Abstract
The ability to control and direct fire is a major evolutionary step in the human story. The development of aceramic cooking technologies is less well understood as they rarely survive in the archaeological record. However, inferential evidence such as fire-cracked rocks, earthen pits and heated bones suggest a variety of cooking methods were used prior to the invention of ceramics. Yet there is a paucity of experimental evidence testing the efficacy of perishable organic containers in tasks involving their use with heat. The study presents experimental results of organic containers and their use for heating water related to cooking. Containers were made from deer hide and pig stomach and water was heated using two different techniques: placing the container directly above a fire and placing hot stones into the container. The results suggest that different organic containers and heating types could attain and maintain a sub-boiling cooking temperature; however, not all could reach boiling point. It is argued that these sub-boiling methods may be as, or perhaps more, desirable than boiling, with potential implications for the development of vessels prior to the adoption of ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thermal alteration and processing of foodstuffs is widely considered to be a significant threshold in human evolution (Wrangham and Conklin-Brittain 2003). It has been argued that predation pressure acting on early hominins may have selected for increasing group size (Coward and Gamble 2008; Hart and Sussman 2005) demanding a larger brain geared towards negotiating a greater number of more complex social relationships, necessitating adaptations such as language (Aiello and Dunbar 1993; Dunbar 1993; Arsuaga and Martínez 1998; Gamble 2002; Gowlett 2006). The expensive tissue hypothesis, which claims that decreasing gut size energetically facilitated increasing brain size, is one explanation as to how the brain could so rapidly expand (Aiello and Wheeler 1995). Cooking may have reduced the caloric cost of digestion, providing the necessary free energy to help stimulate this cognitive advancement across generations (Boback et al. 2007; Aiello and Wheeler 1995; Henrich 2015). Yet, for all the attested importance of pre-ceramic cooking, the physical practices and methods of containment and heating technologies remain relatively under-researched and discussed (Wrangham 2007; Wright 2004).
Speth (2015) explored the mechanism of boiling water in perishable materials and found it to be accessible to Palaeolithic humans. Boiling has unique capabilities—such as fully eradicating pathogenic microbes from meat (Avens et al. 2002) and degrading collagen strands to the point of gelatin formation (Lawrie and Ledward 2006). However, foods that are boiled for long periods will eventually disintegrate. Additionally, the fuel required to raise water to boiling point and maintain it may be unavailable or a prohibitive investment of labour to collect. Perhaps more importantly, it may not be strictly necessary to achieve boiling to wet-cook food. When considering the use of organic containers in wet-cooking, it is therefore important to distinguish between the use of heated vs boiling water. For example, starches can be hydrolyzed at temperatures from 35 °C upwards (Shariffa et al. 2009) and when cooked become significantly more bioavailable (Carmody and Wrangham 2009). Likewise, with meat, heat will denature and unravel complex proteins resulting in a larger number of proteolytic cleavage points and a more thermodynamically efficient digestion process (Carmody et al. 2011). Muscle meat specifically is comprised of a number of structural proteins, including actin, α-actinin and myosin, as well as sarcoplasmic and globular proteins, all of which begin to denature at temperatures between 40 °C and 60 °C (Yu et al. 2017; Cheng and Parrish 1979; Kemp et al. 2009). The benefits of heating water are not limited to cooking. For example, in some plants, this can extend to increasing ease of peeling (Henry 2017) and the extraction of potentially harmful compounds (Ressler et al. 1997). However, an experimental assessment of the aceramic technology used in methods of cooking using a sub-boiling strategy has not been explored. Given that many of the advantages of cooking food can be achieved with sub-boiling temperatures, it is important in any experiment surrounding aceramic cooking technology to avoid focusing solely on boiling temperatures and the methods and materials that can be used to reach and sustain them. Thus, this study aimed to test via a programme of experimental archaeology the feasibility of heating water—both to boiling and sub-boiling temperatures using a range of organic containers, via both direct and indirect heating methods, to inform the question of the development and nature of wet-cooking prior to the adoption of ceramics.
Background
Cooking before the invention of ceramics
The earliest potential use of fire by hominins—the prerequisite for any type of cooking—has been suggested to originate from Africa as early as 1.5–1 mya. Evidence includes baked sediment and heat altered stones recovered from Koobi Fora (Kenya), dating to 1.4–1 mya; concentrations of baked clay in association with tools and animal bones from Chesowanja (Kenya), dating to 1.4–1 mya; burnt bone recovered from Member 3 of Swartkrans (South Africa), dating to 0.8–1 mya; and evidence of routine burning of vegetation from layer 10 of Wonderwerk Cave (South Africa), dating to 1–0.8 mya (Dunbar and Gowlett 2014; James et al. 1989; Gowlett 2015). The earliest substantial evidence for the controlled use of fire is found much later from sites including: Gesher Benot Ya’aqov (Israel) dating to c. 790 kya, where ash, charcoal and burnt flint was recovered (Alperson-Afil and Goren-Inbar 2010); Zhoukoudian Locality 1 (China) dating to c. 670–400 kya, where burnt bone and chipped-stone artefacts were identified; and Beeches Pit (Britain) dating to c. 400 kya, where abundant burned stone tools were found (Gowlett 2006, 2015; James et al. 1989). However, it is not until the transition from Lower to Middle Palaeolithic that evidence of fire control becomes a recurring feature of archaeological sites (Preece et al. 2006; Mallol et al. 2013; Roebroeks and Villa 2011).
Perhaps as a result of the scarcity of available evidence, discussions of Palaeolithic cuisine have traditionally focused on the roasting of meat over open fires (Barkai et al. 2017; Straus 1989; Germonpré and Lbova 1996). ‘Dry-cooking’ techniques of this kind are presumed to dominate in large measure because ‘wet-cooking’ necessitates the surrounding of food in water within enclosed containers, a technology presumed to appear much later in the archaeological record (Gamble 2009). Previous attempts to characterise aceramic cooking technologies have argued for a linear evolutionary model, placing different techniques into a chronological sequence (Thoms 2009; Benison 1999). Yet it remains difficult to identify a clear pattern in the prehistoric record given the limited available evidence. Indeed, Speth (2015) suggests that archaeologists may not have fully appreciated the potential for fragile organic materials to be used for boiling water directly over a fire, which amongst ethnographically documented societies includes containers made from bark, wood, shell, hide, animal organs and stone (Nelson 2010). However, direct and indirect evidence for the use of non-ceramic or ‘aceramic vessels’ has been increasingly reported, which has advanced understanding.
Direct evidence includes the recovery of heated stones and cooking containers dating to the Middle Palaeolithic and Middle Stone Age (MSA) (Carbonell et al. 1996; Oestmo 2013; Bentsen and Wurz 2017, 2019); two wooden vessels from Abric Romani (Spain) dating to 45,000–49,000 BP found in association with a hearth and a possible tripod (Carbonell et al. 1996); wooden troughs from the Mesolithic site of Friesack IV (Germany) dating to between 8170–6990 BP (Gramsch and Kloss 1989); a wooden container and possible containers made of birch bark from the Early Mesolithic site of Star Carr (UK) dating to 9385–9260 cal BC–8555–8380 cal BC (Fletcher et al. 2018; Milner et al. 2018; Taylor et al. 2018); possible containers made from birch bark Nizhny Veretye I (Russia) dating to 9000–8000 BP (Oshibkina 1989) and from the Late Mesolithic site of Szczepanki (Poland) dating to ca. 7000–4500 cal BC (Gumiński 2012; Wacnik et al. 2020). Diverse indirect traces have also been recognised—all of which would likely necessitate some type of organic container. This includes the use of containers to boil bones for grease extraction (Krief et al. 2015; Lupo and Schmitt 1997; Patania and Jaffe 2021); hot stone cooking from Pavlov VI (Czech Republic) dating to 26,000 BP (Svoboda et al. 2009), Shuidonggou Locality 12 (China) dating to 11–12,000 BP (Gao and Dennell 2014; Gao et al. 2014), and several Magdalenian examples (Lucquin 2007; March and Lucquin 2007; Nakazawa et al. 2009; Batchelor 1979; Bolus 1990); circular areas devoid of remains believed to represent the negative spaces left by perishable containers on the floor of the Magdalenian habitation number 1 of Pincevent (France) (Leroi-Gourhan and Brézillon 1966); pits believed to be waterproofed with hide to be used as containers at the Magdalenian site of Gonnersdorf (Bosinski 1981); and hide containers used over direct heat (Speth 2015).
Indirect evidence from recovered food remains can also point to the probability of prehistoric wet-cooking. Charred food aggregates from Franchthi Cave in Greece, dating to between the Bølling-Allerød and early Holocene, revealed a starch-rich matrix made from different wild pulses (Kabukcu et al. 2023). The authors point to the necessity of soaking, heating and possibly boiling the pulses to achieve the observed level of microscopic processing. Such an activity seemingly necessitates a container which could enclose the seeds and foodstuffs, while modifying them using heated water. Given the recovery of both direct and indirect evidence that might represent Palaeolithic and Mesolithic container use and the deep timeframe for hominin fire manipulation, this raises the possibility for an early origin to wet-cooking cuisine, with potential implications for human evolution.
The shift from aceramic to ceramic technology occurred much earlier than previously believed, with the oldest evidence discovered in Southern China, dated to approximately 18–20,000 BP (Kuzmin 2017; Patania et al. 2019; Patania and Jaffe 2021), then Japan and the Russian Far East by 16–10,000 BP (Shoda et al. 2020). A recent geoarchaeological assessment of the early pottery site of Yuchanyan (18,300 cal BP) revealed a sophisticated suite of pyrotechnologies, including clay lined hearths and ceramics, probably deployed for processing and rendering bone fats (Patania and Jaffe 2021). This transition phase seems to have captured otherwise invisible information about aceramic organic containers, such as baskets or string nets, with these potentially serving as a template for producing pots. Cordage and netting impressions on ceramic sherds have been found on the earliest Russian pottery (Zhushchikhovskaya 1999; Hyland et al. 2002) and on fired clay fragments dated from Spanish Palaeolithic-Mesolithic transition (Aura Tortosa et al. 2019). Techniques employed to build Ertebolle pointed base vessels may also have derived from coiled basketry (Povlsen 2013). The contextual evidence and discussion about how and why ceramics emerged and dispersed across the world has been discussed in a number of works over recent decades (Rice 1999; Hayden et al. 1995; Brown 1989; Reid 1984; Sassaman et al. 1995; Murdock and Provost 1973; White et al. 1977; Jordan and Zvelebil 2016; Piezonka 2021; Dolbunova et al. 2022). These debates highlight that both ‘functional’ and ‘social’ driving forces contributed to the development and refinement of ceramics, with both requiring consideration when trying to understand why different societies chose to prioritise pottery over pre-existing aceramic cooking methods. However, a number of presumptions still prevail in these discussions, epitomised here by Jordan and Zvelebil:
With many organic technologies able to perform the roles played by pottery, what, other than direct boiling ability, might have made pottery more attractive? (Jordan and Zvelebil 2016, 57).
The two assumptions here—that aceramic technologies are incapable of direct boiling, and that boiling is in itself the most useful and productive cooking technique—deserve to be interrogated more fully. Despite advances based on the increasing available material evidence for early pottery and their uses, expansion of the discussion to the likely organic technologies that preceded it remains limited. Closer consideration of how and when humans began to use aceramic containers for wet-cooking is therefore required, with experimental archaeology being an important methodological tool for advancing understanding in this area. To address Speth’s (2015) challenge to explore the possibilities and evidence for boiling and aceramic wet-cooking more generally in archaeology, an experimental programme was designed with the aim of testing how aceramic vessels perform and the particular material properties involved in their functionality.
Materials and methods
Methods
In total, six experiments were conducted: in five cases attempting to heat water to boiling and sub-boiling thresholds, and one experiment attempting to reach and sustain a ‘long-time low-temperature’ cooking range of 45–70 °C. Of the six experiments carried out, five used a ‘Direct Heat’ method (defined as the suspension of the container over a heat source to heat directly) and one used a ‘Hot Stone’ method (defined as an indirect heating technique where stones are heated and transported into the container which heats the contents). Two organic container materials were tested: red deer hide and pig stomach. Experiments consisted of:
-
A)
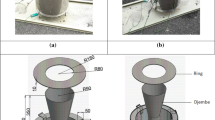
Direct heat via a fire using a suspended pig stomach container (Fig. 1);
-
B)
Direct heat via a fire using a suspended red deer hide container with decreasing distance to the fire (Fig. 1);
-
C)
Direct heat via a fire using a suspended red deer hide container with stable distance from the fire (Fig. 1);
-
D)
Direct heat via embers contained within a pit covered with a red deer hide container (Fig. 2);
-
E)
Indirect heating using hot stones added to a suspended pig stomach container (Fig. 3); 6) and direct heat via a fire using a suspended red deer hide container for over three hours (Fig. 1).
Each experiment consisted of a heating source (direct fire, embers, hot stones), a container and water, allowing for the following variables to be measured:
-
A)
Increase and decrease in water temperature over time;
-
B)
The time taken for the water to reach boiling or sub-boiling point;
-
C)
Fuel consumed to achieve water heating;
-
D)
Whether cooking of the foodstuff was achieved;
-
E)
The type and extent of damage or modification to the organic container
This allowed for contrasts between both material types using the same heat source and for comparisons between the heating methods.
The experiments were conducted in two phases. Phase one was primarily qualitative and aimed to test aceramic boiling using a sample of materials and heating methods discussed by Speth (2015). Phase two expanded on the results of phase one, testing sustained sub-boiling. The temperature of a litre of water was raised to and held between 45–70 °C for 3 h, thus subjecting the container to conditions consistent with ‘long-time, low-temperature’ cooking. An unprocessed red deer hide suspended directly over a fire was used, which allowed for the testing of Speth’s (2015) challenge of using organic perishable containers placed directly into a fire, while also being compatible with the earliest technology available to produce ‘simple containers’, based on early dates for animal skinning (Verheijen et al. 2023).
Experiment 1: Direct Heat methods
In order to establish the efficiency of heating water with the heat source positioned directly beneath the vessel, two container types (pig stomach, red deer hide) and two suspension techniques (pit, tripod) were tested across four vessels: V1–V4. Vessel 1 (V1) was made from pig stomach (Sus scrofa) and was suspended from a tripod over a fire using paracord (Fig. 1). V1 was filled with c. 1 L of water for the experiment. The pig stomach membranes proved ineffective in pre-experiment testing. Water penetrated between the inner and outer membranes, causing a bubble. Instead, the stomachs were used inside out, which prevented this problem during experimentation. The sphincters and mechanical damage were repaired using a leather sewing kit which produced a pouch, into which water could be poured. V2 and V3 used red deer hide containers (Fig. 1) each containing c. 2 L of water and tied with paracord to a tripod built from hazel poles, suspended over a fire. The fur-bearing side of the hide was used as the outside of the container and the interior was filled with water. Hearths were centrally located beneath each tripod and a vessel suspended from each using cord. The experiments differed only in the distance from the fire, which was kept the same in V3 and progressively lowered into the fire until the base of the hide was resting on the coals in V2. V4 used a deer hide container suspended over a c. 50 × 50 × 25 cm pit filled with burning coals, embers and fresh wood. V4 was then placed over the pit with the central depression filled with c. 2 L of water and the edges secured with branches (Fig. 2).
Experiment 2: Hot Stone methods
The Hot Stone method heats the contents of the container by placing heated stones directly inside the vessel. The aim of these experiments was to attempt to heat the water in the containers solely through the indirect transfer of heat via the stones. Pig stomach (V5) was selected for testing due to their ability to hold both water and heated stones. The pig stomach (V5) was prepared as in Experiment One and suspended from a branch with c. 1 L of water added (Fig. 3). In order to raise the temperature, the hot stones needed to be placed into the water and quickly removed from the container before being added to the fire to reheat. To avoid thermal shock and for safety, rapid heating of stones was avoided by placing stones c. 30 cm away from the fire and moving them closer over time to allow for controlled heating to c. 250–500 °C. It was common throughout the experiments for multiple stones to be used simultaneously; typically two, in order to maximise the heating potential.
Experiment 3: Direct Heat ‘long-time, low-temperature’
A single unprocessed red deer hide was modified to create a simple container by periodically piercing the outer edge with a sharp flint flake and threading string through the perforations. The hide was then attached to a simple wooden tripod—made from three pieces of wood tied together—using the string (Fig. 1). A litre of water was added to the hide and an assessment of any damage to the hide container—indicated by leakage—was made prior to the commencement of the experiment. Hide container integrity was actively managed via periodic checking for tears or leakage, involving a reorientation of the hide when this occurred. This experiment placed greater emphasis on quantification to understand the dynamics involved in LTLT (long-time, low-temperature) cooking. Firewood was measured before and after the experiment by measuring out an area in cubic feet, filling the space with the wood before use, and measuring the remainder after the experiment concluded, to quantify total consumption. Thermocouples were used to record temperature data in both the container and the fire. Temperature data was recorded every 5 min on the commencement of the experiment, which for the purpose of timing, was taken as the water in the hide container reached the desired temperature threshold. The experiment proceeded to attempt to maintain this temperature for 3 h to simulate LTLT cooking conditions. Temperature was actively managed by adjusting the distance between the heat source and container using the strings to raise up or lower down the container.
Materials
The experiments were conducted outdoors at the York Experimental Archaeology Research (YEAR) Centre, Department of Archaeology, University of York (UK) over two sessions, one in the autumn and the second in the spring. The vessels which required direct heat had their own separate hearths within the YEAR Centre. An infrared laser digital thermometer (temperature range: − 50–750 °C; accuracy: ± 2 °C) was used to make all temperature recordings for the first session, and a thermocouple set (TM-RS232 Thermometer) was used for the second. Other equipment—tripods, sewing kits, flint, etc.—were supplied by the YEAR Centre. For the first session, firewood was a mix of horse chestnut (Aesculus hippocastanum) and elder (Sambucus nigra) derived from the YEAR Centre itself, supplemented by kiln dried birch (Betula pendula), purchased from a commercial supplier. For the second session, the firewood was mixed hardwood from a commercial supplier. The heating stone selection was balanced against the need for safety whilst maintaining archaeological and ethnographic fidelity. Coarse-grained, commercially shaped and rounded (8 cm × 6 cm × 2 cm) basalt was selected for its resilience to thermal fracture (Shantry 2020; Wilson and DeLyria 1999). To comply with ethical guidelines set out by the university, unprocessed adult male red deer (Cervus elaphus) hides and pig stomachs (Sus scrofa) used in making containers were procured from commercial suppliers.
Results
The results from Experiments 1 and 2 are presented in Table 1 and Fig. 5. Each experiment is discussed in turn, along with a summary comparison of Direct Heat results, Hot Stone results, with the two methods then compared.
Results: Direct Heat method
The results of experiment V1—a pig stomach vessel containing 1 L of water, suspended 50 cm above an open fire—are presented in Table 1 and Fig. 4. Figure 4 presents the change in temperature recorded through time for experiments V1–V5 and shows that the water temperature inside the V1 container rose rapidly, reaching a maximum of 69.1 °C. However, this was not consistently maintained throughout the experiment (Fig. 4). While this experiment failed to achieve a temperature to boil water, a sub-boiling temperature suitable for cooking food or other non-cooking tasks was achieved and maintained. As the experiment continued, physical modifications to the vessel became apparent. The stomach shrank significantly, dropping its water capacity from an initial 1 L to a final capacity of approximately 300 mL, a loss of c. 70% (Fig. 5). Shrinkage of this magnitude may have implications for the types of uses or lengths of time to which a vessel of this kind might be employed. Colour change in the container was also detected, likely as a result of the container beginning to cook after prolonged exposure to the heat source.
Photographic illustrations of damage and wear to the organic vessels during cooking. a Pig stomach (V1) showing signs of discolouration and shrinkage from the heat. b The fur burning and coming away from the red deer hide vessel (V6). c The blackening and scorching of the hide when placed over the fire (V6)
The results of experiment V2—a red deer hide vessel containing 2 L of water, suspended over and gradually lowered into a fire via a tripod—are presented in Table 1 and Fig. 4. Recorded water temperature inside the V2 container initially rose rapidly, eventually reaching a maximum temperature of 63 °C, which was maintained for the remainder of the experiment (Table 1, Fig. 4). In this experiment, the distance between heat source and the base of the container was gradually reduced until the container was in direct contact with the fire and hot coals; nonetheless, the container did not achieve the greatest recorded temperature. The result suggests that both proximity to and structure of the fire are important for conserving and directing the heat into the vessel, rather than losing it into the surrounding air. As the experiment progressed, changes to the container became apparent. This included increasing rigidity of the hide and charring and blackening of the outer surface facing the heat source, but with no evidence of shrinkage (Fig. 5).
The results of experiment V3—a red deer hide vessel containing 2 L of water, suspended over a fire via a tripod—are presented in Table 1 and Fig. 4. The heating pattern for the water in container V3 was markedly different to other experiments, with initial heating occurring gradually, followed by a rapid decline and equally rapid increase, before finally plateauing (Fig. 4). Fuelling of the fire may have contributed; the maximum temperature achieved was the lowest recorded: 48.2 °C across the direct heating experiments (Table 1, Fig. 4). However, the highest temperature recorded for the fire (877 °C) was associated with this experiment: evidencing a dramatic loss of heat in the air space between fire and container. It is probable that the thick deer hide acted as an efficient insulator, limiting heat transfer from fire to water. Observed changes to the vessel were in keeping with V2 (charring, blackening and stiffening), but less marked given the increased distance from the heat source.
The results of experiment V4—a red deer hide vessel containing 2 L of water, suspended over a pit with hot embers—are presented in Table 1 and Fig. 7. The water within the V4 container was rapidly heated over a short period of time (Fig. 4). This is likely due to the small distance between heat source and container, but also because the pit walls and container base acted to trap and channel the heat. Because the heat source could not be replenished, a sharp temperature decline was observed prior to the termination of the experiment (Fig. 4). The greatest temperature of all direct heating experiments (88.6 °C) was achieved in this configuration, likely due to the short distance between container base and heat source and the channelling of heat. Modifications to the deer hide container were again similar to V2, but with no reduction in container capacity. A perforation in the container developed where the hide was not protected by water shortly after the termination of the experiment.
In summary, the results of the Direct Heat methods demonstrate the difficulty of raising water to boiling point within an organic container. Neither hide nor stomach is efficient conductors of heat and the fur on the exterior of the hide may have increased its insulating properties. This is in line with thermal conductivity studies of cow hides showing that hide has a similar efficiency level as air—an extremely poor conductor (Maia et al. 2009). Nonetheless, sub-boiling temperatures were readily attained and maintained. While fuel consumption was not measured quantitatively, it was clear that the tripod method used significantly more firewood than the pit method. Characteristic modifications to the red deer hide containers were observed across experiments, with extent varying with specific experimental parameters, especially proximity to the heat source. In contrast to the pig stomach container, those made from deer hide displayed no visible signs of shrinkage.
Results: Hot Stone method
The results of experiment V5—a pig stomach vessel containing 1 L of water into which hot stones were placed—are presented in Table 1 and Fig. 4. The water temperature within container V5 rapidly and continuously heated throughout the experiment, exceeding the boiling point threshold of 100 °C prior to termination of the experiment (Fig. 4). To achieve this temperature, rapid rotation of heated stones was required to prevent the water cooling in between. A total of six stones were found to be sufficient: two were placed into the container while four heated in the fire. The stones themselves survived multiple rounds of heating and quenching with minimal cracking in the process. Repeated use of stones from the fire did, however, mean that the water began to be contaminated with ash, dirt and other particulates. This could have been mitigated by washing the stones before they were placed into the container after they had been heated and this should be taken into consideration when appraising the efficiency of heating the water. Physical modifications to the stomach were also noted. This included colour change, likely from cooking of the stomach in direct contact with hot stones, and shrinkage of around 50%. Despite this, it remained in a suitable condition to be reused for further heating. Overall the observations noted during this experiment closely matched many of those made by Ryder over 50 years ago, when he attempted to heat water in an animal stomach. The loss of volume through shrinkage, minor water displacement from the addition of hot stones and bubbling over of water at boiling point were observed during both experiments (Ryder 1969).
In summary, the two Hot Stone experiment results demonstrate that water can be heated rapidly and to a higher temperature when compared with Direct Heat methods. The experiments proved to be effective at boiling water (V5). However, there is a problem of contamination due to transfer of stones from a fire. Whilst indirect, the method is also fuel intensive as a fire must be maintained to heat the stones for the duration of the experiment. It should always be acknowledged that actualistic experimentation carries many variables related to the skill and experience of the practitioners, and replication of these experiments by others would help make these results more rigorous. Nonetheless, given that the aim of all experiments was to heat water, and that many forms of cooking do not require boiling water, both sets of results can be said to have achieved their initial goals.
Results: Direct Heat ‘long-time, low-temperature’
The results of experiment V6—a red deer hide vessel containing 1 L of water suspended over a fire for 3 h—are presented in Table 2, Figs. 5, 6 and 7. Results demonstrate the viability of heating water to a consistent sub-boiling temperature for a protracted period without the vessel failing (Table 2; Fig. 6). Charring of the deer hide occurred and this was consistent with other direct heat experiments (Fig. 5). Active management requirements were minimal, with two interventions required: firstly, to rearrange the hide to prevent the water pressure forcing a minor leak, and secondly, to raise the vessel slightly to prevent the water becoming too hot. The volume of water remaining at the conclusion of the experiment was measured and totalled 650 mL. When completely cooled, it became apparent that the substance had gelatinised, suggesting that the water was slowly breaking down the collagen from the interior membranes of the hide container. The fuel usage was approximately 65 kg, which is high for a campfire (Pryor et al. 2016); however, this probably reflects the artificial nature of the experiment and the intentions of the practitioners to keep the fire as high as possible, knowing the temperature read-out from the thermocouple. Under ‘realistic’ circumstances, hunter-gatherer fires are often multipurpose and a cooking vessel placed over a fire would be just one function amongst many (insect repellant, warmth, social gathering, craftwork). Therefore, it is difficult to directly compare the firewood usage for this single experiment with a lifelike scenario.
Calculated temperature curves for the V6 Direct Heat ‘long-time, low-temperature’ experiment. The experimental data is plotted against a theoretical fit, as well as a comparative calculated ceramic curve. Equations 3 and 4 have been included with the corresponding colours for ‘heating’ and ‘cooling’
The temperature data collected using thermocouples allows for characterisation of the thermodynamic system, expressed via a temperature against time curve T(t) model (Fig. 7). As the experiment was actualistic, a number of assumptions were made: 1 kg of water was heated over an open fire using a red deer skin vessel; the skin was approximately 1 mm thick; the fur, predominantly keratin, was assumed to be redundant following its rapid destruction by the fire; and the contact area (A) between the hide and fire was reasoned to be 800 cm2. The T(t) curve was evaluated using Newton’s heating law for the heating phase up to 215 min and after removal of the heat source, Newton’s cooling law was applied to fit the respective part of the curve.
The fit used is based on the following consideration: an instantaneous change in the transferred heat dQ is related to a small change of temperature dT by:
where mwater is the mass of the heated water and cwater the specific heat of water. Hence, the heating rate is
Correspondingly, the heat transfer through the hide is proportional to the temperature gradient dT/dx and depends on its heat conductivity kskin as well as the area A. The corresponding heating rate is then given in one dimension by
combining Eqs. 1 and 2 leads then to
rearranging this we get
This is a first order differential equation in space and time. Assuming now a linear gradient dT/dx = ΔT/d with d being the thickness of the skin this can be simplified to
Integration with the boundary condition Twater either the initial temperature before heating or Tsurface the temperature before cooling. Distinguishing between the heating and cooling phases we find in the case of heating
and in the case of cooling
Equations 3 and 4, respectively, were used to fit the experimental T(t) data for heating and cooling with the parameters A = 800 cm2, kskin = 0.7 W/mK for collagen, cwater = 4184 J/kg for water, d = 1 mm, Twater = 6 °C (temperature of the environment) and Tsurface = 70 °C (temperature of the heating zone above the fire).
The values for the kskin/cwater ratios during the heating and cooling phase are 2.19 10–4 kg/ms and 2.25 10–4 kg/ms, respectively. Given the approximations made, this is in remarkable proximity to the expected value of 1.75 10–4 kg/ms, corroborating the assumptions.
Overall, the measured time dependence of temperature increase and decrease confirm that the key component of the hide acting as vessel for the heated water is the collagenous deer skin. A comparison of the deer hide data with those theoretically expected for a ceramic vessel with a similar thickness revealed that the significantly higher thermal conductivity of ceramics led to attainment of the target temperature of 70 °C more quickly, while the cooling would also be faster. Hence, a deer hide based vessel is less favourable in terms of heating time to reach a targeted temperature but would keep the heated water hot for a longer period of time.
A number of useful observations can be drawn from these results. Firstly, the initial heating requirements are likely much greater to transfer the heat through the hide in comparison to ceramics, but the greater insulation properties suggest that either the fire could be reduced in intensity or even extinguished and the hide continues to cook the foodstuffs for longer. Secondly, the outer layer of fur may contribute little to nothing to both the vessel’s heating and cooling, presumably because the damage from the fire has undermined the molecular properties of the keratin fur matrix. Thirdly, that unprocessed animal hide functions perfectly well as a vessel for LTLT cuisine—by eliminating the stipulation that cooking is synonymous with boiling, this type of direct heat food processing makes best use of the hide’s thermal properties.
Discussion
Recent years have seen an increase in interest in hunter-gatherer aceramic container technologies and the keystone role they have played in our species survival and evolution, with ethnographic data used to inform a non-exhaustive range of uses, including: short and long-term storages and mobility of foodstuffs, water, raw materials, colourants, medicine, tools, equipment in addition to the carrying of infants and burial of corpses (Henrich 2015; Langley and Suddendorf 2020; Suddendorf et al. 2020). Not all, but many of these storage containers are made from materials that can be used for cooking and are at least semi-fire retardant (e.g., shells, plant leaves/fibres, animal skins/organs); however, the equation between containers and cooking is far from being a simplistic one. The right amount of heat, requiring the use of the right type of vessel for cooking a particular foodstuff, has implications for how digestible the food is (e.g., detoxifying, tenderising and breaking down tubers and other fibrous plants; breaking down proteins found in meat), the digestive workload of our mouths, stomachs and colons, and ultimately, the degree of energetic benefits (Carmody and Wrangham 2009; Henrich 2015).
The experimental archaeological results demonstrate the effectiveness, but also key differences, between multiple methods of heating water in organic perishable containers. Experimental replication and use of hide containers with the Direct Heat method indicate that cooking at sub-boiling temperatures is achievable, while boiling may not be. Results from the stone cooking experiments confirm that this technique is more fuel efficient and effective at rapidly raising water temperature than placing the container directly over the heat. It is, however, interesting to consider that these results differ from other research investigating stone cooking. In Holman and Egan’s (1985) experimental work studying the traditional methods of maple syrup production, they record that hot stone cooking was amongst the least efficient methods of reducing the liquid to a syrup. The time taken to make the syrup was measured at between 5 and 6 h: an impractical amount of time to constantly rotate and manoeuvre the stones. This suggests that the efficiency of hot stone heating may be quickly undermined when the cooking procedure requires many hours of heat. This is consistent with previously published work on stone boiling, which suggests the process can be rapid but increases labour costs (Driver and Massey 1957).
Across all experiments, there was appreciable variation in the degree of monitoring and labour required to heat water. Hot stones were effective in raising the internal temperature of the vessel; however, they demanded a higher level of observation, planning, activity and monitoring. The use of animal skins directly over a fire may still require more active engagement than pottery, but for the user it is less involved than using heated stones. Equally, while both methods proved to be viable, the pit method was more fuel efficient than the tripod methods, which may have been a pertinent consideration when selecting the type of method to achieve a specific task. The container material also proved to be an important variable. The pig stomach container was more efficient at initial heat transfer when compared with the red deer hide containers, perhaps indicating that internal organ membranes might be more desirable as a vessel. It is, however, important to stress that containers were not tested to the point of destruction; in which case, its thinness, lower insularity and tendency towards shrinkage may prove less effective for slow cooking. When taken together, the results suggest that the material used in creating the vessel and the configuration used to heat its contents would be prominent variables that were actively considered in diverse tasks in different archaeological contexts. Viewing these results in the context of evolving and developing cooking strategies, it is likely that cooking foods using pottery would change the engagement for the user from active to potentially semi-active or passive. The social implications for users practising different methods of aceramic cooking are deserving of closer consideration in the future (see Wrangham 2010; Dunbar and Gowlett 2014).
Speth (2015) questioned the types of damage and alteration that might be seen in organic containers and the results provide insight. Red deer hides suspended above a fire using a tripod became increasingly hard and inflexible in the time it took to heat water. The fur on the underside of the hides became blackened and scorched but the heat failed to damage the skin tissues. The hide suspended above a pit was damaged soon after the experiment ended when the embers burnt through the sections which were unprotected by the heated water, demonstrating that a level of skill and attention is required to maintain even this method. The stomach containers used for hot stone and direct heating methods shrank considerably during the experiments, losing over half of their usable volume, likely due to the sensitivity of the tissue to heat. This suggests that contrary to Speth (2015), hot stone cooking can be equally as damaging to the container as a direct method, depending on the material used. These findings further support the view that the material from which to make a container was taken into consideration when selecting the cooking method. For example, the capacity for damage from indirect hot stone cooking has been a key factor in identifying the function of different pottery styles (Sassaman et al. 1995).
A related area of consideration is the extent to which inclusions from stone transfer or derived from leaching from the heated stones changes water composition, such as pH. While the experiments made use of basalt for safety reasons, globally it remains a lesser utilised stone and alternatives such as quartz and limestone may have their own effects on the cooking process. It is possible that specialised forms of plant processing relied on the deliberate selection of stones with particular properties. For example, work on a variety of plants has shown that alkaline solutions generated through limestone use can produce dramatic changes in plant and seed biochemistry (Ellwood et al. 2013; Abdel-Gawad 1993). Alongside changes in water composition is the more qualitative and sensory question of taste. Stone boiling has the potential to introduce foreign objects including ash, soil, grit and other particulate matter into the food. This can be avoided by cleaning the stones after they have left the fire, but before they are placed into the water, either through manually brushing or momentarily dipping them into another container of water. Fresh hide is not an instinctively appealing vessel material to contemporary western sensibilities, being a potential vector for zoonotic diseases, and potentially producing unpleasant odours and tastes if not used immediately from the animal. However, this should be balanced against knowledge from the ethnographic record that taste and smell are culturally contingent, and rotting, putrefying or decaying foods can be highly prized and savoured in different cuisines (Speth and Eugène 2022). Therefore, unprocessed animal skin can be considered viable when considering possible material choices throughout prehistory. Of course, other organic materials should also be trialled for their thermal properties in future research. Bark and wood are often noted in the ethnographic record as an alternative to animal-based containers, and an expansion of this type of actualistic experimentation into their working properties is essential to characterising aceramic cooking technologies as a whole.
During phase one of the experimental programme, boiling of water using hot stones and direct heat was only achieved once. In this respect, reaching and maintaining boiling via aceramic methods can be seen to be challenging, but nonetheless, viable. Sub-boiling temperatures that would allow foodstuffs, such as starch or meat, to begin exhibiting biochemical changes that would aid in their processing and digestion were consistently achieved. Phase two of the experimentation demonstrated that such sub-boiling temperatures can also be sustained for protracted periods. Depending on the cuisine, maintaining consistent, low temperatures can be preferable to boiling. For example, cooking meat in water for long periods of time below 60 °C (‘long-time, low temperature’ (LTLT) cooking) results in significant collagen denaturation, while temperatures above this result in the meat being tougher (Latorre et al. 2019; Paul 1963; Bertola et al. 1994). The research suggests that LTLT cooking produces a superior product both in flavour and tenderness (Christensen et al. 2012; Tornberg 2005; Dominguez-Hernandez et al. 2018), which is likely a prominent factor in why many cuisines employ long, slow cooking (Wandsnider 1997). When these insights are applied to prehistoric contexts, it can be suggested that a cooking technology which is unable to attain boiling temperatures, but which can still heat water, may have even been desirable and advantageous. As Fig. 8 demonstrates, the temperature spectrum of water possesses numerous useful sub-boiling thresholds, highlighting that the advantages of wet-cooking begin at lower temperatures than archaeologists have previously acknowledged. The experimental data indicates that Direct Heat cooking might be particularly well suited to harnessing the LTLT effect to process raw meat, and perhaps other types of food, into a bioavailable resource.
The utility of heated water does not begin at boiling point, important biochemical changes occur in different plant and animal products at sub-boiling temperatures for different periods of time. Many of these are crucial to either detoxifying or rendering carbohydrates and proteins available for human digestion. (1) (Shi et al. 2017); (2 & 4) (Hickman et al. 2000); (3) (Shariffa et al. 2009); (5) (Dominguez-Hernandez et al. 2018); (6) (Oke 1983); (7) (Senica et al. 2016); (8) (Thompson et al. 1983); (9) (Ellwood et al. 2013); (10) (Modesto et al. 2019)
This conclusion is consistent with other experiments across diverse contexts. For example, a study by Hanson et al. (2019) of acorn processing tested both stone boiling and simmering in ceramic vessels, concluding that boiling the acorns had the effect of binding the bitter tannins to the nutmeat, while simmering methods instead leached them. Discussing the origins of ceramics during the North American Woodland Period, Skibo et al. (2009) observed that water boiling can bring with it limitations, including overflow into the fire, unsuitability for extracting greases and oils and the bubbling surface can inhibit skimming of fats. It should, however, be noted that boiling can have distinct advantages over heating in other contexts. For example, Ellwood et al. (2013) demonstrate that using heated limestone to cook and nixtamalise maize relies on rapidly heating the water to between 70 and 100 °C, which provides the optimal environment for the kernels to absorb the alkaline water. Arnold’s (1988) argument that the development of pottery was driven by the need to detoxify and make available the stored energy and nutrients in various plant foods remains important. Listing commonly used plants from around the world which require prolonged boiling to be rendered safe and edible also highlights a key advantage of pottery over aceramic cooking methods—the ability to withstand high temperatures for long periods of time. It has been proposed that the appearance of early pottery might be related to the exploitation of seasonal abundance of oily fish species, notably to the processing or storing of ‘prized’ aquatic oil with a highly symbolic value (Hayden 1995). Alternatively, pottery may have been used to cook previously stored commodities such as dried fish, as suggested by organic residue analysis of Incipient and Initial Jomon pottery (Lucquin et al. 2018). Lengthy simmering temperatures would have been easier to obtain using ceramic vessels in addition to providing a more secure and durable container within a context of increasing exploitation of aquatic ecotones (Wang and Sebillaud 2019).
Speth’s (2015) original question of ‘how did humans learn to boil?’ can thus be usefully expanded to ‘how did humans learn to cook?’. Since boiling may be both unnecessary and undesirable in various cooking recipes, separating ‘rapid boiling’ from ‘LTLT’ and recognising them as two specific and distinct methods of processing foods, each with potential advantages depending on the specific context, is important, with potential implications for human evolution, the development of organic container technologies, and the transition to the use of ceramics. Thus, these two methods may have coexisted, targeting different commodities, fulfilling two different roles within a complex system involving diverse forms of food preparation: forming a cuisine.
Conclusions
Results from this experimental programme demonstrate the complexity of engaging with the evolution of material technologies and the benefits of experimentally testing latent assumptions. The results demonstrate that organic, perishable containers placed over direct heat are capable of heating water sufficiently to process foods, bringing into question the presupposition that a cooking technology should be necessarily judged on its ability to heat more quickly and to a higher temperature than another. In some contexts, higher temperatures can be undesirable and instead a lower and more stable temperature may be favoured. The ability to heat water and to boil water should be seen as two separate methods of food processing. Combining the scientific research into ‘long-time, low-temperature’ cooking with the experimental data of direct heat cooking in perishable containers, it is proposed that the need for lengthy simmering temperatures, as opposed to rapid boiling temperatures, may have been a driver in the creation of ceramic vessels during the Upper Palaeolithic and a factor in their increase in dominance thereafter. Future research can more fully test this hypothesis by characterising other common organic vessel materials, such as bark and wood. Finally, the results demonstrate how experimental archaeology is an important method for understanding the functionality of different material properties and container technologies, even for a topic as seemingly mundane as heating water.
Data Availability
The data that support the findings of this study are available from the corresponding author, [Andy Langley], upon reasonable request.
References
Abdel-Gawad AS (1993) Effect of domestic processing on oligosaccharide content of some dry legume seeds. Food Chem 46(1):25–31. https://doi.org/10.1016/0308-8146(93)90070-V
Aiello L, Dunbar R (1993) Neocortex size, group size, and the evolution of language. Curr Anthropol 34(2):184–193
Aiello L, Wheeler P (1995) The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 36(2):199–221
Alperson-Afil N, Goren-Inbar N (2010) The Acheulian site of Gesher Benot Ya’aqov volume II: ancient flames and controlled use of fire. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3765-7
Arnold DE (1988) Ceramic Theory and Cultural Process. Cambridge University Press
Arsuaga JL, Martínez I (1998) The chosen species: the long march of human evolution. Blackwell, Oxford
Aura Tortosa JE, Pérez-Jordà, G, Carrión Marco Y, Seguí Seguí, JR, Jordá Pardo, JF, Miret i EstruchC, Verdasco Cebrián CC (2019) Cordage, basketry and containers at the Pleistocene--Holocene boundary in southwest Europe. Evidence from Coves de Santa Maira (Valencian Region, Spain). Veget Hist Archaeobot 29:581–594. https://doi.org/10.1007/s00334-019-00758-x
Avens JS, Albright SN, Morton A, Prewitt B, Kendall P, Sofos J (2002) Destruction of microorganisms on chicken carcasses by steam and boiling water immersion. Food Control 13(6):445–450. https://doi.org/10.1016/S0956-7135(01)00073-1
Barkai R, Rosell J, Blasco R, Gopher A (2017) Fire for a reason: barbecue at Middle Pleistocene Qesem Cave, Israel”. Curr Anthropol 58(S16):S314–S328. https://doi.org/10.1086/691211
Batchelor D (1979) The use of quartz and quartzite as cooking stones. In: Bosinski G (ed) Die Ausgrabungen in Gönnersdorf 1968–1976 Und Die Siedlungsbefunde Der Grabung 1968. Franz Steiner, Wiesbaden, pp 154–165
Benison CJ (1999) Burned rock complexes, baked clay objects, steatite, and ceramics: evolutionary implications for Plains/Eastern Woodlands cooking technologies. N Am Archaeol 20(4):287–317. https://doi.org/10.2190/DDMQ-H4K7-V9RG-4LM9
Bentsen SE, Wurz S (2017) Towards a better understanding of cooking techniques in the African Middle Stone Age, Primit. Tider 19:101–114. https://doi.org/10.5617/pt.7164
Bentsen SE, Wurz S (2019) Color me heated? A comparison of potential methods to quantify color change in thermally-altered rocks”. J Field Archaeol 44(4):1–19. https://doi.org/10.1080/00934690.2019.1591092
Bertola NC, Bevilacqua AE, Zaritzky NE (1994) Heat treatment effect on texture changes and thermal denaturation of proteins in beef muscle. J Food Process Preserv 18(1):31–46. https://doi.org/10.1111/j.1745-4549.1994.tb00240.x
Boback S, Cox CL, Ott BD, Carmody R, Wrangham RW, Secor SM (2007) Cooking and grinding reduces the cost of meat digestion. Comp. biochem. physiol., Mol. amp; integr. Physiol. 148(3):651–56 https://doi.org/10.1016/j.cbpa.2007.08.014
Bolus M (1990) The internal organization of two Magdalenian structures from Andernach: interpretations based on conjoined quartz material. In: Cziesla E, Eickhoff S, Arts N, Winter D (eds) The big puzzle: International Symposium on Refitting Stone Artefacts, Symposium Monrepos, 1987. Holos, Bonn, pp 331–37
Bosinski G (1981) Gonnersdorf - Eiszeitjager am Mittelrhein, Ausstellung des Landesmuseums Koblenz. Selbstverlag des Landesmuseums, Koblenz
Brown JA (1989) The beginnings of pottery as an economic process. In: van der Leeuw SE, Torrence R (eds) What's New? A Closer Look at the Process of Innovation. Routledge, pp 203–224
Carbonell E, Cebrià A, Allué E, Cáceres I, Castro Z, Díaz R, Esteban M, Ollé A, Pastó I, Rodríguez XP, Rosell J, Sala R, Vallverdú J, Vaquero M, Vergés JM (1996) Behavioural and organizational complexity in the Middle Palaeolithic from the Abric Romaní. In: Carbonell E, Vaquero M (eds) The last Neandertals/the first Anatomically Modern Humans: a tale about human diversity: Cultural change and human evolution: the crisis at 40 KA BP. Universitat Rovira i Virgili, Tarragona, pp 385–434
Carmody RN, Weintraub GS, Wrangham RW (2011) Energetic consequences of thermal and nonthermal food processing. Proc Natl Acad Sci USA 108(48):19199–19203. https://doi.org/10.1073/pnas.1112128108
Carmody RN, Wrangham RN (2009) The energetic significance of cooking. J Hum Evol 57(4):379–391. https://doi.org/10.1016/j.jhevol.2009.02.011
Cheng CS, Parrish FC Jr (1979) Heat-induced changes in, myofibrillar proteins of bovine longissimus muscle. J Food Sci 44(1):22–24. https://doi.org/10.1111/j.1365-2621.1979.tb09995.x
Christensen L, Gunvig A, Tørngren MA, Dall Aaslyng M, Knøchel S, Christensen M (2012) Sensory characteristics of meat cooked for prolonged times at low temperature. Meat Sci 90(2):485–489. https://doi.org/10.1016/j.meatsci.2011.09.012
Coward F, Gamble C (2008) Big brains, small worlds: material culture and the evolution of the mind. Philos Trans R Soc B 363(1499):1969–1979. https://doi.org/10.1098/rstb.2008.0004
Dolbunova E, Lucquin A, McLaughlin R T, Bondetti M, Courel B, Oras E, Piezonka H et al (2022) The transmission of pottery technology among prehistoric European Hunter-Gatherers. Nat Hum Behav. December. https://doi.org/10.1038/s41562-022-01491-8
Dominguez-Hernandez E, Salaseviciene A, Ertbjerg P (2018) Low-temperature long-time cooking of meat: eating quality and underlying mechanisms. Meat Sci 143:104–113. https://doi.org/10.1016/j.meatsci.2018.04.032
Driver HE, Massey WC (1957) Comparative studies of North American Indians. Trans Am Phil Soc 47:165–456
Dunbar R (1993) Coevolution of neocortex size, group size and language in humans. Behav Brain Sci 16:681–735. https://doi.org/10.1017/S0140525X00032325
Dunbar R, Gowlett J (2014) Fireside chat: the impact of fire on hominin socioecology. In: Dunbar R, Gamble C, Gowlett J (eds) Lucy to Language: the benchmark papers. Oxford University Press, Oxford, pp 277–296
Ellwood EC, Scott MP, Lipe WD, Matson RG, Jones JG (2013) Stone-boiling maize with limestone: experimental results and implications for nutrition among SE Utah preceramic groups. J Archeol Sci 40(1):35–44. https://doi.org/10.1016/j.jas.2012.05.044
Fletcher L, Milner N, Taylor M, Bamforth M, Croft S, Little A, Pomstra D, Robson HK, Knight B (2018) The use of birch bark. In: Milner N, Conneller C, Taylor B (eds) Star Carr Volume 2: Studies in Technology, Subsistence and Environment. White Rose University Press, York, p 419–435. https://doi.org/10.22599/book2
Gamble C (2002) The Palaeolithic Societies of Europe. Cambridge University Press, Cambridge
Gamble C (2009) Human display and dispersal: a case study from Biotidal Britain in the Middle and Upper Pleistocene. Evol Anthropol 18(4):144–156. https://doi.org/10.1002/evan.20209
Gao X, Guan Y, Chen F, Yi M, Pei S, Wang H (2014) The discovery of Late Paleolithic boiling stones at SDG 12, North China. Quat Int 347:91–96. https://doi.org/10.1016/j.quaint.2014.07.003
Gao X, Dennell R (2014) Late Pleistocene and Palaeolithic studies in Northeast Asia. Quat Int 347:1–4. https://doi.org/10.1016/j.quaint.2014.08.023
Germonpré M, Lbova L (1996) Mammalian remains from the Upper Palaeolithic site of Kamenka, Buryatia (Siberia). J Arc Sci 23(1):35–57. https://doi.org/10.1006/jasc.1996.0004
Gowlett J (2006) The early settlement of northern Europe: fire history in the context of climate change and the social brain. C R Palevol 5(1–2):299–310. https://doi.org/10.1016/j.crpv.2005.10.008
Gowlett JAJ (2015) Les origines de l’utilisation du feu par les hommes: hypothèses actuelles et indices les plus anciens. In: de Lumley H (ed) Sur le chemin de l’hunanité. Via humanitatis: les grandes étapes de l’évolution morphologique et culturelle de l’Homme: émergence de l’être humain. Colloque international de l’Academie Pontificale des Sciences, Cité du Vatican avril 2013. Académie Pontificale des Sciences/CNRS, Paris, p171–197
Gramsch B, Kloss K (1989) Excavations near Friesack: an Early Mesolithic marshland site in the northern plain of Central Europe. In: Bonsall C (ed) The Mesolithic in Europe: papers presented at the third international symposium, Edinburgh, 1985. John Donald, Edinburgh, p 313–324
Gumiński W (2012) Nowe Wyjątkowe Siedlisko Osadnicze Paraneolitycznej Kultury Zedmar Na Wschodnim Cyplu Wyspy Szczepanki (sektor „A”) Na Mazurach. Światowit 9:87–144
Hanson KE, Bryant PL, Painter AM, Skibo JM (2019) Acorn processing and pottery use in the Upper Great Lakes: an experimental comparison of stone boiling and ceramic technology. Ethnoarchaeol 11(2):170–185. https://doi.org/10.1080/19442890.2019.1642574
Hart D, Sussman W (2005) Man the Hunted: Primates, Predators, and Human Evolution. Westview Press, New York
Hayden B (1995) The emergence of prestige technologies and pottery. In: Barnett WK, Hoopes JW (eds) The Emergence of Pottery: Technology and Innovation in Ancient Societies. Smithsonian Institution Press, Washington, DC, pp 257–266
Hayden B, Barnett WK, Hoopes JW (1995) The Emergence of Pottery. Washington, [DC]: Smithsonian Institution Press; 1-56098-517-8, pp 257-266
Henry AG (2017) Neanderthal cooking and the costs of fire. Curr Anthropol 58(S16):S329–S336. https://doi.org/10.1086/692095
Henrich J (2015) The Secret of Our Success: How Culture is Driving Human Evolution, Domesticating Our Species, and making Us Smarter. Princeton University Press, USA
Hickman D, Sims TJ, Miles CA, Bailey AJ, de Mari M, Koopmans M (2000) Isinglass/collagen: denaturation and functionality. J Biotechnol 79(3):245–257. https://doi.org/10.1016/s0168-1656(00)00241-8
Holman MB, Egan KC (1985) Processing maple sap with prehistoric techniques. J Ethnobiol 5(1):61–75
Hyland DC, Zhushchikhovskaya IS, Medvedev VE, Derevianko, AP, Tabarev AV (2002) Pleistocene textiles in the Russian Far East: impressions from some of the world’s oldest pottery. L’Anthropologie 40(1):1–10
James SR, Dennell RW, Gilbert AS, Lewis HT, Gowlett JAJ, Lynch TF, McGrew WC, Peters CR, Pope GG, Stahl AB, James SR (1989) Hominid use of fire in the Lower and Middle Pleistocene: a review of the evidence [and Comments and Replies]. Curr Anthropol 30(1):1–26
Jordan P, Zvelebil M (2016) Ex oriente lux: the prehistory of hunter-gatherer ceramic dispersals. In: Jordan P Zvelebil M (ed) Ceramics Before Farming, Routledge, pp 33–90
Kabukcu C, Hunt C, Hill E, Pomeroy E, Reynolds T, Barker G, Asouti E (2023) Cooking in caves: Palaeolithic carbonised plant food remains from Franchthi and Shanidar. Antiquity 97 (391): 12–28. https://doi.org/10.15184/aqy.2022.143
Kemp RM, North MF, Leath SR (2009) Component heat capacities for lamb, beef and pork at elevated temperatures. J Food Eng 92(3):280–284. https://doi.org/10.1016/j.jfoodeng.2008.11.006
Krief S, Daujeard C, Moncel M-H, Lamon N, Reynolds V (2015) Flavouring food: the contribution of chimpanzee behaviour to the understanding of Neanderthal calculus composition and plant use in Neanderthal diets. Antiquity 89(344):464–71. https://doi.org/10.15184/aqy.2014.7
Kuzmin YV (2017) The origins of pottery in East Asia and neighboring regions: an analysis based on radiocarbon data. Quat Int 441(B):29–35. https://doi.org/10.1016/j.quaint.2016.10.011
Langley MC, Suddendorf T (2020) Mobile containers in human cognitive evolution studies: understudied and underrepresented. Evol Anthropol 29:299–309. https://doi.org/10.1002/evan.21857
Latorre ME, Palacio MI, Velázquez DE, Purslow PP (2019) Specific effects on strength and heat stability of intramuscular connective tissue during long time low temperature cooking. Meat Sci 153:109–116. https://doi.org/10.1016/j.meatsci.2019.03.016
Lawrie RA, Ledward DA (2006) Lawrie’s Meat Science, Abington. Woodhead Publishing Limited, Cambridge
Leroi-Gourhan A, Brézillon M (1966) L’habitation magdalénienne n°1 de Pincevent, près de Montereau (Seine-et-Marne). Gallia Préhistoire 9:263–371
Lucquin A (2007) Etude Physico-Chimique Des Méthodes de Cuisson Pré et Protohistorique. Dissertation, Université de Rennes 1
Lucquin A, Robson HK, Eley Y, Shoda S, Veltcheva D, Gibbs K, Heron CP, Isaksson S, Nishida Y, Taniguchi Y, Nakajima S, Kobayashi K, Jordan P, Kaner S, Craig OE (2018) The impact of environmental change on the use of early pottery by East Asian hunter-gatherers. Proc Natl Acad Sci USA 115(31):7931–7936. https://doi.org/10.1073/pnas.1803782115
Lupo KD, Schmitt DN (1997) Experiments in bone boiling: nutritional returns and archaeological reflections. Anthropozoologica 25–26:137–144
Maia ASC, da Silva RG, de Souza Junior JBF, da Silva RB, Domingos HGT (2009) Effective thermal conductivity of the hair coat of Holstein cows in a tropical environment. R Bras Zootec 38(11):2218–2223
Mallol C, Hernández CM, Cabanes D, Machado J, Sistiaga A, Pérez L, Galván B (2013) Human actions performed on simple combustion structures: an experimental approach to the study of Middle Palaeolithic Fire. Quat Int 315:3–15. https://doi.org/10.1016/j.quaint.2013.04.009
March RJ, Lucquin, A (2007) Les Activités Réalisées En Lien Avec L’utilisation Du Feu et L'analyse Des Comportements Dans L'espace : Modalités Fonctionnelles, Modalités Saisonnières. In Beyries S, Vaté V (eds) Les Civilisations Du Renne D’hier et D’aujourd’hui - Approches Ethnohistoriques, Archéologiques et Anthropologiques. Actes Des Rencontres, 19–20–21 Octobre 2006 (Palais Des Congrès d’Antibes - Juan-Les-Pins) XXVII: Rencontres Internationales D’archéologie et D’histoire d’Antibes. éditions APDCA, Sophia Antipolis, p 421–38
Milner N, Taylor B, Conneller C, Bayliss A (2018) Interpretative narrative of the history of occupation. In N. Milner, C. Conneller, B. Taylor (Eds.) Star Carr. Volume 1: A Persistent Place in a Changing World. White Rose University Press, York, p 225–244. https://doi.org/10.22599/book1
Modesto J, Nunes E, Chisté RC, da Silva PR (2019) Oven drying and hot water cooking processes decrease HCN contents of cassava leaves. Food Res Int 119(May):517–523. https://doi.org/10.1016/j.foodres.2019.01.029
Murdock GP, Provost C (1973) Factors in the division of labor by sex: a cross-cultural analysis. Ethnology 12(2):203–225. https://doi.org/10.1177/106939717701200101
Nakazawa Y, Straus LG, González-Morales MR, Cuenca Solana D, Caro Saiz J (2009) On stone-boiling technology in the Upper Paleolithic: behavioral implications from an Early Magdalenian hearth in El Mirón Cave, Cantabria, Spain. J Archaeol Sci 36(3):684–693. https://doi.org/10.1016/j.jas.2008.10.015
Nelson K (2010) Environment, cooking strategies and containers. J Anthropol Archaeol 29(2):238–247. https://doi.org/10.1016/j.jaa.2010.02.004
Oestmo S (2013) Digital imaging technology and experimental archeology: a methodological framework for the identification and interpretation of fire modified rock (FMR). J Archaeol Sci 40(12):4429–4443. https://doi.org/10.1016/j.jas.2013.07.011
Oke OL (1983) Processing and detoxification of cassava. In: Cassava, Toxicity and Thyroid: Research and Public Health Issues: proceedings of a workshop held in Ottawa, Canada, IDRC, Ottawa, ON, CA
Oshibkina SV (1989) The material culture of the Veretye-type sites in the region to the east of Lake Onega. In: Bonsall C (ed) The Mesolithic in Europe: papers presented at the third international symposium, Edinburgh, 1985. John Donald, Edinburgh, p 402–413
Patania I, Goldberg P, Cohen DJ, Wu X, Zhang C, Bar-Yosef O (2019) Micromorphological analysis of the deposits at the early pottery Xianrendong Cave site, China: formation processes and site use in the Late Pleistocene. Archaeol Anthropol Sci 11(8):4229–4249. https://doi.org/10.1007/s12520-019-00788-6
Patania I, Jaffe Y (2021) Collaboration, not competition: a geoarchaeological approach to the social context of the earliest pottery. J Anthropol Archaeol 62(1012977): https://doi.org/10.1016/j.jaa.2021.101297
Paul PC (1963) Influence of Methods of Cooking on Meat Tenderness. In: Proceedings Meat Tenderness Symposium. Campbell Soup Company, Camden New Jersey, p 225–42
Piezonka H (2021) A container innovation in the Ice Age: the world’s oldest pottery and its dispersal among North Eurasian hunter-gatherers. In: Klimscha F, Hansen S, Renn J (ed) Contextualizing Ancient Technology. Berlin Studies of the Ancient World, pp 57–82
Povlsen K (2013) The introduction of ceramics in the Ertebølle culture. Dan J Archaeol 2:146–163. https://doi.org/10.1080/21662282.2013.904127
Preece RC, Gowlett JAJ, Parfitt SA, Bridgland DR, Lewis SG (2006) Humans in the Hoxnian: habitat, context and fire use at Beeches Pit, West Stow, Suffolk, UK. J Quat Sci 21(5):485–496. https://doi.org/10.1002/jqs.1043
Pryor AJE, Pullen A, Beresford-Jones DG, Svoboda JA, Gamble CS (2016) Reflections on Gravettian firewood procurement near the Pavlov Hills, Czech Reublic. J Anthropol Archaeol 43(September):1–12. https://doi.org/10.1016/j.jaa.2016.05.003
Reid KC (1984) Fire and ice: new evidence for the production and preservation of Late Archaic fiber-tempered pottery in the middle-latitude lowlands. Am Antiq 49(1):55–76. https://doi.org/10.2307/280512
Ressler C, Tatake JG, Kaizer E, Putnam DH (1997) Neurotoxins in a vetch food: Stability to cooking and removal of γ-Glutamyl-β-Cyanoalanine and β-Cyanoalanine and acute toxicity from common vetch (Vicia Sativa L.) Legumes. J Agric Food Chem 45(1):189–194. https://doi.org/10.1021/jf9603745
Rice PM (1999) On the origins of pottery. J Archaeol Method Theory 6:1–54. https://doi.org/10.1023/A:1022924709609
Roebroeks W, Villa P (2011) On the earliest evidence for habitual use of fire in Europe. Proc Nat Acad Sci USA 108(13):5209–5214. https://doi.org/10.1073/pnas.1018116108
Ryder ML (1969) Paunch cooking. Antiquity 43(171):218–220. https://doi.org/10.1017/S0003598X00107628
Sassaman K (1995) The social contradictions of traditional and innovative cooking technologies in the prehistoric American Southeast. In: Barnett W, Hoopes JW (ed) The emergence of pottery: technology and innovation in ancient societies, Smithsonian Institution Press, pp 223–240
Senica M, Stampar F, Veberic R, and Mikulic-Petkovsek M (2016) Processed elderberry (Sambucus Nigra L.) products: a beneficial or harmful food alternative? LWT - Food Sci Technol 72 (October): 182–88 https://doi.org/10.1016/j.lwt.2016.04.056
Shantry K (2020) The selection of boiling stones from a heterogeneous supply. J Archaeol Sci Rep 29(102030). https://doi.org/10.1016/j.jasrep.2019.102030
Shariffa YN, Karim AA, Fazilah A, Zaidul ISM (2009) Enzymatic hydrolysis of granular native and mildly heat-treated tapioca and sweet potato starches at sub-gelatinization temperature. Food Hydrocoll 23(2):434–440. https://doi.org/10.1016/j.foodhyd.2008.03.009
Shi L, Mu K, Arntfield SD, Nickerson MT (2017) Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J Food Sci Technol 54(4):1014–1022. https://doi.org/10.1007/s13197-017-2519-6
Shoda S, Lucquin A, Yanshina O, Kuzmin Y, Shevkomud I, Medvedev V, Derevianko E, Lapshina Z, Craig OE, Jordan P (2020) Late Glacial hunter-gatherer pottery in the Russian Far East: indications of diversity in origins and use. Quat Sci Rev 229(106124). https://doi.org/10.1016/j.quascirev.2019.106124
Skibo JM, Malainey ME, Drake EC (2009) Stone boiling, fire-cracked rock and nut oil: exploring the origins of pottery making on Grand Island. Wis Archeol 90(1–2):47–64
Speth JD (2015) When did humans learn to boil. PaleoAnthropology 2015:54–67. https://doi.org/10.4207/PA.2015.ART96
Speth, JD, Eugène M (2022) Putrid meat in the tropics: it wasn’t just for inuit. PaleoAnthropol 2022(2). https://doi.org/10.48738/2022.iss2.114
Straus LG (1989) On early hominid use of fire. Curr Anthropol 30(4):488–491
Suddendorf T, Kirkland K, Bulley A, Redshaw J, Langley MC (2020) It’s in the bag: mobile containers in human evolution and child development. Evolut Hum Sci 2:E48. https://doi.org/10.1017/ehs.2020.47
Svoboda J, Králík M, Čulíková V, Hladilová Š, Novák M, Nývltová Fišáková M, Nývlt D, Zelinková M (2009) Pavlov VI: an upper Palaeolithic living unit. Antiq 83(320):282–295. https://doi.org/10.1017/S0003598X00098434
Taylor M, Bamforth M, Robson HK, Watson C, Little A, Pomstra D, Milner N, Carty JC, Colonese AC, Lucquin A, Allen S (2018) The Wooden artefacts. In: Milner N, Conneller C, Taylor B (eds) Star Carr Volume 2: Studies in Technology, Subsistence and Environment. White Rose University Press, York, p 347–366 https://doi.org/10.22599/book2
Thoms AV (2009) Rocks of ages: propagation of hot-rock cookery in western North America. J Archaeol Sci 36(3):573–591. https://doi.org/10.1016/j.jas.2008.11.016
Thompson LU, Rea RL, Jenkins DJA (1983) Effect of heat processing on hemagglutinin activity in red kidney beans. J Food Sci 48(1):235–236. https://doi.org/10.1111/j.1365-2621.1983.tb14831.x
Tornberg E (2005) Effects of heat on meat proteins - implications on structure and quality of meat products. Meat Sci 70(3):493–508. https://doi.org/10.1016/j.meatsci.2004.11.021
Verheijen I, Starkovich B M, Serangeli J, van Kolfschoten T, Conard NJ (2023) Early evidence for bear exploitation during MIS 9 from the site of Schöningen 12 (Germany). J Hum Evol 177 (April):103294. https://doi.org/10.1016/j.jhevol.2022.103294
Wacnik A, Gumiński W, Cywa K, Bugajska K (2020) Forests and foragers: exploitation of wood resources by Mesolithic and para-Neolithic societies in north-eastern Poland. Veget Hist Archeobot 29:717–736. https://doi.org/10.1007/s00334-020-00778-y
Wandsnider L (1997) The roasted and the boiled: food composition and heat treatment with special emphasis on pit-hearth cooking. J Anthropol Archaeol 16(1):1–48. https://doi.org/10.1006/jaar.1997.0303
Wang L, Sebillaud P (2019) The emergence of early pottery in East Asia: new discoveries and perspectives. J World Prehist 32:73–110. https://doi.org/10.1007/s10963-018-9126-y
White DR, Burton ML, Brudner LA (1977) Entailment theory and method: a cross-cultural analysis of the sexual division of labor. Behav Sci Res 12(1):1–24. https://doi.org/10.1177/106939717701200101
Wilson DC, DeLyria DV (1999) The experimental reduction of rock in a camas oven: towards an understanding of the behavioral significance of fire-cracked rock. Archaeol Wash 7:81–89
Wrangham R, Conklin-Brittain N (2003) Cooking as a biological trait. Comp Biochem Physiol A Mol Integr Physiol 136(1):35–46. https://doi.org/10.1016/S1095-6433(03)00020-5
Wrangham RW (2007) The cooking enigma. In: Ungar PS (ed) Evolution of the human diet: the known, the unknown, and the unknowable. Oxford University Press, Oxford, pp 308–323
Wrangham R (2010) Catching fire: how cooking made us human. Profile Books, Great Britain
Wright K (2004) The emergence of cooking in Southwest Asia. Archaeol Int 8:33–37. https://doi.org/10.5334/ai.0810
Yu T-Y, Morton JD, Clerens S, Dyer JM (2017) Cooking-induced protein modifications in meat. Compr Rev Food Sci Food Saf 16(1):141–159. https://doi.org/10.1111/1541-4337.12243
Zhushchikhovskaya IS (1999) Dynamics of prehistoric pottery-making of the Russian Far East. In Jingkun G (ed) The Proceedings of the International Symposium of Asian Ceramics 1999, Shanghai Institute of Ceramics, Shanghai, p 471–84
Acknowledgements
We would like to acknowledge and dedicate this paper to Professor Emeritus John Speth, in particular his 2015 paper ‘When Did Humans Learn To Boil?’ which provided the inspiration for these experiments. We thank Speth for his comments on an earlier draft of this paper. Experimental work was carried out at the York Experimental Archaeology Research Centre, PalaeoHub, Department of Archaeology, University of York.
Funding
Andy Langley would like to thank the White Rose College of Arts and Humanities (WRoCAH), supported by the Arts and Humanities Research Council, for financial support (grant number AH/R012733/1) of his PhD, of which this work is a part.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations
All animal products used in this research were purchased from commercial wholesalers. No animal was killed for the purpose of this research.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Langley, A., Needham, A., Kröger, R. et al. An experimental study of wet-cooking in organic vessels: implications for understanding the evolution of cooking technologies. Archaeol Anthropol Sci 15, 142 (2023). https://doi.org/10.1007/s12520-023-01843-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01843-z