Abstract
The present study deals with diagenesis and dolomitization modeling of Middle Miocene – Quaternary sedimentary sequence outcropped at Ras Banas Peninsula, Egyptian Red Sea Coast. This sequence is comprising successively overlapped clastics, carbonates, and evaporites attaining 254 m total thickness resting on the Pre-Cambrian basement rocks in the Peninsula. Diagenetic history and dolomitization were carefully investigated using several techniques including petrographic microscope, XRD, SEM, cathodoluminescence, atomic absorption, and isotopes of oxygen and carbon. The conspicuous phenomena is the pervasive dolomitization of directly pre- and post evaporite limestone components. These dolomites are mostly nonstochiometric, poorly to moderately ordered crystals. Based on all the results obtained from this study, the dolomitization processes of Ras Banas Peninsula are the result of complex geologic setting, paleo-climate changes, tectonic activity, and sea-level fluctuations. The proposed graphic model describes dolomitization mechanism which refers to mixed meteoric-hypersaline water environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
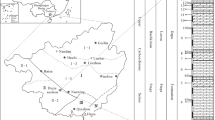
Ras Banas Peninsula is a large tract of land jutting out into the Red Sea (Fig. 1). It extends about 40 km eastward out of the general trend of the Red Sea coast of Egypt, and covers about 400 km2. Geomorphologically, the peninsula is made up of two distinct units: (1) A high land unit covering the central and southern parts of the peninsula, is a long narrow ridge of massive Pre-Cambrian rocks, oriented parallel to the length of the peninsula extending NW–SE direction separating by inliers filled with Neogene sediments, and (2) a gentle sloped coastal plain unit which extends from the foothills of high land seaward, and is covered by sands, gravels, sabkha, and coralline limestones. A lithostratigraphic classification of the Miocene sedimentary sequence along the Red Sea Coast is given by Samuel and Saleeb-Roufaiel (1977) adopted in the present manuscript. A litho-and biostratigraphic description of these sediments in Ras Banas Peninsula is given by (Felesteen et al. 1994). Detailed geomorphic, petrographic geochemical characters of Ras Banas sedimentary sequence integrated with remote sensing interpretation have been previously described (Abou Elmagd and Emam 2012; Abou Elmagd et al. 2013). New insights concerning the geological setting, paleoenviroments and archeology of the whole Red Sea area have been described in Rasul and Stewart. (2019) .
Pervasively dolomitize marine carbonates of Miocene age in several parts along the Egyptian Red Sea Coast were recognized and described by some authors (Khedr 1984; 1990; Aissaoui et al. 1986; Youssef 1986; Coniglio et al. 1988). Pervasive dolomitization was also described from Miocene epoch in Iran attributed to climate changes and sea level fluctuations (Noorian et al, 2022). Several dolomitization models have been summarized in Tucker and Wright (1990). Dolomitization of other carbonates in Egypt has been described by Holail et al. (1988). According to the previous references, both diagenetic sequence and dolomitization models are quite different based on stratigraphic and geologic setting, depositional environments, textural maturity, mineralogical and chemical composition, pore water, and isotopic signature. Comparison between the present pervasive dolomite and some previous dolomite settings and models has been described based on petrographic, chemical, and isotopic signature.
The main goals of this study are to understand dolomitization processes and tracing paleo-climate within an active continental margin. Moreover, investigation of the diagenetic sequence of carbonate rocks in different types of marine, mixed, and meteoric water within a special stratigraphic situation below and above evaporite formation was carried out. Tracing the fine stratigraphic relation between the dolomitized intervals and the host rocks was used to describe the extent and time of dolomitization. A new proposed model of dolomitization is required to describe dolomitization mechanism in such active continental margin setting which involves mixed meteoric- hypersaline water environment.
Materials and methods of study
Multiple techniques were conducted to achieve these purposes which include field relation, petrographic and SEM microscopy, XRD, some trace elements indicator, and isotopic composition of oxygen and carbon. Six overlapped stratigraphic sections perpendicular to the elongation of the peninsula were measured and sampled. The 150 collected samples were used in preparation of many representative thin sections by impregnating the carbonate chips with blue epoxy under vacuum to emphasize pore spaces and diagenetic processes such as cementation and dissolution. These chips were mounted and thin sectioned, then polished using diamond paste and left uncovered in order to be used for further analysis.
Cathodoluminescent properties of the above polished thin sections were studied as described by Marshall (1988) using Maas/Nuclide Luminoscope E/M-3 series model. Cathodoluminescence was brought out by subjecting the sample to an electron beam. The optimum conditions were setting the power at a range of 10 to 20 kv, and 0.5 to 1.0 milliamperes, with slight to nonfocusing.
Bulk mineralogy was determined by XRD according to the method described by (Tucker 1988) Observations of ultra-microstructures were made for polished thin sections and freshly fractured surfaces, using a scanning electron microscope (JEOL, SEM). Sr and Na contents of dolomites were determined using atomic absorption spectrophotometer (Hitachi(R) spectrophotometer). Ca, Mg, Fe, and Mn contents of 40 dolomite samples were determined with an electron-probe microanalyser (JEOL, JSM-840) using re-polished thin sections coated with carbon vapor.
Twenty milligrams of pulverized dolomite and calcite samples was dissolved in 100% phosphoric acid at 25 °C for isotopic analysis. Reaction period of calcite samples was one day, whilst of dolomite samples was one week. The liberated CO2 gas was purified and collected through a vacuum line using liquid nitrogen and dry-ice. Oxygen and carbon isotopic ratios of the purified gas were measured using Finnigan Mat Delta-E mass spectrometer. The standard deviation of independent analyses was 0.05 permil for carbon, and 0.4 permil for oxygen. Isotopic values are reported in the permil notation relative to PDB standard. Additional corrections were applied for dolomite-phosphoric acid fractionation (Craig 1957, 1966). All the described laboratory methods of study have been carried in laboratories of Earth and Planetary Institute, Tokyo University, Japan.
Results
Geological setting
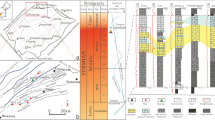
There is a 254-m total thick sequence of sedimentary rocks ranging in age from Middle Miocene to the Recent in Ras Banas Peninsula (Fig. 1). This sequence refers to deposition of the lower unit during one first order of sedimentation including three 3rd order cycles (Fig. 2). The older 3rd order sedimentation cycle started with a continental basal conglomerate, followed upwards by fluvial sediments and intertidal alluvial fan deposits (Ranga Formation) and culminated by shallowing upwards fossiliferous dolomitized limestone of shallow open marine (Um Mahara Formation). This unit has had exposed to subaerial processes indicated by desiccation cracks filled by red laminae of iron oxides and thin sheets of aeolian quartz grains.
The second cycle of sedimentation (Abu Dabbab Formation) is started with sabkha-type gypsum, followed upwards by coastal lagoon sandy and clayey gypsum facies and crowned by subaqueous laminated gypsum. Abu Dabbab sulfate evaporites are followed upwards by highly fossiliferous, shallow open marine, dolostones of Um Gheig Formation indicating a gradual rising of the sea level. The top of Um Gheig dolostones is defined by an undulatory erosional surface indicating a subsequent sudden lowering of the sea level.
In Tortonian age, a transgressive phase was followed and resulted in the deposition of deeping upward, shallow open marine, limestones of Samh Formation (Fig. 2). Local subsidence of Ras Banas Peninsula was most probably occurred and coincided with the 5th rifting phase (Girdler 1969) of the Red Sea.
Many factors are thought to be controlled the cyclicity of sedimentation along the Red Sea coastal strip. To a great extent, lithologies and thickness of these sedimentary sequences were controlled by continental marine affinities in relation to regional tectonic movements (Khedr 1984). Four factors were considered, these are Red Sea rifting movements, sea floor spreading (Girdler 1969, Rasul and Stewart. 2019), westward migration of the asthenosphere (Lowell and Genik 1972), and isostatic readjustment due to global sea level changes (Haq et al. 1987).
Occurrence and petrography of pre-evaporite and post-evaporite dolomites
Based on the stratigraphic profiles of Ras Banas sedimentary sequence, a complete stratigraphic column was constructed (Fig. 2) and a correlation chart was prepared (Fig. 3). The sequence is subdivided into two stratigraphic units: The lower unit of clastic, gypsiferous, and dolomitic carbonate nature comprises five formations (Ranga Formation, Um Mahara Formation, Abu Dabbab Formation, Um Gheig Formation and Samh Formation) of Middle and upper Miocene age. The upper unit includes Shagra Formation of Pliocene age, and Ras Ranga Formation of Pleistocene age, together with the Recent sediments (Fig. 4).
Field photographs showing lithological outcrops of the study rock units. (A) Pinching out limestone wedge replaced by dolomite, pre–evaporite carbonate (Um Mahara Formation). (B) Northward dipping pre-evaporite carbonate (Um Mahara Formation). (C) Cryptalgal laminated gypsum (lower part), and white massive gypsum, upper part (Abu Dabbab Formation). (D) Post-evaporite open marine dolomitized carbonate (Um Gheig Formation). (E) Stromatolite mound limestone (Shagra Formation). (F) Reefal limestones (Ras Ranga Formation)
Distribution of dolomite
Carbonate sediments which belong to Um Mahara Formation and Um Gheig Formation are completely dolomitized in Ras Banas Peninsula along the Egyptian coastal strip of the Red Sea. These two rock units are of Middle Miocene age, and separated by Abu Dabbab evaporite (Figs. 2 and 3). Hence, the terms pre-evaporite dolomite and post-evaporite dolomite are applied here to refer to these dolomitized carbonates respectively. Allochems as well as cements are replaced by dolomite with high degree of fabric preservation, indicating mimetic replacement (Sibley 1980; Sibley and Gregg 1987). Pore filling cements are often formed of dolomite. It is important to notice that the younger carbonate sediments (Samh Fm; Shagra Fm and Ras Ranga Formation) in the study area are devoid of any pervasive dolomitization.
Pre-evaporite dolomite
Pre-evaporite dolomite (Um Mahara Formation) is composed mainly of dolomicrite, dolomitized limestones, and gypsiferous dolostones. It is remarkably fossiliferous, containing many benthic foraminiferal species, in addition to coralline algae, ostracods and microgastropods. Petrographically, it comprises fossilliferous, algal, and ostacodal wackestone and packstone refers to deposition in very shallow restricted marine environment (Figs. 4A, B; 5A, B, C; 6 and 7A).
Photomicrographs of thin sections showing micrite marine cement. A- Algal foraminiferal dolomitized carbonate with micrite envelope and intragranular micrite (Um Mahara Formation). B- Marine micrite rim, intergranular and intragranular micrite cement ((Um Mahara Formation). C- Marine micrite rims and envelopes around bioclastic grains ((Um Mahara Formation). D- Dolomitized foraminiferal algal carbonate cemented with micrite cement (Um Gheig Formation). [Arrows: m, micrite cement; mv, micrite envelope]
Photomicrographs of thin sections showing mixed zone diagenesis of post-evaporite carbonates (Um Gheig Formation). (A) Completely dissolved bioclasts leaving moldic porosity. (B) Dissolved biomolds and later filled with sparry calcite dolomite growing centerward. (C) & (D) Dissolved bioclasts and intragranular cement, isopachous sparry calcite rim cement are conspicuous meteoric diagenetic products. [Arrow: p, pore space; Ic, Isopachous cement]
Photomicrographs of thin sections showing: (A) Near completely filled biomolds with drusy dolomite growing centerward (Um Mahara Formation). (B) Well-preserved coralline algae floating in blocky calcite cement (Shagra Formation). (C) Drusy cement filling pores in stromatolite boundstone (Shagra Formation). (D) Blocky intergranular dolomite cement, with undissolved dolomitized coralline bioclasts (Shagra Formation). [Arrows: BC, blocky cement, DC, Drusy cement]
Post-evaporite dolomite
Post-evaporite dolomite (Um Gheig Formation) is composed of dark brown to grey, very hard, highly fossiliferous peloidal dolostones (Fig. 4D). Petrographically, it is composed mainly of highly fossiliferous dolostones, casts of gastropods, pelecypods, echinoderms, and brachiopods in addition to many benthic foraminiferal species. It comprises bioclastic and foraminiferal packstone and grainstone microfacies that refer to shallow open marine deposition (Figs. 4D, 5D, and 6).
Diagenesis of Ras Banas carbonate sediments
Carbonate sediments of Ras Banas Peninsula exhibit different types of diagenesis including marine cementation, mixed zone and meteoric diagenesis, and dolomitization.
Marine diagenesis
Micrite cementation and micrite envelopes
Micrite cement of different origin is the most common early, intergranular and intragranular, cement type in the studied carbonates. It is represented by superficial and penetrative micritic clots of silt size, pellets, and isopachous rinds (Fig. 5). Micrite cement is usually composed of small and equant crystals of “algal micrite” (Fig. 5C). This is particularly true for micrite formed by blue-green algae (Flugel 1982). Mineralogically, it is now completely dolomitized in Um Mahara Formation and Um Gheig Formation whereas it is composed of low Mg calcite in the upper limestone formations.
Micritization of some skeletal grains is a common feature in sediments of Um Mahara Formation (Fig. 5A, C), which is an early diagenetic process.
Dolomitized micrite envelopes on the some of skeletal grains are common in sediments of Um Mahara Formation (Fig. 5A, C). They are composed of equant crystals of dolomite.
Micritization process indicates a very shallow water (Wilson 1975).The origin of micrite envelopes is possibly attributed to endolithic algae and other micro-organisms which have attacked and bored into the surface of bioclasts and other particles produces, especially in tidal zones, tiny little voids which are infilled by bacterially precipitated micrite after the death of the organisms (Tucker 1981; Flugel 1982). Micritized carbonate bioclasts as well as micrite envelopes can be used as paleobathymetry indicators of water depth, usually postulating water depth of less than 15–20 m (Swinchat 1969), being confirmed in this study, by abundance of miliolid foraminifera associated with these features. The preservation of morphology of dissolved skeletal grains coated with micrite envelopes indicates early marine origin of these envelopes.
Mixed-zone diagenesis
Both dissolution and fabric preservation are widely distributed mixed zone diagenesis of Ras Banas carbonates according to original mineralogy (Sibley 1980 and 1982) or the microstructure (Constanz 1986; Dullo 1986) of different skeletal grains.
Dissolution and fabric preservation modes (Fig. 6)
-
A
Original aragonitic skeletal grains, such as benthic foraminifera and microgastropods which, are important components of the studied carbonates, are dissolved and represented as:
-
(i) Unfilled biomolds (Fig. 6A), and (ii) partially or completely filled biomolds essentially with dolosparite and rarely followed by a late phase of low Mg calcite or gypsum (Fig. 6B). Older, small crystals of dolomite are usually lining the edge of dissolved shells followed by younger, larger size crystals center ward, i.e., drusy fabric cement (Fig. 7B).
-
-
B
Original high Mg calcite skeletal grains, such as ostracods, some benthic foraminifera, and some corals are usually dissolved or dolomitized with well fabric preservation. Amphestiginid foraminifera (Fig. 6C) preserved a radial extinction indicating mimetic retention of original optical c-axis orientations in the replacement dolomite (Sibley 1980 and 1982). Style of biomoldic filling is identical to aragonitic grains.
-
C
Original low Mg calcite skeletal grains, such as coralline algae, some bivalves, and some foraminifera are well preserved, without dissolution (Fig. 7B, D).
-
(i) Entirely dolomitized carbonate rocks include Um Mahara Formation (pre-evaporite dolomite) and Um Gheig Formation (post-evaporite dolomite). These dolomitized carbonates exhibit high degree of preservation of depositional fabric. Consequently, these dolomites are appearing texturally identical to limestones.
-
Meteoric water diagenesis (Fig. 7
Drusy cement
Drusy cement commonly occurs as pore filling of dissolved skeletal grains (Fig. 7A, C). It is formed of anhedral to euhedral crystals, 10 to 300 μm, with increasing crystal size from pore walls to center of pores. Mineralogically, it is dolomite in Um Mahara Formation and Um Gheig Formation (pre- and post evaporite dolomite respectively), whilst it is formed of low Mg calcite in the younger carbonate sediments.
Isopachous cement
Isopachous fringe cement presents only around skeletal grains from topmost part of Um Gheig Formation It is formed of anhedral granular dolomite crystals and followed by the precipitation of micritic marine cement (Fig. 6C, D). The size of individual crystals ranges between 3 and 5 μm.
Blocky calcite cement
Blocky calcite cement predominantly occurs in interparticles within sediments of Shagra Formation and Ras Ranga Formation. It is formed of hypidiotopic crystals of low Mg calcite (Fig. 7B, D). The size of individual crystals of blocky calcite ranges between 100 and 300 μm.
Environments of dolomitization
Cathodoluminescence
Both of limestones and post-evaporite dolomite of Ras Banas area display homogeneous dull cathodoluminescence, whilst pre-evaporite dolomite exhibits weak orange-red CL when viewed under the luminoscope. Exception was found in a bed of post-evaporite dolomite, which displays uniform orange-red to red CL (Fig. 8A–D). No zoning was observed in individual dolomite crystals. This may indicate that the main dolomitization of these two units occurred as a single separate phase with different chemical composition fluid followed by secondary alternative phases. Dull to very weak CL of dolomites are due to the presence of equal or high Fe/Mn ratio (Marshall 1988), which is corresponding to the Fe/Mn ratio of the studied carbonate as measured by EDS microprobe (see later).
Photomicrographs and cathodoluminescent of thin sections of Um Mahara Formation showing: (A, B) dolomitized pelletal packstone with limpid dolomite cement, post evaporite dolomite. (C & D) Cathodoluminescence photomicrographs of (A) & B respectively, both replaced and pore filling dolomite exhibit similar orange-red CL characters indicating similar dololmitizing fluids
Micro-structure of dolomite
Dolomite replacing allochems and cements is generally formed of anhedral to subhedral crystals, their crystal size ranges between 5 and 10 μm. Pore filling dolomite varies from anhedral to euhedral crystals, their size ranges between 20 and 100 μm. Large-sized (Fig. 9A, B), pore filling dolomite is commonly formed of subhedral to euhedral, and clear non-zoned crystals may be classified as limpid dolomite. Petrographic data indicate that skeletal aragonite was dissolved before and during dolomitization because the aragonite fossil molds contain dolomite cements and lack of pre-dolomitization calcite cements (Sibley 1982).
SEM and thin section photomicrograph showing dolomite and post dolomite phases: (A & B) Euhedral dolomite crystals filling pore space, Um Gheig Formation. (C) Photomicrograph of a thin section showing gypsum crystals filling pore space within dolomite, Um Mahara Formation. (D) Pyrite crystals filling pore space in dolomite, Um Mahara Formation
Post dolomite phases
Post dolomitization phases play only a minor role in the diagenetic sequence of the mentioned carbonates. Gypsum and to a less extent low Mg sparry calcite and rarely pyrite occur as fillings of interparticles and intraparticles pore spaces of Miocene carbonates (Fig. 9C, D). Cement stratigraphy indicates that these phases are the later events, because they usually occur towards the centers of biomolds following dolomite cement.
Dolomite ordering and stoichiometry (Figs. 10 and11)
XRD analyses of dolomite also give information on the degree of ordering of dolomite crystals. Degree of ordering can be measured by the relative height of an ordering peak to another peak not directly associated with alternating cation planes. The greater the ratio of the heights of the ordering peak (110) at 2Θ = 35.30 to the diffraction peak (015) at 2Θ = 37.30, the higher the degree of order (Goldsmith and Graf 1958). Applying this method to the studied samples, most of these dolomites have 0.26–0.67 degree of order indicating poorly to not well ordering. The present result is in harmony with the result of dolomite non-stoichiometry. Dolomites which are non-stoichiometric are generally less well ordered than ideal dolomite through the occurrence of some Ca ions in the Mg sheet or vice versa (Hardy and Tuker 1988). This poorly ordered, rich in calcium dolomite is typical of an early diagenetic origin (Bathurst 1976).
The mineral dolomite, CaMg(CO3)2, is commonly nonstochiometric, and so do not have ideal molar ratio of CaCO3/MgCO3 of 50:50 (Ca0.5 Mg0.5 CO3) but has an excess of Ca2+ up to Ca:Mg = 60:40, or rarely an excess of Mg, up to Ca:Mg = 49:51. Ionic substitution of Ca for Mg in the dolomite crystal lattice causes shift of the d104 diffraction peak to lower 2Θ values. The degree of shift is a function of the amount of excess calcium. The Ca excess can be calculated from the equation of Lumsden (1979) relating mole % CaCO3 (NCaCo3) to d104 spacing measured in angstrom units (d):
where M is 333.33 and B is—911.99.
Applying this equation, most of the studied dolomite samples have d104 = 2.901 A0; hence, CaCO3 mole% = 55 indicating non-stoichiometric dolomite.
Diagenetic sequence
The paragenesis of diagenetic sequence of the Middle Miocene dolomitized carbonate sediments is summarized and given in Table 1.
Geochemistry of dolomite
Magnesium and calcium contents in dolomite (Fig. 12)
Tri plot of EDS microprobe analyses of dolomite (in mole %). Plot indicates non-ferroan, non-stoichiometric dolomite except C which is nearly stoichiometric. (A) Dolomite of Um Mahara Formation (pre-evaporite). (B) Dolomite of Um Gheig Formation (post –evaporite). (C) Dolomite of topmost part of Um Gheig Formation
Chemical composition of individual dolomite crystals was investigated by EDS microprobe analyses. The general trends are summarized in the following paragraphs:
Pre-evaporite dolomite exhibits 51.0 to 55.7 mol % CaCO3, 42.2 to 48.4 mol % MgCO3, 0.0 to 0.7 mol % FeCO3, and 0.2 to 1.1 mol % MnCO3 (Fig. 12A). Post-evaporite dolomite shows 55.5 to 59.9 mol % CaCO3, 39.2 to 44.3 mol % MgCO3, 0.0 to 0.4 mol % FeCO3, and 0.0 to 0.4 mol % MnCO3 (Fig. 12B). Topmost part of Um Gheig Formation displays a limited range between 53.5 and 49.3 mol % CaCO3, and 46.0 to 49.9 mol % MgCO3 indicating a more stoichiometric dolomite upwards (Fig. 12c).
Strontium
Concentration of Sr2+ in Ras Banas dolomites varies from 62 to 1112 ppm with average 277 ppm as determined using atomic absorption technique. Strontium was considered among the most useful trace elements in evaluating the salinity of dololmitizing fluids due to the nature of the partitioning between dolomite crystals and the waters from which they precipitate and designing the dolomitization model (Land 1980; Veizer 1983). Sr2+ largely substitutes Ca rather than Mg ions in the dolomite lattice (Behrence and Land 1972).
In comparison, the present Sr content is higher than the Plio-Pleistocene dolomites of the Caribbean-Bahamas region, 70–250 ppm Sr (e.g., Land 1973a; Supko 1977) but remarkably lower than those given by Youssef (1986) for Middle Miocene dolomites along the Red Sea Coast, 232–19,880 ppm Sr, both examples had been modeled as mixing zone dolomitization. Modern supratidal dolomite from the Arabian Gulf, Bahamas and Florida keys, has around 600 ppm Sr (Behrence and Land 1972, Land and Hoops 1973).
Sodium
Concentration of Na + in Ras Banas dolomites varies from 342 to 4544 ppm with average 2397 ppm determined by atomic absorption technique. Sodium also considered valuable in determining the nature of dololmitizing fluids but it is more difficult to interpret than Sr in dolomites. The reason arises from the uncertainties of where the Na+ is located in the dolomite crystal lattice or occurs as fluid inclusions (Land 1980). Relation between Sr+2 and Na+ is shown in Fig. 13.
In comparison, recent dolomites of Arabian Gulf have 3050 ppm Na + (Kinsman and Patterson 1973) and recent dolomites of Bahamas have average of 2080 ppm Na+ (Land and Hoops 1973). Most ancient dolomites have much lower values such as Hope Gate Formation, Pleistocene, 350 ppm Na+ (Land 1973b), Bonaire dolomites, Pliocene, 280 ppm Na+ (Sibley 1980), Red Sea dolomites, Middle Miocene, 143–775 ppm Na+ (Youssef 1986), the Bire and Gesher Formation dolomites associated with gypsum, Neogene, 400–2700 ppm Na+ (Sass and Bein 1988). All these examples had been interpreted as imprints of mixing or modified sea water dolomitization.
Stable isotope composition
Limestones
Oxygen isotopic composition of calcite released from bulk undolomitized limestone samples of Shagra Formation and Ras Ranga Formation (Table 2, and Fig. 14) reflects fresh water diagenesis. Blocky calcite cement is very abundant within these limestones (Fig. 7). These two rock units are of Pliocene to Pleistocene age, and have relatively wide outcrops in the study area (Fig. 1), and transected by many dry valleys flooded by fresh water in irregular wet periods.
δ18O values of these calcites range between − 1.3 and − 9.5 0/00 PDB. Applying these results in the calcite-water temperature equation, and considering δ18O of meteoric water is − 4 0/00 as discussed later, it is concluded that the range of theoretical temperature of precipitation ranges between + 6 and + 44 °C. The minimum and maximum temperatures recorded at Ras Banas during the last two decades are + 12 and + 39 °C respectively.
δ18O signature of these sediments may reach − 9.5 0/00 PDB confirming meteoric fresh water diagenesis. Alternatively, it is probable that the most depleted δ18O sign the imprints of dissolved ice sheet during late Pleistocene. Glaciations at Red Sea area during late Pleistocene is a common concept Milliman et al. 1969). Pluvial episodes prevailed in Egypt during this period give an additional probable interpretation (Said 1990).
Dolomites
Results of stable carbon and oxygen isotope analyses of pre-evaporite and post-evaporite dolomite of Ras Banas Peninsula are summarized in Table 2 and separately in details (Fig. 15). Stable oxygen isotope ratios for Middle Miocene dolomites are relatively consistent, with ranges from + 0.14 0/00 to + 4.03 0/00 averaging + 2.46 0/00 PDB for bulk dolomite samples, and from − 0.27 to + 4.33 0/00 averaging + 1.95 0/00 PDB for microdrilled dolomite samples of cement (Fig. 15).
Stable oxygen isotopic values of Ras Banas dolomites are comparable to Pleistocene dolomitized coral reef of Sinai, Egypt (Strasser et al. 1992), + 2 to + 4 0/00 PDB, but heavier than Youssef (1986), − 1.85 to − 8.25 0/00 PDB, for Miocene dolomite associated with gypsum at Mersa Alam area along Egyptian Red Sea Coast, and slightly heavier than Aissaoui et al.(1986), Coniglio et al. (1988) for Middle Miocene dolomites, Gulf of Suez area. These dolomites were attributed to mixing-zone dolomitization. The present oxygen isotopic data of dolomites are closely similar to those given by Land (1973a), Choquette and Steinen (1980), Sibley (1980), Ward and Halley (1985), but quite heavier than those recorded by Matsumoto et al. (1988) and Farr (1992). All the above works modeled as mixing zone dolomitization.
Discussion
Occurrence and geochemistry
The presence of complete sulfate evaporite sequence (Abu Dabbab Formation) between two pervasively dolomitized shallow marine carbonate facies is likely to suggest variable and changing conditions along shallow waters. Marine cementation of these dolomitized carbonates is confined only in synsedimentary and very early diagenetic phases, and extensive dissolution of aragonitic and high Mg calcite in these carbonates strongly suggests meteoric water diagenesis. On the other hand, relatively high Na+ content of the present dolomite, 342–4544 ppm, indicates an evaporitic condition, i.e., hypersaline water (Sass and Bein 1988). Also, Sr content ranges between 62 and 1112 ppm suggests hypersaline condition, at least for some dolomites. Above line of evidence, coupled with the occurrence of massive sulfate evaporite sequence between the two dolomitized sequences likely indicates variable and changing conditions between meteoric freshwater to hypersaline conditions for the entire Abu Dabbab Formation.
Oxygen isotopic composition
Oxygen isotopic composition of calcite widely ranges from − 9.5 to − 1.3‰ and from − 0.27 to + 4.33 ‰、suggesting variable environmental conditions. The relation among the temperature (T 0C), oxygen isotopic composition of calcite ( δ18Oc), and water ( δ18Ow) is presented by Epstein et al. (1953) as:
On the other hand, isotopic fractionation between calcite and dolomite (δ18Od-c) has been assumed to be + 3 ‰ (Matsumoto et al. 1988). Hence, the relation among the T 0C δ18Od) and water ( δ18Ow) is given as:
The equilibrium between isotopic composition of calcite, dolomite, and the specified fluids can be tested by Fig. 6A, B, and C, in which the possible range of temperature and δ18O of water are given.
Sea water of Red Sea at Late Miocene-Pleistocene ranges between about − 3.0 and + 3.0 0/00 relative to SMOW (Lawrence 1974). An extremely heavy oxygen water, approximately + 11 0/00 were recognized in solar lakes at Gulf of Aqaba (Aharon et al., 1977). Assuming that the possible range of δ18O from − 3.0 to + 11.0 ‰, possible environmental conditions are shown in Fig. 6A, B, and C.
The average value of δ180 for world-wide meteoric water precipitation around 25° Latitude is about − 4.0 0/00 relative to SMOW (Craig and Gordon 1965). The oxygen isotopic composition of the present Red Sea water is 1.8 0/00 (SMOW) at 40 0/00salinity (Craig 1966).
The mean temperature of the Red Sea surface water ranges between 22 and 32 °C all over the year (Robinson et al. 1979). Temperatures in inshore waters of the Red Sea coastal strip, at least 8 km wide where the depth is less than 50 m, may reach 36–38 °C at midsummer, and may reach 45 °C in the lagoons behind the fringing reefs (Edwards 1987).
Based on the last considerations, assuming that the temperature of dolomitization fluid ranges between 22 and 50 °C, and δ18Ow ranges between − 4.0 and + 11.0 0/00, the rectangles inside Fig. 16A and B represent the possible range of the temperatures and oxygen isotopic composition of the normal and mixed-meteoric-hypersaline water.
(A) Relation between δ180 of calcite, water, and temperature of precipitation. (B) Relation between δ180 of dolomite, water, and temperature of precipitation. (C) Method of estimating of δ180 of diagenetic water and the mixing ratio of mixed meteoric-hypersaline water. HP hypersaline water, SW normal Red Sea water, and FW fresh water around 25° Latitude. See text for detailed description
Carbon isotopic composition
Carbon isotopic composition of Ras Banas calcite anddolomites ranges from + 0.30 to + 3.69 0/00 and from + 0.13 to + 2.69‰respectively. This implies that carbonate diagenesis occurred in near surface water with minimum influence of degradation or fermentation of organic matter (Matsumoto, et al. 1988).
Ratio of mixing dolomitization fluids
The range of δ18O in diagenetic fluids which precipitated dolomite is given by the segment of respective curves enclosed in the rectangles (Fig. 16C). Dolomites of Ras Banas Peninsula are estimated to have precipitated from mixture of 15–70% hypersaline water at a temperature range of 22–50 °C.
Dolomitization mechanism and modeling
Based on the last discussion of both temperature, δ18O of Red Sea water, and the recorded isotopic values of the studied dolomite, a mixed meteoric-hypersaline water dolomitization model was constructed for the below and above evaporite dolomites (Fig. 8A, B).
Mechanism of mixed meteoric-hypersaline dolomitization (Fig. 17)
The basis of this model is that it is easier to precipitate dolomite from a dilute solution, so that if seawater (or hypersaline) with its high Mg/Ca molar ratio is mixed with freshwater, the high Mg/Ca ratio is maintained and some of the kinetic obstacles due to the high ionic strength of water are removed (Folk and Land 1975). Slightly different mechanisms for dolomitization of pre-evaporite and post-evaporite sequences are proposed as follows:
-
A.
Pre-evaporite carbonate dolomitization (Fig. 17A);
-
1-
Penetration of fresh meteoric water into limestones, as a result of subaerial exposure.
-
2-
Reflux of evaporitic seawater with high Mg/Ca ratio, as a result of gypsum-anhydrite sedimentation, into the underneath limestones.
-
3-
Mixing of the two fluids in the phreatic zone resulting in dolomitization of Um Mahara limestones.
-
1-
-
B.
Post-evaporite carbonate dolomitization (Fig. 17B);
-
1-
Pumping of evaporitic pore waters with high Mg/Ca ratio, as a result of evaporite compaction, into overlaying limestones.
-
2-
Penetration of fresh meteoric water into limestones, as a result of outcropping evidenced by unconformity surface at top of Um Gheig Formation
-
1-
Timing of dolomitization
Assuming validity of the above supposed mechanisms of dolomitization of pre-evaporite and post-evaporite limestones at Ras Banas Peninsula, it is concluded that dolomitization of each of these units occurred as a separate single event, followed by secondary alternative events. Slight similarities of the mineralogical, chemical, and isotopic signature between these dolomites suggest that both dololmitizing fluids are not quite different.
Conclusion
Diagenetic sequence of middle Miocene pervasive dolomitization of Ras Banas Peninsula, Red Sea Coast involves marine, mixed, and meteoric stages. Dolomitization processes of Ras Banas Peninsula are the result of complex geologic setting, paleo-climate changes, tectonic activity, and sea-level fluctuations. A new proposed model of pre- and post evaporite dolomitization was constructed graphically and interpreted as mixed meteoric-hypersaline water at mixed rate between 15 and 70% respectively.
References
Abou Elmagd K, Emam A, Ali-Bik MW (2013) Chemostratigraphy, petrography and remote sensing characterization of the Middle Miocene – Holocene Sediments of Ras Banas Peninsula, Red Sea Coast Egypt. Carpathian J Earth Environ Sci 8(3):27–42
Abou Elmagd K, Emam A (2012) Geomorphology and drainage network of Ras Banas peninsula, Egyptian Red Sea Coast - a model of coastal threshold. Arab J Geosci, Published online: 31 August 2012, https://doi.org/10.1007/s12517-012-0660-0.
Aharon P, Kolodny Y, Sass E (1977) Recent hot brine dolomitization in the “Solar Lake” Gulf of Elat, isotopic, chemical and mineralogical study. Jour Geology 85:27–48
Aissaoui D, Coniglio M, James NP, Purser BJ (1986) Diageneses of a Miocene Carbonate Platform: Jebel Abu Shaar, Gulf of Suez, Egypt. In: Schroeder JH, Purser BH (eds) Reef Diagenesis. Springer-Verlag, Berlin, pp 112–131
Bathurst RGC (1976) Carbonate Sediments and their Diagenesis (2nd edition). Elsevier Publ. Co., Amesterdam, p 658
Behrence EW, Land LS (1972) Subtidal Holocene dolomite, Baffin Bay. Texas Jour Sed Petrol 42:155–161
Choquette PW, Steinen RP (1980) Mississippian non- supratidal dolomite, Ste. Genevieve Limestones, Illinois Basin: evidence for mixed- water dolomitization. In Zenger, D.H., Dunham, J.B., and Ethington, R.L.,eds., Concepts and Models, of Dolomitization. Soc. Econ. Paleont. Mineralog., Spec. Publ. No. 28, P. 163–196.
Coniglio M, James NP, Aissaoui DM (1988) Dolomitization of Miocene carbonates, Gulf of Suez. Egypt Jour Sed Petrology 58(1):100–119
Constanz BR (1986) The primary surface area of corals and variations in their susceptibility to diagenesis. In: Schroeder JH, Purser BH (eds) Reef Diagenesis. Springer-Verlag, Berlin, Heidelberg, pp 53–76
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectro-metric analysis of carbon dioxide. Geochem Cosmochem Acta 12:133–149
Craig H (1966) Isotopic composition and origin of the Red Sea and Salton Sea geothermal brines. Science 154:1544–1548
Craig H, Gordon LI (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. In: E. Tongiorgi, ed., Stable Isotopes in Oceanographic Studies and Paleotemperatures: Consiglio Nazionale delle Richerche, laboratorio di Geologia Nucleare, Pisa, p. 9–130.
Dullo W-C (1986) Variations in diagenetic sequence: an example from Pleistocene coral reefs, Red Sea, Saudi Arabia. In: Schroeder JH, Purser BH (eds) Reef Diagenesis. Springer-Verlag, Berlin, Heidelberg, pp 77–90
Edwards FJ (1987) Climate and oceanography. In: Edwards AJ, Head SM (eds) Red Sea. Pergamon Press, pp 45–69
Epstein S, Buchsbaum R, Lowenstam HA, Urey HC (1953) Revised carbonate water isotopic temperature scale. Geol Soc Am Bull 64:1315–1326
Farr MR (1992) Geochemical variation of dolomite cement within the Cambrian Bonneterre Formation, Missouri: Evidence for fluid mixing. Jour Sed Petrol 62(4):636–651
Felesteen AW, Khedr ES, Abou Elmagd K (1994) The Neogene - Quaternary sequence of Ras Banas Peninsula: (1) Stratigraphical studies. Egypt Jour Geology 38:267–287
Flugel E (1982) Microfacies analysis of limestones. Christenson. Springer-Verlag, Berlin, Heidelberg, New York, Translated by K, p 633
Folk RL, Land LS (1975) Mg/Ca ratio and salinity : two controls over crystallization of dolomite. Am Assoc Petrol Geol Bull 59:60–68
Girdler RW (1969) The Red Sea- a geophysical background. In: Degens E, Ross D (eds) Hot Brines and Recent Heavy Metal Deposits in the Red Sea. Springer-Verlag, New York, pp 38–58
Goldsmith JR, Graf DL (1958) Relation between lattice constraints and composition of the Ca-Mg carbonates. Am Mineral 43:84–101
Haq BU, Hardenbol J, Vail PR (1987) Chronology of fluctuating sea levels since the Triassic. Science 235:1156–1167
Hardy RG, Tuker ME (1988) X-ray powder diffraction of sediments. In: Tuker ME (ed) Techniques in Sedimentology. Blackwell Sci. Publ, Oxford, London, pp 191–228
Holail H, Lohman KC, Sanderson I (1988) Dolomitization and dedolomitization of upper Cretaceous carbonates: Bahariya Oasis, Egypt. In:Shukla, V., and Baker, P.A., eds. Sedimentology and Geochemistry of dolostones. SEPM. Spec. Publ.No 43, p. 191- 207.
Khedr ES (1984) Sedimentological evolution of the Red Sea continental margin of Egypt and its relationship to sea level changes. J Sed Geology 39:71–86
Khedr ES (1990) Recent coastal sabkhas from the Red Sea: A model of sabkhitization. Egypt J Geol 33(1–2):87–120
Kinsman DJJ, Patterson RJ (1973) Dolomitization process in sabkha environment (abs.). Am Assoc Petrol Geol Bull 57:788–789
Land LS (1973) Contemporaneous dolomitization of middle Pleistocene reefs by meteoric water. North Jamaica Marine Sci Bull 23:64–92
Land LS (1973) Holocene meteoric dolomitization of Pleistocene limestones, North Jamaica. Sedimentology 20:411–424
Land LS, Hoops GK (1973) Sodium in carbonate sediments and rocks: a possible index to the salinity of diagenetic solutions. Jour Sed Petrol 43:614–617
Land LS (1980) The isotopic and trace element geochemistry of dolomite : the state of the art. In: Zenger, D.H., Dunham, J.B., and Ethington, R.L., eds., Concepts and Models of Dolomitization. Soc. Econ.Paleont. Mineralog., Spec. Publ. No. 28, p. 87–110
Lawrence JR (1974) Stable oxygen and carbon isotopic variations in the pore waters, carbonates and silicates, Sites 225 and 228 Red Sea. Initial Reports of the Deep Sea Drilling Project. 23, Washington, U.S., Government Printing Office, p. 923–937.
Lowell DJ, Genik JD (1972) Sea-floor spreading and structural evolution of Southern Red Sea. Am Assoc Petrol Geol Bull 56(2):247–259
Lumsden DN (1979) Discrepancy between thin section and X-ray estimates of dolomite in limestones. Jour Sed Petrol 49:429–436
Marshall DJ (1988) Cathodoluminscence of geological materials. Boston Unwin Hyman Ltd. 146 p. https://doi.org/10.1002/gj.3350260409.
Matsumoto R, Iijima A, Katayama T (1988) Mixed-water and hydrothermal dolomitization of the Pliocene Shirahama limestones, Izu Peninsula, Central Japan. Sedimentology 35:979–998
Milliman JD, Ross DA, Ku TL (1969) Precipitation and lithification of deep sea carbonates in the Red Sea. Jour Sed Petrology 39:724–736
Noorian Y, Moussavi-Harami R, Hollis C, Reijmer JJG, Mahboubi A, Omidpour A (2022) Control of climate, sea-level fluctuations and tectonics on the pervasive dolomitization and porosity evolution of the Oligo-Miocene Asmari Formation (Dezful Embayment, SW Iran), Sedimentary Geology. V. 246, 106048. https://doi.org/10.1016/j.sedgeo.2021.106048.
Rasul NMA, Stewart ICF Editors (2019) Geological setting, Palaeoenvironment, and Archaeology of the Red Sea. Saudi, Geological Survey. Springer Nature Switzerland. 803 p.
Robinson MK et al (1979) Atlas of N. Atlantic - Indian Ocean monthly mean temperatures and mean salinities of the surface layer. United States Naval Oceanographic Office. Reference Publication 18.
Said R ed (1990) The geology of Egypt. A.A.Blakema/ Rotterdam/ Brookfield. 731 pp.
Samuel MD, Saleeb-Roufaiel GS (1977) Lithostratigraphy and petrological analysis of the Neogene sediments at Abu Ghusun- Um Mahara Area, Red Sea Coast, Egypt. Freiberger Forschungshefte, C 323 Geowissen Schaften - Geologie, p. 45–56.
Sass E, Bein A (1988) Dolomite and salinity: a comparative geochemical study. In:Shukla, V. and Baker, P.A., eds. Sedimentology and Geochemistry of Dolostones. SEPM, Spec. Publ. No. 43, p.223–233.
Sibley DF (1980) Climatic control of dolomitization, Seroe Domi Formation (Pliocene), Bonaire, N.A.. In:Zenger D.H., Dunham, J.B., and Ethington, R.L., eds., Concepts and Models of Dolomitization. Soc. Econ. Paleont. Mineralog., Spec. Pupl. No. 28, p. 247–258.
Sibley DF (1982) The origin of common dolomite fabrics: clues from the Pliocene. Jour Sed Petrol 52:1087–1100
Sibley DF, Gregg JM (1987) Classification of dolomite rock textures. Jour Sed Petrol 57(6):967–975
Strasser A, Strohmenger C, Davaud E, Bach A (1992) Sequential evolution and diagenesis of Pleistocene coral reefs (South Sinai, Egypt). Sed Geology 78:59–79
Supko PR (1977) Subsurface dolomite. San Salvador. Bahamas Jour Sed Petrol 47:1063–1077
Swinchat JP (1969) Algal boring: a possible depth indicator in carbonate rocks and sediments. Geol Soc Amer Bull 80(7):1391–1396
Tucker ME (1981) Sedimentary petrology: an introduction. Blackwell Scientific Publ, Oxford, p 252
Tucker ME ed (1988) Techniques in sedimentology. Blackwell Sc. Publ., Oxford, London, pp. 387.
Tucker ME, Wright VP (1990) Carbonate sedimentology. Blackwell, Oxford, 482. https://doi.org/10.1002/9781444314175
Veizer J (1983) Chemical diagenesis of carbonates: theory and application of trace element technique. In: Stable Isotopes in Sedimentary Geology. SEPM, Short Course No. 10, p. 3–1--3–100.
Ward WC, Halley RB (1985) Dolomitization in a mixing zone of near-seawater composition, Late Pleistocene Northeastern Yucatan Peninsula. Jour Sed Petrology 55(3):407–420
Wilson JL (1975) Carbonate facies in geological history. Springer-Verlag, Berlin, Heidelberg, New York, p 471
Youssef EAA (1986) Depositional and diagenetic models of some Miocene evaporites on the Red Sea coast Egypt. Sed Geology 48:17–36
Acknowledgements
The authors deeply thank the respected editor and two anonymous reviewers for their constructive comments during revision of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Attila Ciner
Highlights
• A sequence of cyclic sedimentary rocks attains 254 m thickness resting on Pre-Cambrian basement rocks, at Ras Banas Peninsula, Red Sea Coast, Egypt.
• Two pervasively dolomitized carbonates formations occurred below and above sulphate evaporite sequence.
• Detailed investigations of dolomite including petrographic, mineralogical, Cathodoluminescence chemical, and isotopic signatures were performed.
• Dolomitization processes of Ras Banas Peninsula are the result of complex geologic setting, paleo-climate changes, tectonic activity, and sea-level fluctuations.
• A proposed model of dolomitization is constructed which involves mixed meteoric- hypersaline water environment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abou Elmagd, K., Matsumoto, R. Diagenesis and pervasive dolomitization of pre- and post evaporite shallow marine carbonates, Ras Banas Peninsula, Red Sea Coast, Egypt. Arab J Geosci 15, 1403 (2022). https://doi.org/10.1007/s12517-022-10648-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10648-8