Abstract
Late Middle Palaeolithic Neandertals in France are known to have engaged in the collection and grinding of black minerals rich in manganese dioxide (MnO2), generally presumed for symbolic use as powdered pigments. However, lab-based experiments conducted by Heyes and colleagues (Sci Rep 6: 22159, 2016) have shown that the addition of powdered MnO2 to wood turnings both reduces the temperature required for combustion by ca. 80–180 °C and significantly increases the rate of combustion. This special pyrotechnic property of powdered MnO2 may have been observed and leveraged by Neandertals to aid in fire making—a technology known to Neandertals in this region by at least 50,000 years ago. To test this idea, a series of actualistic fire-making experiments were performed to determine the practical applicability of MnO2 as a tinder-enhancing additive. The flint-and-pyrite percussive fire-making method was employed to produce sparks that were directed onto eight different types of tinder common to temperate Northwest Europe to determine if and to what degree the addition of MnO2 powder improved their ability to capture sparks that then propagate into glowing embers. The results show that MnO2 does indeed considerably improve the ignition efficiency of tinder material over untreated tinder, both in terms of the point of first ignition and the total number of ignitions achieved. It was observed, however, that the incidental addition of pyrite dust onto a tinder over the course of an experiment also appeared to improve its ability to capture sparks. Supplemental experiments using tinder pre-mixed with powdered pyrite confirmed this hypothesis, suggesting pyrite powder similarly expedites fire production. While this finding may raise questions regarding the need for collecting MnO2 for this purpose, its potential utility may lie in (1) its relative softness compared to pyrite, making it much easier to grind or scrape into powder, and (2) the greater potential for MnO2-bearing deposits to yield larger quantities of usable raw material compared to pyrite-bearing outcrops, making it relatively more abundant in some areas. Thus, when available, it is clear that adding MnO2 to tinder would have noticeably reduced the time and energy required to produce fire, making it a potentially novel Neandertal innovation complementary to the fire-making process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence for early humans utilizing fire as a tool—for processing food and raw materials, modifying their (micro)environment, and for social functions (for a comprehensive list of possible uses for fire and relevant sources, see Rolland 2000)—is largely sporadic until second half of the Middle Pleistocene (roughly 400–300 kya), with combustion traces beginning to appear with increased frequency right around the time the Neandertal morphotype begins to enter the scene (Roebroeks and Villa 2011a; Shimelmitz et al. 2014; Meyer et al. 2016; Zanolli et al. 2018; MacDonald et al. 2021). This upward trend carries on through to the Late Pleistocene, with fire use by Neandertals becoming a (semi-)regular practice up until their disappearance approximately 40 kya (Higham et al. 2014; Djakovic et al. 2022). It is thus not all that controversial to say that Neandertals were competent users of fire. There is debate, however, surrounding the degree and complexity of Neandertal fire use (see Sorensen 2019 and Allué et al. 2022 for an overview).
Beyond the relatively commonplace presence of simple combustion features or fire-affected artifacts at Middle Palaeolithic sites, there are a number of archaeological findings that attest to Neandertal pyrotechnical prowess. These includes the occasional construction of more complex pit hearths or hearths structured with stones (e.g., Goldberg and Bar-Yosef 2002; Meignen et al. 2007; Shahack-Gross et al. 2014; Monnier et al. 2016; Leierer et al. 2020), purposeful burning of the landscape to create conditions favourable for preferred food resources (Pop et al. 2016; Roebroeks et al. 2021), charring the surfaces of wooden tools to make them easier to shape with stone tools (Aranguren et al. 2018), and boiling (as opposed to simply roasting) their food (Henry et al. 2010), the latter possibly suggesting Neandertal use of perishable containers (Speth 2015) and suspension systems for cooking over a fire (Castro-Curel and Carbonell 1995). Another strong indicator of sophisticated use of fire by Neandertals is the series of artifacts suggesting they were producing birch bark tar, an adhesive used as a hafting material for stone tools and generally considered the first “synthetic” material produced by humans (Koller et al. 2001; Mazza et al. 2006; Wragg Sykes 2015; Niekus et al. 2019). The exact methods Neandertals may have use to synthesize birch bark tar are presently unknown, though researchers continue to experiment with different “low-tech” methods that would have been available to Neandertals, with varying levels of complexity and rates of success (Groom et al. 2013; Kozowyk et al. 2017; Schenck and Groom 2018; Schmidt et al. 2019; Koch and Schmidt 2022). Fire may have even served a ritual purpose among some Neandertals, as suggested by the discovery at Bruniquel Cave (France) of two enigmatic circles of broken and stacked stalagmites (~ 400) constructed on the floor of a chamber more than 300 m from the cave entrance, and upon which 20 fires were built (Jaubert et al. 2016).
Perhaps the most fundamental skill necessary for fire mastery among any groups of humans, however, is the ability to produce fire. Whether or not Neandertals possessed this skill remains a contentious issue, largely due to the heavy reliance on proxy evidence for this practice (i.e., the presence/absence or relative abundance of fire traces at Middle Palaeolithic archaeological sites), with some authors believing the evidence for fire use is sufficiently abundant to suggest Neandertals could make fire (Roebroeks and Villa 2011b; Shimelmitz et al. 2014; Sorensen 2017; Sorensen and Scherjon 2018), while others remain sceptical (Sandgathe et al. 2011a, b; Dibble et al. 2017, 2018; Sandgathe 2017; Abdolahzadeh et al. 2022). However, microwear analysis of lithic artifacts appears to provide a means by which to overcome some of these interpretive differences by identifying direct evidence of fire production in the archaeological record, i.e., the fire-making tools themselves—specifically, flint ‘strike-a-lights’ bearing use traces consistent with having been struck against the mineral pyrite to produce sparks (Collin et al. 1991; Stapert and Johansen 1999; Weiner and Floss 2004; Sorensen et al. 2014). A number of such tools have been identified from Middle Palaeolithic contexts in France, including a few dozen Mousterian of Acheulean Tradition (MTA) flint bifaces dating to at least 50 kya (Sorensen et al. 2018), and another possible earlier example (a Levallois point) dating to about 75–85 ka BP (Rots 2011; Sorensen and Rots 2014). While the origins, distribution and continuity of this technology remain nebulous, these findings lend support to the idea that at least some Neandertals were able to produce fire as needed.
MnO2: a pyrotechnological pigment?
Mineral pigments—specifically, the manganese dioxide (MnO2)-rich mineral pyrolusite—may provide an additional twist to the possibility and complexity of Neandertal fire production. These dark-gray to black-colored MnO2 blocks are sometimes found in abundance at various Middle Palaeolithic archaeological sites (Demars 1992; Soressi et al. 2008; Dayet et al. 2014, 2019; Pitarch Martí and d’Errico 2018). Some possess grinding facets or scoring traces that suggest these blocks were being powdered (Fig. 1), possibly for use as body paint (Soressi et al. 2008; Bodu et al. 2014; Pitarch Martí et al. 2019), while rounded edges and facets on softer fragments have been interpreted as their having been used as crayons on skin or hides (Bordes 1972; Soressi and d’Errico 2007). It is not universally accepted that the collection and processing of MnO2 is related to symbolic expression by Neandertals (Chase and Dibble 1987; Velo and Kehoe 1990), as this mineral likely has other practical uses, as well (see Supplementary Information (SI) 1 for a longer discussion of Neandertal pigment use and symbolic thinking).
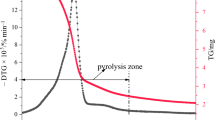
In an effort to test for possible wider applications of powdered mineral materials beyond use as pigments, a series of experiments were performed by Heyes and colleagues (2016) to explore their pyrotechnic properties. Various metal oxide powders (Mn, Fe, Zn, Ti, Al) mixed with beech wood turnings were combusted and monitored via infrared camera and thermogravimetric analysis (TGA). It was found that the addition of MnO2 powder (both commercially produced, and powder produced by grinding blocks recovered during archaeological excavation) uniquely reduced the temperature required for the combustion of the beech wood by ca. 80–180 °C (depending on the ratio of MnO2 powder to wood turnings) and provided a sevenfold increase in the rate of char combustion.
These findings help to elucidate the oxidizing and catalytic properties of MnO2 that promote combustion, thereby highlighting its potential utility for prehistoric fire lighting. However, Heyes and colleagues do not really delve into the real-world practicalities of this application. In some of the experiments performed by the authors, cotton wool or elm seed tinder were used to ignite wood turnings mixed with MnO2 powder, but these tinders were not themselves mixed with MnO2. Thus, in this setup, the wood turnings function more as a fine kindling than as a tinder. So while mixing MnO2 with fine kindling might also be useful, it could be argued that there is more benefit to be gained from instead mixing the MnO2 powder with the tinder. It is here that the most crucial moment in the fire-making process takes place: the initial capture and propagation of a spark into an ember.
Powdered charcoal and gunpowder meal (dust) have been similarly applied to tinder in the past by indigenous peoples (Ray 1885; Segers 1891; Murdoch 1892). This then begs the question: Does adding MnO2 powder to a tinder improve its ability to capture sparks produced using materials and methods available to prehistoric humans (i.e., flint and pyrite)? And if so, is the superior performance of treated over untreated tinder great enough to warrant targeted selection of MnO2 for this purpose by Neandertals?
In a subsequent pilot study, Sorensen (2020) conducted a limited number of actualistic fire-making experiments in a first attempt to test these questions. The results showed shredded tinder fungus (Fomes fomentarius) mixed with MnO2 powder performed noticeably better than untreated tinder, exhibiting increases in both the speed and the frequency at which ignitions were achieved. Given the encouraging results and limited scope of the initial study, it was determined that further exploration was necessary.
Materials and methods
The study presented here attempts to replicate the findings of Sorensen (2020) using an expanded experimental dataset of different tinder types of variable quality to further test the efficacy of MnO2 as a fire-starting aid. The experimental protocol described below closely follows that of the original pilot study.
Tinder types tested
Eight different types of tinder common to various habitats in temperate Northwest Europe were used to test the hypothesis posed by Heyes et al. (2016) that MnO2 powder was potentially used by Neandertals as a tinder additive to improve its fire production capacities. These included three species of tree-dwelling fungi, two terrestrial plant species, one (semi-)aquatic plant species, one tree species, and one species of moss. The fungus species included Fomes fomentarius (SI Fig. 1), Ganoderma adspersum (SI Fig. 2), and Daldinia concentrica (SI Fig. 3). The terrestrial plant species included Cirsium arvense (SI Fig. 4) and Tussilago farfara (SI Fig. 5). The (semi-)aquatic plant, tree and moss species tested were Typha latifolia (SI Fig. 6), Populus nigra (SI Fig. 7) and Brachythecium sp. (SI Fig. 8), respectively. All of the tinders tested in this study were collected in the Netherlands and will hereafter be referred to by their genus names. This selection of tinders is by no means exhaustive, but it does provide a cross-section of easily obtainable tinder materials with different physical attributes and qualities. Moreover, these specific tinders (or those with comparable properties) would likely have been available in Northwest Europe in the past under both warmer interglacial and colder glacial conditions. For more detailed physical and ecological descriptions of the tinders used here (and of tinder, in general), please refer to SI 1.
Tinder preparation
The majority of the tinders selected for this study required little to no processing and could be used as is after being allowed to air-dry at room temperature—in this case, for a number of weeks, but in practice, many of these tinders could have been used shortly after harvesting (SI Figs. 1–8). Most of the residual stems and seed capsules incorporated into the collected mass of Populus seed tufts were removed and discarded. Only the fungi species required minor processing prior to use. While intact fragments of Daldinia are typically used by modern primitive technologists/bushcrafters to make fire, for the sake of consistency and measurability between experiments, the interior portion of the fruiting body was ground into a homogenous powder in a mortar and pestle. For both the Fomes and Ganoderma, the fruiting bodies were bisected with a knife while still fresh to exposed in the inner, velvety trama layers and then allowed to dry (SI Fig. 1). These were then scraped with a flint flake to produce the fluffy masses used as tinder.
The dried and processed tinders were weighed and placed into six Minigrip® bags per tinder type, each containing 0.3 g of material. Three of the six bags (per tinder type) were left as is to serve as the control group. For the experimental group, 0.1 g of commercially manufactured MnO2 powder (specifically, pyrolusite; Sigma-Aldrich, product reference number 310,700) was added to each of the remaining three bags and mixed thoroughly.
Fire-making experiments
Fire-making experiments were conducted using an actualistic approach, using only materials that would have been available to Neandertals. However, various measures were taken to limit variability between experiments. All fire-making experiments were performed by the author indoors at the Leiden University Faculty of Archaeology Laboratory for Artefact Studies to ensure a level of consistency in the environmental conditions between experiments, thus avoiding the variability introduced outdoors by shifts in temperature, relative humidity and wind speeds that can have significant effects on the fire production process and potentially skew the results. Moreover, the ability to maintain relatively low lighting conditions allowed for easier observation of the sparks produced.
For each experiment, one bag of prepared tinder was emptied into a small ceramic bowl (diameter: 4.5 cm; depth: 2.2 cm) to control for the surface area exposed to the sparks (Fig. 2). The tinder was tamped down into the bowl to form a continuous and more or less uniformly thick bed (3–5 mm) to increase connectivity between the tinder fibers and ensure a level consistency between experiments.
The same fire-making set was used throughout the study and consisted of a crested blade strike-a-light crafted from Grand-Pressigny flint (source in Central France) and a halved pyrite nodule (collected from a beach near Cap Gris-Nez, Northwest France). To provide greater manual control, a strong friction gesture was employed in lieu of a percussive striking gesture, whereby the tip of the strike-a-light was forcefully rubbed/scraped in an upwards motion against the broken surface of the pyrite module fragment to produce sparks that were directed into the bowl of tinder (Fig. 2). It should be noted that both elements of the fire-making set had been used previously by the author to make fire. This was done to ensure that the rounded and flattened morphologies of the respective strike-a-light and pyrite contact surfaces would remain largely similar between the first and final experiments. The experiments were conducted over the course of a couple of weeks to ensure fatigue did not create disparities between experiments performed earlier and later in the fire-making sessions.
Experimental program
Six experiments were performed for each tinder type, three treated with MnO2 and three controls, yielding a total of 48 experiments performed for this study. Since not all scrapes of the strike-a-light against the pyrite (hereafter, “strokes”) will necessarily produce sparks, an effort was made to construct an experimental design that would ensure each tinder experiment was exposed to a comparable number sparks. Therefore, each experiment was comprised of 100 spark-producing strokes (i.e., where sparks were observed making contact with the tinder) subdivided into 20 sets of five strokes. The total number of strokes required to achieve this target per set was also recorded but is not particularly important for the analyses (e.g., it may require eight strokes in total to produce sparks five times). The total number of times a spark was captured by the tinder (designated here as “ignitions”) was recorded for each set of five spark-producing strokes. To gauge how the ignition frequency might change over the course of an experiment, the sets were grouped into four groups of five sets and the probability of achieving at least one ignition within each group calculated (P(Ignition)). Table 1 provides a visual representation of the experimental setup and how data was recorded. These data were analyzed and plotted using Microsoft Excel. Figures were produced using Microsoft Excel and Adobe Illustrator.
Results
The addition of MnO2 powder to tinder greatly improves its ability to capture sparks, both in terms of reliability (total ignitions over the course of the experiments) and speed (point of first ignition). Looking at the average results across all tested tinder types for both these parameters, tinder mixed with MnO2 powder proved to be around twice as effective as untreated tinder. A full accounting of the experimental data for the individual tinders tested, along with a detailed comparison of their performance, can be found in SI 1 and SI Table 1. It should be noted that the results obtained in this or similar studies are likely contingent upon the proficiency and experience of the individual conducting the fire-making experiments, as well as the nature and quality of the fire-making tools utilized. Nevertheless, it is reasonable to posit that the relative difference in efficacy between a particular tinder with and without added MnO2 would exhibit consistency across different experimenters.
In terms of total ignitions achieved, the control tinder group produced an average of 8.92 ignitions per 100 spark-producing strokes (range: 1–27.67 ignitions for Brachythecium and Fomes, respectively), while the experimental group produced an average of 17.34 (range: 2.67–46.67 ignitions for Brachythecium and Fomes, respectively), a 1.94x increase in ignitions overall (Fig. 3a), with different tinder types showing increases ranging from 1.57x (Typha) and 2.67x (Brachythecium) (SI Fig. 10). In a normal fire-making scenario, however, it is unlikely that anyone would proceed beyond the first ignition, assuming the attempt at producing flames was ultimately successful. Therefore, it is important to discuss the influence of MnO2 on achieving this crucial first ignition. In this case, the first ignition for the control group was attained after 11.29 sets, or approximately 55 sparks (range: 7.33–17.67 sets for Fomes and Brachythecium, respectively), while the experimental group achieved ignition after 5.33 sets, or approximately 26.67 sparks (range: 1–11.33 sets for Fomes/Ganoderma and Brachythecium, respectively), meaning sparks can be captured, on average, 2.12x faster using MnO2 (Fig. 3b). For the individual tinders tested, the increase in the rate of ignition stemming from the addition of MnO2 varies between 1.32x (Brachythecium) and 9.33x (Ganoderma) (SI Fig. 11).
Comparison of ignition effectiveness between tinder treated with manganese dioxide (MnO2) and untreated tinder. The bar graphs present combined average values for all tinder types tested in terms of (a) the total number of successful ignitions, and (b) the point at which ignition was first achieved (1 Set = 5 spark-producing strokes)
An additional finding of these experiments was that the tinders appeared to become more effective at capturing sparks the longer they were used. This progressive increase in ignition frequency is clearly indicated by the increasing slope of trendlines (line graphs) and by increases in ignition probability (bar graphs) over the course of the experiments, for both the experimental groups treated with MnO2 and the untreated control groups (average of all tinders, Fig. 4; averages of individual tinders, SI Fig. 9). A limited series of supplemental experiments were performed in an effort to explain this phenomenon.
Combined average results of fire-making experiments testing the effects of adding manganese dioxide powder (MnO2) to tinder for all tinder types tested. (a) The line graph plots the average number of successful ignitions per 5 spark-producing strokes (20 sets, 100 strokes per experiment), with trendlines indicating change in ignition frequency over time. (b) The bar graph provides an alternative visualization of these changes in ignition frequency, expressed as the probability of achieving at least 1 ignition within each grouping of 5 sets
Pyrite powder experiments
The increased rate of ignitions over the course of the experiments was presumed to be related to the gradual accumulation of pyrite dust onto the surface of the tinder, based both on personal experience, but also following observations made by Collin and colleagues (1991) and Roussel (2005). However, it could also be due to an increased presence of charred areas in the tinder from previous ignitions, these likely being more accepting of sparks than uncharred material. In an effort to test both of these hypotheses simultaneously, two additional sets of experiments were performed using Fomes and Typha, where pyrite (FeS2) powder (produced by scraping the same pyrite nodule used to produce sparks) was mixed with prepared tinder using the same protocol as for the MnO2 experiments (0.1 g of powder to 0.3 g of tinder).
The experiments appear to show pyrite powder added to tinder indeed improves the fire-making process, often performing even slightly better than tinder treated with MnO2 (Fig. 5; SI Table 1). There was a clear increase in average total ignitions over the controls for both the Fomes (n = 48.67, a 1.76x increase) and Typha (n = 19.33, a 2.76x increase) experiments (Fig. 5a). The point of first ignition was also achieved more quickly by pyrite-treated tinder compared to untreated controls (Fig. 5b), with Fomes only requiring around 1.67 sets (~ 8.33 sparks) and Typha 5.67 (~ 28.35 sparks), while corresponding controls required 7.33 sets (~ 36.67 sparks) and 11 sets (~ 55 sparks). This translates to a 7.33x and 1.94x reduction in the number of sparks needed to start a fire, respectively.
Comparison of ignition effectiveness between tinder treated with powdered pyrite (FeS2), manganese dioxide (MnO2), and untreated tinder. The bar graphs present the average values for two tinder types tested (Fomes fomentarius and Typha latifolia) in terms of (a) the total number of successful ignitions, and (b) the point at which ignition was first achieved (1 Set = 5 spark-producing strokes)
The trend lines and ignition probabilities presented in Fig. 6 demonstrate the rates of ignition again improved over the course of the pyrite experiments. This may provide some support to the idea that the increase in charred surface area in a tinder at least partially contributes to the improved chances for spark capture over time. However, the fact that treated tinder achieved first ignition more quickly prior the accumulation of any charred material strongly suggests the addition of pyrite powder is the main factor influencing the improvement in the tinder performance, both initially and progressively during the fire-making process.
Results of supplemental fire-making experiments testing the effects of adding pyrite powder (FeS2) to tinder (Fomes fomentarius and Typha latifolia), compared to their respective experimental manganese dioxide (MnO2) and control groups. The line graphs plot the average number of successful ignitions per 5 spark-producing strokes (20 sets, 100 strokes per experiment), with trendlines indicating change in ignition frequency over time. The bar graphs provide an alternative visualization of these changes in ignition frequency, expressed as the probability of achieving at least 1 ignition within each grouping of 5 sets
Discussion
In agreement with those produced during an earlier pilot study (Sorensen 2020), the findings of this study confirm the model laid out by Heyes and colleagues (2016) by demonstrating that the addition of MnO2 to a tinder material greatly improves its combustion potential by making it much more effective at quickly and consistently capturing sparks and allowing them to propagate, regardless of tinder type (Figs. 3 and 4; SI Fig. 9). The variability in effectiveness observed between tinder types likely reflects the relative thickness of the individual fibers comprising each tinder type (see SI 1). Coarser tinders require greater and/or longer heat inputs to overcome their respective ignition thresholds, while finer tinder fibers also allow for a greater degree of compactibility, which in turn provides increased connectivity between fibers (i.e., reduced porosity), facilitating ember propagation. In addition to the chemical benefits offered by MnO2, as documented by Heyes and colleagues—namely, a ca. 80–180 °C reduction in the temperature required for combustion and a sevenfold increase in the rate of char combustion—it is suggested here that the powder itself can act as a physical bridge between individual tinder fibers by infilling void space, thereby increasing the connectivity between fibers and allowing an ember to propagate more effectively. Interestingly, this catalytic effect was similarly leveraged in the 19th century to improve the lighting efficiency of cotton lighter wicks, which were impregnated with MnO2 to provide a safer alternative to wicks traditionally impregnated with highly toxic lead chromate (Monier 1876; Moser-Gautrand 1984).
Another finding of the current study was that all of the tinder experiments—with and without added MnO2—showed increased spark capturing capacities as the experiment progressed (Fig. 4; SI Fig. 9). Additional experiments using a subsample of tinders mixed with pyrite powder appear to confirm the hypothesis that this phenomenon is primarily related to the gradual and incidental production of pyrite dust during the fire-making process, which appears to improve the effectiveness of a tinder through physical and possibly chemical means similar to those described above for MnO2 (Figs. 5 and 6). Replicating the experiments and analyses performed by Heyes and colleagues (2016) using pyrite powder would likely shed further light on the chemical mechanisms underlying this process. Localized ignition of the surficial accumulation of pyrite dust appears to extend the life of a spark to the point that it can overcome the initial energy required to ignite the underlying tinder material, as has been surmised in previous fire-making studies (Collin et al. 1991; Roussel 2005) and noted during my own fire-making experience. But to my knowledge, this phenomenon has not been empirically demonstrated until now. Thus, the possible implications of these findings with regard to Neandertal use of MnO2 for fire making are discussed in more detail below.
The results from this study appear to run counter to the recent findings of Langley and Needham (2021), who found that the addition of MnO2 to tinders comprised of birch bark (strips and powdered) or powdered charcoal failed to improve their respective abilities to capture sparks produced by a modern ferrocerium fire-starting rod, which produces sparks with temperatures around 3,000\(\:^\circ\:\)C when scraped with a steel edge—much hotter than those produced by pyrite, estimated to be between ca. 680–780 °C (Weiner 2012) and 760–870 °C (Bois 2004). However, their findings are limited by their lack of success in getting the birch bark and charcoal tinders to ignite at all (both with and without added MnO2). This may be due to poor experimental design and execution. Ethnographic data alludes to the addition of powdered charcoal to tinders like moss, willow catkins or downy bird feathers to improve their efficacy (Ray 1885; Segers 1891; Murdoch 1892), but not charcoal used on its own. Anecdotally, a cursory survey of fire-making videos on YouTube did not yield any instances where charcoal powder was used as a tinder, only occasional examples where whole fragments of charred punk wood were used to capture sparks from a fire-steel or ferrocerium rod. Furthermore, Langley and Needham state they used “ground” or “powdered” birch bark in some of their experiments, but it is unclear how exactly this was produced. Bushcrafters often use with great success the finely shaved/shredded outer, more papery layers of birch bark to start fires with ferrocerium rods, usually achieving ignition within a few strokes. That the authors only managed one ignition out of 30 strokes might speak to either improper preparation of the birch bark tinder (i.e., too coarse), or a lack of experience with the method; a more detailed description of the fire-making gesture used might have shed more light on this issue. Additionally, the relative coarseness of the birch bark and charcoal tinders (at least compared to those tested in this study) may largely negate the physical benefits conferred by MnO2 powder. So in the end, this study does little to validate or refute the model put forward by Heyes and colleagues (2016).
The above findings are applicable to any region or period where MnO2 was being collected by prehistoric peoples (e.g., during the Upper Palaeolithic in Southwest France, see Pitarch-Martí and d’Errico, 2018). However, the archaeological focus of this and previous studies on the Middle Palaeolithic is primarily due to the higher prevalence of MnO2 collection by Neandertals and the ongoing debate surrounding their use of fire (see above). Indeed, the idea that MnO2 may have been collected by Neandertals to aid in fire starting has been challenged by Dibble and colleagues (2018). In their study, the authors compare the relationship between the relative abundance of fire proxy evidence (i.e., heated lithic artifacts) and MnO2 fragments present in different archaeological layers at the Middle Palaeolithic site of Pech de l’Azé IV (Dordogne, France). Figure 8 from this study shows an inverse relationship between heated lithics and the number of MnO2 fragments recovered per layer, with the prevalence of MnO2 rising in layers deposited under colder climatic conditions as the percentages of heated lithics steadily decline. These authors interpret this negative correlation as evidence that the presence of MnO2 is unrelated to fire making, as one would expect a greater prevalence of fire evidence in layers where fires were being produced.
However, this evidence remains open to interpretation and may not be as straightforward as Dibble and colleagues suggest. For starters, it should be mentioned that the majority of the Middle Palaeolithic archaeological layers from sites in Southwest France known to contain MnO2 fragments also exhibit at least some evidence of fire having been used on site (see SI 1 Table 1 in Heyes et al. 2016, and references therein). The trend observed at Pech de l’Azé IV, and similar increases in the prevalence of MnO2 in Quina and Ferrassie Mousterian layers deposited during Marine Isotope Stage (MIS) 4 at Combe Grenal and Caminade (Demars 1992; Dayet et al. 2019), suggest an increased reliance on this material during this colder climatic period that could be interpreted as evidence in support of fire making at these sites using MnO2. Increased pressure to reliably and quickly produce fire during the MIS 4 cold stage could account for the increased presence of MnO2 in these layers. Moreover, limited access to wood fuel may have made fire-making activities more frequent (i.e., due to the reduced ability to retain one’s flame simply through continuously feeding a fire), while fire signals would have nevertheless become weaker through the use of shorter term, task specific fires for the sake of fuel economization (Sorensen 2017). Thus, the apparent relationship between cold climate and MnO2, while not absolute, could provide tacit support for the hypothesis that Neandertals used MnO2 to aid in fire production.
The archaeological focus of the study by Heyes and colleagues (2016) was Layer 4 at Pech de l’Azé I (Dordogne, France), which was deposited under more temperate climatic conditions ca. 50 kya and contains an artifact assemblage attributed to the Mousterian of Acheulean Tradition, or MTA (Soressi 2002; Soressi et al. 2008). This layer not only produced a large number of MnO2 blocks, but also strong fire evidence, including multiple combustion features, an abundance of heated bone fragments and charcoal (Soressi et al. 2008), but interestingly, only a small number of heated lithics (M. Soressi, personal communication), and at least one biface bearing percussive and frictive microwear traces consistent with use as a strike-a-light (Sorensen et al. 2018). This association between the abundance of MnO2 fragments and strong signals of fire use, which runs counter to the apparent inverse relationship between these lines of evidence presented by Dibble and colleagues (2018) for Pech de l’Azé IV, may or may not be related. The high abundance of MnO2—much more than one would need for fire making alone—recovered from this and other MTA layers in Southwest France (e.g., at Le Moustier, Pech de l’Azé IV; Soressi et al. 2008; Pitarch Martí et al. 2019), could signal a retention of this practice by later Neandertal groups, or that these sites may simply have been close to ancient sources of MnO2, allowing for easy aggregation prior to being carried outwards into the landscape.
An alternative and more probable explanation likely lies in the versatility of MnO2, with its ability to aid in fire making being only one of a number of potential uses for this highly valued resource. In addition to the various symbolic and utilitarian functions outlined in the introduction and SI 1, there are two other possible practical applications for MnO2 powder that may have benefitted Neandertals during glacial periods (or under winter conditions, more generally). The first is as an eye-blacking agent, similar to those used by professional athletes today. When smeared under or around the eyes (ideally mixed with animal fat/grease to improve adhesion and spreadability), eye black would have helped to mitigate (snow) glare and improve contrast sensitivity (de Broff and Pahk 2003; Powers 2005), thereby enhancing one’s ability to track moving objects (e.g., prey) on sunny winter days. Such a glare-reduction strategy (but instead, using charcoal) has been recommended for arctic or desert survival situations when suitable eye protection is not available (Wiseman 2009). In a similar vein, smearing the same MnO2 and grease mixture over the entirety of the face could have served the dual purpose of protecting against both sunburn (Rifkin et al. 2015a; b) and chapped skin caused by moisture loss in cold, windy conditions, in the very least, while perhaps even providing a thin layer of insulation against the elements when additionally applied to other unclothed parts of the body (for ethnographic analogues, see Moore 1842; Densmore 1929; Gusinde 1931; Gusinde and Schütze 1937; Chapman 1982).
There are a number of other factors to consider when evaluating the likelihood that MnO2 powder was at least an occasional addition to the Neandertal fire-making kit. Assuming Neandertals did in fact use MnO2 for this purpose, reduced access to higher quality tinder during cold stages may have been a driving factor in the collection of MnO2 during these periods, allowing Neandertals to improve the reliability of less suitable tinders. However, since MnO2 is not a requisite element of the fire-making toolkit—indeed, all tinders tested in this study, even those of poor quality, were able to capture sparks eventually without the addition of MnO2 powder (see SI Fig. 9)—its use for this task may only have been employed when readily available in the landscape, or already on hand from collection for other uses. Moreover, the experimental findings from this study have shown that adding pyrite powder to tinder (either purposefully or incidentally through use) can also expedite the fire-making process, perhaps providing an argument against the need to collect MnO2 for this purpose. Nevertheless, it should be pointed out that pyrite, which is rated 6–6.5/10 on the Moh’s scale of mineral hardness (Mindat 2022a), is much harder and therefore more difficult to grind into powder than the softer, fine-grained blocks of MnO2 usually collected by Neandertals, with pyrolusite in its massive (as opposed to crystalline) form rating ~ 2/10 on the Moh’s scale (Mindat 2022b). MnO2-bearing deposits, of which there are numerous known occurrences in Southwest France, can sometimes yield large quantities of material see Pitarch Martí et al. 2019). Given the necessity of pyrite in the fire-making process and its relative sparseness in some landscapes, grinding MnO2 over tinder may have allowed Neandertals to limit attrition to their pyrite by reducing the number of strikes needed to capture a spark, thereby extending the use-life of the pyrite.
Conclusion
To sum up, previous studies have provided evidence suggesting the following:
-
1)
Neandertals were regular and proficient users of fire;
-
2)
they were occasional and, at times, avid collectors of MnO2-rich rocks;
-
3)
some of these MnO2-rich rocks exhibit use wear traces indicating they had been ground or scraped to produce powder;
-
4)
the addition of MnO2 powder to a tinder material accelerates its combustion under controlled laboratory conditions;
-
5)
Neandertals were capable of producing fire using flint and pyrite.
The aim of this article has been to explore the next logical question in this sequence: How effective is MnO2-treated tinder at capturing sparks during actualistic percussive fire-making experiments? The results of these experiments definitively show MnO2 powder improves the spark capturing efficiency of treated over untreated tinder, regardless of the type and relative quality of the tinder, thereby reducing the time and energy required to produce fire. It was also observed that both treated and untreated tinders became more receptive of sparks as the experiment progressed, a phenomenon presumed to be related to pyrite dust being gradually incorporated into the tinder. This was confirmed through additional experiments that showed pre-mixing pyrite powder into tinder also greatly facilitated spark capture and propagation. While this finding could be used to argue against fire making being the primary motivation for collecting MnO2, its relative softness compared to pyrite greatly facilitates powdering, making it an attractive (and effective) alternative for this purpose. Moreover, the addition of powdered materials to tinder to facilitate combustion (e.g., charcoal, gunpowder meal) is a known strategy for increasing fire-making success among various indigenous groups, lending tacit support to the idea that MnO2 may have been used similarly in the past. At the moment, however, it remains difficult to say definitively that Neandertals used MnO2 powder as a tinder-enhancing agent based on currently available archaeological data. Indeed, it is difficult to surmise what specific set of evidences or conditions would be required to do so. But given the pyrotechnical prowess of Neandertals, their long history of collecting and powdering MnO2-rich rocks, and the intersection of these two aspects of Neandertal daily life at numerous Middle Palaeolithic sites, it seems entirely reasonable to presume that they cultivated through experimentation and observation an intimate knowledge of the various applications of MnO2, including its use as a tinder additive that improved their chances for quickly and easily producing fire.
Data availability
All relevant data are provided in the manuscript and Supplementary Information.
Code availability
Not applicable.
References
Abdolahzadeh A, McPherron SP, Sandgathe DM et al (2022) Investigating variability in the frequency of fire use in the archaeological record of late Pleistocene Europe. Archaeol Anthropol Sci 14:62. https://doi.org/10.1007/S12520-022-01526-1
Allué E, Mallol C, Aldeias V et al (2022) Fire among neanderthals. Updating neanderthals. Elsevier, pp 227–249
Aranguren B, Revedin A, Amico N et al (2018) Wooden tools and fire technology in the early neanderthal site of Poggetti Vecchi (Italy). Proc Natl Acad Sci U S A 115:2054–2059
Bodu P, Salomon H, Leroyer M et al (2014) An open-air site from the recent Middle Palaeolithic in the Paris Basin (France): Les Bossats at Ormesson (Seine-et-Marne). Quat Int 331:39–59. https://doi.org/10.1016/j.quaint.2013.10.029
Bois S (2004) Principe et fonctionnement d’un briquet médiéval. Maîtrise De Physique. Université Claude Bernard, Lyon, p 26
Bordes F (1972) A tale of two caves. Harper and Row, New York
Castro-Curel Z, Carbonell E (1995) Wood Pseudomorphs from Level I at Abric Romani, Barcelona, Spain. J Field Archaeol 22:376–384. https://doi.org/10.1179/009346995791974206
Chapman A (1982) Drama and power in a hunting society: the Selk’nam of Tierra Del Fuego. Cambridge University Press, Cambridge
Chase PG, Dibble HL (1987) Middle paleolithic symbolism: a review of current evidence and interpretations. J Anthropol Archaeol 6:263–296. https://doi.org/10.1016/0278-4165(87)90003-1
Collin F, Mattart D, Pirnay L, Speckens J (1991) L’obtention Du Feu par percussion: approche expérimentale et tracéologique. Bull Des Chercheurs De La Wallonie 31:19–49
Dayet L, d’Errico F, Garcia-Moreno R (2014) Searching for consistencies in Châtelperronian pigment use. J Archaeol Sci 44:180–193. https://doi.org/10.1016/j.jas.2014.01.032
Dayet L, Faivre J-P, le Bourdonnec F-X et al (2019) Manganese and iron oxide use at Combe-Grenal (Dordogne, France): a proxy for cultural change in neanderthal communities. J Archaeol Sci Rep 25:239–256. https://doi.org/10.1016/j.jasrep.2019.03.027
de Broff BM, Pahk PJ (2003) The ability of periorbitally applied antiglare products to improve contrast sensitivity in conditions of sunlight exposure. Arch Ophthalmol 121:997–1001
Demars PY (1992) Les colorants dans le Moustérien Du Périgord. L’apport Des fouilles de F. Bordes. Bull Soc Préhist Fr 47:185–194
Densmore F (1929) Chippewa customs. United States Government Printing Office, Washington
Dibble HL, Abodolahzadeh A, Aldeias V et al (2017) How did Hominins Adapt to Ice Age Europe without Fire? Curr Anthropol 58:S278–S287. https://doi.org/10.1086/692628
Dibble HL, Sandgathe D, Goldberg P et al (2018) Were western European neandertals able to make fire? J Paleolithic Archaeol 1:54–79. https://doi.org/10.1007/s41982-017-0002-6
Djakovic I, Key A, Soressi M (2022) Optimal linear estimation models predict 1400–2900 years of overlap between Homo sapiens and Neandertals prior to their disappearance from France and northern Spain. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-19162-z
Goldberg P, Bar-Yosef O (2002) Site formation processes in Kebara and Hayonim caves and their significance in levantine prehistoric caves. Neandertals and modern humans in Western Asia. Springer, pp 107–125
Groom P, Schenck T, Pedersen GM (2013) Experimental explorations into the aceramic dry distillation of Betula pubescens (downy birch) bark tar. Archaeol Anthropol Sci 7:47–58. https://doi.org/10.1007/s12520-013-0144-5
Gusinde M (1931) The Fuegian indians: volume 1. The Selk’nam, on the life and thought of a Hunting people of the Great Island of Tierra Del Fuego. Verlag Der Internationalen Zeitschrift, Mödling Bei Wien. eHRAF World Cultures. http://ehrafworldcultures.yale.edu
Gusinde M, Schütze F (1937) The Fuegian indians: volume 2. The Yahgan: the life and thought of the Water nomads of Cape Horn. Anthropos-Bibliothek, Mödling Bei Wein. eHRAF World Cultures. http://ehrafworldcul-tures.yale.edu
Henry AG, Brooks AS, Piperno DR (2010) Microfossils in calculus demonstrate consumption of plants and cooked foods in neanderthal diets (Shanidar III, Iraq; spy I and II, Belgium). Proc Natl Acad Sci U S A 108:486–491. https://doi.org/10.1073/pnas.1016868108
Heyes PJ, Anastasakis K, de Jong W et al (2016) Selection and use of Manganese Dioxide by neanderthals. Sci Rep 6:22159. https://doi.org/10.1038/srep22159
Higham T, Douka K, Wood R et al (2014) The timing and spatiotemporal patterning of neanderthal disappearance. Nature 512:306–309. https://doi.org/10.1038/nature13621
Jaubert J, Verheyden S, Genty D et al (2016) Early neanderthal constructions deep in Bruniquel Cave in southwestern France. Nature 534:111–114. https://doi.org/10.1038/NATURE18291
Koch TJ, Schmidt P (2022) A new method for birch tar making with materials available in the Stone Age. Sci Rep 12:1–8. https://doi.org/10.1038/s41598-021-04161-3
Koller J, Baumer U, Mania D (2001) High-tech in the middle Palaeolithic: neandertal-manufactured pitch identified. Eur J Archaeol 4:385–397. https://doi.org/10.1179/EJA.2001.4.3.385
Kozowyk PRB, Soressi M, Pomstra D, Langejans GHJ (2017) Experimental methods for the palaeolithic dry distillation of birch bark: implications for the origin and development of neandertal adhesive technology. Sci Rep 7:8033. https://doi.org/10.1038/s41598-017-08106-7
Langley A, Needham A (2021) A spark of inspiration: experimentally testing manganese Dioxide as a fire lighting aide. EXARC Journal 2021/1https://doi.org/https://exarc.net/ark:/88735/10557
Leierer L, Carrancho Alonso Á, Pérez L et al (2020) It’s getting hot in here – microcontextual study of a potential pit hearth at the Middle paleolithic site of El Salt, Spain. J Archaeol Sci 123:105237. https://doi.org/10.1016/J.JAS.2020.105237
MacDonald K, Scherjon F, van Veen E et al (2021) Middle Pleistocene fire use: the first signal of widespread cultural diffusion in human evolution. Proc Natl Acad Sci U S A 118:e2101108118. https://doi.org/10.1073/PNAS.2101108118
Mazza PPA, Martini F, Sala B et al (2006) A new palaeolithic discovery: tar-hafted stone tools in a European mid-pleistocene bone-bearing bed. J Archaeol Sci 33:1310–1318. https://doi.org/10.1016/j.jas.2006.01.006
Meignen L, Goldberg P, Bar-Yosef O (2007) The hearths at Kebara Cave and their role in site formation processes. In: Bar-Yosef O, Meignen L (eds) Kebara Cave, Mt. Carmel, Israel the Middle and Upper Paleolithic Archaeology. Part 1. American School of Prehistoric Research, Peabody Museum, pp 91–122
Meyer M, Arsuaga J-L, de Filippo C et al (2016) Nuclear DNA sequences from the Middle Pleistocene Sima De Los Huesos hominins. Nature 531:504–507. https://doi.org/10.1038/nature17405
Mindat (2022b) Pyrolusite. https://www.mindat.org/min-3318.html. Accessed 19 May 2022
Mindat (2022a) Pyrite. https://www.mindat.org/min-3314.html. Accessed 19 May 2022
Monier E (1876) Note sur un nouveau procédé pour préparer les mèches à Briquet, sans substances vénéneuses. C R Hebd Seances Acad Sci 83:386
Monnier J-L, Ravon A-L, Hinguant S et al (2016) Menez-Dregan 1 (Plouhinec, Finistère, France): un site D’habitat Du Paléolithique inférieur en grotte marine. Stratigraphie, structures de combustion, industries riches en galets aménagés. Anthropologie 120:237–262. https://doi.org/10.1016/j.anthro.2016.05.003
Moore GF (1842) A descriptive vocabulary of the Language in Common Use Amongst the aborigines of Western Australia. Wm. S. Orr & Company, London
Moser-Gautrand C (1984) Créer, conserver, transporter Le Feu. Les briquets. Musée Ernest Rupin, Brive
Murdoch J (1892) Ethnological results of the Point Barrow expedition. In: Ninth Annual Report of the Bureau of Ethnology, 1887–1888. Smithsonian Institution, Washington, DC, pp 19–441
Niekus MJLT, Kozowyk PRB, Langejans GHJ et al (2019) Middle paleolithic complex technology and a neandertal tar-backed tool from the Dutch North Sea. Proc Natl Acad Sci U S A 116:22081–22087. https://doi.org/10.1073/pnas.1907828116
Pitarch Martí A, d’Errico F (2018) Seeking black. Geochemical characterization by PIXE of palaeolithic manganese-rich lumps and their potential sources. J Anthropol Archaeol 50:54–68. https://doi.org/10.1016/j.jaa.2018.03.004
Pitarch Martí A, d’Errico F, Turq A et al (2019) Provenance, modification and use of manganese-rich rocks at Le Moustier (Dordogne, France). PLoS ONE 14:e0218568. https://doi.org/10.1371/journal.pone.0218568
Pop E, Kuijper W, van Hees E et al (2016) Fires at Neumark-Nord 2, Germany: an analysis of fire proxies from a last Interglacial Middle Palaeolithic basin site. J Field Archaeol 41:603–617. https://doi.org/10.1080/00934690.2016.1208518
Powers BR (2005) Why do athletes Use Eye Black? Inq J 10. https://doi.org/https://scholars.unh.edu/inquiry_2005/10
Ray PH (1885) Ethnographic sketch of the natives of Point Barrow. In: Ray PH, Murdoch J, Riley CV et al (eds) Report of the International Polar Expedition to Point Barrow, Alaska: in response to the resolution of the [US] House of Representatives of December 11. U.S. Government Printing Office, Washington D.C., pp 35–88, p 1884
Rifkin RF, Dayet L, Queffelec A et al (2015a) Evaluating the Photoprotective effects of Ochre on Human skin by in vivo SPF Assessment: implications for human evolution, adaptation and dispersal. PLoS ONE 10:e0136090. https://doi.org/10.1371/JOURNAL.PONE.0136090
Rifkin RF, D’Errico F, Dayet-Boulliot L, Summers B (2015b) Assessing the photoprotective effects of red ochre on human skin by in vitro laboratory experiments. S Afr J Sci 111:1–8. https://doi.org/10.17159/SAJS.2015/20140202
Roebroeks W, Villa P (2011a) On the earliest evidence for habitual use of fire in Europe. Proc Natl Acad Sci U S A 108:5209–5214. https://doi.org/10.1073/pnas.1018116108
Roebroeks W, Villa P (2011b) Reply to Sandgathe et al.: neandertal use of fire. Proc Natl Acad Sci U S A 108:E299. https://doi.org/10.1073/PNAS.1108129108
Roebroeks W, MacDonald K, Scherjon F et al (2021) Landscape modification by last interglacial neanderthals. Sci Adv 7:eabj5567. https://doi.org/10.1126/sciadv.abj5567
Rolland N (2000) Cave Occupation, Fire-making, Hominid/Carnivore Coevolution, and Middle Pleistocene Emergence of Home-Base Settlement systems. Acta Anthropologica Sinica 19:209–217
Rots V (2011) Tool use and hafting in the western European Middle Palaeolithic. In: Toussaint G, Di Modica K, Pirson S (eds) Le Paléolithique Moyen en Belgique. Mélanges Marguerite Ulrix-Closset. ERAUL 128. University of Liège, Liège, pp 277–287
Roussel B (2005) La production Du Feu par percussion de la pierre: Préhistoire, ethnographie, expérimentation. Editions Monique Mergoil, Montagnac
Sandgathe DM (2017) Identifying and describing pattern and process in the evolution of hominin use of fire. Curr Anthropol 58:S360–S370. https://doi.org/10.1086/691459
Sandgathe DM, Dibble HL, Goldberg P et al (2011a) Timing of the appearance of habitual fire use. Proc Natl Acad Sci U S A 108:E298. https://doi.org/10.1073/pnas.1106759108
Sandgathe DM, Dibble HL, Goldberg P et al (2011b) On the Role of Fire in Neandertal Adaptations in Western Europe: Evidence from Pech de l’Azé and Roc de Marsal, France. PaleoAnthropology 216–242. https://doi.org/10.4207/PA.2011.ART54
Schenck T, Groom P (2018) The aceramic production of Betula pubescens (downy birch) bark tar using simple raised structures. A viable neanderthal technique? Archaeol Anthropol Sci 10:19–29. https://doi.org/10.1007/s12520-016-0327-y
Schmidt P, Blessing M, Rageot M et al (2019) Birch tar production does not prove neanderthal behavioral complexity. Proc Natl Acad Sci U S A 116:17707–17711. https://doi.org/10.1073/PNAS.1911137116
Segers PA (1891) Tierra Del Fuego: Hábitos Y Costumbres De Los Indios Aonas. Bol Del Instituto Geográfico Argentino 12:56–82
Shahack-Gross R, Berna F, Karkanas P et al (2014) Evidence for the repeated use of a central hearth at Middle Pleistocene (300 ky ago) Qesem Cave, Israel. J Archaeol Sci 44:12–21. https://doi.org/10.1016/j.jas.2013.11.015
Shimelmitz R, Kuhn SL, Jelinek AJ et al (2014) Fire at will: the emergence of habitual fire use 350,000 years ago. J Hum Evol 77:196–203. https://doi.org/10.1016/j.jhevol.2014.07.005
Sorensen AC (2017) On the relationship between climate and Neandertal fire use during the last glacial in south-west France. Quat Int 436:114–128. https://doi.org/10.1016/j.quaint.2016.10.003
Sorensen AC (2019) The Uncertain origins of Fire-making by humans: the state of art and smouldering questions. Mitteilungen Der Gesellschaft für Urgeschichte 28:11–50. http://mgfuopenaccess.org
Sorensen AC (2020) Neandertal advice for improving your tinder profile: a pilot study using experimental archaeology to test the usefulness of manganese dioxide (MnO2) in palaeolithic fire-making. In: Klinkenberg V, van Oosten R, van Driel-Murray C (eds) A human environment: studies in honour of 20 years Analecta editorship by prof. dr. Corrie Bakels, Analecta P. Sidestone, Leiden, Netherlands, pp 29–38. https://www.sidestone.com/books/a-human-environment
Sorensen AC, Rots V (2014) Testing the ‘expedient strike-a-light model’: An experimental assessment based on the first identified Middle Palaeolithic fire-maker from Bettencourt (France). Union Internationale des Sciences Préhistoriques et Protohistoriques (UISPP) XVII 1005–1006
Sorensen AC, Scherjon F (2018) fiReproxies: a computational model providing insight into heat-affected archaeological lithic assemblages. PLoS ONE 13:e0196777. https://doi.org/10.1371/journal.pone.0196777
Sorensen AC, Roebroeks W, van Gijn A (2014) Fire production in the deep past? The expedient strike-a-light model. J Archaeol Sci 42:476–486. https://doi.org/10.1016/j.jas.2013.11.032
Sorensen AC, Claud E, Soressi M (2018) Neandertal fire-making technology inferred from microwear analysis. Sci Rep 8:10065. https://doi.org/10.1038/s41598-018-28342-9
Soressi M (2002) Le Moustérien de tradition Acheuléenne du Sud-Ouest de la France. Discussion sur la signification du faciès à partir de l’étude comparée de quatre sites: Pech-de-l’Azé I, Le Moustier, La Rochette et la Grotte XVI. Doctoral dissertation, University of Bordeaux I
Soressi M, d’Errico F (2007) Pigments, gravures, parures: les comportements symboliques controversés des Néandertaliens. In: Vandermeersch B, Maureille B (eds) Les Néandertaliens. Biologie et cultures. Comité des Travaux Historiques et Scientifiques (Documents Préhistoriques 23), Paris, pp 297–309
Soressi M, Rendu W, Texier J-P et al (2008) Pech-de-l’Azé I (Dordogne, France): nouveau regard sur un gisement moustérien de tradition acheuléenne connu depuis le XIX siècle. In: Jaubert J, Bordes J-G, Ortega I (eds) Les sociétés Paléolithiques D’un grand Sud-Ouest: nouveaux gisements, nouvelles méthodes, nouveaux résultats. Société Préhistorique française, Paris, pp 95–132
Speth JD (2015) When did humans learn to boil? PaleoAnthropology 2015:54–67. https://doi.org/10.4207/PA.2015.ART96
Stapert D, Johansen L (1999) Flint and pyrite: making fire in the Stone Age. Antiquity 73:765–777. https://doi.org/10.1017/S0003598X00065510
Velo J, Kehoe AB (1990) Red ocher in the paleolithic. In: Lecron Foster M, Botscharow L (eds) The life of symbols. Westview, San Francisco, pp 101–111
Weiner J (2012) Feuerschlagsteine und Feuererzeugung. In: Floss H (ed) Steinartefakte Vom Altpaläolithikum bis in die Neuzeit. Kerns, Tübingen, pp 943–960
Weiner J, Floss H (2004) Eine Schwefelkiesknolle aus dem Aurignacien Vom Vogelherd, Baden-Württemberg - Zu Den Anfängen Der Feuererzeugung Im europäischen Paläolithikum. Archaologische Informationen 27:59–78
Wiseman J (2009) SAS Survival Handbook: the Ultimate Guide to surviving Anywhere. Collins, London
Wragg Sykes RM (2015) To see a world in a hafted tool: birch pitch composite technology, cognition and memory in neanderthals. In: Coward F, Hosfield R, Pope M, Wenban-Smith F (eds) Settlement, Society and Cognition in Human evolution: landscapes in the mind. Cambridge University Press, Cambridge, pp 117–137
Zanolli C, Martinón-Torres M, Bernardini F et al (2018) The Middle Pleistocene (MIS 12) human dental remains from Fontana Ranuccio (Latium) and Visogliano (Friuli-Venezia Giulia), Italy. A comparative high resolution endostructural assessment. PLoS ONE 13:e0189773. https://doi.org/10.1371/JOURNAL.PONE.0189773
Acknowledgements
Funding was provided by the Netherlands Organization for Scientific Research (Grant # VI.Veni.191H.045). Thank you to Marie Soressi for making the Pech de l’Azé IV manganese dioxide blocks accessible for photography, and to Alexander Verpoorte and Wei Chu for their constructive feedback on an earlier version of this article.
Funding
Funding was provided by the Netherlands Organization for Scientific Research (Grant # VI.Veni.191 H.045).
Author information
Authors and Affiliations
Contributions
A.C.S. wrote and prepared the text, figures, and supplementary materials for this manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sorensen, A.C. Lucky strike: testing the utility of manganese dioxide powder in Neandertal percussive fire making. Archaeol Anthropol Sci 16, 134 (2024). https://doi.org/10.1007/s12520-024-02047-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-024-02047-9