Abstract
The Early Pleistocene archeological site of Fuente Nueva-3 (FN3) preserves some of the oldest evidence of hominin presence in Western Europe, including a huge assemblage of Oldowan tools and evidence of butchering and marrow processing of large mammal bones. Moreover, there is also evidence of the regular presence of carnivores at the site, including a small proportion of bones that show tooth marks, the majority of which can be attributed to the giant, short-faced hyena Pachycrocuta brevirostris, and there are 220 coprolites, most of them from the Upper Archeological Level. In order to identify the defecating agent, we analyze here the coprolites and compare them with other specimens from the literature and with scats from zoo spotted hyenas (Crocuta crocuta). The morphology, color, size, and chemical composition of the FN3 coprolites allow us to attribute them to the hyena P. brevirostris, which is also represented at the site by fossil specimens. In addition, we evaluate the origin of the accumulation of coprolites and discuss on the role played by the scavenging hyenas in the accumulation and modification of the bone remains unearthed at the site, which allows evaluating the contribution of the giant hyena to this Early Pleistocene site. Finally, based on the lithology of layer 5 of the Upper Archeological Level, fine sands and clays deposited in a salt-lake environment, we hypothesize that this layer may have acted as a quicksand where large-sized animals like elephants were trapped and their carcasses lured scavenging carnivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The late Early Pleistocene of the Orce area (Baza Basin, SE Spain; Fig. 1) is mainly represented by the paleontological site of Venta Micena (VM), which is dated to 1.5–1.6 Ma (Palmqvist et al. 2022a), and by the archeological sites of Fuente Nueva-3 (FN3) and Barranco León (BL). The latter two localities, with a chronology close to 1.4 Ma (Duval et al 2012; Toro-Moyano et al. 2013; Palmqvist et al. 2016), preserve one of the oldest records of hominin presence in the European subcontinent, based on the finding of huge assemblages of Oldowan tools and skeletal remains of large mammals that preserve evidence of butchering and marrow processing. The tools, which represent the whole reduction sequence, are composed of abundant small flakes, blocks and cobbles, cores, and debitage made of flint, limestones, and calcarenites from the surroundings of the sites. Flint was largely exploited for flake production while limestone, although also used for flake production, was mostly employed for percussion tasks (Tixier et al. 1995; Turq et al. 1996; Martínez-Navarro et al. 1997; Oms et al. 2000; Palmqvist et al. 2005; Barsky et al. 2010, 2015a, 2015b; Toro-Moyano et al. 2011, 2013; Titton et al. 2018, 2021). The tools are associated with abundant remains of large mammals, some of which show cut and percussion marks related to defleshing, butchering, and marrow processing (Espigares et al. 2013, 2019; Yravedra et al. 2021, 2022a; Palmqvist et al. 2023 and references therein). Moreover, a deciduous tooth of Homo sp. has been unearthed from BL, which represents the oldest hominin fossil from Western Europe (Toro-Moyano et al. 2013).
a Geological context of the Guadix-Baza Depression in the Betic Cordillera, SE Spain. The box encloses the Orce-Fuente Nueva sector of the Baza Basin; b geological map of the Orce-Fuente Nueva sector showing the location of the FN3 site; c stratigraphic series of the FN3 site; d photographs of three in situ coprolites (1, 2, 3) that surround an elephant carcass; e detailed view of one of them (3).
Espigares et al. (2019) analyzed the evidence of anthropogenic activity in both sites, where carnivore modifications are also present but at low frequencies (see also Yravedra et al. 2021, 2022a; Courtenay et al. 2023). However, the Upper Archeological Level (UAL) of FN3 shows a high accumulation of hyena coprolites, which suggests that carnivore activity was particularly high at this level. In contrast, there are a low number of coprolites in the Lower Archeological Level (LAL) of FN3 and a high density of non-flaked, allochthonous blocks and cobbles from the Jurassic limestones of the site surroundings. Nearly half of these blocks display percussion marks (Barsky et al. 2015b, 2022), which suggests that hominin activity was important at this level.
Coprolites are mineralized or desiccated feces preserved in caves, shelters, and open-air sites (Reinhard and Byant 1992). The term coprolite was first used by Buckland to describe rounded black phosphatic structures, which corresponded to mineralized ichthyosaur feces of Early Jurassic (Lyas) age that contained fish scales and bones (Buckland 1829; Pemberton 2012). However, the first fossil scats recognized as hyena coprolites came from Late Pleistocene deposits of Kirkdale Cave (Buckland 1822, 1824).
Coprolites are an excellent source of information, because they can provide insights on the taphonomic history of an assemblage. As ichnofossils (i.e., trace fossils), coprolites are key to understanding aspects of the behavior of the defecating agent related to food processing, consumption, digestion, or excretion, which result in differences in the morphology and composition of coprolites (Hunt et al. 2012; Marinova et al. 2013).
The high preservational quality of the coprolites, sometimes considered as a Konservat Lagerstätten, provides a unique information on the diet and digestive system of the producing agent, which is not found elsewhere (Seilacher et al. 2001; Bull et al. 2002; Hollocher and Hollocher 2012; Qvarnström et al 2016). The feces of certain types of organisms are more durable than others, thus being more prone to fossilization. In particular, the coprolites excreted by carnivores, and especially by those like hyenas, whose diet includes a large amount of bones, have a preservation advantage due to their phosphatic composition and bone fragments content, which facilitates their fossilization. This explains why the coprolites of carnivores are much better preserved and largely outnumber those of herbivores (Chin 2002; Hollocher and Hollocher 2012; Linseele et al. 2013).
Coprolite size is also very indicative, as fecal volumes roughly scale with the body mass of the animal. By this reason, modern feces allow to infer, approximately, the size of the organism that produced them (Chin 2002; Farlow et al. 2010; Fig. 11). In addition, inclusions within coprolites provide information on the prey consumed by carnivores and omnivores, including humans (Reinhard et al. 2007): different species of mammals, birds, reptiles, fish, mollusks, and crustaceans have been identified in coprolites (Zangerl and Richardson 1963; Speden 1969; Waldman 1970; Bishop 1977; Parris and Holman 1978; Sohn and Chatterjee 1979; Martin 1981; Stewart and Carpenter 1990; Coy 1995; Meng and Wyss 1997; Meng et al 1998; Chin 2002; Rhode 2003; Harrison 2011; Qvarnström et al. 2019; Bellusci et al. 2021). Coprolites can also provide evidence on predator–prey relationships, digestive efficiency, parasitism, or for identifying fauna not represented in the fossil record, e.g., through the identification of hairs included in coprolites (Chin 2002; Taru and Backwell 2013; Qvarnström et al. 2016).
Another interesting aspect of the study of coprolites is the floristic characterization of the environment through the study of pollen and spores, which are usually preserved within them. Many studies have reported on the presence of fossil pollen in coprolites (Scott 1987; Carrión et al. 2001; Scott et al. 2003; Diedrich 2012; Pineda et al. 2017). Unfortunately, pollen and palynomorphs are scarce and poorly preserved in the sediments of FN3 (Jiménez-Moreno 2003) and only one recent study has reported on them (Ochando et al. 2022). In the case of the coprolites from the Orce sites, efforts to locate pollen have not yielded positive results (Carrión, 2002; Carrión et al. 2009). In addition to pollen, others components not directly related to the diet that can be present in the coprolites are parasites, minerals, or phytoliths (Reinhard and Bryant 1992; Lewis 2011).
Hyena coprolites are easily distinguishable from the excrements of other carnivores because they contain a high proportion of CaO and P2O5, which results from the consumption and digestion of bones (Kruuk 1972, 1976; Larkin et al. 2000). Hyena coprolites consist of aggregates that show a predominance of spheroidal and globular forms, with a whitish color and gritty texture (Horwitz and Goldberg 1989; Saunders and Dawson 1998; Larkin et al. 2000). Diedrich (2012) characterized the morphology of the feces and coprolites attributed to recent (Crocuta crocuta) and Late Pleistocene spotted hyenas (Crocuta spelaea) from Central Europe found in caves and open-air sites. He found that these coprolites are composed of aggregates of various individual elements (pellets), which he classified in seven shape types (see Diedrich 2012).
As noted above, coprolite size is very informative, because it usually correlates with the size of the defecating agent. However, other factors may also play an important role in the final morphology, size, and composition of the coprolite, including season of year, health and age of the individual, or type and amount of food ingested. As result, carnivore species of different sizes produce scats that overlap metrically (Farrell et al. 2000; Harrison 2011; Linseele et al. 2013; Taglioretti et al. 2014; Shillito et al. 2020), although the low dietary variability in the bone-cracking hyenas favors less variability in fecal morphology than in other carnivores with more varied diets (Stuart and Stuart 1998; Chin 2002; Chame 2003; Diedrich 2012). In their analysis of spotted hyena droppings, Larkin et al. (2000) concluded that they are hard and durable, sink rapidly in water, and withstand considerable trampling in the sediment while retaining a consistent shape, thus being recognizable for a long time.
In addition to shape and consistency, color is one of the most characteristic features of hyena feces, which turn white when dried, constituting the classic “album graecum” (Buckland 1824).
Finally, given that hyenas are bone-cracking carnivores, their feces contain small bone fragments and/or teeth that show evidence of digestion, pollen (mainly from the stomach and intestines of their prey), and sometimes also hairs, which can be present as inclusions in the coprolites (González-Sampériz et al. 2003; Backwell et al. 2009). The droppings of extant spotted hyenas use to preserve small bone fragments with evidence of digestion and very small amounts of organic matter (Kruuk 1972; Larkin et al. 2000). In the case of the striped hyena (Hyaena hyaena), which has a more varied diet, the drops generated have more organic contents (Horwitz and Goldberg 1989).
Fossil coprolites are common in many paleontological sites. Their earliest record, attributed to an invertebrate, dates back to the Cambrian (Chen et al. 2007; Hunt et al. 2012), and vertebrate coprolites are frequently reported through the Phanerozoic Era (Northwood 2005; Backwell et al. 2009; Owocki et al. 2012; Vajda et al. 2016). Hunt and Lucas (2019) provided a list of more than 230 sites that preserve Pleistocene sloth and hyena coprolites. Pineda et al. (2017) published the presence of two latrines in the Early Pleistocene deposits of the Iberian Peninsula, at La Mina (Unit II, Barranc de la Boella, Tarragona) and TD6.1 (Sierra de Atapuerca, Burgos). In the case of FN3, Espigares et al. (2013) identified 34 hyena coprolites, attributed to P. brevirostris, which surrounded an elephant (Mammuthus meridionalis) carcass unearthed in the year 2002. Since this finding, the number of coprolites recovered in the site until the year 2015 has increased to 220, 3 in the LAL and 217 in the UAL.
The giant, short-faced hyena Pachycrocuta brevirostris is commonly recorded in the Early Pleistocene sites of the Orce area (Palmqvist et al. 2011). This species is considered by some authors as more closely related (and in all probability also as ethologically closer) to the extant brown hyena (Parahyaena brunnea) and striped hyena, which both behave more as a scavenger than as a hunter and have a more omnivorous diet than the spotted hyena (Kruuk 1972, 1976; Stuart 1982; Turner 1990, 1992). A study of jaw biomechanics of P. brevirostris has shown that this extinct hyena was able of exerting a substantially greater bite force during bone fracturing compared to the living hyenas (Palmqvist et al. 2011). The Venta Micena site in Orce has been interpreted as a number of bone accumulations originated by P. brevirostris around their breeding dens. Taphonomic studies have shown a high degree of bone modification, as many of the remains accumulated by the hyenas are fractured and important anatomical portions are missing (Arribas and Palmqvist 1998; Arribas 1999; Palmqvist and Arribas 2001; Espigares 2010; Palmqvist et al. 2022a; Yravedra et al. 2022b), and there are some bones with evidence of digestion (Espigares 2010; Palmqvist et al. 2022a). These data, together with the enormous size estimated for P. brevirostris (~ 110 kg; Palmqvist et al. 2011), suggest that the coprolites of this species should resemble more closely the ones of the extant C. crocuta than those of the other two living bone-cracking hyenas.
In this paper, we analyze the accumulation of coprolites in FN3 with the goal of characterizing their mode of accumulation and obtaining paleoecological and ethological inferences about the putative defecating agent, the giant hyena P. brevirostris.
Scent marking and latrines in hyenas
Latrines, which have been reported in numerous animal species, are generated by the repetitive use of the same place for defecation. This usually results in the accumulation of between two and several hundred of feces (Buesching and Jordan 2019). Latrines are used not only for defecation and urination, but also for the deposition of glandular secretions useful for scent marking of the territory (Macdonald 1980; Gorman and Mills 1984; Mills and Gorman 1987; Buesching and Jordan 2019). Several hypotheses have been suggested for explaining the use of latrines in different species, including protection of food resources, information center, reproductive advertisement, predator–prey interactions, and landmarks that facilitate orientation (Buesching and Jordan 2019).
Hyena latrines are frequent in open-air settings and use to be associated with environmental features, including dry pools, tracks or roads, on top of a dune, at a tree, at a kill, in the open, or at tall thick vegetation (Gorman and Mills 1984; Mills and Gorman 1987). However, latrines can also be found in other contexts, such as caves (Brain 1981; Berger et al. 2009; Pineda et al. 2017). Differences have been observed in the proportions in which these environmental features are chosen depending on the hyaenid species. For example, a high percentage of latrines of brown hyenas are located near a tree or shrub, while spotted hyenas prefer a track or road, a dry pool, or on top of a dune (Gorman and Mills 1984; Mills and Gorman 1987). However, intraspecific variation resulting from different ecological pressures is also observed, as shown by two populations of C. crocuta in the southern Kalahari Desert and Ngorongoro Crater, respectively (Mills and Gorman 1987).
Latrines play an important role for hyaenids, as all living species (including C. crocuta, H. hyaena, and P. brunnea, but also the aardwolf Proteles cristata) scent mark their territories depositing feces at latrines and secretions produced by their anal pouch onto the grass (Gorman and Mils, 1984).
Bearder and Randall (1978) distinguished between “temporary latrines” and “long-term latrines.” The formers tend to develop in the short-term around sites of interest, such as the surroundings of large ungulate carcasses (as in the case of the elephant carcass of FN3; Espigares et al. 2013), whereas the latter relate to prominent environmental features scattered throughout the territory, which the hyenas visit regularly during long periods of time. In a study on the spatial use and seasonal patterns of C. crocuta communal latrines, Vitale et al. (2020) documented several latrines used during two or three consecutive years and observed an annual cycle of latrine use related to seasonal rainfall: the scats were mainly accumulated during the dry season, while they largely disappeared due to rainfall during the wet season.
Despite the use of latrines by all extant hyaenids, some interspecific variations can be noted. Skinner and Van Aarde (1981) documented differences between P. brunnea and C. crocuta latrines: the ones of P. brunnea are smaller and clearly demarcated, being probably used by single individuals; in contrast, the latrines of C. crocuta spread over an area of about 100 m2 and the feces are deposited communally by all clan members, with the individuals defecating or emitting glandular secretions (paste) on top of other preexisting feces or pastes. The result is a mixture of scents produced by different individuals, which can be detected from at least 2 km downwind (Mills and Gorman 1987).
The site of Fuente Nueva-3
FN3 is an open-air site that lies in the NE sector of the Guadix-Baza Depression (Orce, SE Spain) (Fig. 1a–b), a postorogenic Neogene-Quaternary intramontane basin that extends over an area of ~ 4000 km2 and preserves a thick sedimentary record composed of lacustrine and fluvial deposits (García-Aguilar and Palmqvist 2011; García-Aguilar et al. 2014, 2015; Palmqvist et al. 2022b). The depression was subject to intense hydrothermal activity, which provided mild and productive environments for the terrestrial fauna during the Early Pleistocene (García-Aguilar et al. 2014, 2015), the remains of which were preserved in abundant paleontological localities in the vicinities of the town of Orce (Martínez-Navarro 1991; Arribas and Palmqvist 1998; Palmqvist et al. 2011, 2022a, 2022b; Martínez-Navarro et al. 2018; Granados et al. 2021).
Chronology
The age of the fertile levels of FN3 has been estimated in 1.19 ± 0.21 Ma using a combined approach based on biostratigraphy, magnetostratigraphy, and electron spin resonance (ESR) (Duval et al. 2012; Toro-Moyano et al. 2013). In addition, an age of 1.50 ± 0.31 Ma has been derived for FN3 based on cosmogenic nuclides (Álvarez et al. 2015). Moreover, suids are absent from Europe in the biochronological range comprised between the post Tasso Faunal Unit, which marks the base of the Late Villafranchian (~ 1.8 Ma), and their arrival in Western Europe at layer TE9 from Sima del Elefante (1.22 Ma) (Martínez-Navarro et al. 2015). Therefore, their absence from FN3 suggests an age older than 1.22 Ma for this site. According to all these lines of evidence, the most parsimonious age estimate for FN3 is ~ 1.4 Ma (Palmqvist et al. 2016).
Stratigraphy
Twelve layers, numbered from bottom to top, have been described in the slightly sub-horizontal stratigraphy of the FN3 site (Oms et al. 2011). This stratigraphy consists of a succession of layers constituted by nodular whitish limestones (layers 1, 4, 11, and 12), irregular layers of yellowish marly lutites (layers 2, 3, 6, 7, 8, 9, and 10), and a layer of dark greenish sands in intermediate position (layer 5) (Figs. 1c and 2). These layers can be grouped into three sedimentary cycles (Fig. 1c). Each cycle shows a lower part constituted by levels of colored marly lutites and clays, as well as levels of sands with frequent iron oxides and carbonate nodules, and an upper part characterized by limestones with evidence of microkarstification, together with intraclasts and diagenetic markers. Subaerial pedogenesis is evident in all stratigraphic levels. Therefore, the first cycle is only represented by its upper part, layer 1; the second cycle comprises layers 2 to 4, and the third cycle is formed by layers 5 to 12 (Figs. 1c and 2). These cycles were deposited in a lutitic-carbonate, shallow lacustrine to swampy environment, which was variable in depth and facies type, and sporadically subject to fluvial influence.
a Stratigraphic scheme of the layers of the FN3 site (modified from Oms et al. 2011). b Image showing the morphology of the upper layers (5 to 12). c Image showing the morphology of the lower layers (1 to 5). Red lines indicate faulting.
Most of the layers of the FN3 site have a variable thickness, showing irregular boundaries between them due to soft-sediment deformation structures and erosive processes. Layers 1, 2, and 3 show plastic deformation, specially in the case of layer 1, which has a rounded surface, similar to pillows (Fig. 2). These layers are also affected by two small syn-sedimentary normal faults, with a vertical throw of a few centimeters (Fig. 2). These soft-sediment deformation structures are generally explained as the result of earthquakes (Oms et al. 2011) and have been described in other parts of the Baza Basin (Alfaro et al. 1997, 2010). Layer 4 appears to have been less affected by these deformations and its deposition fits the paleosurface, while its top is irregular and eroded by layer 5. Layer 5 consists of sands with lenticular shapes that can be interpreted as the result of low-energy fluvial flows. These flows may have eroded the unconsolidated top of layer 4 and, then, by decreasing the energy of the flows, the channels would be filled, flattening the surface. Very low energy flows would deposit silts and fine sands in level 5, where most of the fossil remains are found. Later, a succession of marly lutites and clays were deposited, forming layers 6 to 10. The boundary between layers 7 and 8 shows an important irregularity, which can be indicative of an erosive process and, therefore, a subaerial event in the lake margin. Finally, the stratigraphic section of FN3 ends with the deposition of horizontal limestones (layers 11 and 12). It is worth noting that the layers with mammal bones and lithic tools do not show evidence of noticeable transport by traction currents (Oms et al. 2011).
From an archeological point of view, the fertile stratum of FN3 has a thickness of about 1.5–2 m and comprises layers 1 to 7, which have been grouped in two main archeological levels (Fig. 1c): the Lower Archeological Level (LAL, layers 1–3) and the Upper Archeological Level (UAL, layers 4–7) (Turq et al. 1996; Martínez-Navarro et al. 1997; Oms et al. 2010, 2011; Espigares et al. 2013, 2019; Reinoso et al., 2020; Palmqvist et al. 2023). Although bone remains and lithic tools have been recovered from six different layers, most of them come from layers 2, 3, and 5 (Table 1).
Ancient anthropic presence is documented in FN3 by the presence of huge tool assemblages (up to the 2015 excavation season, 1367 Mode 1 lithic artifacts + 375 stones, most of which were probably intentionally brought to the site by the hominins). The tools are mainly composed of flakes and debris made on flint and limestone, lithologies that outcrop in the site surroundings (Barsky et al. 2010, 2015a, 2015b; Toro-Moyano et al. 2011), and are associated to a rich fossil assemblage with remains (n = 9041) of 19 species of large mammals. The taxonomic spectrum includes a proboscidean (M. meridionalis), three perissodactyls (rhino Stephanorhinus hundsheimensis and horses Equus altidens and Equus suessenbornensis), seven artiodactyls (hippo Hippopotamus antiquus, bison Bison sp., water buffalo Hemibos sp. cf. gracilis, goat Hemitragus albus, sheep Ammotragus europaeus, megacerine deer Praemegaceros cf. verticornis, and fallow deer Metacervocerus rhenanus), and eight carnivores (lynx Lynx cf. pardinus, hyena P. brevirostris, painted dog Lycaon lycaonoides, jackal Canis mosbachensis, fox Vulpes alopecoides, bear Ursus etruscus, weasel Martellictis ardea, and badger Meles meles) and some remains of probably two sabertooth cats. In addition, remains of small mammals (15 spp., including a porcupine), herpetofauna (23 taxa), and some avian fossils are also preserved (Martínez-Navarro et al. 1997, 2003, 2010; Espigares et al. 2019; Bartolini-Lucenti and Madurell-Malapeira 2020; Ros-Montoya et al. 2021; Martínez-Monzón et al. 2022; Palmqvist et al. 2023 and references therein).
Taphonomic analyses concluded that the subsistence strategies of the FN3 hominins included the exploitation of carcasses of medium-to-large sized animals for obtaining meat, fat, and bone marrow. These activities are evidenced by the preservation of cut marks resulting from defleshing, evisceration, disarticulation, and skinning, as well as from periosteum removal. Intentional breakage of bones is based on evidence of percussion marks, pits, notches, impact flakes, and negative flake scars produced during bone fracturing to access the medullary cavities (for details, see Espigares et al. 2019; Palmqvist et al. 2023). Some bones (~ 3%; Yravedra et al. 2021) preserve tooth marks, although modification by carnivores is less frequent than that of anthropogenic origin (Espigares et al. 2019; Yravedra et al. 2021; Palmqvist et al. 2023). Size and morphology of most tooth marks suggest that they are the result of a large carnivore, probably the hyena P. brevirostris, although some marks of a small carnivore have been also identified (Espigares et al. 2013, 2019), probably the medium-sized canid C. mosbachensis, which tooth marks have been identified in the bone assemblage from BL (Courtenay et al. 2023). Moreover, there are some marks made by porcupines (Espigares et al. 2019). In addition to the tooth marks, carnivore activity is manifested by the presence of abundant coprolites tentatively attributed to P. brevirostris, which are especially abundant in the UAL (Espigares et al. 2013, 2019; Yravedra et al. 2021).
The rich lithic assemblage from FN3 and the inferences on resource availability and competition intensity among the members of the carnivore guild provide interesting data on the subsistence strategies of the earliest hominin population of Western Europe (Rodríguez-Gómez et al. 2016; Espigares et al. 2019; Palmqvist et al. 2022b).
Materials and methods
The coprolites analyzed in this study mainly belong to layer 5 of the UAL (n = 216) although one coprolite was found in layer 7 and three coprolites are from LAL (Table 1). In addition, coprolites from the site of BL (n = 31) have also been included in the study (Fig. 3). The coprolites were unearthed during systematic excavations and fieldwork at FN3 between the years 2001 and 2015. The preservational state of coprolites from open-air sites is frequently worse than those recovered from caves. In the case of FN3, some specimens were recovered in good state of preservation at the time of excavation, but most of them presented a variable state of disintegration. This made it necessary to use an acrylic resin, Paraloid B 72, dissolved in acetone during the extraction of the coprolites for preserving them. Other coprolites were severely disaggregated at the time of excavation and it was only possible to recover the fragmented material in plastic bags. Moreover, feces of extant individuals of C. crocuta provided by the Research Department of OASYS Parque Temático del Desierto de Tabernas (OASYS-PTDT, Almería, Spain) have been analyzed using different techniques to compare them with the results obtained for the FN3 and BL coprolites.
In this study, the dimensions, morphology, and composition of the coprolites were analyzed. Due to the preservational state of coprolites, their length, width, and thickness was measured in 101 of them; in other 25 coprolites, only their length and width could be measured. Morphological analysis was performed following Diedrich (2012), who differentiated seven pellet morphotypes (conical, disk, oval, long-oval, round, irregular, and drop) (Fig. 4). Following Chin (2002), coprolite color was determined with the Munsell Soil-Color Charts (Munsell Color 1975).
The spatial distribution (x, y, z) of bones, coprolites, lithic artifacts, and stones was recorded using a Cartesian coordinate system. Scatter diagrams of the remains in both the vertical section (X–Z) and plant (X–Y) of the excavated surface of FN3 were created using Origin2021b software. In order to analyze the orientation of the elements, these data were represented in rose diagrams using the Stereonet software.
For components determination and coprolite mineralogical and chemical composition, ten samples of coprolites from FN3 and one from BL were selected. They were studied by X-ray diffraction (XRD), X-ray fluorescence (XRF), X-ray microscopic computed tomography (X-µCT), and scanning electron microscopy (SEM).
XRD patterns were recorded using a Philips X’Pert PRO MPD at the Servicio Centralizado de Apoyo a la Investigación (SCAI) of the University of Málaga (UMA), with CuKα1 radiation and Ge monochromator, operated at 45 mA and 35 kV, with 0.01° 2θ step size and 2 s counting time. Randomly oriented samples were used to determine the semi-quantitative principal mineral composition, using the X’Pert High Score Software, based on Rietveld analysis, and the PDF2-2003 database (International Center for Diffraction Data).
Whole-rock analyses of the major elements of some samples were carried out using XRF in an ARL PERFORM’X (Thermo Fisher) spectrometer (SCAI, UMA). Glass beads with lithium tetraborate were employed to minimize the preferential orientation of phyllosilicates. The detection limit for major elements was 0.4 wt.%. Ignition loss (LOI) was determined from 0.65 g of powdered sample, first dried at 105 °C and then heated at 950 °C for 2 h.
Sample 7 was analyzed with SKYSCAN 2214 (Bruker) X-ray computed microtomography (X-µCT; SCAI, UMA). Images were obtained with an X-ray tube using a W filament (operated at 130 kV and 66 µA) and employing a 1-mm Cu foil. The geometrical settings included a source to object distance of 37.696 mm and an object to detector distance of 313.309 mm. A set of 1276 images was acquired over a 360° rotation at 0.3° rotation steps, with an exposure time of 2.8 s and a resolution of 2276 × 2880. Voxel size at the end of the image cleaning and enhancement process was 0.0748 isometric voxels (same value on the x-, y-, and z-axes).
The resulting image stacks were enhanced, removing background noise and potential artifacts resulting from data acquisition. For doing this, different algorithms and digital filters were implemented in specific virtual reconstruction and image processing software such as 3D Slicer v 4.9.0 (Kikinis et al. 2014) and ImageJ (Rueden et al. 2017). Subsequently, the enhanced images were segmented by thresholding the histogram of gray values (Pertusa 2010). This process is very sensitive and depends on material properties such as bone density and mineralization (Pérez-Ramos and Figueirido 2020).
Some selected coprolite samples were studied with a scanning electron microscope (SEM) to obtain textural feature images and punctual chemical composition of coprolites. These observations were carried out using a JEOL JSM-6490 LV SEM equipped with an X-ray energy-dispersive (EDX) system (OXFORD INCA Energy 350) at an accelerating voltage of 20 kV, 70 nA beam current, and counting time of 100 s per step, using internal available standards in microanalyses system. Samples were gold sputter coated (220 nm thick) to obtain secondary electron (SE) images at 100 × , 500 × , 1500 × , 2000 × , 3000 × , 3500 × , 5000 × , and 10,000 × magnification.
Coprolite surfaces were analyzed with a digital microscope (DINO-LITE Model AM4115TL) for detecting and photographing small fragments of digested bones included in them.
The dimensions, morphology, and composition of the coprolites were compared with modern spotted hyena scats from Colchester Zoo (Larkin et al. 2000), OASYS-PTDT (original data), as well as with other modern and fossil scats of spotted hyenas (C. crocuta) and brown hyenas (P. brunnea) (Fernández-Jalvo et al. 2010) and hyena coprolites from European and African sites with a similar chronology, including BL (Larkin et al. 2000; Keiler 2001; Harrison 2011; Pesquero et al. 2011; Bennet et al. 2016; Sanz et al. 2016; Pineda et al. 2017; Espigares et al. 2019).
Coprolites were not intentionally disaggregated during the taphonomic analysis. The high disaggregation state of most of them allowed the observation of internal features, the identification of inclusions, and the sampling for mineralogical and chemical analysis.
Results
Morphological analysis
Almost all coprolites from FN3 are composed of isolated pellets (Fig. 3a–b), but at least six are formed by several associated pellets (Fig. 3c–e), one of them linked to the carcass of M. meridionalis (Figs. 1d–e, 3). Three coprolites formed by several pellets (two composed of four irregular or drop-shaped pellets and one composed of two pellets with a rounded and irregular morphology) correspond to the posterior region of the coprolite. Two coprolites, composed of two pellets with long-oval and rounded morphologies, correspond to the central part of a coprolite. One coprolite, composed of two pellets with conical and disk-shaped morphologies, is from the anterior part of a coprolite (see Diedrich 2012: Fig. 5). Isolated pellets present a predominance of rounded, oval, and disk-shaped morphologies (Figs. 3 and 4). Coprolites are scarce at BL (N = 31), but their morphology is consistent with the characteristics described in FN3 (Fig. 3f).
Comparison of the length and width of the FN3 coprolites with the scats of modern Crocuta crocuta and the hyena coprolites from Europe and Africa. 1: La Roma 2 (Pesquero et al. 2011); 2: FN3 (original data); 3: La Mina II (Pineda et al. 2017); 4: Untermassfeld (Keiler 2001); 5: West Runton (Larkin et al. 2000); 6: Cova del Coll Verdaguer (Sanz et al. 2016); 7 OASYS-PTDT (original data); 8: Colchester Zoo (with pellets) (Larkin et al. 2000); 9: Colchester Zoo (without pellets) (Larkin et al. 2000); 10: TD6 (Pineda et al. 2017); 11: Ubeidiya (Gaudzinski 2004); 12: Equus Cave (Fernández-Jalvo et al. 2010)
Dimensions
The pellets from FN3 are very variable in size. These differences can be attributed to the dissimilarities between the seven pellet-shape types defined by Diedrich (2012): pellets with conical, disk, oval, and long-oval shapes are larger than those with rounded, irregular, and drop shapes. In addition, the presence of some fussed pellets can mask their size (see Fig. 3). Size values from FN3 pellets are provided in Table 2.
The size of the FN3 coprolites is similar to those from the late Early Pleistocene (Jaramillo) site of Untermassfeld, where P. brevirostris has been conclusively identified (Keiler 2001), and also to the coprolites from la Mina II and West Runton, sites in which it has been suggested that this hyena was probably present (Larkin et al. 2000; Pineda et al. 2017). These coprolites are clearly larger than those produced by spotted hyenas (C. crocuta) and especially by brown hyenas (P. brunnea) (Fig. 5). Given that there is a rough correlation between feces size and the body size of the defecating agent, the larger dimensions of the FN3 coprolites can be attributed to the huge body mass of P. brevirostris, which was a large and extremely powerful hyaena with a body mass in excess of 100 kg (Turner and Antón, 1996) compared to the living C. crocuta and P. brunnea, which have a mass of 55 kg and 42 kg, respectively (Palmqvist et al. 2011).
Color
The color of hyena feces is a distinctive feature that facilitates their recognition. As noted before, the lower part of the hyena’s intestine contains the album graecum, a green paste which mixture with the mineral component of bone (calcium hydroxyapatite) results in the typical whitish color of the coprolites when they are in contact with the atmosphere (Buckland 1824; Matthews 1939).
According to the Munsell Color Chart for Soils, the FN3 coprolites show mostly white and very pale brown tonalities (Fig. 6). Sometimes the coprolites may be of darker colors. This evidences an abundance of organic matter inclusions, which are produced when the hyenas eat large quantities of flesh and grease (Matthews 1939), for example, when they find the carcass of a large-bodied ungulate (Bearder 1977). The 34 coprolites that surround a carcass of M. meridionalis at FN3 evidence this, showing darker tones than others found in the same stratigraphic level and more distantly placed from the elephant carcass, which tend to be lighter (Espigares et al. 2013).
Inclusions
Coprolite contents can have different origins (animal, plant, mineral, or fungal) and can range broadly in size (Shillito et al. 2020). Macroscopic and digital magnification observation allowed to identify bone fragments of large mammals inside many FN3 coprolites (Fig. 7). These fragments show diagnostic features, such as polishing, rounding, or thinning of the cortical surfaces, which evidence digestion. Bone fragments range between 1.1 and 19.2 mm. As previously mentioned, no fossil palynomorphs have been found in the coprolites from FN3.
Analytics data
X-ray data and chemical bulk-rock composition
According to the XRD patterns, the studied coprolites mainly consist of calcium phosphate (> 80%; mostly fluor-apatite and hydroxyapatite, and minor contents of carbonate-apatite) and quartz (~ 10%), accompanied occasionally by calcite [Fig. 8 (1–11) and Table 3]. The very high quartz contents (36%) and the existence of muscovite, feldspars, and dolomite in sample 8 (Fig. 8) suggest the contamination of this coprolite by the surrounding sediment. The mineralogical composition of the studied coprolites is the same than in the feces of living hyenas from OASYS Parque Temático del Desierto de Tabernas (XRD diagram 12 of Fig. 8). Nevertheless, an increase of the high of the baseline and a bad signal/noise ratio are observed in the XRD diagrams from the hyenas’ feces, due to the presence of amorphous material as organic matter.
X-ray diffraction diagrams of the studied coprolites. The numbers are the laboratory references of each sample. Abbreviations for minerals following Warr (2021). 1–10: Samples from FN3; 11: sample from BL; 12: sample of modern Crocuta crocuta provided by the Research Department of Oasys Parque Temático del Desierto de Tabernas (Almería, Spain). Ap, apatite; Qz, quartz; Cal, calcite; Ms, muscovite; Kfs, K-feldspar; Dol, dolomite; Pl, plagioclase
Chemical analyses of major elements in some samples are given in Table 4. The most obvious difference is observed in the SiO2 contents, which depend on the quartz content of the sample. Sample 8 shows the highest quartz contents (Table 4; Fig. 8, XRD diagram 8). Furthermore, Al2O3 and K2O contents relate with the presence of K-feldspars and muscovite in sample 8 (Tables 3 and 4), which is due to the contamination of this sample by the surrounding sediment. Likewise, sample 6 shows high Al2O3 and K2O contents (Table 4), which are also suggestive of the presence of sediment remains, even though the index minerals do not appear in the XRD diagram (they probably appear as trace contents). Also, there is a positive correlation between the amounts of CaO and P2O5 and the fluor- and hydroxyapatite contents of the samples (Tables 3 and 4), with the CaO to P2O5 ratio being higher in those samples containing carbonate minerals (samples 6 and 8, Table 4). A high abundance of P2O5 in the coprolites reflects the carnivorous diet of the defecating animal (Chin et al. 2003; Hollocher et al. 2010). In the FN3 coprolites, their high P2O5 contents were probably derived from dissolved bone, microorganisms, and animal soft tissues.
X-µCT study
Figure 9 shows that many bone particles of small (< 10 mm thick, cold colors) and large size (22 to 24 mm thick, warm colors) can be observed in the three coprolite fragments analyzed in the study by X-µCT. The abundance of bone particles of ≤ 24 mm indicates the efficiency in bone chewing and bone cracking of the agent that produced the coprolites, and correlates with the composition of hyena coprolites observed in previous studies (Kruuk 1972; Holekamp et al. 1997; Van Valkenburgh and Binder 2021).
Bone densitometric analysis and bone thickness distribution of a coprolite from FN3. a Bone densitometry: the three regions marked by squares show distinctive patterns. b Analysis of the distribution of bone thickness in each region depicted in a. Cold colors (thickness ≤ 1 mm); warm colors (thickness ≥ 1 mm). c Plots of the distribution frequencies of bone thickness by region
SEM study
The secondary electrons (SE) images of coprolites from FN3 and BL show the presence of spherical or globular structures (Fig. 10a–d, black arrows) of 1 to 3 µm, which sometimes reach up 5 or 6 µm (Fig. 10a). Moreover, a porous fabric or spherical cavities is also frequent (Fig. 10a–d, red arrows). These spherical or globular structures, which have been named in many studies as microspherulites (e.g., Canti 1999; Hollocher et al. 2010; Owocki et al. 2012; Pesquero et al. 2014; Pineda et al. 2017), are commonly found in hyena feces and coprolites. The BL coprolite shows abundant large cavities (Fig. 10d) and small elongated and circular voids (Fig. 10d, white arrows). The circular voids are microspherulites and the elongated ones resemble rod-shaped bacteria (Owocki et al. 2012; Pesquero et al. 2014; Pineda et al. 2017).
Secondary electrons (SE) images of coprolites from FN3 (a–c) and BL (d). a–b Typical aspect of coprolites from the archeological site of Fuente Nueva-3 (FN3), which are composed of microspherulites (black arrows) and spherical cavities (red arrows). c Details of microspherulitic structures and spherical cavities in the FN3 coprolites. d Typical aspect of coprolites from the site of Barranco León (BL), which are composed of microspherulites (black arrows), small voids that resemble bacteria (white arrows), and large cavities
Some EDX spectra show high calcium and phosphorus peaks, which are indicative of the presence of hydroxyapatite, the major mineral phase of bone (Fig. 11a). Some of them also show a fluorine peak, which evidences the presence of fluorine apatite (Fig. 11b), a mineral phase that replaces in part hydroxyapatite in many fossils from the Orce sites (Arribas and Palmqvist 1998). Nevertheless, most spectra display a variable percentage of carbon (Fig. 11c). The chemical analyses of the coprolites, obtained by SEM study, agrees with the presence of mineral contents of the higher apatite-group obtained from XRD data (Fig. 8, 1 to 11; Table 3) and with the high CaO and P2O5 percentages detected in the XRF analyses (Table 4).
The composition of the FN3 coprolites was compared with extant and fossil feces attributed to different hyena species (Table 5) among which the values of a coprolite from BL was also included. The coprolites from FN3 and BL show similar Ca and P contents, with the exception of sample number 8, which is contaminated by sediment. These values are very similar to those of La Roma II site, where Lycyaena chaeretis is documented (Pesquero et al. 2011), and to one of the two coprolites published by Larkin et al. (2000) from Boxgrove, which are attributed to C. crocuta. The values in this table show Ca and P contents that are overall lower in the coprolites and feces of C. crocuta and especially in those of H. hyaena, which is by far the species with the lowest Ca contents compared to the coprolites attributed to P. brevirostris.
Spatial distribution
Figures 12 and 13 show separate longitudinal (X–Z) and plant (X–Y) sections, respectively, of the spatial distribution of skeletal remains, lithic tools, stones (i.e., limestone blocks that were presumably brought to the site by the hominins, half of which show evidence of impact; Espigares et al. 2013; Barsky et al. 2015b, 2022), hyena coprolites, and remains of M. meridionalis. As mentioned above, two main archeological levels can be distinguished in FN3, the Lower Archeological Level (LAL) and the Upper Archeological Level (UAL). In both levels, bones and lithic tools are frequently recorded. Stones predominate in the LAL. In contrast, coprolites and remains of M. meridionalis are mainly documented in the UAL. As a result, there is a band without skeletal remains in the X–Z projection (Fig. 12), which allows a visual differentiation between the LAL and UAL. It must be noted that the partial carcass of elephant that appears in Fig. 1d was unearthed within a block of sediment; for this reason, the Z-coordinates of these skeletal remains were not recorded and they are not included in Fig. 12. The orientation of the bones has also been analyzed. Three rose diagrams have been produced, one for all bones for which orientation data are available, including the two archeological levels (Fig. 14a), and two others for the UAL and LAL considered independently (Fig. 14b, c). The rose diagram for all bones and that of the UAL show no preferred orientation of the skeletal remains, which allows to discard their allochthonous deposition and resedimentation. In contrast, the directions N-S, E-W, and to a lesser extent NE-SW and NW–SE are more represented in the LAL. However, it must be noted that these orientations are somewhat overrepresented. The reason is that in the excavation seasons prior to the year 2005, from which most of the records of layers 2 and 3 come from, direction measurements were not taken with a compass. In any case, no preferential directions of accumulation related to bone transport by currents are evident, an interpretation also reinforced by the absence of polishing and rounding on the bone surfaces (Espigares 2010; Oms et al. 2011).
Spatial distribution in a longitudinal section (X–Z) of bones, lithic tools, stones, coprolites, and remains of elephant M. meridionalis in FN3. The Z-axis is represented vertically and the X-axis horizontally. The histogram for bone frequencies marks the approximate boundary between the UAL and LAL. Points represent the central position of bones, and their dimensions have not been considering in this representation
Discussion
This paper reports on the finding of a huge accumulation of coprolites in the UAL of FN3. These coprolites have been previously attributed to the giant hyena P. brevirostris, the only species of hyena documented in the Early Pleistocene sites of Orce (Espigares et al. 2013, 2019), of which several isolated teeth have also been recovered at the FN3 site.
Our results show that the morphology, size, color, mineralogical, and chemical composition (presence of apatite-group minerals, main inorganic constituent of bones, and high CaO and P2O5 contents) of the coprolites, as well as the inclusions of bone fragments and the presence of microspherulites in them, match with those expected in the coprolites produced by a large hyena, which confirms their previous ascription to P. brevirostris.
Why are the coprolites recorded mainly in the UAL? The more plausible interpretation for this accumulation is that it corresponds to a P. brevirostris latrine.
Other latrines have been published at the latest Early Pleistocene sites of La Mina II (Barranc de La Boella) and TD6 (Atapuerca) (Pineda et al. 2017), both in Spain. In addition, abundant coprolites attributed to P. brevirostris have also been recovered from the Epivillafranchian site of Untermasfeld (Germany), probably representing the remains of latrines with a territorial marking function (Keiler 2001). Similarly, Coil (2016) published in the early Late Villafranchian site of Dmanisi (Georgia, Caucasus) (Block 2) a concentration of 93 coprolites of P. brevirostris in 4 m2, surrounded by a high number of bones with tooth marks, which probably represents also a latrine. Other Late Villafranchian and Epivillafranchian sites with coprolites attributed to P. brevirostris are West Runton (Parfitt and Larkin 2010), Cueva Victoria (Carrión et al. 2007), Trlica Cave (Argant and Dimitrejevic 2007), Vallparadis (Madurell-Malapeira et al. 2017), Ceyssaguet (Argant and Bonifay 2011), Taurida Cave (Lavrov et al. 2021), Poggio Rosso (Mazza et al. 2004), Pirro Nord (Arzarello et al. 2007), Venta Micena (Palmqvist et al. 2022a), and Zhoukoudian (Boaz et al. 2000).
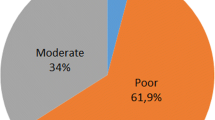
The latrine identified at FN3 corresponds to an open-air setting. This type of setting is frequently used by extant hyenas (Kruuk 1972; Gorman and Mills 1984; Mills and Gorman 1987; Vinuesa 2018) and is also documented in the fossil record (Pesquero et al. 2011; Pineda et al. 2017). Figure 15, based on data from Mills and Gorman (1987), shows the percentage of different settings in which modern open-air latrines of C. crocuta are placed. As previously mentioned, environmental characteristics are key to choosing the latrine position and ecological pressures can determine differences between populations of the same species (Gorman and Mills 1984; Mills and Gorman 1987).
Frequencies of settings in which modern open-air latrines of Crocuta crocuta appear, based on data from Mills and Gorman (1987) (total number of latrines = 116)
Among the different locations shown in Fig. 15, latrines situated at a kill site are relatively frequent (7.2%) and can be classified in the category of “temporary latrines” described by Bearder and Randall (1978), which develop near short-term sites of interest, such as carcasses. In contrast, “long-term latrines” are associated to environmental landmarks and are visited repeatedly over a long period of time, including drinking places (Macdonald 1980).
The discussion that follows focuses on the type of latrine represented by the FN3 coprolites, as deduced from the spatial distribution of fossils.
Bones are the elements most frequently documented at the FN3 site and their abundance is similar in both archeological levels (UAL and LAL), which indicates an intense occupation of the region by many species, especially ungulates. As in the case of bones, lithic tools are frequently preserved in both levels and appear associated with bones bearing cut marks and evidence of marrow processing (Espigares et al. 2013, 2019; Yravedra et al. 2021; Palmqvist et al. 2023). This suggests that the hominins were present in the area during the formation of both archeological levels, especially during the deposition of layers 2–3 and 5. However, there is a marked difference if we look at the spatial distribution of stones and coprolites (Fig. 12c–d). Most stones appear in the LAL and the scarce ones found in the UAL are mostly associated with the partial carcass of M. meridionalis (Espigares et al. 2013). Some of these stones have been interpreted as manuports and were presumably brought to the site by the hominins (Barsky et al. 2015a, 2015b; 2022). In contrast, the coprolites are almost entirely located in the UAL (Figs. 12d and 13b). This marks two distinctive scenarios from the point of view of territory use by hyenas: during the period of time in which the LAL was formed the region was not regularly frequented by hyenas, while the high frequency of coprolites in the UAL evidences an area frequently occupied by hyenas. As a result, during the UAL there was a spatial (and probably also temporal) coexistence of hyenas (more nocturnal) and hominins (predominantly diurnal), whose presence is recorded by abundant lithic tools and the finding of anthropogenic marks on the skeletal remains of large mammals (Espigares et al. 2019; Yravedra et al. 2021).
Another significant aspect of the spatial distribution of fossils is the abundance of proboscidean remains, which are much more frequently represented in the UAL than in the LAL, including a partial carcass of elephant which belonged to an old female (as deduced by the morphology of the jaw symphysis and the advanced degree of wearing of the third lower molar, respectively) and two very large tusks, probably of a male individual, measuring > 4 m in length and with a diameter at its widest part of > 30 cm. The proboscidean remains found in FN3 until the year 2015 correspond to, at least, ten individuals of all ages, from newborns to old adults (Ros-Montoya 2010; Espigares et al. 2019). Most of these remains appear in the UAL and seem to have been accumulated in a small space, within a surface area of 40 m2 and a depth of only 1 m.
The data presented above can help to establish whether the coprolites recovered at the UAL of FN3 represent a temporary or a stable latrine. Coprolites, as well as elephant bones and teeth, are concentrated in the UAL. In addition, one event with 34 coprolites surrounding the elephant carcass has been identified (Espigares et al. 2013). However, despite the large number of skeletal remains of proboscideans at the UAL of FN3 and with the only exception of this carcass, no accumulations of coprolites associated with elephant remains have been located at the site. This could be due to the fact that teeth are the most frequently recovered anatomical elements of M. meridionalis. According to Haynes (1988), an accumulation of more than one elephant carcass is invariably not the result of serial predation but of die-offs at water sources during seasonal droughts in the course of several decades. Elephants were therefore a relatively frequent resource in the UAL, which could be exploited by both the hominins and hyenas (Espigares et al. 2013).
This situation allows us to speculate on a more permanent use of this area by the hyenas as a latrine, linked to the relatively frequent presence of elephant carcasses. It is noteworthy the association in the fossil record of hyena coprolites and proboscidean remains, as in the case of Untermassfeld (Keiler 2001), West Runton (Larkin et al. 2000), or La Mina II (Pineda et al. 2017). Cozzi et al. (2015) showed that the presence of an elephant carcass influences the ranging and feeding behavior of C. crocuta for at least 10–14 days, although carcass consumption may take up to 50 days after the proboscidean death, constituting a point of attraction and contact for multiple species of scavengers and for individuals of neighboring hyena clans. However, the coprolites of FN3 are not exclusively related to elephant remains, being associated with other skeletal remains of large mammals very variable in size, in a region with a permanent source of water (Espigares et al. 2019; Palmqvist et al. 2023).
Given the differences between the two archeological levels of FN3, the relative abundance of herbivore species in them can be used to infer further behavioral aspects of P. brevirostris. If these relative abundances are calculated from the NMIs (data from Espigares et al. 2019), the species abundances are more balanced in the UAL than in the LAL, where there is a high frequency of Equus (Fig. 16). In addition, there is another interesting difference between both levels: the UAL shows a clear predominance of megaherbivores, which represent 36.1% of the ungulate individuals identified compared to 11.4% recorded in the LAL (Fig. 16). Although these differences are probably a consequence of the agents involved in the modification and accumulation of the skeletal remains in both archeological levels, they can also emerge from differences in the sedimentary context, which need to be analyzed in greater depth.
Relative abundance of ungulate species recorded in the UAL and LAL of FN3 according to the minimum number of individuals (MNI) (data from Espigares et al. 2019)
More specifically, there are lithological differences between the LAL and UAL of FN3 (Oms et al. 2011). The fertile levels of the LAL (layers 2–3; Figs. 1 and 2) are composed of green clays (layer 2) and brown whitish clays with nodules (layer 3). In contrast, the fertile levels of the UAL (mainly layer 5, and to a lesser extent layers 6 and 7; Figs. 1 and 2) are composed of greenish sands and marly mudstones (layer 5), dark brown clays (layer 6), and greenish-brown dark marly mudstones (layer 7). Given their composition, fine sands and clays, the layer 5 of the UAL could have behaved as quicksand that became unstable when it was forced to move by the pressure exerted by large-sized animals like proboscideans: experimental data on quicksand from salt-lake environments composed of fine sand, clay, and salt water, similar to those found in Orce (García-Aguilar et al. 2014, 2015; Palmqvist et al. 2022b), show that the quicksand acts as a trap for the animals caught, which find it very hard to escape (Khaldoun et al. 2005). This quicksand shows extreme sensitivity to small variations in stress due to liquefaction of the materials. The higher the stress, the more liquid and viscous the quicksand becomes, so movement by a trapped body causes it to sink (Khaldoun et al. 2005). However, the objects with a density of 1 g ml−1 (i.e., the average density of mammal tissues) are not completely sucked beneath the surface but sink halfway into the quicksand (Khaldoun et al. 2005). This scenario would explain the abundance of skeletal remains of megafauna in the UAL, particularly elephants, which halfway sunk carcasses would regularly attract the scavenging hyenas (Espigares et al. 2013).
The increased exposure of elephants to the risk of entrapment in a quicksand results from the high weight per unit area supported by their limbs. Specifically, data from Pasenko (2017) on body mass and footprint diameters of the manus and pes of a number of African and Indian elephants allows estimating the weight supported per unit area by their feet: 0.93 kg/cm2 in both an adult male and an adult female of 4899 kg and 3962 kg, respectively; 0.77 kg/cm2 in a young adult female of 2386 kg; 0.57 kg/cm2 in a 5 years old male of 1515 kg; and 0.35 kg/cm2 in a 1.5 years old male elephant of 726 kg. In the case of the black rhino (~ 1500 kg), the diameters of the fore- and hindfeet measured in the footprints from Laetoli (data from Leakey and Hay 1979) provide an estimate of 0.80 kg/cm2. In contrast, the weight per unit area supported by the feet of the Laetoli hyena (probably Hyaena bellax, a synonym of P. brevirostris; Palmqvist et al. 2011) is lower, 0.32 kg/cm2, and is even more so in the case of Australopithecus afarensis, 0.17 kg/cm2. These data suggest that while adult elephants could occasionally become trapped in the quicksand of layer 5 of FN3, the hyenas and hominins would have been much less exposed to this risk, thus being able to scavenge on these carcasses. In the case of the very young elephants, which remains are also represented in the UAL of FN3, their lower ratios of weight to foot area may tentatively suggest that these animals would not entrap themselves, but would stay in the vicinity of their mother’s trapped carcass, where they would also eventually die.
Conclusions
The Upper Archeological Level (UAL) of the Early Pleistocene site of FN3 preserves 217 coprolites. The morphology, color, mineralogical, and chemical composition of these coprolites, as well as the presence of inclusions in them, agree with those produced by hyenas. Their average size is larger than in the case of those coprolites produced by the extant C. crocuta. The features of the coprolites and the faunal remains from the site allow to ascribe these coprolites to P. brevirostris. As a result, the UAL of FN3 represents a fossil latrine probably related to the frequent presence of proboscidean carcasses at the site, with at least one event in which the coprolites are clearly associated with a carcass of megafauna, as there is a partial elephant skeleton surrounded by 34 coprolites. However, the coprolites are also associated with other ungulate remains, mainly equids, bovids, and large-sized cervids.
The data presented here allow to stablish differences between the two occupation levels of FN3. In the Lower Archeological Level (LAL), the presence of hyenas is scarce. In contrast, they are well represented in the UAL by some dental remains, tooth marks, and coprolites. The lithology of layer 5 of the UAL, fine sands and clays deposited in a salt-lake environment, suggests that this level could have acted as a quicksand in which large-sized animals, particularly elephants, were trapped and halfway sunk, thus attracting the scavengers.
Data availability
Data are available on request to the corresponding author.
Code availability
Not applicable.
Change history
30 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12520-023-01797-2
References
Alfaro P, Moretti M, Soria JM (1997) Soft-sediment deformation structures induced by earthquakes (seismites) in the Pliocene lacustrine deposits (Guadix-Baza basin, Central Betic Cordillera). Eclogae Geologica Helvetica 90:531–540
Alfaro P, Gibert L, Moretti M, García-Tortosa FJ, Sanz de Galdeano C, Galindo-Zaldívar J, López-Garrido AC (2010) The significance of giant seismites in the Plio-Pleistocene Baza palaeo-lake (S Spain). Terra Nova 22:172–179. https://doi.org/10.1111/j.1365-3121.2010.00930.x
Álvarez C, Parés JM, Granger D, Duval M, Sala R, Toro I (2015) New magnetostratigraphic and numerical age of the Fuente Nueva-3 site (Guadix-Baza basin, Spain). Quat Int 389:224–234. https://doi.org/10.1016/j.quaint.2015.04.044
Argant J, Bonifay MF (2011) Les coprolithes de hyène (Pachycrocuta brevirostris) de la couche 2 du site villafranchien de Ceyssaguer (Lavoûte-sur-Loire, Haute-Loire, France): analyse pollinique et indications paléonvironnementales. Quaternayre 22(1):3–11. https://doi.org/10.4000/quaternaire.5808
Argant J, Dimitrijevic V (2007) Pollen analyses of Pleistocene hyena coprolites from Montenegro and Serbia. Ann Geol Peninsule Balk 68:73–80. https://doi.org/10.2298/GABP0701073A
Arribas A, Palmqvist P (1998) Taphonomy and paleoecology of an assemblage of large mammals: hyaenid activity in the Lower Pleistocene site at Venta Micena (Orce, Guadix-Baza Basin, Granada, Spain). Geobios 31(suppl):3–47. https://doi.org/10.1016/S0016-6995(98)80056-9
Arribas A (1999). Análisis y Modelización de la Tafonomía del Yacimiento de Venta Micena (Orce, Granada) y su estudio comparativo con otras localidades españolas del Plio-Pleistoceno continental. PhD Dissertation, Universidad Complutense de Madrid, 344 pp
Arzarello M, Marcolini F, Pavia G, Pavia M, Petronio C, Petrucci M, Rook L, Sardella R (2007) Evidence of earliest human occurrence in Europe: the site of Pirro Nord (Southern Italy). Naturwissenschaften 94:107–112. https://doi.org/10.1007/s00114-006-0173-3
Backwell L, Pickering R, Brothwell D, Berger L, Witcomb M, Martill D, Penkman K, Wilson A (2009) Probable human hair found in a fossil hyaena coprolite from Gladysvale cave, South Africa. J Archaeol Sci 36:1269–1276. https://doi.org/10.1016/j.jas.2009.01.023
Barsky D, Celiberti V, Cauche D, Grégire S, Lebègue F, Lumley H, Toro I (2010) Raw material discernment and technological aspects of the Barranco León and Fuente Nueva 3 stone assemblages (Orce southern Spain). Quat Int 223–224:201–219. https://doi.org/10.1016/j.quaint.2009.12.004
Barsky D, Sala R, Menéndez L, Toro-Moyano I (2015a) Use and re-use: re-knapped flakes from the Mode 1 site of Fuente Nueva 3 (Orce, Andalucía, Spain). Quat Int 361:21–33. https://doi.org/10.1016/j.quaint.2014.01.048
Barsky D, Vergès JM, Sala R, Menéndez L, Toro I (2015b) Limestone percussion tools from the late Early Pleistocene sites of Barranco León and Fuente Nueva 3 (Orce, Spain). Philos Trans R Soc B 370:20140352. https://doi.org/10.1098/rstb.2014.0352
Barsky D, Titton S, Sala-Ramos R, Bargalló A, Grégoire S, Saos T, Serrano-Ramos A, Oms O, Solano García JA, Toro-Moyano I, Jiménez-Arenas JM (2022) The significance of subtlety: contrasting lithic raw materials procurement and use patterns at the Oldowan sites of Barranco León and Fuente Nueva 3 (Orce, Andalusia, Spain). Front Earth Sci 10, https://doi.org/10.3389/feart.2022.893776
Bartolini-Lucenti S, Madurell-Malapeira J (2020) Unraveling the fossil record of foxes: an updated review on the Plio-Pleistocene Vulpes spp. from Europe. Quat. Sci. Rev. 236:106296. https://doi.org/10.1016/j.quascirev.2020.106296
Bearder SK (1977) Feeding habits of spotted hyaenas in a woodland hábitat. E Afr Wild J 15:263–280. https://doi.org/10.1111/j.1365-2028.1977.tb00408.x
Bearder SK, Randall RM (1978) The use of fecal marking sites by spotted hienas and civets. Carnivore 1:32–48
Bellusci A, Fernández FJ, Beltrame MO (2021) Carnivore coprolites from “Gruta del Indio” site as source of paleoparasitological and paleoecological evidences (late Pleistocene-Holocene, Mendoza, Argentina). Archaeolog Anthopol Sci 13:29. https://doi.org/10.1007/s12520-021-01272-w
Bennet EA, Gorgé O, Grange T, Fernández-Jalvo J., Geigl E-M (2016) Coprolites, paleogenomics and bone content analysis. In Fernández-Jalvo et al. (eds) Azokh Cave and the Transcaucasian Corridor, Vertebrate paleobiology and paleoanthropology, Springer, pp. 271–286. https://doi.org/10.1007/978-3-319-24924-7_12
Berger LR, Pickering R, Kuhn B, Backwell L, Hancox PJ, Kramers JD, Boshoff P (2009) A Mid-Pleistocene in situ fossil brown hyena (Parahyena brunnea) latrine from Gladysvale Cave, South Africa. Palaeogeogr, Palaeoclimatol, Palaeoecol 279:131–136. https://doi.org/10.1016/j.palaeo.2009.05.004
Bishop GA (1977) Pierre feces: a scatological study of the Dakoticancer assemblage, Pierre Shale (Upper Cretaceous) of South Dakota. J Sediment Petrol 47(1):129–136. https://doi.org/10.1306/212F7112-2B24-11D7-8648000102C1865D
Boaz NT, Ciochon RL, Xu Q, Liu J (2000) Large mammalian carnivores as a taphonomic factor in the bone accumulation at Zhoukoudian. Acta Anthrop Sinica 19:224–234
Brain CK (1981) The hunters or the hunted? University of Chicago Press, Chicago, An introduction to African cave taphonomy, p 365
Buckland W (1822) Account of an assemblage of fossil teeth and bones of elephant, rhinoceros, hippopotamus, bear, tiger and hyaena, and sixteen other animals; discovered in a cave at Kirkdale, Yorkshire, in the year 1821; with a comparative view on five similar caverns in various parts of England, and others on the continent. Philos Trans R Soc Lond 112:171–236
Buckland W (1824) Reliquiae diluvianae; or, observations on the organic remains contained in caves, fissures, and diluvial gravel, and on other geological phenomena, attesting the action of an universal deluge. John Murray, London, p 303
Buckland W (1829) On the discovery of coprolites, of fossil feces, in the Lias at Lyme Regis, and in other formations. Geol Soc London, Transactions Series 2(3), 1:223–36
Buesching CD, Jordan N (2019) The social function of latrines: a hypothesis-driven research approach. In: Buesching CD (ed) Chemical signals in vertebrates 14, Springer: pp 94–103. https://doi.org/10.1007/978-3-030-17616-7_8
Bull ID, Matthew J, Lockheart J, Elhmmali MM, Roberts DJ, Evershed RP (2002) The origin of faeces by means of biomarker detection. Environ Int 27:647–654. https://doi.org/10.1016/s0160-4120(01)00124-6
Canti MG (1999) The production and preservation of faecal spherulites: animal, environment and taphonomy. J Archaeol Sci 26:251–258. https://doi.org/10.1006/jasc.1998.0322
Carrión JS (2002) A taphonomic study of modern pollen assemblages from dung and surface sediments in arid environments of Spain. Rev Palaeobot Palynol 120:217–232. https://doi.org/10.1016/S0034-6667(02)00073-8
Carrión JS, Riquelme JA, Navarro C, Munuera M (2001) Pollen in hyena coprolites reflects late glacial landscape in southern Spain. Palaeogeogr Palaeoclimatol Palaeocol 176:193–205. https://doi.org/10.1016/S0031-0182(01)00338-8
Carrión JS, Scott L, Arribas A, Fuentes N, Gil-Romera G, Montoya E (2007) Pleistocene landscapes in central Iberia inferred from pollen analysis of hyena coprolites. J Quat Sci 22(2):191–202. https://doi.org/10.1002/jqs.1024
Carrión JS, Fernández S, González-Sampériz P, Leroy SAG, Bailey GN, López-Sáez JA, Burjachs F, Gil-Romera G, García-Antón M, Gil-García MJ, Parra I, Santos L, López-García P, YII EI, Dupré M (2009) Quaternary pollen analysis in the Iberian Peninsula: the value of negative results. Internet Archaeol 25:1–53. http://intarch.ac.uk/journal/issue25/5/toc.html
Chame M (2003) Terrestrial mammal feces: a morphometric summary and description. Mem Inst Oswaldo Cruz 98:71–94
Chen J, Waloszek D, Maas A, Braun A, Huang D, Wang X, Stein M (2007) Early Cambrian Yangtze Plate Maotianshan Shale macrofauna biodiversity and the evolution of predation. Palaeogeogr Palaeoclimatol Palaeoecol 254:250–272. https://doi.org/10.1016/j.palaeo.2007.03.018
Chin K (2002) Analyses of coprolites produced by carnivorous vertebrates. Paleontol Soc Pap 8:43–50. https://doi.org/10.1017/S1089332600001042
Chin K, Eberth DA, Schweitzer MH, Rando TA, Sloboda WJ, Horner JR (2003) Remarkable preservation of undigested muscle tissue within a Late Cretaceous tyrannosaurid coprolite from Alberta, Canada. Palaios 18:286–294. https://doi.org/10.1669/0883-1351(2003)018%3c0286:RPOUMT%3e2.0.CO;2
Coil RA (2016) Spatial approaches to site formation and carnivore-hominin interaction at Dmanisi. PhD Dissertation, University of Minnesota
Color M (1975) Munsell soil-color charts, 1975th edn. Macbeth Division of Kollmorgen Corporation, Baltimore, Maryland
Courtenay LA, Yravedra J, Herranz-Rodrigo D, Rodríguez-Alba JJ, Serrano-Ramos A, Estaca-Gómez V, González-Aguilera D, Solano JA, Jiménez-Arenas JM (2023) Deciphering carnivoran competition for animal resources at the 1.46 Ma early Pleistocene site of Barranco León (Orce, Granada, Spain). Quat Sci Rev 300:107912. https://doi.org/10.1016/j.quascirev.2022.107912
Coy CE (1995) The first record of spiral coprolites from the Dinosaur Park Formation (Judith River Group, Upper Cretaceous) southern Alberta. Canada J Paleontol 69(6):1191–1194. https://doi.org/10.1017/S0022336000038208
Cozzi G, Börger L, Hutter P, Abegg D, Beran C, McNutt JW, Ozgul A (2015) Effects of trophy hunting leftovers on the ranging behaviour of large carnivores: a case study on spotted hyenas. Plos One 10(3):e0121471. https://doi.org/10.1371/journal.pone.0121471
Diedrich CG (2012) Typology of ice spotted hyena Crocuta crocuta spelaean (Goldfuss, 1823) coprolite aggregate pellets from the European Late Pleistocene and their significance at dens and scavenging sites. In: Hunt (ed) Vertebrate coprolites, New Mexico Museum of Natural Hystory and Science, Bulletin 57:369 –376
Duval M, Falguères C, Bahain JJ, Grün R, Shao Q, Aubert M, Dolo JM, Agustí J, Martínez-Navarro B, Palmqvist P, Toro-Moyano I (2012) On the limits of using combined U-series/ESR method to date fossil teeth from two Early Pleistocene archaeological sites of the Orce area (Guadix-Baza basin, Spain). Quat Res 77:481–482. https://doi.org/10.1016/j.yqres.2012.01.003
Espigares MP, Martínez-Navarro B, Palmqvist P, Ros-Montoya S, Toro I, Agustí J, Sala R (2013) Homo vs. Pachycrocuta: earliest evidence of competition for an elephant carcass between scavengers at FuenteNueva-3 (Orce, Spain). Quat Int 295:113–125. https://doi.org/10.1016/j.quaint.2012.09.032
Espigares MP, Palmqvist P, Guerra-Merchán A, Ros-Montoya S, García-Aguilar JM, Rodríguez-Gómez G, Serrano FJ, Martínez-Navarro B (2019) The earliest cut marks of Europe: a discussion on hominin subsistence patterns in the Orce sites (Baza basin, SE Spain). Sci Rep 9:15048. https://doi.org/10.1038/s41598-019-51957-5
Espigares MP (2010) Análisis y modelización del contexto sedimentario y los atributos tafonómicos de los yacimientos pleistocénicos del borde nororiental de la Cuenca de Guadix-Baza. PhD Dissertation. Universidad de Granada, 533 pp
Farlow JO, Chin K, Argast A, Poppy S (2010) Coprolites from the Pipe Creek Sinkhole (Late Neogene, Grant County, Indiana, USA). Vertebr Paleontol 30:959–969. https://doi.org/10.1080/02724631003762906
Farrell LE, Roman J, Sunquist ME (2000) Dietary separation of sympatric carnivores identified by molecular analysis of scats. Mol Ecol 9(10):1583–1590. https://doi.org/10.1046/j.1365-294x.2000.01037.x
Fernández-Jalvo Y, Scott L, Carrión JS, Gil-Romera G, Brink J, Neumann F, Rossouw L (2010) Pollen taphonomy of hyaena coprolites: an experimental approach. In: Baquedano E, Rosell J (eds) 1st international meeting on hyaena dens (and other carnivores) in archaeological sites of the Iberian Peninsula. Museo Arqueológico Regional, Alcalá de Henares, 148–156
García-Aguilar JM, Palmqvist P (2011) A model of lacustrine sedimentation for the Lower Pleistocene deposits of Guadix-Baza basin (southeast Spain). Quat Int 243:3–15. https://doi.org/10.1016/j.quaint.2011.02.008
García-Aguilar JM, Guerra-Merchán A, Serrano F, Palmqvist P, Flores-Moya A, Martínez-Navarro B (2014) Hydrothermal activity and its paleoecological implications in the latest Miocene to Middle Pleistocene lacustrine environments of the Baza Basin (Betic Cordillera, SE Spain). Quater Sci Rev 96:204–221. https://doi.org/10.1016/j.quascirev.2013.07.009
García-Aguilar JM, Guerra-Merchán A, Serrano F, Flores-Moya A, Delgado-Huertas A, Espigares MP, Ros-Montoya S, Martínez-Navarro B, Palmqvist P (2015) A reassessment of the evidence for hydrothermal activity in the Neogene-Quaternary lacustrine environments of the Baza basin (Betic Cordillera, SE Spain) and its paleoecological implications. Quat Sci Rev 112:226–235. https://doi.org/10.1016/j.quascirev.2015.02.001
Gaudzinski S (2004) Subsistence patterns of Early Pleistocene hominid in the Levant-taphonomic evidence from the ‘Ubeidiya Formation (Israel). J Archaeol Sci 31:65–75. https://doi.org/10.1016/S0305-4403(03)00100-6
González-Sampériz P, Montes L, Utrilla P (2003) Pollen in hyena coprolites from Gabasa Cave (northern Spain). Rev Palaeobot Palynol 126:7–15. https://doi.org/10.1016/S0034-6667(03)00033-2
Gorman ML, Mills MGL (1984) Scent marking strategies in hyaenas (Mammalia). J Zool 202:535–547. https://doi.org/10.1111/j.1469-7998.1984.tb05050.x
Granados A, Oms O, Anadón P, Ibanez-Insa J, Kaakinen A, Jiménez-Arenas JM (2021) Geochemical and sedimentary constraints on the formation of the Venta Micena Early Pleistocene site (Guadix-Baza basin, Spain). Sci Rep 11(1):1–13. https://doi.org/10.1038/s41598-021-01711-7
Harrison T (2011) Coprolites: taphonomic and paleoecological implications. In: Harrison T (ed) Paleontology and geology of Laetoli: human evolution in context, vol 1. geology, geochronology, paleoecology and environment. Springer, Vertebrate paleobiology and paleoanthropology series, New York, pp 279–292
Haynes G (1988) Longitudinal studies of African elephant death and bone deposits. J Archaeol Sci 15:131–157. https://doi.org/10.1016/0305-4403(88)90003-9
Holekamp KE, Smale L, Berg R, Cooper SM (1997) Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J Zool 242(1):1–15
Hollocher KT, Hollocher TC, Keith Rigby Jr JK (2010) A phosphatic coprolite lacking diagenetic permineralization from the Upper Cretaceous Hell Creek Formation, Northeastern Montana: importance of dietary calcium phosphate in preservation. Palaios 25:132–140. https://doi.org/10.2110/palo.2008.p08-132r
Hollocher K, Hollocher TC (2012) Early process in the fossilization of terrestrial feces to coprolites, and microstructure preservation. In: Hunt (ed) Vertebrate coprolites, New Mexico Museum of Natural Hystory and Science, Bulletin 57:79–92
Horwitz LK, Goldberg P (1989) A study of Pleistocene and Holocene hyena coprolites. J Archaeol Sci 16:71–94. https://doi.org/10.1016/0305-4403(89)90057-5
Hunt AP, Lucas EG, Milàn J, Spielmann J (2012) Vertebrate coprolite studies: status and prospectus. In: Hunt (ed) Vertebrate coprolites, New Mexico Museum of Natural Hystory and Science, Bulletin 57:5–24
Hunt AP, Lucas SG (2019) Hyena hegemony: biogeography and taphonomy of Pleistocene vertebrate coprolites with description of a new mammoth coprolite ichnotaxon. Ichnos 27(2):111–121. https://doi.org/10.1080/10420940.2019.1612393
Jiménez-Moreno, G (2003). Análisis polínico de las secciones de Barranco León y Fuente Nueva de Orce (Granada). Primeros resultados. In: Toro I., Agustí, J, Martínez-Navarro, B (eds), El Pleistoceno inferior de Barranco León y Fuente Nueva 3, Orce (Granada). Memoria Científica Campañas 1999–2002. Junta de Andalucía. Consejería de Cultura. E.P.G. Arqueología Monográfico, 173–181
Keiler JA (2001) Die koprolithen aus dem Unterpleistozän von Untermaßfeld. In: Kahlke RD (Ed.) Das Pleistozän von Untermaßfeld bei Meiningen (Thüringen) Teil 2 pp 691–698
Khaldoun A, Eiser E, Wegdam G, Bonn D (2005) Liquefaction of quicksand under stress. Nature 437:635. https://doi.org/10.1038/437635a
Kikinis R, Pieper, SD, Vosburgh, K (2014). 3D Slicer: a platform for subject-specific image analysis, visualization, and clinical support in Jolesz F (ed) Intraoperative imaging and image-guided therapy (New York, NY: Springer) 277–289. https://doi.org/10.1007/978-1-4614-7657-3_19
Kruuk H (1972) The spotted hyena a study of predation and social behaviour. The University of Chicago Press, Wildlife Behavior and Ecology Series, p 335
Kruuk H (1976) Feeding and social behaviour of the striped hyaena (Hyaena vulgaris Desmaret). E Afr Wildl J 14:91–111. https://doi.org/10.1111/j.1365-2028.1976.tb00155.x
Larkin NR, Alexander J, Lewis M (2000) Using experimental studies of recent faecal material to examine hyaena coprolites from de West Runton Freshwater Bed, Norfolk, U.K. J Archaeol Sci 27:19–31. https://doi.org/10.1006/jasc.1999.0437
Lavrov AV, Gimranov DO, Startsev DB, Lopatin AV (2021) Giant hyena Pachycrocuta brevirostris (Hyaenidae, Carnivora) from the Lower Pleistocene of Taurida Cave, Crimea. Dokl Biol Sci 496:5–8. https://doi.org/10.1134/S0012496621010087
Leakey MD, Hay RL (1979) Pliocene footprints in the Laetoli Beds at Laetoli, Northern Tanzania. Nature 278:317–323. https://doi.org/10.1038/278317a0
Lewis MD (2011) Pleistocene hyaena coprolite palynology in Britain: implications for the environments of early humans. In: Ashton NM, Lewis SG, Stringer CB (eds) The ancient human occupation of Britain. Elservier, Developments in quaternary science 14:263–278
Linseele V, Reimer H, Baeten J, De Vos D, Marinova E, Ottoni C (2013) Species identification of archaeological dung remains: a critical review of potential methods. Environ Archael 18(1):5–17. https://doi.org/10.1179/1461410313Z.00000000019
Macdonald DW (1980) Patterns of scent marking with urine and faeces amongst carnivore communities. Symp Zool Soc Lond 45:107–139
Madurell-Malapeira J, Alba DM, Espigares MP, Vinuesa V, Palmqvist P, Martínez-Navarro B, Moyà-Solà S (2017) Were large carnivorans and great climatic shifts limiting factors for hominin dispersals? Evidence of the activity of Pachycrocuta brevirostris during the Mid-Pleistocene Revolution in de Vallparadís Section (Vallès-Penedès Basin, Iberian Peninsula). Quat Int 431:42–52. https://doi.org/10.1016/j.quaint.2015.07.040
Marinova E, Linseele V, Kühn M (2013) Bioarchaeological research on animal dung-possibilities and limitations. Environ Archaeol 18(1):1–3. https://doi.org/10.1179/1461410313Z.00000000023
Martin JE (1981) Contents of coprolites from Hemphillian sediments in northern Oregon, and their significance in paleoecological interpretations. Proc S D Acad Sci 60:105–115
Martínez-Monzón A, Sánchez-Bandera C, Fagoaga A, Oms O, Agustí J, Barsky D, Solano-García J, Jiménez Arenas JM, Blain H-A (2022) Amphibian body size and species richness as a proxy for primary productivity and climate the Orce wetlands (Erarly Pleistocene, Guadix-Baza Basin, SE Spain). Palaeogeogr Palaeoclimatol Palaeoecol. 586:110752. https://doi.org/10.1016/j.palaeo.2021.110752
Martínez-Navarro B, Turq A, Agustí J, Oms O (1997) Fuente Nueva-3 (Orce, Granada, Spain) and the first human occupation of Europe. J Hum Evol 33:611–620. https://doi.org/10.1006/jhev.1997.0158
Martínez-Navarro B, Madurell-Malapeira J, Ros-Montoya S, Espigares MP, Medin T, Hortolà P, Palmqvist P (2015) The Epivillafranchian and the arrival of pigs into Europe. Quat Int 389:131–138. https://doi.org/10.1016/j.quaint.2015.09.039
Martínez-Navarro B, Espigares MP, Ros S (2003) Estudio preliminar de las asociaciones de grandes mamíferos de Fuente Nueva-3 y Barranco León-5 (Orce, Granada, España). Informe de las campañas de 1999–2002. in: Toro I, Agustí J, Martínez-Navarro B (eds) Geología, Paleontología, Paleoecología y Arqueología de la Depresión Guadix-Baza durante el Plio-Pleistoceno. Consejería de Cultura, Junta de Andalucía, 115–136
Martínez-Navarro B, Palmqvist P, Madurell-Malapeira J, Ros-Montoya S, Espigares M P, Torregrosa V, Pérez-Claros JA (2010) La fauna de grandes mamíferos de Fuente Nueva-3 y Barranco León-5: Estado de la cuestión In: Toro I, Martínez-Navarro B, Agustí J (eds) Ocupaciones humanas en el Pleistoceno inferior y medio de la Cuenca de Guadix-Baza, Sevilla: Junta de Andalucía, Consejería de Cultura, 197–236
Martínez-Navarro B, Ros-Montoya S, Espigares MP, Madurell-Malapeira J, Palmqvist P (2018). Los mamíferos del Plioceno y Pleistoceno de la Península Ibérica. Revista PH 94, 206–249. 10.33349/2018.0
Martínez-Navarro B (1991) Revisión sistemática y estudio cuantitativo de la fauna de macromamíferos del yacimiento de Venta Micena (Orce, Granada). Ph D dissertation. Spain, Univ. Autónoma de Barcelona, 264 pp
Matthews LH (1939) The bionomics of the spotted hyaena, Crocuta crocuta Erxl. Proc Zool Soc Lond Ser A 109:43–56. https://doi.org/10.1111/j.1096-3642.1939.tb00046.x
Mazza P, Bertini A, Magi M (2004) The Late Pliocene site of Poggio Rosso (Central Italy): taphonomy and paleoenvironment. Palaios 19:227–248. https://www.jstor.org/stable/3515810
Meng J, Zhai R, Wyss AR (1998) The late Paleocene Bayan Ulan fauna of Inner Mongolia, China, p. 148–185. In: Beard KC and Dawson MR (Eds.) Dawn of the age of mammals in Asia. Bull Carnegie Mus Nat Hist, 34, Pittsburgh
Meng J, Wyss AR (1997) Multituberculate and other mammal hair recovered from Palaeogene excreta. Nature 385:712–714. https://doi.org/10.1038/385712a0
Mills MGL, Gorman ML (1987) The scent-marking behaviour of the spotted hyena Crocuta crocuta in the southern Kalahari. J Zool 212:483–497. https://doi.org/10.1111/j.1469-7998.1987.tb02919.x
Nel JW (1967) An ashing technique to determine Ca, P and Mg from one ashing. Proc s Afr Soc Anim Prod 6:242–243
Northwood C (2005) Early Triassic coprolites from Australia and their palaeobiological significance. Palaeontology 48:49–68. https://doi.org/10.1111/j.1475-4983.2004.00432.x
Ochando J, Carrión J, Altolaguirre Y, Munuera M, Amorós G, Jiménez-Moreno G, Solano-García J, Barsky D, Luzón C, Sánchez-Bandera C, Serrano-Ramos A, Toro-Moyano I, Saarinen J, Blain HA, Bocherens H, Oms O, Agustí J, Fortelius M, Jiménez-Arenas JM (2022) Palynological investigations in the Orce Archaeological Zone, Early Pleistocene of Southern Spain. Rev Palaeobot Palynol 304:104725. https://doi.org/10.1016/j.revpalbo.2022.104725
Oms O, Parés JM, Martínez-Navarro B, Agustí J, Toro I, Martínez-Fernández G, Turq A (2000) Early human occupation of Western Europe: paleomagnetic dates for two Paleolithic sites in Spain. Proc Natl Acad Sci USA 97:10666–10670. https://doi.org/10.1073/pnas.180319797
Oms O, Anadón P, Agustí J, Julià R (2011) Geology and chronology of the continental Pleistocene archeological and paleontological sites of the Orce area (Baza basin, Spain). Quat Int 243:33–43. https://doi.org/10.1016/j.quaint.2011.03.048
Oms O, Agustí J, Parés JM (2010) Litoestratigrafía, magnetoestratigrafía y bioestratigrafía de los yacimientos de Barranco Léon 5 y Fuente Nueva 3 (Cuenca de Guadix-Baza, España). In Toro I, Martínez-Navarro B, Agustí J (eds) Ocupaciones humanas en el Pleistoceno inferior y medio de la Cuenca de Guadix-Baza. Arqueología Monografías. Junta de Andalucía, Consejería de Cultura pp 107–119
Outram AK (2001) A new approach to identifying bone marrow and grease exploitation: why the “indeterminate” fragments should not be ignored. J Archaeol Sci 28:401–410. https://doi.org/10.1006/jasc.2000.0619
Owocki K, Niedźwiedzki G, Sennikov AG, Golubev VK, Janiszewska K, Sulej T (2012) Upper Permian vertebrate coprolites from Vyazniki and Gorokhovets, Vyatkian Regional Stage. Russian Platform Palaios 27(12):867–877. https://doi.org/10.2110/palo.2012.p12-017r
Palmqvist P, Arribas A (2001) Taphonomic decoding of the paleobiological information locked in a lower Pleistocene assemblage of large mammals. Paleobiology 27:512–530. https://doi.org/10.1666/0094-8373(2001)027%3C0512:TDOTPI%3E2.0.CO;2
Palmqvist P, Martínez-Navarro B, Toro I, Espigares MP, Ros-Montoya S, Torregrosa V, Pérez-Claros JA (2005) Réévaluation de la présence humaine au Pléistocène inférieur dans le Sud de l’Espagne. L’anthropologie 109:411–450. https://doi.org/10.1016/j.anthro.2005.06.001
Palmqvist P, Martínez-Navarro B, Pérez-Claros JA, Torregrosa T, Figueirido B, Jiménez-Arenas JM, Espigares MP, Ros-Montoya S, De Renzi M (2011) The giant hyena Pachycrocuta brevirostris: modelling the bone-cracking behavior of an extinct carnivore. Quat Int 243:61–79. https://doi.org/10.1016/j.quaint.2010.12.035
Palmqvist P, Duval M, Diéguez A, Ros-Montoya S, Espigares MP (2016) On the fallacy of using orthogenetic models of rectilinear change in arvicolid teeth for estimating the age of the first human settlements in Western Europe. Hist Biol 28:734–752. https://doi.org/10.1080/08912963.2015.1025390
Palmqvist P, Espigares MP, Pérez-Claros JA, Figueirido B, Guerra-Merchán A, Ros-Montoya S, Rodríguez-Gómez G, García-Aguilar JM, Granados A, Martínez-Navarro B (2022a) Déjà vu: a reappraisal of the taphonomy of quarry VM4 of the Early Pleistocene site of Venta Micena (Baza Basin, SE Spain). Sci Rep 12:705. https://doi.org/10.1038/s41598-021-04725-3
Palmqvist P, Rodríguez-Gómez G, Bermúdez de Castro JM, García-Aguilar JM, Espigares MP, Figueirido B, Ros-Montoya S, Granados A, Serrano FJ, Martínez-Navarro B, Guerra-Merchán A (2022b) Insights on the Early Pleistocene hominin population of the Guadix-Baza Depression (SE Spain) and a review on the ecology of the first peopling of Europe. Front Ecol Evol 10:881651. https://doi.org/10.3389/fevo.2022.881651
Palmqvist P, Rodríguez-Gómez G, Martínez-Navarro B, Espigares MP, Figueirido B, Ros-Montoya S, Guerra-Merchán A, Granados A, García-Aguilar JM, Pérez-Claros JA (2023) Déjà vu: on the use of meat resources by sabretooth cats, hominins, and hyaenas in the Early Pleistocene site of Fuente Nueva 3 (Guadix-Baza Depression, SE Spain) J Archaeol Anthropol Sci 15:17. https://doi.org/10.1007/s12520-022-01712-1
Parfitt SA, Larkin NR (2010) Appendix: exceptionally large hyaena coprolites from West Runton and the possible presence of the giant short-faced hyaena (Pachycrocuta brevirostris). Quat Int 228:131–135
Parris DC, Holman JA (1978) An Oligocene snake from a coprolite. Herpetologica 34(3):258–264. https://www.jstor.org/stable/3891549
Pasenko MR (2017) Quantitative and qualitative data of footprints produced by Asian (Elephas maximus) and African (Loxodonta africana) elephants and with a discussion of significance towards fossilized proboscidean footprints. Quat Int 443:221–227. https://doi.org/10.1016/j.quaint.2017.05.030
Pemberton SG (2012) William Buckland (1784–1856) and Henry De la Beche (1796–1855): The early history of coprolites. In: Hunt AP (ed) Vertebrate coprolites, New Mexico Museum of Natural Hystory and Science, Bulletin 57:29–44
Pérez-Ramos A, Figueirido B (2020) Toward an “ancient” virtual world: improvement methods on X-ray CT data processing and virtual reconstruction of fossil skulls. Front Earth Sci 8:345. https://doi.org/10.3389/feart.2020.00345
Pertusa JF (2010) Técnicas de Análisis de Imagen. Aplicaciones en Biología [Image analysis techniques. Applications in biology], 2nd edn. Publicacions de la Universitat de València, Valencia
Pesquero MD, Salesa MJ, Espílez E, Mampel L, Siliceo G, Alcalá L (2011) An exceptionally rich hyaena coprolites concentration in the Late Miocene mammal fossil site of La Roma 2 (Teruel, Spain): taphonomical and palaeoenvironmetal inferences. Palaeogeogr Palaeoclimatol Palaeocol 311:30–37. https://doi.org/10.1016/j.palaeo.2011.07.013
Pesquero MD, Souza-Egipsy V, Alcalá L, Ascaso C, Fernández-Javlo Y (2014) Calcium phospate preservation of faecal bacterial negative moulds in hyaena coprolites. Acta Paleontol Pol 59(4):997–1005. https://doi.org/10.4202/app.2012.0067
Pickering TR, Egeland CP (2006) Experimental patterns of hammerstone percussion damage on bones: implications for inferences of carcass processing by humans. J Archaeol Sci 33:459–469. https://doi.org/10.1016/j.jas.2005.09.001
Pineda A, Saladié P, Expósito I, Rodríguez-Hidalgo A, Cáceres I, Huguet R, Rosas A, López-Polín L, Estalrichh A, García-Tabernero A, Vallverdú J (2017) Characterizing hyena coprolites from two latrines of the Iberian Peninsula during Early Pleistocene: Gran Dolina (Sierra de Atapuerca, Burgos) and la Mina (Barranc de la Boella, Tarragona). Palaeogeogr Palaeoclimatol Palaeocol 480:1–17. https://doi.org/10.1016/j.palaeo.2017.04.021
Qvarnström M, Niedźwiedzki G, Žigaitė Z (2016) Vertebrate coprolites (fossil faeces): an underexplored Konservat-Lagerstätte. Earth-Sci Rev 162:44–57. https://doi.org/10.1016/j.earscirev.2016.08.014
Qvarnström M, Wernström JV, Piechowski R, Tałanda M, Ahlberg PE, Niedźwiedzki G (2019) Beetle-bearing coprolites possibly reveal the diet of a Late Triassic dinosauriform. R Soc open sci 6:181042. https://doi.org/10.1098/rsos.181042
Reinhard KJ, Ambler JR, Szuter CR (2007) Hunter-gatherer use of small animal food resources: coprolite evidence. Int J Osteoarchaeol 17:416–428. https://doi.org/10.1002/oa.883
Reinhard KJ, Bryant Jr VM (1992) Coprolite analysis a biological perspective on archaeology. Archaeol Method Theory 4:245–288. http://www.jstor.org7stable720170225
Reinoso-Gordo JF, Barsky D, Serrano-Ramos A, Solano-García JA, León-Robles CA, Luzón-González C, Titton S, Jiménez-Arenas JM (2020) Walking among mammoths. Remote sensing and virtual reality supporting the study and dissemination of Pleistocene archaeological sites the case of Fuente Nueva 3 in Orce, Spain. Sustainability 12:4785 https://doi.org/10.3390/su12114785
Rhode D (2003) Coprolites from Hidden Cave, revisited: evidence for site occupation history, diet and sex of occupants. J Archaeol Sci 30:909–922. https://doi.org/10.1016/S0305-4403(02)00270-4
Rodríguez-Gómez G, Palmqvist P, Rodríguez J, Mateos A, Martín-González JA, Espigares MP, Ros-Montoya S, Martínez-Navarro B (2016) On the ecological context of the earliest human settlements in Europe: resource availability and competition intensity in the carnivore guild of Barranco León-D and Fuente Nueva-3 (Orce, Baza Basin, SE Spain). Quat Sci Rev 134:69–83. https://doi.org/10.1016/j.quascirev.2016.05.018
Ros-Montoya S, Bartolini-Lucenti S, Espigares, MP, Palmqvist P, Martínez-Navarro, B (2021) First review of Lycondontini material (Mustelidae, Carnivora, Mammalia) from the Lower Pleistocen archaeo-palaeontological sites of Orce (Southeastern Spain). Riv Ital Paleontol S 127(1):33–47. https://doi.org/10.13130/2039-4942/15132
Ros-Montoya S (2010) Los Proboscídeos del Plio-Pleistoceno de las Cuencas de Guadix-Baza y Granada. PhD Dissertation. Universidad de Granada, 410 pp
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18:529. https://doi.org/10.1186/s12859-017-1934-z
Sanz M, Daura J, Égüez N, Brugal JP (2016) Not only hyenids: a multi-scale analysis of Upper Pleistocene carnivore coprolites in Cova del Coll Verdaguer (NE Iberian Peninsula). Palaeogeogr Palaeoclimatol Palaeocol 443:249–262. https://doi.org/10.1016/j.palaeo.2015.11.047
Saunders JJ, Dawson BK (1998) Bone damage patterns produced by extinct hyena Pachycrocuta brevirostris (Mammalia: Carnivora) at the Haro River Quarry, Northwestern Pakistan. In: Tomida Y, Flynn LJ, Jacobs LL (eds) Advances in vertebrate paleontology and geochronology. Nat Sci Mus Monographs 14, Tokyo 215–242
Scott L (1987) Pollen analysis of hyena coprolites and sediments from Equus Cave, Taung, Southern Kalahari (South Africa). Quat Res 28:144–156. https://doi.org/10.1016/0033-5894(87)90039-1
Scott L, Fernández-Jalvo Y, Carrión J, Brink J (2003) Preservation and interpretation of pollen in hyaena coprolites: taphonomic observations from Spain and southern Africa. Paleont Afr 39:83–91
Seilacher A, Marshall C, Skinner HCW, Tsuihiji T (2001) A fresh look at sideritic “coprolites.” Paleobiology 27:7–13. https://doi.org/10.1666/0094-8373(2001)027b0007:AFLASCN2.0.CO;2
Shillito LM, Blong JC, Green EJ, van Asperen EN (2020) The what, how and why of archaeological coprolite analysis. Earth Sci Rev 207:103196. https://doi.org/10.1016/j.earscirev.2020.103196
Skinner JD, van Aarde RJ (1981) The distribution and ecology of the brown hyaena Hyaena brunnea and spotted hyaena Crocuta crocuta in the central Namib Desert. Madoqua 4:231–239. https://hdl.handle.net/10520/AJA10115498_214
Sohn EG, Chatterjee S (1979) Freshwater ostracodes from Late Triassic coprolites in Central India. J Paleontol 53(3):578–586. https://www.jstor.org/stable/1303999
Speden IG (1969) Predation on New Zealand Cretaceous species of Inoceramus (Bivalvia). N Z J Geol Geophys 14(1):56–70. https://doi.org/10.1080/00288306.1971.10422456
Stavrova T, Borel A, Daujeard C, Vettese D (2019) A GIS based approach to long bone breakage patterns derived from marrow extraction. PLoS ONE 14(5):0216733. https://doi.org/10.1371/journal.pone.0216733
Stewart JD, Carpenter K (1990) Examples of vertebrate predation on cephalopods in the Late Cretaceous of the Western Interior, p. 205–207. In: Boucot AJ (ed) Evolutionary paleobiology of behavior and coevolution. Elsevier, Amsterdam, p 750
Stuart C, Stuart T (1998) A field guide to the tracks and signs of Southern and East African wildlife. Southern Books Publishers, Cape Town, p 310
Stuart A J (1982) Pleistocene vertebrates in the British Isles, 212 pp. Longman, London
Taglioretti V, Sardella NH, Fugassa MH (2014) Morphometric analysis in modern faeces as a tool to identify artiodactyls’ coprolites. Quat Int 352:64–67. https://doi.org/10.1016/j.quaint.2013.12.055
Taru P, Backwell L (2013) Identification of fossil hairs in Parahiena brunnea coprolites from Middle Pleistocene deposits at Gladysvale cave, South Africa. J Archaeol Sci 40:3674–3685. https://doi.org/10.1016/j.jas.2013.04.031
Titton S, Barsky D, Bargalló A, Vergès JM, Guardiola M, García-Solano J, Jiménez Arenas JM, Toro-Moyano I, Sala-Ramos R (2018) Active percussion tools from the Oldowan site of Barranco León (Orce, Andalusia, Spain): the fundamental role of pounding activities in hominin lifeways. J Archaeol Sci 96:131–147. https://doi.org/10.1016/j.jas.2018.06.004
Titton S, Oms O, Barsky D, Bargalló A, Serrano-Ramos A, García-Solano J, Sánchez-Bandera C, Yravedra J, Blain H-A, Toro-Moyano I, Jiménez Arenas JM, Sala-Ramos R (2021) Oldowan stone knapping and percussive activities on a raw material reservoir deposit 1.4 million years ago at Barranco León (Orce, Spain). Archaeol Anthropol Sci 13:108. https://doi.org/10.1007/s12520-021-01353-w
Tixier J, Roe D, Turq A, Gibert J, Martínez-Navarro B, Arribas A, Gibert L, Gaete R, Maillo A, Iglesias A (1995) Presence d’industries lithiques dans le Pléistocène inférieur de la région d’Orce (Grenade, Espagne): etat de la question. C R Acad Sci Ser IIa:71–78
Toro-Moyano I, Barsky D, Cauche D, Celiberti V, Grégoire S, Lebegue F, Moncel MH, de Lumley H (2011) The archaic stone tool industry from Barranco León and Fuente Nueva 3 (Orce, Spain): evidence of the earliest hominin presence in southern Europe. Quat Int 243:80–91. https://doi.org/10.1016/j.quaint.2010.12.011
Toro-Moyano I, Martínez-Navarro B, Agustí J, Souday C, Bermúdez de Castro JM, Martinón-Torres M, Fajardo B, Duval M, Falguères C, Oms O, Parés JM, Anadón P, Julià R, García-Aguilar JM, Moigne A-M, Espigares MP, Ros-Montoya S, Palmqvist P (2013) The oldest human fossil in Europe, from Orce (Spain). J Hum Evol 65(1):1–9. https://doi.org/10.1016/j.jhevol.2013.01.012
Turner A (1990) The evolution of the guild of larger terrestrial carnivores during the Plio-Pleistocene in Africa. Geobios 23:349–368. https://doi.org/10.1016/0016-6995(90)80006-2
Turner A (1992) Large carnivores and earliest European hominids: changing determinants of resource availability during the lower and middle Pleistocene. J Hum Evol 22:109–126. https://doi.org/10.1016/0047-2484(92)90033-6
Turner A, Antón M (1996) The giant hyena, Pachycrocuta brevirostris (Mammalia, Carnivora, Hyaenidae). Geobios 29:455–468. https://doi.org/10.1016/S0016-6995(96)80005-2
Turq A, Martínez-Navarro B, Palmqvist P, Arribas A, Agustí J, Rodríguez-Vidal J (1996) Le Plio-Pléistocène de la région d’Orce, province de Grenada, Espagne: Bilan et perspectivas de Recherche. Paléo, Revue D’archéologie Préhistorique 8:161–204
Vajda V, Pesquero Fernández MD, Villanueva-Amadoz U, Lehsten V, Alcalá L (2016) Dietary and environmental implications of Early Cretaceous predatory dinosaur coprolites from Teruel, Spain. Palaeogeogr Palaeoclimatol Palaeoecol 464:134–142. https://doi.org/10.1016/j.palaeo.2016.02.036
Van Valkenburgh B, Binder WJ (2021). Biomechanics and feeding behaviour in carnivores: comparative and ontogenetic studies. In Blake RW. Domenici P (eds) Biomechanics in animal behaviour, pp 223–235. Garland Science. https://doi.org/10.1201/9781003210801
Villa P, Castel JC, Beauval C, Bourdillat V, Goldberg P (2004) Human and carnivore sites in the Eurepean Middle and Upper Paleolithic: similarities and differences in bone modification and fragmentation. Rev De Paleobiologie 23(2):705–730
Vinuesa V (2018) Bone-cracking hyenas (Carnivora, Hyenidae) from the European Neogene and Quaternary: taxonomy, paleobiology and evolution. PhD Dissertation. Universitat Autònoma de Barcelona. 196 pp
Vitale JD, Jordan NR, Gilfillan GD, McNutt JW, Reader T (2020) Spatial and seasonal patterns of communal latrine use by spotted hyenas (Crocuta crocuta) reflect a seasonal resource defense strategy. Behav Ecol Sociobiol 74:120. https://doi.org/10.1007/s00265-020-02895-0
Waldman M (1970) Comments on a Cretaceous coprolite from Alberta, Canada. Can J Earth Sci 7:1008–1012. https://doi.org/10.1139/e70-093
Warr LN (2021) IMA-CNMNC approved mineral symbols. Mineral Mag 85:291–320. https://doi.org/10.1180/mgm.2021.43
Yravedra J, Solano JA, Courtenay LA, Saarinen J, Linares-Matás G, Luzón C, Serrano-Ramos A, Herranz-Rodrigo D, Cámara JM, Ruiz A, Titton S, Rodríguez-Alba JJ, Mielgo C, Blain H-A, Agustí J, Sánchez-Bandera C, Montilla E, Toro-Moyano I, Fortelius M, Oms O, Barsky D, Jiménez-Arenas JM (2021) Use of meat resources in the Early Pleistocene assemblages from Fuente Nueva 3 (Orce, Granada, Spain). Archaeol Anthropol Sci 13:213. https://doi.org/10.1007/s12520-021-01461-7
Yravedra J, Solano JA, Herranz-Rodrigo D, Linares-Matás GJ, Saarinen J, Rodríguez-Alba JJ, Titton S, Serrano-Ramos A, Courtnay LA, Mielgo C, Luzón C, Cámara J, Sáncjez-Bandera C, Montilla E, Toro-Moyano I, Barsky D, Fortelius M, Agustí J, Blain HA, Oms O, Jimenez-Arenas JM (2022a) Unravelling hominin activities in the zooarchaeological assemblage of Barranco León (Orce, Granada, Spain). J Paleo Arch 5:6. https://doi.org/10.1007/s41982-022-00111-1
Yravedra J, Courtenaqy LA, Herranz-Rodrigo D, Linares-Mata G, Rodríguez-Alba JJ, Estaca-Gómez V, Luzón C, Serrano-Ramos A, Maté-González MA, Solano JA, González-Aguilera D, Jiménez-Arenas JM (2022b) Taphonomic characterisation of tooth marks of extinct Eurasian carnivores through geometric morphometrics. Sci Bull 67:1644–1648. https://doi.org/10.1016/j.scib.2022.07.017
Zangerl R, Richardson ES Jr (1963) The paleoecological history of two Pennsylvanian black shales. Fieldiana Geol Mem, 4. Chicago Nat Hist Mus, Chicago, p 352
Acknowledgements
We acknowledge to OASYS Parque Temático del Desierto de Tabernas, Almería, Spain, for providing us C. crocuta faces for comparison with the coprolites from FN3. We gratefully acknowledge the insightfull comments and suggestions provided by two anonymous reviewers.
Funding
Funding for open access publishing: Universidad Málaga/CBUA. This research has been authorized by the “Consejería de Cultura” of the “Junta de Andalucía” (ref. EXP: BC.03.174/19 10153) and carried out with financial support of program FEDER ANDALUCÍA 2014–2020 (project reference: UMA18-FEDERJA-188), by Research Groups RNM-146 and RNM-199 from “Junta de Andalucía” and 2021SGR 01238 (AGAUR) from “Generalitat de Catalunya,” PAIDI 2020 postdoctoral grants from “Junta de Andalucía” to APR and IC, “Programa de Atracción de Talento” postdoctoral grant from “Comunidad de Madrid” to GRG and the Spanish Ministry of Science and Innovation through the “María de Maeztu” excellence accreditation (CEX2019-000945-M) to BMN.
Author information
Authors and Affiliations
Contributions
MPE, PP, MDRG, and BMN wrote the main manuscript text and all co-authors made contributions. MPE prepared Figs. 4, 5, 6, 12, 13, 14, and 15 and Tables 1, 2, and 5; SRM prepared Figs. 3 and 7; MDRG prepared Figs. 8, 10, and 11 and Tables 3 and 4. AGM, JMGA, AG, and IC prepared Figs. 1 and 2, APR prepared Fig. 9, and GRG prepared Fig. 16. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors agree to participate.
Consent for publication
All authors agree to publish the paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that in page 23 of the original version of this article, unfortunately, there was an error in the scale of the weight supported per unit area by the feet of several species of large mammals. The data published, which are referred to kg/m2, should have appeared in kg/cm2.
The original article has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Espigares, M.P., Palmqvist, P., Rodríguez-Ruiz, M.D. et al. Sharing food with hyenas: a latrine of Pachycrocuta brevirostris in the Early Pleistocene assemblage of Fuente Nueva-3 (Orce, Baza Basin, SE Spain). Archaeol Anthropol Sci 15, 81 (2023). https://doi.org/10.1007/s12520-023-01784-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01784-7