Abstract
During a recent reef coral survey at the submarine Saba Bank (Eastern Caribbean), an uncommon and diverse assemblage of unattached scleractinian corals (coralliths) was encountered, which has not been reported from the Atlantic before. Four different types of these free-living (unattached) corals were distinguished. They were observed on a relatively flat seafloor (15–20 m deep) with poor coral cover and full exposure to oceanic swell. Much of the substratum was not consolidated and consisted mainly of sand and fragments of branching coralline algae. One of the four types is the (1) anthocyathus stage in the life history of the free-living species Manicina areolata and Meandrina danae. The other three are coralliths formed as ecophenotypic varieties: (2) spheroidal–amoeboidal (= globular and (sub)massive) in Porites astreoides, Siderastrea radians, S. siderea, and Stephanocoenia intersepta; (3) tumbleweed-like (= globular and ramose) in Porites divaricata and P. furcata; and (4) discoidal (flat and circular with short branches) in Madracis decactis and possibly in M. cf. auretenra. This assemblage of free-living corals is likely related to a combination of abiotic factors consisting of wave exposure (swell), depths that waves can reach, a horizontal sea floor with little relief, an unconsolidated substratum, and low coral cover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
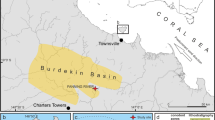
Saba Bank is a large submarine carbonate platform located to the west of the volcanic islands Saba and St. Eustatius, eastern Caribbean, at roughly 17° 30′ N, 63° 30′ W (Fig. 1). It is 65 km long and 40 km wide. Its upper surface is generally flat and slightly tilting (practically horizontal) with depths in eastward direction generally rising from 50 to 15 m. The southeastern ridge reaches up to 12 m depth (Chart 2020, Royal Netherlands Navy), although one source mentions 7 m (Macintyre et al. 1975). This ridge is more exposed to currents than other parts of the bank, while the presence of sand waves on the western part of Saba Bank at 30–40 m depth suggests that the whole platform is wave-swept because of swell (Van der Land 1977). Owing to these conditions, the Saba Bank is not always accessible by divers using SCUBA (self-contained underwater breathing apparatus), which hampers scientific research.
The stony coral fauna (Scleractinia, Milleporidae, Stylasteridae) of Saba Bank was studied during two exploratory expeditions by use of SCUBA, in 1972 (Van der Land 1977) and in 2006 (McKenna and Etnoyer 2010). Coral specimens were sampled as reference material for identification, and they were deposited in museum collections for follow-up studies (Hoeksema et al. 2011). During an earlier expedition in 1971, corals were dredged from 15 to 100 m depths but this did not result in other species records (Macintyre et al. 1975). Stony coral species were also mentioned in a report of a post-hurricane assessment in 1999 (Klomp and Kooistra 2003) and in a report on a benthic survey that took place in 2007 (Toller 2008), but these did not include any remarkable records for Saba Bank. The stony corals of Saba Bank were also subject of more extensive surveys in 1996, 2011, and 2013, which eventually resulted in a total record of 39 species (Meesters et al. 1996; Van Beek and Meesters 2013, 2014).
In addition to bottom depth and substrate, wave energy may be a major factor influencing the variation in species composition on Saba Bank (Van der Land 1977; McKenna and Etnoyer 2010). Water movement can also affect the shape of corals (Todd 2008), but earlier Saba Bank studies did not take that into account and only remarked on the relatively small size of the corals (Macintyre et al. 1975; Van der Land 1977). Available coral collections from Saba Bank (Hoeksema et al. 2011) did not reveal clear ecophenotypic variation as observed in various coral collections from elsewhere (e.g., Wijsman-Best 1974; Best et al. 1984; Hoeksema and Moka 1989; Hoeksema 1993; Amaral 1994; Gittenberger and Hoeksema 2006; Sorauf and Harries 2010; Hoeksema 2012a).
During a recent reef coral survey (November 2015) on Saba Bank, some scleractinian corals were found on unconsolidated substratum and appeared to be free-living (unattached) instead of attached. Stony corals may become detached from their original substratum in various ways, and as such, they are less commonly known from reefs in the Atlantic than in the Indo-Pacific (Hoeksema 1993). Corals of some species developed free-living life history traits, which are most common in the Indo-Pacific, where over 45 of such species are recognized (Best and Hoeksema 1987; Hoeksema 1993; Gittenberger et al. 2011; Benzoni et al. 2012). In the Atlantic, only three recent free-living shallow-water coral species are known, i.e., Manicina areolata (Linneaus, 1758), Meandrina danae (Milne-Edwards and Haime, 1848), and Meandrina brasiliensis (Milne-Edwards and Haime, 1848), forming a remnant of a richer Neogene free-living scleractinian fauna (Klaus et al. 2011, 2013; Pinzón and Weil 2011; Meesters et al. 2013). Free-living corals may also become detached after budding, as seen in most mushroom corals (Kramarsky-Winter and Loya 1996; Gilmour 2004; Hoeksema 2004; Hoeksema and Yeemin 2011) and Goniopora stokesi Milne Edwards and Haime, 1851 on Indo-Pacific reefs (Boschma 1923; Hoeksema and Waheed 2011a), and as observed in various species of deep sea corals (Cairns 1988). Small corals belonging to the genera Heteropsammia (Dendrophylliidae) and Heterocyathus (Caryophylliidae) become free living by entirely overgrowing and incorporating gastropod shells inhabited by a sipunculan worm of the genus Aspidosiphon (Hoeksema and Best 1991; Hoeksema and Matthews 2015; Igawa et al. 2017). Both coral genera are traditionally known from the Indo-Pacific, but Heterocyathus has recently also been found in the Caribbean (Reyes et al. 2009; Santodomingo et al. 2013). Corals that are usually attached to the substrate may become detached by mechanical force or overgrow loose substrate and continue to live as so-called coralliths with a subspherical, globular, or amoeboidal growth form (Glynn 1974; Dullo and Hecht 1990; Roff 2008; Capel et al. 2012; Tortolero-Langarica et al. 2016), also known as circumrotatory or just rotatory corals (Kissling 1973; Sorauf and Harries 2009; Sorauf 2010). The shape of unattached reef corals can also be affected by fragmentation, a means of asexual reproduction that has been observed on Indo-Pacific reefs (Littler et al. 1997; Yamashiro and Nishihira 1998; Feingold 2001; Hoeksema and Gittenberger 2010; Hoeksema and Waheed 2011b), in South Atlantic rock pools (Hoeksema 2012a; Hoeksema and Wirtz 2013), and in deep waters (Cairns 1988). Unattached corals are known to be mobile, in a passive way either by external force (Glynn 1974; Jokiel and Cowdin 1976; Hubman et al. 2002) or by auto-locomotion (Chadwick-Furman and Loya 1992; Yamashiro and Nishihira 1995; Hoeksema and De Voogd 2012; Hoeksema and Bongaerts 2016). All examples above concern scleractinian species (Anthozoa), but fire corals (Hydrozoa: Milleporidae) have also been observed to become detached and continue life as free-living corals, both in the Caribbean (Edmunds 1999; Castro et al. 2006) and in the Indo-Pacific (Razak and Hoeksema 2003).
The free-living coral fauna observed on Saba Bank appeared to consist of corals that were detached either by life history strategy as anthocyathus stage, which is free living as opposed to the attached anthocaulus stage (Wells 1966; Hoeksema 1989: Fig. 42), or by corallith forming after detachment from the substrate. Some of them belonged to species previously unreported to become free living, and one of the observed corallith shapes has not been described before. The present report serves to document this unique assemblage of free-living corals, which may be related to Saba Bank’s physical setting as a wave-swept environment.
Material and methods
The stony coral fauna (Scleractinia, Milleporidae, Stylasteridae) of Saba Bank was surveyed during three SCUBA dives using the roving diver or timed-swim method (ca. 1 h) at 15–25 m depths in November 2015. This is an ideal method for the in situ recording of as many coral species as possible over a large area in a limited time frame, including free-living and small species (Hill and Wilkinson 2004; Hoeksema and Koh 2009). All three dives were at a locality called Tertre de Fleur (17° 23′ 04″ N, 63° 17′ 23″ W), which is situated at the southeastern side of Saba Bank (Fig. 1b).
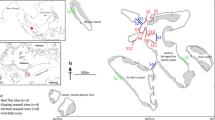
Tertre de Fleur is a disc-like hard bottom carbonate outcrop with a diameter of 600–700 m surrounded by sandy substratum with rubble (21 m depth). The outcrop is slightly elevated (up to 14 m depth) in the centre and smoothly drops to the surrounding sandy plains. It is located approximately 4 km from the steep, swell-exposed eastern edge of the bank (Fig. 1b). The outcrop itself is characterized by a low-relief hard-bottom rugosity colonized by fleshy brown and calcareous algae, sponges, and some corals (Fig. 2). It has a slightly undulating bottom which is interspersed with sand patches and shallow troughs with sand and rubble. Branching red calcareous algae are locally very abundant on Saba Bank (see Van Beek and Meesters 2013). Such algae are brittle, break easily, and become detached, forming maerl deposits and rhodoliths (Peña et al. 2014; Brasileiro et al. 2016; Riosmena-Rodríguez 2017; Sletten et al. 2017). Rhodoliths have been reported earlier from Saba Bank, but they were not taxonomically identified (Littler et al. 2010).
The sea floor at the research site Tertre de Fleur showing low coral cover and only a few large sedimentary animals, like sponges (a) and octocorals (b), among relatively small sessile invertebrates and algae; c 1.5 m2 surface area viewed from above with cover predominantly consisting of sand (sa), branching coralline algae (ca), sponges (sp), and brown algae (ba; scale bar 10 cm)
The sea floor at the dive site was predominantly flat with scarce coral growth. The substratum was not consolidated and consisted mainly of sand (Fig. 2). The stony coral cover (Scleractinia and Milleporidae) measured in 2011 and 2013 at 16 m depth was 2.6 and 1.2%, respectively, which was low compared to 10 other Saba Bank sites (17–30 m deep) with coral cover ranging 3.8–15.6% and 3.1–15.0% in the same years (Van Beek and Meesters 2014). Free-living corals were recorded with their depth, identified, and photographed. Because the number of dives at this site was limited to three during the 2015 survey, there was not sufficient time for the measurement of their densities.
Historical collection material at Naturalis Biodiversity Center (catalogued as RMNH Coel.) was used to verify earlier species records (Van der Land 1977). Coral identifications are based on information given by two field guides (Bright and Lang 2013; Humann and DeLoach 2013).The nomenclature of species mentioned in earlier records is updated according to the World Register of Marine Species (WoRMS; Hoeksema and Cairns 2015).
Results and discussion
The total number of stony coral species (Scleractinia, Milleporidae, Stylasteridae) recorded from Saba Bank is nearly 50 (Table 1). Records of various observation years (Table 1) cannot directly be compared with each other because some concern species names that are currently unaccepted (Hoeksema and Cairns 2015) (like Colpophyllia breviserialis and Dichocoenia stellaris), Brazilian species that previously have not been recorded in the Caribbean (like Favia leptophylla and M. brasiliensis), and species preferring shallow depths (see Roos 1971; Bak 1975) that probably do not occur at Saba Bank, such as Favia fragum, which may resemble juvenile Dichocoenia stokesii. Such records could not be verified without access to collected specimens or photographic documentation. Some congeneric species (e.g., within either Mycetophyllia or Scolymia) may be difficult to distinguish from each other and even from species of related genera (e.g., Wells 1964; Fenner 1993; Budd et al. 2012), which may have resulted in records of misidentified specimens and overestimated species numbers. On the other hand, some small azooxanthellate species may have been overlooked and remained unrecorded, such as two caryophylliids recently found at nearby St. Eustatius (Fig. 1), Colangia immersa Pourtalès, 1871 and Rhizosmilia maculata (Pourtalès, 1874) (Hoeksema and Van Moorsel 2016).
In the present survey, 20 species of scleractinian corals were recorded on Saba Bank with a note mentioning whether they were observed attached or unattached (Table 1). The unattached form was either a coral represented by a free-living anthocyathus stage or a detached coral belonging to as species that is normally attached. Some species have not been recorded before in unattached form. Those represented by coralliths could be categorized in three different ecomorphs, one of which was not distinguished before.
M. danae was represented by a single specimen in anthocyathus stage (Table 2, Fig. 3). It was previously recorded from Saba Bank only once, as M. brasiliensis, by McKenna and Etnoyer (2010), a species that is actually an endemic of Brazil (Pinzón and Weil 2011). M. danae has recently been recorded from the nearby island of St. Eustatius (Hoeksema and Van Moorsel 2016). This species may be rare, but because of its relatively small size, it can easily be overlooked or it can be confused with juveniles of its congener M. meandrites.
Another Caribbean coral with a free-living anthocyathus phase, M. areolata, was not found in 2015, but it was encountered during three previous surveys (Table 1, Fig. 4a–d). In the Saba Bank coral collection of 1972, 33 specimens were present that were collected from 21 to 41 m depths at 12 out of 17 sampling stations. One of these stations (Sta. 142 in Van der Land 1977) was relatively close to the present study site. These large numbers of corals and sampling sites in 1972 suggest that the species was common at that time or that it was a preferred collecting item. Whether the species has become less common at Saba Bank since 1972 is not certain. In 2015, a large specimen of the same species was encountered on coarse sediment at 30 m depth off the adjacent island of St. Eustatius (Fig. 4e). This species has been subject of various studies dealing with its morphology in relation to mobility and sediment rejection (Fabricius 1964; Johnson 1988; Hubman et al. 2002; Uhrin et al. 2005; Sorauf and Harries 2010).
Anthocyathus stage of Manicina areolata. Two corals collected from Saba Bank in 1972 (Van der Land 1977), showing upper and lower surface. a, b RMNH Coel. 8701 from 22 m depth (Sta. 142, 17° 27′ N, 63° 21′ W). c, d RMNH Coel. 8650 from 21 m depth (Sta. 46, 17° 30′ N, 63° 28′ W). e Live specimen off St. Eustatius at 30 m depth on sea floor next to Charles L. Brown shipwreck (17° 27′ 51″ N, 62° 55′ 36″ W), 9 June 2015. Scale bars 1 cm
Corals of four species showed a predominantly spherical or amoeboidal coral shape (Table 2, Fig. 5): Stephanocoenia intersepta (n = 2), Porites astreoides (n = 1), Siderastrea radians (n = 1), and S. siderea (n = 7). Their maximum observed diameter was 5 cm (Fig. 5c, d). Siderastrea radians and S. siderea were previously known to form coralliths (Kissling 1973; Schuhmacher 1976; Lewis 1989; Sorauf and Harries 2009) as well as their congeners S. stellata Verrill, 1868 in Brazil (Lima and Coutinho 2016), S. savignyana Milne Edwards and Haime, 1850 in Madagascar, identified as S. radians by Pichon (1974), and S. glynni Budd and Guzmán, 1994 in the eastern Pacific (Budd and Guzman 1994), which turned out to be a S. siderea population introduced from the Atlantic (Glynn et al. 2016). Specimens of the massive scleractinian P. astreoides in Yucután, Mexico, were observed as epibionts on the axis of gorgonians, which eventually broke off, and in this way also became free living (Rodríguez-Martínez and Jordán-Dahlgren 1999). Other massive Caribbean species known to form coralliths but not found in free-living form during the present study are Solenastrea bournoni and D. stokesii (Kissling 1973). Both species have been recorded from Saba Bank (Table 1). F. fragum has also been mentioned as occurring in corallith shape, but no original source and locality were mentioned (Glynn 1974).
Free-living specimens of two species resembled tumbleweeds by showing long, slender branches directing in various directions, Porites divaricata and P. furcata (Table 2, Fig. 6). Fourteen of such specimens were found, and their maximum observed diameter was 21 cm (Fig. 6f, g). These species have previously not been described as coralliths. Their congener, Porites sverdrupi (Durham, 1947), an endemic of the Gulf of California, has been reported to show a similar kind of ramose corallith but its branches appear to be shorter, less straight, and more compactly arranged (Reyes-Bonilla et al. 1997; López-Pérez 2013; Paz-Garcia and Balart 2016). Examples of tumbleweed coralliths from the Indo-Pacific are, for example, regenerated branch fragments of Acropora (Riegl et al. 1996; Yusuf and Budiyanto 2012), Montipora (Shaish et al. 2010), Pavona (Scoffin et al. 1985), Pocillopora (Glynn 1974; Roff 2008), and Psammocora (Feingold 1996; Denis et al. 2015; Randall 2015).
Twelve out of 18 observed Madracis colonies appeared to be free living. Most of them belonged to Madracis decactis. Such coralliths showed a discoidal form when full grown, with short stubby branches. Their lower and upper surfaces showed less growth than their lateral periphery (Fig. 7). The lower surface was in direct contact with the substrate while the upper surface seemed to suffer from sediment smothering. Smaller coralliths consisted of loose fragments that were not flattened (Fig. 7a, b). The largest specimen was 14 cm wide (Fig. 7g, h). A 11.5-cm wide specimen was 2 cm thick at its centre (Fig. 7i, j). It may have belonged to Madracis auretenra because it was more ochre and not as green as regular M. decactis corals, and although M. auretenra (previously known as Madracis mirabilis) usually has thin, long branches that easily break (Bak and Criens 1981), it may also form thick and short branches (Fenner 1993; Bruno and Edmunds 1997). Both species have corallites with 10 septa and can normally be distinguished by coloration and branch shape (Bright and Lang 2013; Humann and DeLoach 2013). The latter species is commonly known as “yellow pencil coral” (Humann and DeLoach 2013), but it is unknown whether a yellow or ochre coloration is also possible in M. decactis. The flattened upper surface of the Madracis coralliths may be related to sediment accumulation between the short branches on top of the corals. Sediment was reported to negatively affect growth and survival in fragments of branching Madracis at Curaçao (Nagelkerken et al. 2000). In southeastern Brazil, M. decactis has been observed to form subspheroid coralliths up to 15 cm wide (Capel et al. 2012). The shape of discoidal coralliths resembles the form of large free-living mushroom corals (see Hoeksema and Matthews 2011; Hoeksema and Benzoni 2013) and some extinct scleractinian and non-scleractinian corals with analogue shapes (Gill and Coates 1977; Höfling 1989; Webb 1994; Scrutton 1996; Plusquellec et al. 1999; Pandey et al. 2011).
The Saba Bank assemblage of unattached corals consists of at least nine species (Table 2). Two of these are known to be represented by an anthocyathus free-living phase. In the other seven species, a corallith shape appears to happen by way of ecophenotypic variation, which may be most common in Siderastrea spp. Massive coralliths develop by forceful detachment from the sea floor or by overgrowing and incorporating a loose piece of substrate. In branching corals, corallith-forming can be a result of fragmentation and regeneration, which serves as a survival and reproduction strategy (Bak and Criens 1981; Highsmith 1982; Wallace 1985). It can also operate as dispersal mechanism if coral fragments do not self-attach (Guest et al. 2011) or fuse (Heyward and Collins 1985; Nothdurft and Webb 2012). Although a larger proportion of coral cover may be detached from the substrate than appears at first sight, its mobility may depend on how much consolidated the fragments are (Hoeksema 1988).
The occurrence of a multi-species assemblage of coralliths hints at a shared environmental connection. Assemblages of free-living coral species are known as colonizers of sandy substrates (Goreau and Yonge 1968; Fisk 1983; Hoeksema 2012b; Meesters et al. 2013). On the swell-exposed Saba Bank site, all coralliths were encountered on a more or less horizontal carbonate outcrop <20 m deep, where coral growth was limited in size and cover (Fig. 2). Wave force is known to break and dislodge sedentary organisms and also to limit their size (Dollar 1982; Denny et al. 1985; Madin et al. 2014). In various areas, wave action and currents have been recognized as the cause of corallith formation (Scoffin et al. 1985; Roff 2008; Sorauf and Harries 2009; Kersting et al. 2017a, 2017b), although in others places, bioturbation was seen as the driving force (Glynn 1974; Capel et al. 2012). Considering the swell and the apparent absence of burrowing animals, wave action imposed on Saba Bank has most likely caused some corals to break loose and to continue life as coralliths.
The presence of abundant unattached branching red coralline algae, forming rhodoliths and maerl deposits at Saba Bank, is consistent with this observation. Rhodoliths were not reported from similar depths (>15 m) at the more sheltered reefs of the adjacent island St. Eustatius (Van der Loos and Prud’homme van Reine 2016; Van der Loos et al. 2017). Actually a single rhodolith was found here (Van der Loos, pers. comm), which is a rare observation and therefore still consistent with the difference in wave exposure between Saba Bank and St. Eustatius.
Coral colonies are composed of multiple polyps that may continue to multiply by budding until the corallum has reached a maximum size. All observed coralliths show such a modular corallum architecture. Continuous fragmentation and regeneration may enhance their chances of survival (Highsmith 1982) and help them to postpone and perhaps overcome determinate growth caused by size-related physiological constraints (Hoeksema 1991). As unattached and mobile corals, they may undergo sessile dispersal over the sea floor and spread the risk of mortality (Jackson 1986). However, modular growth is not a condition for fragmentation because corals consisting of a single polyp can also break into fragments, regenerate, and continue life as unattached corals (Colley et al. 2002; Hoeksema and Waheed 2011b; Tokuda et al. 2017).
The present report indicates that Saba Bank offers a habitat to a rarely encountered assemblage of corals, which supports the idea that Saba Bank can serve an essential ecological role in the Eastern Caribbean and that it requires further conservation efforts (Hoetjes and Carpenter 2010; De Bakker et al. 2016).
References
Amaral FD (1994) Morphological variation in the reef coral Montastrea cavernosa in Brazil. Coral Reefs 13:113–117. doi:10.1007/BF00300771
Bak RPM (1975) Ecological aspects of the distribution of reef corals in the Netherlands Antilles. Bijdr Dierk 45:181–190
Bak RPM, Criens SR (1981) Survival after fragmentation of colonies of Madracis mirabilis, Acropora palmata and A. cervicornis (Scleractinia) and the subsequent impact of a coral disease. Proc 4th Int Coral Reef Symp 2:221–227
Benzoni F, Arrigoni R, Stefani F, Reijnen BT, Montano S, Hoeksema BW (2012) Phylogenetic position and taxonomy of Cycloseris explanulata and C. wellsi (Scleractinia: Fungiidae): lost mushroom corals find their way home. Contrib Zool 81:125–146
Best MB, Hoeksema BW (1987) New observations on scleractinian corals from Indonesia: 1. Free-living species belonging to the Faviina. Zool Meded 61:387–403
Best MB, Boekschoten GJ, Oosterbaan A (1984) Species concept and ecomorph variation in living and fossil Scleractinia. Palaeontogr Am 54:70–79
Boschma H (1923) Über die Bildung der jungen Kolonien von Goniopora stokesi durch ungeschlechtliche Fortpflanzung. Zool Anz 57:284–286
Brasileiro PS, Pereira-Filho GH, Bahia RG, Abrantes DP, Guimarães SMPB, Moura RL, Francini-Filho RB, Bastos AC, Amado-Filho GM (2016) Macroalgal composition and community structure of the largest rhodolith beds in the world. Mar Biodivers 46:407–420. doi:10.1007/s12526-015-0378-9
Bright T, Lang J (2013) Picture guide to stony corals of glovers reef atoll. Wildlife Conservation Society, New York
Bruno JF, Edmunds PJ (1997) Clonal variation for phenotypic plasticity in the coral Madracis mirabilis. Ecology 78:2177–2190
Budd AF, Guzman HM (1994) Siderastrea glynni, a new species of scleractinian coral (Cnidaria, Anthozoa) from the eastern Pacific. Proc Biol Soc Wash 107:591–599
Budd AF, Fukami H, Smith N, Knowlton N (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool J Linnean Soc 166:465–529
Cairns SD (1988) Asexual reproduction in solitary Scleractinia. Proc 6th Int Coral Reef Symp 2:641–646
Capel KCC, Segal B, Lindner A, Bertuol P (2012) Corallith beds at the edge of the tropical South Atlantic. Coral Reefs 31:75. doi:10.1007/s00338-011-0818-3
Castro C, Monroy M, Solano OD (2006) Estructura de la comunidad epifaunal asociada a colonias de vida libre del hydrocoral Millepora alcicornis Linnaeus, 1758 en Bahía Portete, Caribe Colombiano. Bol Invest Mar Cost 35:195–206
Chadwick-Furman NE, Loya Y (1992) Migration, habitat use, and competition among mobile corals (Scleractinia: Fungiidae) in the Gulf of Eilat, Red Sea. Mar Biol 114:617–623. doi:10.1007/BF00357258
Colley SB, Feingold JS, Peña J, Glynn PW (2002) Reproductive ecology of Diaseris distorta (Michelin) (Fungiidae) in the Galápagos Islands, Ecuador. Proc 9th Int Coral Reef Symp 1:373–379
De Bakker DM, Meesters EHWG, Van Bleijswijk JDL, Luttikhuizen PC, Breeuwer HJAJ, Becking LE (2016) Population genetic structure, abundance, and health status of two dominant benthic species in the Saba Bank National Park, Caribbean Netherlands: Montastraea cavernosa and Xestospongia muta. PLoS One 11:e0155969. doi:10.1371/journal.pone.0155969
Denis V, De Palmas S, Benzoni F, Chen CA (2015) Extension of the known distribution and depth range of the scleractinian coral Psammocora stellata: first record from a Taiwanese mesophotic reef. Mar Biodivers 45:619–620. doi:10.1007/s12526-014-0299-z
Denny MW, Daniel TL, Koehl MAR (1985) Mechanical limits to size in wave-swept organisms. Ecol Monogr 55:69–102. doi:10.2307/1942526
Dollar SJ (1982) Wave stress and coral community structure in Hawaii. Coral Reefs 1:71–81. doi:10.1007/BF00301688
Dullo WC, Hecht C (1990) Corallith growth on submarine alluvial fans. Senckenb Marit 22:77–86
Edmunds PJ (1999) The role of colony morphology and substratum inclination in the success of Millepora alcicornis on shallow coral reefs. Coral Reefs 18:133–140. doi:10.1007/s003380050167
Fabricius F (1964) Active Lage- und Ortveränderung bei der Koloniekoralle Manicina areolata und ihre paläoökologische Bedeutung. Senckenb Lethaea 45:299–323
Feingold JS (1996) Coral survivors of the 1982–83 El Niño-Southern Oscillation, Galapagos Islands, Ecuador. Coral Reefs 15:108. doi:10.1007/BF01771899
Feingold JS (2001) Responses of three coral communities to the 1997–98 El Niño–Southern Oscillation: Galápagos Islands, Ecuador. Bull Mar Sci 69:61–77
Fenner DP (1993) Species distinctions among several Caribbean stony corals. Bull Mar Sci 53:1099–1116
Fisk DA (1983) Free-living corals: distributions according to plant cover, sediments, hydrodynamics, depth and biological factors. Mar Biol 74:287–294. doi:10.1007/BF00403453
Gill GA, Coates AG (1977) Mobility, growth patterns and substrate in some fossil and Recent corals. Lethaia 10(2):119-134
Gilmour JP (2004) Asexual budding in fungiid corals. Coral Reefs 23:595. doi:10.1007/s00338-004-0426-6
Gittenberger A, Hoeksema BW (2006) Phenotypic plasticity revealed by molecular studies on reef corals of Fungia (Cycloseris) spp.(Scleractinia Fungiidae) near river outlets. Contrib Zool 75:195–201
Gittenberger A, Reijnen BT, Hoeksema BW (2011) A molecularly based phylogeny reconstruction of mushroom corals (Scleractinia: Fungiidae) with taxonomic consequences and evolutionary implications for life history traits. Contrib Zool 80:107–132
Glynn PW (1974) Rolling stones amongst the Scleractinia: mobile coralliths in the Gulf of Panama. Proc 2nd Int Coral Reef Symp 2:183–198
Glynn PW, Grassian B, Kleemann KH, Maté JL (2016) The true identity of Siderastrea glynni Budd & Guzmán, 1994, a highly endangered eastern Pacific scleractinian coral. Coral Reefs 35:1399–1404. doi:10.1007/s00338-016-1470-8
Goreau TF, Yonge CM (1968) Coral community on muddy sand. Nature 217:421–423. doi:10.1038/217421a0
Guest JR, Dizon RM, Edwards AJ, Franco C, Gomez ED (2011) How quickly do fragments of coral “self-attach” after transplantation? Restor Ecol 19:234–242. doi:10.1111/j.1526-100X.2009.00562.x
Heyward AJ, Collins JD (1985) Fragmentation in Montipora ramosa: the genet and ramet concept applied to a reef coral. Coral Reefs 4:35–40. doi:10.1007/BF00302202
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hill J, Wilkinson C (2004) Methods for ecological monitoring of coral reefs. Version 1. A resource for managers. Australian Institute of Marine Science, Townsville
Hoeksema BW (1988) Mobility of free-living fungiid corals (Scleractinia), a dispersion mechanism and survival strategy in dynamic reef habitats. Proc 6th Int Coral Reef Symp 2:715–720
Hoeksema BW (1989) Taxonomy, phylogeny and biogeography of mushroom corals (Scleractinia: Fungiidae). Zool Verh 254:1–295
Hoeksema BW (1991) Evolution of body size in mushroom corals (Scleractinia: Fungiidae) and its ecomorphological consequences. Neth J Zool 41:122–139
Hoeksema BW (1993) Phenotypic corallum variability in Recent mobile reef corals. Cour Forsch Inst Senckenb 164:263–272
Hoeksema BW (2004) Impact of budding on free-living corals at East Kalimantan, Indonesia. Coral Reefs 23:492. doi:10.1007/s00338-004-0402-1
Hoeksema BW (2012a) Extreme morphological plasticity enables a free mode of life in Favia gravida at Ascension Island (South Atlantic). Mar Biodivers 42:289–295. doi:10.1007/s12526-011-0106-z
Hoeksema BW (2012b) Evolutionary trends in onshore-offshore distribution patterns of mushroom coral species (Scleractinia: Fungiidae). Contrib Zool 81:199–221
Hoeksema BW, Benzoni F (2013) Multispecies aggregations of mushroom corals in the Gambier Islands, French Polynesia. Coral Reefs 32:1041. doi:10.1007/s00338-013-1054-9
Hoeksema BW, Best MB (1991) New observations on scleractinian corals from Indonesia: 2. Sipunculan-associated species belonging to the genera Heterocyathus and Heteropsammia. Zool Meded 65:221–245
Hoeksema BW, Bongaerts P (2016) Mobility and self-righting by a free-living mushroom coral through pulsed inflation. Mar Biodivers 46:521–524. doi:10.1007/s12526-015-0384-y
Hoeksema B, Cairns S (2015) Scleractinia. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=1363 on 2016–12-20
Hoeksema BW, De Voogd NJ (2012) On the run: free-living mushroom corals avoiding interaction with sponges. Coral Reefs 31:455–459. doi:10.1007/s00338-011-0856-x
Hoeksema BW, Gittenberger A (2010) High densities of mushroom coral fragments at West Halmahera, Indonesia. Coral Reefs 29:691. doi:10.1007/s00338-010-0616-3
Hoeksema BW, Koh EGL (2009) Depauperation of the mushroom coral fauna (Fungiidae) of Singapore (1860s–2006) in changing reef conditions. Raffles Bull Zool Suppl 22:91–101
Hoeksema BW, Matthews JL (2011) Contrasting bleaching patterns in mushroom coral assemblages at Koh Tao, Gulf of Thailand. Coral Reefs 30:95. doi:10.1007/s00338-010-0675-5
Hoeksema BW, Matthews JL (2015) Partial bleaching in an assemblage of small apozooxanthellate corals of the genera Heteropsammia and Heterocyathus. Coral Reefs 34:1227. doi:10.1007/s00338-015-1314-y
Hoeksema BW, Moka W (1989) Species assemblages and ecomorph variation of mushroom corals (Scleractinia: Fungiidae) related to reef habitats in the Flores Sea. Neth J Sea Res 23:149–160. doi:10.1016/0077-7579(89)90009-4
Hoeksema BW, Van Moorsel GWNM (2016) Stony corals of St. Eustatius. In: Hoeksema BW (ed) Marine biodiversity survey of St, Eustatius, Dutch Caribbean, 2015. Naturalis Biodiversity Center, Leiden, pp 32–37
Hoeksema BW, Waheed Z (2011a) Size-dependent dispersal by Goniopora stokesi corals at Semporna, eastern Sabah, Malaysia. Galaxea J Coral Reef Stud 13:9–10. doi:10.3755/galaxea.13.9
Hoeksema BW, Waheed Z (2011b) Initial phase of autotomy in fragmenting Cycloseris corals at Semporna, eastern Sabah, Malaysia. Coral Reefs 30:1087. doi:10.1007/s00338-011-0807-6
Hoeksema BW, Wirtz P (2013) Over 130 years of survival by a small, isolated population of Favia gravida corals at Ascension Island (South Atlantic). Coral Reefs 32:551. doi:10.1007/s00338-012-1002-0
Hoeksema BW, Yeemin T (2011) Late detachment conceals serial budding by the free-living coral Fungia fungites in the Inner Gulf of Thailand. Coral Reefs 30:975. doi:10.1007/s00338-011-0784-9
Hoeksema BW, Van der Land J, Van der Meij SET, Van Ofwegen LP, Reijnen BT, Van Soest RWM, De Voogd NJ (2011) Unforeseen importance of historical collections as baselines to determine biotic change of coral reefs: the Saba Bank case. Mar Ecol 32:135–141. doi:10.1111/j.1439-0485.2011.00434.x
Hoeksema BW, Van Beusekom M, Ten Hove HA, Ivanenko VN, Van der Meij SET, Van Moorsel GWNM (2017) Helioseris cucullata as a host coral at St. Eustatius, Dutch Caribbean. Mar Biodivers 47:71–78. doi:10.1007/s12526-016-0599-6
Hoetjes PC, Carpenter KE (2010) Saving Saba Bank: policy implications of biodiversity studies. PLoS One 5:e10769. doi:10.1371/journal.pone.0010769
Höfling R (1989) Substrate-induced morphotypes and intraspecific variability in Upper Cretaceous scleractinians of the Eastern Alps (West Germany and Austria). Mem Assoc Australas Palaeontol 8:51–60
Hubman B, Piller WE, Riegl B (2002) Functional morphology of coral shape and passive hydrodynamic self-righting in Recent Manicina areolata. Senckenb Lethaea 82:125–130
Humann P, DeLoach N (2013) Reef coral identification: Florida, Caribbean, Bahamas, 3rd edn. New World Publications, Jacksonville
Igawa M, Hata H, Kato M (2017) Reciprocal symbiont sharing in the lodging mutualism between walking corals and sipunculans. PLoS One 12:e0169825. doi:10.1371/journal.pone.0169825
Jackson JBC (1986) Modes of dispersal of clonal benthic invertebrates: consequences for species’ distributions and genetic structure of local populations. Bull Mar Sci 39:588–606
Johnson KG (1988) Size, meander pattern, and behavior in the Caribbean free-living meandroid coral Manicina areolata (Linnaeus). Proc 6th Int Coral Reef Symp 3:403–408
Jokiel PL, Cowdin HP (1976) Hydromechanical adaptation in the solitary free-living coral Fungia scuturia. Nature 262:212–213. doi:10.1038/262212a0
Kersting DK, Cebrian E, Verdura J, Ballesteros E (2017a) Rolling corals in the Mediterranean Sea. Coral Reefs 36:245. doi:10.1007/s00338-016-1498-9
Kersting DK, Cebrian E, Verdura J, Ballesteros E (2017b) A new Cladocora caespitosa population with unique ecological traits. Medit Mar Sci 18:36–42. doi:10.12681/mms.1955
Kissling DL (1973) Circumrotatory growth form in Recent and Silurian corals. In: Boardman RS, Cheetham AH, Oliver WA (eds) Animal colonies: development and function through time. Dowden, Hutchinson, and Ross, Stroudsburg, pp 43–58
Klaus JS, Lutz BP, McNeill DF, Budd AF, Johnson KG, Ishman SE (2011) Rise and fall of Pliocene free-living corals in the Caribbean. Geology 39:375–378. doi:10.1130/G31704
Klaus JS, Murray ST, Swart PK, McNeill DF (2013) Resource partitioning and paleoecology of Neogene free-living corals as determined from skeletal stable isotope composition. Bull Mar Sci 89:937–954. doi:10.5343/bms.2012.1067
Klomp K, Kooistra DJ (2003) A post-hurricane rapid assessment of reefs in the Windward Netherlands Antilles (stony corals, algae and fishes). Atoll Res Bull 496:404–437
Kramarsky-Winter E, Loya Y (1996) Regeneration versus budding in fungiid corals: a trade-off. Mar Ecol Prog Ser 134:179–185. doi:10.3354/meps134179
Lewis JB (1989) Spherical growth in the Caribbean coral Siderastrea radians (Pallas) and its survival in disturbed habitats. Coral Reefs 7:161–167. doi:10.1007/BF00301594
Lima LFO, Coutinho R (2016) The reef coral Siderastrea stellata thriving at its range limit: population structure in Arraial do Cabo, southeastern Brazil. Bull Mar Sci 92:107–121. doi:10.5343/bms.2015.1029
Littler MM, Littler DS, Brooks BL, Koven JF (1997) A unique coral reef formation discovered on the Great Astrolabe Reef, Fiji. Coral Reefs 16:51–54. doi:10.1007/s003380050059
Littler MM, Littler DS, Brooks BL (2010) Marine macroalgal diversity assessment of Saba Bank, Netherlands Antilles. PLoS One 5:e10677. doi:10.1371/journal.pone.0010677
López-Pérez RA (2013) Species composition and morphologic variation of Porites in the Gulf of California. Coral Reefs 32:867–878. doi:10.1007/s00338-013-1031-3
Macintyre IG, Kinsman DJJ, German RC (1975) Geological reconnaissance survey of Saba Bank, Caribbean Sea. Caribb J Sci 15:11–20
Madin JS, Baird AH, Dornelas M, Connolly SR (2014) Mechanical vulnerability explains size-dependent mortality of reef corals. Ecol Lett 17:1008–1015. doi:10.1111/ele.12306
McKenna SA, Etnoyer P (2010) Rapid assessment of stony coral richness and condition on Saba Bank, Netherlands Antilles. PLoS One 5:e10749. doi:10.1371/journal.pone.0010749
Meesters EH, Nijkamp H, Bijvoet L (1996) Towards sustainable management of the Saba Bank. AIDEnvironment, Amsterdam
Meesters EH, Mueller B, Nugues MM (2013) Caribbean free-living coral species co-occurring deep off the windward coast of Curaçao. Coral Reefs 32:109. doi:10.1007/s00338-012-0960-6
Nagelkerken I, Bouma S, van den Akker S, Bak RPM (2000) Growth and survival of unattached Madracis mirabilis fragments transplanted to different reef sites, and the implication for reef rehabilitation. Bull Mar Sci 66:497–505
Nothdurft LD, Webb GE (2012) Fusion or non fusion of coral fragments in Acropora. Geol Belg 15:394–400
Pandey DK, Fürsich FT, Gameil M, Ayoub-Hannaa WS (2011) Aspidiscus cristatus (Lamarck) from the Cenomanian sediments of Wadi Quseib, east Sinai. Egypt. J Palaeontol Soc India 56:29–37
Paz-García DA, Balart EF (2016) New record of the endemic coral Porites sverdrupi (Gulf of California): do fluctuations in seawater temperature regulate its southernmost range limit? Mar Divers 46:499–502. doi:10.1007/s12526-015-0375-z
Peña V, Rousseau F, De Reviers B, Le Gall L (2014) First assessment of the diversity of coralline species forming maerl and rhodoliths in Guadeloupe, Caribbean using an integrative systematic approach. Phytotaxa 190:190–215. doi:10.11646/phytotaxa.190.1.13
Pichon M (1974) Free living scleractinian coral communities in the coral reefs of Madagascar. Proc 2nd Int Coral Reef Symp 2:173–182
Pinzón JH, Weil E (2011) Cryptic species within the Atlantic-Caribbean genus Meandrina (Scleractinia): a multidisciplinary approach and description of the new species Meandrina jacksoni. Bull Mar Sci 87:823–853. doi:10.5343/bms.2010.1085
Plusquellec Y, Webb G, Hoeksema BW (1999) Automobility in Tabulata, Rugosa, and extant scleractinian analogues: stratigraphic and paleogeographic distribution of Paleozoic mobile corals. J Paleontol 73:985–1001. doi:10.1017/S0022336000030936
Randall RH (2015) A new mesophotic branching coral species of Psammocora from the Mariana Islands Archipelago (Cnidaria: Scleractinia: Psammocoridae). Bishop Mus Bull Zool 9:129–146
Razak TB, Hoeksema BW (2003) The hydrocoral genus Millepora (Hydrozoa: Capitata: Milleporidae) in Indonesia. Zool Verh 345:313–336
Reyes J, Santodomingo N, Cairns S (2009) Caryophylliidae (Scleractinia) from the Colombian Caribbean. Zootaxa 2262:1–39
Reyes-Bonilla H, Riosmena-Rodriguez R, Foster MS (1997) Hermatypic corals associated with rhodolith beds in the Gulf of California, Mexico. Pac Sci 51:328–337
Riegl B, Piller WE, Rasser M (1996) Rolling stones: first report of a free living Acropora anthocercis (Brook) from the Red Sea. Coral Reefs 15:149–150. doi:10.1007/BF01145884
Riosmena-Rodríguez R (2017) Natural history of rhodolith/maërl beds: their role in near-shore biodiversity and management. In: Riosmena-Rodríguez R, Nelson W, Aguirr J (eds) Rhodolith/maërl beds: a global perspective. Springer, Switzerland, pp 3–26. doi:10.1007/978-3-319-29315-8_1
Rodríguez-Martínez RE, Jordán-Dahlgren E (1999) Epibiotic and free-living Porites astreoides. Coral Reefs 18:159–161. doi:10.1007/s003380050172
Roff G (2008) Corals on the move: morphological and reproductive strategies of reef flat coralliths. Coral Reefs 27:343–344. doi:10.1007/s00338-007-0344-5
Roos PJ (1971) The shallow-water corals of the Netherlands Antilles. Stud Fauna Curaçao Caribb Isls 130:1–50
Santodomingo N, Reyes J, Flórez P, Chacón-Gómez IC, van Ofwegen LP, Hoeksema BW (2013) Diversity and distribution of azooxanthellate corals in the Colombian Caribbean. Mar Biodivers 43(1):7–22
Schuhmacher H (1976) Korallenriffe, ihre Verbreitung, Tierwelt, und Ökologie. BLV, München
Scoffin TP, Stoddart DR, Tudhope AW, Woodroffe C (1985) Rhodoliths and coralliths of Muri Lagoon, Rarotonga, Cook Islands. Coral Reefs 4:71–80. doi:10.1007/BF00300865
Scrutton CT (1996) Ecophenotypic variation in the early Silurian rugose coral Palaeocyclus porpita. Proc Yorks Geol Soc 51(1):1–8
Shaish L, Levy G, Katzir G, Rinkevich B (2010) Employing a highly fragmented, weedy coral species in reef restoration. Ecol Eng 36:1424–1432. doi:10.1016/j.ecoleng.2010.06.022
Sletten HR, Andrus CFT, Guzmán HM, Halfar J (2017) Re-evaluation of using rhodolith growth patterns for paleoenvironmental reconstruction: An example from the Gulf of Panama. Palaeogeogr Palaeoclimatol Palaeoecol 465:264–277. doi:10.1016/j.palaeo.2016.10.038
Sorauf JE (2010) Colonial form, free-living corals, and macroborers from the Pleistocene of South Florida. Palaeoworld 19:426–434. doi:10.1016/j.palwor.2010.09.007
Sorauf JE, Harries PJ (2009) Rotatory colonies of the corals Siderastrea radians and Solenastraea spp. (Cnidaria, Scleractinia), from the Pleistocene Bermont Formation, south Florida, USA. Palaeontology 52:111–126
Sorauf JE, Harries PJ (2010) Morphologic variation of Manicina areolata (Cnidaria, Scleractinia) from the Pleistocene of south Florida. J Paleontol 84:505–517
Todd PA (2008) Morphological plasticity in scleractinian corals. Biol Rev 83:315–337. doi:10.1111/j.1469-185X.2008.00045.x
Tokuda Y, Haraguchi H, Ezaki Y (2017) First real-time observation of transverse division in azooxanthellate scleractinian corals. Sci Rep 7:41762. doi:10.1038/srep41762
Toller W (2008) Habitat surveys of Saba Bank, Netherlands Antilles: an assessment of benthic communities and fish assemblages. Saba Bank Project Report 2007
Tortolero-Langarica JJA, Rodríguez-Troncoso AP, Carricart-Ganivet JP, Cupul-Magaña AL (2016) Skeletal extension, density and calcification rates of massive free-living coral Porites lobata Dana, 1846. J Exp Mar Biol Ecol 478:68–76. doi:10.1016/j.jembe.2016.02.005
Uhrin AV, Slade CL, Holmquist JF (2005) Self righting in the free-living coral Manicina areolata (Cnidaria: Scleractinia): morphological constraints. Caribb J Sci 40:277–282
Van Beek IJM, Meesters EHWG (2013) Saba Bank research expedition 2011—progress report. IMARES Wageningen UR, Report C018/13, Wageningen
Van Beek IJM, Meesters EHWG (2014) Saba Bank research expedition 2013—progress report. IMARES Wageningen UR, Report C086/14, Wageningen
Van Bruggen AC (2001) Dr Jacob van der Land, marine biologist extraordinary. Zool Verh 334:7–20
Van Bruggen AC, Hoeksema BW (2012) In memoriam Dr Jacob van der Land (1935–2011), late marine biologist at the Leiden museum. Zool Meded 86:505–514
Van der Land J (1977) The Saba Bank—a large atoll in the northeastern Caribbean. FAO Fish Rep 200:469–481
Van der Loos LM, Prud’homme van Reine WF (2016) Macro algae of Statia. In: Hoeksema BW (ed) Marine biodiversity survey of St. Eustatius, Dutch Caribbean, 2015. Naturalis Biodiversity Center, Leiden, and ANEMOON Foundation, Bennebroek, pp 17–22
Van der Loos LM, Prud’homme van Reine WF, Stokvis FR, Speksnijder AGCL, Hoeksema BW (2017) Beta diversity of macroalgal communities around St. Eustatius, Dutch Caribbean. Mar Biodivers 47:123–138. doi:10.1007/s12526-016-0608-9
Wallace CC (1985) Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus Acropora. Mar Biol 88:217–233. doi:10.1007/BF00392585
Webb GE (1994) Benthic auto-mobility in discoid Palaeacis from the Pennsylvanian of the Ardmore Basin, Oklahoma.? J Paleontol 68(02):223–233
Wells JW (1964) The recent solitary mussid scleractinian corals. Zool Meded 9:375–384
Wells JW (1966) Evolutionary development in the scleractinian family Fungiidae. Symp Zool Soc Lond 16:223–246
Wijsman-Best M (1974) Habitat-induced modification of reef corals (Faviidae) and its consequences for taxonomy. Proc 2nd Int Coral Reef Symp 2:217–228
Yamashiro H, Nishihira M (1995) Phototaxis in Fungiidae corals (Scleractinia). Mar Biol 124:461–465. doi:10.1007/BF00363920
Yamashiro H, Nishihira M (1998) Experimental study of growth and asexual reproduction in Diaseris distorta (Michelin, 1843), a free-living fungiid coral. J Exp Mar Biol Ecol 225:253–267. doi:10.1016/S0022-0981(97)00229-3
Yusuf S, Budiyanto A (2012) New records of Acropora russelli (Wallace 1994) from Wallacea area, Indonesia. In: Jompa J, Edyvane K, Malina AC, Lukman M, Amri K (eds) Proceeding of Wallace-Darwin Science Symposium. Identitas Hasanuddin University, Makassar, pp 1–12
Acknowledgements
The authors thank the crew of Caribbean Explorer II for their assistance and support during the field work. The expedition was partly financed by project grant “Saba Bank Marine Biodiversity” (BO-11-019.02-008) from the Netherlands Ministry of Economic Affairs to the third author (EHWGM) and by project grant “Caribbean Coral Reef Ecosystems” (ALW 858.14.020) from the Netherlands Organisation for Scientific Research to the last author (FCvD). This paper is dedicated to the memory of Dr. Jacob van der Land, leader of various large ship-based expeditions (Van Bruggen 2001; Van Bruggen and Hoeksema 2012). The constructive comments provided by two anonymous reviewers and the editor helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Davis Reimer
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hoeksema, B.W., Hassell, D., Meesters, E.H.W.G. et al. Wave-swept coralliths of Saba Bank, Dutch Caribbean. Mar Biodiv 48, 2003–2016 (2018). https://doi.org/10.1007/s12526-017-0712-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0712-5