Abstract
Nickel has negative human health effects depending on dose and length of exposure; it is classified as a class 1 carcinogen. Nickel pollution threatens Saft Rashin village, Egypt. The area’s crops are highly contaminated with nickel, suggesting them as bioaccumulators. Nickel daily intake exceeds twofold the permissible limits. To fully understand the pollution process, Ni concentrations in every input/output were determined and have been normalized to grams per hectare per year (g ha−1 year−1). Geochemical mass balance (GMB) revealed that dustfall is the major Ni input (88.8%) followed by P-fertilizers (11.2% of the total inputs). Nickel in dust is mainly related to traffic emissions. On the other hand, plant uptake removes about 90% of the total nickel input. The remaining 10% accumulates in the soil with a retention rate of 44.7 g ha−1 year−1. Accordingly, using new diesel models that are more efficient and less polluting can reduce pollution levels; likewise, use eco-friendly fertilizers. Egyptian clover is recommended as a phytoremediator to remove Ni from growth media for its high efficiency and low planting cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollution management initiates by sourcing pollutants (Said et al., 2021), which is efficiently achieved using geochemical mass balance (GMB) model. It is useful technique for fully understanding the pollution process because it determines metal concentrations in every pathway to and from the soil. Hence, it can accurately assess potential pollution sources, sinks, and their contributions (Shi et al., 2018). Moreover, soil dynamics can be assessed based on the mass gap between inputs and outputs, which is not easily obtained in the laboratory. Where GMB model acts as a metal budget, subtracting the total metal input from the total output, the first to apply GMB model in the environmental study was in Netherlands in 1974 (Misseyer and Most, 1993). Then, the model was used successively in Czech (Paces, 1994), Germany and France, (Belon et al., 2012), and in China (Luo et al., 2009, Shi et al., 2018 and Xia et al., 2021). Not all GMB studies have fully addressed the inputs/outputs. Ignoring one pollution pathway leads to an inaccurate assessment of the pollution process, because the absence of one pollution pathway directly amplifies the contributions of others. Hence, the current study took into account determination of nickel in every input/output in the study area.
Long-term exposure to heavy metals poses risks to human and ecological systems. Nickel is one of these heavy metals classified as a group 1 carcinogen (Kim et al., 2015). It is a transitional element widely spread in the environment: water, air, soil, and plants (Kabata-Pendias 2010). The spread of nickel pollution is due to its unique chemical and physical properties that are licensed to enter many industries: alloys, nickel-cadmium batteries, electroplating, car tires, dyes, and catalysts. Environmental pollution with nickel may come from different emission sources. Application of chemical fertilizers and combustion of fossil fuels contribute to the anthropogenic input of Ni into the environment (Said et al., 2021). The largest atmospheric nickel contributor is the fossil fuel combustion in which nickel exists mainly as nickel oxide, nickel sulfate, and oxides of complex nickel-containing metals (EPA, 1986 and Peter et al. 2022). Organic matter (OM) has a high affinity for nickel adsorption, thus nickel is highly concentrated in coal and oil (Kabata-Pendias 2010). In addition to those anthropogenic sources, nickel can be derived from a natural source, where it is found in combination with oxygen and sulfur in the form of oxides and sulfide minerals (Kabata-Pendias 2010). Nickel as a potentially toxic element (PTE) has negative human health effects depending on dose and length of exposure. It has been linked to allergic diseases, kidney, lung, and nasal cancer (Kabata-Pendias and Mukherjee 2007). Generally, the major mechanism of heavy metal-induced cancer is oxidative stress caused by reactive oxygen species (ROS) attacking DNA (Kim et al., 2015). ROS activates transcription, increasing cell proliferation and finally carcinogenesis (Jomova et al., 2011). Such oxidative stress results from an imbalance between the production of free radicals and the generation of antioxidants (Jaishankar et al., 2014), where free radicals overwhelm the body’s antioxidant capacity, resulting in molecular damage.
Nickel can enter the human body through various pathways. Food crops are the main human exposure source, contributing about 90% of total heavy metal intake. The remaining 10% occurs through skin contact and inhalation of contaminated dust (Khan et al. 2015). Plants grown in contaminated environments can accumulate heavy metals in high concentrations and promote them to humans via the food chain, causing negative health effects. These plants with exceptional accumulation capacity of elements are known as hyperaccumulator species (Cho-Ruk et al. 2006). Different definitions for hyperaccumulators in literature (Baker & Brooks, 1989) defined hyperaccumulator species as plants which accumulate > 1000 mg kg−1 of Cu, Co, Cr, Ni, or Pb, or > 10000 mg kg−1 of Mn or Zn. Tangahu et al. (2011) defined hyperaccumulators as plants that exhibit 0.2% on a dry weight basis for toxic elements (Cd, Pb) to 2% for the less toxic ones (Zn, Ni, Cu). Felix et al. (1999) reported that plants able to uptake elements above established background concentrations and more than other species from the same soil are called hyperaccumulators. Generally, hyperaccumulator is a specific term for those plant species containing an exceptionally high concentration of an element. In the current study, the bioaccumulators were described based on Felix et al. (1999) point of view. Bioaccumulator species threaten consumer health if they contain potentially toxic elements (PTEs) above permissible limits in their edible parts. On the other hand, such plant species that accumulate PTEs in elevated concentrations are used in pollution treatment programs, as bio-pumps remove pollutants from the growth media. Use of berseem (Trifolium alexandrinum) as a PTEs-phytoremediator is well known in the scientific literature (Bianchi et al., 2011 and Ali et al., 2012). The paper aims to describe the fate of nickel from soil to food chain using GMB model and to propose a phytoremediation for nickel decontamination.
Materials and methods
Study area
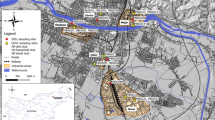
The study area is located about 30 km South West Beni Suef Governorate, Egypt. It extends between latitudes 28° 58′ 00″–28° 59′ 53″ N and longitude longitudes 30° 54′ 22″–30° 56′ 49″ E (Fig. 1). Like most of Egypt’s arable land, it is of alluvial origin transported by the Nile. Saft Rashin village is an extremely agricultural/residential zone with a significant impact on environmental status. Nickel pollution degrades crops and air, endangering human health. Geochemical mass balance approach created by Paces (1994) was applied in an attempt to understand the pollution process and to determine the rate of nickel retention by soil in the study area.
Sampling and analysis
Samples in Fig. 1 were obtained by direct collection using various sampling devices. Nine irrigation water were collected corresponding to nine soil samples and 17 plants (representing four crops grown in the area, collected at the harvesting stage). Five samples of sugar beet, six garlic, four celery, and finally two clover sample. Three drainage water numbered as 6, 7, and 8. Finally, two dustfall samples (A and B) and two samples of the commonly used fertilizers. All samples were analyzed for total Ni according to the standard methods and measured using atomic absorption spectrometry (AAS). Water samples were filtered using 0.45 μm membranes, acidified with HNO3 to pH <2, and kept in the refrigerator for analysis. Plant samples were collected in plastic bags and transferred to the lab. Plant samples were washed with distilled water several times and then washed with 10% hydrochloric acid to ensure the samples’ purity. Each sample was cut into small slices with plastic scissors and dried in the oven at 70 °C for 24 h. The dried plants were ground and preserved in sealed bowls (Liu et al. 2013 and Bhatti et al. 2016). Agricultural soil samples (30 cm depth) were collected using a hand shovel, air-dried, and passed through a 2-mm sieve, and then dried in the oven at 110°C for 3 h. Each sample was milled to pass through 63 mesh sieves and homogenate for analysis. Dust samples were collected for a month in beakers containing 1l of distilled water to prevent sample loss by blowing air. The beaker has a diameter of 15 cm and a height of 45 cm, and is placed at a height of 2 m (Balakrishna et al. 2011) in two sites (Fig. 1) at a distance of 20 m away from the surrounding building. The collected dust was separated by evaporation, dried, and crushed to <63 μm. One gram of each of soil, dust, and plant powder was digested using aqua regia (HNO3:HCl = 1:3). Finally, all treated samples were analyzed for their nickel content using atomic absorption spectrometry (PerkinElmer 400). Quality assurance and control were assessed through replicate analysis and selection of most appropriate absorption wavelength.
Daily intake
Ni daily intake for the studied food plants was calculated as follows:
Ci is the concentration of Ni in edible part of the studied crops (mg kg−1); DC is average daily consumption of sampled crops (g/person/day).
Geochemical mass balance (calculation
Geochemical mass balance (GMB) of an element “e” is described by the following equation (Paces 1994): GMB = (We + Pe + De + Fe + Ie) – (DRe + Be) where We parent rock weathering flux, Pe wet precipitation flux, De dry deposition flux, Fe chemical fertilizers, Ie irrigation water flux, DRe drainage water flux (surface water runoff and deep percolation), and Be biological uptake flux. The positive GMB value points to the element accumulation in the soil, while the negative GMB value indicates the element depletion. When GMB is zero, the element is in a steady-state.
Ni input by atmospheric deposition (g ha−1 year−1)
The soil input of Ni via dustfall (g ha−1 year−1) = measured concentration of Ni in dustfall (g kg−1) × dustfall rate (kg ha−1 year−1).
Ni input by fertilizers (g ha−1 year−1)
The soil input of Ni via fertilizer (g ha−1 year−1) = measured Ni concentration in fertilizer (g kg−1) × fertilization rate (kg ha−1 year−1). Since fertilization rates vary for different crops, the fertilization influx (g ha−1 year−1) for each crop was calculated separately and then the average fertilization influx (g ha−1 year−1) was taken.
Soil influx of Ni from irrigation water (g ha−1 year−1)
The soil input of element via irrigation water was neglected because all water samples are below detection limit (BDL) and not contaminated comparing with FAO (1994).
Soil influx of Ni from natural weathering (g ha−1 year−1)
In this study, the input flux from parent rock weathering was neglected in Egypt, as sediments making up soils in the delta no longer reach soil surfaces after the construction of Aswan High Dam (Ahmed and Fawzi 2011).
Soil influx of Ni from wet precipitation
Modeling studies usually neglect the rainfall contribution since it is a rare input due to the arid climate.
Ni output by crops (g ha−1 year−1)
The soil output of Ni by crops (g ha−1 year−1) = measured Ni concentration (g kg−1) in crop category multiplied by its DMY (kg ha−1 year−1). Note: Average plant uptake (g ha−1 year−1) here was calculated as the sum of plant uptake of all studied crops divided by their number (4 crops). Since each crop has a different productivity (kg ha−1 year−1) from the other, plant uptake (g ha−1 year−1) was calculated for each individual crop and then the average was taken.
Ni leached into drainage water (surface runoff and deep percolation)
The soil output of Ni through drainage water was neglected because all water samples are below detection limit (BDL).
Result and discussion
Water and soil quality
All water sampled is below the detection limit (<1 ppb), suitable for irrigation uses compared to FAO (1994). Almost all sampled soil is below detection limit, within the limits of uncontaminated agricultural soils compared with Turekian and Wedepohl (1961) (Ni= 68 mg kg−1). Only samples No. 2 and 12 slightly exceed Turekian and Wedepohl (1961) showing Ni contamination factor of 1.3.
Aerial deposition
Atmospheric deposition contributes to the pollution of environmental compartments (water, soil, vegetation). Table 1 shows the dustfall rate and its Ni content in Saft Rashin village. The mean dustfall rate (4664 kg ha−1 year−1) terrifyingly exceeded the background value of clean areas (1217 kg ha−1 year−1) cited in EEAA (Egyptian Environmental Affairs Agency) (1994). A high rate of deposition (5862 kg ha−1 year−1) was recorded in the village center (sample A), due to the higher activity.
Dustfall has more Ni content than almost all soil samples. This means that atmospheric deposition is one of the external Ni sources in the soil. Ni in dust could be attributed to anthropogenic activities prevailing in the area such as traffic emission (Peter et al. 2022), agricultural activities (agrochemical application), and burning of various wastes. Such anthropogenic origin of Ni supports the role of aerial deposition in the pollution of crops grown in the study area. Because metals of anthropogenic origin are generally more bioavailable than metals of natural origin (Said et al., 2019).
Fertilizer Ni content
Ni concentration in the most commonly used NP-fertilizers in Egypt is 49.5 mg kg−1 in calcium superphosphate (15.5% P2O5) while it was not detected in urea (46% N). Generally, PTE content in different fertilizer types varies with the metal concentration in the source ores and the losses/gains during the manufacturing process (Said et al., 2021).
Crop pollution
Table 2 shows Ni content per dry weight (DW) in the sampled crops, compared to the upper limits of worldwide average reported in Kabata-Pendias (2010), based on Ni content in the edible parts. Accordingly, the studied plants were heavily polluted with Ni, associated with the chemical composition of the growth media, suggesting them as bioaccumulators. On the basis of whole plant nickel content, the tested crops were ordered as follows: clover > celery > garlic > sugar beet. Hence, clover is considered as a hyperaccumulator compared to the remaining crops grown in the same environment (Felix et al., 1999), and is recommended for in situ decontamination of polluted soil. Ni content varied with plant species and plant parts; it is more concentrated in the inedible portion of garlic than in its edible one, and vice versa for sugar beets and celery.
Health assessment
One of the most important environmental problems is related to the quantities of PTEs accumulated in plant parts used as food. Table 3 shows the daily intake (DI) of Ni via the studied crops (edible parts) calculated based on the daily consumption rate of Egyptian food. Average Egyptian diet in grams per person daily is 60.6 g/p/day for garlic and anions (Bulletin of Agriculture Economic 2016), 116.7 g/p/day for vegetables (Abdel-Gawaad and Shams El Dine 1989), and 93 g/p/day for sugar beet (white sugar) according to CCSC (2017). All reference values were normalized to mg/person/daily, based on an average body weight of 60 kg for adults, set by JECFA (2000). The estimated daily intake (DI) was compared with tolerable limit (TL) cited in la Guardia and Garrigues (2015) (Table 3). The results revealed that the crops grown in the study area are not safe for human consumption. Celery and sugar beet significantly exceed the tolerable daily intake in terms of their nickel content. The general order of Ni daily intake via these crops is celery > sugar beet > garlic.
Geochemical mass balance
Ni in every input-output has been normalized to mass per unit area per year; then, geochemical mass balance of Ni was calculated as input-output difference. Fertilization rates of sampled crops (kg ha−1 year−1) were calculated based on data collected from Bulletin of Agriculture Economic (2016) and local farmers. For example, 1191 kg ha−1 year−1 of calcium superphosphate (15.5% P2O5) is applied for garlic planting to obtain its need from P2O5 which is estimated in 184.6 kg ha−1 (i.e., fertilization rate = the amount of phosphorous needed by plant divided by the percentage of P2O5 in the fertilizer and multiplied by 100 = [184.6 × 100]/15.5). Hence, the application rates of superphosphate fertilizers (kg ha−1 year−1) for the studied crops were celery, 238; Egyptian clover, 238; sugar beet, 238; and garlic, 1191. Minimum fertilization rates were introduced in this study, because the old cultivated land is relatively more fertile compared to the newly reclaimed land. Crop productivity was calculated as dry matter yield (DMY) from data published in the literature (Leonardi, 1993; Shipley & Vu, 2002; Wojciechowska and Siwek 2006; Gadel-Hak et al., 2011; Petrova et al. 2014; Starke and Hoffmann, 2014a; Starke and Hoffmann, 2014b; Bulletin of Agriculture Economic, 2016; and El-Naby et al., 2016). Published data presented crop yield by total weight (i.e., moisture content plus dry mass). To calculate dry matter yield (DMY), we subtract the moisture% from 100% (the total weight) and then multiply the result by total weight yield. Accordingly, dry matter yield (DMY) of the studied crops (Ton ha−1 year−1) were garlic, 10.2; sugar beet, 17; celery, 6.0; and Egyptian clover (berseem), 22.1. DMY is defined as the whole-plant productivity per dry weight; it has other synonyms in the literature such as the dry biological yield or mass yield. For example, the biological yield of sugar beets = economic yield (root) + aerial part; similarly, the mass yield of garlic equals the edible part (economic yield) plus the remaining parts. The biological yield in the case of berseem and celery is the economic yield (edible part).
Table 4 shows Ni content of every input/output and their annual fluxes at Saft Rashin village simply sketched in Fig. 2. Atmospheric deposition is the major Ni source, accounting for 88.8% of the total inputs. Burning residual and fuel oil is the most probable anthropic Ni sources (Kabata-Pendias and Mukherjee 2007) as well as brake wear, engine oil leakage, tire wear, and road abrasion (Winther & Slentø, 2010). Inorganic fertilizers contribute by 11.2% of nickel inputs. On the other hand, Ni removed by biomass accounts for 89.7% of the total inputs (Table 4). The remaining 10.3% was accumulated in the soil with a retention rate of 44.7 g h−1 year−1. All crops examined are considered as bioaccumulators and Egyptian clover (berseem) is recommended for on-site decontamination. Clover acts as a bio-pump that removes heavy metals from the soil in large quantities. Based on their ability to remove Ni (g h−1 year−1), these crops can be arranged as follows: berseem (1043) > celery (209) > sugar beet (192) > garlic (108). Egyptian clover (berseem) tops the list as a nickel remover. It is the best recommended phytoremediator, because of its high efficiency and low cultivation cost. Clover is distinguished by a high planting density (number of plant/m2) and potentiality to regrowth (multi-cut feature). High planting density increases the removal rate of pollutant, and multi-cut property reduces processing cost. According to Bulletin of Agriculture Economic (2016), clover planting is relatively inexpensive; it is the least expensive among the studied crops.
Conclusion
Identifying pollutant pathways to and from the soil makes the pollution process well understood and manageable. In this regard, GMB was performed to show the fate of Ni in a small agro-ecosystem, calculated as the total Ni-input minus the total Ni-output. The result is food chain pollution with nickel due to atmospheric deposition and fertilizer. The source of nickel in atmospheric deposition is traffic emissions. Hence, it is recommended to use eco-friendly fertilizers. Using more efficient and less polluting new diesel models can reduce pollution levels, such as cleaner-burning gasoline vehicles and electric cars. In parallel, it is advisable to intensify the cultivation of berseem as a phytoremediator. A further investigation is recommended.
References
Abdel-Gawaad AA, Shams El Dine A (1989) Insecticide residues in total diet samples. J Egypt Soc Toxicol 4:79–84
Ahmed AA, Fawzi A (2011) Meandering and bank erosion of the River Nile and its environmental impact on the area between Sohag and El-Minia, Egypt. Arab J Geosci 4:1–11
Ali H, Naseer M, Sajad MA (2012) Phytoremediation of heavy metals by Trifolium alexandrinum. Int J Environ Sci 2:1459–1469
Baker AJM, Brooks R (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Balakrishna G, Pervez S, Bisht DS (2011) Source apportionment of arsenic in atmospheric dust fall out in an urban residential area, Raipur, Central India. Atmos Chem Phys 11:5141–5151
Belon E, Boisson M, Deportes IZ et al (2012) An inventory of trace elements inputs to French agricultural soils. Sci Total Environ 439:87–95
Bhatti SS, Sambyal V, Nagpal AK (2016) Heavy metals bioaccumulation in Berseem (Trifolium alexandrinum) cultivated in areas under intensive agriculture, Punjab, India. Springerplus 5:1–11
Bianchi V, Masciandaro G, Ceccanti B et al (2011) Phytoremediation of contaminated sediments: evaluation of agronomic properties and risk assessment. Chem Ecol 27:1–11
Bulletin of Agriculture Economic (2016) Ministry of agriculture, Egypt
CCSC (Central Council for Sugar Crops) (2017) annual report. Minist Agric L Reclamation, Cairo, Eygpt
Cho-Ruk K, Kurukote J, Supprung P, Vetayasuporn S (2006) Perennial plants in the phytoremediation of lead-contaminated soils. Biotechnology 5:1–4
EEAA (Egyptian Environmental Affairs Agency) (1994) Egyptian air quality limits values. http://www.eeaa.gov.eg/eimp/limit%20values.html
El-Naby A, Zeinab M, Magda N, et al (2016) Evaluation of quality characters of some Egyptian clover genotypes. Int J Appl Pure Sci Agric 2:
EPA (1986) Health assessment document for nickel and nickel compounds
FAO (Food and Agri- culture Organization) (1994) Water quality for agriculture. https://www.fao.org/3/t0234e/T0234E06.htm
Felix HR, Kayser A, Schulin R (1999) Phytoremediation, field trials in the years 1993-1998. In: International conference on the biogeochemistry of trace elements. pp 8–9
Gadel-Hak SNH, Moustafa YMM, Abdel-Naem GFA, Abdel-Wahab IA (2011) Studying different quantitative and qualitative traits of some white-and colored-bulb garlic genotypes grown under a drip irrigation system. Aust J Basic Appl Sci 5:1415–1427
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60
JECFA (Joint FAO/WHO Expert Committee on Food Additives) (2000) Evaluation of certain food additives and contaminants: fifty-third report of the Joint FAO/WHO Expert Committee on Food Additives
Jomova K, Jenisova Z, Feszterova M et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Kabata-Pendias A (2010) Trace elements in soils and plants. CRC press
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer Science & Business Media
Khan A, Khan S, Khan MA et al (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22:13772–13799
Kim HS, Kim YJ, Seo YR (2015) An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J cancer Prev 20:232
la Guardia M, Garrigues S (2015) Handbook of mineral elements in food. Wiley Online Library
Leonardi C (1993) Dry matter yield and nitrogen content in celery under salt stress conditions. In: International Symposium on Water Quality & Quantity-Greenhouse 458. pp 257–262
Liu X, Song Q, Tang Y et al (2013) Human health risk assessment of heavy metals in soil--vegetable system: a multi-medium analysis. Sci Total Environ 463:530–540
Luo L, Ma Y, Zhang S et al (2009) An inventory of trace element inputs to agricultural soils in China. J Environ Manage 90:2524–2530
Misseyer MP, van der Most PFJ (1993) Decision support systems for environmental impact assessment: addressing the industrial need. In: Proceedings of the IFIP TC5/WG5. 11 Working Conference on Computer Support for Environmental Impact Assessment. pp 301–310
Paces T (1994) Modeling the hydrologic and biogeochemical response of a catchment area to anthropogenic inputs. In: Chemistry of Aquatic Systems: Local and Global Perspectives. Springer, pp 465–495
Peter AE, Menon JS, George M et al (2022) Composition, sources, and health risk assessment of particulate matter at two different elevations in Delhi city. Atmos Pollut Res 13:101295
Petrova I, Petkova N, Kyobashieva K et al (2014) Isolation of pectic polysaccharides from Celery (Apium graveolens var. rapaceum DC) and their application in food emulsions. Turkish J Agric Nat Sci 1:1818–1824
Said I, Hursthouse A, Salman SAE-R (2021) Identification of pollution sources in roadside soils of Cairo-Alexandria Highway, Egypt. Arab J Geosci 14:1–11
Said I, Salman S, Samy Y et al (2019) Environmental factors controlling potentially toxic element behaviour in urban soils, El Tebbin, Egypt. Environ Monit Assess 191:267
Shi T, Ma J, Wu X et al (2018) Inventories of heavy metal inputs and outputs to and from agricultural soils: a review. Ecotoxicol Environ Saf 164:118–124
Shipley B, Vu T-T (2002) Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol 153:359–364
Starke P, Hoffmann C (2014a) Yield parameters of Beta beets as a basis to estimate the biogas yield. Sugar Ind 139:169–176
Starke P, Hoffmann CM (2014b) Dry matter and sugar content as parameters to assess the quality of sugar beet varieties for anaerobic digestion. Sugar Ind 139:232–240
Tangahu BV, Abdullah S, Rozaimah S, et al (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng 2011:. https://doi.org/https://downloads.hindawi.com/journals/ijce/2011/939161.pdf
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Winther, M. & Slentø E (2010) Heavy Metal Emissions for Danish Road Transport
Wojciechowska R and, Siwek P (2006) The effect of shading on nitrate metabolism in stalks and blades of celery leaves (Apium graveolens L. var. dulce). Folia Hortic 18:. https://doi.org/http://www.ptno.ogr.ar.krakow.pl/Wydawn/FoliaHorticulturae/Spisy/FH2006/PDF18022006/fh1802p03.pdf
Xia J, Wang J, Zhang L et al (2021) Mass balance of nine trace elements in two karst catchments in southwest China. Sci Total Environ 786:147504
Acknowledgements
I am grateful to Mr. Mohammed Sayeed Ibrahim and Dr. Ahmed Shaaban (Fayum University), for field guidance and agricultural information. The author also thanks National Research Centre (Egypt).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Said, I. Nickel pollution pathways in small ecosystem, Egypt. Arab J Geosci 15, 988 (2022). https://doi.org/10.1007/s12517-022-10259-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10259-3