Abstract
This study seeks to assess changes over time in the structure of subtidal macroalgal assemblages across depth in the south-eastern Bay of Biscay. The results reveal a large-scale decline in total macroalgal biomass between 1982 and 2014. However, the temporal pattern of shift differs from one depth to another: total biomass decreased at depths from 3 to 10 m, but increased at depths of 2 and 11 m. The strong decrease in biomass detected in the 3–10-m depth range is a consequence of a sharp net decline in large macroalgae biomass which was not offset by increased biomass of small species, mainly corresponding to turf-forming algae. The dominant canopy-forming Gelidium corneum in 1982 had practically disappeared by the end of the study period and its biomass loss was far from being offset by the small increase detected in the fucoid Gongolaria baccata. By contrast, at depths of 2 and 11 m, the most notable result is an increase in large species, mainly Halopithys incurva and Codium decorticatum at 2 m and G. baccata at 11 m; however, at both depth levels, a new canopy was far from being developed. These findings evidence that biomass and habitat provision, two pivotal roles of canopy-forming species in ecosystem functioning, have been altered. Further research into potential changes in primary productivity and biodiversity linked to the shift detected in assemblage structure needs to be conducted in order to get information for conservation and management decisions associated with the loss of habitat-forming macroalgae.
Similar content being viewed by others
Introduction
Canopy-forming macroalgae usually form extensive stands in rocky benthic subtidal communities in most temperate regions (Steneck et al. 2002; Smale et al. 2013; Strain et al. 2014). These large, often perennial macroalgae play a very important role in marine ecosystems as they create structurally complex assemblages analogous to forests on land (Ballesteros et al. 2009; Reed and Foster 2012; Gianni et al. 2013), i.e. they are foundation species. In this regard, these macroalgae increase three-dimensional complexity by providing biogenic habitats and protection for a great variety of marine organisms (Steneck et al. 2002; Wernberg et al. 2011; Smale et al. 2013). They also act as ecosystem engineers, since they substantially modify the local environment by changing light conditions (Wernberg et al. 2005), water flow (Rosman et al. 2007) and sedimentation rates (Eckman et al. 1989) in ways that favour the settlement of other organisms. Canopy-dominated assemblages constitute some of the most diverse and productive ecosystems anywhere in the world, contribute significantly to nearshore primary productivity and also enhance secondary productivity (Mann 2000; Steneck et al. 2002; Tait and Schiel 2011; Smale et al. 2013). In addition, these foundation species supply many valuable ecosystem services such as reducing coastal erosion, CO2 sinking, nutrient cycling and water quality control (Airoldi and Beck 2007; Smale et al. 2013; Wernberg et al. 2016).
However, in the last 30 years, drastic declines in these foundation species have been observed as a consequence of multiple anthropogenic pressures comprising harvesting, pollution, sedimentation, invasive species, overgrazing due to a decline in grazer predators, fishing nets, recreation and ocean warming (Steneck et al. 2002; Serisawa et al. 2004; Connell et al. 2008; Perkol-Finkel and Airoldi 2010; Smale et al. 2013; Mineur et al. 2015; Krumhansl et al. 2016; Wernberg et al. 2016). Whatever is driving the decline, a shift to less structurally complex communities dominated by turf-forming, filamentous or ephemeral macroalgae has been widely documented (Airoldi et al. 2008; Mangialajo et al. 2008; Perkol-Finkel and Airoldi 2010; Tait and Schiel 2011). To date, there has been little evidence of foundation species recovering from disturbances (Dayton et al. 1992, Martínez and Cárdenas 2003), mainly because once less structurally complex communities are established they may inhibit recolonisation by canopy species, resulting in alternative stable states (Strain et al. 2014; Wernberg et al. 2016). Loss of large perennial macroalgae in favour of less structured communities may have consequences for the whole ecosystem, and in this connection, numerous studies have linked the retreat of these ecosystem engineers to a decrease in species richness and abundance (Graham 2004; Norderhaug et al. 2007; Schiel and Lilley 2007; Wikström and Kautsky 2007), homogenisation with neighbouring habitats (Mangialajo et al. 2008) and loss of productivity (Tait and Schiel 2011; Crowe et al. 2013).
In the particular case of the south-eastern Bay of Biscay, local retreats in subtidal perennial canopy-forming Gelidium corneum, Laminaria ochroleuca and Gongolaria baccata (formerly Cystoseira baccata) have been reported in the past few decades (Díez et al. 2012; Borja et al. 2013, 2018; Muguerza et al. 2017, 2020). In addition to canopy decline, there has been an increase over the same period in richness and abundance of warm-affinity species (mainly ephemeral forms with simple morphology), coralline algae and crustose species have become abundant, and non-indigenous species have expanded (Díez et al. 2012; Muguerza et al. 2017). These studies point out that higher temperatures are probably the main driver of the changes observed. Indeed, a warming of 0.26 ± 0.03 °C every 10 years was detected in the Bay of Biscay for 1982–2014 (Costoya et al. 2015). However, other local factors such as nutrient availability, solar radiation, sunlight hours and wave height have been suggested as potential co-acting factors of change in combination with warming (Díez et al. 2012; Borja et al. 2013, 2018; Muguerza et al. 2017).
Most of the aforementioned research papers provide information on species abundance in terms of cover, but little information about loss of biomass due to canopy decline is available for the south-eastern Bay of Biscay. Only Borja et al. (2013) provide data on changes in G. corneum biomass for the eastern Basque coast, where a drastic reduction in its standing stock of about 7800 t across 30 km has been documented for 1993–2012. However, no data on the variability over time of the biomass of other species and areas are available. This paper seeks to assess changes over time (three sampling surveys: 1982, 2007 and 2014) in the structure of subtidal macroalgal assemblages in terms of composition and taxon biomass in the westernmost part of the Basque coast. These assemblages can be considered representative of the south-eastern Bay of Biscay since they are distributed along large stretches of coastline in this region (Gorostiaga et al. 1998; Díez et al. 2003).
Materials and methods
Study area
The study area lies at the eastern end of the Cantabrian Sea (Northern Spain), on the south-eastern Bay of Biscay. It is over 192 km in length and is open to strong waves coming mostly from the NW, with an average height of 1.9 m (Díez et al. 2003; González et al. 2004; Galparsoro et al. 2010). The south-eastern Bay of Biscay is exposed to highly exposed to the prevailing NW swells, with high, mostly erosional, energy. It also features extensive vertical cliffs and abrasion platforms interspersed with sandy beaches. In shallow waters in the study area, the rocky bottom is almost continuous, but it becomes sandy as depth increases (Chust et al. 2011). The flora belongs to the warm temperate NE Atlantic Region according to the biogeographical scheme proposed by van den Hoek and Breeman (1990).
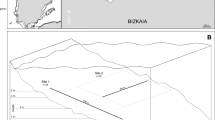
The study was carried out on a stretch of shoreline about 1.8 km long, between the locations of Kobaron and Muskiz (43°35′34″N 03°15′44″W and 43°35′52 ″N 03°12′94″W, respectively), in the province of Bizkaia (Fig. 1).
Study area. Layout of the seven transects along the western end of the coast of Bizkaia. (Source: modified from Díez et al. 2000)
Field sampling and processing
The biomass of taxa composing in macroalgal assemblages was studied at six different depths (2, 3, 6, 9, 10 and 11 m) along seven transects in three sampling surveys (1982, 2007 and 2014). Each transect was set perpendicular to the coastline following a north-south orientation with a starting point at 2 m below extremely low tides (Fig. 1). Within each transect, a surface area of 2000 cm2 was delimited at each depth using quadrats of 40 × 50 cm placed systematically at the midpoint of the cross section of the transect. This means that there were six quadrats per transect across each depth profile and sampling survey. In some transects, it was not possible to sample at certain depths due to a lack of appropriate substrate (continuous bedrock with slight to moderate slopes of <30°). Each surface was destructively sampled, with all macroalgal species within the quadrat being collected except the mostly calcareous crustose layer, which was not sampled. Once in the laboratory, samples were kept frozen in labelled plastic bags. For analysis, samples were thawed and the macroalgae were separated and identified. Algal taxonomy was updated following AlgaeBase (Guiry and Guiry 2021). The dry weight (DW) in grammes (100–110 °C, 24 h) was then obtained for each species.
Statistical analysis
Multivariate analyses were performed in order to explore the spatio-temporal variability of the structure of assemblages in terms of composition and taxon biomass. Prior to analysis, the biomass values for each taxon were square root transformed to reduce the influence of the dominant ones. The similarity between pairs of samples was calculated using the Bray-Curtis index. The hypothesis that time and depth have no influence on the structure of macroalgal assemblages was tested by means of PERMANOVA (permutational multivariate analysis of variance, see Anderson et al. 2008) with an a priori chosen significance level of α = 0.05. The design of the experiment was as follows: time (Year; set with three levels: 1982, 2007 and 2014) and depth (Depth; random with six levels: 2, 3, 6, 9, 10 and 11). Post hoc pairwise comparisons were performed using Gosset’s t-statistic to investigate the significant terms of the PERMANOVA (Anderson et al. 2008). In order to graphically visualise this spatio-temporal variation, a non-metric multidimensional scaling (nMDS) was conducted. Given that PERMANOVA tests the null hypothesis that centroids and/or dispersion of the groups defined by the factors of the experimental design is equivalent, we performed a permutational test for homogeneity of multivariate dispersions (PERMDISP) to check for differences in dispersion between the levels within the Year factor. By applying classification analysis (CLUSTER), samples were segregated into different groups according to their similarities. This analysis was followed by a similarity percentage (SIMPER) analysis to calculate the contribution of each taxon (%) to the dissimilarity between the clustering groups. All statistical analyses and the aforementioned routines were performed using the PERMANOVA+ for PRIMER6 software package (Clarke and Gorley 2006).
Results
The survey carried out in 1982 identified a total of 55 taxa, 94 species were documented in 2007 and 65 in 2014. Across all three surveys, the most widely represented phylum was Rhodophyta, with a total of 92 taxa, followed by Ochrophyta with 13 and Chlorophyta with 12 (Table 4).
PERMANOVA results (Table 1) show the partitioning of sample variation in the multivariate space based on the Bray-Curtis similarity in response to the Year and Depth factors. Differences between depths in the pattern of temporal change were detected, given that the Year x Depth interaction was significant (p = 0.0028). Pairwise comparisons reveal that at a depth of 2 m, significant changes in the structure of assemblages start to occur in 2007. At depths of 3 and 6 m, significant differences are detected from 1982 onwards, whilst at depths from 9 to 11 m, the structure of assemblages changed between 1982 and 2007 but has not changed significantly since then (Table 1). Non-metric multidimensional scaling (nMDS) (Fig. 2) shows the PERMANOVA results in graphic form. A gradual shift in the structure of assemblages is found across the sampling surveys (1982, 2007 and 2014). The permutational test for homogeneity of multivariate dispersions (PERMDISP) for the Year factor shows an increase in multivariate dispersion from 1982 to 2007, indicating a rise in the heterogeneity of vegetation. However, no differences in the dispersion of samples are found between 2007 and 2014 (Table 2; Fig. 2).
In the dendrogram resulting from classification analysis (CLUSTER) of samples, two main groups, A and B, are distinguished at a similarity level of 14% (Fig. 3). Group A (average similarity between samples 42.9%) consists mainly of samples from 1982 but also contains some samples from 2007 and one from 2014. The samples from 2007 are all from depths of 3 and 6 m except for two which come from 2 and 9 m (Fig. 3). Group B (average similarity between samples 24.8%) comprises the rest of the samples from 2007 and 2014 and two samples from 1982 taken at a depth of 2 m (Fig. 3). The similarity percentage (SIMPER) analysis points to Gelidium corneum as the main species responsible for the separation of these two groups, with a contribution of 26.9%. The mean biomass of this rhodophyte is higher in group A. Apart from G. corneum, other species which make noteworthy contributions (>2%) to group A are Pterosiphonia complanata, Plocamium cartilagineum, Asparagopsis armata and Dictyopteris polypodioides. Of these species, P. complanata, P. cartilagineum and D. polypodioides are more abundant in group A, whilst the biomass of A. armata is similar in both groups. In addition to the lower presence of G. corneum, group B also shows a mosaic distribution of many species, dominated by Gongolaria baccata, Codium decorticatum, Halopithys incurva and Corallina spp. It is also noteworthy that this group shows a greater abundance of morphologically simple forms such as Aphanocladia stichidiosa, Lychaete pellucida, Aglaothamniom pseudobyssoides or Microcladia glandulosa, among others (Table 3).
Dendrogram resulting from classification analysis of samples based on taxon square root transformed biomass data and Bray-Curtis similarity index. Black lines represent significantly different groups, whilst red lines denote groups that do not differ significantly according to the SIMPROF test. Black triangles: 1982; grey circles: 2007; white squares: 2014
Group A is divided into two subgroups (A1 and A2) at a similarity level of 24% (Fig. 3). Subgroup A1 consists of samples from 1982 and the aforementioned samples from 2007 and 2014, mostly from depths of 3 and 6 m. These samples are characterised by the dominance of G. corneum accompanied mainly by P. cartilagineum (Table 3). By contrast, subgroup A2 comprises samples from 1982, mainly from deeper waters. In this case, the abundance of G. corneum is notably lower and the species that shows the greatest abundance is P. complanata (Table 3). Other species that show considerable biomass values are A. armata, D. polypodioides, Calliblepharis ciliata and Heterosiphonia plumosa, all of which are more abundant in subgroup A2 than in subgroup A1. The latter subgroup (samples from depths of 3 and 6 m) is divided, in turn, into two groups (A1.1 and A1.2) at a similarity level of 59% (Fig. 3). Subgroup A1.1 consists of samples from 1982 plus two samples from 2007, whilst subgroup A1.2 comprises the remaining samples from 2007 and the single sample from 2014. The dominant species in both subgroups is G. corneum, but its abundance in subgroup A1.1 is greater. Other significant macroalgae in terms of biomass in the latter subgroup are P. cartilagineum, P. complanata, D. polypodioides and Desmarestia ligulata. In subgroup A1.2, the taxa with notable abundances are G. baccata, Callithamnion tetragonum, P. cartilagineum, Dictyota dichotoma, A. armata, Rhodymenia pseudopalmata and Corallina spp. The first two of these species are not found in subgroup A1.1 whilst the others all show higher biomass values in subgroup A1.2 (Table 3).
Group B is divided into two subgroups (B1 and B2) at a similarity level of 17% (Fig. 3). Subgroup B1 is represented mainly by deeper samples from 2007 and 2014, with G. baccata as the dominant species (Table 3). Other species with noteworthy biomass values include Phyllophora crispa, and Corallina spp. Subgroup B2 mainly comprises shallower samples from 2007 and 2014, and is characterised by higher values of C. decorticatum, H. incurva, Corallina spp. and Jania rubens (Table 3). This latter heterogeneous group is further divided into different subgroups with no defined spatio-temporal pattern (Fig. 3).
Mean total biomass decreases from 106.75 g DW · 2000 cm−2 in 1982 to 70.82 g DW · 2000 cm−2 in 2007, but remains nearly constant in 2014 (72.68 g DW · 2000 cm−2). Biomass corresponding to small species (<5 cm) (3.07 vs. 10.73 g DW · 2000 cm−2) and medium-size species (5–15 cm) (7.59 vs. 9.18 g DW · 2000 cm−2) increases slightly between 1982 and 2014, whereas that of aggregated large-size (>15 cm) species shows a noteworthy decline (96.08 vs. 52.77 g DW · 2000 cm−2) (Table 4). Changes in both the total biomass and the thallus size vary in relation to depth (Table 5 in the Appendix).
The trend over time across depths of the mean total biomass and the biomass of those species that SIMPER indicates contribute most (>7%) to the groups detected in the CLUSTER which is shown in Fig. 4. The highest total biomass figures for 1982 are found at depths of 3 and 6 m with 194.56 and 154.47 g DW · 2000 cm−2, respectively (Fig. 4). Intermediate values are found at depths of 9 and 10 m (77.36 and 71.51 g DW · 2000 cm−2) and the lowest values at depths of 2 and 11 m (46.18 and 30.18 g DW ·2000 cm−2). These biomass records in 1982 at depths of 3, 6, 9 and 10 m are consistent with the high abundance of G. corneum (189.36, 149.51, 63.74 and 43.67 g DW ·2000 cm−2, respectively). However, at 2 m, H. incurva is the most abundant species (18.65 g DW ·2000 cm−2), whilst at 11 m, the biggest contributor to biomass is P. complanata (7.31 g DW · 2000 cm−2). In 2007 and 2014, the total biomass does not exceed 93 g DW · 2000 cm−2 at any depth and it is more homogeneously distributed than in 1982.
As for temporal changes in biomass (Fig. 4), at a depth of 2 m, the total biomass increases from 1982 to 2007 (78.17 g DW · 2000 cm−2), with no changes being shown in 2014 (78.18 g DW · 2000 cm−2). This increase is mainly associated with C. decorticatum (0 vs. 24.66 g DW · 2000 cm−2; in 1982 and 2007, respectively) and H. incurva (18.66 vs. 45.24 g DW · 2000 cm−2; in 1982 and 2014, respectively). At depths of 3 and 6 m, the total biomass sharply declines to 92.99 and 58.85 g DW · 2000 cm−2, respectively, between 1982 and 2014. In this regard, the increases from 1982 to 2014 in C. decorticatum (0 vs. 21.79 g DW · 2000 cm−2) and H. incurva (0.00 vs. 15.10 g DW · 2000 cm−2) at a depth of 3 m and G. baccata (0.00 vs. 25.19 g DW · 2000 cm−2) at 6 m do not offset the drastic decrease in biomass detected for G. corneum towards the end of the study period (1.21 and 13.37 g DW · 2000 cm−2; for depths of 3 and 6 m, respectively). At depths of 9 and 10 m, the total biomass decreases to 53.44 and 62.05 g DW · 2000 cm−2, respectively, between 1982 and 2014. G. corneum sharply decreases at both depths (0.33 and 0.45 g DW · 2000 cm−2; for depths of 9 and 10 m, respectively). To a lesser extent, P. complanata also decreases (9 m: 3.92 vs. 0.44; 10 m: 14.67 vs. 0.19 g DW · 2000 cm−2; in 1982 and 2014, respectively). By contrast, G. baccata moderately increases between 1982 and 2014 (0.00 vs. 28.14 and 0.00 vs. 33.45 g DW · 2000 cm−2; for depths of 9 and 10 m, respectively). The deepest assemblages (11 m) show an increase in total biomass (86.87 g · 2000 cm−2) by the end of the study period (2014), mainly related to the development of G. baccata (0.00 vs. 66.73 g DW · 2000 cm−2, in 1982 and 2014, respectively). The greatest decrease at this depth is detected for P. complanata (7.31 vs. 0.37 g DW · 2000 cm−2, in 1982 and 2014, respectively). Mean biomass figures for all taxa over the years are shown in Table 4.
Discussion
This research reveals a large-scale decline in total macroalgal biomass between 1982 and 2014. However, the temporal pattern of the shift differs from one depth to another: total biomass decreased at depths from 3 to 10 m but increased at 2 and 11 m. The strong decrease in biomass detected in the 3–10-m depth range is a consequence of the sharp net decline in large macroalgal biomass, which was not offset by the increased biomass of small species, mainly turf-forming forms. The dominant large macroalga in 1982, the canopy-forming G. corneum, had practically disappeared by the end of the study, and its biomass loss was far from offset by the small increase detected in the fucoid Gongolaria baccata. By contrast, at depths of 2 and 11 m, the most notable result is the increase in large species, mainly Halopithys incurva and the annual chlorophyte C. decorticatum at 2 m, and G. baccata at 11 m; however, at both depth levels, a new canopy is far from being developed.
The decline of G. corneum detected in this study is in line with that recorded along the Basque coast in the last 30 years (Díez et al. 2012; Muguerza et al. 2017). Increased water temperature, irradiance and wave height are suggested as being among the main drivers underlying this regression (Díez et al. 2012; Borja et al. 2013; Muguerza et al. 2017; Quintano et al. 2019). The loss of the biomass and canopy functions provided by G. corneum in 1982 in the 3–10-m depth range may have severe, long-lasting consequences for benthic assemblages, as no other large macroalga has shown conspicuous development in this depth range. At the end of the study period, scattered individuals and small patches of the large macroalga G. baccata were detected. However, the ability of this fucoid to colonise shallow rocky reefs in coastal stretches exposed to strong waves is rather limited (Díez et al. 2003). Accordingly, the increase in wave energy detected since the early 1990s on the Basque coast (Borja et al. 2013) is suggested as the main factor of change explaining the decline of G. baccata in some pristine locations along this coast (Muguerza et al. 2017). This macroalga is a warm-temperate species whose distribution is expected to expand northwards in the context of ongoing climate change (Hiscock et al. 2004). It has a high capacity for acclimation to increased temperature and irradiance levels (Miguel-Vijandi et al. 2010), which may be the reason for the lengthening of its growth period and the increase in biomass detected in semi-exposed coastal stretches of the southern Bay of Biscay since 2007 (Méndez-Sandín and Fernández 2016). Likewise, the physiological traits of G. baccata may explain the increase detected in the present study at a depth of 11 m, where the bottom friction exerted by waves is low enough for G. baccata to cope with it, whilst the increases in water temperatures and irradiance registered in the study area (Quintano et al. 2019) may have favoured its development.
Concurrently with the strong net decrease in canopy-forming macroalgae at depths of 3–10 m, turf-forming species comprising articulated coralline macroalgae (Corallina spp., J. rubens) and morphologically simple and ephemeral algal forms (Aphanocladia stichidiosa, Lychaete pellucida, Aglaothamniom pseudobyssoides, M. glandulosa) have expanded. By contrast, at a depth of 2 m, where the warm-temperate affinity species H. incurva and C. decorticatum increased, and at the depth of 11 m where G. baccata increased, no expansion of turf-forming species was detected. Large macroalgae competitively exclude some species by monopolising resources, particularly space (Maggi et al. 2009), and numerous studies indicate that opportunistic species readily colonise space made available by canopy loss (Benedetti-Cecchi et al. 2001; Bulleri et al. 2002; Airoldi et al. 2008). The net loss of biomass linked to the shift detected in assemblage structure may be long-lasting (Tait and Schiel 2011; Crowe et al. 2013), since once turfing vegetation is well established, it may inhibit canopy-forming macroalgae from recruiting (Airoldi et al. 2008). In this regard, a shift to stable turfing assemblages in the vicinity of the study area has already been documented (Díez et al. 2014).
Canopy-forming macroalgae act as ecosystem engineer species and directly or indirectly modulate the availability of resources (Steneck et al. 2002). One consequence of canopy loss is a decline in the richness and abundance of the associated flora and fauna (Steneck et al. 2002; Graham 2004; Norderhaug et al. 2007; Schiel and Lilley 2007; Wikström and Kautsky 2007). In the study reported here, this functional role provided by G. corneum in 1982 was not replaced in 2014 by the expansion of H. incurva and C. decorticatum at a depth of 2 m, or by G. baccata at 11 m, since at both these levels, a new canopy was far from being developed. The complex habitat formed by G. corneum is essential for the functioning of the ecosystem since it preserves understory and epiphytic assemblages of smaller macroalgae, as well as sessile and vagile invertebrates (Borja et al. 2004; Bustamante et al. 2017). This habitat has become extinct in most of the study area, which may have consequences for the whole benthic ecosystem (Bustamante et al. 2017).
Branched canopies of species of the genus Cystoseira (G. baccata was formerly Cystoseira baccata) increase coastal primary production (Ballesteros et al. 2009), preserve biodiversity (Bianchelli et al. 2016), offer nursery areas for juvenile fish (Cheminée et al. 2013) and provide a home for outstanding species richness and density in coastal fish assemblage (Orlando-Bonaca and Lipej 2005). Therefore, the potential development of deepwater forests of G. baccata may take over the ecosystem functional role previously played by G. corneum. However, at present, this functional replacement has not yet occurred, since G. baccata stands are poorly developed. In addition, the newly established turf-forming macroalgae typically consist of species with less ecological and functional value than those replaced (Crowe et al. 2013). A decrease in the richness and abundance of associated organisms may therefore be expected. Furthermore, recent research in the study area has shown a sharp decrease in invertebrate taxonomic and functional density and diversity after canopy loss (Bustamante et al. 2014, 2017). Although species richness increased only slightly by the end of the study period for this research, significant increases (mainly in ephemeral forms) have been detected along the Basque coast (Muguerza et al. 2017, 2020). The latter finding has been related to the intermediate disturbance hypothesis, which predicts maximal diversity at intermediate levels of disturbance (Connell 1978).
Foundation species are very important for marine food webs as they facilitate the capture and export of carbon (Dayton 1985; Krumhansl and Scheibling 2012; Smale et al. 2013). Moreover, much of their biomass is not consumed directly by herbivores, so canopy species are a major source of nutrition for other nearshore ecosystems (Duggins and Eckman 1997, Mann 2000, Steneck et al. 2002). Although no specific productivity measurements are made in this study, there may be a high risk of future primary productivity being significantly lower if the biogenic habitat previously provided by G. corneum is not recovered. Thus, previous experimental research on the impact of canopy loss on ecosystem functioning (Tait and Schiel 2011) has reported long-term reductions in primary productivity of macroalgal assemblages following canopy removal.
In addition to changes in ecosystem functions, canopy loss and subsequent decrease in macroalgal biomass in the study area could also impair the ecosystem services that canopy species provide. As mentioned above, these ecosystem engineers provide refuge for numerous other species, including many that are economically important for humans (Graham 2004; Smale et al. 2013). In this regard, the fact that the species that have replaced G. corneum cannot perform the same functional role means that the exploitation of some species in the study area might decline. G. corneum used to be the main raw material for agar extraction along the Atlantic shores of Spain, Portugal and Morocco (McHugh 1991). In the particular case of the study area, this resource has not been exploited since 1999 due to its decline (Borja et al. 2013).
In summary, the findings reported here evidence that two pivotal roles of canopy-forming species in coastal ecosystem functioning (biomass and habitat provision) have been altered in shallow rocky bottoms in the south-eastern Bay of Biscay. It seems that at present there are no native canopy-forming species that can adapt to the environmental conditions in the 3–10-m depth range where G. corneum previously thrived. Further research on potential changes in primary productivity and biodiversity linked to the shift detected in assemblage structure needs to be conducted to obtain information of elementary importance for conservation and management decisions associated with the loss of habitat-forming macroalgae.
References
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol 45:345–405. https://doi.org/10.1201/9781420050943
Airoldi L, Balata D, Beck MW (2008) The grey zone: relationships between habitat loss and marine diversity and their applications in conservation. J Exp Mar Biol Ecol 366:8–15. https://doi.org/10.1016/j.jembe.2008.07.034
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth, Plymouth
Ballesteros E, Garrabou J, Hereu B, Zabala M, Cebrian E, Sala E (2009) Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the northwestern Mediterranean: insights into assemblage structure and population dynamics. Estuar Coast Shelf Sci 82:477–484. https://doi.org/10.1016/j.ecss.2009.02.013
Benedetti-Cecchi L, Pannacciulli F, Bulleri F, Moschella P, Airoldi L, Relini G, Cinelli F (2001) Predicting the consequences of anthropogenic disturbance: large-scale effects of loss of canopy algae on rocky shores. Mar Ecol Prog Ser 214:137–150. https://doi.org/10.3354/meps214137
Bianchelli S, Buschi E, Danovaro R, Pusceddu A (2016) Biodiversity loss and turnover in alternative states in the Mediterranean Sea: a case study on meiofauna. Sci Rep 6:34544. https://doi.org/10.1038/srep34544
Borja A, Aguirrezabalaga F, Martínez J, Sola JC, Garcia-Arberas L, Gorostiaga JM (2004) Benthic communities, biogeography and resources management. In: Borja A, Collins M (eds) Oceanography and marine environment of the Basque Country. Elsevier, Amsterdam, pp 455–492
Borja A, Fontán A, Muxika I (2013) Interactions between climatic variables and human pressures upon a macroalgae population: implications for management. Ocean Coast Manag 76:85–95. https://doi.org/10.1016/j.ocecoaman.2013.02.023
Borja A, Chust G, Fontán A, Garmendia JM, Uyarra MC (2018) Long-term decline of the canopy-forming algae Gelidium corneum, associated to extreme wave events and reduced sunlight hours, in the southeastern Bay of Biscay. Estuar Coast Shelf Sci 205:152–160. https://doi.org/10.1016/j.ecss.2018.03.016
Bulleri F, Benedetti-Cecchi L, Acunto S, Cinelli F, Hawkins SJ (2002) The influence of canopy algae on vertical patterns of distribution of low-shore assemblages on rocky coasts in the northwest Mediterranean. J Exp Mar Biol Ecol 267:89–106. https://doi.org/10.1016/S0022-0981(01)00361-6
Bustamante M, Tajadura J, Gorostiaga JM, Saiz-Salinas JI (2014) Response of rocky invertebrate diversity, structure and function to the vertical layering of vegetation. Estuar Coast Shelf Sci 147:148–155. https://doi.org/10.1016/j.ecss.2014.06.001
Bustamante M, Tajadura J, Díez I, Saiz-Salinas JI (2017) The potential role of habitat-forming seaweeds in modeling benthic ecosystem properties. J Sea Res 130:123–133. https://doi.org/10.1016/j.seares.2017.02.004
Cheminée A, Sala E, Pastor J, Bodilis P, Thiriet P, Mangialajo L, Cottalorda JM, Francour P (2013) Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J Exp Mar Biol Ecol 442:70–79. https://doi.org/10.1016/j.jembe.2013.02.003
Chust G, Borja A, Caballero A, Irigoien X, Sáenz J, Moncho R, Marcos M, Liria P, Hidalgo J, Valle M, Valencia V (2011) Climate change impacts on coastal and pelagic environments in the southeastern Bay of Biscay. Clim Res 48:307–322. https://doi.org/10.3354/cr00914
Clarke KR, Gorley RN (2006) PRIMER v6: User manual/Tutorial. PRIMER-E Ltd, Plymouth
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Connell SD, Russell BD, Turner DJ, Shepherd SA, Kildea T, Miller D, Airoldi L, Cheshire A (2008) Recovering a lost baseline: missing kelp forests from a metropolitan coast. Mar Ecol Prog Ser 360:63–72. https://doi.org/10.3354/meps07526
Costoya X, de Castro M, Gómez-Gesteira M, Santos F (2015) Changes in sea surface temperature seasonality in the Bay of Biscay over the last decades (1982-2014). J Mar Syst 140:91–101. https://doi.org/10.1016/j.jmarsys.2015.06.002
Crowe TP, Cusson M, Bulleri F, Davoult D, Arenas F et al (2013) Large-scale variation in combined impacts of canopy loss and disturbance on community structure and ecosystem functioning. PLoS One 8(6):e66238. https://doi.org/10.1371/journal.pone.0066238
Dayton PK (1985) Ecology of kelp communities. Annu Rev Ecol Syst 16:215–245
Dayton PK, Tegner MJ, Parnell PE, Edwards PB (1992) Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol Monogr 62:421–445. https://doi.org/10.2307/2937118
Díez I, Secilla A, Santolaria A, Gorostiaga JM (2000) The North coast of Spain. In Seas at the millenium: an environmental evaluation. C. Sheppard. Elsevier, Amsterdam, pp. 135-150
Díez I, Santolaria A, Gorostiaga JM (2003) Relationships of environmental factors to the structure and distribution of subtidal seaweed vegetation of the western Basque coast (N. Spain). Estuar Coast Shelf Sci 56:1041–1054. https://doi.org/10.1016/S0272-7714(02)00301-3
Díez I, Muguerza N, Santolaria S, Ganzedo U, Gorostiaga JM (2012) Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci 99:108–120. https://doi.org/10.1016/j.ecss.2011.12.027
Díez I, Santolaria A, Muguerza N, Gorostiaga JM (2014) Capacity for recovery of rocky subtidal assemblages following pollution abatement in a scenario of global change. Mar Pollut Bull 86:197–209. https://doi.org/10.1016/j.marpolbul.2014.07.018
Duggins DO, Eckman JE (1997) Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites. Mar Biol 128:489–495. https://doi.org/10.1007/s002270050115
Eckman JE, Duggins DO, Sewell AT (1989) Ecology of under story kelp environments. I. Effects of kelps on flow and particle transport near the bottom. J Exp Mar Biol Ecol 129:173–187. https://doi.org/10.1016/0022-0981(89)90055-5
Galparsoro I, Borja A, Legorburu I, Hernández C, Chust G, Liria P, Uriarte A (2010) Morphological characteristics of the Basque continental shelf (Bay of Biscay, northern Spain); their implications for Integrated Coastal Zone Management. Geomorphology 118:314–329. https://doi.org/10.1016/j.geomorph.2010.01.012
Gianni F, Bartolini F, Airoldi L, Ballesteros E, Francour P, Guidetti P, Meinesz A, Thibaut T, Mangialajo L (2013) Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of marine protected areas. Adv Oceanogr Limnol 4:83–101. https://doi.org/10.1080/19475721.2013.845604
González M, Uriarte A, Fontán A, Mader J, Gyssels P (2004) Marine dynamics. In: Borja A, Collins M (eds) Oceanography and marine environment of the Basque Country. Elsevier Oceanography Series 70, Amsterdam, pp 133–157
Gorostiaga JM, Santolaria A, Secilla A, Díez I (1998) Sublittoral benthic vegetation of eastern Basque coast (N Spain): structure and environmental factors. Bot Mar 41:455–465. https://doi.org/10.1515/botm.1998.41.1-6.455
Graham MH (2004) Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems 7:341–357. https://doi.org/10.1007/s10021-003-0245-6
Guiry MD, Guiry GM (2021) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. https://www.algaebase.org; searched on 25 September 2021
Hiscock K, Southward A, Tittley I, Hawkins S (2004) Effects of changing temperature on benthic marine life in Britain and Ireland. Aquat Conserv Mar Freshw Ecosyst 14:333–362. https://doi.org/10.1002/aqc.628
Krumhansl K, Scheibling RE (2012) Production and fate of kelp detritus. Mar Ecol Prog Ser 467:281–302. https://doi.org/10.3354/meps09940
Krumhansl K, Okamoto D, Rassweiler A, Novak M, Bolton J, Cavanaugh K, Connell S, Johnson C, Konar B, Ling S, Micheli F, Norderhaug K, Perez-Matus A, Sousa-Pinto I, Reed D, Salomon A, Shears N, Wernberg T, Anderson R, Byrnes J (2016) Global patterns of kelp forest change over the past half-century. P Natl Acad Sci USA 113:10.1073
Maggi E, Bertocci I, Vaselli S, Benetti-Cecchi L (2009) Effects of changes in number, identity and abundance of habitat-forming species on assemblages of rocky seashores. Mar Ecol Prog Ser 381:39–49. https://doi.org/10.3354/meps07949
Mangialajo L, Chiantore M, Cattaneo-Vietti R (2008) Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar Ecol Prog Ser 358:63–74. https://doi.org/10.3354/meps07400
Mann KH (2000) Ecology of coastal waters. Blackwell, Malden
Martínez E, Cárdenas L (2003) Recovery and genetic diversity of the intertidal kelp Lessonia nigrescens (Phaeophyceae) 20 years after El Niño 1982/83. J Phycol 39:504–508. https://doi.org/10.1046/j.1529-8817.2003.02191.x
McHugh DJ (1991) Worldwide distribution of commercial resources of seaweeds including Gelidium. Hydrobiologia 221:19–29. https://doi.org/10.1007/BF00028359
Méndez-Sandín M, Fernández C (2016) Changes in the structure and dynamics of marine assemblages dominated Bifurcaria bifurcata and Cystoseira species over three decades (1977–2007). Estuar Coast Shelf Sci 175:4–56. https://doi.org/10.1016/j.ecss.2016.03.015
Miguel-Vijandi C, Márquez-Garrido E, Gorostiaga JM, Korbee N, Figueroa FL (2010) Acclimation to the stress factors (high irradiance, temperature and low nutrient availability) of marine macroalgae from the Bay of Biscay: possible relation to the changes in the algal distribution. In: XII International Symposium on Oceanography of the Bay of Biscay, Brest, France
Mineur F, Arenas F, Assis J, Davies AJ, Engelen AH, Fernandes F, Malta E-J, Thibaut T, Nguyen TV, Vaz-Pinto F, Vranken S, Serrao EA, De Clerck O (2015) European seaweeds under pressure: consequences for communities and ecosystem functioning. J Sea Res 98:91–108. https://doi.org/10.1016/j.seares.2014.11.004
Muguerza N, Díez I, Quintano E, Bustamante M, Gorostiaga JM (2017) Structural impoverishment of the subtidal vegetation of southeastern Bay of Biscay from 1991 to 2013 in the context of climate change. J Sea Res 130:166–179. https://doi.org/10.1016/j.seares.2017.06.006
Muguerza N, Bustamante M, Díez I, Quintano E, Tajadura FJ, Saiz-Salinas JI, Gorostiaga JM (2020) Long-term surveys reveal abrupt canopy loss with immediate changes in diversity and functional traits. Mar Biol 167:61. https://doi.org/10.1007/s00227-020-3675-1
Norderhaug KM, Christie H, Fredriksen S (2007) Is habitat size an important factor for faunal abundances on kelp (Laminaria hyperborea)? J Sea Res 58:120–124. https://doi.org/10.1016/j.seares.2007.03.001
Orlando-Bonaca M, Lipej L (2005) Factors affecting habitat occupancy of fish assemblage in the Gulf of Trieste (Northern Adriatic Sea). Mar Ecol 26(1):42–53. https://doi.org/10.1111/j.1439-0485.2005.00037.x
Perkol-Finkel S, Airoldi L (2010) Loss and recovery potential of marine habitats: an experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLoS One 5:e10791. https://doi.org/10.1371/journal.pone.0010791
Quintano E, Celis-Plá PSM, Martínez B, Díez N, Muguerza N, Figueroa FL, Gorostiaga JM (2019) Ecophysiological responses of a threatened red alga to increased irradiance in an in situ transplant experiment. Mar Environ Res 144:166–177. https://doi.org/10.1016/j.marenvres.2019.01.008
Reed DC, Foster MS (2012) The effects of canopy shadings on algal recruitment and growth in a giant kelp forest. Ecology 65:937–948. https://doi.org/10.2307/1938066
Rosman JH, Koseff JR, Monismith SG, Grover J (2007) A field investigation into the effects of a kelp forest (Macrocystis pyrifera) on coastal hydrodynamics and transport. J Geophys Res 112:C02016. https://doi.org/10.1029/2005JC003430
Schiel DR, Lilley SA (2007) Gradients of disturbance to an algal canopy and the modification of an intertidal community. Mar Ecol Prog Ser 339:1–11. https://doi.org/10.3354/meps339001
Serisawa Y, Imoto Z, Ishikawa T, Ohno M (2004) Decline of the Ecklonia cava population associated with increased seawater temperatures in Tosa Bay, southern Japan. Fish Sci 70:189–191. https://doi.org/10.1111/j.0919-9268.2004.00788
Smale DA, Burrows MT, Moore P, O’Connor N., Hawkins S.J. (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3(11):4016–4038. https://doi.org/10.1002/ece3.774
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459. https://doi.org/10.1017/S0376892902000322
Strain E, Thomson RJ, Micheli F, Mancuso FP, Airoldi L (2014) Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob Chang Biol 20:3300–3312. https://doi.org/10.1111/gcb.12619
Tait LW, Schiel RD (2011) Legacy effects of canopy disturbance on ecosystem functioning in macroalgal assemblages. PLoS One 6(10):e26986. https://doi.org/10.1371/journal.pone.0026986
van den Hoek C, Breeman AM (1990) Seaweed biogeography of the North Atlantic: where are we now? In: Garbary DJ, South GR (eds) Evolutionary biogeography of the marine algae of North Atlantic. Springer-Verlag, Berlin, pp 55–86
Wernberg T, Kendrick GA, Toohey BD (2005) Modification of the physical environment by an Ecklonia radiata (Laminariales) canopy and its implications to associated foliose algae. Aquatic Ecol 39:419–430. https://doi.org/10.1007/s10452-005-9009
Wernberg T, Thomsen MS, Tuya F, Kendrick GA (2011) Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. J Exp Mar Biol Ecol 400:264–271. https://doi.org/10.1016/j.jembe.2011.02.017
Wernberg T, Bennett S, Babcock RC, Bettignies T, Cure K, Depczynski M, Dufois F, Fromont J, Fulton CJ, Hovey RK, Harvey ES, Holmes TH, Kendrick GA, Radford B, Santana-Garcon J, Saunders BJ, Smale DA, Thomsen MS, Tuckett CA et al (2016) Climate-driven regime shift of a temperate marine ecosystem. Science 353:6295. https://doi.org/10.1126/science.aad8745
Wikström SA, Kautsky L (2007) Structure and diversity of invertebrate communities in the presence and absence of canopy-forming Fucus vesiculosus in the Baltic Sea. Estuar Coast Shelf Sci 72:168–176. https://doi.org/10.1016/j.ecss.2006.10.009
Acknowledgements
The authors are most grateful to Julen Ibinaga for his invaluable help during the field sampling surveys and would like to thank María Bustamante and Javier Tajadura, the zoologists of the Marine Benthos Research Group at the University of the Basque Country (UPV/EHU), for their valuable support during the field sampling. We are very grateful to the reviewers for their helpful comments and recommendations on improving the quality of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
JMG, ID and NM conceived and designed this research. NM, EQ and JMG conducted data collection and field work. NM analysed data. NM, ID and EQ wrote the manuscript. All authors read the manuscript and contributed to make improvements for approval and submission to Marine Biodiversity.
Additional information
Communicated by A. F. Bernardino
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muguerza, N., Díez, I., Quintano, E. et al. Decades of biomass loss in the shallow rocky subtidal vegetation of the south-eastern Bay of Biscay. Mar. Biodivers. 52, 28 (2022). https://doi.org/10.1007/s12526-022-01268-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-022-01268-2