2020; Boulart et al. 2022). These intermediate sites, as well as others yet to be discovered, likely support the genetic connectivity of the species across its global range.
Conclusion
This study provides new evidence for a single cosmopolitan holothurian species restricted to deep-sea reducing environments. This is in direct contrast with the distinct regional biogeographical patterns of the majority of other species at chemosynthetic habitats, especially hydrothermal vents, highlighting the importance of considering understudied, peripheral taxa, such as holothurians, when studying the biogeography, connectivity, and speciation of these insular deep-sea habitats.
References
Ahrens D, Ahyong ST, Ballerio A et al (2021) Is it time to describe new species without diagnoses?—a comment on Sharkey et al. (2021). Zootaxa 5027:151–159. https://doi.org/10.11646/zootaxa.5027.2.1
Audzijonyte A, Vrijenhoek RC (2010) When gaps really are gaps: statistical phylogeography of hydrothermal vent invertebrates. Evolution 64:2369–2384. https://doi.org/10.1111/j.1558-5646.2010.00987.x
Baco AR, Etter RJ, Ribeiro PA et al (2016) A synthesis of genetic connectivity in deep-sea fauna and implications for marine reserve design. Mol Ecol 25:3276–3298. https://doi.org/10.1111/mec.13689
Boissin E, Hoareau TB, Paulay G, Bruggemann JH (2017) DNA barcoding of reef brittle stars (Ophiuroidea, Echinodermata) from the southwestern Indian Ocean evolutionary hot spot of biodiversity. Ecol Evol 7:11197–11203. https://doi.org/10.1002/ece3.3554
Boulart C, Rouxel O, Scalabrin C et al (2022) Active hydrothermal vents in the Woodlark Basin may act as dispersing centres for hydrothermal fauna. Commun Earth Environ 3:1–16. https://doi.org/10.1038/s43247-022-00387-9
Bribiesca-Contreras G, Solís-Marín FA, Laguarda-Figueras A, Zaldívar-Riverón A (2013) Identification of echinoderms (Echinodermata) from an anchialine cave in Cozumel Island, Mexico, using DNA barcodes. Mol Ecol Resour 13:1137–1145. https://doi.org/10.1111/1755-0998.12098
Cleland J, Kazanidis G, Roberts JM, Ross SW (2021) Distribution of megabenthic communities under contrasting settings in deep-sea cold seeps near Northwest Atlantic Canyons. Front Mar Sci 8:692851. https://doi.org/10.3389/fmars.2021.692851
Copley JT, Marsh L, Glover AG et al (2016) Ecology and biogeography of megafauna and macrofauna at the first known deep-sea hydrothermal vents on the ultraslow-spreading Southwest Indian Ridge. Sci Rep 6:39158. https://doi.org/10.1038/srep39158
Dellicour S, Flot J-F (2018) The hitchhiker’s guide to single-locus species delimitation. Mol Ecol Resour 18:1234–1246. https://doi.org/10.1111/1755-0998.12908
Dueñas LF, Puentes V, León J, Herrera S (2021) Fauna associated with cold seeps in the deep Colombian Caribbean. Deep-Sea Res I Oceanogr Res Pap 173:103552. https://doi.org/10.1016/j.dsr.2021.103552
Dupuis JR, Roe AD, Sperling FAH (2012) Multi-locus species delimitation in closely related animals and fungi: one marker is not enough. Mol Ecol 21:4422–4436. https://doi.org/10.1111/j.1365-294X.2012.05642.x
Fernández-Rodríguez I, Arias A, Anadón N, Acuña JL (2019) Holothurian (Echinodermata) diversity and distribution in the central Cantabrian Sea and the Avilés Canyon System (Bay of Biscay). Zootaxa 4567:293–325. https://doi.org/10.11646/zootaxa.4567.2.5
Georgieva MN, Wiklund H, Bell JB et al (2015) A chemosynthetic weed: the tubeworm Sclerolinum contortum is a bipolar, cosmopolitan species. BMC Evol Biol 15:280. https://doi.org/10.1186/s12862-015-0559-y
Gubili C, Ross E, Billett DSM et al (2017) Species diversity in the cryptic abyssal holothurian Psychropotes longicauda (Echinodermata). Deep-Sea Res II Top Stud Oceanogr 137:288–296. https://doi.org/10.1016/j.dsr2.2016.04.003
Hilário A, Metaxas A, Gaudron SM et al (2015) Estimating dispersal distance in the deep sea: challenges and applications to marine reserves. Front Mar Sci 2:6. https://doi.org/10.3389/fmars.2015.00006
Hoareau TB, Boissin E (2010) Design of phylum-specific hybrid primers for DNA barcoding: addressing the need for efficient COI amplification in the Echinodermata. Mol Ecol Resour 10:960–967. https://doi.org/10.1111/j.1755-0998.2010.02848.x
ICZN (2012) Article 73. Name-bearing types fixed in the original publication (holotypes and syntypes). In: International Code of Zoological Nomenclature, London, p 206
Kremenetskaia A, Gebruk A, Alt C, Budaeva N (2021) New and Poorly Known Species of Peniagone (Holothuroidea, Elpidiidae) from the Northwest Pacific Ocean with Discussion on Phylogeny of the Genus. Diversity 13:541. https://doi.org/10.3390/d13110541
Layton KKS, Corstorphine EA, Hebert PDN (2016) Exploring Canadian echinoderm diversity through DNA barcodes. PLoS ONE 11:e0166118. https://doi.org/10.1371/journal.pone.0166118
Liu R, Liu J, Zhang H (2021) Positive selection analysis reveals the deep-sea adaptation of a hadal sea cucumber (Paelopatides sp.) to the Mariana Trench. J Ocean Limnol 39:266–281. https://doi.org/10.1007/s00343-020-0241-0
Lundsten L, Barry JP, Cailliet GM et al (2009) Benthic invertebrate communities on three seamounts off southern and central California, USA. Mar Ecol Prog Ser 374:23–32. https://doi.org/10.3354/meps07745
Miller AK, Kerr AM, Paulay G et al (2017) Molecular phylogeny of extant Holothuroidea (Echinodermata). Mol Phylogenet Evol 111:110–131. https://doi.org/10.1016/j.ympev.2017.02.014
Pawson DL, Vance DJ (2004) Chiridota heheva, new species, from Western Atlantic deep-sea cold seeps and anthropogenic habitats (Echinodermata: Holothuroidea: Apodida). Zootaxa 534:1–13. https://doi.org/10.11646/zootaxa.534.1.1
Pedraza-Marrón C d R, Silva R, Deeds J et al (2019) Genomics overrules mitochondrial DNA, siding with morphology on a controversial case of species delimitation. Proc R Soc B Biol Sci 286:20182924. https://doi.org/10.1098/rspb.2018.2924
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Mol Ecol Resour 21:609–620. https://doi.org/10.1111/1755-0998.13281
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21:1864–1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x
Reid WDK, Wigham BD, Marsh L et al (2020) Trophodynamics at the Longqi hydrothermal vent field and comparison with the East Scotia and Central Indian Ridges. Mar Biol 167:141. https://doi.org/10.1007/s00227-020-03755-1
Rogacheva AV (2007) Revision of the Arctic group of species of the family Elpidiidae (Elasipodida, Holothuroidea). Mar Biol Res 3:367–396. https://doi.org/10.1080/17451000701781880
Rogers AD, Tyler PA, Connelly DP et al (2012) The discovery of new deep-sea hydrothermal vent communities in the Southern Ocean and implications for biogeography. PLoS Biol 10:e1001234. https://doi.org/10.1371/journal.pbio.1001234
Smirnov AV, Gebruk AV, Galkin SV, Shank T (2000) New species of holothurian (Echinodermata: Holothuroidea) from hydrothermal vent habitats. J Mar Biol Assoc U K 80:321–328. https://doi.org/10.1017/S0025315499001897
Sun S, Sha Z, Xiao N (2021) The first two complete mitogenomes of the order Apodida from deep-sea chemoautotrophic environments: new insights into the gene rearrangement, origin and evolution of the deep-sea sea cucumbers. Comp Biochem Physiol Part D Genomics Proteomics 39:100839. https://doi.org/10.1016/j.cbd.2021.100839
Thomas EA, Liu R, Amon D et al (2020) Chiridota heheva—the cosmopolitan holothurian. Mar Biodivers 50:110. https://doi.org/10.1007/s12526-020-01128-x
Van Dover CL, German CR, Speer KG et al (2002) Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295(5558):1253–1257. https://doi.org/10.1126/science.1067361
Wang X, Guan H, Qiu J-W et al (2022) Macro-ecology of cold seeps in the South China Sea. Geosystems Geoenvironment 1:100081. https://doi.org/10.1016/j.geogeo.2022.100081
Ward RD, Holmes BH, O’hara TD (2008) DNA barcoding discriminates echinoderm species. Mol Ecol Resour 8:1202–1211. https://doi.org/10.1111/j.1755-0998.2008.02332.x
Xu H, Du M, Li J et al (2020) Spatial distribution of seepages and associated biological communities within Haima cold seep field, South China Sea. J Sea Res 165:101957. https://doi.org/10.1016/j.seares.2020.101957
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29:2869–2876. https://doi.org/10.1093/bioinformatics/btt499
Zhang L, He J, Tan P et al (2022) The genome of an apodid holothuroid (Chiridota heheva) provides insights into its adaptation to a deep-sea reducing environment. Commun Biol 5:1–11. https://doi.org/10.1038/s42003-022-03176-4
Zhou Y, Zhang D, Zhang R et al (2018) Characterization of vent fauna at three hydrothermal vent fields on the Southwest Indian Ridge: Implications for biogeography and interannual dynamics on ultraslow-spreading ridges. Deep Sea Res Part I Oceanogr Res Pap 137:1–12. https://doi.org/10.1016/j.dsr.2018.05.001
Acknowledgements
We thank colleagues who have supported this study, and we acknowledge Shao’e Sun, Zhongli Sha, and Ning Xiao, the authors of the paper that published the first mitogenomes for chiridotids at deep-sea chemosynthetic environments, which provided the basis for this study. We thank the Editor, Sabine Stöhr, and three anonymous reviewers for providing valuable comments to improve this manuscript. This is contribution number 2 from the Senckenberg Ocean Species Alliance.
Funding
EAT is supported by a studentship awarded by the Faculty of Medicine, Health and Life Sciences, Queen’s University Belfast. This study was partially funded by a Systematics Research Fund grant from the Systematics Association and Linnean Society, awarded to EAT. JDS is supported by the Hong Kong Branch of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Availability of data and material
Molecular data deposited in GenBank: https://www.ncbi.nlm.nih.gov/genbank/
Author contribution
EAT conceived and designed the research and conducted data analyses. SJH and JDS provided input on data analyses and results. All authors contributed to writing and have approved the manuscript.
Additional information
Communicated by S. Stöhr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 116 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, E.A., Sigwart, J.D. & Helyar, S.J. New evidence for a cosmopolitan holothurian species at deep-sea reducing environments. Mar. Biodivers. 52, 63 (2022). https://doi.org/10.1007/s12526-022-01298-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-022-01298-w