Abstract
Neoproterozoic Abu Ghalaga ilmenite-bearing mafic rocks were subjected to geological, petrographical and geochemical studies integrated with mineral chemistry of ilmenite, pyrite and magnetite in order to deduce their petrogenesis. Abu Ghalaga mafic intrusions hosting the largest ilmenite reserve in the Egyptian part of the Arabian-Nubian Shield. Field observations revealed that the intrusions impound ilmenite ore, which occurs as interlayer of massive bands or disseminated within gabbroic rocks. Petrographically, Abu Ghalaga mafic intrusions include different kinds of gabbro; olivine pyroxene gabbro, pyroxene gabbro, pyroxene hornblende gabbro, altered gabbro and Fe-Ti-rich gabbro. They have calc-alkaline to tholeiitic magmas, which are depleted in high field strength elements (e.g., Ta, Nb and Th) relative to low field strength elements (e.g., Ba, Sr and Rb), and exhibit light rare earth elements enrichment relative to heavy rare earth elements with positive Eu anomaly (Eu/Eu*= 0.8-2.4). Variable mineralogical and wide variation of bulk rock geochemistry are ascribed to fractional crystallization and hydrothermal overprinting. Abu Ghalaga gabbroic rocks are derived from fractional crystallization of depleted mantle magmas that were modified by ascending fluids from subducted slab in volcanic arc setting. Fe-Ti oxides required high oxygen fugacity (ƒO2) and water contents by crystal settling of Ti-rich mantle which is applicable with subduction setting. It can be inferred that Abu Ghalaga mafic rocks are neither related to ophiolite nor to Alaskan type but to one of the layered intrusions hosting Fe-Ti oxides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Late Proterozoic mafic-ultramafic rocks are widely distributed in Central and Southern Eastern Desert of Egypt in most northwestern part of the Arabian-Nubian Shield (ANS) (Azer and Stern 2007; Lasheen et al. 2021a; Kamar et al. 2022; Saleh et al. 2022). ANS is the northern expansion of Mozambique belt, which developed during Neoproterozoic Era by amalgamation of West and East Gondwana to form the supercontinent Gondwana during the Pan-African orogeny (Stern et al. 2004; Lasheen et al. 2021b; Hamdy et al. 2022; Khaleal et al. 2022). The accretion of arcs and oceanic terrains led to crustal evolution of ANS (Abdelsalam and Stern 1996; Lasheen et al. 2022). The ultramafic exposures constitute 5.3% form the Egyptian crystalline outcrops mostly as allochthonous sheets (Dixon 1979).
According to tectonic regime, mafic-ultramafic rocks can be subdivided into three types: I) deformed/metamorphosed ophiolite with different tectonic setting but mostly supra-subduction zone (forearc) in the south Eastern Desert (SED) (Azer and Stern 2007; Lasheen et al. 2021a; Hamdy et al. 2022; Khaleal et al. 2022), II) roots of arc related so-called mafic-ultramafic of Alaskan-type that are un metamorphosed and concentrically zoned (Farahat and Helmy 2006; Khedr and Arai 2016) and III) layered mafic-ultramafic intrusions of extensional post orogenic related (Abdel Halim et al. 2016). Furthermore, they can be subdivided based on their significant ore deposits as: (type I) the mafic-ultramafic of ophiolite related. These rocks generally host talc, chromite, magnesite and gold-bearing listwanite (e.g., Lasheen et al. 2021a; Hamdy et al. 2022). Alaskan-type (type II) includes valuable platinum group (PGEs), S and Cu (Helmy 2004). Layered intrusion (type III) impound economic Fe-Ti oxides such as ilmenite (Khedr et al. 2020).
Fe-Ti oxides deposits are widely distributed in Eastern Desert including Egyptian black sand deposits, Abu Dahr, Hamra Dome, Umm Effin, Korab Kansi and Abu Ghalaga area. Abu Ghalaga area is considered the first rank that hosts the largest ilmenite reserve ore in Egypt (Makhlouf et al. 2008; Khedr et al. 2020). Amin (1954) studied the ilmenite deposit of Abu Ghalaga area. He concluded that the ilmenite ore is formed by accumulation of ilmenite-rich residual fluid that occur as sheet-like zone on the top of gabbros mass. Abu Ghalaga ilmenite ore represents the largest reserve in Egypt, whereas the second one is recorded in Korab Kansi (Khedr et al. 2020).
The present work aims to study the geology, petrography and geochemistry of the Neoproterozoic Abu Ghalaga mafic intrusions integrated with Fe-Ti oxides chemistry in order to deduce their petrogenesis as well as the tectonic regime of the host rocks.
Geological setting
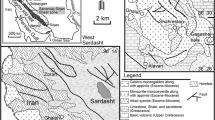
Abu Ghalaga ilmenite mine lies about 25km Southwest of Abu Ghusun City on the Red Sea Coast, at the intersection of Lat. 24° 21' 00" N and long. 35° 3' 35" E and far about 125km from Marsa Alam city. It is accessible from the Red Sea along Wadi Ranga (Fig. 1). The dominant Neoproterozoic rocks of Abu Ghalaga area are superstructure rocks comprising island arc metavolcaniclastics, gabbroic intrusion (rich in ilmenite bands) and syn- to late-granitic intrusions. The island arc metavolcaniclastics crop out as large belts extending nearly NW-SE (cover about 30 km2) and injected by Abu Ghalaga gabbroic rocks and their contents are observed along Wadi Abu Ghalaga. Late granitic rocks crop out as low to moderate relief of isolated masses in the extreme western part of the mapped area with NNW-SSE trend.
a Geographic map of Egypt showing location of Abu Ghalaga. b Geological map Abu Ghalaga area modified from Amin (1947) and Saleh (2012)
Abu Ghalaga gabbroic rocks are medium- to coarse-grained dark green to black color due to high contents of mafic minerals. They crop out at the central part of the mapped area (Fig. 2a) forming the highest topographic feature herein and decrease to small masses at the western side of Wadi Abu Ghalaga. Generally, the contact between Abu Ghalaga intrusion and the syn- to late- granitic intrusions that are exposed in the southern part of the mapped area is gradational in a zone of few meters. Occasionally, this zone is characterized by hybrid diorites.
They hosted Fe-Ti oxides (ilmenite) with different sizes (Fig. 2a). Ilmenite mine of Abu Ghalaga is considered as one of the oldest and largest ilmenite mines in the ED (Amin 1954; Mahmoud 2021) (Fig. 2b,c). The ilmenite ore occurs either as massive bands or disseminated within the gabbroic rocks. Massive bands occur as sheeted-like body trending NNW-SSE and dipping (20°-60°) to the NNE and having length ranging between 50 m and 600 m, and width ranging from 20 m to 300 m with NW-SE trends, conformable with the primary layering of gabbroic rocks (Figs. 2d,e,f).
Petrography
Unmetamorphosed Abu Ghalaga gabbroic rocks comprising (5 varieties), olivine pyroxene gabbro, pyroxene gabbro, pyroxene hornblende gabbro, altered gabbro and Fe-Ti-rich pyroxene gabbro depending on abundance of constituent minerals.
Olivine pyroxene gabbros are mainly composed of plagioclase (55-65 vol.%), clinopyroxene (22-35 vol.%) and olivine (6-8 vol.%) minerals. The main accessory minerals are ilmenite, magnetite and pyrite. Plagioclase crystals are the dominant mineral, commonly pristine tabular laths and rarely saussuritized. They occur as euhedral to subhedral ranging in size from 0.4 × 0.5 to 2.5 × 7 mm in length. Occasionally, they show zoning and pericline but lamellar twinning is common, cumulus textures and corroded clinopyroxene crystals. Clinopyroxene (augite) occurs as anhedral crystals and sometimes enclose numerous ilmenite and plagioclase minerals. Therefore subophitic and ophitic textures can be observed. Fresh olivine occurs as subrounded grains with perfect cracks and represents intercumulus phases (Fig. 3a).
Photomicrographs are taken under crossed polarized (except g and i) of Abu Ghalaga gabbroic intrusion: Abbreviations of minerals are after Whitney and Evans (2010). a Granoblastic olivine (Ol) surrounded by zoned and pericline plagioclase. b Cumulus of plagioclase (Pl) associated with amphiboles. c Plagioclase crystals ophitically and subophitically enclosed in clinopyroxene (Cpx)s. d Clinopyroxene oikocryst enclosing apatite (Ap) minerals and rimed by hornblende (Hbl) forming corona structure. e f Partially to completely saussuritized plagioclase with euhedral and cubic forms of pyrite surrounded by clinopyroxene. g Backscattered image (BSI) of cubic and fractured pyrite crystals. h Intercumulus and disseminated ilmenite (Ilm) with clinopyroxene. i BSI reveals disseminated ilmenite associated with clinopyroxene
Pyroxene gabbros are the dominant rocks type that mainly consist of cumulus of plagioclase (Fig. 3b), and clinopyroxene. Little amounts of hornblende are observed. Ophitic and subophitic textures are common. Corona textures (Cpx is partially transformed into hornblende) are observed (Fig. 3c). Oriented, rod-like of opaques is widely distributed along two intersecting planes (cleavage planes) of clinopyroxene minerals forming schiller structures.
Pyroxene hornblende gabbros are mainly formed of plagioclase, clinopyroxene, brown hornblende and opaques. Brown hornblende (10-15 vol.%) is observed as primary minerals and secondary after clinopyroxene forming corona texture, mostly occurs as brown big oikocrysts enclosing small plagioclase crystals (Fig. 3d).
Altered gabbros are mainly consist of saussuritized plagioclase and intercumulus clinopyroxene that are transformed to hornblende. It is noticed that the opaques are disseminated (Fig. 3e). In addition, pyrite is the main sulfide minerals, which mostly reveals well cubic form and large cluster crystals surrounded by saussuritized plagioclase (Figs. 3f,g).
Fe-Ti oxides-rich pyroxene gabbros are mainly composed of clinopyroxene (45-57 vol.%), opaques (35-46 vol.%) and subordinate plagioclase minerals. Ilmenite and magnetite are the dominant opaque minerals, occurring as anhedral, disseminated or as aggregates in thin section and commonly associated with/ within augite as inclusions (Figs. 3h,i).
Analytical techniques
Microprobe analyses were carried out at the University of New Brunswick (UNB), Canada. Samples were analyzed with a JEOL JXA-733 Electron Probe Microanalysis, equipped with ds spec and Qant32 automation (Geller Micro Analytical Labs). An accelerating voltage of 15 kV and a probe current of 30 nA. Peak counting times were 30 s (Al and Si), 90 s (Ti, V, Cr and Cs), and 120 s (Na, Mg, K, Ca, Mn, Fe and Ni. Bulk rock analysis (major, trace and rare earth elements) were carried out by (ICP-MS) in ACME Labs, Vancouver, Canada (Some samples were tested in Nuclear Material Authority), following fusion with lithium tetraborate/metaborate with digestion by nitric acid. Calibration of the ICP-MS was carried out using standards (STD OREAS45E and STD OREAS25A-4A). Detection limits for trace elements and major oxides were 0.01–0.5 ppm and 0.001 wt%-0.04 wt%, respectively. The analytical precision, as calculated from replicate analyses, was 0.5% for major oxides and varied from 2% to 20% for trace elements.
Environmental scanning electron microscope (ESEM) investigations were made at the Nuclear Material Authority (NMA). The ESEM is equipped with Link Analytical AN-1000/855 energy dispersive X-ray spectrometer (EDX) calibrated using natural standards to identify elements and detect, semi quantitatively, the chemical compositions of minerals, especially Fe-Ti minerals. The accelerating voltage during analysis was 25-30 kV.
Results
Mineral Chemistry
Pyrite
Electron probe microanalyses (EPMA) of Abu Ghalaga pyrite show narrow variation in their composition (Table 1). Trace element concentrations are close to the detection limit such as Zn, Ag and Cu or below of it like Sn, Sb, Pb, Bi, Cd and As. Microprobe study coupled with Ore microscopy revealed that no gold grains were detected within this pyrite.
EDX such as EPMA analyses of the examined pyrite gave the same results (Fig. 4a). The main element in Abu Ghalaga pyrite is sulfur that ranges from 53.17 to 55.66 with average 54 wt.%. Fe in the examined Abu Ghalaga pyrites ranges from 44.86 to 46.19 with an average value of 45.68 wt.%. It can be inferred that the Abu Ghalaga pyrites are deficient in Fe content and contain excess of S relative to theoretical values of pyrite (Fe = 46.55; S = 53.45 wt.%; Baiwah et al. 1987; and Xu et al. 2020). Fe/S and S/Fe ratios of the investigated Abu Ghalaga pyrites vary from 0.809 to 0.862 and 1.15 to 1.23, respectively.
Fe-Ti oxides
Chemical compositions of Abu Ghalaga magnetite and ilmenite samples are listed in Tables 2 and 3, respectively. EDX and EMPA of magnetite (Fig. 4b) revealed that they have high content of FeO* (av. 94 wt.%). They possess ulvite less than 0.5 mole% based on Stromer (1983) (Fig. 5a).
TiO2 varies from 45.98 to 50.37 wt.% and FeO* varies from 40.27 to 45.19wt.% with their corresponding average values 48 wt.% and 43 wt.%, respectively, for the examined ilmenites (Fig. 4c). The average values of SiO2, Al2O3 as well as MgO are 1.62 wt.%, 1.83 and 3.14, respectively. Furthermore, MnO, Na2O, K2O, Cr2O3, ZnO, NiO and V2O5 contents are less than unity. Abu Ghalaga ilmenites contain lower contents of MgO, TiO2 and high FeO* than those of Korab Kansi (7.8, 57.2 and 35.3 wt.%, respectively) (Khedr et al. 2020). According to Stromer (1983), ilmenite components are calculated. Their compositions are close to pure ilmenite since they vary from 89.69% to 97.99% with an average value of 94.25%.
Whole-rock geochemistry
Major oxides
Chemical analyses of representative samples from Abu Ghalaga gabbroic rocks were carried out for major oxides, trace elements and REEs and are given in Table 4. The examined rocks belong to gabbroic rocks (Fig. 5b) as indicated by bivariate Zr/TiO2*0.0001 versus Nb/Y ratios of Winchester and Floyd (1977). They range from clac-alkaline to tholeiitic on AFM ternary diagram of Irvine and Baragar (1971; Fig. 5c).
Variable mineralogical contents of Abu Ghalaga gabbroic rocks as well as major, trace and REEs of the present study (as discussed below) are due to fractional crystallization processes.
Generally, the examined gabbroic rocks show a wide variation in their chemical composition with respect to SiO2 (46.55 - 50.12), Al2O3 (13.26 - 17.49 wt.%), Fe2O3 (9.66 - 13.65 wt.%), MgO (4.62 - 8.57 wt.%), CaO (8.79 - 11.14 wt.%), total alkali from 3.24 to 4.86 wt.%, high ratios of Na2O/K2O (1.22 - 6.71) with average value 2.79 wt.%, Al2O3/TiO2 (7.73 - 19.05) with average value 11.09 wt.% and CaO/TiO2 (4.83-10.77, with mean 7.05 wt.%). Fresh samples are indicated by low LOI (1.6-2.8; av. 2.076 wt.%). They contain Mg# (Fe/Fe+Mg) that varies from 54.82 to 72.26. Slightly high concentrations of TiO2 (0.86 - 1.86, 1.42 wt.%) as a result of abundance of opaque minerals and fractionation processes (Sun and Nesbitt 1977) and high content of CaO are due to enrichment of Ca-plagioclase/clinopyroxene. Fe2O3/MgO exhibits narrow and low content from 1.21 to 2.61 wt.%, and CaO/Al2O3 ranges from 0.54 to 0.84 wt.% with mean values 1.71 wt.% and 0.64 wt.%, respectively (Table 4).
Trace elements and rare earth elements (REEs)
Abu Ghalaga gabbroic rocks exhibit enrichment and wide variation of low field strength elements (LFSEs) such as Ba (120-265, 164.1 ppm) and Sr (145-620, 325.53 ppm), high field strength elements (HFSEs) such as Zr (49-151, 91.2 ppm) and Y (21-49, 29.26 ppm) and transition elements as like V (195-450, 319 ppm), Co (35-162, 84.93 ppm), Ni (20-132, 76.6 ppm), Zn (29-97, 69.2 ppm) and Cu (31-175, 99.8 ppm). Table 4 reveals that the hornblende pyroxene gabbros possess the lowest Rb (8 ppm), Sr (~159 ppm), Zr (~70 ppm), Zn (60 ppm), Cu (60 ppm) and Ni (~33 ppm) concentrations relative to other gabbroic rocks.
Primitive-mantle normalized trace elements (Fig. 6a) reveal strong Ni negative anomaly as a result of immobile feature during partial melting (McCulloch and Gamble 1991) and enrichment of LFSEs relative to HFSEs, which is ascribed to mantle melt at arc with low partial melting (Jaques and Green 1980). Furthermore, small negative anomaly of Nb is observed which may be related to the role of crustal contamination of parent gabbro as well as depletion of Nb relative to enriched LFSEs (Taylor and McLennan 1985).
a Spider diagrams of trace elements normalized to the primitive mantle (PM). b Rare earth element spider diagrams normalized to chondrite (Sun and McDonough 1989)
Abu Ghalaga rocks possess high contents of light rare earth elements (LREEs) (39.14 - 44.56, 41.76 ppm relative to heavy rare earth elements (HREEs, 9.06 - 21.42), with LREEs/ HREEs ratio (2.46) (Table 4). Primitive-normalized REEs of the studied rocks reveal smooth shape (Fig. 6b). They reveal good fractionated REE patterns (La/Yb)N av.= (1.62). Light and heavy rare earth elements are well fractionated (La/Sm av.= 1.34) and (Gd/Yb av.= 1.04), respectively. It is noticeable (Table 4) that the positive Eu anomaly (Eu/Eu*= 0.8-2.4) may be related to accumulations of plagioclase that cause also LREEs enrichment than HREEs.
Discussion
Crustal contamination and fractionation
Mantle-derived magmas can be contaminated during ascending to shallow levels by crustal sediments (Hora et al. 2009). Some trace elements and their ratios (La/Zr, Nb/Zr and Y/Nb,) can be used to manifest the possible crustal contamination (Lippard 1973). Low and narrow ranges of these ratios reflect negligible effect of crustal contamination. Crustal contamination played a minor role on the examined rocks, where these ratios are low with narrow variations (0.178-0.146 of La/Zr; 0.026-0.1 of Nb/Zr and 3.62-15 of Y/Nb). Fractional crystallization is indicated by variable factors as indicated by petrographic studies (as cumulate textures) and bulk rock geochemistry (e.g., wide variance of Ni (20-132 ppm) as well as relatively low contents of Cr2O3).
Partial melting and oxygen fugacity
REEs abundance can be used to measure the partial melting degree of the mantle sources (Zhu et al. 2008). Enrichment/depletion mantle degrees can be manifested by using binary diagram of Aldanmaz et al. (2009) and compared with melting curves of Bezard et al. (2011). The examined rocks revealed a horizontal trend on spinel + lherzolite sources with 5 to 30% partial melting degree (Fig. 7a). Fractionation of clinopyroxene and amphiboles has a positive relationship with Zr, while fractionation of olivine and plagioclase is not modified with Zr/Y ratio (Floyd 1993). There are two partial melting curves according to Drury (1983); curve (I) has 60% olivine +20% opx +10% cpx +10% plagioclase, and curve (II) has 60% olivine +20% opx +10% cpx +10% garnet. The examined gabbroic samples show a positive relationship on Zr against Zr/Y diagram and plot near or follow curve II, suggesting medium to high partial melting degree of mafic phases ± plagioclase from clinopyroxene fractionation according to Drury (1983) (Fig. 7b). Fractional crystallization vs partial melting arrows in Ce/Yb-Ce relationship. The examined samples plot follow the fractional crystallization trend (Fig. 7c). The investigated Abu Ghalaga layered intrusions are modified by fluids from subducted slab that play a significant role in transporting of some elements such as Sr, Nd and Yb (Fig. 7d).
a Binary Sm/Yb vs. La/Sm diagram after Aldanmaz et al. (2009). Curves of melting degree after Bezard et al. (2011). b Zr/Y–Zr diagram, fractional crystallization after Floyd (1993) and partial melting curves I after Drury (1983). c Bivariate Ce/Yb vs. Ce. d Binary variation diagram between Sr/Nd and Th/Yb
High oxygen fugacity (ƒO2) is indicated by massive layers of ilmenite intercalated with the host rocks (Fig. 2), and intercumulus of ilmenite and magnetite (Figs. 3h,i) reflects crystallization under high oxidizing conditions from Ti-rich melts (e.g., Toplis and Carroll 1995).
Tectonic setting
HFSE and transition elements mostly used for discriminating tectonic setting of igneous and metamorphic rocks due to their immobility during low grade of metamorphism (Manya 2014; Lasheen et al. 2021a). Abu Ghalaga gabbroic rocks are enriched in LFSEs than in HFSEs, which is consistent with subduction affinity. In addition, slightly enrichments of LREEs relative to HREEs (Table 4) may be ascribed to the nature of subduction zone since they are added from hydrous fluids to the mantle source during subduction (Wehrmann et al. 2014; Khedr et al. 2020). It is known that the HFSEs and HREEs are immobile; therefore, they can be used as an indicator for magma from which Abu Ghalaga gabbroic rocks were derived.
Volcanic arc affinity can be inferred by some ratios since the examined rocks have average values of Hf/Ta ratios 5.67 (>5), Nb/La ratios 0.74 (≤1), Hf/Th ratios of 2.17 (<8) and Th/Yb ratios 0.65 (>0.1), similar to those of Condie (1989) and Azer et al. (2014). In addition, ARC, MORB and OIB can be distinguished as manifested in Nb/U versus Nb diagram of O’Neill and Jenner (2012). The majority of the studied samples straddling arc field (Fig. 8a). According to Pearce and Gale (1997) diagrams, the examined samples occupy volcanic arc field using SiO2 vs. Nb binary diagram (Fig. 8b).
Why Abu Ghalaga gabbroic rocks are considered as layered intrusion not ophiolitic?
Calc-alkaline magmatism can be subdivided into two groups. The first is older and deformed of syn-/late orogenic, while the second is undeformed post-collision and commonly related to post-collision (Be’eri-Shlevin et al. 2011). Based on the geology, petrography and bulk rock geochemistry, the examined Abu Ghalaga mafic rocks are neither related to ophiolite nor to Alaskan type. Moreover, ophiolitic mafics-ultramafics are deformed/metamorphosed and hosting chromite, magnesite and talc, when compared with Abu Ghalaga gabbroic rocks (not hosting these deposits). Alaskan mafic rocks are concentrated and enriched in Cu, PGEs and S in contrast to Abu Ghalaga gabbroic rocks host Fe-Ti oxides. Mg# of the Abu Ghalaga rocks [54.82 - 64.96 (except pyroxene hornblende gabbro>70)] is lower than that of ophiolitic gabbroic rocks (61- 81; Ali et al. 2010) but consistent with the Egyptian layered intrusion (Mg# <68.76; Essawy et al. 1997). In view of that, the examined Abu Ghalaga mafic rocks are neither related to ophiolite nor to Alaskan type.
Genesis of Pyrite
Pyrite is the main sulfide minerals present in various systems, especially hydrothermal one. It is resistant to different physical and chemical conditions and is reliable for gold mineralization (Deditius et al. 2011; Xu et al. 2020). Pyrite genesis can be inferred through using transition elements and their ratios such as Co and Ni (Cook et al. 2009; Zhang et al. 2014; Bierlein et al. 2016; Adam et al. 2020).
There is a good relationship between Co of pyrite and its temperature, since high-temperature pyrite contains Co>1000 ppm, 100-1000 ppm for the medium-temperature one, and less than 100 ppm for the low-temperature one (Li et al. 2017). Furthermore, high Co/Ni ratio reflects high-temperature pyrite (Mao et al. 2009; Li et al. 2017). Abu Ghalaga pyrite contains Co concentration more than 1000 ppm and ranges from 2114 to 10826 ppm with average value of 7062 ppm, suggesting high-temperature pyrites. In addition, Co/Ni ratio of the examined Abu Ghalaga pyrites varies from 7.86 to 30.84 with corresponding mean 15.14. As in the examined pyrite is below the detection limit since it may replace S in pyrite. As is enriched in low-temperature regime and flushed out in high-temperature pyrites (Yan et al. 2014; Leng 2017).
Depth of pyrite at which it is formed can be inferred from Fe/(S + As) ratio. Deep depth contains 0.846, medium depth contains 0.863, and the shallower ones contain 0.926 according to Zhou et al. (2005). In Abu Ghalaga pyrite, Fe/(S + As) ratio ranges from 0.809 to 0.862 with an average value 0.848, reflecting deep-depth. Moreover, Ni content of Abu Ghalaga pyrite varies from 0. 0116 to 0.0793 wt.% with average value 0.0514 wt.%, close to that of the mantle (2200 ± 500×10–6), indicating their mantle origin.
Source of pyrite
Abu Ghalaga pyrite is depleted in Zn, Ag, Cu, Bi, Cd, Sn, Sb, Mn, Se, Pb, Ni and As that may be attributed to the depletion of these elements in the initial/parent rock or fluids (Reich et al. 2016). Moreover, their concentrations depend on conditions at which pyrite is recrystallized and nature of pyrites refractory (Hazarika et al. 2013; Zhang et al. 2015). Metamorphic pyrite encompasses Co<100 ppm and Co/Ni<1, while hydrothermal one contains Co>400ppm and Co/Ni>1 according to Carstens (1941). Sedimentary pyrite contains Co/Ni<1, whereas Co/Ni>1 refers to magmatic/hydrothermal origin according to Clark et al. (2004). Occasionally, Co/Ni ratio is less than unity in hydrothermal pyrite as a result of interaction of fluids and wall rocks (Bajwah et al. 1987; Bierlein et al. 2016). Abu Ghalaga pyrite has Co/Ni ratio ranging from 7.86 to 30.84 with average value 15, suggesting their magmatic/hydrothermal nature, which is consistent with their host rocks (gabbro) (Fig. 8c).
In addition, nature of fluids that precipitate pyrite is indicated by Ni content (Zhao et al. 2011). Ni content in mafic rocks (as in the examined gabbro of Abu Ghalaga) reaches up to 150 ppm (Hofmann 1988), and < 50 ppm in felsic igneous rocks (Rudnick and Gao 2003). In the present study, Ni content of the studied pyrite ranges from 116 to 793 ppm, suggesting that the ascending fluids may have interact with mafic wall rock.
The investigated pyrite contains sulfur range from 53.17 to 55.66 wt.%, which may be related to different sources such as seawater, hydrothermal genesis and syngenetic sulfides (Zoheir et al. 2019). Using the dominant element in the examined pyrites (sulfur), hydrothermal type is depleted in S, while the sedimentary one is enriched in S (Xu and Shao 1980). The deficient character of S/Fe ratio of the examined pyrites (1.159–1.235 with mean 1.178) reflects their hydrothermal origin. From the previous, it can be deduced that the Abu Ghalaga pyrites are of magmatic/hydrothermal nature sources.
Tectonic modelling and petrogenesis of Fe-Ti oxides
Fe-Ti oxides can be developed by fractionation of Fe-Ti mantle magma or by partial melting of Fe-Ti mantle magma. Fe-Ti oxides of Abu Ghalaga gabbroic rocks are widely accepted to be formed by fractional crystallization of Fe-Ti-rich minerals by crystal settling (gravitational separation of the earlier formed minerals (Basta and Takla 1968). This takes place as a result of fluids derived from the subducted slab in lithosphere, which yields molten magma enriched with Fe-Ti oxides. The crystal settling of the earlier minerals leads to accumulation and formation of massive layer of ilmenite intercalated within gabbroic rocks (Fig. 9). In addition, Fe-Ti oxides enrichment in Abu Ghalaga layered intrusions may be related to Ti enrichment in the slab-derived fluids leading to heterogeneity of ANS mantle (Jennings et al. 2016). The formation of Fe-Ti oxides requires high oxygen fugacity (ƒO2) and water contents, which is applicable with subduction signature (Howarth and Prevec 2013).
Schematic model for Fe-Ti oxide-bearing gabbroic intrusions. Oceanic plate collides with a continental one; therefore, the oceanic plate subducted beneath the continental one. Water and fluids enriched in LFSEs were driven from the subducted slab leading to partial melting mantle rocks. Fe-Ti oxides are formed by crystal settling processes associated with fractional crystallization of these magma-forming gabbroic rocks.
Conclusions
The current study discusses field, petrographic and bulk rock geochemistry of Abu Ghalaga mafic intrusions coupled with mineral chemistry of Fe-Ti oxides and pyrite. They host significant Fe-Ti oxides as massive layer or disseminated in gabbroic rocks. The examined intrusion exhibits enrichment of LFSEs relative to HFSEs, Nb, Ta negative anomalies and positive Eu anomaly, suggesting arc signature. Moreover, volcanic arc affinity can be inferred by some ratios such as Hf/Ta, Nb/La, Hf/Th and Th/Yb ratios. Crustal contamination played a minor role in the crystallization of the examined Abu Ghalaga gabbros, as indicated by low and narrow variations of La/Zr, Nb/Zr and Y/Nb. Fractional crystallization of Fe-Ti mantle is a responsible factor as indicated by petrographic study (as cumulate textures) and wide variation in bulk rock geochemistry. Abu Ghalaga pyrite has Co/Ni ratio more than the unity, suggesting their hydrothermal/magmatic nature. Fe oxide is magnetite that contains FeO* (av. 94 wt.%) with less than 0.5 mole% ulvite, while Ti oxide is ilmenite that contains TiO2 elevated from 45.98 to 50.37 wt.% and FeO* from 40.27 to 45.19wt.%. Abu Ghalaga gabbroic rocks are of layered intrusion rather than those of Alaskan and ophiolitic types.
References
Abdel Halim AH, Helmy HM, Abd El-Rahman YM, Shibata T, El Mahallawi MM, Yoshikawa M, Arai S (2016) Petrology of the Motaghairat mafic-ultramafic complex, Eastern Desert, Egypt: a high-Mg post-collisional extension-related layered intrusion. J Asian Earth Sci 116:164–180
Abdelsalam MG, Stern RJ (1996) Sutures and shear zones in the Arabian–Nubian Shield. J Afr Earth Sci 23:289–310
Adam MMA, Lv X, Abdel Rahman AA, Stern RJ, Abdalrhman AA, Ullah Z (2020) In-situ sulfur isotope and trace element compositions of pyrite from the Neoproterozoic Haweit gold deposit, NE Sudan: Implications for the origin and source of the sulfur. Ore Geol Rev 120:103–405. https://doi.org/10.1016/j.oregeorev.2020.103405
Aldanmaz E, Schmidt M, Gourgaud A, Meisel T (2009) Mid-ocean ridge and supra-subduction geochemical signatures in spinel– peridotites from the Neotethyan ophiolites in SW Turkey: implications for upper mantle melting processes. Lithos 113:691–708
Ali KA, Azer MK, Gahlan HA, Wilde SA, Samuel MD, Stern RJ (2010) Age constraints on the formation and emplacement of Neoproterozoioc ophiolites along the Allaqi-Heiani suture, South Eastern Desert of Egypt. Gondwana Res 18:583–595
Amin MS (1954) The ilmenite deposit of Abu Ghalaga, Egypt. Report, 5 pp
Azer MK, Stern RJ (2007) Neoproterozoic (835-720 Ma) serpentinites in the Eastern Desert, Egypt: Fragments of Fore-arc mantle. J Geol 15:457–472
Azer MK, Obeid MA, Gahlan HA (2014) Late Neoproterozoic layered mafic intrusion of arc-affinity in the Arabian-Nubian Shield: A case study from the Shahira layered mafic intrusion, southern Sinai, Egypt. Geologica Acta, 14: 2 3 7 - 2 5 9.
Bajwah ZU, Seccombe PK, Offler R (1987) Trace element distribution Co: Ni ratios and genesis of the Big Cadia iron-copper deposit, New South Wales, Australia. Mineral Deposita 22(4):292–300
Basta EZ, Takla MA (1968) Petrological studies on Abu Ghalaga ilmenite occurrence, Eastern Desert. Egypt J Geol 12:43–72
Be’eri-Shlevin Y, Samuel MD, Azer MK, Rämö OT, Whitehouse MJ, Moussa HE (2011) The Ediacaran Ferani and Rutig volcano-sedimentary successions of the northernmost Arabian–Nubian Shield (ANS): New insights from zircon U-Pb geochronology, geochemistry and O-Nd isotope ratios. Precambrian Res 188:21–44
Bezard R, He’bert R, Wang C, Dostal J, Dai J, Zhong H (2011) Petrology and geochemistry of the Xiugugabu ophiolitic massif, western Yarlung Zangbo suture zone, Tibet. Lithos 125:347–367
Bierlein FP, Reynolds N, Arne D, Bargmann C, McKeag S, Bullen W (2016) Petrogenesis of a Neoproterozoic magmatic arc hosting porphyry Cu-Au mineralization at Jebel Ohier in the Gebeit Terrane, NE Sudan. Ore Geol Rev 79:133–154. https://doi.org/10.1016/j.oregeorev.2016.05.010
Brill B (1989) Trace-element contents and partitioning of elements in ore minerals from the CSA Cu–Pb–Zn deposit, Australia. Can Mineral 27:263–274
Carstens CW (1941) Om geokjemiske undersøkelser av malmer. Norsk Geol Tids 21:213–221
Clark C, Grguric B, Mumm AS (2004) Genetic implications of pyrite chemistry from the Palaeoproterozoic Olary Domain and overlying Neoproterozoic Adelaidean sequences, northeastern South Australia. Ore Geol Rev 25(3–4):237–257
Condie KC (1989) Geochemical changes in basalts and andesites across the Archean – Proterozoic boundary: identification and significance. Lithos 23(1-2):1–18
Cook NJ, Ciobanu CL, Mao J (2009) Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China Craton (Hebei Province, China). Chem Geol 264(1–4):101–121
Deditius AP, Utsunomiya S, Reich M, Kesler SE, Ewing RC, Hough R, Walshe J (2011) Trace metal nanoparticles in pyrite. Ore Geol Rev 42(1):32–46
Dixon TH (1979) Age and chemical characteristics of some pre-Pan-African rocks in the Egyptian Shield. Precambrian Res 14:119–133
Drury SA (1983) The petrogenesis and tectonic setting of Archaean metavolcanics from Karnataka state, south India. Geochim Cosmochim Acta 47:317–329
Farahat ES, Helmy HM (2006) Abu Hamamid Neoproterozoic Alaskan-type complex, south Eastern Desert, Egypt. J Afr Earth Sci 45:187–197
Floyd PA (1993) Geochemical discrimination and petrogenesis of alkaline basalt sequences in part of the Ankara mélange, Central Turkey. J Geol Soc Lond 150:541–550
Hamdy MM, Lasheen ESR, Abdelwahab W (2022) Gold-bearing listwaenites in ophiolitic ultramafics from the Eastern Desert of Egypt: Subduction zone-related alteration of Neoproterozoic mantle? J Afr Earth Sci 104574. https://doi.org/10.1016/j.jafrearsci.2022.104574
Hazarika P, Mishra B, Saravanan Chinnasamy S, Bernhardt HJ (2013) Multi-stage growth and invisible gold distribution in pyrite from the Kundarkocha sediment-hosted gold deposit, eastern India. Ore Geol Rev 55:134–145
Helmy HM (2004) Cu-Ni-PGE mineralization in the Genina Gharbia mafic-ultramafic intrusion, Eastern Desert Egypt. Canad Mineral 42:351–370
Hofmann A (1988) Chemical differentiation of the Earth: the relationship between mantle, continental crust, and oceanic crust. Earth Planet Sci Lett 90:297–314
Hora JM, Singer BS, Wörner G, Beard BL, Jicha BR, Johnson CM (2009) Shallow and deep crustal control on differentiation of calc-alkaline and tholeiitic magma. Earth Planet Sci Lett 285(1-2):75–86
Howarth GH, Prevec SA (2013) Hydration vs. oxidation: Modelling implications for Fe-Ti oxide crystallization in mafic intrusions, with specific reference to the Panzhihua intrusion, SW China. Geosci Front 4:555–569
Irvine TN, Baragar WR (1971) A guide to chemical classification of the common volcanic rocks. Can J Earth Sci 8:523–548
Jaques AL, Green DH (1980) Anhydrous melting of peridotite at 0–15 Kb pressure and the genesis of tholeiitic basalts. Contrib Mineral Petrol 73:287–310
Jennings ES, Holland TJ, Shorttle O, Maclennan J, Gibson SA (2016) The composition of melts from a heterogeneous mantle and the origin of ferropicrite: application of a thermodynamic model. J Petrol 57:2289–2310
Kamar MS, Salem IA, El-Aassy IE, El-Sayed AA, Awad HA, Tekin HO, Alzahrai AM, Lasheen ESR (2022) Petrology and geochemistry of multiphase post-granitic dikes: A case study from the Gabal Serbal area, Southwestern Sinai, Egypt. Open Chem 20:169–181. https://doi.org/10.1515/chem-2022-0136
Khaleal FM, Saleh GM, Lasheen ESR, Alzahrani AM, Kamh SZ (2022) Exploration and Petrogenesis of Corundum-Bearing Pegmatites: A Case Study in Migif-Hafafit Area, Egypt. Front Earth Sci 10:869828. https://doi.org/10.3389/feart.2022.869828
Khedr MZ, Arai S (2016) Petrology of a Neoproterozoic Alaskan-type complex from the Eastern Desert of Egypt: implications for mantle heterogeneity. Lithos 263:15–32
Khedr MZ, El-Awady A, Arai S, Hauzenberger C, Tamura A, Stern RJ, Morishita T (2020) Petrogenesis of the ~740 Korab Kansi mafic-ultramafic intrusion, South Eastern Desert of Egypt: Evidence of Ti-rich ferropicritic magmatism. Gondwana Res 82:48–72. https://doi.org/10.1016/j.gr.2019.12.013
Lasheen ESR, Saleh GM, Khaleal FM, Alwetaishi M (2021a) Petrogenesis of Neoproterozoic Ultramafc Rocks, Wadi Ibib—Wadi Shani, South Eastern Desert, Egypt: Constraints from Whole Rock and Mineral Chemistry. Appl Sci 11:10524. https://doi.org/10.3390/app112210524
Lasheen ESR, Rashwan MA, Osman H, Alamri S, Khandaker MU, Hanfi MYM (2021b) Radiological Hazard Evaluation of Some Egyptian Magmatic Rocks Used as Ornamental Stone: Petrography and Natural Radioactivity. Materials 14:7290. https://doi.org/10.3390/ma14237290
Lasheen ESR, Azer MK, Ene A, Abdelwahab W, Zakaly HMH, Awad HA, Kawady NA (2022) Radiological hazards and natural radionuclide distribution in granitic rocks of Homrit Waggat area, Central Eastern Desert, Egypt. Materials 15:4069. https://doi.org/10.3390/ma15124069
Li JL, Zhang YM, Gu XX, Meng FJ, Gao HJ, Wang L (2017) Geological characteristics of the Xiyi MVT-type Pb-Zn ore deposit in Yunnan and EPMA analysis of the sulfides. Geol Explor 53:23–34 (In Chinese)
Lippard SJ (1973) The petrology of phonolites from the Kenya rift. Lithos 6:217–234
Mahmoud MAM (2021) The Environmental and Radiological Impacts in Abu Ghalaga Ilmenite Mine, South Eastern Desert, Egypt. Inter J Mining Sci 7:10–19
Makhlouf A, Beniamin NY, Mansour MM, Mansour SA, El-Shrbeni H (2008) Mafic-ultramafic intrusion of South Korab Kansi area with emphasis on titanomagnetite ores, south Eastern Desert, Egypt. Annals Geol Surv Egypt. XXXV:1–20
Manya S (2014) Geochemistry of the Palaeoproterozoic gabbros and granodiorites of the Saza area in the Lupa Goldfield, southwestern Tanzania. J Afr Earth Sci 100:401–408
Mao JW, Shao YJ, Xie GQ, Zhang JD, Chen YC (2009) Mineral deposit model for porphyry-skarn polymetallic copper deposits in Tongling ore dense district of Middle-Lower Yangtze Valley metallogenic belt. Mineral Deposita 28:209–219 (In Chinese)
McCulloch MT, Gamble JA (1991) Geochemical and geodynamical constraints on subduction zone magmatism. Earth Planet Sci Lett 102:358–374
O’Neill HSC, Jenner FE (2012) The global pattern of trace-element distributions in ocean floor basalts. Nature 491:698
Pearce JA, Gale GP (1977) Identification of ore deposition environment from trace elements geochemistry of associated igneous host rocks. In: Volcanic Processes in Ore Genesis 7. Inst. Geological Society of London, Special Publication, pp. 14–24
Reich M, Simon AC, Deditius A, Barra F, Chryssoulis S, Lagas G (2016) Trace element signature of pyrite from the Los Colorados iron oxide-apatite (IOA) deposit, Chile: a missing link between Andean IOA and iron oxide copper-gold systems? Econ Geol 111(3):743–761
Rudnick RL, Gao S (2003) The composition of the continental crust. In: Rudnick RL (ed) The Crust. Elsevier-Pergamon, Oxford, pp 1–64
Saleh GM (2012) Geochemical characteristic of of some mineralization in Wadi El Gemal-Abu Ghusun area, South Eastern Desert, Egypt. Report, pp. 45
Saleh GM, Khaleal FM, Lasheen ESR (2022) Geochemistry and paleoweathering of metasediments and pyrite-bearing quartzite during the Neoproterozoic Era, Wadi Ibib-Wadi Suwawrib, South Eastern Desert, Egypt. Arab J Geosci 15:51. https://doi.org/10.1007/s12517-021-09141-5
Stern RJ, Johnson PJ, Kröner A, Yibas B (2004) Neoproterozoic ophiolites of the Arabian–Nubian Shield. In: Kusky T (ed) Precambrian Ophiolites. Elsevier, Amsterdam, pp 95–128
Stromer JR (1983) The effects of recalculation on estimates of temperatures and oxygen fugacity from analyses of multicomponent iron-titanium oxides. Am Mineral 68:586–594
Sun SS, McDonough WF (1989) Chemical and isotopic systematics of oceanic basalts: implication for mantle composition and processes. In A. D. Saunder, and M. J. Norry (Eds.), Magmatism in the Ocean Basins, 42, 313-345. Journal of Geological Society of London, Special Publications. https://doi.org/10.1144/GSL.SP.1989.042.01.19
Sun SS, Nesbitt RW (1977) Chemical heterogeneity of the Archaean mantle, composition of the bulk earth and mantle evolution. Earth Planet Sci Lett 35:429–448
Taylor SR, McLennan SM (1985) The continental crust: Its composition and evolution (Oxford: Blackwell scientific field), P. 312
Toplis MJ, Carroll MR (1995) An experimental study of the influence of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J Petrol 36:1137–1170
Wehrmann H, Hoernle K, Jacques G, Garbe-Schönberg D, Schumann K, Mahlke J, Lara L (2014) Sulphur and chlorine geochemistry of mafic to intermediate tephras from the Chilean Southern Volcanic Zone (33–43°S) compared with those from the Central American Volcanic Arc. Int J Earth Sci 103:1945–1962
Whitney DL, Evans BW (2010) Abbreviations for names of rock-forming minerals. Am Mineral 95:185–187. https://doi.org/10.2138/am.2010.3371
Winchester JA, Floyd PA (1977) Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem Geol 20:325–343
Xu GF, Shao JL (1980) Typomorphic characteristics of pyrite and its significance. Int Geol Rev 26:541–546 (In Chinese)
Xu N, Wu C, Li S, Xue B, Xiang He Yan-long Yu, Liu J (2020) LA-ICP-MS in situ analyses of the pyrites in Dongyang gold deposit, Southeast China: Implications to the gold mineralization, China Geol 3: 230-246. https://doi.org/10.31035/cg2018123
Yan Y, Zhang N, Li S, LiY (2014) Mineral chemistry and isotope geochemistry of pyrite from the Heilangou gold deposit, Jiaodong Peninsula, Eastern China. Geosci Front 5: 205-213. https://doi.org/10.1016/j.gsf.2013.05.003
Zhang J, Deng J, Chen H, Yang L, Cooke D, Danyushevsky L, Gong Q (2014) LA-ICP-MS trace element analysis of pyrite from the Chang'an gold deposit, Sanjiang region, China: Implication for ore-forming process. Gondwana Res 26: 557-575. https://doi.org/10.1016/j.gr.2013.11.003
Zhang Y, Kusky T, Wang L, Jianwei J, Feng P, Huang Y, Giddens R (2015) Occurrence of gold in hydrothermal pyrite, western Taupo Volcanic Zone. Geodinamica Acta, New Zealand. https://doi.org/10.1080/09853111.2015.1113024
Zhao HX, Frimmel HE, Jiang SY, Dai BZ (2011) LA-ICP-MS trace element analysis of pyrite from the Xiaoqinling gold district, China: implications for ore genesis. Ore Geol Rev 43:142–153
Zhou XW, Li SR, Lu L, Li JJ, Wang JZ (2005) Study of Pyrite typomorphic characteristics of Wulong quartz-vein-type gold deposit in Dandong, Liaoning Province, China. Geosci 19:231–238
Zhu D, Mo X, Pan G, Zhao Z, Dong G, Shi Y, Liao Z, Wang L, Zhou C (2008) Petrogenesis of the earliest Early Cretaceous mafic rocks from the Cona area of the eastern Tethyan Himalaya in south Tibet: Interaction between the incubating Kerguelen plume and the eastern Greater India lithosphere? Lithos 100:147–173
Zoheir BA, Johnson PR, Goldfarb RJ, Klemm DD (2019) Orogenic gold in the Egyptian Eastern Desert: Widespread gold mineralization in the late stages of Neoproterozoic orogeny. Gondwana Res 75:184–217. https://doi.org/10.1016/j.gr.2019.06.002
Acknowledgements
The authors sincerely thank Prof. Dr. D.R. Lentz, Department of Earth Sciences, University of New Brunswick, Fredericton, NB, Canada, for his help during the EPMA analysis. The authors would like to thank the guest editors and Prof. Dr. Abdullah M. Al-Amri (editor in chief) and Prof. Dr. Zakaria Hemimi (Gest editor) for handling the manuscript and an anonymous referee for their constructive comments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Gehad M. Saleh, Farrage M. Khalil and El Saeed R. Lasheen are contributed in conceptualization, data curation, writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The present authors declare that the work has no competing interests.
Consent for publication
The present authors declare that consent for publication was obtained.
Additional information
This article is part of the Topical Collection on New Advances and Research Results on the Geology of Africa
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleh, G.M., Khaleal, F.M. & Lasheen, E.S.R. Petrogenesis of ilmenite-bearing mafic intrusions: A case study of Abu Ghalaga area, South Eastern Desert, Egypt. Arab J Geosci 15, 1508 (2022). https://doi.org/10.1007/s12517-022-10782-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10782-3