Abstract
Besides nitrate deposits located in the Atacama Desert of Chile and the Mojave Desert of California, the present work documents, for the first time, the occurrence of potassium nitrate as oval-suboval-shaped aggregations associated with the Late Maastrichtian-Early Paleocene Dakhla Shale and the Paleocene-Early Eocene Esna Shale encountered in the northeastern part of the Kharga Oasis, particularly at G. Um El-Ghanayem and G. Ghaneima. Consequently, integrated petrographic, mineralogical, and geochemical investigations were carried out for shale deposits and nitrate salts to reconstruct the paleoenvironmental conditions of shale, reveal the extent to which nitrate salts are genetically related to the paleoenvironment of shale deposits, and build up a complete scenario about the source and the formation mechanism of nitrate salts. The overall results showed that the studied shale deposits were sourced from mafic igneous and quartzose sedimentary provenances where humid climatic conditions were dominant; the transported detrital particles were then settled down under oxidizing bottom water and shallow depositional conditions. Moreover, the nitrate salts are of an epigenetic origin and sourced from the microbial nitrification of organic matter and the wet atmospheric deposition that is believed to be triggered by the active volcanic eruptions during the Late Eocene/Early Oligocene transition where warm climatic conditions prevailed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen is considered to be one of the most critical nutrients for many different life forms on the Earth’s surface due to its vital role in the synthesis of DNA, proteins, and chlorophyll. It largely occurs as dinitrogen gas (N2) that comprises about 72% of the ambient atmosphere and accounts for 90% of the global N reservoir. Besides dinitrogen gas, nitrogen is also found in other forms (e.g., NO2, NO3, NH3, and NH4), which are cycled and exchanged between the atmosphere, water bodies, soils, and rocks through what so-called the global nitrogen cycle. The latter is driven by diverse consortia of microorganisms (e.g., bacteria, algae, and fungi), as well as plants, and encompasses three main transformational processes of the atmospheric nitrogen: N-fixation, nitrification, and denitrification (Widdison and Burt 2008; Bernhard 2010).
In addition to the Earth’s atmosphere, nitrogen was estimated in different rock types and shale-driven soils, including igneous (e.g., 0.3 mg N kg−1 in ultramafic xenoliths, USA, and 243 mg N kg−1 in granites of central Spain), low-grade metamorphic (e.g., 422 mg N kg−1 in Moine metasediments, Scotland, and 738 mg N kg−1 in phyllite, Erzegeberge, Germany), sedimentary rocks (e.g., 15,976 mg N kg−1 in coal deposits of the Appalachian region, USA, and up to 163,000 mg N kg−1 in the Chilean caliche-type deposits), and California shale-sourced soil (e.g., 3000 mg N kg−1) (Ericksen 1981; Li and Daniels 1994; Hall et al. 1996; Boyd and Philippot 1998; Mohapatra and Murty 2000; Mingram and Bräuer 2001; Bingham et al. 2021). For sedimentary rocks, in particular shales, nitrogen occurs as inorganic and organic compounds. Ammonium ions “NH4+” represent the common inorganic nitrogen forms that are incorporated into the crystal structure of clay minerals (e.g., illite and vermiculite). They substitute for K+ ions and compensate the negative charge resulting from the isomorphic substitution of Al for Si in the tetrahedral sheets of clay minerals (Stevenson 1962; Itihara et al. 1986). Besides the lattice-bound N, the recalcitrant organic matter of muddy rocks is considered the main depository of organic nitrogen that can be microbially transferred to the terrestrial ecosystem more readily than the clay minerals-fixed nitrogen (Morford et al. 2016; Bingham et al. 2021). The transformation of N from seawater into shale deposits can be summarized through three main steps (Peterson 1981; de Leeuw and Largeau 1993; Mayer 1994a, b; Macko et al. 1994;Holloway and Dahlgren 2002; Knicker 2004; Thompson et al. 2012; Delmint et al. 2018; Pajares and Ramos 2019) as follows: (a) N-fixation and nitrification from the surface seawater by diazotrophs (e.g., diverse group of bacteria, algae, and diatoms) which convert N2 into bioavailable NH4+ and NO3− stored in the living tissues as peptides and amino acids; (b) as diazotrophs become dead, the nitrogen-bearing organic compounds accumulate downward with the settling clay particles; (c) during sediment diagenesis, some of the deposited N-organic compounds are microbially degraded under anoxic conditions into NH4+ that encapsulated into the silicate structure of clay minerals, while the other fraction is stabilized into the recalcitrant organic matter. In addition to the foregoing literature, other studies (e.g., Mingram et al. 2003; Schneider-Mor et al 2012; Ristic et al. 2016; Li et al. 2019; Zhu et al. 2021) utilized nitrogen isotope composition to reconstruct the paleoenvironmental conditions of carbonaceous shales and to investigate the behavior of nitrogen-organic compounds during diagenesis and shale gas generation.
Depending on the above-mentioned studies, it is clear that the occurrence of nitrogen salt minerals, particularly nitrate minerals, in shale deposits has attracted little attention. This may be stemmed from the absence of such minerals in the studied shales due to their water solubility. Until the present day, the only preserved nitrate minerals are well-known from caliche-type deposits located at the Atacama Desert of Chile and the Mojave Desert of California (Ericksen 1981; Ericksen et al. 1988). Through this context, the current study reports and sheds more light on the occurrence of nitrate minerals in Late Maastrichtian-Early Paleocene Dakhla Shale and the Paleocene-Early Eocene Esna Shale deposits located in the Western Desert of Egypt, particularly at Gebel “G” Um El-Ghanayem and Ghaneima. Also, the nitrate occurrence has to be studied along with the paleoenvironmental conditions of the host shale. In general, the paleoenvironmental conditions of clastic sedimentary rocks (e.g., provenance, plaeoclimate, tectonic setting, and paleoredox conditions) can be inferred using different geochemical approaches among which many types of discrimination diagrams constructed depending on the elemental ratios of major oxides and some trace elements (Alqahtani and Khalil 2021). Besides these geochemical approaches, petrographic and mineralogical characterizations are considered for shale deposits and their nitrated content in an attempt to reveal the paleoenvironmental conditions and the probable genesis of nitrate minerals.
Geology of the study area
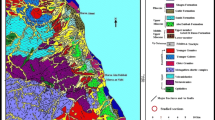
The study area is located in the Western Desert of Egypt, about 19 km to the northeastern part of El-Kharga Town, and represented by two conspicuous hills called Gebel “G” Um El-Ghanayem (25° 35′ 32.9″ N and 30° 43′ 52.1″ E) and G. Ghaneima (25° 26′ 40.1″ N and 30° 44′ 57.6″ E) (Fig. 1). According to many geological studies conducted on the Upper Cretaceous-Tertiary successions in the Western Desert of Egypt (e.g., Awad and Ghobrial 1965; Issawi 1972; Luger 1988; Bassiouni and Luger 1990; Felesteen and Zakhera 1999; Orabi and Khalil 2014), G. Um El-Ghanayem and G. Ghaneima comprise four stratigraphic units well-exposed from base to top as follows: Early Maastrichtian-Early Paleocene Dakhla Formation, Paleocene Tarawan Formation, Paleocene-Early Eocene Esna Formation, and Early Eocene Thebes Formation. The Dakhla Formation consists mainly of shale deposits along with intercalated beds of marl, glauconitic sandy claystone, and phosphatic conglomerate. Depending upon the vertical facies changes, the Dakhla Formation is subdivided into five members ordered from base to top as follows: Mawhoob (gypsiferous brownish-dark grey shale and silty mudstone succession), Baris (argillaceous dolomitic beds with a noticeable dispersion of Exogyra Overwegi), Lower Kharga (dark grey shale occasionally containing bivalves and fish scales), Bir Abu Munqar (coarse-grained, calcareous, phosphatic conglomerate layer), and Upper Kharga members (laminated calcareous shale rich in planktonic foraminifera). It is worth to mention that the Bir Abu Munqar Member remarks a hiatus between the underlying Early Maastrichtian Mawhoob Member and the overlying Early Paleocene Upper Kharga Member. The deposition of Dakhla Formation was terminated during the late Early Paleocene and remarked by a bioturbated conglomeratic bed occurring at the top of the Upper Kharga Member, indicating another hiatus with the overlying fossiliferous chalky limestone of the Tarawan Formation that underlies 70 m thick greenish-grayish gypsiferous shale of the Esna Formation. These successions are eventually topped by thinly bedded Oyster limestone of the Thebes Formation.
Location map of G. Um El-Ghanayem and G. Ghaneima along with the general geology of El-Kharga Oasis (after Faris et al. 2017)
Materials and methods
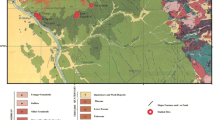
About sixteen representative shale samples were collected from the two studied sections at G. Um El-Ghanayem and G. Ghaneima (Fig. 2). The collected samples were undergone petrographic and stereo-microscopic characterizations to study the sedimentary structures of shale along with the contained weathering products and to give a close-up view of the mode of occurrence of nitrate salts in shale samples. For petrography, thin sections were prepared and examined using transmitted light microscope (Olympus-BX61). Stereomicroscope (Olympus-SZX16) was utilized to study the morphology of nitrate minerals due to their water solubility. Regarding mineralogical and geochemical analyses, the shale samples were pulverized up to −63 µm and then dried overnight at 105 °C. The bulk mineral composition was studied using X-ray diffractometer “XRD-D8 Advance” (ʎ = 1.540 Å, 25 mA, 40 kV), and the mineral phases were identified using the X’Pert High Score Plus software. The distribution of major and trace elements was studied by preparing press pellets that were scanned using X-ray fluorescence “Hitachi-X-Supreme 8000”; the loss on ignition was calculated after heating each shale sample at 1000 °C for 2 h. The total organic carbon “TOC” and total nitrogen contents “TN” were measured using the Walkley-Black and Kjeldahl titrations, respectively, as discussed by vanReeuwijik (2002). The concentration of NO3−, SO42−, and Cl− ion species was determined using UV-Vis. Spectrophotometer, while that of K+ and Na+ was measured by flame photometer.
Results
Petrography
Under the petrographic microscope, shale deposits at Gebel Um El-Ghanayem and Gebel Ghaneima, including Dakhla Shale members and Esna Shale, consist mainly of clay minerals, carbonaceous material, and non-clay minerals. The latter are represented by halloysite, gypsum, quartz, glauconite, jarosite, calcite, and siderite. With an exception for the Upper Kharga Member, the studied shales are characterized by wavy lamination comprising light brown, clay minerals-rich laminae (30–100 µm in thickness) rhythmically alternating with dark colored, organic matter-rich laminae (20–300 µm in thickness) (Fig. 3A–F). In some places, the carbonaceous laminae are gradually obliterated, leaving non-laminated argillaceous matrix behind. The latter is occasionally invaded by gypsum veinlets and also partly altered into brownish creamy spots of halloysite (Fig. 4A–F).
Photomicrographs of the studied shale deposits showing a wavy lamination sedimentary structure detected in the Mawhoob (A and B at G. Um El-Ghanayem and G. Ghaneima, respectively), and Lower Kharga members (C and D) as well as Esna Shale (E and F) and composed of light brown, argillaceous laminae rhythmically alternating with dark carbonaceous laminae
Photomicrographs of the studied shale deposits revealing the role of chemical weathering in obliteration of the carbonaceous wavy laminae and the partial alteration of argillaceous matrix into brownish creamy spots of halloysite (look at arrows) through the Mawhoob (A and B at G. Um El-Ghanayem and G. Ghaneima, respectively) and Lower Kharga members (C and D) along with Esna Shale (G and H)
Besides wavy lamination, parallel laminae of glauconite (52–280 µm in diameter) and quartz grains (30–150 µm in diameter) are well-observed only in the Lower Kharga Shale Member (Fig. 5A–D). Glauconite grains are generally detected in all the studied shale samples as yellowish green, suboval, subrounded, structure-less pellets. However, their abundance is more pronounced in the Lower Kharga Shale Member than in the other samples. On chemical weathering, some glauconite pellets are replaced by hydrated potassium and iron sulfate, known as jarosite (Fig. 5E–F).
Photomicrographs of the Lower Kharga Member (A and B at G. Um El-Ghanayem and C and D at G. Ghaneima) showing the occurrence of parallel lamination, consisting of oval-suboval and sometimes subangular glauconite pellets and quartz, along with a prominent dispersion of rutile grains (look at arrows). In places, glauconite grains are altered into alunite as a response of the imposed chemical weathering (E—PPL and F—XPL)
On the other side, the Upper Kharga Shale Member appears as non-laminated, fossiliferous, light brown, argillaceous matrix, with a frequent appearance of halloysite (Fig. 6A, B).Furthermore, the disseminated fossil shells are subjected to a diagenetic micritization. This is revealed by formation of micrite envelope along the peripheries of fossil shells, and then recrystallized into calcite spar (Fig. 6C, D). Also, reddish brown, well-developed, rhombohedral crystals of siderite are observed inside of the micritized shells and also as disseminations through the argillaceous matrix (Fig. 6E). The growth of siderite is seemed to be at the expense of calcite. This is proved by an increase of the crystallinity of siderite inward from the shell wall, where Fe2+ ions gradually replace Ca2+ ions in calcite crystal lattice (Fig. 6F).
Stereo-microscope characterization
Investigation of shale hand specimens using stereo-microscope revealed the occurrence of white-colored aggregations of nitrate minerals in the collected samples. Morphologically, oval-suboval-shaped clusters, varying in size between 0.5 and 2 mm, along with thin crusts and fracture-infilling aggregates are observed along the external surfaces of fissility planes (Fig. 7).
Photomicrographs showing the occurrence of nitrate minerals as white-colored, oval-suboval-shaped aggregations dispersed on the external surface of shale hand specimens collected from the Mawhoob (A and B at G. Um El-Ghanayem and G. Ghaneima, respectively), Lower Kharga (C and D), and Upper Kharga members (E and F) and Esna Shale (G and H)
Mineralogical characterization
Interpretation of XRD patterns of the collected shale samples indicates the occurrence of illite-smectite mixed-layer, smectite, sepiolite, palygorskite, kaolinite, halloysite, and glauconite. Nitrate-minerals are dominantly represented by potassium-nitrate “niter.” Both clay and nitrate minerals are associated with quartz, gypsum, alunite, jarosite, halite, goethite, siderite, and rutile (Figs. 8 and 9). By comparing the clay mineral species of the Dakhla Shale located at G. Um El-Ghanayem and its counterpart at G. Ghaneima, it is clear that the former is composed of illite-smectite mixed-layer, smectite, sepiolite, palygorskite, kaolinite, halloysite, and glauconite; on the other side, latter is distinguished by kaolinite-dominated clay fraction which can also be traced in the Upper Kharga Member at Gebel Um El-Ghanayem. Moreover, the reflection peaks of glauconite show a noticeable increase in the bulk mineralogy of the Lower Kharga Member. For Esna Shale, its bulk mineralogy at Um El-Ghanayem and Ghaneima areas is largely similar to each other.
Geochemical characterization
Distribution of major oxides
Distribution of major oxides of the studied shale samples is listed in Table 1. SiO2 contents vary between 25.2 and 66.0 wt.%, with averages of 51.28wt.% and 51.19 wt.% at G. Um El-Ghanayem and G. Ghaneima, respectively. Al2O3 contents at G. Ghaneima show a remarkable increment (max. 20.78 wt.%, min. 13.31 wt.%, and avg. 16.36 wt.%) compared to G. Um El-Ghanayem samples (max. 17.21 wt.%, min. 7.51 wt.%, and avg. 13.0 wt.%). At G. Um El-Ghanayem, Al2O3 contents are strongly correlated with SiO2 (r = 0.97) (Supplementary Fig. S1A). However, this relationship has become relatively weak (r = 0.45) at G. Ghaneima (Supplementary Fig. S1A). Other elements are also incorporated into clay minerals and positively correlated with Al2O3, including Fe2O3 (max. 7.27 wt.%, min. 3.39 wt.%, and avg. 5.82 wt.%), MgO (max. 2.07 wt.%, min. 1.21 wt.%, and avg. 1.49 wt.%), K2O (max. 1.86 wt.%, min. 0.52 wt.%, and avg. 1.04 wt.%), and Na2O (max. 0.51 wt.%, min. 0.06 wt.%, and avg. 0.26 wt.%) (Supplementary Fig. S1B–E). It is worthy to mention that the positive correlations between Al2O3 and the contents of K2O and Na2O are detected only in G. Um El-Ghanayem samples. These relationships have become negatively correlated at G. Ghaneima (Supplementary Fig. S1D, E). Another negative relationship was found between Al2O3 and CaO contents (max. 32.8 wt.%, min. 0.18 wt.%, and avg. 8.97 wt.%) in all collected shale samples (Supplementary Fig. S1F). Moreover, CaO is positively correlated with L.O.I. (r = 0.91) (Fig. 10G). From another side, the studied samples are characterized by higher concentrations of TiO2 (max. 3 wt.%, min. 0.86 wt.%, and avg. 1.8 wt.%) than the average crustal shales “ACS” (0.77 wt.%, Turekian and Wedepohl 1961) and the North American Shale Composite “NASC” (0.78 wt.%, Gromet et al. 1984). TiO2 contents of all samples are positively correlated with Al2O3 (r = 0.85 and 0.75) (Supplementary Fig. S1H).
Discrimination plots of TOC, CaO, Fe2O3, SO42−, insoluble N, NO3−, K+, Na+ and Cl− show the positive correlation of TOC with CaO (A) and insoluble N (B); NO3− is positively correlated with K+ (C) and negatively correlated with Na+ (D); SO42− is negatively correlated with TOC (E) CaO (F), and reveals positive and negative correlations with Fe2O3 (G); it is also positively correlated with Cl− (H) and Al2O3 (I); Cl− shows positive and negative correlations withAl2O3 (J)
Distribution of trace elements
Regarding trace elements, contents of Cr, Mn, Zn, Sr, U, and Th were measured (Table 2). By comparing with NASC and ACS, the studied samples are enriched in Cr (max. 580 ppm, min. 360 ppm, and avg. 475 ppm), Zn (max. 427 ppm, min. 67 ppm, and avg. 271 ppm), and Sr (max. 2710 ppm, min. 201 ppm, and avg. 958 ppm) and relatively depleted in Mn (max. 3160 ppm, min. 72 ppm, and avg. 679 ppm), U (max. 3.5 ppm, min. 0.6 ppm, and avg. 2.26 ppm), and Th (max. 7.2 ppm, min. 3.1 ppm, and avg. 4.8 ppm). For the majority of samples, Cr and Zn contents show weak positive and strong negative correlations with Al2O3 (Supplementary Fig. S2A, B). Also, Cr contents exhibit a strong positive relationship with Mn (Supplementary Fig. S2C). Sr is positively correlated with CaO at G. Ghaneima (r = 0.79), while a weak positive correlation is found at G. Um El-Ghanayem (r = 0.24) (Supplementary Fig. S2D). Both U and Th display weak positive and negative correlations with Al2O3 (Supplementary Fig. S2E, F).
Distribution of total organic carbon “TOC,” nitrogen, SO4 2−, Cl−, K+, and Na+
As listed in Table 3, TOC contents vary between 0.56 and 1.9 wt.%, with an average of 1.15 wt.%. The majority of shale samples are generally depleted in TOC contents, which are highly positive correlated with CaO (e.g., r = 0.80 and 0.75) (Fig. 10A).
For total nitrogen “TN,” it is measured between 0.44 and 1.22 wt.%, with an average of 0.82 wt.%. It is noticed that TN contents of the Dakhla Shale samples at G. Ghaneima (1.0–1.22 wt.%) are relatively higher than the counterpart at G. Um El-Ghanayem (0.44–0.68 wt.%). On the other hand, TN contents of Esna Shale at the two studied sections (0.70–0.77 wt.% at G. Um El-Ghanayem and 0.65–0.81 wt.% at G. Ghaneima) are greatly similar. Further, TN contents comprise two main fractions, namely water-insoluble nitrogen and water-soluble NO3−. The former is determined at lower concentrations (max. 0.27wt.%, min. 0.05wt.%, and avg. 0.13wt.%) than NO3− fraction (max. 1.02wt.%, min. 0.36wt.%, and avg. 0.70 wt.%) and is also positively correlated with TOC (Fig. 10B). Although the measured nitrate content is lower than the nitrate deposits of Atacama Desert, Chile (16.64 wt.%) and the Amargosa River Valley, California (5.67–31.66 wt.%) (Ericksen et al. 1988), it can be comparable with the Dawadi potassium nitrate deposits, China (min. 0.09–0.72 wt.% on the eastern and western mining areas, respectively) (Zhang et al. 2021). Moreover, NO3− contents are positively correlated with K+ (r = 0.86 and 0.77) (Fig. 10C) and negatively correlated with with Na+ (r = −0.50 and −0.41) (Fig. 10D).
SO42− contents are negatively correlated with TOC at G. Um El-Ghanayem (r = −0.90) and G. Ghaneima (r = −0.70) (Fig. 10E). Likewise, SO42− contents are negatively correlated with CaO (r = −0.73 and −0.66 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Fig. 10F). Further, SO42− and Fe2O3 contents (r = 0.60) are positively correlated with each other at G. Um El-Ghanayem, whereas a weak negative correlation (r = −0.30) is found at G. Ghaneima (Fig. 10G).
Cl− content is positively correlated with SO42− (r = 0.71 and 0.60 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Fig. 10H). Both Cl− and SO42− contents exhibit a positive correlation with Al2O3 at G. Um El-Ghanayem (r = 0.63 and 0.88, respectively) (Fig. 10I) and a weak relationship at G. Ghaneima (r = −0.16 and 0.12, respectively) (Fig. 10J).
Discussion
Interpretation of the elemental distribution
Distribution of major and trace elements is greatly controlled by the contained mineral phases, and the correlation between them can infer preliminary information about the paleoenvironmental conditions. For instance, the remarkable occurrence of kaolinite in the bulk mineralogy of Dakhla Shale and Esna Shale at G. Ghaneima stands behind the increased Al2O3 contents and the weak correlation of Al2O3 with SiO2, Na2O, and K2O compared to G. Um El-Ghanayem. For all collected shale samples, the negative correlation of CaO with Al2O3 along with its positive relation with L.O.I. indicates the marine origin of calcium, mainly contained in the dispersed foraminiferal shells and represented by carbonates. From another side, TiO2 is strongly correlated with Al2O3, indicating its detrital origin. With respect to trace elements, some of which are strongly confined to Mn contents (e.g., Cr&Zn) rather than Al2O3, implying the adsorption of these elements on Mn-oxides and its weak relation to clay sedimentation due (Termaz 2005; Makoundi 2016). The same scenario can be seen for Th and U contents. Sr is mainly stored into the carbonate fraction as shown by its positive correlation with CaO (Liu et al. 2020).
Regarding TOC, the majority of shale samples are generally depleted in TOC due to the post-depositional chemical oxidation of organic matter, as previously shown by the microscopic examination. TOC contents are highly correlated with CaO that can be confined to the high productivity rates of CaCO3-bearing microorganisms like benthic foraminifera (Nioti et al. 2013). Also, another positive correlation of TOC was found with the insoluble nitrogen content, indicating the importance role of organic matter as repositories of organic nitrogen. On the other hand, the soluble nitrogen fraction, mainly nitrate content, is positively correlated with K + concentrations, supporting the occurrence of niter as the dominant nitrate mineral in the studied samples.
For SO42− fraction, its negative correlation with TOC at G. Um El-Ghanayem and G. Ghaneima can be interpreted depending on the metabolism of sulfate-reducing bacteria within the micro-pores between sediment particles. At these places, the dissolved sulfate is microbially reduced to metabolize the contained organic matter, leading to release H2S that can be oxidized again into SO42−. On repetition, the dissolved oxygen content is consumed, and the micro-pore environment becomes more reducing, resulting in preservation of organic matter (Liu et al. 2020). Likewise, the negative correlation with CaO can be attributed to the microbial sulfate reduction that increases the acidity degree by releasing H2S as a metabolic by-product. The latter was partly consumed in the formation of pyrite as illustrated by the positive correlation between SO42− and Fe2O3 contents (r = 0.60) at G. Um El-Ghanayem, whereas the weak negative correlation (r = −0.30) at G. Ghaneima indicates that no chemical reaction occurred between H2S and the dissolved ferrous iron species to form pyrite. Both SO42− and Cl− are associated with each other as evoporite minerals as manifested by the strong positive correlation between them. Moreover, SO42− and Cl− are positively correlated with Al2O3 that can be attributed to the incorporation of Cl− into the crystal structure of clay minerals (Vassilev et al. 2000) and to the role of clay minerals in the partial inhabitation of the microbial sulfate reduction (Wong et al. 2003).
Implications for the paleoenvironmental conditions
The paleoenvironmental conditions of the studied shale deposits, including paleoclimate, paleoweathering, shale provenance, marine/or non-marine environment, tectonic setting, and paleoredox conditions, were reconstructed depending on the chemical index of weathering “CIX,” the binary plot of K2O/Na2O ratio vs. SiO2, the elemental ratios of major oxides in relative to Al2O3, and U/Th ratio.
Paleoclimate conditions
Like the other clastic sedimentary rocks, shales are formed by weathering processes of the pre-existing rocks. During such processes, there is a differential mobilization of alkali elements (e.g., Na, K, and Ca) in relative to Al. The alkali elements tend to be mobilized, whereas Al is retained in the weathered products (Fedo et al. 1995). The relative proportions of these two element types, expressed as the chemical index of weathering “CIX = 100*[Al2O3/(Al2O3 + Na2O + K2O)],” is therefore used as a proxy for the paleoweathering conditions of the source rocks and the Paleoclimate conditions of the source area (Cullers 2000; Armstrong-Altrin et al. 2021; Ramos-Vázquez et al. 2022). For instance, CIX values < 63, 63–80, and > 80 indicate weak weathering degree under cold and dry conditions, strong weathering associated with warm and humid climate, and extremely intensive weathering under hot and humid conditions, respectively. CIX values of the studied samples were calculated at 90.65–92.74 (Dakhla Shale at G. Um El-Ghanayem), 85.60–95.37 (Dakhla Shale at G. Ghaneima), 88.07–88.71 (Esna Shale at G. Um El-Ghanayem), and 87.55–89.31 (Esna Shale at G. Ghaneima). Accordingly, the sediments load was brought about from strongly weathered source areas under warm climatic conditions. Moreover, the CIX values are plotted on the A “Al2O3”-CN “CaO + Na2O”-K “K2O” ternary diagram to reveal the extent of weathering of the source rocks and to shed more light on the possible K-metasomatism, transformation of kaolinite into illite during diagenesis, of the studied shale samples (Nesbitt and Young 1982; Fedo et al. 1995). As illustrated in Fig. 11, the studied samples plot away the plagioclase-K-feldspar line, indicating that the source rocks experienced an intensive chemical weathering and the detrital products are enriched in clay minerals relative to feldspars. On the other hand, the post-depositional K-metasomatism will result in a deviation of the weathering trend away from the predicted weathering line (red dashed line) toward K2O apex; however, the plotted samples are all encountered along the predicted weathering trend line. Accordingly, the studied shale deposits were not subjected to K-metasomatism of illite during diagenesis.
Shale provenance and tectonic setting
The source area of shale deposits can be inferred from their chemical composition using a number of major, trace, and rare earth elements-based diagrams. The current study employed the discrimination diagram of Roser and Korsch (1988), which determines the provenance of siliciclastic rocks based on the ratios of TiO2, Fe2O3, MgO, Na2O, and K2O with Al2O3. Accordingly, the majority of shale samples, including Esna Shale, Upper Kharga, and Lower Kharga members, are driven from quartzose sedimentary provenance, while shale deposits of the Mawhoob Member are weathered from a felsic igneous provenance (Fig. 12). Regarding tectonic setting, the discrimination diagram of Verma and Armstrong-Altrin (2013) (Fig. 13) indicates that the weathered particles seem to be deposited within a continental rift setting.
Discrimination diagram for shale provenance shows that the majority of samples are driven from mafic igneous provenance, with exception for the Mawhoob Member that is driven from quartzose sedimentary provenance (after Roser and Korsch 1988)
Plot of DF1 vs. DF2 indicates that the continental rift setting is the main depositional basin for the studied shale samples (For equations, see Verma and Armstrong-Altrin 2013)
Paleoredox conditions
Paleoredox conditions of seawater can be reconstructed using U/Th ratios. According to Jones and Manning (1994), U/Th ratios < 0.75 indicate the deposition through oxygenating bottom water, while the reducing conditions are dominant at ratios > 1.25. Also, the content of authigenic U can be employed as another proxy for the paleoredox conditions. It is measured at 12.0, between 12 and 5, and lower than 5 under suboxic-anoxic, dysoxic, and oxidizing conditions, respectively. For the current study, U/Th ratios are calculated between 0.31 and 0.70 and the authigenic U content is measured between averages of 0.94 and 0.42, implying that the paleodepositional environment of the studied shales were prevailed by oxidizing conditions (Fig. 14). Moreover, the dominance of oxygenating seawater is also an indication for the shallow deposition as a result of the relative sea level fall (Anderson et al. 1989).
Binary plot of Th vs. U shows that the majority of shale samples deposited under oxidizing bottom seawater (after Jones and Manning 1994)
Mineralogical variations
The mineralogical variations throughout shale deposits can be reflected by the fluctuations of Si/Al, K/Al, and Na/Al ratios as being a good proxy for the relative abundance of quartz, illite/or illite-smectite mixed-layer, and smectite, respectively (Hofmann et al. 2001). Quartz is a common detrital mineral in the clastic sedimentary rocks; its occurrence causes an increase in the Si/Al ratio of the associated aluminosilicate minerals (e.g., kaolinite1.17, smectite 2.2, and illite 2.0) (Werling et al. 2022). Little variations in the relative quartz content can be observed throughout the studied shale samples at G. Um El-Ghanayem (Si/Al ratio varies between 3.11 and 4.35). On the other side, a remarkable decline in quartz content is found throughout the Lower Kharga (2.47–2.54 Si/Al ratio) and Upper Kharga members (2.41–2.44 Si/Al ratio) compared to the Mawhoob Member (4.27–4.46 Si/Al ratio) and Esna Shale (3.56–3.72 Si/Al ratio) at G. Ghaneima.
Regarding K/Al ratio, its values range between 0.07 and 0.11 at G. Um El-Ghanayem and between 0.04 and 0.13 at G. Ghaneima. In general, the calculated values are lower than the corresponding K/Al ratio (0.25) of illite (Chuhan et al. 2001), indicating a lower illite content throughout the studied shale samples or the occurrence of illite as a mixed-layer (e.g., illite-smectite mixed-layer). Furthermore, the binary plot of Si/Al ratios against K/Al ratios revealed a weak negative correlation (r = −0.40) for Um El-Ghanayem shale samples and a strong positive correlation (r = 0.98) for Ghaneima shale samples (Supplementary Fig. S3A). Accordingly, the contained illite at G. Um El-Ghanayem is attributed to a diagenetic origin, the transformation of smectite into illite-smectite mixed-layer, whereas illite particles at G. Ghaneima are of detrital origin. For Na/Al ratio, its values (0.01–0.05) fall through the corresponding range of smectite (0.01–0.9) (Bishop et al. 2002) and are also relatively constant, indicating the little variation in smectite content of the studied shale samples. In addition, the positive correlation between Si/Al ratios and Na/Al ratios at G. Um El-Ghanayem (r = 0.79) and G. Ghaneima (r = 0.86) suggests the detrital origin of the contained smectite (Supplementary Fig. S3B). From another side, the negative correlation (r = − 0.60) between Na/Al ratios and K/Al ratios at G. Um El-Ghanayem indicates the relative abundance of illite/illite-smectite mixed-layer compared to smectite. It also supports the diagenetic origin of illite as illite-smectite mixed-layer at the expense of smectite, while the strong positive correlation between the same ratios at G. Ghaneima (r = 0.90) indicates that both smectite and illite are associated with each other as detrital components (Supplementary Fig. S3C).
Implications for nitrate origin
First of all, nitrate salts are well-known for their high water solubility that makes the occurrence of nitrate deposits rarely encountered within the sedimentary successions, with an exception for some extremely arid regions such as the Atacama Desert of Chile, McMurdo Dry Valleys of Antarctica, Mojave Desert of the United States, and Dawadi depression of the Lop Nor basin in China (Zhang et al. 2021). As a contribution to such record, the current study documents, for the first time, the occurrence of nitrate deposits hosted by the Maastrichtian-Early Paleocene Dakhla Shale and the Paleocene-Lower Eocene Esna Shale at G. Um El-Ghanayem and G. Ghaneima in the Western Desert of Egypt. Although isotopes study (e.g., δ15N & δ18O) was inaccessible for the current work, a complete scenario about the origin of the Western Desert nitrate deposits was built up by making integration between the obtained microscopic, mineralogical, and geochemical data.
The microscopic investigations indicate that the studied sections experienced an intensive chemical weathering that left some fingerprints behind, including a partial degradation of the dark carbonaceous laminae, destabilization of some glauconite grains to alunite, and partial alteration of clay minerals into halloysite. So, the preservation of nitrate deposits within these weathered sections suggests an epigenetic origin for such deposits. Another evidence supporting this suggestion can be driven from the weak relationships of NO3− content with both Al2O3 (r = 0.26 and −0.40 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Supplementary Fig. S4A) and CIA values (r = 0.22 and 0.20 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Supplementary Fig. S4B). These weak correlations imply that NO3− content is not related to clay minerals during the transportation from the source area or during the sedimentation process. Both Cr and Zn are well-known for their incorporation into the organic matter fraction from the ambient seawater (Termaz 2005). However, Cr and Zn contents reveal weak correlations with NO3− (r = −0.26 and −0.22, respectively) (Supplementary Fig. S4C, D) that may exclude the authigenic origin of the nitrate content. Moreover, the positive correlation between NO3− and Fe2O3 (r = 0.72 and 0.61 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Supplementary Fig. S4E) refers to the adsorption of NO3− anions on the active surfaces of iron oxyhydroxides that are thought to be formed as a result of the imposed post-depositional chemical weathering. The absence of pyrite peaks from the studied XRD patterns supports this hypothesis. Furthermore, NO3− can be incorporated into the crystal lattice of calcite during diagenesis (Kontrec et al. 2004); however, the binary plot of NO3− against CaO reveals a weak negative correlation between NO3− and CaO (r = −0.13 and −0.10 at G. Um El-Ghanayem and G. Ghaneima, respectively) (Supplementary Fig. S4F). This can be considered another clue for the epigenetic origin of nitrate. At this point, it can be said that the detected nitrate salts are not related to the paleodepositional environment of the studied shale deposits.
From a mineralogical perspective, the epigenetic nitrate precipitation is somewhat controlled by the clay mineral species. For instance, the kaolinite-dominated Dakhla Shale samples at G. Ghaneima are higher in NO3− content (0.88–1.02 wt.%) than the counterpart at G. Um El-Ghanayem (0.36–0.60 wt.%). Unlike the other clay minerals whose crystal structure permanently holds a negative charge (e.g., smectite and illite), kaolinite can be negatively and positively charged on the tetrahedral and octahedral sheets, respectively (Tombácz and Szekeres 2006). Consequently, the dissolved NO3− ion species are expected to be attracted by the positively charged side of kaolinite crystal structure.
Considering nitrate source, nitrogen is naturally brought about from different sources among which atmosphere (4 × 109 kg N year−1), mantle via volcanic eruptions (8.4 × 109 kg N year−1), and oceans (5.7 × 105 kg N year−1) (Houlton et al. 2018).The atmospheric and volcanic eruptions-related nitrogen can be converted into nitrate form by the atmospheric photochemical reactions, while the microbial nitrification by the action of ammonia-oxidizing bacteria and/or archea is the main process responsible for the formation of oceanic nitrate (Pajares and Ramos 2019; Zhang et al. 2021). Although the studied shale deposits were settled down under oxygenating water conditions, as aforementioned by U/Th ratios, the oceanic source of nitrate salts is not favorable here. It can be envisaged that the action of nitrifiers, occurring in the micro-environments between sediment particles, will result in dispersion of nitrate salts within clay laminae; however, the reported nitrate salts are accumulated along the external surface of fissility planes. This is considered another indication to exclude the formation of nitrate salts under the same depositional conditions as the host shales.
From another point of view, the occurrence of nitrate salts in the studied sections from Esna Shale at the upper part, passing through Upper Kharga and Lower Kharga members, to Mawhoob Member at the lower part proposes that the nitrate salts formed as a result of the atmospheric deposition. According to Paerl et al. (2002), the atmospheric deposition of nitrogen compounds occurs either as wet (N is dissolved in the falling rain water) or dry deposition (N is settled down as aerosol and gaseous phases). The atmospheric wet deposition is adopted here as being the driven force for the chemical weathering conditions once imposed on the studied shale deposits. Moreover, this atmospheric deposition may be related to the Late Eocene-Early Oligocene volcanism occurred in the Western Desert of Egypt. Such period is considered to be the second most active Cenozoic volcanism, after the Paleocene volcanic activity, detected in several parts of the Western Desert and characterized by greenhouse climate (Orabi et al. 2015). This claim is built upon: (a) the fact that the volcanic eruptions are among the natural sources of atmospheric nitrogen and the subsequent nitrate deposits such as the Chilean nitrate deposits whose source is partly driven from the Neogene volcanism (Pérez-Fodich et al. 2014) and (b) the epigenetic origin of nitrate deposits in relative to the Early Maastrichtian-Early Paleocene Dakhla Shale and the Paleocene-Lower Eocene Esna Shale. Another proxy for the source of nitrate deposits can be inferred from the weak negative correlation between TOC and NO32− (i.e., r = −0.40 and −0.35 for the Dakhla Shale samples at G. Um El-Ghanayem and G. Ghaneima, respectively), implying that the nitrate deposits were partly formed at the expense of oxidation and microbial nitrification of organic matter. This hypothesis is evidenced by C:N ratio estimated at averages of 24.97 and 24.71 for the Dakhla Shale samples at G. Um El-Ghanayem and G. Ghaneima, respectively. These values indicate a partial microbial mineralization of the organic nitrogen content (Craft et al. 1991).
Conclusion
Nitrogenous shale successions, assigned to the Early Maastrichtian-Early Paleocene Dakhla Formation and the Paleocene-Early Eocene Esna Formation, were documented, for the first time in Egypt, at G. Um El-Ghanayem and G. Ghaneima. These successions were studied in terms of petrography, mineralogy, and geochemistry to reconstruct the paleoenvironmental conditions for shale and deduce the possible origin of nitrate deposits. The majority of shale samples exhibit microbially induced wavy lamination, indicating shallow marine depositional conditions under which the studied shale were sourced from felsic igneous (e.g., Esna Shale, Upper Kharga, and Lower Kharga members) and quartzose sedimentary provenances (e.g., Mawhoob Member) where humid climate prevailed (e.g., 85.64–95.35% CIX values). Moreover, the shale deposition seems to be occurred under oxidizing marine conditions (0.31–0.70 U/Th ratio) through continental rift setting.
From another side, the studied shale is characterized by oval-suboval-shaped aggregates and thin crusts of niter randomly dispersed along fissile planes, resulting in NO3− content (0.36–1.02 wt.%) that can be comparable with the Dawadi potassium nitrate deposits, China (min. 0.09–0.72 wt.%). As being highly water-soluble salts, the occurrence of nitrate aggregates throughout partly weathered shale successions can be considered a clue for the epigenetic origin of such salts. Also, the Western Desert nitrate salts are hypothesized to be formed by microbial nitrification along with atmospheric nitrogen deposition. The latter is thought to be occurred during the Late Eocene/Early Oligocene transition characterized by active volcanic eruptions and warm climate after which dry and cold conditions gradually prevailed.
As a recommendation, the Western Desert of Egypt needs reconnaissance and detailed investigations to find out other localities of nitrate deposits and make resource estimation. Moreover, it is advised to perform agronomic experiments for the studied shale deposits on both lab and field scales in an attempt to reveal the potential application of such deposits as a natural nitrogen fertilizer and soil conditioner.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary files.
References
Alqahtani F, Khalil M (2021) Geochemical analysis for evaluating the paleoweathering, plaeoclimate, and depositional environments of the siliciclastic Miocene-Pliocene sequence at Al-Rehaili area, Northern Jeddah, Saudi Arabia. Arab J Geosci 14(239):1–12
Anderson RF, Fleisher MQ, LeHuray AP (1989) Concentration, oxidation state and particulate flux of uranium in the Black Sea. Cosmochim Acta 53(9):2215–2224
Armstrong-Altrin JS, Madhavaraju J, Vega-Bautista F, Ramos-Vázquez MA, Pérez-Alvarado BY, Kasper-Zubillaga JJ, Bessa AZE (2021) Mineralogy and geochemistry of Tecolutal and Coatzacoalcos beach sediments, SW Gulf of Mexico. Appl Geochem 134:1–10
Awad GH, Ghobrial MG (1965) Zonal stratigraphy of the Kharga oasis. Ministry of industry, general Egyptian organization for geological research and mining. Egyptian Geological Survey 34:1–77
Bassiouni MAA, Luger P (1990) Maastrichtian to Early Eocene ostracoda from southern Egypt (paleontology, paleoecology, paleobiogeography and biostratigraphy). Berliner geowissenschaftliche Abhandlungen 120(2):755–928
Bernhard A (2010) The nitrogen cycle: processes, players, and human impact. Nat Educ Knowl 2(2):1–9
Bingham NL, Slessarev EW, Homyak PM, Chadwick OA (2021) Rock-sourced nitrogen in semi-arid, shale-derived California soils. Front For Glob Chang 4:1–16
Bishop J, Madejova J, Komadel P, Fröschl H (2002) The influence of structural Fe, Al and Mg on the infrared OH bands in spectra of dioctahedralsmectites. Clay Miner 37:607–616
Boyd SR, Philippot P (1998) Precambrian ammonium biogeochemistry: a study of the Moine metasediments, Scotland. Chem Geol 144:257–268
Chuhan FD, BjØrlykke K, Lowrey CJ (2001) Closed-system burial diagenesis in reservior sandstones: exanples from the Garn formation at haltenbanken area, offshore Mid-Norway. J Sediment Res 71:15–26
Craft CB, Seneca ED, Broome SW (1991) Loss on ignition and Kjeldahl digestion for estimating organic carbon and total nitrogen in estuarine marsh soils: calibration with dry combustion. Estuaries 14(2):175–179
Cullers RL (2000) The geochemistry of shales, siltstones and sandstones of Pennsylvanian-Permian age, Colorado, U.S.A.: implications for provenance and metamorphic studies. Lithos 51:181–203
de Leeuw JW, Largeau C (1993) A review of macromolecular organic compounds that comprise living organisms and their role in kerogen, coal, and petroleum formation. In: Engel MH, Macko SA (eds) Organic geochemistry: principles and applications. Plenum Press, New York, pp 23–72
Delmint TO, Quince C, Shaiber A, Esen OC, Lee STM, Rappé MS, McLellan SL, Lücker S, Eren AM (2018) Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat Microbiol 3:804–8013
Ericksen GE (1981) Geology and origin of the Chilean nitrate deposits. US Geol Surv Prof Pap 1188:1–37
Ericksen GE, HostermanSt. Amand JWP (1988) Chemistry, mineralogy and origin of the clay-hill nitrate deposits, Amargosa River Valley, Death Valley Region, California, U.S.A. Chem Geol 67:85–102
Faris M, Shabaan M, Shaker F (2017) Calcareous nannofossil bioevents at the Paleocene/Eocene boundary in the Kharga Oasis, Western Desert of Egypt. Geol Cro 70:179–199
Fedo CM, Nesbitt HW, Young GM (1995) Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 23(10):921–924
Felesteen AW, Zakhera MS (1999) Stratigraphy and petrography of some Upper Cretaceous-Lower Tertiary sediments in the Kharga-Dakhla area, Western Desert, Egypt. Ann Geol Surv Egypt XXII:143–163
Gromet LP, Dymek RF, Haskin LA, Korotev R (1984) The “North American shale composite”: its compilation, major and trace element characteristics. Geochim Cosmochim Acta 48:2469–2482
Hall A, Pereira MD, Bea F (1996) The abundance of ammonium in the granites of central Spain, and the behaviour of the ammonium ion during anatexis and fractional crystallization. Mineral Petrol 56:105–123
Hofmann P, Ricken W, Schwark L, Leythaeuser D (2001) Geochemical signature and related climatic-oceanographic processes for early Albian black shales: Site 417D, North Atlantic Ocean. Cretac Res 22:243–257
Holloway JM, Dahlgren RA (2002) Nitrogen in rock: occurrences and biogeochemical implications. Global Biogeochem Cycles 16(4):1–17
Houlton BZ, Morford SL, Dahlgren RA (2018) Convergent evidence for widespread rock nitrogen sources in Earth’s surface environment. Science 360:58–62
Issawi B (1972) Review of upper cretaceous-lower tertiary stratigraphy in central and southern Egypt. Am Assoc Pet Geol 56:1448–1463
Itihara Y, Suwa K, Hoshino M (1986) Organic matter in the Kavirondian sedimentary rocks of Archaean period in Kenya. Geochem J 20:201–207
Jones B, Manning DC (1994) Comparison of geochemical indices used for the interpretation of paleo-redox conditions in Ancient mudstones. Chem Geol 111:111–129
Knicker H (2004) Stabilization of N-compounds in soil and organic-matter-rich sediments—what is the difference. Mar Chem 92:167–195
Kontrec J, Kralj D, Brečević LJ, Falini G (2004) Incorporation of Inorganic Anions in Calcite. Berichte der deutschen chemischen Gesellschaft 23:4579–4585
Li RS, Daniels WL (1994) Nitrogen accumulation and form over time in young mine soils. J Environ Qual 23:166–172
Li M, Chen J, Wang T, Zhong N, Shi S (2019) Nitrogen isotope and trace element composition characteristics of the Lower Cambrian Niutitang Formation shale in the upper-middle Yangtze region, South China. Palaeogeogr Palaeoclimatol Palaeoecol 501:1–12
Liu B, Song Y, Zhu K, Su P, Ye X, Zhao W (2020) Mineralogy and element geochemistry of salinized lacustrine organic-rich shale in the Middle Permian Santanghu Basin: implications for paleoenvironment, provenance, tectonic setting and shale oil potential. Mar Pet Geol 120:1–13
Luger P (1988) Campanian to paleocene agglutinated foraminifera from freshwater influenced marginal marine (deltaic) sediments of southern Egypt. Abhandlungen der Geologischen Bundesanstalt Wien 41:245–254
Macko SA, Engel MH, Qian Y (1994) Early diagenesis and organic matter preservation—a molecular stable isotope perspective. Chem Geol 116:365–379
Makoundi C (2016) Geochemistry of Phanerozoic carbonaceous black shales, sandstones and cherts in Malaysia: insights into gold source rock potential. Ph.D. thesis, University of Tasmania, pp. 71–80
Mayer LM (1994a) Relationship between mineral surfaces and organic carbon concentrations in soil and sediments. Chem Geol 114:347–363
Mayer LM (1994b) Surface area control of organic carbon accumulation in continental shelf sediments. Geochim Cosmochim Acta 58:1271–1284
Mingram B, Bräuer K (2001) Ammonium concentration and nitrogen isotope composition in metasedimentary rocks from different tectonometamorphic units of the European Variscan Belt. GeochemicaetCosmochimicaActa 65(2):273–287
Mingram B, Hoth P, Harlov DE (2003) Nitrogen potential of Namurianshales in the North German Basin. J Geochem Explor 78–79:405–408
Mohapatra RK, Murty SVS (2000) Search for the mantle nitrogen in the ultramafic xenoliths from San Carlos, Arizona. Chem Geol 164:305–320
Morford SL, Houlton BZ, Dahlgren RA (2016) Geochemical and tectonic uplift controls on rock nitrogen inputs across terrestrial ecosystems. Global Biogeochem Cycles 30:333–349
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 199:715–717
Nioti D, Maravelis A, Tserolas P, Zelilidis A (2013) TOC and CaCO3 content in Oligocene shelf deposits on Lemons Island and their relation with depositional conditions. Bull Geol Soc Greece 47(2):852–861
Orabi OH, Khalil HM (2014) Calcareous benthonic foraminifera across the Cretaceous/Paleocene transition of Gebel Um El-Ghanayem, Kharga Oasis, Egypt. J Afr Earth Sci 96:110–121
Orabi H, El Beshtawy M, Osman R, Gadallah M (2015) Larger benthic foraminiferal turnover across the Eocene-Oligocene transition at Siwa Oasis, Western Desert, Egypt. J Afr Earth Sci 105:85–92
Paerl HW, Dennis RL, Whitall DR (2002) Atmospheric deposition of nitrogen: implications for nutrient over-enrichment of coastal waters. Estuaries 25(4b):677–693
Pajares S, Ramos R (2019) Processes and microorganisms involved in the marine nitrogen cycle: knowledge and gaps. Front Mar Sci 6:1–33
Pérez-Fodich A, Reich M, Álvarez F, Snyder GT, Schoenberg R, Vargas G, Muramatsu Y, Fehn U (2014) Climate change and tectonic uplift triggered the formation of the Atacama Desert’s giant nitrate deposits. Geology 42(3):251–254
Peterson BJ (1981) Perspectives on the importance of the oceanic particulate flux in the global carbon cycle. Ocean Sci Eng 6:71–108
Ramos-Vázquez MA, Armstrong-Altrin JS, Madhavaraju J, Gracia A, Salas-de-León DA (2022) Mineralogy and geochemistry of marine sediments in the northeastern Gulf of Mexico. In: Armstrong-Altrin JS, Pandarinath K, Verma SK (eds) Geochemical treasures and petrogenetic processes. Springer, Singapore, pp 153–183
Ristic ND, Djokic MR, Van Geem KM, Marin GB (2016) On-line analysis of nitrogen containing compounds in complex hydrocarbon matrixes. J Vis Exp 114:1–12
Roser BP, Korsch RJ (1988) Provenance signatures of sandstone mudstone suites determined using discriminant function analysis of major-element data. Chem Geol 67:119–139
Schneider-Mor A, Alsenz H, Ashckenazi-Polivoda S, Illner P, Abramovich S, Feinstein S, Almogi-Labin A, Berner Z, Püttmann W (2012) Paleoceanographic reconstruction of the late Cretaceous oil shale of the Negev, Israel: integration of geochemical, and stable isotope records of the organic matter. Palaeogeogr Palaeoclimatol Palaeoecol 319–320:46–57
Stevenson FJ (1962) Chemical state of the nitrogen in rocks. Geochim Cosmochim Acta 26:797–809
Termaz MGMA (2005) Mineralogical and geochemical studies of carbonaceous shale deposits from Egypt. Ph.D. thesis, Technical University of Berlin, pp. 48–53
Thompson AW, Foster RA, Krupke A, Carter BJ, Musat N, Vaulot D, Kuypers MMM, Zehr JP (2012) Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337(6101):1546–1550
Tombácz E, Szekeres M (2006) Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl Clay Sci 34:105–124
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
vanReeuwijik LP (2002) Procedures for soil analysis, 6th edn. International Soil Reference and Information Centre, Netherlands, pp 1–50
Vassilev S, Eskenazy G, Vassileva C (2000) Contents, modes of occurrence and origin of chlorine and bromine in coal. Fuel 79:903–921
Verma SP, Armstrong-Altrin JS (2013) New multi-dimensional diagrams for tectonic discrimination of siliciclastic sediments and their application to precambrian basins. Chem Geol 355:117–133
Widdison PE, Burt TP (2008) Nitrogen cycle. In: Fath BD (ed) Encyclopedia of ecology. Elsevier, pp 2526–2533
Wong D, Suflita JM, McKinley JP, Krumholz LR (2003) Impact of clay minerals on sulfate-reducing activity in aquifers. Microb Ecol 47:80–86
Werling N, Kaltenbach J, Weidler PG, Schumann R, Dehn F (2022) Solubility of clacined kaolinite, montmorillonite, and Illite in high molar NaOH and suitability as precursors for geopolymers. Clay Clay Miner 70:270–289
Zhang Y, Ma L, Wang K (2021) Genesis of the Dawadi potassium nitrate deposit in Lop Nor, China. Sci Rep 11:1–9
Zhu G, Wang P, Li T, Zhao K, Yan H, Li J, Zhou L (2021) Nitrogen geochemistry and abnormal mercury enrichment of shales from the lowermost Cambrian Niutitang Formation in South China: implications for the marine redox conditions and hydrothermal activity. Glob Planet Chang 199:1–12
Acknowledgements
Authors are so grateful for the great efforts made by journal’s editor and reviewers during the revision process and for the guidance and encouragement of Mr. Mohamed El-Namer “Desert Tiger” and Mr. Ahmed El-Sherief along the road to G. Um El-Ghanayem and G. Ghaneima where huge sand dunes are everywhere.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Domenico M. Doronzo
Highlights
• Successions of nitrate-bearing shale are found at G. Um El-Ghanayem and G. Ghaneima.
• The shale deposition occurred under shallow oxygenating bottom water and humid climate.
• Niter is the dominant nitrate mineral.
• The detected nitrate salts are thought to be of atmospheric and microbial origin.
• The Late Eocene/Early Oligocene volcanism is the main driven force for nitrate deposition.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Hakeem, M., El-Habaak, G., El-Kheir, G.A. et al. Petrography, mineralogy, and geochemistry of nitrogenous shale in the Western Desert of Egypt: implications for paleoenvironment. Arab J Geosci 16, 269 (2023). https://doi.org/10.1007/s12517-023-11360-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11360-x