Abstract
In humans, breastfeeding and weaning depend on the infant’s needs and physiology but are also influenced by environmental and cultural factors. While infant feeding strategies vary across different regions and historical eras, the associated transition from breastmilk to solid foods is universally thought to be stressful. However, still little is known about infant feeding practices and possibly associated stress in former times. This also applies to the period of transition from classical antiquity to medieval times, which shaped modern Western civilization. To enhance the understanding of childhood nutrition and stress during this period, we first analyzed stable carbon and nitrogen isotopes in serial dentine samples from the first molars of 38 individuals buried in the region once known as the Roman frontier province of Raetia secunda, now encompassing Southern Bavaria. In addition, we investigated the presence of linear enamel hypoplasia (LEH), known to be a marker of unspecific physiological stress, within their dentition. We used this data to create isotope profiles that display dietary changes in comparison with the occurrence of LEH. We found highly variable δ15N and δ13C values and different shapes of isotope profiles which indicate different nutrition of breastfeeding individuals, complementary foods and post-weaning diets, and individual weaning patterns. For most individuals, the weaning process was completed between the ages of two and three. Interestingly, some females of non-local origin show longer weaning periods, likely displaying the influence of different cultural practices in other communities. We also found that LEH most frequently occurred in the post-weaning phase, which supports the assumption that children were at increased risk once breastfeeding had ceased completely. Furthermore, a change in the post-weaning diet in the seventh century coincided with an increased prevalence of LEH, indicating that the foods chosen or available during this time affected the susceptibility of children to stress. In conclusion, our study unveiled diverse infant feeding strategies practiced across various communities, both in different historical eras and geographical locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early childhood is a critical stage in human life. During these initial years, the development and growth of children are significantly influenced by their living conditions, particularly their diet and exposure to stress (e.g., diseases), two factors that are closely intertwined (Caulfield et al. 2006).
Typically, breastmilk serves as the primary source of nutrition for infants. It offers a secure and hygienic supply of essential macro- and micronutrients such as proteins, fats, carbohydrates, vitamins, and minerals (e.g., Ballard and Morrow 2013; Walker 2010). Moreover, it contains substances that help to protect an infant against infection and inflammation (e.g., Walker 2010), while also contributing to the development of the infant’s immune system (e.g., Lönnerdal 2000; McDade 2003). However, breastmilk alone cannot meet the requirements for optimal development of an infant after approximately six months (e.g., Kramer and Kakuma 2004; Jay 2009; Pérez-Escamilla et al. 2019), necessitating the inclusion of complementary foods.
Weaning is the process that starts with the gradual or immediate introduction of non-breastmilk liquids and solid foods, replacing breastmilk, which can result in nutritional stress and raise the risk of exposure to pathogens from contaminated food (e.g., Humphrey et al. 2008, Black et al. 2008). The tradeoff between these risks and the potential impediment to development associated with prolonged exclusive breastfeeding is often termed the “weanlings dilemma” (e.g., Rowland et al. 1978; Katzenberg et al. 1996). Many early bioarcheological studies have inferred that “weaning stress” represents a significant health risk or even threat to survival to infants (e.g., Katzenberg et al. 1996; Dittmann and Grupe 2000).
However, Kendall et al. (2021) criticize that a lot of these studies underrate the complexity of breastfeeding and the processes involved. They argue that the period of greatest risk to children does not occur during weaning but after complete discontinuation of breastfeeding. Fernández-Crespo et al. (2022) have referred to this as the “post-weanling’s conundrum”: a phase of heightened risk characterized by increasing dietary needs and exposure to pathogens with a simultaneous loss of nutritional and antimicrobial support provided by breastmilk while the child’s immune system is not yet fully developed. Additionally, they point out that food choices are critical to health in the post-weaning phase, but that even continued breastfeeding may not fully protect against health risks stemming from contaminated, unsuitable, or quantitatively insufficient foods during the weaning phase.
In any case, the practices of infant feeding and supposedly associated stress are influenced by various cultural, socioeconomic, and environmental factors (e.g., Fildes 1986; Quandt 1995; Sellen 2009; Britton et al. 2015; Eerkens et al. 2017), including dietary traditions, resource availability, or infant sex and health (Bereczkei 2001, Fernández-Crespo et al. 2018, Tsutaya 2017, Chinique de Armas and Roksandic 2018) which can result in different patterns of weaning and stress across different temporal and geographical contexts.
In this study, we investigate dietary changes and physiological stress experienced during the first ten years of life in a group of 38 individuals buried in Late Roman and Early Medieval times within the former Roman province of Raetia secunda (Raetia II). Although these individuals were buried in the same region, some of them spent their childhood in different places, supposedly adhering to various cultural backgrounds and distinct dietary habits (Velte et al. 2023). We characterize infant feeding strategies through serial stable isotope analysis and assess their correlation with the formation of linear enamel hypoplasia (LEH) as an indicator of stress. This not only provides information on different early life histories of people living in Bavaria between the fourth and seventh centuries but also contributes to the ongoing discussion of “weaning stress” and its timing.
Reconstruction of early childhood diet with serial isotope analysis
Analysis of stable carbon (δ13C) and nitrogen (δ15N) isotopes from preserved human tissues offers a unique opportunity to study breastfeeding and weaning practices in past populations (e.g., Fogel et al. 1989, Fuller et al. 2006, Lösch 2009, Eerkens et al. 2011, Bourbou et al. 2019, Siebke et al. 2019, Ganiatsou et al. 2023). A newborn who is exclusively breastfed after birth exhibits an increase in δ15N by approximately one trophic level of around 3.0‰, compared to the breastfeeding female (e.g., Fogel et al. 1989, Millard 2000, Fuller et al. 2006, Herrscher et al. 2017) (Fig. 1). Some studies also noted a rise of approximately one trophic level of around 1.0‰ in δ13C (e.g., Richards et al. 2002; Fuller et al. 2003, 2006) for exclusively breastfed infants, while others found slightly smaller increases of around 0.5‰ (Herrscher et al. 2017) or no increase at all (Fogel et al. 1989). Nonetheless, increasing consumption of complementary foods leads to a gradual decrease in δ15N and δ13C down to the level of the breastfeeding female, i.e., until weaning is completed (e.g., Fogel et al. 1989, Fuller et al. 2006, Fernández-Crespo et al. 2018) (Fig. 1). However, this pattern can deviate when the infant’s diet significantly differs from that of the breastfeeding female. Still, individual variations in physiological and metabolic processes can also affect δ13C and δ15N in the body tissues (e.g., Neuberger et al. 2013, Fuller et al. 2004, 2005, Crowder et al. 2019) of both the breastfeeding female and the infant.
Employing serial isotope analysis of tooth dentine allows us to monitor the course of isotopic values throughout the period of dentine formation within an individual (e.g., Eerkens et al. 2011; Beaumont et al. 2013; Czermak et al. 2020). The development of first molars starts around birth and continues throughout the initial 10 years of life, providing information about the phase of exclusive breastfeeding, the complementary feeding (weaning) phase, and the post-weaning phase. Isotope data derived from specific dentine sections can be assigned to their approximate formation ages to create isotope profiles (e.g., Czermak et al. 2020). Consequently, this approach enables us to estimate the age at which supplementary foods were introduced and when weaning was completed.
Linear enamel hypoplasia as an indicator of physical stress
Linear enamel hypoplasia (LEH) results from a disruption in the enamel secretion by ameloblasts (e.g., King et al. 2002) during tooth development. Thus, LEH presumably indicate unspecific physiological stress during childhood (e.g., Goodman and Rose 1990, Guatelli-Steinberg and Lukacs 1999, King et al. 2005, Hillson 2008). Since tooth enamel is not remodeled, it is possible to estimate the approximate age at which any observed defect was formed.
Over the last decades, several studies have implied a connection between weaning and the occurrence of LEH. While many of them have remained rather vague about during which phase “weaning stress” occurred (e.g., Goodman et al. 1984, Corruccini et al. 1985, Berbesque and Hoover 2018), some have observed an increase in the number of LEH towards or shortly after the end of complementary feeding (e.g., Moggi-Cecchi et al. 1994, Crowder et al. 2019, Orellana-González et al. 2020).
South Bavaria between Roman times and the Middle Ages
Southern Bavaria was initially integrated into the Roman Empire in the first century, forming a part of the province Raetia. After the province was subdivided in the late fourth century, it became part of Raetia II. During the Roman Imperial period, the region experienced progressing urbanization and intensive trade led to the development of a healthy economy that presumably formed a thriving society in Southern Bavaria (e.g., Fehr 2010). However, this situation potentially changed as several crises began to accumulate, such as recurring attacks of German tribes from the mid-third century onwards and increasing supply shortages during the fourth century. The Roman administration and frontier defense for the entire pre-Alpine region eventually ended in 476 AD when the Germanic leader Odoacer replaced the last Western Roman Emperor. Little is known about the subsequent events in the region that was once Raetia II. But in the middle of the sixth century, Bavaria became a dukedom under Frankish rule, which lasted until 751 AD (e.g., summarized in Haas-Gebhard 2013).
In a previous study, we showed that part of the individuals buried in the former province of Raetia II around 500 AD spent their childhood in a region with differing strontium isotope signatures (Velte et al. 2023). Our findings also indicated that individuals exhibiting unusual dietary habits, e.g., frequent consumption of millet, a C4 plant that was rather uncommon in Bavaria during that time (e.g., Hakenbeck et al. 2010), likely have foreign origins. This was especially notable among some women with artificial cranium deformation (ACD) (Velte et al. 2023). Interestingly, Veeramah et al. (2018) demonstrated that most of these women show a genetic similarity to people living in the South-East European region.
In this study, our aim is to explore whether the profound political, social, and economic changes that occurred during the transition from Antiquity to the Middle Ages, or potential cultural differences related to people’s origins, had any influence on child feeding strategies.
Material
The human remains of the individuals studied (n = 38) were excavated at four burial sites in Bavaria (Fig. 2). Most of these individuals (n = 32) were uncovered at two cemeteries in Erding: Late Roman Erding-Kletthamer Feld (EKF, n = 4) and Early Medieval Altenerding-Klettham (AED, n = 28). The small cemetery of EKF (13 inhumations) dates from the second half of the fourth century AD until the first half of the fifth century AD (Sofeso et al. 2012). The burial site of AED came into use in the second half of the fifth century, and burials there continued well into the seventh century (Sage 1984, Losert and Pleterski 2003). It stands as one of the biggest Early Medieval cemeteries excavated in Bavaria, comprising about 1450 graves. We selected 17 individuals dating around 500 AD, including two women with ACD and five other individuals of documented non-local origin (Veeramah et al. 2018; Velte et al. 2023), and eleven burials dating from the seventh century AD. In addition, we included individuals from two sites located less than 100 km away, namely, Straubing-Bajuwarenstraße (STB) and Burgweinting (BW), due to the presence of ACD in three cases and unusual dietary patterns likely linked to non-local origin (Codreanu-Windauer and Harbeck 2016, Trautmann et al. 2017, Veeramah et al. 2018, Velte et al. 2023). The cemetery STB was occupied from the mid-fifth until the mid-seventh century AD, containing over 800 graves (Geisler 1998). From this site, we included three individuals dating around 500 AD (Veeramah et al. 2018; Velte et al. 2023). Furthermore, we analyzed three individuals of a small grave group (15 inhumations named grave group A) from BW (BWA), dating between the late fifth to the early sixth century AD (Zintl 2012, Codreanu-Windauer and Schleuder 2013).
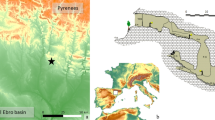
(Left) Map of Germany with its federal states; the study area (shown in detail on the right) is backed by a gray box; dashed lines indicate the extent of the first duchy of Bavaria (after Weiß 2021) and dotted areas the presumed territory of Raetia II (after Haberstroh and Harbeck (2013)). (Right) Part of southern Bavaria with the geographical positions of the archaeological sites included in this study (AED, Altenerding-Klettham; EKF, Erding-Kletthamer Feld; STB, Straubing-Bajuwarenstraße; BWA, Burgweinting Group A). Map data: EU-DEM © European Union, Copernicus Land Monitoring Service 2017, European Environment Agency (EEA).
Many individuals included in this study had been previously investigated, providing valuable data on age at death and sex, the presence of LEH, the presence of ACD, strontium isotopes, genetic ancestry, dating and δ13C and δ15N ratios in bone, and bulk values of first molar dentine (Hakenbeck et al. 2010, Sofeso et al. 2012, Codreanu-Windauer and Schleuder 2013, Codreanu-Windauer and Harbeck 2016, Harbeck et al. 2016, Trautmann et al. 2017, Veeramah et al. 2018, Toncala et al. 2020, Neidich 2023; Velte et al. 2023). Detailed information on all data used and the corresponding literature are listed in the Supplementary (Table S2).
All individuals in the study were adults (> 20 years), except one juvenile individual (AED_1053: 13–20 years). The sample set comprises a slightly higher number of females (n = 21) compared to males (n = 14), with sex being indeterminable for three individuals (AED_100, AED_217, AED_724) due to poor preservation.
Based on the analysis of strontium, carbon, and nitrogen isotopes as well as genetic data, some individuals from around 500 AD in this study (n = 23) can be reasonably assumed to be migrants (n = 13, AED + STB + BWA), which includes five females with ACD (as discussed in Velte et al. 2023). Those individuals who showed no indication of foreign origin are considered part of the “local” population (n = 10, AED), although it cannot be ruled out that unrecognized migrants are among them (also see Velte et al. 2023).
For burials dating from the first half of the seventh century (n = 11, AED), or between the second half of the fourth century and the first quarter of the fifth century (n = 4, EKF), such a classification cannot be made, because the corresponding data are not available for all samples.
Methods
δ13C and δ15N ratios in collagen were analyzed from both bone and serial dentine samples. Bone samples from adults provide information about dietary patterns during a later life stage, due to tissue turnover, but a more precise timespan reflected by measured isotope values cannot be determined due to varying turnover rates (Hedges et al. 2007). In contrast, dentine is not remodeled once formed during early life, which allows us to associate measured values with the specific examination of chronological age (e.g., Czermak et al. 2020).
Bone collagen was extracted following the protocol described in Siebke et al. (2019) (also see Velte et al. 2023 and Supplementary S1.1). Measurements were carried out at the Isolab GmbH in Hanau using an Elementar Vario Cube EL connected to an Isoprime mass spectrometer. Accuracy and precision were verified through replicate analyses of laboratory standards (e.g., Collagen STD R (USGS 89), Collagen STD S, Collagen STD BRA), calibrated to international standards USGS40 and 41. The Isolab GmbH ensures an analytical precision of 0.1‰ for δ13C and 0.2‰ for δ15N.
Serial dentine sampling from the first molars and collagen extraction followed the procedure described in Velte et al. (2023) (for detailed method description see Supplementary S1.1). The samples were analyzed at the GeoCenter (Friedrich-Alexander University, Erlangen-Nürnberg), utilizing a Flash EA 2000 elemental analyzer connected to a ThermoFinnigan Delta V Plus mass spectrometer. Analytical precision ensured by the GeoCenter is 0.1‰ for δ13C and δ15N checked by replicate analyses of laboratory standards (e.g., Casein, Cyclo) calibrated to international standards USGS 40 and 41.
The results are reported in the conventional δ-notation in per mil (‰) relative to internationally accepted standards, VPDB for carbon, and AIR for nitrogen. The collagen quality of each sample was evaluated through the C/N atomic ratio, as well as carbon and nitrogen content.
Chronological age assignment of sampled dentine sections to illustrate changes in stable isotopes over time was made using a scheme developed in a prior study (Velte et al. 2023). This scheme is based on The London Atlas of Tooth Development and Eruption (AlQahtani et al. 2010) and takes potential wear into account for each tooth.
We defined weaning age as the approximate age at which either breastfeeding is finally terminated, reduced to a low amount, and/or when the isotope signal is masked by the consumption of higher protein sources. To estimate individual weaning age, a customized scheme based on the relative decrease in δ15N values at the beginning of the isotope profiles is employed: Starting with the first dentine section, any dentine section showing a depletion in δ15N by at least 0.2‰ (analytical error) compared to the previous section (n1, n2, …, n↓end) (Fig. 3A) is given a new value representing the relative decrease in δ15N (↓δ15Nrel). This value is calculated as the difference between the ratio of the last section in the series with continuously decreasing values (n↓end) and the ratio of the respective section (↓δ15Nrel(n1, n2, …, n↓end) = δ15N(n↓end) − δ15N(n1, n2, …, n↓end)). ↓δ15Nrel values are then plotted against the approximate formation age of dentine sections (Fig. 3B). The intersection of the linear trend line for normal tooth development and the y-axis is determined as the weaning age (line function: f(x) = m × x + t; with t = weaning age, x = decline, m = slope). The error caused by the biological variation in tooth development is determined by using dentine formation ages for both early and late tooth development (Fig. 3B).
Schematic illustration of the model used to estimate weaning ages based on the relative decrease in δ15N ratios of successive dentine sections. A Example of dentine sections from a stable light isotope profile. Only dentine sections with a constant decline in δ15N ratios (by min 0.2‰) at the beginning of the isotope profiles are selected. In this example down to a total drop of 3.2‰. Samples are given a new value, representing the relative decrease in δ15N. This value is calculated as the difference between the ratio of the last section in the series (n↓end) with continuously decreasing values and the ratio of the respective section (↓δ15Nrel(n1, n2, …, n↓end) = δ15N(n↓end) − δ15N(n1, n2, …, n↓end)). B Plotting of the ↓δ15Nrel ratios of dentine sections against the chronological formation age of dentine samples (early, normal, and late development) including linear trend lines. Weaning age is defined as the intersection of the linear trend line for normal development and the y-axis: f(x) = m × x + t, with t = weaning age, x = decline, m = slope/linear trend. The error is shown by the functions for early and late development.
This approach can be used to estimate individual weaning ages (as exemplarily shown in Fig. 3B) but it can also be applied to evaluate weaning age on a population level by combining data from all individuals or groups, enabling the assessment of inter- or intra-populational differences (also see Figure S1.2.2).
Data on linear enamel hypoplasia (LEH) were collected on permanent maxillary and mandibular teeth. Rather than analyzing general stress levels, we aimed to investigate a link between LEH formation and weaning. Thus, we only analyzed teeth that develop during the supposed weaning period: incisors and canines. We recorded the number and position of observable palpable LEH (a rippled pattern was detected by scratching the surface with the fingernail) on teeth from the right side of the mouth, using the corresponding teeth from the left side only if the right tooth was unavailable. Teeth that had lost over 50% of their crown height, e.g., due to wear, or were missing, were marked “not assessable.”
The SNSB, Bavarian State Collection of Anthropology, which houses the human remains, specifies in its osteological record form (Harbeck et al. 2020) that the position of LEH should be assessed by dividing the tooth into three equal parts: top, middle, and bottom. For some of the individuals in our study, this data was already available in this format, and we recorded LEH for the remaining individuals following the same manner. To determine the age span during which these crown parts are formed, we provide enamel growth curves for early, normal, and late tooth development in incisors and canines of the upper and lower jaw, based on AlQahtani et al. (2010) (see S1.3 for more details). Linear trend line equations from these curves were used to calculate the age span during which a crown part is most commonly formed (normal development) and the error resulting from the biological variation in tooth development (early and late development).
Statistical data analysis was performed using IBM SPSS 29.00 and RStudio 1.4.1717 for Windows. Statistical tests on metric data were exclusively non-parametric (independent-sample Mann–Whitney U and Kruskal–Wallis tests) due to small sample sizes. Nominal data was analyzed by a non-parametric chi-square test. For multiple comparisons, significance values have been adjusted by the Bonferroni correction to counteract alpha error accumulation. Graphics were created using QGIS 3.22 and Microsoft Excel for Windows.
All statistical analysis and more detailed results are given in the Supplementary (S1.2–3).
Results
Collagen quality
Collagen quality of dentine (n = 350) and bone specimens (n = 11) was monitored, generally accepting C/N atomic ratios between 2.9 and 3.6 (DeNiro 1985), as well as a content of carbon from 13 to 50% and of nitrogen from 4.8 to 19%) (largely following DeNiro 1985, Ambrose 1990, Rand et al. 2015). Dentine samples (n = 15) with higher contents were excluded.
Isotope profiles
Intra-tooth isotopic patterns (isotope profiles) of all 38 individuals, illustrating changes in stable light isotope ratios throughout the first 10 years of life are shown in Fig. 4. We proceed with the assumption that tooth development was normal and identify changes of isotopy only accepting a minimum difference of at least 0.2‰ for δ13C and δ15N values.
Carbon and nitrogen isotope profiles (n = 38). Colored frames: dating of burials (orange = second half fourth century to first half fifth century AD; blue = around 500 AD; green = seventh century). Data points: δ13C or δ15N values of the first molar dentine sections assuming normal dentine development, small dashed lines highlight changes in δ13C or δ15N between successive sections. Error bars: variations in early or late tooth development. Horizontal red and blue dotted lines represent individual 13C or δ15N bone values. Shaded red and blue areas display intra-populational variability ranges of δ13C or δ15N in Erding (δ13C: − 20.7 to − 18.5‰, δ15N: 7.9 to 11.1‰), Straubing (δ13C: − 20.8 to − 18.9‰, δ15N: 8.6 to 10.3‰), and Regensburg (δ13C: − 21.2 to − 18.7‰, δ15N: 7.8 to 10.5‰) (Velte et al. 2023). Vertical green lines: big dashed line = calculated individual weaning age, smaller dashed lines = variation assuming early or late tooth development. Dental charts show the present teeth (filled teeth), sampled molars (yellow), and evaluated anterior teeth for the presence of LEH (dark grey). The formation of LEH is shown below the isotope profiles. Filled bars represent the age span in which the corresponding crown third was formed (assuming normal enamel development), error bars display variations assuming early or late tooth formation. Red signs indicate individuals with incomplete anterior dentition, so the absence of LEH must be evaluated with caution.
Weaning phase
We observe no period of exclusive breastfeeding, indicated by the first rising δ15N values (Fig. 4). In most isotope profiles (n = 36), δ15N ratios continuously decrease from the beginning of observation (Table 1, Fig. 4), marking the weaning phase with complementary feeding. A concomitant initial decrease in δ13C values is also observed in many profiles (n = 28), but which often ends earlier than the drop in δ15N (n = 13). In contrast, in some individuals, δ13C remains stable (n = 6) or increases (n = 2) in the earliest part of the isotopic profile, while δ15N simultaneously decreases (Table 1).
Overall, values and intra-tooth patterns of δ13C and δ15N are highly variable in the initial part of the profiles. The levels of the first dentine sections, as well as the extent and rate of the continuous decrease over two or more sections, vary markedly (Table 1).
The δ15N ratios of the first dentine sections are almost universally increased compared to both bone ratios (n = 37) and post-weaning levels (n = 38). This is in line with the expectation that the trophic level increase due to breastfeeding can still be observed at the beginning of the intra-tooth isotopic pattern. The δ13C ratios, however, are similar or lower compared to bone (n = 15) and/or post-weaning levels (n = 9) in some cases (Table 1), as anticipated, given the lesser impact of weaning on carbon.
In most isotope profiles, the extent of the observable decrease in δ15N and δ13C (n = 25) falls within the range of the expected trophic level effect of breastfeeding (~ 3.0‰ in δ15N, ~ 1.0‰ in δ13C, e.g., Fuller et al. 2006). In some cases, values decrease to a greater extent in δ15N (≥ 3.2‰, n = 8) and δ13C (≥ 1.2‰, n = 6) (Table 1). Non or minimally decreasing δ15N values (< 1.0‰) are only found in individuals (n = 5) with missing first dentine samples, consequently showing the weaning phase only towards its end or not at all.
The decrease of δ15N and δ13C is generally faster at the beginning and slows down towards the end (Table S2.2). Higher rates are predominately found in individuals whose profiles show a greater total decrease in isotope ratios, while lower rates are mostly found in profiles with a smaller total decrease (Kruskal–Wallis test p(δ15N) < 0.001, p(δ13C) = 0.003, Table S1.2.9). In some cases (AED_343, AED_416, STB_361*, BWA_10254*), δ15N decreases rather gradually at a smaller rate (≤ 1.5‰ per year) but still exhibits a notable extent of decline (1.5 to 2.5‰) (Table S2.2). Nevertheless, in certain cases δ15N (n = 11) or δ13C ratios (n = 5) decrease discontinuously, i.e., ratios decrease after a short stabilization or even increase. However, this second decrease mainly (n = 9) follows a prior constant decline in δ15N (e.g., AED_105, AED_1129), whereas a discontinuous drop in δ13C only occurs if values were either stable or increasing at the very beginning (e.g., AED_211, AED_343) (Table 1, Fig. 4).
Age of complete weaning
At the population level, weaning was completed by the age of 3.0 years (Figure S1.2.1). This corresponds to the average of individual weaning ages (3.0 ± 0.7 years (sd)). Notably, individual weaning ages vary significantly, ranging from as early as 1.9 years to as late as 4.4 years (Table 1). Additionally, the Gaussian kernel density distribution of weaning ages shows two side peaks at over 3.0 years, however, a clear main peak is found around 2.5 years (Fig. 5).
Estimated individual weaning ages considering normal tooth development. Error bars display deviations for early and late tooth development. The Kernel density estimation (KDE) in the lower part is based on a Gaussian kernel. The optimal bandwidth for the Gaussian kernel was calculated using the “solve the equation” method by Sheather and Jones (1991).
Post-weaning phase
Values and intra-tooth patterns of δ13C and δ15N are also significantly variable within the post-weaning phase. Post-weaning levels, assigned as the mean value of dentine ratios covering 3 years following individual weaning ages, show a great range and different extent and direction of fluctuations (Table 1, Fig. 4).
δ15N (n = 3) or δ13C (n = 5) post-weaning levels only rarely match individual bone ratios (± 0.1‰). Instead, they tend to be more or less equally frequent found above (δ15N: by 0.2 to 2.3‰, n = 20; δ13C: by 0.5 to 4.3‰, n = 15) or below bone (δ15N): by − 0.2 to − 2.4‰, n = 15; δ13C: by − 0.2 to − 1.6‰, n = 18) (Table 1). The δ15N values frequently (n = 30) exhibit an increase from the lowest point of the initial decrease, including the observed second drops, towards the end of the profiles, creating the impression of a post-weaning dip (e.g., AED_249, AED_1119) (Fig. 4). However, the visibility of this dip varies; in some cases, it is not clearly visible due to generally more fluctuating ratios (e.g., EKF_1663, BWA_10075), or because the later increase is of lower extent (e.g., EKF_1719, AED_204) or shorter time (e.g., AED_125*, AED_724) (Fig. 4). Four individuals (AED_154, AED_249, AED_501, AED_1053) show covariant decreasing δ13C values during the later increase in δ15N.
Group differences
Intra tooth patterns are highly variable within all defined groups. However, we identified some general characteristics and group differences.
Late Roman individuals (EKF, n = 4) and the “locals” from around 500 AD (AED, n = 10) show similar ranges of ratios in the first dentine sections, as well as the extent or rate of decreasing values, post-weaning levels, and bone ratios (Table 1, Fig. 6). Their post-weaning levels often align or are slightly above their individual bone values (Table 1). In both of these groups, single individuals display increased δ13C values in dentine, at least temporarily (EKF_1663, EKF_1700, AED_154), corresponding with the levels observed in some migrants from around 500 AD.
Group differences of stable light isotope ratios in populations from Erding (EKF, AED). Filled data points show individual values for each stage. Additional comparative bone data are given as unfilled points. Comparative data are taken from Sofeso et al. (2012) for Late Roman individuals (EKF), Hakenbeck et al. (2010), and Velte et al. (2023) for individuals from around 500 AD, and Neidich (2023) for individuals dating from the seventh century (AED). Information about individual’s provenance is taken from Velte et al. (2023).
In contrast, generally lower δ13C dentine ratios are found in individuals dating from the seventh century (AED, n = 11) (Table 1, Fig. 6). The δ13C ratios in their first dentine sections show a trend towards lowered values, often (n = 5) falling below their individual bone values (Table 1), which are found within a range similar to that of earlier times (Table 1, Fig. 6). Furthermore, their post-weaning δ13C levels following the weaning dip are significantly decreased in comparison to the levels of the “locals” from around 500 AD (pairwise comparison p = 0.004, Table S1.1.23). Although the mean difference is even greater when compared to Late Roman individuals (Table 1), it remains non-significant, probably due to the small sample size and increased variability within the group. Additionally, δ15N ratios show similar ranges in the first dentine sections, extent of decrease, and post-weaning levels in comparison to individuals dated into Late Roman times (Table 1, Fig. 6). However, the post-weaning δ15N levels are significantly lower than their individual bone values (paired samples test p = 0.004, Table S1.2.25). Moreover, the bone ratios of individuals from the seventh century are significantly increased compared to Late Roman individuals (pairwise comparison p = 0.002, Table S1.2.38) and the “locals” from around 500 AD (pairwise comparison p = 0.015, Table S1.2.38).
In summary, the estimated weaning ages are remarkably variable across all periods, including prolonged weaning (weaning age > 3.0 years) (Fig. 5). Thus, no significant chronological change in weaning age is found. Instead, chronological differences in δ13C and δ15N ratios result from changes in the seventh century.
However, we found some significant differences linked to individuals’ origin around 500 AD. Migrants (AED + STB + BWA, n = 13) generally exhibit more variable results at the beginning of the observation and in post-weaning levels compared to the “locals” (AED, n = 10). While δ15N ratios of first dentine sections and post-weaning levels tend to differ only slightly from “locals,” δ13C ratios are, on average, significantly increased in the first dentine sections (Mann–Whitney U test p = 0.006, Table S1.2.1) and in post-weaning levels (independent-samples Mann–Whitney U test p = 0.003, Table S1.2.1) (Fig. 6).
Moreover, discontinuously and more strongly decreasing δ13C ratios in the earliest parts of profiles are exclusively found in migrants (Table 1), but also stronger fluctuations in δ13C after weaning were primarily observed in non-locals (Fig. 4). Both δ15N or δ13C values in post-weaning dentine of migrants differ significantly from their bone ratios (paired samples test p(δ15N) = 0.006, p(δ13C) = 0.019, Table S1.2.25), which in turn do not differ from bone ratios of the “locals” (Fig. 6). Differences between post-weaning and bone ratios are more pronounced in female migrants (AED + STB + BWA, n = 10) compared to male migrants (AED, n = 3) (independent-samples Mann–Whitney U test p = 0.028, Table S1.2.19). This difference can be linked to a sex-dependent difference among migrants: δ13C ratios of the first dentine sections of female migrants are, on average, significantly increased compared to male migrants (Mann–Whitney U test p = 0.007, Table S1.2.2), as well as post-weaning δ13C levels (independent-samples Mann–Whitney U test p = 0.049, Table S1.2.19). Additionally, more increased post-weaning δ15N levels are primarily found in female migrants (Table 1). Although we did not find a significant difference between migrants and “locals” in estimated weaning ages, a clear trend towards later weaning in migrants is noticeable. Among the group of non-locals, females, particularly those with ACD, more often show prolonged weaning (weaning age > 3.0 years) (Fig. 5). Thus, the observed differences between migrants and “locals” are primarily based on non-local females, while male migrants and single female migrants do not exhibit major differences to the “local” population from around 500 AD, in which no differences between men (n = 5) and women (n = 5) were found.
Linear enamel hypoplasia (LEH)
The preservation of the anterior dentition was highly variable (Fig. 4). LEH data from individuals with an incomplete dentition (less than three teeth assessable) is labeled with an exclamation mark (n = 6) in Fig. 4. One individual with a completely missing dentition was excluded from the analysis (Table 2). The incidence of LEH varies, with the highest count being 12. Most individuals have fewer than four (n = 14), averaging to 1.9 ± 3.0 (sd) LEH. Slightly over half of the individuals display at least one LEH (n = 21). The development of LEH happened between 0.8–1.9 years and 4.5–5.9 years, on average at age 3.0–4.2.
The number of LEH significantly correlates with the mean formation age (midpoint of determined formation span) of the first LEH (Pearson correlation p = 0.014, Table S1.3.7), thus, individuals with LEH appearing at an early age tended to develop more LEH.
The average age at the formation of the first LEH (2.7–3.8 years, Table 2) corresponds well with the mean age at the cessation of weaning. However, on an individual level, the initial formation of LEH only rarely matches the estimated weaning age (n = 5, Fig. 4). In most individuals, the first LEH developed with a temporal offset (≥ 0.5 years) relative to the estimated weaning ages, either before (n = 8) or after (n = 8) weaning (Fig. 4).
Regarding the total number of LEH, only a few defects appear during the weaning phase (n = 13, 17.6%), primarily observed in individuals (n = 6, 75.0%) with noticeable late weaning (≥ 3.4 years). The largest number of LEH formed around the age of complete weaning (n = 25, 33.8%) or in the post-weaning phase (n = 36, 48.7%). Apparently, they most frequently occur during the supposed post-weaning dip in δ15N ratios (Fig. 4).
Group differences
We found no indications of differences in the presence, number, or timing of LEH associated with individuals’ origin or between sexes among the “locals” from around 500 AD (Table 2). Regarding the timing of defects, there is also no clear difference between the diachronic time phases (Table 2).
However, we observe a difference in individuals from the seventh century (n = 11, AED) compared to earlier diachronic phases (Late Roman: n = 3, EKF; “Locals” ~ 500 AD: n = 10, AED), at least in the presence and number of LEH. They display LEH more frequently (Table 2) and with significantly higher prevalence (independent samples Kruskal Wallis test p = 0.026, Table S1.3.10), compared to the “local” individuals from around 500 AD (pairwise comparison p = 0.027, Table S1.3.11), but not in comparison to Late Roman individuals, most likely due to the small sample size (Table 2).
Moreover, it is noteworthy that the two individuals from the seventh century with the highest numbers of LEH (AED_213: 11, AED_100: 12) died in early adult to adult age (Fig. 4).
Discussion
General observations and limitations
Unsurprisingly, we could not detect the phase of exclusive breastfeeding or the introduction of complementary foods in any individual in our sample (see Fig. 1), as our observation begins only after the age of 6 months. From that age onward, complementary food is necessary to satisfy the growing need of the infant for energy and nutrients (e.g. Michaelsen et al. 2000, Dewey and Brown 2003). Nevertheless, the weaning process, indicated by the “weaning dip” becomes visible in almost all profiles: it is clearly noticeable in δ15N but less obvious in δ13C. As the trophic level effect is smaller and the impact of supplementary foods is stronger on δ13C, the values align faster to the breastfeeder’s value (e.g., Fuller et al. 2006).
The observed variability of both isotope ratios within the weaning phase can be attributed to various factors, including the potential onset of the weaning before observation starts, the progression of the weaning process after the start of observation, the nutrition and physiology of the breastfeeding female, different breastfeeding practices, different weaning foods, as well as variations in the physiology and health of the infant (e.g., Fuller et al. 2006; Crowder et al. 2019; Henderson et al. 2022). However, this variability increased significantly with the inclusion of individuals who grew up in different geographic locations or during different historical periods.
A distinct pattern is apparent in most profiles in all groups: increasing δ15N ratios after cessation of weaning creates the impression of a “post-weaning dip.” Depleted δ15N values in the post-weaning phase before a subsequent rise were observed in many other studies across diverse populations from various periods and socio-economic backgrounds (Beaumont et al. 2012; Eerkens et al. 2011; Fernández-Crespo et al. 2018; Henderson et al. 2014). This pattern indicates that the proportion of animal protein only later reaches a level comparable to that of adults. However, post-weaning values below bone level must not exclusively be linked to diet but may also be influenced by a general cause, such as physiological features (e.g., Henderson et al. 2022) e.g., related to growth (e.g., Waters-Rist and Katzenberg 2010). In our study, δ15N values of the “post-weaning dip” drop less frequently below individual adult bone values in contrast to previous studies, but we assume that although less clear, this is still comparable.
In our method for calculating the weaning age, we include the lowest point of the δ15N curve in the calculation. Since this point often lies within the post-weaning dip, which is also influenced by factors that are independent of breastfeeding, it can be assumed that the weaning age may be overestimated. Furthermore, some patterns within profiles can be misinterpreted using our method, e.g., discontinuously decreasing δ15N ratios during the weaning process. These values may result from variable breastfeeding frequencies, changing trophic levels of weaning foods, or physiological stress (Fuller et al. 2005; Mekota et al. 2006; Reitsema 2013). Thus, subsequent decreasing δ15N ratios may also belong to the actual weaning dip but are not considered in the calculations of our weaning ages.
However, these problems are not unique to our method. Especially the inclusion of the lowest δ15N ratios of the “post-weaning dip” is a common practice (e.g., Scharlotta et al. 2018). While a heavily pronounced post-weaning dip could also pose an issue for the method recently published by Ganiatsou et al. (2023), it can be assumed that this method is less sensitive to discontinuously decreasing δ15N ratios. The tool they developed, the WEaning Age FiNder tool (WEAN), employs fitted curves that compensate for such fluctuations. Nevertheless, compared to Ganiatsou et al. (2023), our method offers the advantage of presenting results as age ranges, allowing for a better illustration of the uncertainty of weaning ages due to the biological variability of tooth development.
Regardless of the methodological approach, the calculation of weaning ages should only be seen as an approximation for various reasons. Changes in the protein source potentially appear with a temporal offset of several weeks to month in isotope profiles (e.g., Waters-Rist et al. 2006, Chinique de Armas et al. 2022). This individually delayed onset can hardly be reconstructed, making precise weaning age calculations nearly impossible. Additionally, the temporal resolution of the isotope profiles is limited by the sampling method and by the age assignment of the samples, resulting in rather broad age ranges for certain data points or events derived (Figs. 4 and 5).
The latter also applies to the calculation of LEH formation ages. The less precise determination of the position of the LEH used here (see Methods) further increases the error beyond normal biological variability.
Weaning time and process
Most individuals exhibit a rather abrupt weaning process, with rapidly decreasing δ15N ratios. A rapid decline can result from low-trophic complementary foods with limited protein supply leading to less expressed fractionation of 15N/14N and thus to a decrease in δ15N of the body’s nitrogen pool (e.g., Henderson et al. 2022). Another possibility is that higher amounts of protein in complementary foods result in a faster decline of δ15N as it more efficiently dilutes the infant’s nitrogen pool (e.g., Fuller et al. 2006). However, we consider the first scenario to be more likely, as many typical weaning foods (e.g., bread and cereal porridge) are high in carbohydrates and low in protein (e.g., Sellen and Smay 2001), which is also consistent with Roman and Medieval written sources (e.g., Prowse et al. 2008, Bourbou and Garvie-Lok 2009, Dasen 2015).
We observe a high variability in weaning ages within predefined groups from Late Roman times, around 500 AD and the seventh century. Although some of this variation may be related to the methodological limitations mentioned above, it underscores that breastfeeding and weaning practices can differ significantly within a population, e.g., through adaptation to infant’s health and needs, the availability of resources probably dependent on the season, but also social ideals and status, etc. (e.g., Bourbou and Garvie-Lok 2009, Bourbou et al. 2013, Bourbou et al. 2019). Our findings indicate that most individuals are fully weaned around the middle of the third year, although we also observe more extended weaning periods of up to 4 years.
This pattern is consistent with the historical context of the complete cessation of breastfeeding at around 3 to 4 years of age in the Roman provinces, as recommended in ancient texts by the Greek medic Galen, while at the Center of the Roman Empire, breastfeeding typically ceased earlier around 2 years, following the recommendations of another Greek physician, Soranus, which is corroborated by bioarcheological data from the Classical, Hellenistic, and Roman periods (summary in Fulminante 2015). Although mainly shorter weaning ages between 1 and 2 years were observed in Continental Europe during the Early Middle Ages (Fulminante 2015), some studies suggest that breastfeeding also ended rather late around the age of three in some communities (Haydock et al. 2013, Herrscher 2003). Dittmann and Grupe (2000) calculated the weaning age based on bone collage values from the remains of deceased children buried in a cemetery from the same time and region of our study. Interestingly, they also found a rather late weaning age of 3 years for these “non-survivors.” In contrast, it is often assumed that individuals who were not breastfed or only for a short period tend to have a lower survival rate (e.g., Stuart-Macadam & Dettwyler 1995; Bourbou et al. 2019; Fernández-Crespo et al. 2022). Consequently, one might expect to observe a higher average weaning age in our study, which in turn exclusively examined survivors.
This underscores the need for additional data on subadults and adults from the same graveyards to get a more detailed picture of infant diet and associated stress in Late Roman and Early Medieval Europe.
Weaning stress
LEH observed in our sample indicates that stress, which manifests as enamel defects, only rarely occurs during the progressing replacement of breastmilk with non-breastmilk liquids and foods. This demonstrates that the weaning process does not represent an intrinsic stress factor for infants (Kendall et al. 2021), but also that continued breastfeeding may not fully protect them from all stressors. The increase in LEH formed at the end of the weaning process or in the post-weaning phase in our sample supports the assumption that the final cessation of breast milk as a source of nutrients and immune protection initiates a high-risk phase, a “weaning conundrum,” as suggested by Fernández-Crespo et al. (2022).
An increase in δ15N (Fuller et al. 2005; Mekota et al. 2006; Reitsema 2013), especially with a simultaneous decrease in δ13C (Beaumont et al. 2018; Beaumont and Montgomery 2016, Craig-Atkins et al. 2020) is often considered a sign of physiological stress. In our study, this pattern is observed in only a few individuals and is never clearly associated with LEH formation. This correlation is perhaps not necessarily to be expected, since different types, severities, and durations of physiological stress do not necessarily have the same effect on enamel formation and isotope composition. However, LEH formation is often correlated with the observed post-weaning dip in δ15N ratios, suggesting that infants may be more susceptible to stressors during this phase, possibly related to growth or a low-trophic diet (see above). Nevertheless, far more data is needed to confirm this connection.
Furthermore, we found a positive correlation between the formation age of the first LEH and the total frequency of LEH, a trend also reported previously (e.g., Berbesque and Hoover 2018). This supports the hypothesis that individuals exposed to stressors earlier in life may be less resilient to subsequent crises due to damaged immunological competence (Goodman and Armelagos 1989; Armelagos et al. 2009, Larsen 2015). Previous studies have also linked higher LEH frequencies to an earlier age-at-death (e.g., King et al. 2005; Boldsen 2007; Armelagos et al. 2009), which could explain why the two individuals with the highest numbers of LEH died at early (adult) ages (AED_100, AED_213).
Nutrition and early life histories: links to diachronic changes
We did not find significant differences in weaning patterns or weaning ages related to the considered diachronic phases.
However, we observe variations in δ13C and δ15N values in dentine as well as bone collagen values among individuals who potentially grew up in the same region around Erding but during different chronological times. We assume that this is probably related to diachronic changes in infant feeding practices and/or economics.
While the isotope ratios of diachronic groups generally show great overlap (Fig. 6), we only observe increased δ13C ratios in the dentine of single individuals dating into Late Roman times or around 500 AD (Figs. 4 and 6). This could be related to millet consumption, either indirectly through breastmilk or directly as weaning foods. Hence, this may also be a potential hint at a previously undetected non-local origin of these individuals. However, sporadic millet cultivation cannot be ruled out, although millet was not commonly used in Bavaria during this time (e.g., Hakenbeck et al. 2010; Velte et al. 2023).
People living in Bavaria between Roman and Medieval times primarily consumed a C3 plant-based terrestrial diet (e.g., Hakenbeck et al. 2010, Sofeso et al. 2012, Velte et al. 2023). The ratio of animal protein to plant content can only be estimated by comparing contemporaneous material, ideally from the archaeological context. However, only animal remains are available for the Late Roman site EKF, where concentration-dependent mixture models suggest an average of 25% animal vs. 75% plant content in the human diet (Harbeck and von Heyking 2014). Similar human bone isotope values of individuals from AED dating around 500 AD suggest comparable ratios if we assume the same “baseline” with unchanged δ15N values of the consumed plants and thus animals.
Assuming an unchanged baseline, the significant increase in δ15N bone ratios of individuals from AED dating into the seventh century indicates a higher proportion of animal protein. Increased δ15N bone ratios were also found in other burial sites in Bavaria dating to the seventh century (Strott 2007, Czermak 2011) and were interpreted as an increase in protein content associated with intensification of livestock and dairy farming (e.g., Fehr 2019). However, this increase can just as well display a baseline shift related to changes in mixed farming practices through the beginning agricultural revolution (e.g., Fehr 2019), e.g., more intensive land use and increased fertilization (e.g., Bogaard et al. 2007; Fraser et al. 2011). Confirmation of a baseline shift would require sufficient data of different animal genera from the site, which is currently lacking.
The two interpretations of the bone collagen isotopes (“higher protein” vs. “different baseline hypothesis”) also affect the understanding of the observed differences from the post-weaning diet. In individuals from Late Roman times and around 500 AD, we see post-weaning δ15N levels within the range of adult collagen ratios, suggesting that infants had an adult-like diet during this time (e.g., Fournier et al. 2022). However, for the seventh century, we observe post-weaning δ15N levels that are lower than the comparatively increased values we observe in adults of this time but consistent with the post-weaning δ15N levels in children from the earlier times. If we follow the “higher protein hypothesis,” i.e., the increased bone levels in adults result from a higher amount of animal protein, it raises the question of why the protein intake increased in adulthood but not in childhood in the seventh century compared to earlier times. Additionally, the post-weaning diet in the seventh century appears not different from the previous century in δ15N values but in δ13C ratios, which are lower. If we consider the “higher protein hypothesis,” additional explanations are needed. Lower δ13C values can indicate a changing plant spectrum, e.g., towards a greater diversification (e.g., Lewit 2009, Zach 2019) or smaller amounts of specific plants (e.g., millet) or plant parts with increased δ13C (e.g., cereal grains see Cernusak et al. 2009), but this should then also be reflected in bone collagen ratios, at least to some extent, which is not observed.
If we follow the “different baseline hypothesis,” in which higher environmental δ15N levels are assumed for the seventh century, the observed pattern becomes more straightforward to explain. In this scenario, there was no increase in the protein content of the human diet, and the δ15N values of children’s post-weaning diet can be interpreted as a low protein diet because they fall below adult bone collagen ratios (e.g., Eerkens et al. 2011). This also explains the decreased post-weaning δ13C levels compared to earlier times, potentially influenced by a change in plant foods but masked by the higher proportion of animal protein and thus not visible in bone isotope values. Furthermore, the observed increase in LEH in the seventh century can also be explained by a low-trophic diet, which is not supported by the “higher protein hypothesis.” A low-trophic post-weaning diet may not be sufficient to meet the needs of children, especially during growth phases (e.g., Henderson et al. 2022), and may also make them more susceptible to external stressors.
In summary, the hypothesis that an increase in environmental δ15N occurred in the seventh century and thus children of this period had a lower protein diet compared to adults, which can in turn be related to the increased formation of the LEH, seems more reliable. However, further research in the region on human remains and especially on animal bones is needed to validate this conclusion.
Early life histories: links to a foreign origin
Profiles of migrants who spent their childhood in other geographical locations, with divergent ecogeographic conditions and thus distinct isotopic baselines, as well as probably different dietary traditions, show the most variable isotope profiles. While some of the increased δ15N ratios are possibly related to regions with higher nitrogen baselines, e.g., marine areas (Fig. 6), the fluctuating δ13C ratios at significantly higher levels suggest a more frequent consumption of C4 plants such as millet (Fig. 4). However, these unusual signals are primarily limited to the diet during childhood, whereas most of the bone collagen ratios of migrants adapted to the common signal of a C3 plant-based terrestrial diet (Fig. 6) (for detailed discussion see Velte et al. 2023).
Despite differences in the nutritional spectrum of migrants during childhood, it is notable that only about half of the non-local individuals were completely weaned within the third year of life, all males included. For the remaining women, especially those with ACD, the cessation of weaning was later. Additionally, three female migrants, including two with ACD, show a rather gradual weaning transition curve, which was only observed in four individuals in our sample. The difference between non-local men and women, along with the remarkable variability within the group of migrants, can be attributed to the fact that those migrants originate from different geographical regions in which people likely had a different lifestyle and culture (Velte et al. 2023). Although some migrants potentially grew up in the same cultural environment as people from southern Bavaria, women with ACD likely spent their childhood in a community with different traditions.
Cases of intentional cranial modification in pre- and early history Europe are known since the fifth to fourth centuries bc but become more widespread with the arrival of nomadic populations from the Eurasian Steppe. Thus, ACD is interpreted as an indication of nomadic incomers, e.g., the historically documented Huns and other nomadic groups associated with a pastoralist way of life (for review see Hakenbeck 2009). Some studies suggest that prolonged weaning times are found in such non-sedentary populations (e.g., Clayton et al. 2006, Waters-Rist et al. 2011). Rationales are still debated, ranging from a lack of appropriate weaning foods and the distribution of subsistence activities and workload to fertility control (e.g., Sellen and Smay 2001; Fouts et al. 2005; Sellen 2007; Bocquet-Appel 2011). Studies of pastoral societies indicate that infant diets are supplemented with animal products, including milk, meat, and blood rather than cereals or nuts (Ventresca Miller et al. 2014, 2017, Sellen 2001, Sellen and Smay 2001), which can result in a more gradual weaning curve. Furthermore, a study of pastoralists from Bronze Age Eurasia found that weaning was completed by the age of four (Ventresca Miller et al. 2017). Isotope data from an Early Medieval nomadic culture of Tuva (Southern Siberia) also suggests weaning after two to three years of age (Milella et al. 2022). Consequently, growing up in a community with a pastoralist and/or nomadic lifestyle is a possible explanation for the observed more gradual weaning pattern and prolonged weaning times observed in several migrants. However, to our knowledge, no data on weaning patterns of Early Medieval nomadic-pastoralists or individuals with ACD other than those presented here are available to substantiate this assumption.
To date, no men with ACD or who have a genetic origin or dietary pattern similar to these women have been identified in Bavarian cemeteries. This may indicate that men and women came to the region from different areas, at least partly as a result of distinct, gender-specific migration processes.
Conclusion
The analysis of weaning patterns and childhood stress through stable light isotope analysis of serial dentine samples is an intricate task, with many aspects that are still far from being sufficiently understood.
However, our study has revealed that more abrupt weaning occurred within the third year of life of most of the individuals buried between Roman and Medieval Southern Bavaria. Although we found no differences in weaning ages between males and females or diachronic time phases, we observed a trend towards prolonged weaning in some migrants.
Moreover, we found some evidence for a diachronic change in isotope baselines linked to changes in economic practices, which likely also affect infant feeding strategies, reflected in the post-weaning diet and stress level in the seventh century. If individuals experienced stress associated with the weaning process, it manifests itself primarily in the post-weaning phase, after the complete cessation of breastfeeding. This demonstrates that the choice of foods during this time is important as low-trophic foods can potentially facilitate the susceptibility of infants to stressors.
In summary, we were able to show different weaning practices in various communities, both by chronological distance and cultural differences, although weaning patterns within a community can also vary significantly. However, more data are needed including the remains of subadults and animals to obtain a more detailed and complete picture of infant feeding strategies and related stress.
Data availability
All data analyzed in this study are included in the submitted supplementary information files.
References
AlQahtani SJ, Hector MP, Liversidge HM (2010) Brief communication: the London atlas of human tooth development and eruption. Am J Phys Anthropol 142(3):481–490
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17(4):431–451
Armelagos GJ, Goodman AH, Harper KN, Blakey ML (2009) Enamel hypoplasia and early mortality: bioarcheological support for the Barker hypothesis. Evol Anthropol 18:261–271
Ballard O, Morrow AL (2013) Human milk composition. Pediatr Clin North Am 60:49–74
Beaumont J, Montgomery J (2016) The Great Irish Famine: identifying starvation in the tissues of victims using stable isotope analysis of bone and incremental dentine collagen. Bondioli L, editor. PLoS ONE. 11(8):e0160065
Beaumont J, Geber J, Powers N, Wilson A, Lee-Thorp J, Montgomery J (2012) Victims and survivors: stable isotopes used to identify migrants from the Great Irish Famine to 19th century London. Am J Phys Anthropol 150(1):87–98
Beaumont J, Gledhill A, Lee-Thorp J, Montgomery J (2013) Childhood diet: a closer examination of the evidence from dental tissues using stable isotope analysis of incremental dentine. Archaeometry 55:277–295
Beaumont J, Craig-Atkins E, Buckberry J, Haydock H, Horne P, Howcroft R et al (2018) Comparing apples and oranges: why infant bone collagen may not reflect dietary intake in the same way as dentine collagen. Am J Phys Anthropol 167(3):524–540
Berbesque JC, Hoover KC (2018) Frequency and developmental timing of linear enamel hypoplasia defects in Early Archaic Texan hunter-gatherers. PeerJ 6:e4367
Bereczkei T (2001) Maternal trade-off in treating high-risk children. Evol Hum Behav 22:197–212
Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, Maternal and Child Undernutrition Study Group (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371(9608):243–60
Bocquet-Appel JP (2011) When the world’s population took off: the springboard of the Neolithic demographic transition. Science 333:560–561
Bogaard A, Heaton THE, Poulton P, Merbach I (2007) The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J Archaeol Sci 34:335–343
Boldsen JL (2007) Early childhood stress and adult age mortality—a study of dental enamel hypoplasia in the medieval Danish village of Tirup. Am J Phys Anthropol 132:59–66
Bourbou C, Garvie-Lok S (2009) Breastfeeding and weaning patterns in Byzantine times: evidence from human remains and written sources. In: Papaconstantinou A, Talbot AM (eds) Becoming Byzantine: Children and Childhood in Byzantium. Harvard University Press, Washington, DC, pp 65–83
Bourbou C, Fuller BT, Garvie-Lok SJ, Richards MP (2013) Nursing mothers and feeding bottles: reconstructing breastfeeding and weaning patterns in Greek Byzantine populations (6th–15th centuries AD) using carbon and nitrogen stable isotope ratios. J Archaeol Sci 40:3903–3913
Bourbou C, Arenz G, Dasen V, Lösch S (2019) Babes, bones, and isotopes: a stable isotope investigation on nonadults from Aventicum, Roman Switzerland (first–third century CE). Int J Osteoarchaeol 29:974–985
Britton K, Fuller BT, Tütken T, Mays S, Richards MP (2015) Oxygen isotope analysis of human bone phosphate evidences weaning age in archaeological populations: bone phosphate oxygen isotope ratios evidence weaning. Am J Phys Anthropol 157:226–241
Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE (2006) Stunting, wasting, and micronutrient deficiency disorders. In: Jamison DT et al (eds) Disease control priorities in developing countries, vol 28, 2nd edn. The International Bank for Reconstruction and Development/The World Bank. Washington DC, New York, pp 551–76
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A et al (2009) Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36(3):199–213
Chinique de Armas Y, Roksandic M (2018) Breastfeeding and weaning in ancient populations: challenges and future directions. Int J Osteoarchaeol. 28:477–78
Chinique de Armas Y, Mavridou AM, Garcell Domínguez J, Hanson K, Laffoon J (2022) Tracking breastfeeding and weaning practices in ancient populations by combining carbon, nitrogen and oxygen stable isotopes from multiple non-adult tissues. PLoS ONE. 17:e0262435
Clayton F, Sealy J, Pfeiffer S (2006) Weaning age among foragers at Matjes river rock shelter, South Africa, from stable nitrogen and carbon isotope analyses. Am J Phys Anthropol 129:311–317
Codreanu-Windauer S, Harbeck M (2016) Neue Untersuchungen zu Gräbern des 5. Jahrhunderts: Der Fall Burgweinting - In: Geisler H, editor. Wandel durch Migration? Büchenbach: Dr. Faustus. 243–260
Codreanu-Windauer S, Schleuder R (2013) Gräber des 5. Jahrhunderts von Regensburg-Burgweinting aus archäologischer und anthropologischer Sicht. Berichte der Bayerischen Bodendenkmalpflege 54:351–64
Corruccini RS, Handler JS, Jacobi KP (1985) Chronological distribution of enamel hypoplasias and weaning in a Caribbean slave population. Hum Biol 57(4):699–711
Craig-Atkins E, Jervis B, Cramp L, Hammann S, Nederbragt AJ, Nicholson E et al (2020) The dietary impact of the Norman Conquest: a multiproxy archaeological investigation of Oxford, UK. PLoS ONE 15(7):e0235005
Crowder KD, Montgomery J, Gröcke DR, Filipek KL (2019) Childhood “stress” and stable isotope life-histories in Transylvania. Int J Osteoarchaeol 29(4):544–653
Czermak A, Fernández-Crespo T, Ditchfield PW, Lee-Thorp JA (2020) A guide for an anatomically sensitive dentine microsampling and age-alignment approach for human teeth isotopic sequences. Am J Phys Anthropol 173(4):776–783
Czermak AM. Soziale Stratifizierung im frühen Mittelalter: Aussage- und Nachweismöglichkeiten anhand von biologischen Indikatoren. PhD Thesis, LMU München. 2011; 290 p.
Dasen V (2015) Le sourire d'Omphale. Maternité et petite enfance dans l'Antiquité. Rennes: Presses Universitaires de Rennes. 408
DeNiro MJ (1985) Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317(6040):806–809
Dewey KG, Brown KH (2003) Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food Nutr Bull 24:5–28
Dittmann K, Grupe G (2000) Biochemical and paleopathological investigation and infant mortality in the early Middle Ages. Anthropol Anz 58(4):345–355
Eerkens JW, Berget AG, Bartelink EJ (2011) Estimating weaning and early childhood diet from serial micro-samples of dentin collagen. J Archaeol Sci 38(11):3101–3111
Eerkens JW, Washburn E, Greenwald AM (2017) Weaning and early childhood diets at two early period sites: implications for parental investment and population growth in Central California. Calif Archaeol 9(2):199–222
Fehr H (2010) Am Anfang war das Volk? Die Entstehung der bajuwarischen Identität als archäologisches und interdisziplinäres Problem. In: Pohl W, Mehofer M (eds) Archäologie der Identität. Österreichische Akademie der Wissenschaften, Wien, pp 211–231
Fehr H (2019) Agrartechnologie, Klima und Effektivität frühmittelalterlicher Landwirtschaft. In: Haberstroh J, Heitmeier I, editors. Gründerzeit. Siedlung in Bayern zwischen Spätantike und Frühmittelalter. St. Ottilien. 219–45
Fernández-Crespo T, Czermak A, Lee-Thorp JA, Schulting RJ (2018) Infant and childhood diet at the passage tomb of Alto de la Huesera (north-central Iberia) from bone collagen and sequential dentine isotope composition. Int J Osteoarchaeol 28:542–551
Fernández-Crespo T, Schulting RJ, Czermak A, Ordoño J, Lorenzo JI, Rodanés JM (2022) The “post-weanling’s conundrum”: exploring the impact of infant and child feeding practices on early mortality in the Bronze Age burial cave of Moro de Alins, north-eastern Iberia, through stable isotope analysis. Archaeol Anthropol Sci 14:196
Fildes V (1986) Breasts, Bottles and babies: a history of infant feeding. Edinburgh University Press, Edinburgh, p 462
Fogel M, Tuross N, Owsley DW (1989) Nitrogen isotope tracers of human lactation in modern and archaeological populations. Carnegie Inst Wash Yearbook. 88:111-17
Fournier NA, Kennedy Thornton E, Arellano MV, Leventhal A (2022) Stable isotopic reconstruction of weaning and childhood diet during times of change: an examination of life history and health of San Francisco Bay Area juveniles. J Archaeol Sci Rep 44:103495
Fouts HN, Hewlett BS, Lamb ME (2005) Parent-offspring weaning conflicts among the Bofi farmers and foragers of Central Africa. Curr Anthropol 46:29–50
Fraser RA, Bogaard A, Heaton T, Charles M, Jones G, Christensen BT et al (2011) Manuring and stable nitrogen isotope ratios in cereals and pulses: towards a new archaeobotanical approach to the inference of land use and dietary practices. J Archaeol Sci 38:2790–2804
Fuller BT, Richards MP, Mays SA (2003) Stable carbon and nitrogen isotope variations in tooth dentine serial sections from Wharram Percy. J Archaeol Sci 30:1673–1684
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges RE (2004) Nitrogen balance and δ15N: why you’re not what you eat during pregnancy. Rapid Commun Mass Spectrom 18(23):2889–2896
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges REM (2005) Nitrogen balance and δ15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19(18):2497–2506
Fuller BT, Fuller JL, Harris DA, Hedges REM (2006) Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am J Phys Anthropol 129:279–293
Fulminante F (2015) Infant feeding practices in Europe and the Mediterranean from prehistory to the Middle Ages: a comparison between the historical sources and bioarchaeology. Child Past 8(1):24–47
Ganiatsou E, Souleles A, Papageorgopoulou C (2023) WEaning Age FiNder (WEAN): a tool for estimating weaning age from stable isotope ratios of dentinal collagen. Archaeol Anthropol Sci 15:50
Geisler H (1998) Das frühbairische Gräberfeld Straubing Bajuwarenstraße I. Rahden: M. Leidorf. 2
Goodman AH, Armelagos GJ (1989) Infant and childhood morbidity and mortality risks in archaeological populations. World Archaeol 21:225–243
Goodman AH, Rose JC (1990) Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Am J Phys Anthropol 33(S11):59–110
Goodman AH, Armelagos GJ, Rose JC (1984) The chronological distribution of enamel hypoplasias from prehistoric Dickson mounds populations. Am J Phys Anthropol 65(3):259–266
Guatelli-Steinberg D, Lukacs JR (1999) Interpreting sex differences in enamel hypoplasia in human and non-human primates: developmental, environmental, and cultural considerations. Am J Phys Anthropol 110:73–126
Haas-Gebhard B (2013) Die Baiuvaren: Archäologie und Geschichte. Friedrich Pustet. 200
Haberstroh J, Harbeck M (2013) 2013 Nekropolen des 5. Jahrhunderts n. Chr. in Bayern – Eine Projektskizze aus archäologischer und anthropologischer Perspektive. Ber. Bayer. Bodendenkmalpflege. 54:325–38
Hakenbeck SE (2009) ‘Hunnic’ modified skulls: physical appearance, identity and the transformative nature of migrations. In: Sayer D, Williams H (eds) Mortuary practices and social identities in the middle ages. Exeter University Press, Exeter, pp 64–80
Hakenbeck SE, McManus E, Geisler H, Grupe G, O’Connell T (2010) Diet and mobility in Early Medieval Bavaria: a study of carbon and nitrogen stable isotopes. Am J Phys Anthropol 143(2):235–249
Harbeck M, Codreanu-Windauer S, McGlynn G, Müller R, Haberstroh J (2016) Living at the outskirts of the Roman Empire after the fall. A Study of 5th Century Bavarian Burials. IANSA 7(1):123–35
Harbeck M, von Heyking K (2014) Die Ernährung menschlicher Bevölkerungen in Spätantike und Mittelalter auf dem Gebiet der ehemaligen Raetia secunda und des Herzogtums Baierns - Welche Hinweise können Isotopenanalysen geben? In: Drauschke J, Reis A, Prien R, editors. Küche und Keller in Antike und Frühmittelalter. Tagungsbeiträge der Arbeitsgemeinschaft Spätantike und Frühmittelalter. 197–222
Haydock H, Clarke L, Craig-Atkins E, Howcroft R, Buckberry J (2013) Weaning at Anglo-Saxon Raunds: Implications for changingbreastfeeding practice in Britain over two millennia. Am J Phys Anthropol 151(4):604–612
Hedges R, Clement J, Thomas C, O’Connell T (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am J Anthopol 133:808–816
Henderson RC, Lee-Thorp J, Loe L (2014) Early life histories of the London poor using δ13C and δ15N stable isotope incremental dentine sampling. Am J Phys Anthropol 154:585–593
Henderson RC, Zarina G, Czermak A, Schulting RJ, Henderson PA, Legzdina D et al (2022) Life histories at stone age Zvejnieki based on stable isotope profiles of tooth dentine. J Archaeol Sci Rep 44:103496
Herrscher E (2003) Alimentation d’une population historique: Analyse des données isotopiques de la nécropole Saint-Laurent de Grenoble (XIIIeeXVe siècle, France). Bull Mem Soc Anthropol Paris 3e4:145e320
Herrscher E, Goude G, Metz L (2017) Longitudinal study of stable isotope compositions of maternal milk and implications for the Palaeo-diet of infants. Bull Mem Soc Anthropol Paris 29:131–139
Hillson S (2008) Dental Pathology. In: Katzenberg MA, Saunders SR (eds) Biological anthropology of the human skeleton. John Wiley & Sons, Inc., pp 299–340
Humphrey LT, Dean MC, Jeffries TE, Penn M (2008) Unlocking evidence of early diet from tooth enamel. Proc Natl Acad Sci 105:6834–6839
Jay M (2009) Breastfeeding and weaning behaviour in archaeological populations: evidence from the isotopic analysis of skeletal materials. Child Past 2(1):163–178
Katzenberg MA, Herring D, Saunders SR (1996) Weaning and infant mortality: evaluating the skeletal evidence. Yearb Phys Anthropol 39:177–199
Kendall E, Millard A, Beaumont J (2021) The, “weanling’s dilemma” revisited: evolving bodies of evidence and the problem of infant paleodietary interpretation. Yearb Phys Anthropol 175:57–78
King T, Hillson S, Humphrey LT (2002) A detailed study of enamel hypoplasia in a post-medieval adolescent of known age and sex. Arch Oral Biol 47(1):29–39
King T, Humphrey LT, Hillson S (2005) Linear enamel hypoplasias as indicators of systemic physiological stress: evidence from two known age-at-death and sex populations from postmedieval London. Am J Phys Anthropol 128:547–559
Kramer MS, Kakuma R (2004) The optimal duration of exclusive breastfeeding: a systematic review. In: Pickering LK, Morrow AL, Ruiz-Palacios GM, Schanler RJ (eds) Protecting Infants through Human Milk, Advances in Experimental Medicine and Biology. Springer, US, Boston, MA, pp 63–77
Larsen CS (2015) Bioarchaeology: Interpreting behavior from the human skeleton, 2nd edn. Cambridge University Press, Cambridge, p 654
Lewit T (2009) Pigs, presses and pastoralism: farming in the fifth to sixth centuries. Early Mediev Eur 17:77–91
Lönnerdal B (2000) Breast milk: a truly functional food. Nutrition 16:509–511
Lösch S (2009) Paläopathologisch-Anthropologische und molekulare Untersuchungen an mittelalterlichen und frühneuzeitlichen Bevölkerungsgruppen. PhD Thesis, LMU München. 376
Losert H, Pleterski A (2003) Altenerding in Oberbayern: Struktur des frühmittelalterlichen Gräberfeldes und "Ethnogenese" der Bajuwaren. Berlin, Bamberg, Ljubljana. 700
McDade TW (2003) Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol 122:100–125
Mekota AM, Grupe G, Ufer S, Cuntz U (2006) Serial analysis of stable nitrogen and carbon isotopes in hair: monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun Mass Spectrom 20(10):1604–1610
Michaelsen KF, Weaver L, Branca F, Robertson A (2000) Feeding and nutrition of infants and young children: guidelines for the WHO European Region, with emphasis on the former Soviet countries. WHO Regional office for Europe. WHO Regional Publications, European Series No. 87. 296
Milella M, Caspari G, Laffranchi Z, Arenz G, Sadykov T, Blochin J, Keller M, Kapinus Y, Lösch S (2022) Dining in Tuva: social correlates of diet and mobility in Southern Siberia during the 2nd–4th centuries CE. Am J Biol Anthropol 178:124–139
Millard AR (2000) A model for the effect of weaning on nitrogen isotope ratios in humans. In: Goodfriend G, Collins M, Fogel M, Macko S, Wehmiller J (eds) Perspectives in amino acid and protein geochemistry. Oxford University Press, New York, NY, pp 51–59
Moggi-Cecchi J, Pacciani E, Pinto-Cisternas J (1994) Enamel hypoplasia and age at weaning in 19th-century Florence, Italy. Am J Phys Anthropol 93:299–306
Neidich D (2023) Bioarchaeology of identity formation in Late Antique and Migration Period Bavaria, Germany. PhD Thesis, University of Pittsburgh, p 292
Neuberger FM, Jopp E, Graw M, Püschel K, Grupe G (2013) Signs of malnutrition and starvation: reconstruction of nutritional life histories by serial isotopic analyses of hair. Forensic Sci Int 226:22–32
Orellana-González E, Sparacello VS, Bocaege E, Varalli A, Moggi-Cecchi J, Dori I (2020) Insights on patterns of developmental disturbances from the analysis of linear enamel hypoplasia in a Neolithic sample from Liguria (northwestern Italy). Int J Paleopathol 28:123–136
Pérez-Escamilla R, Buccini GS, Segura-Pérez S, Piwoz E (2019) Perspective: should exclusive breastfeeding still be recommended for 6 months? Adv Nutr 10:931–943
Prowse TL, Saunders SR, Schwarcz HP, Garnsey P, Macchiarelli R, Bondioli L (2008) Isotopic and dental evidence for infant and young child feeding practices in an imperial Roman skeletal sample. Am J Phys Anthropol 137:294–308
Quandt SA (1995) Sociocultural aspects of the lactation process. In: Stuart-Macadam P, Dettwyler KA (eds) Breastfeeding: biocultural perspectives. Aldine de Gruyter, New York, pp 127–138
Rand AJ, Bower M, Munkittrick J, Harris A, Burchell M, Grime V (2015) Comparison of three bone collagen extraction procedures: the effect of preservation on δ13C and δ15N values. North Atlantic Archaeol 4:93–113
Reitsema LJ (2013) Beyond diet reconstruction: stable isotope applications to human physiology, health, and nutrition. Am J Hum Biol 25(4):445–456
Richards MP, Mays S, Fuller BT (2002) Stable carbon and nitrogen isotope values of bone and teeth reflect weaning age at Medieval Wharram Percy site, Yorkshire, UK. Am J Phys Anthropol 119:205–210
Rowland MGM, Barrell RAE, Whitehead RG (1978) The Weanling’s dilemma: bacterial contamination in traditional Gambian weaning foods. Lancet 311(8056):136–138
Sage W (1984) Das Reihengräberfeld von Altenerding in Oberbayern. I: Katalog der anthropologischen und archäologischen Funde und Befunde. Germ. Denkmäler Völkerwanderungszeit A 14. Berlin: Mann. 356
Scharlotta I, Goude G, Herrscher E, Bazaliiskii VI, Weber AW (2018) Shifting weaning practices in Early Neolithic Cis-Baikal, Siberia: New insights from stable isotope analysis of molar microsamples. Int Journal Osteoarchaeol 28(5):579–598
Sellen DW (2001) Weaning, complementary feeding, and maternal decision making in a rural east African pastoral population. J Hum Lact 17:233–244
Sellen DW (2007) Evolution of infant and young child feeding: implications for contemporary public health. Annu Rev Nutr 27:123–148
Sellen DW (2009) Evolution of human lactation and complementary feeding: implications for understanding contemporary cross-cultural variation. In: Goldberg G, Prentice A, Filteau S, Simondon K (eds) Breast-Feeding: Early Influences on Later Health, Advances in Experimental Medicine and Biology. Springer, Netherlands, Dordrecht, pp 253–282
Sellen DW, Smay DB (2001) Relationship between subsistence and age at weaning in “preindustrial” societies. Hum Nat 12(1):47–87
Sheather SJ, Jones MC (1991) A reliable data-based bandwidth selection method for kernel density estimation. J R Stat Soc Series B Stat Methodol 53(3):683–690
Siebke I, Moghaddam N, Cunningham CA, Witzel C, Lösch S (2019) Those who died very young—Inferences from δ15N and δ13C in bone collagen and the absence of a neonatal line in enamel related to the possible onset of breastfeeding. Am J Phys Anthropol 169(4):664–677
Sofeso C, Vohberger M, Wisnosky A, Päffgen B, Harbeck M, Burger J et al (2012) Verifying archaeological hypotheses: investigations on origin and genealogical lineages of a privileged society in Upper Bavaria from Imperial Roman times (Erding, Kletthamer Feld). In: Kaiser E, Burger J, Schier W, editors. Population dynamics in prehistory and early history. New approaches using stable isotopes and genetics. Topoi Berlin Studies of the Ancient World, Vol. 5. Berlin, Boston: Walter de Gruyter. 113–30
Strott N (2007) Paläodemographie frühmittelalterlicher Bevölkerungen Altbaierns - Diachrone und allopatrische Trends. PhD Thesis, LMU München. 331
Stuart-Macadam P, Dettwyler KA (1995) Breastfeeding: biocultural perspectives. Aldine de Gruyter, New York, p 430
Toncala A, Trautmann B, Velte M, Kropf E, McGlynn G, Peters J et al (2020) On the premises of mixing models to define local bioavailable 87Sr/86Sr ranges in archaeological contexts. Sci Total Environ 745:140902
Trautmann B, Haas-Gebhard B, Boos A, Rott A, Gross M, Burger J et al (2017) Eine Reevaluation artifiziell deformierter Schädel des Frühen Mittelalters aus Bayern. Archäologisches Korrespondenzblatt 47:263–282
Tsutaya T (2017) Post-weaning diet in archaeological human populations: a meta-analysis of carbon and nitrogen stable isotope ratios of child skeletons. Am J Phys Anthropol 164:546–557
Veeramah KR, Rott A, Groß M, van Dorp L, López S, Kirsanow K et al (2018) Population genomic analysis of elongated skulls reveals extensive female-biased immigration in Early Medieval Bavaria. PNAS 115(13):3494–3499
Velte M, Czermak A, Grigat A, Haas-Gebhard B, Gairhos A, Toncala A et al (2023) Between Raetia Secunda and the dutchy of Bavaria: exploring patterns of human movement and diet. PLoS ONE 18(4):e0283243
Ventresca Miller A, Usmanova E, Logvin V, Kalieva S, Shevnina I, Logvin A et al (2014) Subsistence and social change in central Eurasia: stable isotope analysis of populations spanning the Bronze Age transition. J Archaeol Sci 42:525–538
Ventresca Miller A, Hanks BK, Judd M, Epimakhov A, Razhev D (2017) Weaning practices among pastoralists: new evidence of infant feeding patterns from Bronze Age Eurasia. Am J Phys Anthropol 162:409–422
Walker A (2010) Breast milk as the gold standard for protective nutrients. J Pediatr 156(2 Suppl):3–7
Waters-Rist AL, Katzenberg MA (2010) The effect of growth on stable nitrogen isotope ratios in subadult bone collagen. Int J Osteoarchaeol 20:172–191
Waters-Rist AL, Bazaliiskii VI, Weber AW, Katzenberg MA (2011) Infant and child diet in neolithic hunter-fisher-gatherers from Cis-Baikal, Siberia: intra-long bone stable nitrogen and carbon isotope ratios. Am J Phys Anthropol 146:225–241
Waters-Rist AL, Bazaliiskii VI, Weber A, Goriunova OI, Katzenberg MA (2006) Dental enamel hypoplasia in Holocene Siberian hunter-gatherers: evaluating evidence for ‘‘weaning stress’’ and ‘‘seasonal stress.’’ Annual Meeting of the Canadian Association for Physical Anthropology. Peterborough, ON
Weiß DJ (2021) Bayern (Raum). In: Historisches Lexikon Bayerns, URL: http://www.historisches-lexikon-bayerns.de/Lexikon/Bayern_(Raum). Accessed 27 June 2023
Zach B (2019) Äcker und Gärten im frühmittelalterlichen Bayern. In: Haberstroh J, Heitmeier I, editors. Gründerzeit. Siedlung in Bayern zwischen Spätantike und Frühmittelalter. St. Ottilien: Eos. 205–18
Zintl S (2012) Wiedergeöffnete Gräber der Merowingerzeit im Umland von Regensburg. In: Chytráček M, Gruber H, Michálek J, Schmotz K, Sandner R, editors. Fines Transire. 21.Treffen der Archäologischen Arbeitsgemeinschaft Ostbayern/West- und Südböhmen/Oberösterreich in Stříbro. Rahden/Westf. 189–97
Acknowledgements
The authors are grateful to Silvia Codreanu-Windauer (BLfD) for information regarding the individuals from Burgweinting. We thank George McGlynn and Albert Zink for their comments on our results. The authors also thank Gabriele Arenz for their support with the collagen extraction of some specimens and Joris Peters for providing lab space and equipment.
Funding
Open Access funding enabled and organized by Projekt DEAL. We thank the Bavarian State Office for Monument Protection and the town of Erding for financial support.
Author information
Authors and Affiliations
Contributions
Study design: M. Harbeck, M. Velte
Methodology: A. Czermak, M. Velte
Formal analysis and investigation: M. Velte, A. Grigat, D. Neidich, B. Trautmann, S. Lösch
Writing – original draft preparation: M. Harbeck, M. Velte, A. Czermak
Writing – review and editing: S. Lösch, D. Neidich
Funding acquisition: B. Päffgen, M. Harbeck
Conceptualization: B. Päffgen, M. Harbeck
Supervision: M. Harbeck, A. Czermak
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velte, M., Czermak, A., Grigat, A. et al. Tracing early life histories from Roman times to the Medieval era: weaning practices and physiological stress. Archaeol Anthropol Sci 15, 190 (2023). https://doi.org/10.1007/s12520-023-01882-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01882-6