Abstract
Background
Rotavirus is the primary cause of gastroenteritis in children worldwide and is a leading cause of gastroenteritis in children, with a significant burden. Rotavirus vaccine became available in Ireland in 2016. This study aimed to investigate hospital admissions and seasonal characteristics of rotavirus gastroenteritis in a pre- and post-vaccination period in a single district general hospital.
Methods
In the post-vaccination year, from November 18th 2016 to November 18th 2017, all children up to 3 years of age who presented to Mayo University Hospital with vomiting and diarrhea, were recruited and had their stool tested for rotavirus. Retrospective analysis of hospital data of children of the same age during pre-vaccination years (2014–2016) were used for comparison.
Results
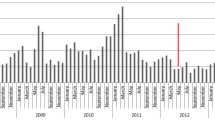
Compared with the pre-vaccination years (2014–2016), the median percentages of reduction of rotavirus positive stool requests and hospital admissions were high, 48.5% and 73%, respectively. In the post-vaccination year, the median percentage of reduction of emergency department presentation (stool requests) with gastroenteritis was 9%. No delay in the onset of RV season or reduction of the peak of RV infection was noted in the post-vaccination year. The duration of rotavirus season in 2016/2017 was short.
Conclusions
Compared with 3 pre-vaccination years, the total number of gastroenteritis presentations, gastroenteritis hospital admissions, and rotavirus positive gastroenteritis cases were all reduced, and the duration of the rotavirus season was shorter.



Similar content being viewed by others
Data availability statement
Available upon reasonable request.
References
Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine. 2015;33:2097–107.
Weidemann F, Dehnert M, Koch J, Wichmann O, Höhle M. Modelling the epidemiological impact of rotavirus vaccination in Germany–a Bayesian approach. Vaccine. 2014;32:5250–7.
Uhlig U, Kostev K, Schuster V, Koletzko S, Uhlig HH. Impact of rotavirus vaccination in Germany: rotavirus surveillance, hospitalization, side effects and comparison of vaccines. Pediatr Infect Dis J. 2014;11:299–304.
Vesikari T, Uhari M, Renko M, Hemming M, Salminen M, Torcel-Pagnon L, et al. Impact and effectiveness of RotaTeq® vaccine based on 3 years of surveillance following introduction of a rotavirus immunization program in Finland. Pediatr Infect Dis J. 2013;12:1365–73.
Martinón-Torres F, Aramburo A, Martinón-Torres N, Cebey M, Seoane-Pillado MT, Redondo-Collazo L, et al. A reverse evidence of rotavirus vaccines impact. Hum Vaccine Immunother. 2013;9:1289–91.
Koch J, Wiese-Posselt M, Remschmidt C, Wichmann O, Bertelsmann H, Garbe E, et al. Background paper to the recommendation for routine rotavirus vaccination of infants in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:957–84.
Tilson L, Jit M, Schmitz S, Walsh C, Garvey P, McKeown P, et al. Cost-effectiveness of universal rotavirus vaccination in reducing rotavirus gastroenteritis in Ireland. Vaccine. 2011;29:7463–73.
Macartney KK, Power M, Dalton D, Cripps T, Maldigri T, Isaacs D, et al. Decline in rotavirus hospitalisations following introduction of Australia’s national rotavirus immunisation programme. J Paediatr Child Health. 2011;47:266.
O’Ryan M, Lucero Y, Linhares AC. Rotarix®. Vaccine performance 6 years post licensure. Expert Rev Vaccines. 2011;12:1645–59.
Tiemessen CT, Nel MJ. Detection and typing of subgroup F adenoviruses using the polymerase chain reaction. J Virol Methods. 1996;59:73–82.
Paulke-Kronek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospetalisationrates in Austrian children. Pediatr Infect Dis J. 2010;29:319–23.
Buttery JP, Lamber SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in Rotavirus- associated gastroenteritis following introdiction of rotavirus vaccine into Australia’s national childhood vaccine schedule. Pediatr Infect Dis J. 2011;30:S25–S29.
Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TT, Maldonado YA, Spoulou V, et al. Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin. 2011;7:734–48.
Gray J. Rotavirus vaccines: safety, efficacy and public health impact. J Intern Med. 2011;270:206–14.
Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J. 2011;30:S21–4.
Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, et al. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008–2009. J Infect Dis. 2013;208:790.
Osborne CM, Montano AC, Robinson CC, Robinson CC, Schultz-Cherry S, Dominguez SR. Viral gastroenteritis in children in Colorado 2006–2009. J Med Virol. 2015;87:931.
Hall AJ, Rosenthal M, Gregoricus N, Greene SA, Ferguson J, Henao OL, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis. 2011;17:1381.
Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20:14.
Dennehy PH. Viral gastroenteritis in children. Pediatr Infect Dis J. 2011;30:63.
Lee RM, Lessler J, Lee RA, Rudolph KE, Reich NG, Perl TM, et al. Incubation periods of viral gastroenteritis: A systematic review. BMC Infect Dis. 2013;13:446.
Public Health Agency of Canada. Adenovirus (serotypes 40 & 41). Pathogen safety data sheet-infectious substances 2010. Accessed 10 Sept 2019
Koh H, Beak SY, Shin JY, Chung KS, Jee YM. Coinfection of viral agents in Korean children with acute watery diarrhoea. J Korean Med Sci. 2008;23:937–40.
Macartney KK, Power M, Dalton D, Cripps T, Maldigri T, Isaacs D, et al. Decline in Rotavirus hospitalisations following introduction of Australia’s national rotavirus immunisation programme. J Paediatr Child Health. 2011;47:266–70.
Miren Iturriza-Gomara, Daniel Humgerford. European rotavirus surveillance network. Annual report 2017. Accessed 10 Sept 2019
Hungerford D, Vivancos R, Read JM, Iturriza-Gomara M, French N, Cunliffe NA. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med. 2018;16:10.
Zellar M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28:7507–1.
Acknowledgements
I greatly thank Ms Zoe Yandle (Clinical Scientist at the National Virus Reference Laboratory-Dublin) for her kind assistance in relation to performing laboratory investigations and was happy with submission for publication.
Funding
None.
Author information
Authors and Affiliations
Contributions
ZB was responsible for conceptualization, study design, study protocol, data collection and management, literature search, writing original draft, review & editing, and submitting the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No financial or non- financial benefits have been received or will be received from any part related directly or indirectly to the subject of this article.
Ethics approval
Obtained from Ethical Committee at Mayo University Hospital prior to study commencement (Ref TOM/DP).
Consent to participate
Obtained from carers of children.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barsoum, Z. Regional hospitalisation and seasonal variations of Pediatric rotavirus gastroenteritis pre- and post-RV vaccination: a prospective and retrospective study. World J Pediatr 18, 404–416 (2022). https://doi.org/10.1007/s12519-022-00546-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-022-00546-0