Abstract
Background
Advances in cancer treatments, particularly the development of radiation therapy, have led to improvements in survival outcomes in children with brain tumors. However, radiation therapy is associated with significant long-term neurocognitive morbidity. The present systematic review and meta-analysis aimed to compare the neurocognitive outcomes of children and adolescents with brain tumors treated with photon radiation (XRT) or proton therapy (PBRT).
Methods
A systematic search was conducted (PubMed, Embase, Cochrane, and Web of Science from inception until 02/01/2022) for studies comparing the neurocognitive outcomes of children and adolescents with brain tumors treated with XRT vs. PBRT. The pooled mean differences (expressed as Z scores) were calculated using a random effects method for those endpoints analyzed by a minimum of three studies.
Results
Totally 10 studies (n = 630 patients, average age range: 1–20 years) met the inclusion criteria. Patients who had received PBRT achieved significantly higher scores (difference in Z scores ranging from 0.29–0.75, all P < 0.05 and significant in sensitivity analyses) after treatment than those who had received XRT for most analyzed neurocognitive outcomes (i.e., intelligence quotient, verbal comprehension and perceptual reasoning indices, visual motor integration, and verbal memory). No robust significant differences (P > 0.05 in main analyses or sensitivity analyses) were found for nonverbal memory, verbal working memory and working memory index, processing speed index, or focused attention.
Conclusions
Pediatric brain tumor patients who receive PBRT achieve significantly higher scores on most neurocognitive outcomes than those who receive XRT. Larger studies with long-term follow-ups are needed to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain tumors are the second leading cause of cancer in children and adolescents and the leading cause of cancer death in this population [1]. The first attempts to use X-ray therapy (roentgen therapy) were made by Percival Bailey and Harvey Cushing in the 1930s. They found that a subset of patients at autopsy had no evidence of medulloblastoma but rather radiation necrosis [2]. This led other groups to explore radiation therapy as a treatment after surgery in pediatric brain tumors [2]. Later, Paterson in the 1950s began administering craniospinal radiation to pediatric patients with medulloblastoma [3]. These achievements raised the survival outcomes of children with brain tumors but also increased the neurocognitive sequalae of these patients. Since then, evidence has shown that pediatric brain tumor survivors treated with cranial radiotherapy have a remarkable risk of neurocognitive impairment, not only in global intellectual functioning [e.g., full-scale intelligent quotient (FSIQ)] but also in specific cognitive domains such as executive function, attention, memory, processing speed, and fine motor control [4,5,6,7,8,9,10,11]. In fact, there is meta-analytical evidence confirming the neurocognitive decline associated with photon radiation (XRT) in children and adolescents with brain tumors [7].
Many strategies have been developed to decrease the neurocognitive side effects of these children, including the use of chemotherapy to reduce radiation doses in many pediatric brain tumors [12] and the reduction in the radiation boost volume [13]. However, the greatest advances have probably been made in radiotherapy techniques, which have sought to deliver intended doses to the target tumor while reducing the exposure of surrounding healthy brain tissue, with the goal of decreasing radiation-induced long-term complications.
Proton therapy (PBRT) is becoming widely used in high-income countries across the world. It was first available in the US for children at the Harvard Cyclotron after 1974. Children with central nervous system (CNS) tumors were treated in 1992 at Loma Linda University Medical Center (CA, USA) [14]. However, proton therapy started to become more available for children in 2000 (e.g., Boston 2000, MD Anderson 2006) [15]. The potential advantage of PBRT over XRT is the ability to reduce the exposure of healthy tissue around the target area, with the potential to reduce its deleterious effects on neurocognitive outcomes. However, few studies have compared the effects of XRT and PBRT on neurocognitive outcomes in pediatric patients with brain tumors. To date, these studies have been retrospective, as it is challenging to conceive a study for children with brain tumors randomized to photons or protons due to ethical concerns [16], as dosimetric studies have repeatedly shown the superiority of PBRT, and clinical studies have demonstrated benefits including lower late endocrine deficits, reduced radiation-induced neoplasia, and cardiac mortality [17,18,19]. In addition, there is a significant cost difference between XRT and PBRT, which makes these results very important. Proton therapy may be justified not only because of the already known dosimetric benefits [20] but also in neurocognitive outcomes and later quality of life.
In this context, the present systematic review and meta-analysis aimed to compare the neurocognitive outcomes of children and adolescents with brain tumors treated with XRT or PBRT.
Methods
This review is registered in PROSPERO (CRD42020204102). We followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses [21].
Data sources and search strategies
Two authors (AL, JSM) independently conducted a systematic search in the electronic databases PubMed, Web of Science, Embase and Cochrane for relevant articles written in English (from inception to February 1, 2022) using the following search strategy: (proton) AND (child* OR pediatric OR pediatric OR infant OR adolescen*) with no filters for language, article type or any other filter. An example of the search is available in Supplementary Table 1. The search was supplemented by a manual review of reference lists from included studies and review articles to find additional studies on the subject.
Study selection
Citations were first retrieved and preliminarily screened by title and abstract, and the full texts of those studies that met the inclusion criteria were assessed. Disagreements between authors were resolved through consensus or after consultation with a third reviewer (PLV). Studies were eligible for inclusion if they met the following criteria: (1) included one group of survivors of pediatric brain tumors treated with PBRT; (2) compared with a control group of survivors of pediatric brain tumors treated with XRT; and (3) assessed neurocognitive outcomes.
Data extraction
The following data were extracted from each study: number of participants in each group, characteristics of the participants, socioeconomic status, cancer and treatment characteristics, cognitive domain measures, and neurocognitive-related results. Data were extracted, when available, as the mean and standard deviation (SD) for each study group at both baseline and postintervention, although all studies provided only postintervention data. When data were provided using other measures of dispersion [e.g., 95% confidence interval (CI)], the required information was estimated following the guidelines reported elsewhere [22]. When the standard error was reported instead of the SD, the latter was obtained through the formula of Altman and Bland [23]. Endpoint data were transformed into Z scores [mean (M) = 0; standard deviation = 1] from each norm test value to homogenize them and enable comparisons between tests obtained using different types of measurement. We also contacted the authors of four studies [24,25,26,27] because the required data were not reported. The authors of three studies [24, 25, 27] provided the required information.
Outcomes
Tests used for general neurocognitive abilities measures included the intelligent quotient (IQ) (Wechsler Scales of Intelligence, Bayley Scales of Infant and Toddler Development, Stanford-Binet Intelligence Scales, Reynolds Intellectual Assessment Scales, Woodcock-Johnson Tests of Cognitive Ability, Leiter International Performance Scale, Differential Abilities Scales and Raven’s Progressive Matrices), as well as Wechsler Intelligence indices for verbal comprehension index, verbal quotient (verbal IQ), perceptual reasoning index, fluid reasoning index, working memory index, and processing speed index. Wechsler indices were merged; specifically, verbal comprehension index and verbal IQ scores were analyzed as verbal comprehension scores, and perceptual reasoning and fluid reasoning indices were analyzed as perceptual reasoning scores. For specific neurocognitive abilities, tests examined visual motor integration (Beery-Buktenica Developmental Test of Visual Motor Integration), verbal memory (California Verbal Learning Test Children’s Edition, and California Verbal Learning Test Second Edition delayed recall test, Wide Range Assessment of Memory and Learning-2, and Children’s Memory Scale delayed story memory test), nonverbal memory (A Developmental Neuropsychological Assessment—Second Edition and Wechsler Memory Scales-IV memory for designs tests), verbal working memory (Wechsler Digit Span subtest) and focused attention/information processing speed (Wechsler Coding subtest). The tests carried out in each study are specified in Table 1.
Quality assessment
Two authors (JSM, PLV) independently assessed the methodological quality of the included studies with an adapted form of the Newcastle Ottawa Scale for cross-sectional studies [28]. Studies were given a maximum score of four stars for selection, two stars for comparability, and three stars for outcome. A third author (AL) resolved any potential disagreement.
Statistical analyses
To minimize the issues found when employing a meta-analytic approach with a small number of studies, we only performed a meta-analysis when a minimum of three studies assessed a given outcome. Pooled mean differences (MD) between groups (expressed as Z scores unless otherwise specified, along with 95% CI) were computed using a random effects model (Dersimonian and Laird model) [29]. When two studies shared part of the same sample, only the study with the largest sample was included in the analyses. Begg’s test was used to determine the presence of publication bias, and I2 statistics were used to assess heterogeneity across studies. Sensitivity analyses were performed by removing one study at a time to confirm our results. Sensitivity analyses were also performed by including adjusted data or results from multivariable analyses in studies that reported both nonadjusted and adjusted results. All statistical analyses were performed using Comprehensive Meta-analysis 2.0 (Biostat; Englewood, NJ) setting the level of significance at 0.05.
Results
Study selection
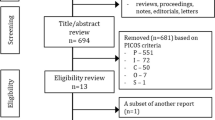
From the retrieved articles, 10 studies including 630 survivors of pediatric brain tumors (of whom 53% were treated with PBRT) were included in the systematic review (Supplementary Fig. 1) [24,25,26,27, 30,31,32,33,34,35]. The characteristics of the included studies are summarized in Table 1. Two studies [24, 25] shared part of the same sample, and thus, only the study with the largest sample was included to compute the total number of participants.
Quality assessment
The quality of the included studies was moderate overall (Table 1). Out of a maximum 10-point score, two studies had a quality score of seven [26, 32], two of six [24, 33], three of five [25, 34, 35], and the remaining studies had a quality score of four or lower [27, 30, 31].
Study characteristics
The included studies involved between 8 and 150 participants (average of 75 participants) whose average age ranged between 1 and 20 years (Table 1). All studies included both male and female participants (39% of the participants were female).
The most frequently analyzed tumor histologies were craniopharyngioma, medulloblastoma/primitive neuroectodermal tumors, ependymoma, germinoma, astrocytoma, and ependymoma, and the most frequently reported tumor location was infratentorial, followed by supratentorial. The total radiation dose ranged between 30–60 Gy and 20–59 Gy for PBRT and XRT, respectively, and the total craniospinal irradiation (CSI) dose ranged between 15 Gy and 40 Gy for both PBRT and XRT. Most studies reported no differences in major demographic/clinical variables between groups (e.g., age, sex, socioeconomic status, tumor histology or location). However, some studies did find differences in some variables, such as tumor location, histology or total radiotherapy dose to the tumor [25, 26, 31, 32, 34].
Outcomes
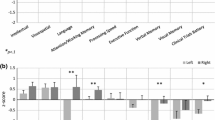
A summary of the meta-analyzed outcomes is shown in Table 2. Patients who had received PBRT achieved significantly higher Z scores (all P < 0.05) than those who had received XRT for most analyzed neurocognitive outcomes, including IQ (evaluated by means of the Full-Scale IQ and other intelligence scores), verbal comprehension, perceptual reasoning and processing speed indices, verbal working memory and working memory index, visual motor integration, focused attention, and verbal memory (Fig. 1). The only domain for which no differences were observed was nonverbal memory (P = 0.367). Forest plots for each outcome are available in Supplementary Fig. 2. No signs of heterogeneity (all I2 values < 5%) or risk of bias (all Begg’s P values > 0.10) were found for any of the analyzed outcomes.
Some studies reported not only nonadjusted data but also adjusted data/multivariable analyses for some outcomes (including different variables in the model such as tumor location, interval between radiotherapy and evaluation, total radiation dose, or CSI dose). Sensitivity analyses were conducted when possible, using adjusted data, and all results remained essentially unchanged except for processing speed index (P = 0.089) and focused attention (P = 0.163), which became nonsignificant (Supplementary Table 2). Sensitivity analyses by removing one study at a time also confirmed significant differences for most outcomes except for verbal working memory and working memory index, processing speed index and focused attention, which suggests that these results were mostly driven by some individual studies. Despite significant benefits on verbal working memory and working memory index on the overall analysis (P = 0.016 and P = 0.012, respectively), the results became nonsignificant when removing the study by Kahalley et al. [24], although with a trend toward significance (P = 0.072 and P = 0.057, respectively). It should be noted that scores extracted from the meta-analyzed papers for these outcomes are heterogeneous, as they include scores from auditory and visual sensory modalities, and this issue may affect the variance of these variables. Sensitivity analysis also showed no consistent benefits for the processing speed index, which despite being significant in the main analysis (P = 0.046), became nonsignificant when removing almost every single study (e.g., Kahalley et al. [24], Peterson et al. [30], Yip et al. [27], Yang et al. [31], Eaton et al. [33], or Weusthof et al. [35]), except for Gross et al. [34] (P = 0.028), and Child et al. [32] (P = 0.03). Similarly, focused attention also became nonsignificant when removing the studies of Gross et al. [34], Yip et al. [27], Eaton et al. [33], or Child et al. [32].
Other neurocognitive-related outcomes could not be included in the meta-analyses, as they were assessed by two or fewer studies. These outcomes included the general ability index (Weschler Intelligence Scales), fine motor function (Pegboard Groove), visuoconstructive praxis and memory (Rey-Osterrieth Complex Figure Test), categorical and lexical word fluency (Regensburger Word Fluency Test), executive function (Behavior Rating Inventory of Executive Function and Preschool-Version, Delis-Kaplan Executive Function System and Behavior Assessment System for Children Second and Third Edition), attention [Continuous Performance Test (CPT) Second Edition-II, Behavior Assessment System for Children Second and Third Edition and Bayley Scales of Infant and Toddler Development Third Edition], academic skills (Wechsler Individual Achievement Test, Woodcock-Johnson Tests of Achievement Third and Fourth Edition, and Woodcock-Johnson Tests of Cognitive Abilities), social cognition (Adaptive Behavior Assessment System Second Edition) and adaptative behavior (Adaptive Behavior Assessment System Second Edition). For these outcomes, Gross et al. reported that compared with XRT, PBRT was associated with a higher general ability index with no differences reported for the other outcomes [34].
Discussion
The main finding of the present systematic review and meta-analysis, which included 10 studies and more than 600 participants, was that patients who received PBRT seem to demonstrate significantly higher scores than those who received XRT on a varied number of neurocognitive outcomes (i.e., full-scale IQ, verbal comprehension, perceptual reasoning and processing speed indices, verbal working memory and working memory index, visual motor integration, verbal memory and focused attention). Sensitivity analyses confirmed significant differences for full-scale IQ, verbal comprehension and perceptual reasoning indices, visual motor integration, and verbal memory.
Numerous variables can affect neurocognitive function in children and adolescents with brain tumors, notably age [36, 37], surgery [35], hydrocephalus [38, 39], chemotherapy [40] or postoperative cerebellar mutism syndrome [41], among others. However, it is well documented that radiation, especially craniospinal radiation, confers the greatest neurocognitive risk [9, 25]. Additionally, regarding radiation, there are many factors that affect the neurocognitive development of these patients, such as radiation field, focal/CSI, boost volume or CSI dose. In this regard, although modern XRT techniques that enable tighter conformality of the administered dose around targets [e.g., intensity-modulated radiotherapy (RT), tomotherapy, etc.] seem to have improved intellectual benefits [42,43,44], these benefits do not seem to yet be as significant as those with PBRT [45]. All patients in this meta-analysis who received photon therapy were treated after 2000 with modern techniques.
In line with our findings, a recent systematic review on cognitive changes following PBRT or XRT in pediatric brain tumor patients found significantly poorer cognitive outcomes—particularly worse general cognition and working memory—among patients treated with XRT compared with PBRT [45]. Craniospinal irradiation was consistently associated with poorer cognitive outcomes, while focal therapy was associated with minor cognitive changes [45]. However, to the best of our knowledge, this is the first meta-analysis that quantitatively compares the neurocognitive outcomes of pediatric survivors of brain tumors after treatment with XRT or PBRT.
The most homogeneous study included in the present meta-analysis evaluated patients with medulloblastoma treated contemporaneously on comparable treatment protocols that differed only in RT modality (PBRT or XRT) [24]. This study revealed significantly different scores between the PBRT and XRT groups in global IQ, perceptual reasoning and working memory indices favoring the PBRT group [24]. At four years after RT, patients treated with PBRT exhibited overall stable performance over time in all neurocognitive domains except for the processing speed index. In contrast, patients treated with XRT exhibited a significant decline in global IQ, working memory and processing speed scores [24]. Even in the context of CSI, patients treated with PBRT showed stable intellectual outcomes in most domains and experienced significantly better long-term outcomes in global IQ, perceptual reasoning and working memory indices compared with patients treated with XRT [24]. These findings were also confirmed by Eaton et al. in very homogeneous standard-risk medulloblastoma patients matched 1:1 based on demographic and clinical characteristics [33]. Patients treated with PBRT demonstrated higher scores for intelligence after treatment than their counterparts treated with XRT, with the former scoring about 1.5 SD (between 22 and 23 points) higher than the XRT group for FSIQ, verbal and nonverbal outcomes.
The remaining studies included in the present meta-analysis involved different pediatric brain tumors. Kahalley et al. [25] compared the IQ scores of 150 patients (90 receiving PBRT) with different tumor histologies. In the PBRT group, no change in IQ over time was identified, whereas in the XRT group, IQ declined by 1.1 points per year. IQ was lower in the XRT group (by 8.7 points) than in the PBRT group. Among the 82 patients treated with CSI, FSIQ was 12.5 points lower in the XRT group than in the PBRT group, and although IQ remained stable over time among PBRT patients, IQ decreased, on average, by 1.57 points per year in the XRT patients. Gross et al. [34] compared neuropsychological outcomes of different brain tumor histologies in 125 patients who underwent XRT or PBRT. On multivariable analysis, PBRT was associated with higher full-scale IQ and processing speed index relative to XRT, with a trend toward higher verbal IQ and general adaptive functioning. Weusthof et al. [35] evaluated neurocognitive outcomes in 56 pediatric brain tumor patients who received PBRT vs. XRT. There were no alterations in long-term neurocognitive abilities after PBRT, whereas declines in the processing speed index, nonverbal intelligence, and visuospatial abilities were observed after XRT.
In the study by Child et al. [32], patients treated with focal PBRT scored within normal limits on most cognitive measures and generally performed comparably to normative samples of typically developing children. Only mild challenges in processing speed index, fine motor, and academic fluency skills were seen in this cohort. The focal XRT cohort showed worse results than expected for age on global intellectual functioning. This study also confirmed that CSI radiation confers the greatest neurocognitive risk [9, 25]. After a long follow-up, the CSI XRT group was severely impaired, with 76% of the patients showing clinically impaired global intellectual functioning and 53%–88% demonstrating impaired performance across all cognitive and academic fluency tasks.
The processing speed index has been reported as the most vulnerable domain regarding neurocognitive outcome in pediatric brain tumor patients [24, 32, 46,47,48]. This domain shows a decrease in longitudinal development with a below-average IQ in patients treated with surgery only, XRT or PBRT [35]. Processing speed depends on intact white matter connections, and its tasks reflect both cognitive efficiency and fine motor functioning. White matter tracts can be harmed by surgery or radiation [49,50,51]. Interestingly, in three out of the nine studies meta-analyzed for this domain, patients receiving PBRT experienced significantly less processing speed index decline when compared to XRT.
Socioeconomic status (SES) has been demonstrated to be a predictor of cognitive outcomes for pediatric brain tumor patients both at treatment initiation and over time. Higher SES appears to serve as a protective factor mitigating the harmful effects of treatment on cognitive functioning. SES may represent a useful focal point for improving interventions, as those in low SES groups may be better served through broad policy change, education, and support [52]. In some countries, proton therapy is only available to patients with certain types of insurance or with wealth to be able to pay for the treatment (and travel to a proton center if there isn´t one nearby). SES could not be meta-analysed, as only three out of the nine studies had taken this factor into account but with different methods of assessing it.
A major strength of the present meta-analysis is that, to our knowledge, it is the first to compare the effects of PBRT and XRT on neurocognitive outcomes in children and adolescents with brain tumors. Several limitations must, however, be acknowledged, notably the small number of available studies, which impeded us from performing subanalyses attending to major variables, including patient (e.g., age, tumor histology or location, socioeconomic status) and treatment characteristics [e.g., treatment dose, timing, modality of radiotherapy (focal or CSI)]. Indeed, several studies did not adjust for these clinical/descriptive variables in their analyses, which might be viewed as a potential bias and would also affect the present results. It must be noted, however, that we attempted to perform sensitivity analyses by including adjusted data when available, and the results were confirmed. The heterogeneity found in the methods used for the assessment of neurocognitive outcomes can also be considered a limitation, as well as the heterogeneity found in participants’ characteristics. To bypass this limitation, we analyzed all outcomes as Z scores instead of absolute scores, although the latter could have provided more accurate information. Moreover, data from some studies could not be meta-analyzed because the necessary data were not available despite contacting the corresponding authors, which might be regarded as a potential bias.
Despite the abovementioned limitations, the present meta-analysis has relevant clinical implications. Our findings highlighted the potential of PBRT for the improvement of long-term psychosocial functioning in adult survivors of pediatric brain tumors by mitigating multiple neuropsychological sequelae of radiation treatment. It must be noted, nonetheless, that although patients treated with PBRT may have less neurocognitive impairment than those treated with XRT, the former are vulnerable to post-RT side effects, and therefore, these patients should also be closely monitored and encouraged to participate in interventions aimed at improving their neurocognitive functioning.
In conclusion, patients who have received PBRT achieve significantly higher scores on most analyzed neurocognitive outcomes (including IQ, verbal comprehension and perceptual reasoning indices, visual motor integration, and verbal memory) than those who have received XRT. These results can be used to guide treatment planning and indicate targets for monitoring and neurocognitive intervention. Future high-quality research is warranted to identify how patient (e.g., age, tumor histology or location) and treatment characteristics [e.g., treatment dose, timing, modality of radiotherapy (focal or CSI)] might affect neurocognitive outcomes of children and adolescents with brain tumors treated with PBRT. Larger studies with long-term follow-ups are needed to confirm these results.
Data availability
Data are available upon request.
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22:iv1–96.
Ingraham FD, Bailey OT, Barker WF. Medulloblastoma cerebelli; diagnosis, treatment and survivals, with a report of 56 cases. N Engl J Med. 1948;238:171–4.
Paterson E, Farr RF. Cerebellar medulloblastoma: treatment by irradiation of the whole central nervous system. Acta radiol. 1953;39:323–36.
Edelstein K, Spiegler BJ, Fung S, Panzarella T, Mabbott DJ, Jewitt N, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13:536–45.
Mabbott DJ, Spiegler B, Greenberg M, Rutka J, Hyder D, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–63.
Ullrich NJ, Embry L. Neurocognitive dysfunction in survivors of childhood brain tumors. Semin Pediatr Neurol. 2012;19:35–42.
Robinson KE, Kuttesch JF, Champion JE, Andreotti CF, Hipp DW, Bettis A, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55:525–31.
Padovani L, Andre N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol. 2012;8:578–88.
Mabbott DJ, Monsalves E, Spiegler BJ, Bartels U, Janzen L, Guger S, et al. Longitudinal evaluation of neurocognitive function after treatment for central nervous system germ cell tumors in childhood. Cancer. 2011;117:5402–11.
Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–6.
Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–8.
Packer RJ. Craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. Curr Neurol Neurosci Rep. 2007;7:130–2.
Michalski JM, Janss AJ, Vezina LG, Smith KS, Billups CA, Burger PC, et al. Children’s oncology group phase III trial of reduced-dose and reduced-volume radiotherapy with chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2021;39:2685–97.
Fuss M, Hug EB, Schaefer RA, Nevinny-Stickel M, Miller DW, Slater JM, et al. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys. 1999;45:1117–26.
Tian X, Liu K, Hou Y, Cheng J, Zhang J. The evolution of proton beam therapy: current and future status. Mol Clin Oncol. 2018;8:15–21.
Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26–44.
Eaton BR, Esiashvili N, Kim S, Patterson B, Weyman EA, Thornton LT, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016;18:881–7.
Zhang R, Howell RM, Taddei PJ, Giebeler A, Mahajan A, Newhauser WD. A comparative study on the risks of radiogenic second cancers and cardiac mortality in a set of pediatric medulloblastoma patients treated with photon or proton craniospinal irradiation. Radiother Oncol. 2014;113:84–8.
Mak DY, Siddiqui Z, Liu ZA, Dama H, MacDonald SM, Wu S, et al. Photon versus proton whole ventricular radiotherapy for non-germinomatous germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2022;2022:e29697.
Stokkevag CH, Indelicato DJ, Herfarth K, Magelssen H, Evensen ME, Ugland M, et al. Normal tissue complication probability models in plan evaluation of children with brain tumors referred to proton therapy. Acta Oncol. 2019;58:1416–22.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JPT AD, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors. Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane; 2017.
Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331:903.
Kahalley LS, Peterson R, Ris MD, Janzen L, Okcu MF, Grosshans DR, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38:454–61.
Kahalley LS, Ris MD, Grosshans DR, Okcu MF, Paulino AC, Chintagumpala M, et al. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol. 2016;34:1043–9.
Ali JS, Ashford JM, Swain MA, Harder LL, Carlson-Green BL, Miller JM, et al. Predictors of cognitive performance among infants treated for brain tumors: findings from a multisite, prospective, longitudinal trial. J Clin Oncol. 2021;39:2350–8.
Yip ATT, Huynh-Le MP, Crawford J, Kaner R, MacEwan I, Hattangadi-Gluth JA. Comparing endocrine and neurocognitive outcomes in pediatric brain tumor patients treated with proton vs photon radiation. Int J Radiat Oncol Biol Phys. 2020;108:E237–8.
Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13:154.
DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45.
Peterson RK, Katzenstein JM. Working memory and processing speed among pediatric brain tumor patients treated with photon or proton beam radiation therapy. Childrens Health Care. 2019;48:131–41.
Yang CC, Lin SY, Tseng CK. Maintenance of multidomain neurocognitive functions in pediatric patients after proton beam therapy: a prospective case-series study. Appl Neuropsychol Child. 2019;8:389–95.
Child AE, Warren EA, Grosshans DR, Paulino AC, Okcu MF, Ris MD, et al. Long-term cognitive and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiotherapy. Pediatr Blood Cancer. 2021;68: e29125.
Eaton BR, Fong GW, Ingerski LM, Pulsifer MB, Goyal S, Zhang C, et al. Intellectual functioning among case-matched cohorts of children treated with proton or photon radiation for standard-risk medulloblastoma. Cancer. 2021;127:3840–6.
Gross JP, Powell S, Zelko F, Hartsell W, Goldman S, Fangusaro J, et al. Improved neuropsychological outcomes following proton therapy relative to X-ray therapy for pediatric brain tumor patients. Neuro Oncol. 2019;21:934–43.
Weusthof K, Luttich P, Regnery S, Konig L, Bernhardt D, Witt O, et al. Neurocognitive outcomes in pediatric patients following brain irradiation. Cancers. 2021;2021:13.
Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17:287–98.
Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys. 2015;93:400–7.
Scott MA, Fletcher JM, Brookshire BL, Davidson KC, Landry SH, Bohan TC, et al. Memory functions in children with early hydrocephalus. Neuropsychology. 1998;12:578–89.
Rourke BP, Ahmad SA, Collins DW, Hayman-Abello BA, Hayman-Abello SE, Warriner EM. Child clinical/pediatric neuropsychology: some recent advances. Annu Rev Psychol. 2002;53:309–39.
Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52:159–64.
Camara S, Fournier MC, Cordero P, Melero J, Robles F, Esteso B, et al. Neuropsychological profile in children with posterior fossa tumors with or without postoperative cerebellar mutism syndrome (CMS). Cerebellum. 2020;19:78–88.
Ursache A, Noble KG, Pediatric Imaging N, Genetics S. Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016;6:e00531.
Merchant TE, Kiehna EN, Li C, Xiong X, Mulhern RK. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–54.
Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85:1301–6.
Yahya N, Manan HA. Neurocognitive impairment following proton therapy for paediatric brain tumour: a systematic review of post-therapy assessments. Support Care Cancer. 2021;29:3035–47.
Antonini TN, Ris MD, Grosshans DR, Mahajan A, Okcu MF, Chintagumpala M, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124:89–97.
Kahalley LS, Conklin HM, Tyc VL, Hudson MM, Wilson SJ, Wu S, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22:1979–86.
Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159–68.
Law N, Bouffet E, Laughlin S, Laperriere N, Briare M-E, Strother D, et al. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. Neuroimage. 2011;56:2238–48.
Law N, Greenberg M, Bouffet E, Laughlin S, Taylor MD, Malkin D, et al. Visualization and segmentation of reciprocal cerebrocerebellar pathways in the healthy and injured brain. Hum Brain Mapp. 2015;36:2615–28.
Morris EB, Phillips NS, Laningham FH, Patay Z, Gajjar A, Wallace D, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132:3087–95.
Torres VA, Ashford JM, Wright E, Xu J, Zhang H, Merchant TE, et al. The impact of socioeconomic status (SES) on cognitive outcomes following radiotherapy for pediatric brain tumors: a prospective, longitudinal trial. Neuro Oncol. 2021;23:1173–82.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a postdoctoral contract granted by Instituto de Salud Carlos III (Sara Borrell, CD21/00138 to PLV) and Junta de Andalucia (PAIDI 2020, POSTDOC_21_00725 to JSM).
Author information
Authors and Affiliations
Contributions
AL, JSM: conceptualization, data curation, formal analysis, writing–original draft. EP, FC: conceptualization. BE: data curation, formal analysis, PLV: data curation, formal analysis, writing–original draft. AL and JSM contributed equally to this work. All the authors revised, edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethic approval
Not applicable as this is a systematic review and meta-analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lassaletta, Á., Morales, J.S., Valenzuela, P.L. et al. Neurocognitive outcomes in pediatric brain tumors after treatment with proton versus photon radiation: a systematic review and meta-analysis. World J Pediatr 19, 727–740 (2023). https://doi.org/10.1007/s12519-023-00726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-023-00726-6