Abstract
Although there is robust evidence that revascularisation of non-culprit vessels should be pursued in patients presenting with an acute coronary syndrome (ACS) and multivessel coronary artery disease (MVD), the optimal timing of complete revascularisation remains disputed. In this systematic review and meta-analysis our results suggest that outcomes are comparable for immediate and staged complete revascularisation in patients with ACS and MVD. However, evidence from randomised controlled trials remains scarce and cautious interpretation of these results is recommended. More non-biased evidence is necessary to aid future decision making on the optimal timing of complete revascularisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the majority of patients presenting with acute coronary syndromes (ACS), percutaneous coronary intervention (PCI) is the preferred modality of reperfusion [1, 2]. Up to 60% of patients presenting with ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation acute coronary syndrome (NSTE-ACS) have multivessel coronary artery disease (MVD) on coronary angiography [3, 4]. Patients with MVD have a worse prognosis compared with patients with single-vessel disease [3, 4]. Both the 2017 European Society of Cardiology STEMI and the recently published 2020 NSTE-ACS guidelines encourage complete revascularisation in patients presenting with MVD, class IIa, level of evidence (LOE) A and C respectively [2, 5]. The recommendation in the STEMI guidelines was based on several randomised controlled trials (RCTs), which demonstrated that complete revascularisation is superior to a culprit-only strategy in terms of major adverse cardiovascular events (MACE), but the beneficial effect was driven by the need for revascularisation and reduction in angina [6,7,8,9]. The recently published COMPLETE trial was the first to demonstrate the superiority of complete revascularisation with respect to the primary endpoint of myocardial infarction (MI) or cardiovascular mortality [10].
Complete multivessel revascularisation seems the preferred strategy, but timing remains unclear. Complete revascularisation can be performed during the index procedure, after treatment of the culprit lesion. Alternatively, operators can perform culprit-only revascularisation during the index procedure, and staged complete revascularisation, either during index hospitalisation or even in an ambulatory setting. The STEMI guidelines do not contain recommendations on the timing of revascularisation, as data are limited: there are only three old and small RCTs in STEMI patients (n = ~300 patients). Based upon the SMILE study, the NSTE-ACS guidelines state that complete revascularisation during the index procedure may be considered, class IIB, LOE B [2, 5].
This systematic review and meta-analysis compares immediate complete revascularisation during the index procedure versus staged complete revascularisation in patients presenting with ACS (including STEMI and NSTE-ACS) and MVD. Only studies in which both immediate and staged complete revascularisation were compared were included. Because of the limited data, this systematic review included both RCTs and non-randomised trials.
Methods
Search strategy and study selection
The protocol for this systematic review and meta-analysis was developed and registered in the PROSPERO database (CRD42019124604) and was reported in accordance with the PRISMA statement guidelines [11]. The search strategy was developed using the main parameters (Table 1), and PubMed, EMBASE, Cochrane Library and MEDLINE online databases were searched for publications between 2000 and 1 April 2020. An example of the search strategy for EMBASE can be found in Table S1 (Electronic Supplementary Material). Pre-defined inclusion criteria were based on the main parameters and included head-to-head comparisons of immediate revascularisation of non-culprit arteries with staged revascularisation in patients with ACS and MVD. Both RCTs and non-randomised comparisons were included. The records obtained were assessed on the title and abstract and were excluded if one of the following was applicable: case reports, observational studies without a comparison group, reviews, meta-analyses, lack of relevant outcomes or study question and conference abstracts. Selected full text records were analysed by two independent reviewers (WKD and PAV) to determine if the inclusion criteria were fulfilled. Any disagreements were resolved by consensus. If > 1 publication was based on the same cohort or population and reported the same outcomes, only the most recent or comprehensive publication was included. In addition, a manual reference search of relevant literature was performed to ensure completeness.
Data extraction
A standardised and pre-piloted form was used for extracting data of the studies included. The following study characteristics were collected: age, sex, cardiac risk factors (diabetes mellitus, hypertension, smoking, hypercholesterolaemia, family history of premature cardiovascular disease), type of ACS (STEMI/NSTEMI/unstable angina), localisation of MI, and two- or three-vessel disease. The pre-specified primary outcomes collected were 30-day and 1‑year all-cause mortality. Secondary outcomes were unplanned revascularisation, MI, disabling stroke and MACE. For completeness, we also tried to contact authors via e‑mail or telephone to obtain further information that had not been reported in their published articles.
Risk of bias assessment
After data extraction, quality assessment of each included record was performed. For RCTs, the risk of bias tool 2.0 [12] and for non-randomised analyses the ROBINS-I tool was used [13]. Risk of bias was assessed per study per domain. The distributions of (small study) treatment effects were evaluated in comparison with the obtained pooled effect using funnel plots. Asymmetrical funnel plots were further evaluated to determine if asymmetry was a result of selective outcome reporting or publication bias, or due to poor methodological quality, true heterogeneity or chance.
Qualitative and statistical analysis
Data were included in the meta-analysis if there was no critical risk of bias. For each study included, treatment effect was reported as odds ratio (OR) with 95% confidence intervals (CI) for both primary and secondary outcomes. Heterogeneity was assessed visually and between-study heterogeneity using the I2 statistic. If collected data were considered appropriate for data synthesis based on the risk of bias assessment, a random-effects model was used, with the Sidik-Jonkman estimator for τ to estimate the between-study variance. Statistical analysis was performed using R (R Development Core Team, Vienna, Austria) with meta and metafor packages, and STATA 15.1 (StataCorp, College Station, TX, USA) with metan and confunnel packages.
Results
Study selection and characteristics
The results of the search process are presented in a PRISMA flow chart (Fig. S1, Electronic Supplementary Material), and after applying exclusion criteria 20 studies were selected for the systematic review [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. A total of 10,737 patients were included: 4835 (45%) patients underwent immediate revascularisation of non-culprit arteries and 5902 (55%) staged revascularisation. Five RCTs were included but accounted for only 916 (9%) patients [15, 18, 20, 25, 28]. Most patients (9116, 85%) presented with a STEMI. Patients presenting with cardiogenic shock at the time of the index PCI were not excluded in 6 studies [14, 16, 17, 19, 21, 26] and in 4 of these studies significantly more patients with cardiogenic shock were included in the immediate complete revascularisation group [14, 16, 17, 19]. An overview of individual study and baseline characteristics is shown in Table 2.
Risk of bias
Risk of bias was assessed for all individual studies. No studies were considered at critical risk of bias, but a substantial risk of bias was present in all non-randomised studies. Several non-randomised studies used propensity score matching to reduce confounding [17, 19, 24, 26, 29, 30, 32], but even in these studies a risk of residual confounding remained because of the inability to correct for unobserved factors. Funnel-plot analyses demonstrated an asymmetrical distribution, most likely due to heterogeneity of the studies included. Publication bias seemed unlikely, due to the location of the evidence gap: there was no suggestion of missing studies in the area of non-significance (Fig. 1a,b).
Clinical outcomes
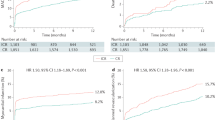
Ten studies (6957 patients, 65%; 2 RCTs and 8 non-randomised studies) reported on 30-day mortality outcomes and the data were suitable for data synthesis to determine the 30-day mortality risk. The 30-day mortality risk was comparable in the RCTs (OR 3.4, 95% CI 0.2–55.1), but this was based on 2 studies with a limited number of events (Fig. 2a). In the non-randomised studies, the risk appeared to be significantly higher for patients who underwent immediate revascularisation of non-culprit arteries compared with patients who underwent staged revascularisation (OR 4.2, 95% CI 2.6–7.0), as is shown in Fig. 2a.
Thirty-day (a) and 1‑year (b) mortality risk of patients with acute coronary syndrome who underwent immediate or staged revascularisation of non-culprit arteries. c One-year mortality risk of patients with acute coronary syndrome who underwent immediate or staged revascularisation of non-culprit arteries with or without exclusion of patients in cardiogenic shock (CS) (RCT randomised controlled trail, OR odds ratio, CI confidence interval)
Sixteen studies (8296 patients, 77%) were included in the data synthesis to determine the 1‑year mortality risk. Of these, 11 studies were non-randomised and 5 studies were RCTs (7380 patients and 916 patients respectively). One-year mortality was comparable between immediate and staged complete revascularisation in the RCTs (OR 0.8, 95% CI 0.3–2.2), and was increased for patients that underwent immediate complete revascularisation in the non-randomised trials (OR 2.9, 95% CI 1.9–4.4; Fig. 2b).
However, if studies that included patients with cardiogenic shock were excluded, 1‑year mortality was comparable with both strategies (OR 1.7, 95% CI 0.9–3.1; Fig. 2c). There was no difference between immediate and staged revascularisation as regards the risk of unplanned revascularisation: OR 0.88 (95% CI 0.52–1.47) in non-randomised studies and OR 0.71 (95% CI 0.26–1.95) in randomised studies (see Figure S 3a in Electronic Supplementary Material [ESM]) or MI: OR 0.91 (95% CI 0.54–1.54) in non-randomised studies and OR 0.92 (95% CI 0.23–3.61) in randomised studies (see Figure S 3b in Electronic Supplementary Material [ESM]). There were insufficient data to compare the two strategies regarding other outcomes such as stroke, urgent revascularisation or MACE.
Discussion
In this systematic review and meta-analysis, we compared immediate complete revascularisation with staged complete revascularisation in patients with ACS and MVD. In a pooled analysis of the RCTs there was no difference in 30-day and 1‑year mortality between the two revascularisation strategies. In non-randomised studies, mortality was higher in patients who underwent immediate complete revascularisation compared to staged complete revascularisation. However, these registries were prone to important confounding bias, as more patients in cardiogenic shock underwent ad hoc complete revascularisation. Indeed, the increased mortality risk was no longer present when studies that also allowed cardiogenic shock were excluded.
There is compelling evidence that complete revascularisation rather than culprit-only revascularisation is beneficial among patients with STEMI and MVD [6,7,8,9,10]. The COMPLETE trial showed a clear benefit of complete revascularisation in terms of prognostically relevant clinical endpoints (mortality and MI). Nevertheless, the optimal timing of complete revascularisation remains disputed. Complete revascularisation can be achieved during the index procedure or in a staged fashion during index hospitalisation or even after discharge. Immediate complete revascularisation prevents exposure to a second invasive procedure with all its associated risks and costs. Conversely, in the index procedure there might be an overestimation of the severity of non-culprit lesions due to higher vascular tone, a higher risk of stent thrombosis and peri-procedural MI due to the prothrombotic milieu, a higher risk of contrast nephropathy due to the use of more contrast and a higher risk of arrhythmia. The COMPLETE timing sub-study showed that the benefit of complete revascularisation was irrespective of whether complete revascularisation was performed during index hospitalisation or after discharge [34]. Notably, the trial did not allow complete revascularisation during the index procedure.
Data comparing the timing of complete revascularisation in ACS patients with MVD are ambiguous and RCTs are scarce. Although meta-analyses have been performed previously, they all used different inclusion criteria and outcomes than ours. In particular, a direct comparison between immediate complete revascularisation and staged complete revascularisation is lacking, as most meta-analyses also included studies that compared immediate or staged complete revascularisation with culprit-only revascularisation and subsequently performed network or pairwise analyses. Vlaar et al. performed a pairwise and network meta-analysis including 4 prospective and 14 retrospective studies [35]. They found that immediate complete multivessel primary PCI for STEMI was associated with the highest mortality rates for both short-term and long-term outcomes. These findings were confirmed by two subsequent meta-analyses by Tarantini et al. and Li et al., also showing an increased risk of mortality associated with immediate complete revascularisation [36, 37]. However, three meta-analyses including only RCTs showed no difference in mortality or MACE between immediate complete or staged complete revascularisation, but lower rates of recurrent MI in the immediate complete group [38,39,40]. Gaffar et al. did perform a meta-analysis that included only studies comparing immediate complete revascularisation and staged complete revascularisation in STEMI and NSTEMI patients [41]. However, they included only RCTs, so this yielded no more than 4 RCTs with a total of 853 patients. The risk of unplanned repeat revascularisation was significantly lower in the immediate complete group, while there was also a trend toward a lower risk of MACE.
In contrast to these earlier meta-analyses, we included both STEMI and NSTE-ACS patients, as well as RCTs and non-randomised studies. Moreover, we included studies up until April 2020 and only those in which both immediate and staged complete revascularisation were performed, creating a more homogeneous study population and allowing true head-to-head analysis.
Our meta-analysis revealed different outcome effects with immediate complete revascularisation in the RCTs as compared to the non-randomised studies. Cardiogenic shock was excluded from the RCTs but allowed in registries. Although complete revascularisation in ACS and MVD seems beneficial, this beneficial effect seems to be restricted to patients with ACS and MVD in the absence of cardiogenic shock [42]. The CULPRIT-SHOCK trial demonstrated that for patients in cardiogenic shock and with MVD at the time of acute MI, PCI of the culprit lesion only was superior to immediate complete revascularisation at 30 days, with a significantly lower mortality in the culprit-only PCI group [43]. For patients in cardiogenic shock, the longer procedure time and increased contrast volume do not outweigh the possible reduction in peri-infarct ischaemia or early recurrent ischaemia. Current guidelines do not advocate routine multivessel PCI in acute MI complicated by cardiogenic shock. An unequal or unknown distribution of patients with cardiogenic shock in non-randomised studies may present an important source of bias and could lead to unfair interpretation of results. As a matter of fact, when cardiogenic shock was excluded, there was also no difference in mortality between immediate complete revascularisation and staged revascularisation in the registries.
The unequal inclusion of cardiogenic shock patients illustrates the most important limitation of this meta-analysis: the shortage of data stemming from RCTs (916 patients, 8%). Non-randomised studies are always at an increased risk of bias, and although some studies reduced bias by performing propensity score matching [17, 19, 24, 26, 29, 30, 32] this cannot correct for unmeasurable confounders. Especially in patients presenting with STEMI, it is feasible that in retrospective analyses there were reasons not explicitly stated in the reports why operators preferred to perform complete revascularisation during the index procedure.
To bridge the noticeable evidence gap, we have initiated the BIOVASC trial (NCT03621501) [44]. In this study, all patients presenting with ACS (STEMI and NSTE-ACS) and MVD are being randomised to immediate complete revascularisation or culprit-only PCI plus staged revascularisation within 6 weeks after the index procedure. Significant coronary artery disease is defined as at least 70% stenosis in a vessel ≥ 2.5 mm by visual estimation or positive coronary physiology testing. Principal exclusion criteria are cardiogenic shock, no clear culprit lesion, prior coronary artery bypass surgery or the presence of a chronic total occlusion. The primary endpoint is a composite of death from any cause, MI, unplanned ischaemia-driven revascularisation or cerebrovascular events after 1‑year follow-up. Enrolment of 1525 patients was completed in October 2021 and the first results are expected at the end of 2022. BIOVASC aims to provide further insights into the clinical implications of immediate complete revascularisation across the entire ACS spectrum, to evaluate different effects in STEMI versus NSTE-ACS patients, and to explore the impact of the timing of revascularisation on early and late quality of life. The iMODERN (NCT03298659) and MULTISTARS AMI (NCT03135275) [45] studies are also investigating the timing of revascularisation, but there are some marked differences compared with the BIOVASC trial. iMODERN is a European multicentre trial that compares instantaneous wave-free (iFR)-guided immediate complete revascularisation with staged stress perfusion cardiac MRI-guided complete revascularisation (within 6 weeks after STEMI). Thus, unlike the BIOVASC trial, physiological assessment by iFR or cardiac MRI is mandatory. Furthermore, the iMODERN trial includes only STEMI patients, and those with complex bifurcation lesions and left main stenosis of ≥ 50% are excluded. Patients in cardiogenic shock are also excluded, as in the BIOVASC trial. A total of 1146 patients will be enrolled and the primary endpoint is a composite of all cause death, recurrent MI and hospitalisation for heart failure at 1 year. Enrolment is expected to be completed at the beginning of 2022. MULTISTARS AMI is also a European multicentre trial that compares immediate complete revascularisation with staged complete revascularisation in STEMI patients. The staged procedure has to be performed at least 19 days after the index procedure, but within 45 days. Lesions are considered significant if they cause a ≥ 70% diameter stenosis by visual estimation. Like the iMODERN study, patients with left main stenosis of ≥ 50% and those in cardiogenic shock are excluded. The primary endpoint is a composite of death, non-fatal MI, ischaemia-driven revascularisation hospitalisation for heart failure and stroke at 1 year. Although the anticipated number of patients to be enrolled was 840, this figure will probably not be reached as patient inclusion is planned only until the end of 2022, while after 3.5 years only 393 patients had been enrolled. The contribution of these three trials could help mitigate the limitations of this meta-analysis and aid future clinical decision-making.
In conclusion, this overview and meta-analysis suggests similar outcomes with an immediate or staged complete revascularisation strategy in patients with ACS and MVD without cardiogenic shock. However, these findings are mainly driven by non-randomised studies with a significant risk of bias and therefore support ongoing randomised trials on this topic to determine the optimal timing of complete revascularisation.
References
Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevationThe Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77.
van der Schaaf RJ, Timmer JR, Ottervanger JP, et al. Long-term impact of multivessel disease on cause-specific mortality after ST elevation myocardial infarction treated with reperfusion therapy. Heart. 2006;92:1760–3.
Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28:1709–16.
Collet J‑P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367.
Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–23.
Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–71.
Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–72.
Smits PC, Abdel-Wahab M, Neumann F‑J, et al. Fractional flow reserve—guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–44.
Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–21.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
Higgins J, Sterne J, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Corpus RA, House JA, Marso SP, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. Am Heart J. 2004;148:493–500.
Ochala A, Smolka GA, Wojakowski W, et al. The function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute myocardial infarction. J Invasive Cardiol. 2004;16:699–702.
Varani E, Balducelli M, Aquilina M, et al. Single or multivessel percutaneous coronary intervention in ST-elevation myocardial infarction patients. Catheter Cardiovasc Interv. 2008;72:927–33.
Hannan EL, Samadashvili Z, Walford G, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv. 2010;3:22–31.
Politi L, Sgura F, Rossi R, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart. 2010;96:662–7.
Kornowski R, Mehran R, Dangas G, et al. Prognostic impact of staged versus “one-time” multivessel percutaneous intervention in acute myocardial infarction: analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2011;58:704–11.
Maamoun W, Elkhaeat N, Elarasy R. Safety and feasibility of complete simultaneous revascularization during primary PCI in patients with STEMI and multi-vessel disease. Egypt Heart J. 2011;63:39–43.
Mohamad T, Bernal JM, Kondur A, et al. Coronary revascularization strategy for ST elevation myocardial infarction with multivessel disease: experience and results at 1‑year follow-up. Am J Ther. 2011;18:92–100.
Jensen LO, Thayssen P, Farkas DK, et al. Culprit only or multivessel percutaneous coronary interventions in patients with ST-segment elevation myocardial infarction and multivessel disease. EuroIntervention. 2012;8:456–64.
Kim MC, Jeong MH, Park KH, et al. Three-year clinical outcomes of staged, ad hoc and culprit-only percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction and multivessel disease. Int J Cardiol. 2014;176:505–7.
Manari A, Varani E, Guastaroba P, et al. Long-term outcome in patients with ST segment elevation myocardial infarction and multivessel disease treated with culprit-only, immediate, or staged multivessel percutaneous revascularization strategies: Insights from the REAL registry. Catheter Cardiovasc Interv. 2014;84:912–22.
Tarasov RS, Ganyukov VI, Protopopov AV, Barbarash OL, Barbarash LS. Six month results of randomized clinical trial: Multivessel stenting versus staged revascularization for ST-elevation myocardial infarction patients with second generation drug eluting stents. Clin Med Res. 2014;3:125–9.
Chung W‑Y, Seo J‑B, Choi D‑H, et al. Immediate multivessel revascularization may increase cardiac death and myocardial infarction in patients with ST-elevation myocardial infarction and multivessel coronary artery disease: data analysis from real world practice. Korean J Intern Med. 2016;31:488–500.
Khan JN, Nazir SA, Greenwood JP, et al. Infarct size following complete revascularization in patients presenting with STEMI: a comparison of immediate and staged in-hospital non-infarct related artery PCI subgroups in the CvLPRIT study. J Cardiovasc Magn Reson. 2016;18:85.
Sardella G, Lucisano L, Garbo R, et al. Single-staged compared with multi-staged PCI in multivessel NSTEMI patients: the SMILE trial. J Am Coll Cardiol. 2016;67:264–72.
Yu XF, Li Y, Wang QC, et al. Staged versus “one-time” multivessel intervention in elderly patients with non-ST-elevation acute coronary syndrome. J Geriatr Cardiol. 2016;13:760–7.
Iqbal MB, Nadra IJ, Ding L, et al. Culprit vessel versus multivessel versus in-hospital staged intervention for patients with ST-segment elevation myocardial infarction and multivessel disease: stratified analyses in high-risk patient groups and anatomic subsets of nonculprit disease. JACC Cardiovasc Interv. 2017;10:11–23.
Kim I, Kim MC, Jeong HC, et al. Optimal timing of percutaneous coronary intervention for nonculprit vessel in patients with ST-segment elevation myocardial infarction and multivessel disease. Korean Circ J. 2017;47:36–43.
Doğan C, Bayram Z, Çap M, et al. Comparison of 30-Day MACE between immediate versus staged complete revascularization in acute myocardial infarction with multivessel disease, and the effect of coronary lesion complexity. Ann Univ Mariae Curie Sklodowska [Med]. 2019;55:51.
Tovar Forero MN, Scarparo P, den Dekker W, et al. Revascularization strategies in patients presenting with ST-elevation myocardial infarction and multivessel coronary disease. Am J Cardiol. 2020;125:1486–91.
Wood DA, Cairns JA, Wang J, et al. Timing of staged nonculprit artery revascularization in patients with ST-segment elevation myocardial infarction: COMPLETE trial. J Am Coll Cardiol. 2019;74:2713–23.
Vlaar PJ, Mahmoud KD, Holmes DR, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol. 2011;58:692–703.
Tarantini G, D’Amico G, Brener SJ, et al. Survival after varying revascularization strategies in patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease. JACC Cardiovasc Interv. 2016;9:1765–76.
Li Z, Zhou Y, Xu Q, Chen X. Staged versus one-time complete revascularization with percutaneous coronary intervention in STEMI patients with multivessel disease: a systematic review and meta-analysis. PLoS ONE. 2017;12:e169406.
Bangalore S, Toklu B, Stone GW. Meta-analysis of culprit-only versus multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction and multivessel coronary disease. Am J Cardiol. 2018;121:529–36.
Elgendy IY, Mahmoud AN, Kumbhani DJ, Bhatt DL, Bavry AA. Complete or culprit-only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2017;10:315–24.
Shah R, Berzingi C, Mumtaz M, et al. Meta-analysis comparing complete revascularization versus infarct-related only strategies for patients with ST-segment elevation myocardial infarction and multivessel coronary artery disease. Am J Cardiol. 2016;118:1466–72.
Gaffar R, Habib B, Filion KB, Reynier P, Eisenberg MJ. Optimal timing of complete revascularization in acute coronary syndrome: a systematic review and meta-analysis. J Am Heart Assoc. 2017; https://doi.org/10.1161/JAHA.116.005381.
de Waha S, Jobs A, Eitel I, et al. Multivessel versus culprit lesion only percutaneous coronary intervention in cardiogenic shock complicating acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2018;7:28–37.
Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–32.
den Dekker WK, Van Mieghem NM, Bennett J, et al. Percutaneous complete revascularization strategies using sirolimus eluting biodegradable polymer coated stents in patients presenting with acute coronary syndrome and multivessel disease: rationale and design of the BIOVASC trial. Am Heart J. 2020;227:111–7.
Stähli BE, Varbella F, Schwarz B, et al. Rationale and design of the MULTISTARS AMI Trial: a randomized comparison of immediate versus staged complete revascularization in patients with ST-segment elevation myocardial infarction and multivessel disease. Am Heart J. 2020;228:98–108.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P.A. Vriesendorp, J.M. Wilschut, R. Diletti, J. Daemen, I. Kardys, F. Zijlstra, N.M. Van Mieghem, J. Bennett, G. Esposito, M. Sabate and W.K. den Dekker declare that they have no competing interests.
Supplementary Information
12471_2022_1687_MOESM2_ESM.tif
Unplanned revascularisation (a) and myocardial infarction (b) risk of patients with acute coronary syndrome who underwent immediate or staged revascularisation of non-culprit arteries.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vriesendorp, P.A., Wilschut, J.M., Diletti, R. et al. Immediate versus staged revascularisation of non-culprit arteries in patients with acute coronary syndrome: a systematic review and meta-analysis. Neth Heart J 30, 449–456 (2022). https://doi.org/10.1007/s12471-022-01687-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-022-01687-7