Abstract
Over the last few decades, research has significantly enhanced our understanding of the role played by shellfish in human subsistence during the Mesolithic period along the Atlantic coast of Europe. Instrumental to this advance has been the analysis of stable oxygen isotope measurements (δ18O) from mollusc shells, which offers valuable insights into the seasonality of shellfish exploitation. The δ18O values serve as reliable indicators of past seasonal seawater temperature variations experienced by the molluscs, enabling us to accurately determine the time of year when past humans collected them. Recent studies using this approach have successfully identified seasonal exploitation patterns of the topshell Phorcus lineatus (da Costa, 1778) in Atlantic Europe. However, uncertainties remain regarding the exploitation schedule of Patella depressa Pennant 1777, one of the most abundantly collected species in Mesolithic sites. Here we used shell δ18O values of P. depressa from the Mesolithic sites of El Mazo (Northern Iberia) to determine its seasonality of exploitation. Our study reveals that P. depressa was prevalently exploited during colder months. Modern specimens of the same limpet species were also collected during two annual cycles to assess potential seasonal changes in meat yield return. Results obtained has profound implications for better understanding how last coastal foragers managed available littoral resources throughout the year.
Similar content being viewed by others
Introduction
Coastal areas and marine resources played a significant role in the subsistence strategies of past hominins around the world (Bicho and Esteves 2022; Colonese et al. 2011; Jerardino and Marean 2010; Ramos-Muñoz et al. 2016). The use of shellfish as a key resource has been argued to have been especially pronounced during periods of adverse climate conditions (García-Escárzaga et al. 2022a; Klein et al. 2021; Marean et al. 2007). During the Mesolithic period, in the first millennia of the Holocene, human populations residing along the coasts of Europe extensively harvested intertidal resources, resulting those in the formation of large shell deposits, mainly in caves and rock shelters, but in open air sites as well (Astrup et al. 2021; Bicho et al. 2010; Gutiérrez-Zugasti et al. 2011; Milner et al. 2007). This kind of archaeological assemblage is particularly abundant in Northern Iberia (Arias et al. 2015; Fano 2007, 2019). Recent investigations in the Cantabrian region (littoral area located in Northern Iberia), have advanced our understanding of the lifeways of the last foragers along the Atlantic coast of Europe. These studies have highlighted the role of molluscs in the subsistence strategies of Mesolithic human groups (García-Escárzaga and Gutiérrez-Zugasti 2021; Gutiérrez-Zugasti et al. 2016; Portero et al. 2022), the importance of mollusc shells for reconstructing shellfish collection patterns (Álvarez-Fernández 2011; Gutiérrez-Zugasti 2009, 2011; Gutiérrez-Zugasti et al. 2011; García-Escárzaga 2020; García-Escárzaga et al. 2021), as well as the impact of abrupt climate changes, such as the 8.2 ka cal BP cold event, on marine ecology and human populations (García-Escárzaga 2020; García-Escárzaga et al. 2022a). These studies have also been instrumental in determining the technological and symbolics uses of shell remains (Álvarez-Fernández 2006; Cuenca-Solana 2015; Rigaud and Gutiérrez-Zugasti 2016), among other relevant issues.
Analyses of stable oxygen isotope values (δ18O) in marine mollusc shells have been extensively applied to archaeological assemblages to establish the season(s) when molluscs were collected by the past human groups (e.g., Branscombe et al. 2021; Hausman and Meredith-Williams, 2017; Leclerc et al. 2023; Prendergast et al. 2016). For example, studies have shown that Mesolithic groups in the Northern Iberia collected the topshell Phorcus lineatus (da Costa, 1778) primarily during the coldest months of the year (Bailey et al. 1983; Deith 1983; Deith and Shackleton 1986; García-Escárzaga et al. 2019). Interestingly, studies around the Mediterranean basin also reached similar conclusions for the topshell Phorcus turbinatus (Born, 1778), which was found to have been mostly exploited during the coldest months of the year by distinct Mesolithic populations (Colonese et al. 2018; Mannino et al. 2011; Prendergast et al., 2016). A consensus thus appears to emerge that most intertidal resources were exploited during the colder months of the year. Although different explanations have been proposed (e.g., absence of hunter-gatherers in coastal areas during the warmer seasons, scarcity of food supply during colder months, high mobility along the coast, social or religious reasons, etc.), thus far the driving factor remains unclear based on available data (Deith 1983; Colonese et al. 2009; García-Escárzaga et al. 2019; Mannino et al. 2011). Recently, García-Escárzaga et al. (2019) demonstrated throughout an experimental programme, in which modern live-collected molluscs were harvested, boiled, and weighed, that P. lineatus offered higher meat profitability during colder months due to gonadal development, therefore suggesting that cost-benefit principles may have driven seasonal exploitation patterns.
Although we have made significant progress in our understanding of the role of some marine mollusc species in human diets, significant gaps still exist for other taxa that were also abundantly collected in the past. This is the case of the limpet Patella depressa Pennant, 1771, an open coast species which dominates shell assemblages in some Late Mesolithic sites in the Southern Atlantic Europe (Bello-Alonso et al. 2015; García-Escárzaga 2020; García-Escárzaga et al. 2021, 2022a; Stiner et al. 2003; Valente 2012). Although a preliminary investigation conducted by Bailey and Craighead (2003) suggested throughout a study of the shell annual growth lines that limpets were harvested year-round during the Mesolithic in Northern Iberia, further evidence are still needed due to the uncertainties of the method used to establish the season of capture. A sclerochronological investigation using live-collected P. depressa shells from Northern Iberia has recently highlighted that shell δ18O values of this species accurately reflect seasonal temperature variations year-round, and thus can act as a robust paleothermometer (García-Escárzaga et al. 2020), as well as a proxy for the season of collection by human foragers. Here, we present the results obtained from stable oxygen isotope analyses conducted on sub-fossil P. depressa shells recovered from El Mazo (Northern Iberia), a Mesolithic shell midden site in the European Atlantic façade with highly resolved chronological and sedimentary deposits (García-Escárzaga et al. 2022a). These results are combined with those previously obtained from P. lineatus topshells recovered from the same archaeological site (García-Escárzaga et al. 2019), which is crucial to understand how last foragers scheduled littoral exploitation annual patterns. In addition to isotopic data, we have also conducted an experimental program involving the collection of modern P. depressa samples for two years. By combining these approaches, we are able to explore the principles that drove seasonal mollusc exploitation patterns in this region. The study advances our understanding of forager adaptation to Early Holocene environmental conditions in Western Europe.
Background
El Mazo shell midden site
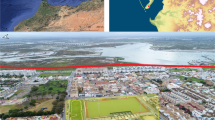
El Mazo is in the municipality of Llanes (Asturias, Northern Spain) (43° 24′ 4″ N, 4° 42′ 42″ W) (Fig. 1a). Currently, this archaeological site is situated approximately 1 km away from the seashore. Sea level fluctuations during the Mesolithic period would have caused this distance to vary, although previous studies on the Cantabrian region suggest that it would not have been greater than three kilometres over the course of the past 9,000 years (Leorri et al. 2012; Gutiérrez-Zugasti et al. 2013). The shell midden was formed along the 18-meter-long rock shelter, but only a small area near the walls has been preserved to this day (Fig. 1b). In 2009 and 2010, a test pit measuring 2 × 1 m was excavated in squares V15 and V16, and the resulting north profile was sampled in a limited area (2 m in the east-west axis x 0.25 m in the north-south axis) in 2012 (squares X15 and X16) (Fig. 1c) (Gutiérrez-Zugasti and González-Morales 2013). A total of 25 stratigraphic units (SUs) were identified in squares X15 and X16 (Fig. 1d), providing important insights into the site’s history of occupation. The excavation of the rock shelter area produced several stratigraphic units composed mainly of shell deposits (Gutiérrez-Zugasti and González-Morales 2013). The modelled radiocarbon chronology of the deposit constrained the formation process to the Early and Middle Holocene, between 9.0 and 7.4 ka cal BP (García-Escárzaga et al. 2022a, b) (Fig. 2). This is a critical time interval marked by the abrupt 8.2 ka cal BP cold event (García-Escárzaga et al. 2022a).
a) Location of the study area (Cantabrian region, Northern Iberia) and El Mazo shell midden site. b) External view of the El Mazo rockshelter. c) Squares excavated during several archaeological campaigns (2009–2017), highlighting in yellow that area sampled during 2012. d) Stratigraphy of the inner test pit (squares X15 and X16). Stratigraphic units from which the shell analysed herein were recovered (107, 105 and 101B) are framed by a red square
Bayesian modelling of a total of 65 radiocarbon dates obtained from the shell midden site of El Mazo cave (Asturias, N Spain) calibrated in OxCal v.4.4.4 (Bronk Ramsey 2009a, b) using IntCal20 (Reimer et al. 2020) and Marine20 (Heaton et al. 2020) curves and the ΔR values published by García-Escárzaga et al. 2022b for Patella vulgata Linnaeus, 1758 and P. lineatus mollusc species. Figure published by García-Escárzaga et al. 2022a and modified in this study to add red dashed rectangles to indicate the units from which marine samples analysed herein were recovered
Biology and ecology of Patella depressa Pennant, 1777
The limpet P. depressa (Fig. 3a) is a marine gastropod that geographically extends from Southwestern England and Wales to Northern Africa (Fretter and Graham 1976). A previous study conducted on the Portuguese coast showed that P. depressa has a life span of no longer than three years (Guerra and Gaudencio 1986). P. depressa is a microphagous herbivore that feeds on microscopic plants and macroalgae by grazing the rocky substrate using a horny tongue (radula) (Moore et al. 2007). The length and morphology of this species varies depending on its position in the intertidal zone. Shells in the upper intertidal zone are usually steeply conical and very thick, whereas at lower intertidal levels, they are polygonal, flattened, and thin (Orton and Southward 1961). Sclerochronological investigations previously conducted on this species using modern samples from the Northern Iberia show that the species grows almost year-round in this middle latitude (ca. 43° N). Despite the observation of short stoppages in summer and winter, because of surpassing its thermal tolerance, these growth cessations were no longer than six weeks and did not prevent correct deduction of the season of mollusc collection (García-Escárzaga et al. 2020). Their spawning and gonadal development stages vary depending on latitude (Orton and Southward 1961; Ribeiro et al. 2009). In Northern Iberia, mature gonads are found all year-round except for the summer months, reaching their maximum prior to the two main spawning events, which occur in January/February and April/June (Fernández et al. 2016).
P. depressa from the shell midden site of El Mazo. Dashed lines show the cutting axis from which two think sections (c and e) were obtained. b) Inner side of P. depressa shell. Arrows indicate the shell edge area from where the calcium carbonate powder micro-sample representative of the last shell growth were taken. c) Thick section of a limpet shell. d) Edge portion of a think section showing the visible growth lines and the calcite layer from which calcium carbonate micro-samples were extracted. e) Thick section stained with Mutvei’s solution. f) Mutvei’s-stained cross section displaying major and minor growth lines/increments
Materials and methods
Material
Archaeological shells were recovered from three stratigraphic units from squares X15 and X16 (excavated in 2012): SUs 107, 105 and 101B (Fig. 2). These stratigraphic units were selected to cover different time intervals, as well as different environmental conditions before, during and after the 8.2 ka cal BP cold event. The Bayesian model indicates that the selected units covered a time interval of ca. 700 years through the Early and Middle Holocene (Fig. 2) (García-Escárzaga et al. 2022a). SU 107 is in the lower part of the shell midden and was formed between 8,550 and 8,330 cal BP (95% confidence interval). SU 105 is located approximately in the middle part of the sequence and had radiocarbon dated between 8,315 and 8,185 cal BP (95% confidence interval), partially corresponding to the 8.2 ka cold event (García-Escárzaga et al. 2022a). An increase in the percentage of P. vulgata shells, a species adapted to cold climate conditions, revealed a significant impact of the 8.2 ka cold event during the stratigraphic unit formation (García-Escárzaga et al. 2022a). In addition, a decrease of mollusc shell sizes in SU 105 also suggests a higher human pressure over the intertidal resources at that time (García-Escárzaga et al. 2022a). Finally, SU 101B is situated in the upper part of the shell midden and was formed at the first centuries of the Middle Holocene, between 7,945 and 7,795 cal BP (95% confidence interval). A total of 20 P. depressa shells were analysed per stratigraphic unit (Table 1; Supplementary Table 1) to determine the season(s) when this species was harvested by Mesolithic populations. The amount of P. depressa isotopically analysed against the total individuals recovered from each stratigraphic unit is low (SU 107 = 0.5%; SU 105 = 0.4%; SU 101B = 0.9%). However, limpets studied herein were randomly selected from a volume of 2.5 L, and the results from a previous investigation on seasonality of shell collection (García-Escárzaga et al. 2019) were very similar when using shells from different squares of the same unit. Although the variance in length between specimens of the same stratigraphic unit is low (Supplementary Table 1), according to a Kruskall-Wallis test, there are significant differences in mean sizes (p < 0.01) between units. However, all of the specimens are smaller than 30 mm in length, which is the only size criterion that could impact our seasonal estimations following the criteria derived from the sclerochronological investigation recently conducted on modern limpets of this species from the same coastal location (García-Escárzaga et al. 2020). The selection of individuals smaller than 30 mm in length was conducted to limit the effect of an ontogenetic slowing down of shell growth rate.
Sampling and stable oxygen isotope analysis
Building on previous studies on limpets, including P. depressa, such as Fenger et al. (2007), García-Escárzaga et al. (2020), Gutiérrez-Zugasti et al. (2017), and Prendergast and Schöne (2017), two complementary methods were employed to obtain calcium carbonate (CaCO3) for stable oxygen isotope analysis. Firstly, a CaCO3 micro-sample was manually taken from the shell edge using a dentist microdrill with a 1 mm diamond drill bit by milling along the perimeter of the innermost part of the shell edge. This was done to prevent loss of the isotope signal corresponding to the last growth of the shell during the next sampling phase in case the limpet margin was broken (Fig. 3b). Secondly, the remaining CaCO3 micro-samples were sequentially taken along the axis of shell growth to obtain δ18O value profiles across the lifespan of the mollusc. To achieve this, the specimens were partially coated with a metal epoxy resin along the axis of maximum growth, from the anterior to the posterior margin, to prevent shell breakage when sectioned using a Buehler Isomet low speed saw and a diamond wheel. Two thick sections (~ 3 mm each) were obtained per sample. They were fixed onto a glass microscope slide with metal epoxy resin. The sections were then ground on glass plates using 600 and 800 SiC grit powder and polished with 1 μm diamond suspension grit until the internal growth lines and increments were visible (Fig. 3c). One of these thick sections was used for studying shell growth patterns, and the other was employed for taking CaCO3 micro-samples from the calcite layer using a New Wave MicroMill and a 1 mm diamond drill bit. The sampling was conducted in a direction parallel to the visible growth increments and from the edge to the apex of the shell (Fig. 3d).
The sampling approach conducted here aimed to obtain long and short isotope series. It involved taking the maximum CaCO3 micro-samples possible (n = 40‒73) from nine shells (three per stratigraphic unit) (Supplementary Table 1). Additionally, 15 CaCO3 micro-samples of the final stage of shell growth were taken on 17 shells per unit (Supplementary Table 1). Both long and short isotope sequences were used for estimating the maximum and minimum δ18O values and the intra-annual δ18O range for each archaeological assemblage and the season when P. depressa species were harvested by Mesolithic groups. The width of the sampling paths in both cases was measured using the New Wave MicroMill software. The weight of each carbonate powder sample obtained was more than 200 µg, and they were stored in glass vials. The δ18O analyses were carried out using a Thermo Fisher Scientific DELTA V IRMS coupled to a Gas Bench II Interface at the Department of Archaeology, Max Planck Institute of Geoanthropology (formerly for Science of Human History) Jena, Germany. Each powder sample was dissolved in 100% concentrated phosphoric acid at 70 ºC in helium flished vials. The raw isotopic ratios of the resulting CO2 gases were normalized using a three-point calibration against international standards NBS-18 (-23.2‰), IAEA-603 (-2.37‰), and IAEA-CO-8 (-22.7‰). The results are reported as δ18O (‰) relative to the Vienna Pee Dee Belemnite (VPDB) standard. The analytical error of the instrument was determined to be ± 0.1‰.
Determination of Patella depressa collection season(s)
To determine the season(s) when the molluscs were collected, the quartiles system proposed by Mannino et al. (2003, 2007) was used. This method has been also successfully adopted by other scholars working on other European contexts (Branscombe et al. 2021; Colonese et al. 2009, 2018; Kimball et al. 2009; Prendergast et al. 2016), including Northern Iberia (García-Escárzaga et al. 2019). The maximum and minimum δ18O values and the intra-annual δ18O range for each stratigraphic unit were determined by combining the δ18O values from the long and short isotope sequences. Then, the intra-annual δ18O range obtained for each unit was divided into four quartiles. Each quartile approximately represents a season. The upper quartile (i.e., higher values) represents cool conditions (i.e., winter) and the lower quartile (i.e., lower values) represents warm conditions (i.e., summer). Shell edge δ18O values falling into the upper and lower quartiles indicate that the specimens were collected in winter and summer, respectively. The intermediary quartiles represent cooling (i.e., autumn) and warming (i.e., spring) periods. When shell edge δ18O values fall into these quartiles, it is possible to distinguish between collection events that occurred in spring or autumn based on the δ18O trend just before the death of the mollusc. If δ18O values increase during the last weeks/months of the shell growth, specimens were collected in a cooling period (i.e., autumn). In contrast, if results report a decrease in δ18O values before the individual dying, it was harvested in a warming season (i.e., spring).
Shell growth lines/increments study
To accurately estimate the collection season, we examined the shell growth lines and increments, with particular focus on those located near the shell ventral margins, in every shell to determine if CaCO3 precipitation ceased during the final months of the mollusc’s lifespan, even if the specimen stopped growing. This information allows for a more precise estimation of the season of collection. To achieve this objective, one of the thick sections obtained from each limpet was polished until the internal growth lines and increments were clearly visible. The sections were then immersed in Mutvei’s solution for 20 min at 37‒40 ºC to increase the visibility of the growth lines and increments (Schöne et al. 2005) (Fig. 3e). Growth lines and increments were analysed using a sectoral dark-field illumination under a Leica S8APO stereoscopic microscope (8−50x magnification) at the IIIPC – University of Cantabria (Spain), coupled to a Leica MC190HD digital camera (10MP), following identification criteria previously established for this species (García-Escárzaga et al. 2020).
Modern specimens and relative meat yield calculation
To determine variations (if any) in the profitability of P. depressa, living specimens were collected every ca. 45 days from Langre Beach (Ribamontán al Mar, Cantabria), in Northern Iberia (Fig. 1a). The area exhibits similar oceanographic conditions to those littoral areas located near the archaeological site where Mesolithic specimens were collected. Shell collection was carried out year-round throughout two non-consecutive years (from August 2014 to July 2015 and from March 2016 to June 2017), in the beginning and during the middle part of each season during a total of 19 collection events (Supplementary Table 2). The collections were suppressed during seven months (from July 2015 to March 2016) to maintain the local population.
During the experimental programme, a total of 950 samples were collected, 50 specimens from both lower and higher intertidal zones per collection event. Their soft parts were extracted from the shell, and the mollusc meat was weighed. Shells were also weighed to obtain a direct comparison between the weight of the shell and the mollusc meat. The relative meat yield (RMY) was estimated following Thomas and Mannino (2017) and García-Escárzaga and Gutiérrez-Zugasti (2021). The results obtained were compared with sea surface temperature (SST) measured from August 2014 to June 2017 by the Aquaculture Facility of Santander’s Oceanographic Centre (Cantabria, N Iberia) (Fig. 1a).
Results
Shell δ18O values
Both short and long sequences obtained from sub-fossil samples demonstrate a sinusoidal pattern along the shell growth axis. The time span covered by the isotope series was variable, spanning from a few months to one year in the case of the short sequences (Supplementary Fig. 1) and from one to two years in the case of the long sequences (Supplementary Fig. 2). The maximum and minimum δ18O values were + 2.99‰ and − 0.17‰, respectively. The maxima and minima, and therefore also the intra-annual δ18O range resulting from the dataset, differed between individuals and stratigraphic units (Table 2). The maxima and minima values obtained for each stratigraphic unit level ranged between + 2.99‰ and + 2.78‰ and − 0.05‰ and − 0.17‰, respectively. Thus, intra-annual ranges for each unit vary between 3.15‰ and 2.90‰. These values were used to calculate the four quartiles for each stratigraphic unit (Table 2; Supplementary Fig. 1).
Seasonality of collection
The δ18O profiles obtained from the 60 shells retrieved from the three stratigraphic units demonstrate a consistent collection pattern over time. All specimens were collected between autumn, winter, and spring, except one sample from unit 105 that indicated a death in summer (Fig. 4). Most specimens, however, were collected during the winter season, as evident in the three units under study (SU 101B: n = 12, 60%; SU 105; n = 9, 45%; 107: n = 13, 65%). A detailed study in which lower δ18O values of winter quartiles and δ18O values obtained from the last portion of growth of those shells assigned to autumn and spring were considered allowed us to estimate the moment of the season when molluscs died more precisely. Many of the individuals included in the intermediate seasons were collected at the end of autumn and at the beginning of spring, suggesting that P. depressa were predominantly harvested during the colder months of the year. (Supplementary Figs. 1 and 2). Specifically, around 87% of the specimens analysed were gathered between November and April. The number of specimens collected in the warmest period of the year (including late spring and early autumn) was minimal, comprising only three out of 60 shells (5%).
Shell growth cessation and major growth lines
The analysis of growth increments revealed that the species grew almost continuously throughout the year during the Early/Middle Holocene. Only a few specimens exhibited significant growth cessation in the form of major growth lines, which was likely caused by exceeding upper and lower thermal tolerances during summer and winter, respectively. For instance, specimen MA.101B.53 displayed a major growth line located 5.3 mm from the shell edge (Fig. 5a). This distinct line coincided with the minimum isotope value of the long series (+ 0.68‰), which was assigned to the summer season based on lower quartile values for unit 101B (−0.07‰ to + 0.80‰). Specimen MA.107.56 also showed a growth cessation at 0.9 mm from the ventral margin (Fig. 5b), and similarly the isotope value around the major growth line (+ 0.52‰) is also assigned to the summer quartile (−0.12‰ to + 0.61‰). In both cases, the minimum values of the series are significantly higher than minimum intra-annual δ18O value estimated for those units, therefore suggesting that there were probably lengthy growth stoppages in these individuals due to exceeding the upper thermal tolerance during summer. Major growth lines were also observed very close to the shell edge (less than 0.4 mm from the margin) on two other specimens (MA.105.66 and MA.107.62), indicating that shell growth ceased a few weeks before the molluscs were collected (Fig. 5c-d). While δ18O values prior to the shell growth cessation (MA.105.66 = + 2.79‰; MA.107.62 = + 2.67‰) were clearly assigned to winter quartiles (Table 2), δ18O values after major growth lines (MA.105.66 = + 1.98‰; MA.107.62 = + 1.89‰) were ascribed to intermediary quartiles. These results reported, therefore, a shell growth cessation in the coldest moment of the year because of exceeding the lower thermal tolerance of these two specimens and a resuming of the calcium carbonate deposition in the early spring after the temperature increase.
Relative meat yield (RMY)
Seasonal variation in the average RMY of 950 modern specimens revealed that protein return per unit effort changes throughout the year (Fig. 6). Highest RMY values were always recorded in middle winter (ca. February) and early spring (ca. March). By contrast, the lowest RMY values were always observed in middle summer months (ca. August). Nevertheless, lower RMY were also obtained in late spring, autumn, and even early winter (December 2014). RMY values exhibited a significant correlation with SST variations throughout the three annual cycles (R2 = 0.72, p < 0.0001), suggesting that coldest months (ca. from February to April) were more profitable for collecting P. depressa limpets.
Variation in annual relative meat yield (RMY) from modern P. depressa (diamonds) and current sea surface temperature (SST) (empty grey circles). The colours in the meat yield series represent the season of collection (red = summer; brown = autumn; blue = winter; green = spring). The upper and lower bars show the period of maximum gonadal development (ca. December to April) (yellow rectangles) and the months when P. depressa were primarily collected by the Mesolithic populations in Northern Iberia (green rectangles), respectively
Discussion and conclusion
P. depressa growth patterns in Northern Iberia during the Early and Middle Holocene
A previous study on P. depressa, using live-collected specimens in Northern Iberia, suggested that this species grows all year-round but not continuously throughout all seasons (García-Escárzaga et al. 2020). Our results extend these conclusions to archaeological specimens collected during the first millennia of the Holocene (from ca. 9 ka to 7.4 ka cal BP). Prominent major growth lines in specimens MA.101B.53 and MA.107.56 likely indicate shell growth stoppages during the year’s warmest months. The position of the minima δ18O values of these two profiles (Fig. 5a-b) within the summer quartile ranges for each unit (Table 2) suggest that these individuals stopped growing for several weeks during the warmest months of the year. Nevertheless, in both cases, the growth stoppages would not have prevented us from accurately deducing a possible collection in that summer. This is because the last δ18O values before shell growth cessation could have been assigned to the warmest season quartile (i.e., summer). The same conclusion can be derived from those shells that have reported a growth stoppage during the winter (Fig. 5c-d). Despite shells were not growing during the coldest week of the year and, therefore, recording the minimum annual seawater temperatures, a collection in winter would have been correctly deduced since the last δ18O value prior to growth stoppages had assigned to the upper quartile (i.e., winter). Furthermore, no analysed shell had reported a growth cessation longer than three months, and all minima and maxima values of all profiles (Supplementary Figs. 1 and 2) are consistent with winter and summer quartiles (Table 2), respectively.
Examining and studying the shell growth patterns near the shell edge was also a very helpful tool for accurately determining the season when the specimens were collected. Mutvei’s-stained cross sections revealed major growth lines and short-term stoppages in samples MA.105.66 and MA.107.62 located at 0.35 and 0.30 mm from the shell edge, respectively (Fig. 5c-d). The last δ18O values before mollusc shell growth stoppages (MA.105.66 = + 2.79‰; MA.107.62 = + 2.67‰) were ascribed in both specimens to winter. However, δ18O values after resuming carbonate deposition were assigned to spring. The possibility of deducing a cessation in shell growth close to the edge is crucial for accurately estimating the proper season of mollusc collection. Our observation of a growth stoppage of presumably several weeks because of colder temperatures in MA.105.66 and MA.107.62 has allowed us to read this decrease in δ18O values as a change of season and not only as a possible short warm event in the middle of the cold season. These two molluscs stopped shell growth at the end of winter and then resumed it just before their collection by Mesolithic humans during early spring.
Seasonal shellfish collection during the Mesolithic in N Iberia
P. depressa was primarily collected during colder months of the year by Mesolithic populations that inhabited El Mazo from ca. 8.5 ka to 7.8 ka cal BP. This marked seasonal pattern is very similar to that previously observed in Mesolithic occupations in caves and rock shelters along the Northern Iberian coast (Bailey et al. 1983; Deith 1983; Deith and Shackleton 1986), including El Mazo (García-Escárzaga et al. 2019), but also in other places located in Northern Atlantic Europe, such as Great Britain (Mannino et al. 2003). Likewise, a similar seasonality pattern has been documented in the Mediterranean coastal areas (Mannino et al. 2007, 2011, 2014; Colonese et al. 2009, 2018; Prendergast et al. 2016), even though some shellfish exploitation during summer was also observed during the Mesolithic along the Mediterranean coast (Branscombe et al. 2021; Mannino et al. 2007; Prendergast et al. 2016). Our study therefore contributes to the robust body of evidence suggesting that Mesolithic groups that occupied caves and rock shelters in Atlantic Europe and the Mediterranean Basin had a strong preference for consuming intertidal molluscs during the colder months of the year.
Previous studies proposed that such a pattern could reflect low summer human population density along the coast, or scarcity of terrestrial food resources in coastal areas in winter, among others (Deith 1983; Deith and Shackleton 1986; Mannino et al. 2007, 2011; Prendergast et al. 2016). However, the available information so far from the Northern Iberia appears to refute these explanations. Zooarchaeological studies revealed that El Mazo was inhabited year-round (Marín-Arroyo et al. 2020), thus confirming that other prey was available in summer and that humans were locally exploiting them. Traditionally, molluscs have been considered an emergency food supply alternative during periods of scarcity in higher meat return resources (Marín-Arroyo et al., 2013; Moss 2013). However, studies have shown that this may not have always been the case. The role of shellfish in human subsistence strategies along Atlantic Europe has been recently re-evaluated from an approach based on meat yield estimations, concluding that the contribution of this littoral resource to human diets during the Mesolithic could be even higher than terrestrial mammals in some littoral archaeological contexts (García-Escárzaga and Gutiérrez-Zugasti 2021). Moreover, experimental studies demonstrated that winter collections coincide with the period of higher mollusc meat yield, suggesting that winter Mesolithic collections reflected optimization, based on cost-benefit principles, which does not agree with the idea that shellfish were only an emergency food supply.
Our results from modern P. depressa broadly support this optimization argument. The relative meat yield variation year-round confirms that a higher meat yield would have been attained in the late winter and early spring, coinciding with that annual period in which gonads reached their maximum size (Fernández et al. 2016). Although late autumn and early winter did not show maximum meat yield values, they consistently reported a significant increase in relative meat yield compared to summer. This rise in meat yield return that occurs in November probably justified why Mesolithic populations started to collect P. depressa limpets again after the summer break at the end of autumn. The results suggest, therefore, that mollusc collection in Southern Europe during the Mesolithic very likely responded to cost-benefit principles supported by a deep human knowledge of local marine ecosystems.
Littoral resource management over time
Previous investigations carried out along the Atlantic façade of Europe have documented an increase in the intensification of littoral resource exploitation patterns from the Palaeolithic to the Late Mesolithic, attending to the evolution of mollusc shell sizes throughout time (Álvarez-Fernández et al. 2011; Gutiérrez-Zugasti 2011; Mannino and Thomas 2001). Biometric analyses conducted from marine shells recovered from El Mazo also revealed short-term changes in the intensification of shellfish collection during the Mesolithic (García-Escárzaga et al. 2022a). Shell sizes and, consequently, the meat grams extracted from each mollusc specimen of the main species represented throughout the El Mazo sequence (García-Escárzaga and Gutiérrez-Zugasti 2021) demonstrated a significant decrease in those stratigraphic units formed around the 8.2 ka cal BP cold event due to higher human pressure on marine resources (García-Escárzaga et al. 2022a). Deciphering seasonality in shellfish collection patterns could also be a helpful tool for inferring how past human managed the use of intertidal resources. For example, Mannino et al. (2007) observed that while molluscs were exclusively harvested during colder months in the Early Mesolithic, their collection expanded to all seasons during the Late Mesolithic. They considered these as a sign of an increase in the intensification of rocky shore mollusc consumption just before of adopting a Neolithic economy.
The results obtained herein showed that P. depressa was primarily exploited from late autumn to early spring throughout the sequence (Fig. 4), including during the formation of stratigraphic unit 105, which was dated to during the 8.2 ka cal BP cold event (Fig. 2) and where a significant shell size and meat return decrease has been argued to have been linked to increasing human exploitation of these resources (García-Escárzaga et al. 2022a). These data suggest that despite existing periods of higher anthropic pressure over coastal resources, which provoked a very relevant shell size decrease, this higher intensification in shellfish exploitation did not change the annual subsistence strategies scheduled by Mesolithic populations to mainly collect molluscs during the seasons of higher meat yield return. A conclusion that highlights how last foragers were able to develop and maintain throughout time complex ecological subsistence strategies for not overexploiting available food sources despite periods in which the pressure over marine resources significantly increased, such as during 8.2 ka cal BP cold event.
References
Álvarez-Fernández E (2006) Los objetos de adorno-colgantes del paleolítico superior y del mesolítico en la cornisa cantábrica y en el valle del Ebro. Tesis Doctoral, Universidad de Salamanca, Salamanca
Álvarez-Fernández E, Chauvin A, Cubas M, Arias P, Ontañón R (2011) Mollusc shell sizes in archaeological contexts in Northern Spain (13 200 to 2600 cal BC): new data from la Garma A and los Gitanos (Cantabria). Archaeometry 53(5):63–985
Álvarez-Fernández E (2011). Humans and marine resource interaction reappraised: Archaeofauna remains during the late Pleistocene and Holocene in Cantabrian Spain. J Anthropological Archaeology 30(3):327–343. https://doi.org/10.1016/j.jaa.2011.05.005
Arias P, Cubas M, Fano MÁ, Jordá Pardo JF, Salzmann C, Teichner F, Teira LC (2015) Where are the ‘Asturian’ dwellings? An integrated survey programme on the Mesolithic of northern Spain. Antiquity 89(346):783–799. https://doi.org/10.15184/aqy.2015.49
Astrup PM, Benjamin J, Stankiewicz F, Woo K, McCarthy JK, Wiseman C, Baggaley P, Jerbic, Fowler M, Skriver C, Bailey G (2021) A drowned mesolithic shell midden complex at Hjarnø Vesterhoved, Denmark and its wider significance. Q Sci Rev 258:106854
Bailey GN, Craighead AS (2003). Late Pleistocene and Holocene coastal palaeoeconomies: a reconsideration of the molluscan evidence from northern Spain. Geoarchaeology Int J 18(2):175–204. https://doi.org/10.1002/gea.10057
Bailey GN, Deith MR, Shackleton NJ (1983) Oxygen isotope analysis and seasonality determinations: limits and potential of a new technique. Am Antiq 48(2):390–398
Bello-Alonso P, Ozkorta-Escribano L, Gutiérrez-Zugasti I (2015) Un Acercamiento Al Aprovechamiento De Los recursos Litorales Durante El Mesolítico: Los invertebrados marinos del abrigo de El Toral III (Llanes, Asturias). La Investigación Arqueomalacológica en la Península Ibérica: Nuevas aportaciones. Nadir Ediciones, Santander, pp 91–99
Bicho N, Esteves E (2022) Pleistocene hunter-gatherer coastal adaptations in Atlantic Iberia. Front Earth Sci. 10https://doi.org/10.3389/feart.2022.957214
Bicho N, Umbelino C, Detry C, Pereira T (2010) The emergence of Muge Mesolithic Shell Middens in Central Portugal and the 8200 cal yr BP Cold Event. J Island Coastal Archaeol 5(1):86–104. https://doi.org/10.1080/15564891003638184
Branscombe T, Schulting R, Lee-Thorp J, Leng M (2021) The potential of marine bivalve Spisula sachalinensis as a marine temperature record. Palaeogeogr Palaeoclimatol Palaeoecol 582:110634. https://doi.org/10.1016/j.palaeo.2021.110634
Bronk Ramsey C (2009a) Bayesian analysis of Radiocarbon dates. Radiocarbon 51:337–360. https://doi.org/10.1017/S0033822200033865
Bronk Ramsey C (2009b) Dealing with outliers and offsets in Radiocarbon Dating. Radiocarbon 51(3):1023–1045. https://doi.org/10.1017/S0033822200034093
Colonese AC, Troelstra S, Ziveri P, Martini F, Lo Vetro D, Tommasini S (2009) Mesolithic shellfish exploitation in SW Italy: seasonal evidence from the oxygen isotopic composition of Osilinus turbinatus shells. J Archaeol Sci 36(9):1935–1944. https://doi.org/10.1016/j.jas.2009.04.021
Colonese AC, Mannino MA, Bar-Yosef Mayer DE, Fa DA, Finlayson JC, Lubell D, Stiner MC (2011) Marine mollusc exploitation in Mediterranean prehistory: an overview. Quatern Int 239(1):86–103. https://doi.org/10.1016/j.quaint.2010.09.001
Colonese AC, Vetro DL, Landini W, Di Giuseppe Z, Hausmann N, Demarchi B, d´Angelo C, Leng MJ, Incarbona A, Withwood AC, Martini F (2018) Late pleistocene-holocene coastal adaptation in central Mediterranean: snapshots from Grotta d’Oriente (NW Sicily). Quatern Int 493:114–126. https://doi.org/10.1016/j.quaint.2018.06.018
Cuenca-Solana D (2015) The use of shells by hunter-fisher-gatherers and farmers from the early Upper Palaeolithic to the neolithic in the European Atlantic façade: a technological perspective. J Island Coastal Archaeol 10:52–75. https://doi.org/10.1080/15564894.2014.934491
Deith MR (1983) Seasonality of shell collecting, determined by oxygen isotope analysis of marine shells from asturian sites in Cantabria. Anim Archaeol 2:67–76
Deith M, Shackleton N (1986) Seasonal exploitation of marine molluscs: oxygen isotope analysis of shell from La Riera cave. La Riera Cave. Stone age hunter-gatherer adaptations in northern Spain, pp 299–313
Fano MÁ (2007) Un nuevo tiempo: el Mesolítico en la región cantábrica. In: Las sociedades del Paleolítico en la región cantábrica. Kobie Anejo, 8. Diputación Foral de Bizkaia, Bilbao, pp. 337–401
Fano M (2019) The mesolithic asturian culture (North Iberia), one century on. Quatern Int 515:159–175. https://doi.org/10.1016/j.quaint.2017.12.025
Fenger T, Surge D, Schöne B, Milner N (2007) Sclerochronology and geochemical variation in limpet shells (Patella vulgata): a new archive to reconstruct coastal sea surface temperature. Geochem Geophys Geosyst 8(7). https://doi.org/10.1029/2006GC001488
Fernández N, Alborés I, Aceña-Matarranz S (2016) Spatial variability of the reproductive cycle and physiological condition of Patella spp. (Mollusca Gastropoda) in the NW of the Iberian Peninsula: implications for exploitation. Fish Res 179:76–85. https://doi.org/10.1016/j.fishres.2016.02.010
Fretter V, Graham A (1976) The Prosobranch molluscs of Britain and Denmark. Part 1: Pleurotomariacea, Fissurellacea and Patellacea. Malacological Society of London, London
García-Escárzaga A (2020) Paleoclima y aprovechamiento de recursos costeros durante el Mesolítico en la región cantábrica (N de Iberia). BAR International Series, vol 2976. BAR Publishing, Oxford. https://doi.org/10.30861/9781407356723
García-Escárzaga A, Gutiérrez-Zugasti I (2021) The role of shellfish in human subsistence during the Mesolithic of Atlantic Europe: an approach from meat yield estimations. Quatern Int 584:9–19. https://doi.org/10.1016/j.quaint.2020.03.003
García-Escárzaga A, Gutiérrez-Zugasti I, Cobo A, Cuenca-Solana D, Martín-Chivelet J, Roberts P, González-Morales MR (2019) Stable oxygen isotope analysis of Phorcus Lineatus (Da Costa, 1778) as a proxy for foraging seasonality during the Mesolithic in northern Iberia. Archaeol Anthropol Sci 11(10):5631–5644. https://doi.org/10.1007/s12520-019-00880-x
García-Escárzaga A, Gutiérrez-Zugasti I, González-Morales M, Arrizabalaga A, Zech J, Roberts P (2020) Shell sclerochronology and stable oxygen isotope ratios from the limpet Patella depressa pennant, 1777: implications for palaeoclimate reconstruction and archaeology in northern Spain. Palaeogeography Palaeoclimatology Palaeoecology 560:110023. https://doi.org/10.1016/j.palaeo.2020.110023
García-Escárzaga A, Gutiérrez-Zugasti I, Cuenca-Solana D, Cobo A, González-Morales M (2021) Coastal resource exploitation patterns and climatic conditions during the early mesolithic in the cantabrian region (northern Iberia): preliminary data from the shell midden site of El Mazo. Foraging assemblages, vol 2. Serbian Archaeological Society, Belgrade, pp 382–387
García-Escárzaga A, Gutiérrez-Zugasti I, Cuenca-Solana D, González-Morales MR, Hamann C, Roberts P, Fernandes R (2022a) Bayesian estimates of marine radiocarbon reservoir effect in northern Iberia during the early and middle holocene. Quat Geochronol 67:101232. https://doi.org/10.1016/j.quageo.2021.101232
García-Escárzaga A, Gutiérrez-Zugasti I, Marín-Arroyo AB, Fernandes R, de la Núñez S, Cuenca-Solana D, Iriarte E, Simões C, Martín-Chivelet J, González-Morales MR, Roberts P (2022b) Human forager response to abrupt climate change at 8.2 ka on the Atlantic coast of Europe. Sci Rep 12(1):6481. https://doi.org/10.1038/s41598-022-10135-w
Guerra MT, Gaudencio MJ (1986) Aspects of the ecology of Patella spp. on the Portuguese coast. Hydrobiologia 142:57–69
Gutiérrez-Zugasti I (2009) La explotación de moluscos y otros recursos litorales en la región cantábrica durante el Pleistoceno final y el Holoceno inicial. Santander: Publican, Ediciones Universidad de Cantabria
Gutiérrez-Zugasti I (2011) Changes in Molluscan Exploitation patterns during the late pleistocene and early holocene in Eastern Cantabria (Northern Spain). Trekking the Shore. Changing Coastline and the antiquity of Coastal Settlement. Springer, New York, pp 179–201. DOI: https://doi.org/10.1007/978-1-4419-8219-3_8.
Gutiérrez-Zugasti I, González-Morales MR (2013) Intervención arqueológica en la cueva de El Mazo (Llanes, Asturias): campañas de 2009, 2010 y 2012. Excavaciones arqueológicas en Asturias 2007–2012. Gobierno del Principado de Asturias, Oviedo, pp 159–168
Gutiérrez-Zugasti I, Andersen SH, Araújo AC, Dupont C, Milner N, Monge-Soares AM (2011) Shell midden research in Atlantic Europe: state of the art, research problems and perspectives for the future. Quatern Int 239(1):70–85. https://doi.org/10.1016/j.quaint.2011.02.031
Gutiérrez-Zugasti I, Cuenca-Solana D, González-Morales MR, García-Moreno A, Ortíz-Menéndez JE, Risseto J, De Torres T (2013) Back to the Asturian: first result from the Mesolithic shell midden site of El Mazo (Asturian, Northern Spain). In: Ancient maritime communities and the relationship between people and environment along the European Atlantic coasts. BAR International Series, 2570, BAR Publishing, Oxford, pp. 483–490
Gutiérrez-Zugasti I, Tong E, García-Escárzaga A, Cuenca-Solana D, Bailey G, González-Morales M (2016) Collection and consumption of echinoderms and crustaceans at the mesolithic shell midden site of El Mazo (northern Iberia): opportunistic behaviour or social strategy? Quatern Int 407:118–130. https://doi.org/10.1016/j.quaint.2015.11.149
Gutiérrez-Zugasti I, Suárez-Revilla R, Clarke L, Schöne B, Bailey G, González-Morales M (2017) Shell oxygen isotope values and sclerochronology of the limpet Patella vulgata Linnaeus 1758 from northern Iberia: implications for the reconstruction of past seawater temperatures. Palaeogeography Palaeoclimatology Palaeoecology 484:162–175. https://doi.org/10.1016/j.palaeo.2017.04.012
Hausmann N, Meredith-Williams M (2017) Exploring Accumulation Rates of Shell deposits through Seasonality Data. J Archaeol Method Theory 24:776–795. https://doi.org/10.1007/s10816-016-9287-x
Heaton T, Köhler P, Butzin M, Bard E, Reimer R, Austin W, Bronk Ramsey C, Grootes PM, Hughen KA, Kromer B, Reimer PJ, Adkins J, Burke A, Cook MS, Olsen J, Skinner L (2020) Marine20—The Marine Radiocarbon Age Calibration curve (0–55,000 cal BP). Radiocarbon 62:1–42. https://doi.org/10.1017/RDC.2020.68
Jerardino A, Marean CW (2010) Shellfish gathering, marine paleoecology and modern human behavior: perspectives from cave PP13B, Pinnacle Point, South Africa. J Hum Evol 59(3):412–424. https://doi.org/10.1016/j.jhevol.2010.07.003
Kimball MJ, Showers W, McCartan S, Genna BJ (2009) 18O analysis of Littorina littorea shells from Ferriter¹s Cove, Dingle Peninsula: preliminary results and interpretations. Prehistoric Soc Res Paper 1:189–197
Klein C, Jackson LS, Parker DJ, Marsham JH, Taylor CM, Rowell DP, Guichard F, Vischel T, Famien AM, Diedhiou A (2021) Combining CMIP data with a regional convection-permitting model and observations to project extreme rainfall under climate change. Environ Res Lett 16(10):104023. https://doi.org/10.1088/1748-9326/ac26f1
Leclerc N, Kuehn S, Clark T, Burchell M, Coupland G, Schöne BR (2023) Investigation of seasonal settlement and clam harvest pressure in the Sechelt Inlet System, British Columbia, Canada, through sclerochronology and stable oxygen isotope analyses. Environ Archaeol 1–12. https://doi.org/10.1080/14614103.2022.2154452
Leorri E, Cearreta A, Milne G (2012) Field observations and modelling of Holocene sea-level changes in the southern Bay of Biscay: implication for understanding current rates of relative sea-level change and vertical land motion along the Atlantic coast of SW Europe. Q Sci Rev 42:59–73. https://doi.org/10.1016/j.quascirev.2012.03.014
Mannino MA, Thomas KD (2001) Intensive mesolithic exploitation of coastal resources? Evidence from a shell deposit on the Isle of Portland (Southern England) for the impact of human foraging on populations of intertidal rocky shore molluscs. J Archaeol Sci 28(10):1101–1114. https://doi.org/10.1006/jasc.2001.0658
Mannino M, Spiro B, Thomas K (2003) Sampling shells for seasonality: oxygen isotope analysis on shell carbonates of the inter-tidal gastropod Monodonta lineata (Da Costa) from populations across its modern range and from a mesolithic site in southern Britain. J Archaeol Sci 30(6):667–679. https://doi.org/10.1016/S0305-4403(02)00238-8
Mannino M, Thomas K, Leng M, Piperno M, Tusa S, Tagliacozzo A (2007) Marine resources in the mesolithic and neolithic at the Grotta dell’Uzzo (Sicily): evidence from isotope analyses of marine shells. Archaeometry 49:117–133. https://doi.org/10.1111/j.1475-4754.2007.00291.x
Mannino MA, Thomas KD, Leng MJ, Di Salvo R, Richards MP (2011) Stuck to the shore? Investigating prehistoric hunter-gatherer subsistence, mobility and territoriality in a Mediterranean coastal landscape through isotope analyses on marine mollusc shell carbonates and human bone collagen. Quatern Int 244(1):88–104. https://doi.org/10.1016/j.quaint.2011.05.044
Mannino MA, Thomas KD, Crema ER, Leng MJ (2014) A matter of taste? Mode and periodicity of marine mollusc exploitation on the Mediterranean island of Favignana (Ègadi Islands, Italy) during its isolation in the early Holocene. Archaeofauna 23(1):133–147
Marean CW, Bar-Matthews M, Bernatchez J, Fisher E, Goldberg P, Herries AIR, Zenobia J, Jerardino A, Karkanas P, Minichillo T, Nilssen PJ, Thompson E, Watts I, Williams HM (2007) Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature 449(7164):905–908. https://doi.org/10.1038/nature06204
Marín-Arroyo AB (2013) Human response to Holocene warming on the Cantabrian Coast (northern Spain): an unexpected outcome. Q Sci Rev 81:1–11. https://doi.org/10.1016/j.quascirev.2013.09.006
Marín-Arroyo AB, Gutiérrez-Zugasti FI, Cuenca-Solana D (2020) Patrones De Subsistencia De Los grupos mesolíticos en la cueva de El Mazo (Asturias). Sagvntvm-Extra 21:277–290
Milner N, Craig O, Bailey G (2007) Shell middens in Atlantic Europe. Oxbow Books, Oxford
Moore P, Thompson RC, Hawkins SJ (2007) Effects of Grazer identity on the probability of escapes by a canopy-forming macroalga. J Exp Mar Biol Ecol 344(2):170–180. https://doi.org/10.1016/j.jembe.2006.12.012
Moss ML (2013) Beyond subsistence: the social and symbolic meanings of shellfish in northwest coast societies. Shells energy. Mollusc shells as coastal resources. Oxbow Books, Oxford, pp 7–20
Orton JH, Southward AJ (1961) Studies on the biology of limpets IV. The breeding of Patella depressa pennant on the north Cornish coast. J Mar Biol Association United Kingd 41(3):653–662
Portero R, Cueto M, Fernández-Gómez MJ, Álvarez-Fernández E (2022) Surf and turf. Animal resources in the human diet in cantabrian Spain during the Mesolithic (11.5–7.5 Ky cal. BP). J Archaeol Science: Rep 45:103635. https://doi.org/10.1016/j.jasrep.2022.103635
Prendergast AL, Schöne BR (2017) Oxygen isotopes from limpet shells: implications for palaeothermometry and seasonal shellfish foraging studies in the Mediterranean. Palaeogeogr Palaeoclimatol Palaeoecol 484:33–47. https://doi.org/10.1016/j.palaeo.2017.03.007
Prendergast A, Stevens R, O’Connell T, Hunt C, Schöne B, Barker G (2016) Changing patterns of eastern Mediterranean shellfish exploitation in the late glacial and early holocene: oxygen isotope evidence from gastropod in Epipaleolithic to neolithic human occupation layers at the Haua Fteah cave, Libya. Quatern Int 407:80–93. https://doi.org/10.1016/j.quaint.2015.09.035
Ramos-Muñoz J, Cantillo-Duarte JJ, Bernal-Casasola D, Barrena-Tocino A, Domínguez-Bella S, Vijande-Vila E, Clemente-Conte I, Gutiérrez-Zugasti I, Soriguer-Escofet M, Almisas-Cruz S (2016) Early use of marine resources by Middle/Upper Pleistocene human societies: the case of Benzú rockshelter (northern Africa). Quatern Int 407:6–15
Reimer PJ, Austin WEN, Bard E, Bayliss A, Blackwell PG, Ramsey B, Butzin C, Cheng M, Edwards H, Friedrich RL, Grootes M, Guilderson PM, Hajdas TP, Heaton I, Hogg TJ, Hughen AG, Kromer KA, Manning B, Muscheler SW, Palmer R, Pearson JG, van der Plicht C, Reimer. J, Richards RW, Scott DA, Southon EM, Turney JR, Wacker CSM, Adolphi L, Büntgen F, Capano U, Fahrni M, Fogtmann-Schulz SM, Friedrich A, Köhler R, Kudsk P, Miyake S, Olsen F, Reinig J, Sakamoto F, Sookdeo M, Talamo A, S (2020) The IntCal20 Northern Hemisphere Radiocarbon Age Calibration curve (0–55 cal kBP). Radiocarbon 62(4):725–757. https://doi.org/10.1017/RDC.2020.41
Ribeiro P, Xavier R, Santos A, Hawkins S (2009) Reproductive cycles of four species of Patella (Mollusca: Gastropoda) on the northern and central Portuguese coast. J Mar Biol Assoc U K 89:1215–1221. https://doi.org/10.1017/S0025315409000320
Rigaud S, Gutiérrez-Zugasti I (2016) Symbolism among the last hunter–fisher–gatherers in northern Iberia: personal ornaments from El Mazo and El Toral III mesolithic shell midden sites. Quatern Int 407:131–144. https://doi.org/10.1016/j.quaint.2015.10.029
Schöne B, Dunca E, Fiebig J, Pfeiffer M (2005) Mutvei’s solution: an ideal agent for resolving microgrowth structures of biogenic carbonates. Palaeogeogr Palaeoclimatol Palaeoecol 228:149–166. https://doi.org/10.1016/j.palaeo.2005.03.054
Stiner MC, Bicho NF, Lindly J, Ferring R (2003) Mesolithic to neolithic transitions: new results from shell-middens in the western Algarve, Portugal. Antiquity 77(295):75–86. https://doi.org/10.1017/S0003598X00061366
Thomas K, Mannino M (2017) Making numbers count: beyond minimum numbers of individuals (MNI) for the quantification of mollusc assemblages from shell matrix sites. Quatern Int 427:47–58. https://doi.org/10.1016/j.quaint.2015.10.066
Valente MJ (2012) Mesolithic and Neolithic shell middens in Western Algarve: issues in ecology, taphonomy and economy. In: Proceedings of the First Zooarchaeology Conference in Portugal, University of Lisbon, Lisbon, Portugal, pp. 24–32
Acknowledgements
We would like to thank Roberto Suárez-Revilla (IIIPC), Adolfo Cobo-Garcia (UC), Miguel Ángel Fano-Martínez (UR) and Alvaro Arrizabalaga (UPV/EHU) for their scientific support, and Patricia Bello-Alonso (UVigo) for helping us during the experimental collection programme. We are also grateful to Lucia Agudo-Pérez (UC) for their technical support.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona. This research was performed as part of the projects HAR2016-75605-R and HAR2017-86262-P, funded by the Spanish Ministry of Economy and Competitiveness, MINECO, and PID2021-124059NB-I00 and PID2021-124589NA-I00, funded by the Spanish Ministry of Science and Innovation. This investigation was also supported by the Alexander von Humboldt Foundation thought a Humboldt Research Fellowship (no code available). This work was also funded by EarlyFoods (Evolution and impact of early food production systems) (grant number 2021_SGR_00527). During the development of this research AGE was funded by the Basque Country Postdoctoral Programme (grant number POS_2020_2_0032), by the University of La Rioja through a posdoctoral grant (no code available), by the Catalonia Postdoctoral Programme through a Beatriu de Pinós fellowship (grant number 2020 BP 00240) and he is currently working in the framework of a Marie Skłodowska Curie – Postdoctoral Fellowship (grant reference: HEU-101064225-NEARCOAST), funded by the European Union. RAM is supported by the University of Cantabria (grant number: CVE:2019–8461). This work contributes to the ICTA-UAB “María de Maeztu’’ Programme for Units of Excellence of the Spanish Ministry of Science and Innovation (CEX2019-000940-M). PR would like to thank the Max Planck Society for funding.
Open Access Funding provided by Universitat Autonoma de Barcelona.
Author information
Authors and Affiliations
Contributions
We confirm that all authors have approved the final version of the manuscript and have made substantial contributions. AGE and IGZ designed the isotopic project. AGE, IGZ, DCS and MRGM excavated El Mazo cave. AGE and RAM extracted the calcium carbonate samples from Patella depressa shells. AGE, JI and ML conducted the isotopic analyses at the Max Planck Institute for Science of Human History. All authors interpreted the data. All authors wrote and provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García-Escárzaga, A., Gutiérrez-Zugasti, I., Arniz-Mateos, R. et al. Seasonal exploitation of intertidal resources at El Mazo (N Iberia) reveals optimized human subsistence strategies during the Mesolithic in Atlantic Europe. Archaeol Anthropol Sci 16, 76 (2024). https://doi.org/10.1007/s12520-024-01960-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-024-01960-3