Abstract
Dung has been an important material used by humans since at least the early Neolithic Period. It accumulated within domesticated animal enclosures and it was used as fuel and fertiliser as well as construction material. While the formers were studied in details, to date, the use of dung as a construction material received less attention. Here, we present a geo-ethnoarchaeological pilot study aimed at understanding the archaeological formation processes of outdoor dung-plastered floors and the possibility to identify dung markers. We studied two house terrace in a rural village from a humid tropical environment in South India (Western Ghats). Sediment samples were collected from the plastered terrace surfaces, the terraces embankment and from forest soil controls. Multi-proxy analysis of the samples included infrared spectroscopy, phytolith and dung spherulite quantification, loss on ignition, elemental analysis and micromorphological analysis. The plastering of the floors was made by mixing a quantity of dung with water and by spreading the slurry unevenly across the terrace. This result in formation of a 0.1- to 0.5-mm-thick dung crust that the analyses showed to be rich in humified organics but with very low concentrations of phytoliths and dung spherulites. The careless spreading of the dung slurry, however, resulted in localised deposition of dung lumps that displayed relatively high concentrations of phytoliths, dung spherulites, organic matter, phosphorus and strontium. The generally low preservation of dung markers seems to be related to pre- and post-depositional processes. Forest arboreal plants are low phytoliths producer, having therefore little input of these siliceous bodies in the animal faeces. Post depositional processes included trampling, sweeping and water runoff that caused severe mechanical weathering, resulting in the heavy decay of the dung crust and the removal of dung residues from the terrace surfaces. In addition, the acidic conditions of a humid tropical environment likely promoted the complete dissolution of dung spherulites. This study provides new data and insights on the potentials and limitations of dung identification in outdoor settings in humid tropical environments. We suggest possible directions for advancing the study of archaeological dung used as construction materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remains of dung found in archaeological sites bear important information regarding human-animal relations, animal domestication, human-environment interaction, paleo-environmental reconstruction, subsistence practices and economy among other things. The use of dung marks a technological development and broader exploitation of resources derived from animals. It can be used as fertiliser (McCann 1997; Bogaard et al. 2013; Gur-Arieh et al. 2013), as fuel (Anderson and Ertug-Yaras 1998; Milek 2012; Portillo et al. 2012, 2014, 2017; Gur-Arieh et al. 2013, 2014; Spengler III et al. 2013, 2014a, b, 2016; Spengler III and Willcox 2013; Doumani et al. 2015) and also as construction material (Mbae 1990; Reddy 1998; Boivin 2000; Matthews 2005, 2010; Karkanas 2006; Macphail et al. 2007; Viklund et al. 2013; Portillo et al. 2014; Berna 2017). The domestication of herd animal, beginning at the middle of the 11th millennium BP in the Near East (Vigne 2011) increased the accumulation and availability of animal dung to prehistoric societies. Evidence for dung accumulation in animal enclosures and for the use of dung as fuel can be found as early as the pre-pottery Neolithic A (PPNA) in Turkey and the pre-pottery Neolithic B (PPNB) in the Levant and the Caucasus (Portillo et al. 2009, 2014; Stiner et al. 2014; Kadowaki et al. 2015). From the Neolithic period onwards, the use of dung became more common, particularly in arid environments where wood is scarce (Kramer 1982).
The identification of dung in the archaeological record is not always an easy task and it requires laboratory-based analysis. Dung is identified based on several proxies from the macro- to the micro-scale such as charred macro-botanical remains as seeds (usually with higher representation of wild plants) and chaff (Miller 1984a, b, 1996), insects (Smith et al. 2014), high concentrations of grass phytolith (silicified plant cells) (Albert et al. 2008) and the presence of dung spherulites that are monohydrocalcite spheres created in herbivore guts (Brochier 1983; Canti 1997; Shahack-Gross et al. 2003). In such cases of good organic matter preservation, there can be the presence of intestinal parasites typical of herbivores and/or high levels of δ15N (Shahack-Gross 2011), as well as the dung itself (e.g. for goat/sheep dung: Rasmussen 1993; Rosen et al. 2005; for cow dung: Akeret and Rentzel 2001; camel dung: Zhang et al. 2013). The different ways in which dung has been used and deposited in archaeological sites (e.g. on surfaces of animal enclosures, in combustion features as fuel and as construction material) and the different chemical environments and taphonomic processes, result in variable archaeological indicators for the formation of dung as an archaeological material.
Many geoarchaeological researchers working on the identification of dung turn to ethnoarchaeological contexts to study the use of dung and its impact on the formation of archaeological evidence (see Friesem 2016 for overview on geo-ethnoarchaeology). The use of dung as fertiliser was recorded ethnographically by Watson (1979) and by Kramer (1982) in Iran. Shahack-Gross et al. (2003, 2004) studied animal enclosures in Maasai sites in Kenya and the results were later used to identify Neolithic pastoral sites in East Africa by means of stable nitrogen and carbon isotopes analysis and soil micromorphology (Shahack-Gross et al. 2008). Macphail et al. (2004) who studied animal enclosures in an experimental farm in England, identified similar patterns of formation and degradation processes of dung to the ones identified by Shahack-Gross et al. (2008). Reddy (1998) and Lancelotti and Madella (2012) studied modern dung cakes in India to investigate their macro- and micro-remains in order to assess their potential as proxies for the use of dung in antiquity. Zapata Peña et al. (2003), Gur-Arieh et al. (2013) and Portillo et al. (2017) studied the use of dung as fuel in Morocco, Uzbekistan and Tunisia, respectively, to gain a better understanding of the use and fuelling practices of ancient mud-constructed ovens by means of phytolith analysis, micromorphology and Fourier transform infrared (FTIR) spectroscopy. Lancelotti et al. (2017) investigated fuel practices based on dung through integrated analysis of phytoliths and chemical elements of samples collected in a domestic compound in North Gujarat, India.
While the indicators for dung accumulation in animal enclosures and the use of dung as fuel were studied in ethnographic and archaeological contexts (e.g. McCann 1997; Simpson et al. 2003; Macphail et al. 2004; Zapata Peña et al. 2003; Shahack-Gross et al. 2005; Portillo et al. 2009, 2014; Portillo and Albert 2011; Gur-Arieh et al. 2013, 2014; Lancelotti et al. 2014), evidence for the use of dung as a construction material is relatively less studied. Shahack-Gross et al. (2004) and Mbae (1990) documented the use of dung mixed with ash to cover wooden framed walls of Maasai huts. Goodman-Elgar (2008) identified the use of dung as temper in abundant earthen dwelling in the Bolivian Andes using micromorphology. Reddy (1998) and Boivin (2000) documented the use of dung to plaster floors in India, while Berna (2017) conducted a micromorphological study of dung plaster floors in South Africa. Lanzhe (2013) documented the use of yak dung in the Tibetan Plateau as the main construction material used to build animal enclosures and even children’s toys.
The use of dung as construction material has been so far identified in the archaeological record mostly as part of plaster floors. Karkanas (2006) identified, by using micromorphology, dung ashes mixed with red clay to form floors in Neolithic caves in Greece. Portillo et al. (2014) identified the presence of dung spherulites in red clay plastered floors and gypsum plastered features (a bin and a channel) in the PPNB site of Tell Seker al-Aheimar in Syria. Love (2012) observed dung spherulites in mud bricks from Neolithic Çatalhöyük, although it is not clear whether the dung was added to the matrix as ashes or in fresh form. Macphail et al. (2007) and Viklund et al. (2013) recognised the use of dung as wall daub from sites located in NW Europe, where the cold humid environment favoured the preservation. What seems to be a relative underrepresentation of dung being used as construction material raises questions regarding the reasons for this absence; is it because of human choice, where dung was only used as fuel and fertiliser until more recent times, or is it due to post depositional, taphonomic or analytical processes that result in less obvious archaeological signature? This absence is especially striking, as many ethnographic works demonstrate the widespread use of dung as an invaluable construction material (Mbae 1990; Reddy 1998; Boivin 2000; Shahack-Gross et al. 2004; Lanzhe 2013; Berna 2017).
This problem calls for more research on the use of dung as construction material in general, and for plastering floors in particular as this use has been documented ethnographically, and in very few exceptional examples also identified in archaeological contexts. Better understanding of human behaviour related to the formation and degradation processes of dung plaster surfaces is needed in order to define the best indicators (markers) for the archaeological identification of dung. In addition, it would be important to trace these markers to distinguish dung plaster floor surfaces from other dung deposits such as dung surfaces in animal enclosures and dung heaps. So far, no work has highlighted the markers for dung use in humid tropical environments even if dung plaster floor surfaces were sometime archaeologically identified in temperate, semi-arid and arid environments. The aims of this geo-ethnoarchaeological pilot study are therefore twofold (1) to understand the archaeological formation processes of dung-plastered outdoor surfaces, and (2) to assess the influence of humid tropical environments on the preservation of dung residues.
Materials and methods
Ethnoarchaeological fieldwork and sampling
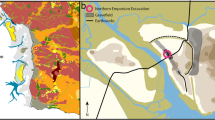
Fieldwork was carried out in a contemporary rural village located in the hills of the Western Ghats in South India at an altitude of ca. 900 m above sea level (Fig. 1a). The area has a humid tropical climate with temperature range from 17 to 37 °C and average annual precipitations of 2600 mm. Most precipitation falls during the monsoon season, from June to September (Jayakumar and Nair 2013).
Location of site and samples. a Map showing the location of the study area (in white circle) in the mountain ridge of the Western Ghats, south India. The Blue Marble Next Generation data is courtesy of Reto Stockli (NASA/GSFC) and NASA’s Earth Observatory. NASA/Goddard Space Flight Center Scientific Visualisation Studio. The country data is taken from the CIA World DataBank II. b The exterior terrace in locality A with location of the bulk sediment samples. c The exterior terrace in locality B with the location of the bulk sediment samples. Samples B1–3 represent the location block sediment samples for micromorphological analysis
The geology of the area is characterised by plutonic and metamorphic rocks (e.g. charnockites and enderbites) (Sahoo et al. 2016). Sediments originating from the weathering of these rocks undergoing pedogenesis below the forest canopy produce laterite-type soils (latosols), reddish in colour and composed mainly of clay, quartz and iron/aluminium oxides (Friesem et al. 2016).
Two localities (A and B) belonging to two different households within the same village and representing dung-plastered terraces were sampled in 2012 and 2015, respectively (Table 1, Fig. 1b, c). The terraces were artificially built to create flat activity areas. They were covered with a layer of dung plaster as part of the household routine maintenance. Sediment samples were collected from the exterior part of the terraces, adjacent to each house. Bulk sediment samples (locality A, n = 7; locality B, n = 18) were collected by opening small sections in the terrace surface and sampling between 3 and 10 g of sediments using a metal spoon from (1) the uppermost crust (a few mm thick), (2) just below the crust (to a depth of few cm) and (3) the lower terrace sediment (to a depth of up to 10 cm). Three undisturbed sediment samples for micromorphology were collected from the terrace floor of locality B. A control sample for dung was collected from a dung lump left on the terrace surface as a result of the heterogeneous nature of the dung slurry (see description in ‘Ethnographic observations’). Regional sediment samples (n = 2) from outside the site were collected as controls. The samples were registered, photographed, and inserted into plastic bags. The inhabitants of each house were interviewed (unstructured interviews) about the activities associated with the spreading of dung on the terrace surfaces. Our aim was to test if the amount of phytoliths coupled with spherulites, elemental composition and micromorphology of floor sediments can be used as markers for outdoor dung-plastered surfaces under humid tropical environments.
Mineralogical analysis via FTIR spectroscopy
All samples (Table 1; n = 27) were analysed using Fourier transform infrared (FTIR) spectroscopy in order to identify the mineral and organic components. The spectra were collected using the KBr method (Weiner 2010) between 4000 and 250 cm−1, at 4 cm−1 resolution using a Thermo Nicolet 380 spectrometer and interpreted using an internal library of infrared spectra of archaeological materials at the Kimmel Center for Archaeological Sciences at the Weizmann Institute of Science.
Loss on ignition
The percentage of organic matter within the samples (Table 1; n = 26) was calculated based on weight loss on ignition. Samples were heated in a furnace oven for 4 h at 500 °C and the difference between the initial weight and the weight following the burning was calculated.
Phytolith analysis
Phytoliths are hydrated silica (opal- SiO2·nH2O) microfossils that form within and between the cells of living plants (Piperno 2006). Bulk sediment samples (Table 1; n = 26) were analysed for phytolith concentrations following Katz et al. (2010), a method that was chosen in order to provide phytolith concentration in units comparable to dung spherulite concentrations (see below). In order to eliminate organic matter, samples were first heated in a furnace for 4 h at 500 °C before extraction. The samples were scanned using a light microscope (Zeiss Axio) to count individual phytoliths in about 30–40 fields at ×400 magnification. Multi cells (MC) articulated phytoliths were counted according to the number of single cells inside them to avoid a possible bias, where better-preserved samples appear to have lower concentrations than they actually have. At this stage, we did not carry out an analysis of phytolith taxonomy since there is currently no detailed reference collection for the area and no data was collected regarding the animals’ diet.
Dung spherulites analysis
Dung spherulites are spherical bodies that form in the intestines of animals, especially ruminants. Their size ranges from 5 to 20 μm and they are composed of radially crystallised monohydrocalcite (Canti 1997, 2017; Shahack-Gross 2011). They appear as a bluish-pinkish transparent sphere under plane-polarised light (PPL), and can be identified by their permanent cross of extinction and low-order white to second-order orange interference colours under crossed-polarised light (XPL) (Brochier 1983; Shahack-Gross 2011). Dung spherulite concentrations in the samples were determined using the method developed by Gur-Arieh et al. (2013). About 100 mg of sample (n = 26) was burnt in 500 °C for several minutes to remove organic material. The sediments were then sieved with a 150-μ mesh size sieve and placed into a 0.5 ml Eppendorf tube. After adding 500 μl sodium polytungstate (SPT) at 2.4 g/ml density, the samples were sonicated for 10 min. After sonication, they were vortexed for about 3 s and immediately, a 50-μl aliquot was placed on a slide that was scanned under the microscope (A1 Zeiss Axioscope) in XPL at ×400 magnification. About 30 fields of view were observed in each sample slide.
Elemental analysis using inductively coupled plasma-atomic emission spectrometry
Multi-element analysis was carried out using inductively coupled plasma-atomic emission spectrometry (ICP-AES) to provide quantitative data on the chemical elements. Due to the limited number of samples with a sufficient amount of sediment (>3 g) only a subset of samples from locality B (Table 2; n = 17) was sent to ALS Laboratory Group in Seville (Spain), where they were pre-treated with aqua-regia digestion and ICP-AES analysed to measure the concentration of 35 main elements from aluminium to zinc (see Table 2).
Micromorphological analysis
Thin sections for micromorphological analysis were made from undisturbed floor samples. The samples were dried in an oven at 30 °C and then impregnated using a 9:1 mixture of polyester resin with acetone and 1% v/v MEKP. Pre-cut sample slices were ground to 30-μm thickness thin sections. Thin sections were studied with a petrographic microscope at magnifications ranging from ×4 to ×200 with PPL and XPL.
Ethnographic observations
The studied village belongs to a small rural community of ten households; each house in the village is situated on a flat terrace built by cutting into the forested hill-slope. Traditionally, the local population had a subsistence economy involving foraging from the forest, small-scale animal husbandry and agriculture, also supplemented by some work in neighbouring farms (Hockings 2012). Currently, most people work at the nearby plantations for daily wages; several households own a couple of cows, which are kept in sheds near their house. A few own chickens, and some cultivate tea, pepper and coffee. Recent initiatives by local non-governmental organisations (NGOs) and the government resulted in the construction of new houses built of concrete, bricks and clay tiles that replaced the traditional ones made of forest timber and mud. Women carry out most of the domestic maintenance, while both men and women work in the plantations and tend the domestic animals. Cows are mainly used for milk but dung is an important household resource. According to our informants, the cows graze freely in the village area and their food is mainly composed of forest vegetation, though we did not record the type of plants the cows grazed on. Traditionally, cow dung is used for construction (mainly for plastering floors and walls) and fuel (although forest timber is the major fuel source used by these groups). Today, as part of the NGOs’ initiatives, dung is also employed to produce biogas for cooking hobs installed in the new houses.
The exterior terrace surface of each house was covered with a thin layer of dung plaster. The production of the dung plaster started by sourcing the raw material from a pile of dung stored in the cowshed. The pile of fresh dung was placed on the terrace and then mixed with water to make a dense suspension that was spread in a thin layer all over the terrace using a broom made of grass stems (Fig. 2a). Where the dung pile is initially placed on the terrace surface, few unmixed lumps often remain and dry in place. The spreading of the dung slurry formed a few-millimetre-thick layer of dung on the terrace surface. After the water evaporated the layer became solid and with a crusty appearance (Fig. 2b). In some cases, the vegetal fibres originating from the dung could clearly be observed (Fig. 2c). The spreading of dung was not always carried out homogeneously, leaving in some parts a relatively thick deposition of dung lumps (Fig. 2d).
The dung plaster on the terrace floor surface. a Spreading fresh dung by adding water to fresh dung and smearing it on the terrace floor surface. b The small trench made in the terrace surface for sampling. Note the upper crusty thin layer overlain a brown-red sediment below. Scale bar = 20 cm. c Close-up on the terrace floor surface showing thin dung crust with fibres. Note how the dung crust is deteriorated in the centre of the photograph revealing the lower brown sediment. Scale bar = 20 cm. d The location of a sample (A_5) identified in the field as a dung lump. Note the relative concentrated deposit of dung material. Scale bar = 20 cm
The reason mentioned for spreading dung on the terrace floor was that the dung attracts flies and other insects, keeping them away from the house. A few people even mentioned the addition of garlic to the mixture, apparently for the same purpose. Sweeping of the terrace was carried out on a daily basis. After several days, the thin grey dung crust began to deteriorate revealing the reddish local sediment below. According to our informants, when the terrace floor acquired again a red colour, a new layer of dung is applied, and this occurred from every week to every few weeks. In average, every 10 days, a new dung mixture was prepared and applied on the terrace surfaces. The practice of plastering activity surfaces with dung was observed in many different communities in the region and should not be regarded as a specific local practice. However, the reasons for which this plastering is carried out can be very different, from (as in here) relief from insect to create working surfaces, or simply to diminish the amount of dirt brought into the house.
Results
All sediment samples have similar mineralogical composition with kaolinite (a clay mineral, Al2Si2O5(OH)4) as the major component shown by absorbance bands at 1031, 1008, 1111, 912, 797, 754, 694, 536, 471, 3697 and 3620 cm−1 (Fig. 3a, b). FTIR analysis also highlighted that samples collected below the dung surface from both localities A and B are identical to the regional sediment control samples (Fig. 3b), this being the natural soil of the forest. The most striking differential characteristic of the terrace dung crust was a mix composition of kaolinite (from the forest sediments) and organic matter (from the dung). Organic matter was identified based on the presence of absorbance bands at 2922, 2852 and 1641 cm−1, as well as a broad band in the range 1600 and 1350 cm−1. Minor absorbance band at 1383 cm−1 is associated with sodium nitrate, a soluble salt that precipitates at the surface due to water evaporation (Weiner 2010). The presence of phytoliths, originating from the plant material in the dung, is evidenced by characteristic opal absorbance bands (Fig. 3c). A sample of fresh dung (A_5) deposited on the terrace surface (Fig. 2d) provided a comparative infrared spectrum for this component, which showed high concentrations of organic matter and opal (Fig. 3d).
FTIR spectra of representative samples. a Regional sediment control sample showing kaolinite as the major mineral component with absorbance bands at 3697, 3620, 1031, 1008, 1111, 912, 797, 754, 694, 536 and 471 cm−1. b Terrace brown-red sediment below dung crust showing identical spectrum to the regional sediment. c Upper dung crust showing moderate amount of organic matter, as evident form the absorbance bands at 2922 and 2852 cm−1, higher band at 1641 cm−1 and a broad band in the range between 1700 and 1300 cm−1. The absorbance band at 1383 cm−1 is associated with sodium nitrate. The presence of phytolith, originating from the dung is shown by absorbance bands characteristic to opal at 1094 cm−1 and the fact that the silicate doublet at 797 and 778 cm−1 is less defined, probably as a result of the opal broad band at 800 cm−1. d Dung lump showing very high concentrations of organic matter (evident by high absorbance bands at 2922 and 2852 cm−1 and in the range between 1700 and 1300 cm−1) and clear presence of opal at 1093 cm−1 and possibly also hydrated calcium oxalate (whewellite—CaC2O4·H2O) evident by the absorbance band at 781 cm−1
The results for organic matter content and phytolith concentration are presented in Table 1 and Fig. 4. The regional sediment showed a range of 14–17 weight percentage of organic matter (henceforth wt%OM), low phytolith concentration in the range of 0.39–0.57 million phytoliths per 1 g of sediment (henceforth M phyt/g) and no dung spherulites were observed. The terrace sediments from well below the dung surface (n = 5) exhibited characteristics and values similar to the regional sediments with a range of 9–15 wt%OM and 0.19–0.70 M phyt/g, and no dung spherulites observed. Sediment samples were also collected just below (in contact with) the dung crust. This group of samples (n = 7, excluding sample B_2 composed of red sediment aggregates that was too little for phytolith analysis) yielded a range of 7–12 wt%OM, 0.14–0.64 M phyt/g; again, none of the samples had dung spherulites. The last group of samples (n = 12) was collected from the surface of the terrace floor, representing the thin millimetric layer of dung crust (Fig. 2c). This set of samples had the highest concentrations of organic matter with 10–28 wt%OM. However, the phytolith concentrations were very variable with three of the samples having very low concentrations (< 0.3 M phyt/g) and five samples having between 0.5 and 1 M phyt/g. Two samples, on the other hand, had relatively high concentration of phytoliths with 1.58 and 2.08 M phyt/g and one sample reaching 13.63 M phyt/g. The crust sample identified as a dung lump had 70 wt%OM, typical of fresh dung (Shahack-Gross 2011), and very high phytolith concentration of 69 M phyt/g. It is also the only sample where dung spherulites were identified, with a concentration of 1.6 M per 1 g of sediment (Table 1, Fig. 4).
Representative samples from locality B (n = 15) were analysed by ICP-AES for elemental composition. In addition, the two regional sediment control samples were measured with one of them (B_20) in duplicate to test the precision of the analysis. All the results are presented in Table 2. For comparison between the different sample groups we used elements associated with dung such as: phosphorus (P), potassium (K), strontium (Sr) and calcium (Ca) (Lancelotti and Madella 2012). Overall, the results showed that while the regional sediment presented the lowest concentrations of these elements, the samples from the crust on the top of the terrace floor surface presented significant enrichment of these elements. The samples collected just below the crust showed higher values than the ones lower down and these latter had higher concentrations than the regional sediment (Fig. 5). Among the crust samples one sample (B_12) showed relatively low concentrations of the indicative elements suggesting advanced decay of the dung crust. Within the group of samples collected just below the dung crust, one sample (B_7), presented high concentrations similar to the dung crust samples. This may hint to the preservation of a previous dung layer just below the sampled one.
Micromorphological analysis described the terrace sediment to be composed of a clay-rich groundmass with a speckled b-fabric. The coarse fraction presented silt to coarse sand-sized grains of quartz at 10% abundance, alongside other minerals such as hornblende, biotite, and plagioclase, all with abundance of less than 5%. The sediment showed a porphyric c/f related distribution and a complex microstructure with moderate degrees of bioturbation. Micromorphological analysis of thin sections made from the terrace floor divided the samples into two types of micro-facies (Fig. 6). The lower facies, ca. 2 mm below surface, did not exhibit high abundance of anthropogenic residues, besides scarce charcoal fragments. The upper facies ranging between 0.5- and 2-mm thick, is characterised by compaction and elevated concentration of humified organic matter which decreased with depth. The compaction and the presence of sub-horizontal cracks are both indicative features for trampling (Gé et al. 1993; Rentzel et al. 2017). While the lower part of the upper facies showed a diffuse contact with the lower facies, the top of the upper facies presented a very thin layer, ranging between 0.1- and 0.5-mm thick, of humified laminated organic matter with localised yet intensive disaggregation. This thin layer can be associated with the dung crust at the top of the terrace surface. Among the three block sediment samples collected for micromorphological analysis, only two presented an upper organic-rich facies while the third sample showed only the regional sediment fabric.
Micromorphological analysis of the terrace floor. a Microphotograph of the terrace floor presenting the lower facies (1), showing characteristics of natural sediment, and the upper facies (2), showing elevated concentrations of humified organics, sub-horizontal cracks due to trampling and diffuse contact with the lower facies. The top of the upper facies represents the dung crust with compact humified organic (arrow) and localised intensive disintegrations. The rectangular shows the area photographed (c). Photograph taken in plane-polarised light (PPL). b Same photograph taken in crossed-polarised light (XPL). c Zoom in on the upper facies and the dung crust. Note the disintegration of the crust and the diffuse contact between facies 1 and 2. Photograph taken in PPL; d same photograph taken in XPL
Discussion
Deposition pattern
In our case study, the spreading of dung is carried out unevenly and the slurry of dung and water forms a very thin (0.1–0.5-mm thick) and discontinuous crust. This heterogeneous depositional pattern is reflected in the diversity of dung signal highlighted by the analysed samples. Our results show that, although the terrace was plastered regularly (in our case about 10 days before sampling), the signal for the presence of dung is evident only within the first millimetre of deposit, directly related to the dung plaster. The samples from the outermost surface, which are related to the dung crust, floor have a variable composition in respect to the dung component. Some showed moderate concentrations in phytoliths and organic matter while in other these components were non-relevant. At the same time, all the samples but one showed higher concentrations of P, K, Sr and Ca in comparison to the sediments below the crust (the forest soil). The micromorphological analysis was able to identify a thin layer rich in humified organics with signs of trampling in the uppermost part of the terrace floor samples. However, this feature cannot be used as an unequivocal evidence for dung-plastered floors since other human activities may result in a similar signal. One of our samples (B_7), originating from below the dung crust, showed a relatively high concentration of P, K, Sr and Ca, which suggests the preservation of a previous layer of plaster. While overall the majority of surface samples exhibited a moderate to low dung signal, the dung lump left on the terrace surface during the hasty plastering of the floor had very high dung markers (e.g. sample B_5). Therefore, the archaeological deposition of such dung lumps has high potential for preserving clear dung signals. It is also possible that the combination of a very humid and hot climate and the practice of mixing with water to produce a slurry facilitate the quick dissolution of dung spherulites.
The type of floor and practices we report here differ from the plastering practices described by Boivin (2000) and Berna (2017), where the dung plaster mixture is carefully prepared and several plaster layers could be identified in a sequence. Boivin (2000) observed the seasonal floor plastering indoors (therefore less exposed to the elements) as part of a carefully performed ritual. Berna (2017), who worked on floor plaster inside a church, noticed that in areas where trampling was more frequent the top dung plaster was abraded in a few years. The areas that were less exposed to trampling exhibited a millimetre-thick layer of amorphous organic matter with grass stems in which the author identified cellulose and phytoliths. However, Berna (2017) examined a recent context where diagenesis was not an issue, and it is reasonable to assume that with time the volume of this plaster layer will reduce significantly (Shahack-Gross et al. 2005). In addition, the plastering activities observed by Boivin (2000) and Berna (2017) used more refined plasters, where dung was mixed with water and clay to produce a much finer and plastic paste than the one observed in our case study.
It should be noted that signal intensity for archaeological dung would be also affected by the original compositional characteristic of the dung used for plastering. The presence of phytoliths and spherulites in dung can vary depending on the animals’ diet, environmental conditions, seasonality and even the animal age and sex (Canti 1999; Lancelotti and Madella 2012). In our case study, we suggest that the animal diet is based mostly on plants with a low phytolith production (such as the tree and shrubs of a tropical forest), which in turn can explains the generally low phytoliths concentrations even in the samples composed mostly of dung when compared to previous studies in other parts of the world (Lancelotti and Madella 2012; Gur-Arieh et al. 2013; Portillo et al. 2017). Future studies should investigate the effect of animal diet in humid tropical forests on the quantity and quality of phytoliths and spherulites.
Post depositional and taphonomic processes
The post depositional dynamics affecting the preservation of dung-plastered surfaces can be related to a set of processes: (1) anthropogenic erosive processes (the removal of material due to human activity during the life of the floor or after abandonment), (2) natural erosive processes, e.g. (rain and water flow); (3) the chemistry of the depositional environment and (4) bioturbation. In our ethnographic setting, trampling and sweeping were the main anthropogenic mechanical activities that removed dung material from the surface during the floor lifetime. Repetitive trampling, either by people or by the livestock, resulted in the deterioration of the thin dung crust (see also Berna 2017). Daily sweeping contributed to the removal from the terrace surface of the plaster debris produced by trampling (see also Friesem and Lavi 2017 for the effect of sweeping on the preservation of microscopic floor deposits among foragers in South India and Milek 2012 among farmers in Iceland). Our observations indicate that significant deterioration of the dung plaster can develop in less than a month from the new plastering episode, although this process is uneven and we never witnessed a complete abrasion of dung remains from the entire terrace surface.
Besides the people’s use and maintenance practices, natural erosion, the chemistry of the depositional environment and bioturbation all have a significant influence on the preservation of the dung component and our ability to archaeologically identify dung plaster. Shahack-Gross (2011) reviewed the general taphonomic processes affecting dung materials and divided dung remains found in archaeological contexts into organic-rich and organic-poor remains. The organic-rich remains may retain the original volume/dimensions, but will only preserve in environmental conditions with low bacterial or fungal activity such as in desiccated, waterlogged or permafrost environments (Shahack-Gross 2011). In other cases, the organic matter would decompose immediately after the deposition and, unless it is buried rapidly in an anaerobic environment, it will decompose completely. The decomposition of the organic matter will release acids that eventually dissolve the calcitic components of the dung, such as dung spherulites and calcium oxalates (Shahack-Gross 2011). Dung spherulites are especially soluble due to high surface to bulk ratio and dissolution can already start at pH 7 (Brochier et al. 1992; Canti 1999; Shahack-Gross 2011; Gur-Arieh et al. 2014). The decomposition of the organic matter would also result in volume reduction and the formation of authigenic minerals (minerals that form in situ) such as calcium phosphates, gypsum and sylvite (see also Macphail et al. 2004; Milek 2012; Friesem et al. 2014). In neutral and alkaline conditions, phosphate minerals are relatively stable and they can be used as an indicator of decomposed dung (Shahack-Gross 2011). In the case of dung burning, the organic matter is consumed resulting in a better preservation of the calcitic micro-remains in the archaeological record.
In the humid tropical environment of the Western Ghats forests, where the soil pH can be lower than 7, carbonates dissolve rapidly (Friesem et al. 2016, 2017). Thus, the calcitic micro-remains such as spherulites, calcium-phosphate nodules and calcium oxalates tend to rapidly disappear. Phytoliths on the other hand preserve better in acidic conditions as long as the pH is above 3 (Cabanes et al. 2011). While the acidic conditions are favourable, the high monsoon precipitation and temperatures may facilitate partial dissolution and runoff water act as a mechanical taphonomic agent, washing away the phytoliths from the terrace surfaces. High humidity and temperatures in the depositional environment may also accelerate the leaching of soluble salts and elemental markers such as P, K and Sr. Friesem et al. (2016, 2017), who examined both contemporary and abandoned sites of foragers in the same environmental settings, made evident that the enrichment of P, K, Sr, Mg, Na, Zn and Ba detected in the contemporary (lived in) sites was significantly reduced after 30 years of abandonment.
Markers for dung plaster floor surfaces in humid tropical environment
To date, very few studies investigated markers of dung use for plastering floors, especially in outdoor contexts. Our pilot geo-ethnoarchaeological study highlights the challenges and potentials for the archaeological identification of dung used for plastering outdoor surfaces in comparison to other contexts (Table 3). Considering that most studies to date analysed dung remains in temperate, arid and semi-arid environments in which grasses and crop by-products are often available, it was a worthwhile exercise to examine the potential of preservation of dung markers in outdoor floors from humid tropical environments. The results from the current study show that contexts in such environments seem to have a diminished signal in respect to the generally accepted dung markers. Under the environmental conditions of our sites (and by extension in an archaeological context in the same environment) we would not expect to find calcitic micro-remains such as dung spherulites, calcium-phosphate nodules or other soluble elements, which quickly dissolve under humid tropical acidic conditions. In such environmental settings, less-soluble elements like P and Sr and the concentration of phytoliths is probably a more reliable marker for dung, even when the input of phytoliths in the dung is lowered by the animal diet being characterised by non-grass plants with low phytolith production. From a morphological perspective, micromorphological analysis supplied evidence for the built-up of a crust composed of humified organics with signs of trampling. However, the quick decay of the crust observed in the contemporary context pose serious doubts about the long-term preservation of this morphological characteristic.
The high variability of dung markers in the floor samples suggests that the plastering of outdoor surfaces may be detected only in localised palimpsests. For example, only two samples (A_5 and A_6) among the dung crust samples yielded significantly higher concentrations of phytoliths compared to the regional sediment samples (Table 1, Fig. 4). All the other samples, did not differ significantly in their phytolith concentrations and organic matter content from the sediment below the crust and from the regional sediment (Table 1, Fig. 4). Furthermore, none of the crust samples had spherulites. The elemental analysis, however, can help in separating between the dung crust samples (n = 7) and the regional control samples, and the sediment below the crust a (Table 2, Fig. 5). Micromorphological analysis showed that the crust is characterised by a very thin 0.1–0.5-mm-thick layer rich in humified organic that correlate to the elevated distinctive elements. Our current results demonstrated that the only samples with full-spectrum dung markers are the dung lumps resulting from the uneven plastering practice. These dung lumps form a micro-environment in which dung markers seem to have the possibility to preserve. The structure of the lump clearly protects the components from the major agents of chemical (pH levels) and mechanical (trampling and runoff water) weathering. Finally, it is important to note, that while the concentration of phytoliths in the dung lumps in our study are significantly higher than in other samples, they are lower than samples from different environments where animal are fed mainly on grasses and crops by-products (Lancelotti and Madella 2012; Gur-Arieh et al. 2013).
Conclusions
Although dung was, and in some cases still is, a major source of construction material, the use of dung in antiquity has not been properly approached in the archaeological discourse. This might be due to the ‘invisibility’ of such resource in the archaeological record. Whether this invisibility is due to the level of use of this material in the past, a low preservation in the archaeological record or the difficulty to identify such material (and the originating human practices) in the recovered contexts, we believe that a more comprehensive approach is required. Most of the studies carried out so far to understand the use of dung in archaeological contexts were in arid or semi-arid environments; therefore, more work is needed to explore the wide range of environments in which dung was used and deposited to advance our understanding of the range and intensity of taphonomic processes involving dung.
In the current study, we tested the usefulness of different established proxies for the identification of dung-plastered outdoor floor surfaces in ethnoarchaeological contexts of humid tropical environments. We have described the domestic and mundane practices of floor plastering, which involve the spreading of a dung slurry resulting in an uneven and rather fragile thin surface. Post depositional processes on this surface include mechanical decay of the plaster by trampling and sweeping as well as natural diagenesis processes, which are intensified by the humid tropical settings and characterised by dissolution of carbonates and leaching out of chemical elements.
Our case demonstrates that identification of outdoor dung-plastered floor in humid tropical environment, is highly challenging. Archaeologically, systematic spatial sampling could potentially detect palimpsest of localised preservation of concentrated dung (e.g. dung lumps), although their unambiguous attribution to plastering activities may be impossible. We suggest that the best way to approach an understanding of the different archaeological deposits involving dung is by a combined appraisal: (1) the microscopic structure and depositional patterns of the context (using micromorphology), (2) the intensity of dung markers (by studying phytoliths and elemental composition), and (3) the spatial distribution of such markers (e.g. Lancelotti et al. 2017). In cases where the local flora and animal diet are well documented, phytolith taxonomic analysis may also contribute to the identification of dung deposits. In addition, more proxies for dung identification should be developed. One such possibility would be the use of gas chromatography mass spectrometry (GC-MS) for identifying faecal lipids components such as stanols and sterols (Evershed et al. 1997; Sistiaga et al. 2014) and δ15N stable isotope enrichment (e.g. Shahack-Gross et al. 2008).
References
Akeret Ö, Rentzel P (2001) Micromorphology and plant macrofossil analysis of cattle dung from the Neolithic lake shore settlement of Arbon Bleiche 3. Geoarchaeology 16:687–700
Albert RM, Shahack-Gross R, Cabanes D, Gilboa A, Lev-Yadun S, Portillo M, Sharon I, Boaretto E, Weiner S (2008) Phytolith-rich layers from the late Bronze and Iron ages at Tel Dor (Israel): mode of formation and archaeological significance. J Archaeol Sci 35:57–75
Anderson S, Ertug-Yaras F (1998) Fuel fodder and faeces: an ethnographic and botanical study of dung fuel use in Central Anatolia. Environ Archaeol 1:99–109
Berna F (2017) Geo-ethnoarchaeology study of the traditional Tswana dung floor from the Moffat mission church, Kuruman, north Cape Province, South Africa. Archaeol Anthropol Sci 1–9
Bogaard A, Fraser R, Heaton THE, Wallace M, Vaiglova P, Charles M, Jones G, Evershed RP, Styring AK, Andersen NH, Arbogast RM, Bartosiewicz L, Gardeisen A, Kanstrup M, Maier U, Marinova E, Ninov L, Schäfer M, Stephan E (2013) Crop manuring and intensive land management by Europe’s first farmers. Proc Natl Acad Sci 110:12589–12594
Boivin N (2000) Life rhythms and floor sequences: excavating time in rural Rajasthan and Neolithic Catalhoyuk. World Archaeol 31:367–388
Brochier JE (1983) Combustion et parcage des herbivores domestiques. Le point de vue du sedimentologue. Bull Soc Prehistorique Fr 80:143–145
Brochier JE, Villa P, Giacomarra M (1992) Shepherds and sediments: geo-ethnoarchaeology of pastoral sites. J Anthropol Archaeol 11:47–102
Cabanes D, Weiner S, Shahack-Gross R (2011) Stability of phytoliths in the archaeological record: a dissolution study of modern and fossil phytoliths. J Archaeol Sci 38:2480–2490
Canti MG (1997) An investigation of microscopic calcareous spherulites from herbivore dungs. J Archaeol Sci 24:219–231
Canti MG (1999) The production and preservation of faecal spherulites: animals, environment and taphonomy. J Archaeol Sci 26:251–258
Canti MG (2017) Faecal spherulites. In: Nicosia C, Stoops G (eds) Archaeological soil and sediment micromorphology. Wiley, Hoboken, pp 51–54
Doumani PN, Frachetti MD, Beardmore R, Schmaus TM, Spengler RN III, Mar'yashev AN (2015) Burial ritual, agriculture, and craft production among Bronze Age pastoralists at Tasbas (Kazakhstan). Archaeol Res Asia 1–2:17–32
Égüez N, Zerboni A, Biagetti S (in press) Microstratigraphic analysis on a modern central Saharan pastoral campsite. ovicaprine pellets and stabling floors as ethnographic and archaeological referential data. Quat Int
Evershed RP, Bethell PH, Reynolds PJ, Walsh NJ (1997) 5β-Stigmastanol and related 5β-stanols as biomarkers of manuring: analysis of modern experimental material and assessment of the archaeological potential. J Archaeol Sci 24:485–495
Friesem DE (2016) Geo-Ethnoarchaeology in action. J Archaeol Sci 70:145–157
Friesem DE, Lavi N (2017) Foragers, tropical forests and the formation of archaeological evidences: an ethnoarchaeological view from South India. Quat Int 448:117–128
Friesem DE, Tsartsidou G, Karkanas P, Shahack-Gross R (2014) Where are the roofs? A geo-ethnoarchaeological study of mud brick structures and their collapse processes, focusing on the identification of roofs. Archaeol Anthropol Sci 6:73–92
Friesem DE, Lavi N, Madella M, Ajithprasad P, French C (2016) Site formation processes and hunter-gatherers use of space in a tropical environment: a geo-ethnoarchaeological approach from South India. PLoS One 11:e0164185
Friesem DE, Lavi N, Madella M, Boaretto E, Ajithprasad P, French C (2017) The formation of fire residues associated with hunter-gatherers in humid tropical environments: a geo-ethnoarchaeological perspective. Quat Sci Rev 171:85–99
Gé T, Courty MA, Matthews W, Wattez J (1993) Sedimentary formation processes of occupation surfaces. In: Goldberg P, Nash DT, Petraglia MD (eds) Formation processes in archaeological context. Prehistory Press, Madison, pp 149–163
Goodman-Elgar M (2008) The devolution of mudbrick: ethnoarchaeology of abandoned earthen dwellings in the Bolivian Andes. J Archaeol Sci 35:3057–3071
Gur-Arieh S, Mintz E, Boaretto E, Shahack-Gross R (2013) An ethnoarchaeological study of cooking installations in rural Uzbekistan: development of a new method for identification of fuel sources. J Archaeol Sci 40:4331–4347
Gur-Arieh S, Shahack-Gross R, Maeir AM, Lehmann G, Hitchcock LA, Boaretto E (2014) The taphonomy and preservation of wood and dung ashes found in archaeological cooking installations: case studies from Iron Age Israel. J Archaeol Sci 46:50–67
Hockings P (2012) Encyclopaedia of the Nilgiri Hills. Manohar, New Delhi
Jayakumar R, Nair KKN (2013) Species diversity and tree regeneration patterns in tropical forests of the western Ghats, India. ISRN Ecology 2013:1–14. https://doi.org/10.1155/2013/890862
Kadowaki S, Maher L, Portillo M, Albert RM, Akashi C, Guliyev F, Nishiaki Y (2015) Geoarchaeological and palaeobotanical evidence for prehistoric cereal storage in the southern Caucasus: the Neolithic settlement of Göytepe (mid 8th millennium BP). J Archaeol Sci 53:408–425
Karkanas P (2006) Late Neolithic household activities in marginal areas: the micromorphological evidence from the Kouveleiki caves, Peloponnese, Greece. J Archaeol Sci 33:1628–1641
Katz O, Cabanes D, Weiner S, Maeir AM, Boaretto E, Shahack-Gross R (2010) Rapid phytolith extraction for analysis of phytolith concentrations and assemblages during an excavation: an application at tell Es-Safi/Gath, Israel. J Archaeol Sci 37:1557–1563
Kramer C (1982) Village ethnoarchaeology: rural Iran in archaeological perspective. Academic Press, New York
Lancelotti C, Madella M (2012) The ‘invisible’ product: developing markers for identifying dung in archaeological contexts. J Archaeol Sci 39:953–963
Lancelotti C, Balbo AL, Madella M, Iriarte E, Rojo-Guerra M, Royo JI, Tejedor C, Garrido R, García I, Arcusa H, Pérez Jordà G, Peña-Chocarro L (2014) The missing crop: investigating the use of grasses at Els Trocs, a Neolithic cave site in the Pyrenees (1564 m asl). J Archaeol Sci 42:456–466
Lancelotti C, Ruiz-Pérez J, García-Granero JJ (2017) Investigating fuel and fireplaces with a combination of phytoliths and multi-element analysis; an ethnographic experiment. Veg Hist Archaeobotany 26:75–83
Lanzhe (2013) Yak Dung, Through Their Eyes, Rural Culture Research Centre Nyanpo Yuzee Environmental Protection Association. https://youtu.be/ZfpTHOhExGI. Accessed: 10.2.2017
Love S (2012) The geoarchaeology of mudbricks in architecture: a methodological study from Çatalhöyük, Turkey. Geoarchaeology 27:140–156
Macphail RI, Cruise GM, Allen MJ, Linderholm J, Reynolds P (2004) Archaeological soil and pollen analysis of experimental floor deposits; with special reference to Butser ancient farm, Hampshire, UK. J Archaeol Sci 31:175–191
Macphail R I, Crowther J, Cruise G M (2007) Microstratigraphy: soil micromorphology, chemistry and pollen. In: D Bowsher, T Dyson, N Holder, I Howell (eds) The London guildhall. An archaeological history of a neighbourhood from early medieval to modern times, volume MoLAS monograph 36: Museum of London Archaeological Service, London, pp 18, 25-6, 35, 39, 55-6, 57, 59, 76, 90, 97, 98, 134, 154–5, 428–430
Matthews W (2005) Life-cycle and life-course of buildings. In: Hodder I (ed) Catalhoyuk perspectives: themes from the 1995–9 seasons. McDonald Institute for Archaeological Research and British Institute of Archaeology at Ankara, Cambridge, pp 125–151
Matthews W (2010) Geoarchaeology and taphonomy of plant remains and microarchaeological residues in early urban environments in the ancient near east. Quat Int 214:98–113
Mbae NB (1990) The ethnoarchaeology of Maasai settlements and refuse disposal patterns in the Lemek area. In: Robertshaw P (ed) Early pastoralists of south-western Kenya, British Institute in Eastern Africa Memoir. British Institute in Eastern Africa, Nairobi, pp 279–292
Mccann JC (1997) The plow and the forest: narratives of deforestation in Ethiopia, 1840-1992. Environ Hist 2:138–159
Milek KB (2012) Floor formation processes and the interpretation of site activity areas: an ethnoarchaeological study of turf buildings at Thverá, Northeast Iceland. J Anthropol Archaeol 31:119–137
Miller NF (1984a) The use of dung as fuel: an ethnographic example and an archaeological application. Paleorient 10:71–79
Miller NF (1984b) The interpretation of some carbonized cereal remains as remnants of dung cake fuel. Bull Sumer Agric 1:45–47
Miller NF (1996) Seed eaters of the ancient near east: human or herbivore? Curr Anthropol 37:521–528
Piperno DR (2006) Phytoliths: a comprehensive guide for archaeologists and paleoecologists. AltaMira Press, Lanham
Portillo M, Albert RM (2011) Husbandry practices and livestock dung at the Numidian site of Althiburos (el Médéina, kef governorate, northern Tunisia): the phytolith and spherulite evidence. J Archaeol Sci 38:3224–3233
Portillo M, Albert RM, Henry DO (2009) Domestic activities and spatial distribution in Ain Abū Nukhayla (Wadi rum, southern Jordan): the use of phytoliths and spherulites studies. Quat Int 193:174–183
Portillo M, Valenzuela S, Albert RM (2012) Domestic patterns in the Numidian site of Althiburos (northern Tunisia): the results from a combined study of animal bones, dung and plant remains. Quat Int 275:84–96
Portillo M, Kadowaki S, Nishiaki Y, Albert RM (2014) Early Neolithic household behavior at tell Seker al-Aheimar (upper Khabur, Syria): a comparison to ethnoarchaeological study of phytoliths and dung spherulites. J Archaeol Sci 42:107–118
Portillo M, Belarte MC, Ramon J, Kallala N, Sanmartí J, Albert RM (2017) An ethnoarchaeological study of livestock dung fuels from cooking installations in northern Tunisia. Quat Int 431(Part A):131–144
Rasmussen P (1993) Analysis of goat/sheep Faeces from Egolzwil 3, Switzerland: evidence for branch and twig foddering of livestock in the Neolithic. J Archaeol Sci 20:479–502
Reddy SN (1998) Fueling the hearths in India: the role of dung in paleoethnobotanical interpretation. Paléorient 24:61–69
Rentzel P, Nicosia C, Gebhardt A, Brönnimann D, Pümpin C, Ismail-Meyer K (2017) Trampling, poaching and the effect of traffic. In: Nicosia C, Stoops G (eds) Archaeological soil and sediment micromorphology. Wiley, Hoboken, pp 281–297
Rondelli B, Lancelotti C, Madella M, Pecci A, Balbo A, Pérez JR, Inserra F, Gadekar C, Ontiveros MÁC, Ajithprasad P (2014) Anthropic activity markers and spatial variability: an ethnoarchaeological experiment in a domestic unit of Northern Gujarat (India). J Archaeol Sci 41:482–492
Rosen SA, Savinetsky AB, Plakht Y, Kisseleva NK, Khassanov BF, Pereladov AM, Haiman M (2005) Dung in the desert: preliminary results of the Negev Holocene ecology project. Curr Anthropol 46:317–326
Sahoo AK, Krishnamurthi R, Vadlamani R, Pruseth KL, Narayanan M, Varghese S, Pradeepkumar T (2016) Genetic aspects of gold mineralization in the southern granulite terrain, India. Ore Geol Rev 72:1243–1262
Shahack-Gross R (2011) Herbivorous livestock dung: formation, taphonomy, methods for identification, and archaeological significance. J Archaeol Sci 38:205–218
Shahack-Gross R (2017) Animal gathering enclosures. In: Nicosia C, Stoops G (eds) Archaeological soil and sediment micromorphology. Wiley, Hoboken, NJ, p 265–280
Shahack-Gross R, Marshall F, Weiner S (2003) Geo-ethnoarchaeology of pastoral sites: the identification of livestock enclosures in abandoned maasai settlements. J Archaeol Sci 30:439–459
Shahack-Gross R, Marshall F, Ryan K, Weiner S (2004) Reconstruction of spatial organization in abandoned Maasai settlements: implications for site structure in the pastoral Neolithic of East Africa. J Archaeol Sci 31:1395–1411
Shahack-Gross R, Albert RM, Gilboa A, Nagar-Hilman O, Sharon I, Weiner S (2005) Geoarchaeology in an urban context: the uses of space in a Phoenician monumental building at Tel dor (Israel). J Archaeol Sci 32:1417–1431
Shahack-Gross R, Simons A, Ambrose SH (2008) Identification of pastoral sites using stable nitrogen and carbon isotopes from bulk sediment samples: a case study in modern and archaeological pastoral settlements in Kenya. J Archaeol Sci 35:983–990
Simpson IA, Vésteinsson O, Adderley WP, McGovern TH (2003) Fuel resource utilisation in landscapes of settlement. J Archaeol Sci 30:1401–1420
Sistiaga A, Berna F, Laursen R, Goldberg P (2014) Steroidal biomarker analysis of a 14,000 years old putative human coprolite from paisley cave, Oregon. J Archaeol Sci 41:813–817
Smith D, Nayyar K, Schreve D, Thomas R, Whitehouse N (2014) Can dung beetles from the palaeoecological and archaeological record indicate herd concentration and the identity of herbivores? Quat Int 341:119–130
Spengler RN III, Willcox G (2013) Archaeobotanical results from Sarazm, Tajikistan, an early bronze Age Village on the edge: agriculture and exchange. J Environ Archaeol 10:211–221
Spengler RN III, Frachetti MD, Fritz GJ (2013) Ecotopes and herd foraging practices in the steppe/mountain Ecotone of Central Asia during the Bronze and Iron Ages. J Ethnobiol 33:125–147
Spengler RN III, Cerasetti B, Tengberg M, Cattani M, Rouse LM (2014a) Agriculturalists and pastoralists: Bronze Age economy of the Murghab alluvial fan, southern Central Asia. Veg Hist Archaeobotany 23:805–820
Spengler RN III, Frachetti MD, Doumani PN (2014b) Late Bronze Age agriculture at Tasbas in the Dzhungar Mountains of eastern Kazakhstan. Quat Int 348:147–157
Spengler III RN, de Nigris I, Cerasetti B, Carra M, Rouse LM, (2016) The breadth of dietary economy in Bronze Age Central Asia: case study from Adji Kui 1 in the Murghab region of Turkmenistan. J Archaeol Sci Rep
Stiner MC, Buitenhuis H, Duru G, Kuhn SL, Mentzer SM, Munro ND, Pöllath N, Quade J, Tsartsidou G, Özbaşaran M (2014) A forager–herder trade-off, from broad-spectrum hunting to sheep management at Aşıklı Höyük, Turkey. Proc Natl Acad Sci 111:8404–8409
Vigne JD (2011) The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C R Biol 334:171–181
Viklund K, Linderholm J, Macphail R I (2013) Integrated palaeoenvironmental study: micro- and macrofossil analysis and geoarchaeology (soil chemistry, magnetic susceptibility and micromorphology). In: L-E Gerpe (ed) E18-prosjektet Gulli-Langåker. Oppsummering og arkeometriske analyser, Volume Bind 3: Bergen, Fagbokforlaget, pp 25–83
Watson PJ (1979) Archaeological ethnography in Western Iran. University of Arizona Press, Tucson
Weiner S (2010) Microarchaeology. Beyond the visible archaeological record. Cambridge University Press, New York
Zapata Peña L, Peña-Chocarro L, Esté JJI, Urquijo JG (2003) Ethnoarchaeology in the Moroccan Jebala (western Rif): Woodand dung as fuel. In: Neumann K, Butler A, Kahlheber S (eds) Food, fuels and fields—progress in African Archaeobotany. Heinrich -Barth - Institut, Köln, pp 163–175
Zhang J, Lu H, Wu N, Qin X, Wang L (2013) Palaeoenvironment and agriculture of ancient Loulan and Milan on the silk road. The Holocene 23:208–217
Acknowledgements
We are grateful and thankful to the people who accepted us into their home and allowed us to collect information and samples. This research could not have been possible without their invaluable collaboration and generosity. We thank P. Ajithparasad for his help with permits for fieldwork, S. Weiner and E. Boaretto for allowing us to use the FTIR of the Kimmel Center at the Weizmann Institute of Science and R. Shahack-Gross for helpful comments on an earlier draft.
Funding
Open access funding provided by Max Planck Society. This research was partly funded by the People Programme (Marie Skłodowska-Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007–2013) under REA agreement no. 623293 granted to D.E.F. at the McDonald Institute for Archaeological Research, University of Cambridge. The work of S.G.A. was supported by the Max Planck Society.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gur-Arieh, S., Madella, M., Lavi, N. et al. Potentials and limitations for the identification of outdoor dung plasters in humid tropical environment: a geo-ethnoarchaeological case study from South India. Archaeol Anthropol Sci 11, 2683–2698 (2019). https://doi.org/10.1007/s12520-018-0682-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12520-018-0682-y