Abstract
The genus Leptochiton Gray, 1847 is a paraphyletic group of small, pale, and usually deepwater chitons. They lack some of the morphological shell characters that are important in the systematics of Polyplacophora, and species identification within this genus is challenging. One species complex in the North Pacific includes at least 11 species that were historically synonymized or confused with Leptochiton rugatus (Carpenter in Pilsbry, 1892). Scrutiny of ecological and biogeographical patterns has helped to separate species lineages within this clade and support morphological diagnoses. Based on morphological and molecular studies, a new species is described that was previously confused with both Leptochiton assimilis (Thiele, 1909) and Leptochiton rugatus. This new species is widely distributed in the summer-warmed bays of the southern Primorye, Moneron Island, Northern Japan, Kunashir, and the Yellow Sea. This species is at least ~8% different in the COI barcode region from Leptochiton rugatus s.s. Morphological differences between the new species and closely related species are presented.
Similar content being viewed by others
Introduction
Polyplacophoran molluscs, chitons, represent around 1000 living species divided into two distinct orders: Lepidopleurida and Chitonida. Lepidopleuran chitons are mainly found in deepwater environments, but a number of species are distributed in shallow seas and even in the intertidal. This group is characteristically small, pale in color, and a challenge for systematists (Sigwart et al. 2011). Microscopic characters of the shells, girdle scale, and radula are clearly diagnostic and can separate species; however, so far, no morphological diagnosis has been found to support clades within the paraphyletic genus Leptochiton. There are over 120 species of Leptochiton Gray, 1847 and one hotspot for diversity in this genus is the North Pacific and particularly the northwestern Pacific region (e.g., Sirenko 2017).
The species composition of the chiton fauna of the genus Leptochiton in the NW Pacific Ocean from Sagami Bay to the Bering Strait is quite rich (Sirenko 2017). There are eleven known species of this genus in the region (Thiele 1909; Is. Taki 1938; Jakovleva 1952; Klimova and Sirenko 1976; Sirenko 1976, 1978, 1990, 1994, 2013, 2017; Sirenko and Scarlato 1983; Saito 2000, 2017). Of these, five species live in shallow water: Leptochiton assimilis (Thiele, 1909), L. alascensis (Thiele, 1909), L. hakodatensis (Thiele, 1909), L. lukini Sirenko, 1990, and L. rugatus (Carpenter in Pilsbry, 1892). Another six species live at great depths: Leptochiton batialis Sirenko, 1979, L. belknapi Dall, 1878, L. commandorensis Sirenko, 2017, L. fuliginatus (Reeve, 1847), L. incubatus Sirenko, 2017, and L. kaasi Sirenko, 1990. Several of these species have ranges that extend to the northeast Pacific, but understanding the range and biogeography of each species and the group has been confounded by confusion over their taxonomy (Sigwart and Chen 2018).
Many existing names were “lumped” under the epithet Leptochiton rugatus in a major revision (Ferreira 1979). In fact, Leptochiton rugatus represents a widespread species complex. Several of the subsumed names were subsequently recognized as valid, whereas others have required new names. While the species are superficially similar, there are sufficient characteristics to separate them confidently based on morphology where microanatomical descriptions and SEM visualizations are available. The current state of taxonomy is such that every similar Leptochiton specimen is identified as “Leptochiton rugatus” sensu lato, unless it can be matched to one of the lineages that have been recognized since the revision of Ferreira (1979). Thus, we use Leptochiton rugatus s.s. as restricted to material from the coasts of California and Mexico in the region near the type locality in Baja California. However, Leptochiton rugatus s.l. refers to other material which has not yet been confidently associated with a well-described species from the entire range of the species complex across the North Pacific.
Among the NW Pacific Leptochiton fauna, four shallow-water species (L. assimilis, L. alascensis, L. lukini, L. rugatus s.l.) can be considered to belong to a common group based on their morphological characteristics. Members of this “assimilis”-like group have a similar sculpture of the tegmentum in the region of the central areas of the intermediate valves, a radula with small, numerous teeth, and dorsal scales with longitudinal ribs. Several species in this same group are found off the Pacific coast of North America, including at least one additional species: Leptochiton cascadiensis Sigwart & Chen, 2018. The strong similarity in radula and girdle characteristics often led to confusion in defining species. At present, the range attributed to Leptochiton rugatus includes the whole North Pacific, but this is likely not being the true range of the Leptochiton rugatus s.s. Another important point of confusion is the species Leptochiton alascensis, which has an ambiguous type locality (Sigwart and Chen 2018); however, its inclusion here as a valid species in the NW Pacific is recognized based on morphological comparisons with the type material. The type specimens of Leptochiton alascensis represent a diagnosable distinct morphology, although its type locality and therefore its geographic range are not currently known.
Previous work also confused particularly Leptochiton assimilis and L. rugatus, although they have significant differences in the sculpture of the tegmentum, shape, and ribbing of the dorsal scales, and the shape of the central teeth radula, indicating that these are two different species (Kaas and Van Belle 1994). Nonetheless, Ferreira (1979) and later Kaas and Van Belle (1985) decided to refer all Leptochiton rugatus–like specimens found in Asia together with real Leptochiton assimilis to Leptochiton rugatus, the species found off the coast of North America. Leptochiton assimilis was subsequently recognized as a valid species in a later volume of the Monograph of Living Chitons (Kaas and Van Belle 1994). The species described here as new has been conflated with both Leptochiton assimilis (Is. Taki 1938, Jakovleva 1952 and others) and Leptochiton rugatus. At present, prior to the present study, the NE Pacific is home to Leptochiton rugatus s.s., and the NW Pacific Leptochiton fauna includes both L. rugatus s.l. and L. assimilis.
Examination by the first author of the morphological features of both Asian and North American specimens of L. rugatus s.l. revealed several significant and consistent morphological differences, which we describe here. The second author had conducted molecular studies (Sigwart et al. 2011; Sigwart and Chen 2018) which demonstrate that the records of Leptochiton rugatus s.l. found off the coast of Asia are clearly different from the species living off North America. Given the combined evidence of morphological and molecular differences, we can describe it as a new species.
Material and methods
The material was collected for about 90 years in western and eastern parts of the Sea of Japan in Vostok, Peter the Great and Possjet Bays, near Moneron Island, near Kunashir Islands, and in the Yellow Sea and was sent from Hokkaido and Honshu by Hiroshi Saito. A total of 24 samples were collected, and about 250 specimens of this species were identified in the collections of the Zoological Institute of the Russian Academy of Sciences, St. Petersburg (ZISP).
The holotype of the new species and a comparative specimen of Leptochiton rugatus s.s. (identified based on morphological characteristics) from Baja California, Mexico (31° 35′ N, 116° 40′ W, collected by J McLean) were boiled for 5–7 min in 7% KOH solution to remove all organic material. Then the valves I, IV, V, and VIII and half of the girdle cuticle (perinotum and hyponotum) and half of the radula were used for a study with a FEI SEM Quanta 250, while the remaining half of the girdle and radula was put in Canada Balm to be examined under a light microscope. Type specimens are kept in ZISP. Taxonomy is based on Sirenko and Ivanov 2005, Sigwart et al. 2011, and more recently published descriptions of related species.
We used previously prepared shell sections to examine the presence of growth rings in cross section, to determine the approximate age of adult specimens of the new species. We further used data for the cytochrome oxidase subunit 1 (COI) fragment of mitochondrial DNA, which is commonly used as a DNA barcode, to compare specimens we attribute to the new species with other species in the same morphological grouping, based on previously published results (Sigwart et al. 2011; Sigwart 2017; Sigwart and Chen 2018).
Taxonomy
Class Polyplacophora Gray, 1821
Subclass Neoloricata Bergenhayn, 1955
Order Lepidopleurida Thiele, 1909
Family Leptochitonidae Dall, 1889
Genus Leptochiton Gray, 1847
Type species: Chiton cinereus Montagu, 1803 (Linnaeus, 1767) = Leptochiton asellus (Gmelin, 1791) fide Lovén, 1846, subsequent designation by Gray 1847.
Genus distribution: Worldwide, Carboniferous—recent.
Leptochiton subrugatus sp. nov.
http://zoobank.org/8738B668-1264-46D3-A02C-DAC25BB4ACCC (Figs. 1, 2, 3, and 4)
Lepidopleurus assimilis; Is. Taki 1938: 328, pl. XIV, fig. 2 pl. XVI, figs. 5, 9–13, 15, pl. XVII, figs. 9–11; Kuroda and Kinoshita 1951: 7; Huang and Xu 1964: 3; Jakovleva 1952: 55, fig. 16, pl. 1, fig. 4; Klimova and Sirenko 1976: 78, fig. 181; Sirenko 1976: 88 (non-Lepidopleurus assimilis Thiele, 1909, part)
Leptochiton assimilis; Sirenko and Scarlato 1983: 3; Sirenko 1985: 347; Saito 1994: 94; 1995: 100; Saito and Tsuchida 1998: 24; Saito 2000: 5, figs. 1a,b, 2017: 728, pl. 2, fig. 3 (non–Lepidopleurus assimilis Thiele, 1909, part)
Leptochiton (Leptochiton) rugatus; Ferreira 1979; Kaas and Van Belle 1985: 85, fig. 37, map 15; Dell’Angelo et al. 1990: 32, fig. 2, pl. 1, figs. 1–6, pl. 2, figs. 1–7; Sirenko and Ivanov 2005: Xu 2008: 456; Sigwart et al. 2011; Sirenko 2013: 148 (non–Lepidopleurus rugatus Carpenter in Pilsbry, 1892 part)
Leptochiton sp. 2; Sigwart and Chen 2018
Type material: Holotype (ZISP 2385) body length 9.0 mm now disarticulated consisting of SEM stub of valves I, IV, V, VIII, part of perinotum and radula, mount of part of perinotum, radula, and vial with other valves.
Type locality: Sea of Japan, Possjet Bay, Novgorodskaya Inlet, 42° 39′ 50″ N, 130° 40′ 51″ E, 1.2–1.8 m, old shells of oysters.
Etymology: From Leptochiton rugatus which is morphologically most similar.
Material examined: Sea of Japan, Peter the Great Bay, Vostok Bay, 42° 54′ 01″ N, 132° 22′ 13″ E, 1.0 m, 13 spms, body length 5.0–8.0 mm, 21.10.1930; 42° 44′ 30″ N, 132° 50′ 31″ E, 6.0–7.0 m, 1 spm, body length 4.0 mm, 17.08.1980, leg. B. Sirenko; 42° 53′ 02″ N, 132° 43′ 58″ E, 0.5 m, 63 spms, body length 6.5–10.0 mm, 22.08.1980, leg V. Potin; 42° 53′ 04″ N, 132° 43′ 32″ E, 4.0–5.0 m, 102 spms, body length 3.0–9.0 mm, 25.08.1980, leg. B. Sirenko; 42° 54′ 20″ N, 132° 45′ 25″ E, 0.5–2.0 m, 7 spms, body length 6.0–8.5 mm, 03.09.1980, leg. B. Sirenko. 42° 53′ 20″ N, 132° 44′ 05″ E, 1.5–2.5 m, 22 spms, body length 6.0–8.5 mm, 06.08.1981, leg. B. Sirenko; Vityaz Bay, 42° 35′ 52″ N, 131° 09′ 50″ E, 12.0 m, 2 spms, body length 3.5 mm, 19.07.1980, leg A. Kafanov; Possjet Bay, 42° 36′ 54″ N, 130° 51′ 11″ E, 2.0–4.0 m, 10 spms, body length 5.0–10.0 mm, 12.08.1971, leg. B. Sirenko; 42° 38′ 52″ N, 130° 47′ 51″ E, 3.0–3.5 m, 4 spms, body length 6.0–10.5 mm, 11.08.1981, leg. B. Sirenko; 42° 36′ 11″ N, 130° 51′ 04″ E, 7.0–9.0 m, 49 spms, body length 4.0–9.0 mm, 18.08.1981, leg. B. Sirenko; 42° 35′ 44″ N, 130° 51′ 20″ E, 0.7–18.0 m, 39 spms, body length 3.5–9.2 mm, 07.09.1982, leg. B. Sirenko; Moneron Island, 46° 15′ 07″ N, 141° 14′ 08″ E, 0.2 m, 1 spm, body length 10.5 mm, 24.08.1972, leg. O. Kussakin; 46° 15′ 26″ N, 141° 12′ 17″ E, 3–4 m, 5 spms, body length 7.0–11.0 mm, 24.08.1972, leg. B. Sirenko; 46° 14′ 57″ N, 141° 12′ 10″ E, 0–1 m, 1 spm, body length 10.0 mm, 27.08.1972, leg. B. Sirenko; 46° 15′ 14″ N, 141° 12′ 03″ E, 30–32 m, 1 spm, body length 4.0 mm, 30.08.1972, leg. B. Sirenko; 46° 14′ 55″ N, 141° 12′ 15″ E, 5–7 m, 2 spms, body length 10.0 mm, 07.10.1972, leg. B. Sirenko; 46° 15′ 14″ N, 141° 12′ 20″ E, 30 m, 3 spms, body length 5.0–8.0 mm, 08.10.1972, leg. B. Sirenko; 46° 15′ 17″ N, 141° 14′ 10″ E, 20 m, 1 spm, body length 7.0 mm, 15.07.1977, leg. V. Lukin; South Kurile Islands, Kunashir Island, 43° 49′ 02″ N, 145° 24′ 08″ E, 21–26 m, 3 spms, body length 4.0–6.0 mm, 03.07.1969, leg. B. Sirenko; 44° 20′ N, 146° 22′ E, 5–8 m, 1 spm, body length 6.0 mm, 06.09.1972, leg. B. Sirenko; 44° 20′ 01″ N, 146° 01′ 26″ E, 18–20 m, 6 spms, body length 6.0–9.0 mm, 07.09.1972, leg. B. Sirenko; Japan, Hokkaido, near Oshoro, 2.0 m, 10 spms, body length 6.0–8.0 mm, 25.07.1983, leg. H. Hoshikawa; Yellow Sea, near Tsingtao, 36° 03′ 57.78″ N, 120° 22′ 9.80″ E, 1.0–2.0 m, 2 spms, body length 4.0–5.0 mm, 05.12.2016, leg. B. Sirenko.
Diagnosis: Animal of a small size. Shell low elevated, rounded. Intermediate valves almost rectangular. Tail valve distinctly wider than the head valve, mucro anterior, posterior slope almost straight. Tegmentum with rounded oval granules arranged in radial rows in the head valve, in lateral and postmucronal areas, and in longitudinal rows in the central area of intermediate valves and the antemucronal area. Each granule with five pores of aesthetes. Girdle narrow, dorsal scales wide, curved, bluntly pointed with thickened base, and 15–18 longitudinal ribs, ventral scales elongate oval with six–nine longitudinal ribs in upper part. Central teeth of radula long, narrow in the upper part and extended in the lower part, and major lateral teeth with narrow unidentate cusp.
Description: Holotype 9.0 mm. Shell elongate oval, low elevated (elevation ratio in valve V 0.28). Valves thin, rounded, not beaked, side slopes convex, lateral areas not raised, the color of tegmentum white, the surface of tegmentum, and scales covered with creamy environmental deposits.
Head valve semicircular, hind margin V-shaped. Intermediate valves almost rectangular, anterior and posterior margins in valves II–VII nearly straight, lateral margins rounded. Tail valve distinctly wider than head valve, with anterior mucro, antemucronal and postmucronal slopes about straight. The ratio of the width of the tail valve to the width of the head valve 1.14.
Tegmentum sculptured with rounded oval densely spaced granules, arranged in implicit radial rows in the head valve, in lateral and postmucronal areas, and in about 60 more or less distinct longitudinal rows in the central area of intermediate valves and in about 50 longitudinal rows in antemucronal area of the tail valve. Each granule has one megalaesthete and four micraesthetes.
Articulamentum slightly developed, apophyses short, narrow, width of apophyses about 2.5 times less than the width of the jugal sinus. The entire ventral surface of the intermediate valves with numerous pores except for two wedge-shaped thickenings from the apophysis to the apex. Pores arranged in a random manner; at the edges of the valves, 2 and 3 rows of noticeably larger pores.
Girdle narrow, dorsally covered with wide, curved, bluntly pointed scales (75–80 × 60–65 μm near the base) with thickened base and 15–18 longitudinal ribs, those near sutures longer (110 × 35 μm). Intersegmental area with long smooth needles up to 215 × 24 μm. Marginal needles of two kinds: like intersegmental needles but shorter up to 190 × 18 μm and needles (100 × 20 μm) with 16 ribs around. Ventrally girdle covered with elongate pointed scales (70–100 × 28 μm) with 6–9 longitudinal ribs.
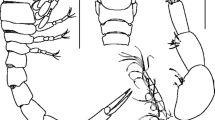
Radula of holotype 3.1 mm long with 115 transverse rows of mature teeth. Central tooth long, narrow in the upper part and extended in the lower part, first lateral tooth long and aliform, major lateral teeth with narrow unidentate cusp.
Twelve long and thin gills per side arranged from valve VI to the anus.
Remarks: The similarity in the structure of the radula, the sculpture of the shell, and the armament of the perinotum among the new species, Leptochiton assimilis and Leptochiton rugatus, has long been the main reason for the mixing of these three species (Taki 1938; Jakovleva 1952; Sirenko 1976; Saito 1994, and others). However, as our research of the available extensive material has shown, we have three good species not only with different morphological features but also with different attitudes to environmental factors.
Leptochiton subrugatus sp. nov. differs from L. rugatus (Figs. 5 and 6) by having always anterior mucro, meaning that the length of the antemucronal area is smaller than the length of postmucronal area (vs mucro median or posterior or length of antemucronal area is equal or larger than the length of postmucronal area in L. rugatus); five aesthete pores in each granule (vs seven pores in each granule in L. rugatus). COI sequences attributed to Leptochiton rugatus s.s. (see Sigwart and Chen 2018) are 7.6–8.6% different in pairwise comparisons to the sequences of the two available specimens of Leptochiton subrugatus sp. nov.
The new species differs from Leptochiton assimilis and Leptochiton alascensis by having bluntly pointed scales with thickened base and 15–18 longitudinal ribs (vs sharply pointed scales with not thickened base and 5 and 7 longitudinal ribs in L. assimilis and L. alascensis); ventral scales with 6 and 7 longitudinal ribs (vs ventral scales smooth in L. assimilis and L. alascensis); and central tooth of the radula long (vs central tooth of radula short in L. assimilis and L. alascensis). There are no published sequences that can be confidently attributed to either of these two species.
Leptochiton alascensis was studied by the senior author (BIS) using syntypes which are kept in ZISP (the label reads: Coll. Russo-amer, Dr Behse, 1856?). Leptochiton alascensis differs from L. subrugatus by having one aesthete in granule (vs five aesthete pores in each granule in L. subrugatus), narrow shape of dorsal scales with 5 and 6 ribs, short central teeth of radula, distribution of granules in head valve in a random manner (vs radial rows of granules in L. subrugatus), and subcarinated valves.
Distribution: The Sea of Japan: Vostok, Peter the Great and Possjet bays, Moneron Island, Southwest Sakhalin, Kunashir Island, Hokkaido and North Honsu, and the Yellow Sea.
Ecology and life history: The new species inhabits depths from 0.2 to 30 m mainly on slightly silted rocky and stony bottoms, often with an admixture of sand. The chitons feed on detritus. In the digestive tract, in addition to detritus, there were a few foraminifera and sand. Females with a body length of more than 5.5 mm and gonads filled with eggs with a diameter of 220–240 μm were observed in early September in the open part of Possjet Bay.
Discussion
Temperature is apparently an important factor in this species’ distribution. Shallow-water lepidopleuran chitons may be tolerant of relatively wide ranges of temperatures in short-term exposure, such as Leptochiton asellus, maintained experimentally over a range from 10 to 20°C (Sigwart et al. 2011); however, tolerance of extremes and overall temperature profile in the long term will control species ranges. The known localities for Leptochiton subrugatus sp. nov. range from –1.7 (in winter) to 24°C (in summer) and a salinity of 28–34, 5%. However, we consider this to be a relatively warmwater species. Although it is distributed in relatively high latitudes, the localities in the northern part of its range depend on the summer warming of shallow waters or the penetration of warmwater currents to the north. In Peter the Great Bay, Leptochiton subrugatus sp. nov. lives at depths of 0.4–18 m, apparently preferring depths of 2–8 m. Moneron Island has the largest depth range of the species, 0.2–30 m, but even there the chitons are most often found at depths of 1–10 m. Near the coast of southwestern Sakhalin, this species lives at depths from 5 to 10 m, and on the Kunashir Island from 5 to 21 m. We speculate that in the latter three areas, warmwater currents facilitate the distributional spread of Leptochiton subrugatus sp. nov. to the north. Although it is not possible to measure directly in this case, it nonetheless seems very likely that the distribution of the species and the settlement of larvae are controlled more by temperature than by depth per se. It is difficult to compare with other species in the genus Leptochiton, but this is commonly found in marine species, where shallow-water species are distributed to much greater depth in warm oceanic basins (e.g., Young et al. 1997).
In the Possjet Bay, the density of settlement of this species can reach up to 100 ind./m2 which equates to a wet weight biomass of up to 1.5 g/m2 as estimated by one of the authors (BIS, unpub. obs.). This is also found in some other shallow-water chitons; for example, Katharina tunicata (Wood, 1815) in the order Chitonida, which is much larger (body size up to 12 cm), can occur at densities up to 100 ind./m2 (Dethier and Duggins 1984). High density is more unusual for lepidopleuran chitons, which usually occur in relatively low abundance. The northeast Pacific lepidopleuran Leptochiton cascadiensis occurs in dense but small and very isolated patches, and this was speculated to be correlated with brooding life history (Sigwart and Chen 2018). In the case of the majority of chitons which are not brooders, high density is associated with high resource provisioning and a strong ecological function of chiton grazing (Dethier and Duggins 1984; Littler et al. 1995).
Despite the small size of the body, the age of Leptochiton subrugatus sp. nov. can reach 10 years, based on our examination of shell thin sections. Their growth rate is apparently low and the age at their maximum body size is 10 and 11 years. Two other large-bodied chitons in the North Pacific have had growth rates estimated using shell rings: maximum age of 17 years for Katharina tunicata and 40 years for Cryptochiton stelleri (Middendorf, 1847), the largest chiton species (Lord 2012). Marine animals have indeterminant growth in terms of volume, yet the rate of expansion of the valves in chitons decreases with age (e.g., Baxter and Jones 1978; Lord and Shanks 2012). The largest specimens of Leptochiton subrugatus sp. nov., with a body length of 10.5–11.0 mm, were collected in well-warmed bays of Peter the Great Bay and at depths of up to 10 m near Moneron Island.
The taxonomic identification of this species resolves one small part of the larger “Leptochiton rugatus” species complex and demonstrates the importance of integrating molecular and morphological evidence in systematics. Molecular sequence data are still lacking for the majority of species in this clade, and for the genus Leptochiton more broadly, which frustrates efforts at wide-scale systematic revision (Sigwart et al. 2011). Comprehensive revision must also include examination of the morphological features from type material of all available names. However, a comprehensive view of all possible members of a clade is not needed to justify the diagnosis and description of a distinct new species. Two specimens of Leptochiton subrugatus sp. nov. were included in previous molecular phylogenetic analyses of Lepidopleurida but previously identified as Leptochiton rugatus (Sigwart et al. 2011). After two other large-scale molecular studies contributed sequence data for Leptochiton rugatus s.l. from the northeast Pacific (Kelly et al. 2007; Layton et al. 2014), it became clear that these represented multiple distinction lineages (Kelly et al. 2007; Sigwart 2017). Three of these can be identified as Leptochiton rugatus s.s., L. cascadiensis, and now L. subrugatus sp. nov.
The material in the present study is an example of this complicated problem. Leptochitons from Vostok Bay, with depths of 2–2.5 m, first were identified as L. assimilis (Sirenko 1976), but later they were reidentified as L. rugatus (Sirenko 1994: pp. 161–162). We now understand that L. assimilis is a cold water chiton that inhabits the Sea of Japan at depths from 40 to 50 m and deeper. Leptochiton subrugatus is a warmwater species and lives in the same area at depths less than 20–30 m (usually 2–8 m).
Untangling the biogeography of the Leptochiton rugatus complex in the North Pacific will require further work and especially molecular data for additional species lineages within this group. From available results, it is clear that the higher latitude lineages are more closely related to each other: Leptochiton subrugatus sp. nov., Leptochiton cascadiensis, and an undescribed species referred to (probably erroneously) as Leptochiton alascensis by Layton et al. (2014), forming a clade (Sigwart 2017). That analysis showed that this high-latitude clade split from Leptochiton rugatus s.s. in the Cretaceous, around 112 million years ago (95% confidence interval: 163.9–65.5 Mya), whereas Leptochiton cascadiensis and Leptochiton subrugatus sp. nov. share a common ancestor dating to around 24 Mya (Sigwart 2017).
The members of this species complex have not only subtly but also distinctly different morphology and clear genetic separation, as well as differing ecology and distribution ranges. Leptochiton subrugatus sp. nov. was previously confused with Leptochiton rugatus s.s. and the two species are indeed extremely similar, which is why molecular sequences and the disjunct geographic ranges are also important parts of the evidence to establish the new species name. Understanding the range restrictions on species is critical for informing conservation actions and predicting the impacts of climate change (e.g., Butt and Gallagher 2018). Chitons, among other invertebrates, are too often neglected in a conservation context (Hochkirch et al. 2020). This issue was highlighted in the description of Leptochiton cascadiensis, because of its patchy restricted distribution where a single localized disturbance event could inadvertently eradicate a local population (Sigwart and Chen 2018). Here, the critical importance of temperature to the distribution of a high-latitude species could also be a cause for concern. Dependency on relatively warm waters in high latitudes does not ensure that a species would be protected from stress of global heating; for example, it may be especially vulnerable to compete with other warmer water species shifting poleward. The previous status quo starting in the 1970s (Ferreira 1979) was to consider all Leptochiton in all coasts across the North Pacific as “Leptochiton rugatus”. Recognizing, and protecting, this group will require ongoing work to highlight the real diversity, one unique species at a time.
References
Baxter IM, Jones AM (1978) Growth and population structure of Lepidochitona cinereus (Mollusca: Polyplacophora) infected with Minchinia chitonis (Protozoa: Sporozoa) at Easthaven, Scotland. Mar Biol 46:305–313
Bergenhayn JRM (1955) Die fossilen schwedischen Loricaten nebst einer vorläufigen Revision der ganzen Klasse Loricata. Kungliga Fysiografiska Sallskapets Handlingar, Lund N.F. 66(8): 1-44 pl. 1-2
Butt N, Gallagher R (2018) Using species traits to guide conservation actions under climate change. Clim Chang 151(2):317–332
Dall WH (1878) Descriptions of new forms of mollusks from Alaska contained in the collections of the National Museum. Proceedings of the US National Museum 1:1–3
Dall WH (1889) Report on the results of dredging, under the supervision of Alexander Agassiz, in the Gulf of Mexico (1877-78) and in the Caribbean Sea (1879-80), by the U.S. coast survey steamer “Blake”, Lieutenant-Commander CD Sigsbee, U.S.N., and Commander JR Bartlett, U.S.N., commanding, 29, Report on the Mollusca, 2, Gastropoda and Scaphopoda. Bulletin of the Museum of Comparative Zoology 18:1–492
Dell’Angelo B, Hong J-S, Van Belle RA (1990) The chiton fauna (Mollusca: Polyplacophora) of Korea, part I: suborder Lepidopleurina and Iscnochitonina. Korean Journal of Systematic Biology 6(1):29–56
Dethier MN, Duggins DO (1984) An indirect commensalism between marine herbivores and the importance of competitive hierarchies. Am Nat 124:205–219
Ferreira AJ (1979) The family Lepidopleuridae (Mollusca: Polyplacophora) in the eastern Pacific. Veliger 22(2):145–165
Gmelin JF (1791) Vermes. In: Gmelin JF (Ed.) Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Ed. 13. Tome 1(6). G.E. Beer, Lipsiae [Leipzig]. pp. 3021-3910.
Gray JE (1847) Additional observations on chitons. Proc Zool Soc London 15:126–127
Hochkirch A, Samways MJ, Gerlach J, Böhm M, Williams P, Cardoso P, Cumberlidge N, Stephenson PJ, Seddon MB, Clausnitzer V, Borges PA (2020) A strategy for the next decade to address data deficiency in neglected biodiversity. Conserv Biol. 35(2):502–509
Huang X, Xu F (1964) Polyplacophora. In: Zhang X, Qi Z, Lou Z, Huang X, Ma X, Liu Y, Xu F (eds) Pictorial guide to animals of China, vol I. Science Press, Beijing, pp 5–10
Jakovleva AM (1952) Pantsyrnye mollyuski morei SSSR, Opredeliteli po faune SSSR (Chitons of the seas of the USSR, guides to the fauna of the USSR). Akad. Nauk SSSR, Moscow, 45
Kaas P, Van Belle RA (1985) Monograph of living chitons. Volume 1. Order Neoloricata: Lepidopleurina. EJ Brill/W Backhuys, Leiden
Kaas P, Van Belle RA (1994) Monograph of living chitons. Volume 5 suborder Ischnochitonina: Ischnochitonidae: Ischnochitoninae, (concluded) Callistoplacinae; Mopaliidae Additions to volumes 1-4. EJ Brill/W Backhuys, Leiden
Kelly RP, Eernisse DJ, Ayre D (2007) Southern hospitality: a latitudinal gradient in gene flow in the marine environment. Evolution 61:700–707
Klimova VL, Sirenko BI (1976) Class Loricata. In: Zhirmunsky AV (ed) Animals and plants of the Peter the Great Bay. Nauka, Leningrad, pp 77–79
Kuroda T, Kinoshita T (1951) A catalogue of marine molluscan shells of Hokkaido. Bulletin of the Hokkaido Regional Fisheries Research Laboratory. Fisheries Agency 2:1–40
Layton KK, Martel AL, Hebert PD (2014) Patterns of DNA barcode variation in Canadian marine molluscs. PLoS One 9(4):e95003
Littler MM, Littler DS, Taylor PR (1995) Selective herbivore increases biomass of its prey: a chiton-coralline reef-building association. Ecology 76(5):1666–1681
Linnaeus C (1767) Systema naturae per regna tria naturae: secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Ed. 12. 1., Regnum Animale. 1 & 2. Holmiae [Stockholm], Laurentii Salvii. pp. 1-532 [1766] pp. 533-1327 [1767]
Lord JP (2012) Longevity and growth rates of the Gumboot chiton, Cryptochiton stelleri, and the black leather Chiton, Katharina tunicata. Malacologia 55(1):43–54
Lord JP, Shanks AL (2012) Continuous growth facilitates feeding and reproduction: impact of size on energy allocation patterns for organisms with indeterminate growth. Mar Biol 159:1417–1428
Lovén SL (1846) Index molluscorum litora Scandinaviae Occidentalia habitantium. Öfvers K Vetensk Akad Förh Stockh 3:134-160
Montagu G (1803) Testacea Britannica, or natural history of British shells, marine, land, and fresh-water, including the most minute: systematically arranged and embellished with figures. - pp. i-xxxvii [= 1-37], [1-2], 1-606, [1-4], Pl. 1-16. London.
Pilsbry HA (1892) Monograph of the Polyplacophora. In: Tryon GW, ed. Manual of Conchology 14:1–128
Reeve L (1847) Descriptions of new species of shells collected in the eastern Archipelago by Capt. Sir Edward Belcher and Mr. Adams during the voyage of HMS Samarang. Proceedings of the Zoological Society. London. 15:24–26
Saito H (1994) The shallow-water chiton fauna of eastern Hokkaido. Mem natn Sci Mus Tokyo (A) 27:93–104
Saito H (1995) The chiton fauna of Onagawa Bay. Mem natn Sci Mus Tokyo (A) 28:99–112
Saito H (2000) Polyplacophora. In: Okutani T (ed) Marine mollusks in Japan. Tokai University Press, Tokyo, pp 4–23
Saito H (2017) Polyplacophora. In: Okutani T (ed) Marine mollusks in Japan, Tokai University Press, Tokyo, pp, vol 46–55, 2nd edn, pp 728–738
Saito H, Tsuchida E (1998) Fauna of marine mollusks of the sea around Otsuchi Bay, Iwate Prefecture (8) Polyplacophora. Otsuchi Mar Res Cent Rep 23:22–35
Sigwart JD (2017) Deep trees: woodfall biodiversity dynamics in present and past oceans. Deep Sea Research Part II: Trop Stud Oceanogr 137:282–287
Sigwart JD, Chen C (2018) Life history, patchy distribution, and patchy taxonomy in a shallow-water invertebrate (Mollusca: Polyplacophora: Lepidopleurida). Mar Biodivers 48(4):1867–1877
Sigwart JD, Schwabe E, Saito H, Samadi S, Giribet G (2011) Evolution in the deep sea: a combined analysis of the earliest diverging living chitons (Mollusca: Polyplacophora: Lepidopleurida). Invertebr Syst 24(6):560–572
Sirenko BI (1976) Chitons from the Vostok Bay (Sea of Japan). In: Kasjanov VL (ed) Biological investigations of Vostok Bay. Institute of Marine Biology, Far Eastern Scientific Center, Vladivostok, pp 87–91
Sirenko BI (1978) On the composition of the family Leptochitonidae Dall, 1889 (= Lepidopleuridae Pilsbry, 1892) (Polyplacophora) with description of a new bathyal species. Proc Zool Inst 80:116–121
Sirenko BI (1979) On the composition of the family Leptochitonidae Dall, 1889 (= Lepidopleuridae Pilsbry, 1892) (Polyplacophora) with description of a new bathyal species. Proceedings of the Zoological Institute, Academy of Sciences USSR 80:116–121
Sirenko BI (1985) Polyplacophora of the shelf of south Sakhalin. In: Golikov AN (ed) Biocoenosis and fauna of the shelf of south Sakhalin, Exploration of the fauna of the seas 30(38):346–367
Sirenko BI (1990) New species of chitons of the genus Leptochiton (Mollusca, Polyplacophora) of the shelf and the slope of the Kurile Islands. Proc Zool Inst 218:96–103
Sirenko BI (1994) Chitons (Polyplacophora) of the continental slope of the Kurile Islands with a brief review of deep water species of the Russian seas. In: Sirenko BI and Vassilenko SV (eds) The fauna of the continental slope of the Kurile Islands, Exploration of the fauna of the seas 46 (54):158–174
Sirenko BI (2013) Class Polyplacophora. In Sirenko BI (ed) Check-list of species of free-living invertebrates of the Russian Far Eastern seas. Exploration of the fauna seas 75(83):148–149
Sirenko BI (2017) Two new species of the genus Leptochiton (Mollusca, Polyplacophora) from north-western Pacific. Rus Journ Mar Biol 43(2):111–117
Sirenko BI, Ivanov DL (2005) Polyplacophora. In Kantor YuI, Sysoev AV. Catalogue of molluscs of Russia and adjacent countries. Moscow: KMK Scientific Press Ltd pp 18–24
Sirenko BI, Scarlato OA (1983) Chitons of the north west Pacific region. La Conchiglia 166–167:3–7
Taki I (1938) Report on the biological survey of Mutsu Bay. 31. Studies on chitons of Mutsu Bay with general discussion on chitons of Japan. Sci Rep Toh Imp Univ, Series 4. Biology 12:323–423
Thiele J (1909) Revision des systems der chitonen, part I. Zoologica, Stuttgart 22:1–70
Xu F (2008) Mollusca: Polyplacophora. In: Liu JY (ed) Checklist of marine biota of China seas, pp 456–459
Young CM, Tyler PA, Fenaux L (1997) Potential for deep sea invasion by Mediterranean shallow-water echinoids: pressure and temperature as stage-specific dispersal barriers. Mar Ecol Prog Ser 154:197–209
Acknowledgements
We are very grateful to Alexey Miroljubov (ZISP) for his technical assistance with SEM procedures and Galina Kuznetsova (ZISP), who prepared the digital plates. The research was completed using equipment of the Core Facilities Centre “Taxon” at the Zoological Institute Russian Academy of Sciences (St Petersburg, Russia). Douglas Eernisse and an anonymous reviewer provided comments that improved an earlier version of this manuscript.
Funding
This work was supported by the State scientific program “Taxonomy, biodiversity and ecology of invertebrates from Russian and adjacent waters of World ocean, continental water bodies and damped areas AAA-A19-119020690072-9.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author contribution
BIS conceived the study. BIS and JDS wrote the manuscript.
Additional information
Communicated by V. Urgorri
This publication is registered in ZooBank: lsid:zoobank.org:pub:A4ACA856-92D9-4999-9B9F-699518140CF3
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/A4ACA856-92D9-4999-9B9F-699518140CF3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sirenko, B.I., Sigwart, J.D. Leptochiton subrugatus sp. nov. (Mollusca: Polyplacophora) from low boreal waters of northern Pacific. Mar. Biodivers. 51, 64 (2021). https://doi.org/10.1007/s12526-021-01190-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01190-z