Abstract
Human activities substantially contribute to the rise of various contaminating ionic levels in the water, which translates in a risk to humans, flora, and fauna. Assessment of groundwater quality is very important to estimate the contaminants to take preventive measures and predict future disasters. The statistical method is very effective in combining this estimation with other tools. As a case study, Aurangabad city of India was chosen and 55 groundwater samples were collected from the dugwells and borewells according to the domestic location of the inhabitants and the location of the various industries. The study aims to create various graphs that expose the human-related health risks posed by the considered contaminants. All the samples were analyzed to trace the amount of a considerable range of pollutants; consequently, a health risk assessment for carcinogenic and non-carcinogenic risks was carried out. A water suitability study for irrigation and drinking purposes found that in the studied samples, nitrate was present as the major pollutant among the others. Industrial wastewaters mainly caused nitrate pollution in the area and solid fertilizers and pesticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human intervention and anthropogenic activities play a pivotal role in modifying hydrogeological and geochemical environments (Brindha et al., 2017; Deepa and Venkateswaran, 2018; Wagh et al., 2019). Groundwater is a consistent part of the few available water supply sources in rural and urban regions of developing countries (Mukherjee and Singh 2018; Arya et al. 2020). Groundwater is a reliable and significant natural water resource in drought-prone areas such as the Deccan plateau, where it is a required choice for irrigation, industrial and home demands, and all other water requirements. The Deccan topography is in a rain shadow region. Thus, relying on rainfall for all of water demands puts in serious scarcity danger. It is to mention that this source has reached the point where it requires restorative work as a result of environmental destruction. The major freshwater supply is groundwater, one of the world’s most important ecological assets (Rokade et al. 2004; Kumar and Kumar 2011; Sharma and Kujur 2012; Neelakantan and Yuvaraj 2012; Kumar 2013).
Groundwater restoration rate is evaluated using groundwater and hydrology data such as topography, precipitation, groundwater depth, geology, and hydraulic characteristics, regardless of the evaluation technique utilized (Alley et al. 1999; Thomas et al. 1999, Sarkar et al. 2021).
Groundwater recharge rates are lower in regions with deep-rooted vegetation—such as woods—than in areas with shallow-rooted vegetation, such as annual crops (Owuor et al. 2016). The restoration or recharge rate of groundwater is influenced by soil hydro-physical qualities such as texture, hydraulic conductivity, bulk density, and porosity, which can be either primary—between grains which are formed by deposition—or secondary—due to processes that take place after deposition, such as fracturing or reprecipitation (Yolcubal et al., 2004). These hydro-physical qualities influence the way precipitation penetrates and is maintained in the soil. It also influences the pace of water transfer and the courses that water takes to reach stream networks (Price et al. 2010). The findings demonstrate that precipitation rates have little impact on groundwater recharge in most Indian river basins, implying that humans influence current recharge rates. The spatial variability in recharge rates could provide critical input for policymakers to develop more sustainable groundwater management in India (Bhanja et al., 2019).
Groundwater-dependent ecosystems (GDEs) are crucial in defining the scope of groundwater extraction limitations: As a rule of thumb, once a backup is provided for indispensable needs of aquatic systems and human scopes, water resources can be allocated (Münch and Conrad 2007). In order to govern their existing composition and functioning, GDEs require the introduction of groundwater. They substantially contribute to social, economic, ecological, and spiritual values (Murray et al. 2003). In aquifer ecosystems, groundwater plays a critical function. Groundwater management and planning require a thorough understanding of these GDEs. The water budget myth suggested a simple balance between recharge and availability. However, we have learned over the last century that the relationships between groundwater-dependent ecosystems, human requirements, and the groundwater system are complex. In terms of ecosystem services, the concept of “safe yield” has given way to “sustainability,” which combines consideration of the negative economic consequences of depletion with the maintenance of discharge flows at levels that support ecosystem reliance on surface water and groundwater from aquifers.
Relatively few studies have been conducted across the vast basins of the Indian subcontinent. As a result, these experiments must be conducted in diverse hydrogeological regimes to create an appropriate groundwater management strategy. A recent study by Gupta et al. showed how contamination by nitrate results in severe outcomes in the Maharashtra region (Gupta et al. 2011). Previous work on groundwater geochemistry from the same study area indicates that the contamination is mainly due to anthropogenic activities (CGWB 2006). However, it is predicted that in the recent future that the additional stress on the existing groundwater resources may lead to further contamination (iPla et al. 2014). As a conclusive result, an excessive groundwater withdrawal from aquifers makes the sources vulnerable to salinization and contamination processes.
In the industrial city of Aurangabad, the inhabitants are majorly dependent on the available groundwater to suffice their domestic, drinking, and agricultural needs. Moreover, the area is an arid type of region: The jointed basaltic rock pattern appears just below a thin layer of black soil, which easily allows the contaminated water to mix with the underground aquifers below, thereby altering the groundwater chemistry. Therefore, it is necessary to ascertain the status of groundwater quality for drinking as it is directly or indirectly associated with public health. The proposed study was initiated to evaluate groundwater’s geochemical signatures by assessing geological and geochemical analyses. This study uses statistical and analytical methods to evaluate the influence of the various anthropogenic factors which alter the water composition. The water quality index (WQI) method was also used to classify the groundwater quality for drinking purposes. The conclusions provided by this study may be helpful for the local governing structures and also help researchers to gather basic information about the region, which is useful to develop sustainable management of groundwater resources in the studied area.
Study area
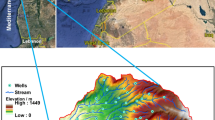
The city is a dry arid type of region where the summer temperature rises to a maximum of 45 °C and a minimum of 28 °C with winter temperatures between 32 and 5 °C, and the summer and winter ranges are quite broad. Rainfalls in the region are scarce and errant. The irrigation resources are rain-dependent rivulets, percolation reservoirs, and water wells. However, due to low rainfall, the natural inflow of freshwater remains dependent on the groundwater flowing through the fractures and joints in the rocks, mostly unjointed basaltic rocks. The area lies between latitude 19° 53′ north and longitude 75° 20′.
Geological settings
Regional geology
Aurangabad is in the heart of Maharashtra. The plains and central highlands display brown to black calcareous loamy clay. The western ghats and the peninsular plateau region exhibit shallow brown to dark brown gravels with loams on high slopes and red to reddish brown laterites and lateritic soils on high lands. The coastal plains on the western side display laterites and lateritic soils with red to reddish brown loam of non-lateritic origin.
Study area geology
The entire city comprises Deccan trap basaltic terrane lava flows belonging to the ages from the upper Cretaceous to the Eocene. Over the Deccan, floods are thin alluvial deposits. The traps have horizontal boundaries, and each flow comprises two separate units. The top layers are vesicular basaltic rocks with zeolite-packed amygdales, whereas the lowest layer is enormous. Because of the linear pattern shown by the darker tone and drainage, several lineaments in satellite photos have been identified as fracture zones. These lineaments are extremely useful for groundwater storage; the soil in the region is black due to a high content of Potash and Montmorillonite. The study was conducted in three different areas. The eastern industrial cluster, which is called Shendra, is located along the basin of river Sukhna, a plain region SZ; the domestic area, which lies in the municipal boundary, includes the city area i.e., a plateau CZ; the western part is a combination of hills and slopes WZ, which is Waluj, an industrial zone that lies in between two river basins: one belonging to river Kham and the other to river Godavari.
Land use/land cover
The human use of land resources gives rise to “land usage,” which varies depending on its function, such as food production, housing provision, recreation, material extraction and processing, and the biophysical qualities of the land itself. Land cover refers to the physical and biological cover on the land’s surface, including water, flora, bare ground, and artificial constructions (Ellis, 2007). The land use and the land cover studies indicate the following order of land use categories: barren land < built-up land < agriculture land < water bodies (Table 6). Our field studies show that many inorganic fertilizers like superphosphate, diammonium phosphate, ammonium nitrate, and urea are routinely applied for agriculture practices in addition to natural fertilizers and biofertilizers. The industrial effluents secreted in the water bodies led to many chemical compounds in the groundwater composition. Changes in land use and land cover have resulted in a decrease in plant cover. Plant cover declines due to a variety of developmental causes, resulting in a host of additional negative environmental repercussions such as biodiversity loss, climate change, and pollution of other natural habitats (Niyogi, et al. 2009). Table 6 clearly shows a huge difference or can be said fall in agricultural land while barren land is increasing compared to earlier years; meanwhile, it is clear that land unproductiveness or infertility is increasing when comparing data from year 2016 with those from year 2021.
Spatial land use and land cover change reflect the effect of biotic and abiotic processes on the region’s primary land use and land cover. The force of the drivers and variations in driver composition impact changes in the prevalent land use and land cover of an area.
Methods applied
Field observation and sampling methods
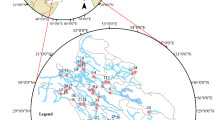
The subsurface samples were obtained at 55 different spots (Table 1), comprising dug wells, borewells, city area wells, industrial sites, and farmland in the pre monsoonal season of 2021. The location of the wells in the research region is depicted in Fig. 1. The groundwater samples collected in 2-L high-density polyethene bottles have been pre-cleaned with a 10% nitric acid solution and accurately cleansed with bi-distilled water. The collected samples were then neutralized with ultrapure nitric acid (0.5% HNO3), cooled down, and carried into the lab for further examination.
Magnesium, calcium, total hardness (TH), and chlorine were all assessed in the field using a water analysis kit. pH, total dissolved solids (TDS), and electrical conductivity (EC) were determined in the field using the pH, TDS, and EC meter (Hanna HI9811-5) (Table 2). Standard techniques were employed for the rest of the cation and anion analysis of the groundwater samples (APHA 1999). Dionex-Dx600 high-performance ion chromatography (HPIC) was used for the determination of anions (HCO3 − , Cl − , SO4 2 − , and NO3 −) and cations (Ca2 + , Mg2 + , Na + , and K +) in groundwater.
Analytical techniques
Sample locations and geology maps of the study area were prepared in ArcGIS 10.1 (Fig. 2). Detailed investigation and digitization were conducted using a geographic information system (GIS), a strong tool for mapping, vulnerability assessments, and making quick choices concerning environmental health issues. The basic digitization was done using the Google Earth application for the location digitization and the toposheet no. M/45 for the Aurangabad city area. The statistical analysis and geochemical plots were prepared using the SPSS software. As a reference for the computation of the WQI, several studies performed by many researchers like Sahu and Sikdar (2008), Brindha and Kavitha (2015), Deepa and Venkateswaran (2018), and Acharya et al. (2018) have been considered.
Human health risk assessment
The human health risk assessment (HHRA) determines the possible consequences of a hazard on the health of an individual, a group of individuals, or a community (Kaur et al. 2020; Thomson 2007). The approach measures the risk of adverse health effects on the human body due to continued consumption of contaminated groundwater (Balamurugan et al. 2020; Kumar and Balamurugan 2019). To calculate the HHRA, four basic steps are taken, explicated by the US EPA (US EPA 2006). The first step is to identify potential hazards and people who may be exposed to them. The amount of the dose and the response can then be determined in the second step considering the concentration of the harmful agent in the area. The third step entails calculating exposure based on how many pollutants people consume and for how long. In the fourth step, these factors are combined to calculate the community’s risk of a health problem. Individuals in the present research were defined based on their age and sex, with discernments such as children, male, and female. The US EPA recommended HHRA for nitrate contamination in groundwater (Singh et al. 2020; Adimalla and Wu 2019); the health risk from oral intake was calculated as:
where in Eq. (1), CDI refers to the daily intake (in mg/(kg*day)); C is the concentration of the groundwater nitrate (in mg/L); I denotes the daily ingestion rate of groundwater (L/day). For both gender, adults, the ingestion of nitrate has a rate which is about 2.5 L/day, and for children, the ingestion rate comes out to be 1 L/day. F denotes the exposure frequency (in day/years), considered 365 days/year for both the gender, adults, and children, and D denotes the exposure duration (a year). ABW is the average body weight. AET is the average exposure rate. HQ is the hazard quotient. Rf denotes the reference dose of nitrate contaminant (in mg/(kg*day)) which is 1.6 mg/(kg*day).
The non-carcinogenic health risk from dermal contact is calculated by the following formulae:
Now in Eq. (3), DAD shows the dermal absorption (in mg/(kg*day)); CT is the time required to acquire contact with the contaminant (in hours per day); A denotes the dermal absorption parameters (in cm/h). CF is the conversion factor, and BF represents the bathing frequency of a person (in times per day). SSA is the skin surface area (in cm2). In Eq. (6), HI is the hazard index so the value of HI denotes the non-carcinogenic human health risk. If HI is greater than 1, there is a potential health risk hazard, while if the value is less than 1, the health risk on humans resides at an acceptable level.
Water quality index
The quality of groundwater and its suitability were assessed using the WQI method. The groundwater quality index (GWQI) is the most accurate technique to evaluate groundwater potability. Hydrochemical parameters such as major cations and anions (Vadiati et al. 2016) and particular characteristics (e.g., land use, geology, and soil type) should be considered in GWQI indexing. Eight parameters (pH, Na, Ca, Mg, Hco3, Cl, SO4, and NO3) were considered to calculate WQI and the WHO drinking water standards were taken into account (WHO 2011). The correlation matrix (Table 3) has a tremendous effect on the (WQI). It finally says that the various parameters show a correlation among themselves. Coherent weights were assigned to compute the WQI values for each groundwater parameter: For TDS, Cl, SO4, and NO3 were assigned higher weights equal to 5, whereas other parameters like Mg, Cl, and Na were given weights between 1 and 4 depending on their presence in the groundwater quality (Magesh et al. 2013; Adimalla et al. 2019). The relative weights (RWi) were calculated using Eq. (1). WQI values were computed using the following Eqs. (2), (3), and (4) and the water quality types for each sample location are given in Table 4. The relative weight (RWi) is calculated with the following equation:
where RWi = relative weight, AWi = assigned weight, and n = number of parameters.
The quality rating scale for each parameter is expressed with the following equation:
where qi = quality rating for the ith parameter.
Ci = concentration of the ith chemical parameter of water sample (mg/L).
Si = permissible standard for the ith parameter set by the WHO.
Sub index (SIi) for each parameter is calculated by Eq. (8):
where SIi = sub index of the ith parameter, qi = rating based on concentration of the ith parameter.
Finally, WQI is computed by the following equation:
The WQI results (Table 5) show that, out of 55 groundwater samples, 89% of the water is not suitable for irrigation or drinking. The high values of WQI at most of the locations have been observed due to elevated concentrations of TH, NO3, TDS, CO3, and HCO3 in the groundwater samples. This pattern of groundwater may be due to the effective leaching of ions to a larger extent impact.
Water quality standards
Water quality standards mean statements and numeric values that describe the water quality and lie within the following three components:
-
1.
Designated uses of the water body related to water supply, aquatic life, agriculture, or recreation.
-
2.
Water quality criteria and general statements describe good water quality and specific numerical concentrations for various parameters.
-
3.
Anti-degradation policy is designated to maintain and protect the existing water uses for each water body.
The water quality index can be calculated for three different uses:
-
a.
Drinking water quality index includes drinking, recreation, irrigation, and livestock watering use.
-
b.
Aquatic water quality index in terms of drinking, recreation, irrigation, and livestock watering use.
-
c.
Overall water quality index which includes the protection of human health, aquatic ecosystems, and wildlife.
Results
Geochemical base parameters
Tables 1 and 2 show the various chemical constituents present in the water samples and the sampling sites and their quantitative descriptions. The amounts of Na, Cl, and HCO3 in the study region are all incredibly high. In all samples, the main ion amounts have been detected in the following order: Ni > HCO3 > Cl > Na > Ca > Mg > NO3 > K > CO3 > SO4. Average HCO3 content contributes 313.77 units of total anions. The average values of chlorine anions make up 251.4 of the total anions. The average Na content influences total anions. The average Ca content contributes 112.4% of total cations. The pH values in all geomorphic units range from 5.18 to 10.8, showing a significant variance in water quality. The EC value ranges from 144.66 to 2654 S/cm, with the highest values seen along the Kham River Basin’s industrial hub. The classification of the total dissolved solids divides samples into two classifications: fresh (less than 1000 mg/L) and saline (more than 1000 mg/L) (Davis and Dewiest, 1966). Low TDS freshwater, which is also characterized by increased HCO3, denotes recent water recharged due to the prevalence of groundwater aquifers near rivers. The saline-type samples showing a TDS value falling between 1459 and 2549 mg/L are reported; the reason behind these values may be linked to some geogenic structures underneath.
Agricultural aspects
When it comes to agriculture (Table 6), the amount of dissolved salts present in the soil is crucial, as groundwater quality is vital for irrigation. When salt builds up in the root zone, salinity can substantially impact crop yield (Jain et al., 2011).
In contemporary agriculture, fertilizers and insecticides are widely employed. Groundwater pollution is more likely due to this, especially when agricultural areas are exposed to harmful chemicals over extended periods of time. Human activities and agriculture have caused groundwater pollution in the area in both direct and indirect ways. The disintegration and transport of excessive amounts of fertilizers and hydrological alterations connected to irrigation and drainage are direct impacts. Increased concentrations of dissolved oxidants, protons, and major ions in soils and aquifers may produce indirect effects such as changes in water–rock processes in soils and aquifers. Agricultural activities have influenced the concentrations of a range of inorganic chemicals in groundwater in the region, including NO3, N2, Cl, SO4, H, K, Mg, Ca, Fe, Cu, B, Pb, and Zn, as well as pesticides and other organic compounds. The high quantities of NO3 and NaCl indicate that the land around the springs has been polluted by untreated sewage and agricultural wastes for a long time (Baba and Tayfur 2011).
Water with a TDS of less than 450 mg/L is deemed well, whereas water with more than 2000 mg/L is regarded unfit for agricultural use (Davis and Dewiest, 1966). EC has a significant impact on crop output since plant water intake decreases as EC rises. A high EC value indicates a high level of salinity. As a result, EC is regarded as a critical water quality criterion in evaluating both drinking and irrigation water. The EC is a commonly used indicator for salinity, and it has also been used to define water as a medium, low, or high salty (Jeyaruba and (Mrs.) Thushyanthy 2009). The incapacity of the plant to compete for water with ions in the soil solution was the principal effect of high EC water on crop productivity. Even if the soil seems wet, the higher the EC, the less water accessible to plants since plants can only transpire “clean” water; usable plant water in the soil solution reduces substantially as EC rises. Because the amount of water transpired by a crop is directly proportional to yield, irrigation water with a high EC lowers yield potential. The categorization for EC is given in the study area (Aher and Gaikwad 2017).
According to the recommendations, an EC value exceeding 3000 S/cm is considered unacceptable. A value of less than 250 S/cm is deemed good for irrigation, while a value of more than 250 S/cm is deemed poor. Few groundwater samples show the content of TDS and EC values between > 10,000 mg/L and 3000 S/cm, respectively, and are considered unsuitable for agriculture.
Total hardness
The magnesium and calcium salts detected in the groundwater sample are included in the total hardness. The hardness of a groundwater sample is a practical value for determining water quality for home, agricultural, and industrial purposes. Soft water contains 0–75 mg/L CaCO3, moderately hard water contains 75–150 mg/L CaCO3, hard water contains 150–300 mg/L CaCO3, and extremely hard water contains > 300 mg/L CaCO3 (1986, US EPA). Although a high level of TH is not detrimental to one’s health, both the extremes of extremely soft (less than 60 mg/L as CaCO3) and excessively hard (more than 180 mg/L as CaCO3) are considered undesirable (Sappa et al., 2014). According to Sawyer and McCartly’s (1967) classification of total hardness, not a single groundwater sample was seen lying in the soft group, whereas 10%, 29%, and 60% fall into the moderate, hard, and extremely hard categories, respectively. The abundant presence of alkaline earth elements such as Ca and Mg cause the water to be hard (Hem, 1985a, b; Freeze and Cherry, 1979), and by these measures show how water has very high values in the study area of hardness. This can be studied more clearly through the Piper plot diagrams (Fig. 3), PL1 shows the water samples of EZ (Shendra MIDC), PL2 shows the parameters of CZ (the city or domestic area), and PL3 shows the various examples of WZ (Waluj MIDC). The PL1 shows higher concentrations of alkaline earth parameters such as Ca and Mg, as shown in Table 1 for comparison.
According to the National Academy of Sciences, hard water does not pose a serious health risk. However, it does supply a modest amount of total calcium and magnesium to the human diet. Dissolved calcium and magnesium levels are typically relatively high in most cases. As a result, it demonstrates that it is an important source of calcium and magnesium in the human diet. In addition, hard water is inconvenient due to mineral accumulation on fixtures and poor soap or detergent efficacy. Almost every cleaning operation, from laundry to dishwashing, bathing, and personal grooming, is affected by hard water. Deposition of salts in pipes can limit water flow (Gupta et al. 2011).
Irrigation aspects
Because groundwater is utilized for irrigation in the region, determine the characteristics responsible for irrigation water quality. The salt absorption ratio (SAR), sodium percentage (Na percent), salinity, permeability index (PI), and Kelly’s index (KI) are the particular characteristics to know the quality of water for irrigation purposes (Kaur et al. 2016). Groundwater plays an important role in agriculture for watering crops and irrigation of dry season crops. It is estimated that more than most irrigation water requirements are met from groundwater sources in the area under study. Due to the contamination, the quality of the groundwater changes along with the depth of the water table. It also keeps to vary with the change in seasons changes and is primarily decided by the distance and composition of dissolved solids present in it. Majorly the instances show that an excessive withdrawal of groundwater results in the parching up of wells, destruction of the ecosystems, land subsidence, salt-water intrusion, and exhaustion of the resources. The rate of fatigue of the groundwater levels and worsening of groundwater quality is of immediate concern in most of the country because of the large-scale disposal of urban and industrial wastes along with the use of chemical fertilizers and pesticides in agricultural fields (CGWB and CPCB 2000; CGWB, 1999, 2000, 2001).
The sodium adsorption ratio (SAR), sodium percentage (percent Na), residual sodium carbonate (RSC), magnesium hazard (MH), permeability index (PI), nitrate, and electrical conductivity were selected for detailed examination in this study to determine if groundwater is acceptable for irrigation (EC). The proportion of different categories of each measure was statistically examined according to the appropriateness categorization for groundwater agricultural irrigation. The Kriging interpolation method in the ArcGIS software was used to create geographical distribution figures for different categories of each indicator. This method frequently studies and interprets groundwater quality spatial variations (Najafzadeh et al., 2021).
The United States Department of Agriculture (USDA) has proposed SAR, which can indicate the relative activity of the alternative adsorption effect between Na + and soil components in groundwater. SAR forecasts the Na + danger of high carbonate waters, especially if no residual alkali is present (Beyene, et al., 2019), and can serve as a useful diagnostic of groundwater alkalinity. The greater the SAR value, the better the groundwater’s alkalization capacity. The permeability and structure of the soil are affected by sodium content, which is commonly stated in percent Na (Raju 2007). Filling the soil with sodium would restrict water and airflow, change the permeability structure, and hinder crop development. RSC is a metric that indicates how damaging carbonate and bicarbonate are to irrigation groundwater quality (Raju 2007). In general, excessive concentrations of carbonate and bicarbonate in groundwater and calcium and magnesium might impair the appropriateness of groundwater for crop raising (Karunanidhi et al., 2020). If the carbonate concentration in groundwater is too high, excess carbonate may mix with sodium ion to produce bicarbonate, affecting the permeability structure of the soil. Szaboles and Darab proposed the magnesium hazard (MH), which was also used to assess water quality for agricultural use. Because of the high MH levels in groundwater, the soil becomes alkaline, resulting in reduced agricultural yields. MH may influence the soil structure and negatively impact crops if the Mg2 + content in irrigation water exceeds a specific threshold. Soil permeability may be affected by salt, calcium, magnesium, and bicarbonate in irrigation water. The soil structure will be disrupted, and crop development will be impacted if high salts collect in the soil (Abboud 2018). Based on the concentrations of Ca2 + , Mg2 + , Na + , and HCO3 (Fig. 4)—the permeability index (PI) may be used to estimate the water movement capability in soil (Chidambaram et al. 2022).
The permeability and drainage capacity of the soil are also assessed using PI as a criteria for water quality appropriateness for agricultural irrigation (Raju 2007). Furthermore, EC is commonly employed as a salinity danger indicator to indicate irrigation water quality. In addition, the irrigation water quality classification standard from the US Department of Agriculture is utilized to classify the groundwater suitability for irrigation using an irrigation water classification diagram (USSL diagram and Wilcox diagram) (USSL Salinity Laboratory. 1954). The USSL diagram depicts the association between EC and SAR, whereas the Wilcox diagram depicts the relationship between EC and percent Na (see Fig. 5).
Salinity
Salinity is related to the total dissolved solids (TDS) and electrical conductivity (EC). A high concentration of TDS and electrical conductivity in irrigation water may increase the soil salinity, affecting the plant’s salt intake. The salts present in the water, besides affecting the growth of the plants directly, also affect the soil structure, permeability, and aeration, which indirectly affect the plant growth. Due to osmotic pressure, soil water passes into the plant through the root zone. As the dissolved solid content of the soil water in the root zone increases, it is difficult for the plant to overcome the osmotic pressure and assimilate water and nutrients through the root membrane (J. Indian Water Resource. Soc., Vol. 32, No. 3–4, July-Oct., 2012). Thus, the dissolved solid content of the residual water in the root zone also has to be maintained within limits by proper leaching. These effects are visible in plants by stunted growth, low yield, discoloration, and even leaf burns at the margin or top.
Sodium adsorption ratio
Salty soil is produced with a high salt concentration in the water, while alkali soil is formed when there is a high sodium concentration. Excess sodium in water harms soil characteristics and permeability (Kelly, 1951). The appropriateness of water is determined by the absolute and relative amounts of Na or alkali cations for irrigational usage, which is expressed as SAR (Richards, 1954). If the irrigation water has a high concentration of Na and a low concentration of Ca, Na may cause the cation-exchange complex to become saturated. Clay particle dispersion may cause soil structural degradation and a reduction in plant growth. If the SAR value is more than 6–9, irrigation water will produce permeability problems on shrinking and expanding clayey soils (Saleh et al., 1999). In the study area, SAR values vary from 2.38 to 59.75, which is regarded as greater than usual. Therefore, the water causes difficulties with permeability and is not suitable for irrigation usage. This means that alkali danger to crops is expected. Because of the dry nature of the environment in the research region, the soil is sandier. The Wilcox diagram shows the higher amount of SAR present in the water samples when we studied them compared to the conductivity calculated in the water sample of the complete study area.
Percent sodium
Sodium is one of the important parameters present in the groundwater which reacts with the soil, and its increasing concentration reduces its permeability. Calculating the percent Na allows determining the groundwater appropriateness for agricultural use. Except for a few samples, Na ranks within good to acceptable values for irrigation usage. The 72% of the samples enter into the good to very good group, 9% enter into the good to a permissible category, 6% enter into the permitted to a questionable category, while 3% is within the range of doubtful to unsuitable and 9% lies to the highly unsuitable category. However, through the point of view of agriculture, the water stands unsuitable.
Permeability index
Long-term water usage affects soil permeability, influenced by Na, Ca, Mg, and HCO3. PI has been widely utilized as an essential measure in determining groundwater suitability for irrigation usage. The PI values of groundwater samples range from 15.78 to 91.86% (Table 1).
Kelly’s index
The KI parameter is calculated using Na measured against Ca and Mg. Waters with KI less than one are appropriate for irrigation, but water with KI more than one indicates an excess of Na in the water and is thus unsuitable for irrigation.
Kelly’s ratio (KR) and sodium percentage (SP) were used by Kelly (1940) and to assess the harmful influence of salt on water quality for irrigation. The Kelly’s ratio and the SP are calculated as follows:
All ionic concentrations are in meql−1.
In groundwater samples, the KI varies from 0.17 to 8.98 (Table 1). Based on the KI readings, it is determined that the 86% of the sample is acceptable for irrigation and the 14% is not. The inappropriate groundwater samples belong to the industrial clusters of WZ and EZ.
Magnesium hazard
Excess of magnesium in the groundwater increases the hardness of water, making it unsuitable for any usage. The presence of an excessive amount of Mg in groundwater raises soil alkalinity, lowering soil quality and, as a result, the overall agricultural production (Kumar et al., 2007). Ca and Mg are in balance in the majority of groundwater (Hem, 1985a, b); generally, it is seen that when studying the system present in the soil, Ca and Mg do not operate similarly, because Mg destroys soil quality, specifically in Na-dominated and very salty streams. The magnesium hazard for irrigation water was proposed by, which is stated as:
with all concentrations in mEq/L.
The MH concentrations in the samples vary from 13.49 to 86.99 (Table 1). According to MH values (Paliwal, 1972), 50% of samples had more than 40% MH values, which might harm agricultural production. Out of it, the maximum examples are from the SZ (Shendra MIDC) area.
Residual sodium carbonate
RSC occurs in irrigation water when the concentrations of CO3 and HCO3 surpass the Ca and Mg levels. The use of RSC-raised water for irrigation will increase Na in the soil (Devi et al., 2015). Black regur soil, which is the specialty of this region, is found in most study areas. A high proportion of bicarbonate in the soil can slightly raise its pH, resulting in black alkali soil. Due to the unique contraction character of the soil, a larger amount of potash and montmorillonite causes the development of large fractures in this black soil. RSC values range from 118 to 4066 mEq/L (see Table 1). According to RSC classification (Eaton, 1950), 60% of the sample is safe, 8% is of questionable quality, and 32% is inappropriate.
The groundwater in the research region may be classified into two chemical dynamic stages: ion exchange and reverse ion exchange. The bulk of the samples suggests an early stage of chemical development instead of the latter, which indicates a late or developed stage of evolution. The ion exchange process, in which anions are replacing most cations, occurs in all geomorphic units, whereas reverse ion exchange occurs mostly in the eastern industrial cluster EZ. The mixing of ions begins in the basin area near the river Kham and is chemically changed to the end member like Cl and Na in the CZ plateau region. The industrial zone has inappropriate samples for irrigational use due to saline mineralization caused by different rocks in the research region, evaporates, halite, and gypsum encrustations in the basaltic aquifers. As a result, the inability of groundwater in the study region to be used for irrigation is entirely due to natural salinization and not to the late or matured stage of chemical dynamics. This can be noted that from the Gibbs diagram (diagram no. 4), the evaporation rate sits on the higher side, increasing the TDS in the groundwater and making it unfit for usage.
Nitrate
Nitrate (NO3) has become a common chemical pollutants in groundwater, which can enter into the groundwater by geogenic processes and anthropogenic sources. Numerous anthropogenic like the indiscriminate release of industrial waste, improper garbage dumping sites, leaching pits, a vast amount of fertilizer usage, poor sewage carriage pipes, leakage in the septic tanks, deforestation, and many more innumerable reasons may be the reason for the nitrate mixing with groundwater.
A titrimetric method was utilized to determine the nitrate concentrations on the azo dye which was concluded from the coupling of diazonized sulfanilamide and N-1 naphthylethylendiamine. After titrating the sample with NaOCl, the following formula was used to find the unknown concentration that can be known by comparison to a nitrate standard.
where u = unknown and std = standard value.
Figure 6 displays the sample collection points and the statistical graph of the presence of nitrate in the sites. At the same time, Fig. 7 shows the temporal changes of the nitrate concentration during 6 years (2016–2021) in the groundwater of the study area, taking the cumulative mean of the collected samples, which gives a clear perspective of the nitrate contamination of the study area taken together cumulatively.
Risk assessed on the human health
This study was based on the average daily intake nitrate content in the study area, and the risk characteristics were described by the hazard quotient (HQ). The hazard quotient is the most common method of calculating quantitative risk. It is usually calculated as the ratio of exposure level to guideline value. The higher the hazard quotient, the higher the health risks involved, and a hazard factor of less than 1 is usually considered low risk. This research aims to glance into the non-carcinogenic health risks for adult males, females, and children heavily reliant on groundwater for drinking. Risk on the citizen’s health, including males, females, and children, has been assessed based on the United States Environmental Protection Agency guidelines. For the different age classes (12 years of children, 67 years for females, and 64 years for males), the findings of HQdermal were slightly lower than zero. At the same time, HQoral ranged from 0.025 to 3.889 with an average of 0.893 for males, 0.035 to 4.597 with an average of 1.055 for the females, and 0.040 to 5.259 with an average of 1.207 for children. The results show that nitrate exposure directly due to drinking water ingestion was higher than the exposure due to dermal interactions in the study area. The reasonable limit for non-carcinogenic health risks is ≤ 1 (HI ≤ 1), based on the USEPA health risk standards. If the hazard index (HI) value is > 1, then the possibility of an adverse risk to human health is very high. HITOTAL values in the study area are varied from 0.029 to 3.900 (average: 0.895) for males, 0.0035 to 4.609 (average: 1.058) for females, and 0.040 to 5.291 (average: 1.214) for children. Out of 55 groundwater samples, 19 samples for males, 20 for females, and 16 samples for infants, the nitrate exposure levels in drinking water were found to expose these age groups to non-carcinogenic effects in the study region intake of higher nitrate concentrations in drinking water. Many other scholars have found that, due to lower body weight and personality characteristics, children are more vulnerable to chronic non-carcinogenic threats than adults.
Implication
Agriculture is the major source of income for the people of Maharashtra plateau, accounts for a significant portion of the overall state domestic product, and employs around 66% of the entire workforce in Maharashtra. This plateau’s geology, hydrogeology, and rainfall make it ideal for agricultural pursuits. The current study discusses evaluating irrigation index parameters in groundwater for its best level utilization. Over 89% of the samples are inappropriate for irrigation and also drinking, based on the current TH categorization. The prominence of Na in Wilcox’s figure (Fig. 5) shows that the groundwater in the study area is doubtful, unsuitable, or highly unsuitable. And per the sample PI, 41% of the samples had poor irrigation quality. However, according to KI, 14% of samples are unsatisfactory for irrigation. MH indicates that 40% of samples had more than 50% MH values, which might harm agricultural production. According to the RSC classification, 60% of the examples are safe, 8% are intermediate quality, and 32% inappropriate. The chemical development of groundwater reveals the Ca–Mg–Cl-HCO3 hydrochemical facies as the dominant type, demonstrating the beginning and intermediate stages of chemical evolution during the ion mixing process. Most inappropriate samples are identified during the early stage of ion mixing within the industrial cluster zone, where the river basin is missing and owing to the different saline aquifers present in the area. Three distinct fields such as precipitation dominance, evaporation dominance, and rock–water interaction dominance areas are shown in the Gibbs diagram (Fig. 4). Thus, examining groundwater factors can help identify inappropriate irrigation regions. Such research will aid in developing and managing groundwater resources in the studied region.
Recommendations and conclusion
Water with such high levels of contaminants does not support crop yield and may induce problems of permeability and thus will reduce the ability of the plant roots to squeeze up the nutrients from the soil below; moreover, such water is not suitable for drinking. Every country like India needs to manage the groundwater resources for urban water supply. Similarly, depending on the above assumptions, some recommendations have been provided for the sustainable management of urban groundwater. This water can be used in all ways when it is pure, non-contaminated, and of good quality to have a healthy society.
The basaltic rock present in the area has a densely jointed structure, which makes it an excellent means to replenish subsurface aquifers. Dykes may be found all across the research region, and the discordant character of the dykes helps to create a water split. Water recharging is aided by the presence of pipe amygdales, pipe vesicles, and porphyritic basalts. If the water has a good quality, it can be used in agriculture and domestic purposes; similarly, when the water is substandard and unusable, it should not be tapped for drinking and other purposes such as cropping.
-
Rainwater recharging strategies are necessary to be adopted in the urbanized environment of the study area as a future investment. At the same time, water conservation structures are needed to improve the groundwater quality and the groundwater quantity of an arid area like Aurangabad.
-
All the concrete pipes have to be checked for leakages, and the sewerages have to be maintained from time to time to reduce groundwater pollution.
-
Domestic waste should not be mixed with domestic sewage to avoid leachate problems.
-
The construction of STPs and ETPs will help conserve water wastage and pollution.
-
Domestic waste disposal has to be controlled as it leads to pollution and contamination of the underground aquifers.
The above recommendations will be helpful to put a strain on the water contamination and increase the quantity and quality of groundwater at the same time.
In the principal component (Table 2) when analyzed, the analytical values obtained from the groundwater samples when plotted on Piper plots (Fig. 3) revealed that the alkaline earth metals (Ca, Mg) are significantly dominant over the alkali (Na), and the strong acidic anions (Cl−, SO4 2−) prevailing over the weak acidic anions (NO3). Furthermore, Piper trilinear diagram classified 93.48 % of the samples from the study area under Ca2–Mg2–Cl−–SO4 2− type and only 6.52 % samples under Ca–Mg–HCO3− type.
The chemical composition of groundwater resources in the Aurangabad region was assessed and their suitability for drinking and industrial applications. The following is a list of the main ions and their relative abundance: HCO3 > Cl > SO42 > CO32 and NO3 > Mg2+ > Ca2+ > Na+ > K+. The massive levels of magnesium present in groundwater come mostly from basalt. The prevalence of nitrate is due to the widespread use of pesticides and chemical insecticides in local agricultural practices and home effluents being combined into groundwater sources like aquifers. Ca–HCO3, Ca–Na–HCO3, and Ca–Mg–Cl were the most common hydrochemical facies.
According to the different analytical methodologies, 55% of the water samples were oversaturated in terms of basalt, 30% in terms of nepheline syenite, and 10% in terms of plagioclase feldspars. The results of the Gibbs diagram (Fig. 4) suggest that feldspar dissolution and reverse ion exchange may be the key processes responsible for the chemical composition of the groundwater in the investigated area. The findings also revealed that the examined groundwater for irrigation has a high salinity hazard (85%, C3 class), a high alkalinity hazard (owing to high concentrations of HCO3 and CO32 and low Ca:Mg molar ratio (97.5%, 1)), is somewhat basic in nature, and is highly hard. According to the findings of this study, the quality of groundwater is unsuitable for irrigation or agriculture, and continued use in the long term may exacerbate salinity and alkalinity issues in the soils. If this water is used for drinking, it may pose health risks in the long run due to higher levels of nitrate. As the water shows contamination with higher amounts of nitrate, it needs to be treated with reverse osmosis and oxidation (Sarkar et al., 2021, Dasgupta et al., 2015, Candamano et al., 2019). The groundwater extraction sources and their surroundings should be properly maintained to ensure hygienic conditions. No sewage or polluted water should be allowed to percolate directly to the groundwater aquifer. To avoid direct wellhead pollution, proper cement platforms should also be constructed surrounding groundwater abstraction sources.
References
Abboud I (2018) Geochemistry and quality of ground-water of the Yarmouk basin aquifer, north Jordan. Environ Geochem Health 40:1405–1435
Acharya S, Sharma SK, Khandegar V (2018) Assessment of ground-water quality by water quality indices for irrigation and drinking in South West Delhi, India. Data Brief 18:2019–2028. https://doi.org/10.1016/j.dib.2018.04.12
Adimalla N, Wu J (2019) Groundwater quality and associated health risks in a semi-arid region of south India: implication to sustainable groundwater management. Hum Ecol Risk Assess Int J 25(1–2):191–216. https://doi.org/10.1080/10807039.2018.1546550
Adimalla N, Venkatayogi S, Das SVG (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh, India. Appl Water Sci 9(4):94. https://doi.org/10.1007/s13201-019-0968-y
Aher, Kailash & Gaikwad, Sharad (2017) Irrigation ground-water quality based on hydrochemical analysis of Nandgaon block, Nashik district in Maharashtra. International Journal of Advanced Geosciences. 5. 1. https://doi.org/10.14419/ijag.v5i1.7116.
Alley WM, Reilly TE, Franke OL (1999) Sustainability of ground-water resources. US Geological Survey Circ 1186
APHA (1999) Standard Methods for the examination of water and waste water. American Public Health Association pp 874
Arya S, Subramani T, Karunanidhi D (2020) Delineation of ground-water potential zones and recommendation of artificial recharge structures for augmentation of ground-water resources in Vattamalaikarai Basin. South India Environ Earth Sci 79:102. https://doi.org/10.1007/s12665-020-8832-9
Baba A, Tayfur G (2011) Ground-water contamination and its effect on health in Turkey. Environ Monit Assess 183:77–94. https://doi.org/10.1007/s10661-011-1907-z
Balamurugan P, Kumar PS, Shankar K, Nagavinothini R, Vijayasurya K (2020) NOn-carcinogenic risk assessment of groundwater in southern part of salem district in Tamilnadu, India. J Chil Chem Soc 65(1):4697–4707. https://doi.org/10.4067/S0717-97072020000104697
Beyene G, Aberra D, Fufa F (2019) Evaluation of the suitability of ground-water for drinking and irrigation purposes in Jimma Zone of Oromia. Ethiopia Groundw Sustain Dev 9:1–11
Bhanja SN, Mukherjee A, Rangarajan R, Scanlon BR, Malakar P, Verma S (2019) Long-term ground-water recharge rates across India by in situ measurements. Hydrol Earth Syst Sci 23:711–722. https://doi.org/10.5194/hess-23-711-2019
Brindha K, Kavitha R (2015) Hydrochemical assessment of surface water and ground-water quality along Uyyakondan channel. South India Environ Earth Sci 73(9):5383–5393
Brindha K, Pavelic P, Sotoukee T, Douangsavanh S, Elango L (2017) Geochemical characteristics and ground-water quality in the Vientiane plain. Laos Exposure and Health 9(2):89–104
Candamano, S., Sgambitterra, E., Lamuta, C., ...Chakraborty, S., Crea, F., 2019 Graphene nanoplatelets in geopolymeric systems: a new dimension of nanocomposites. Materials Letters, , 236, pp. 550–553 Related documents
CGWB (1999) Hydrogeological framework for urban development of Bhopal City, Madhya Pradesh, Central Ground Water Board, North Central Region, Bhopal, India.
CGWB (2000) Ground water quality in urban environment of India, Central Ground Water Board, Faridabad, India.
CGWB (2001) Hydrogeological framework and ground water development prospects in Jabalpur City, M.P., Central Ground Water Board, North Central Region, Bhopal, India.
CGWB (2013) Ground-water information booklet, Technical Report Series D. pp. 10.
Central Ground Water Board-India (2006) Manual on artificial recharge of ground-water. Ministry of Water Resource Board, Government of India, India
Chidambaram S, Prasanna M, Venkatramanan S, Nepolian M, Pradeep K (2022) Groundwater quality assessment for irrigation by adopting new suitability plot and spatial analysis based on fuzzy logic technique. Environ Res 204:1–15
Dasgupta J, Mondal D, Chakraborty S, …Curcio, S., Arafat, H.A. (2015) Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol Environ Saf 121:22–30
Davis SN, Dewiest RJM (1966) Hydrogeology. John Wiley and Sons, New York. Chichester. Brisbane, Toronto, Singapore.
Devi OG, Kumar, Arun, Chandrashekhar AK, Chandrasekharam D (2015) J Geol Soc India 73:229–236
Deepa S, Venkateswaran S (2018) Appraisal of ground-water quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using water quality index (WQI) and multivariate statistical techniques. Modeling Earth Systems and Environment 4(3):1165–1180
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69
Ellis E (2007) Land use and land cover change. Encyclopedia of Earth.
Freeze RA, Cherry JA (1979) Ground-water. Prentice-Hall, Englewood Cliffs
Gupta I, Salunkhe A, Rohra N, Kumar R (2011) Ground-water quality in Maharashtra, India: focus on nitrate pollution. J Environ Sci Eng 53:453–462
Hem JD (1985a) Study and interpretation of the chemical characteristics of natural water. USGS, Water Supply Paper 2254:264
iPla JM, Ghiglieri G, Uras G (2014) Seawater intrusion and coastal ground-water resources management. Examples from two Mediterranean regions: Catalonia and Sardinia. Contributions to science 171–184
Jain MK, Dadhich LK, Kalpana S (2011) Water quality assessment of Kishanpura Dam, Baran, Rajasthan. India Nat Environ Pollut Technol 10:405–408
Jeyaruba, T & Mikunthan (Mrs.) Thushyanthy. (2009). The effect of agriculture on quality of groundwater: a case study. Middle-East Journal of Scientific Research. 4. 110-.
Karunanidhi D, Aravinthasamy P, Kumar D, Subramani T, Roy P (2020) Sobol sensitivity approach for the appraisal of geomedical health risks associated with oral intake and dermal pathways of ground-water fluoride in a semi-arid region of south India. Ecotoxicol Environ Saf 194:1–12
Kaur T, Bhardwaj R, Arora S (2016) Assessment of ground-water quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci, 7 (6). doi: https://doi.org/10.1007/s13201-016-0476-2
Kaur L, Rishi MS, Siddiqui AU et al (2020) Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ Pollut 259:113711. https://doi.org/10.1016/j.envpol.2019.113711
Kelly WP (1940) Permissible composition and concentration of irrigated waters. Proceedings of the ASCF 66:1–607
Kelly WP (1951) Alkali soils—their formation properties and reclamation. Reinhold Publishing Co., New York
Kumar CP (2013) Assessment and strategies for development potential of deeper confined aquifers in India. Asian Acad Res J Multidiscip 1(8):247–258
Kumar B, Kumar U (2011) Ground water recharge zonation mapping and modeling using geomatics techniques. Int J Environ Sci 1(7):1671 Majumder A, Sivaramakrishnan L (2014) Ground-water budgeting in alluvial Damodar fan delta: a study in semi-critical Pandua block of West Bengal, India.
Kumar PS, Balamurugan P (2019) Suitability of ground water for irrigation purpose. In: Omalur Taluk, Salem, Tamil Nadu, India. Indian Journal of Ecology 46(1):1–6
Kumar M, Kumari K, Ramanathan Al, Saxena R (2007) A comparative evaluation of ground-water suitability for irrigation and drinking purposes in two intensively cul-tivated districts of Punjab. India Environ Geol 53:553–574
Hem JD (1985b) Study and interpretation of the chemical characteristics of natural water. USGS, Water Supply Paper 2254:264. Hydrogeol J 15:183–195
Magesh NS, Krishnakumar S, Chandrasekar N, Soundranayagam JP (2013) Groundwater quality assessment using WQI and GIS techniques, Dindigul district, Tamil Nadu, India. Arab J Geosci 6(11):4179–4189. https://doi.org/10.1007/s12517-012-0673-8
Mukherjee I, Singh UK (2018) Groundwater fluoride contamination, probable release, and containment mechanisms: a review on Indian context. Environ Geochem Health 40:2259–2301. https://doi.org/10.1007/s10653-018-0096-x
Münch Z, Conrad J (2007) Remote sensing and GIS based determination of ground-water dependent ecosystems in the Western Cape, South Africa. Hydrogeol J 15:19–28
Murray J, O’Geen AT, McDaniel PA (2003) Development of a GIS database for ground-water recharge assessment of the Palouse Basin. Soil Sci 168(11):759–768
Najafzadeh M, Homaei F, Mohamadi S (2021) Reliability evaluation of ground-water quality index using data-driven models. Environ Sci Pollut Res
Neelakantan R, Yuvaraj S (2012) Evaluation of ground-water using geospatial data—a case study from Sale em taluk, Tamil Nadu. India Int J Remote Sens Geosci 1(2):7
Niyogi D, Mahmood R, Adegoke JO (2009) Land use/land-cover change and its impacts on weather and climate. Boundary Layer Meterology 133(3):297–298
Owuor SO, Butterbach- BK, Guzha AC, Rufino MC, Pelster DE, Diaz- PE, Breuer L (2016) (2016) Ground-water recharge rates and surface runoff response to land cover changes in semi-arid environments. Ecol Process 5:16. https://doi.org/10.1186/s13717-016-0060-6
Paliwal KV (1972) Irrigation with saline water. Water Technology Centre, Indian Agriculture Research Institute, New Delhi, pp. 198
Price K, Jackson CR, Parker AJ (2010) Variation of surficial soil hydraulic properties across land uses in the southern Blue Ridge Mountains, North Carolina, USA. J Hydrol 383:256–268
Raju N (2007) Hydrogeochemical parameters for assessment of ground-water quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh. South India Environ Geol 52:1067–1074
Richards LA (1954) Diagnosis and improvement of saline alkali soils. US Department of Agriculture, Hand Book 60, pp. 160
Rokade VM, Kundal P, Joshi AK (2004) Water resources development action plan for Sasti Watershed, Chadrapur district, Maharashtra using remote sensing and geographic information system. J Indian Soc Remote Sens 32(4):359–368
Sahu P, Sikdar PK (2008) Hydrochemical framework of the aquifer in and around East Kolkata Wetlands, West Bengal. India Environ Geol 55(4):823–835
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sappa G, Ergul S, Ferranti F (2014) Water quality assessment of carbonate aquifers in southern Latium region, central Italy: a case study for irrigation and drinking purposes. Appl Water Sci 4:115–128
Sawyer GN, McCartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw- Hill, New York, p 518
Sharma MP, Kujur A (2012) Application of remote sensing and GIS for ground-water recharge zone in and around Gola block, Ramgargh district, Jharkhand. India Int J Sci Res Publ 2(2):1
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) Multivariate analysis and geochemical signatures of groundwater in the agricultural dominated taluks of Jalandhar district, Punjab, India. J Geochem Explor 208:106395
Thomas A, Sharma PK, Sharma MK, Anil S (1999) Hydrogeomorphological mapping in assessing ground-water by using remote sensing data case study in Lehra Gage Block, Sangrur district, Punjab. J Indian Soc Remote Sens 27:31–42
Thomson B, Nokes C, Cressey P (2007) Intake and risk assessment of nitrate and nitrite from New Zealand foods and drinking water. Food Addit Contam 24:113–121. https://doi.org/10.1080/02652030600934206
USEPA (2006) Risk assessment guidance for superfund Volume I: Human Health Evaluation Manual (Part E)
Vadiati M, Asghari-Moghaddam A, Nakhaei M, Adamowski J (2016) A fuzzy-logic based decision-making approach for identification of ground-water quality based on ground-water quality indices. J Environ Manag. https://doi.org/10.1016/j.jenvman.2016.09.082
Wagh VM, Panaskar DB, Jacobs JA, Mukate SV, Muley AA, Kadam AK (2019) Influence of hydro-geochemical processes on ground-water quality through geostatistical techniques in Kadava River basin. Western India Arab J Geosci 12(1):7
World Health Organization (WHO) (2011) Guideline for drinking water quality. Yidana, S.M., Yidana, A., 2010. An assessment of the origin and variation of ground-water salinity in southeastern Ghana. Environ. Earth Sci. 61 (6), 1259–1273.
Yolcubal I, Brusseau ML, Artiola JF, Wierenga P, Wilson LG (2004) 12 – Environmental physical properties and processes. Environ. Monit. Charact. 207–239.
Funding
Open access funding provided by Università della Calabria within the CRUI-CARE Agreement. This study was financially supported by the Department of Science and Technology (INSPIRE Delhi), DST Inspire/IF170086/Monica.
Author information
Authors and Affiliations
Contributions
All the authors have equally contributed.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research involving human participants and animals
This article does not contain any studies involving animals and human participants performed by any of the authors.
Additional information
Responsible Editor: Santanu Banerjee
Highlights
• Nitrate contamination and risk assessment on the human health risk.
• Groundwater-dependent ecosystems (GDE) in Deccan basaltic terrain (DBT).
• Water quality index (WQI) of the groundwater samples.
• Water quality standards and its human health risk assessment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chakraborty, M., Tejankar, A., Coppola, G. et al. Assessment of groundwater quality using statistical methods: a case study. Arab J Geosci 15, 1136 (2022). https://doi.org/10.1007/s12517-022-10276-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10276-2