Abstract
Introduction
New-onset left bundle branch block (LBBB) following transcatheter or surgical aortic valve replacement (LBBBAVI) implies a proximal pathogenesis of LBBB. This study compares electrocardiographic characteristics and concordance with LBBB definitions between LBBBAVI and non-procedure-induced LBBB controls (LBBBcontrol).
Methods
All LBBBAVI patients at Ghent University Hospital between 2013 and 2019 were enrolled in the study. LBBBAVI patients were matched for age, sex, ischaemic heart disease and ejection fraction to LBBBcontrol patients in a 1:2 ratio. For inclusion, a non-strict LBBB definition was used (QRS duration ≥ 120 ms, QS or rS in V1, absence of Q waves in V5-6). Electrocardiograms were digitally analysed and classified according to three LBBB definitions: European Society of Cardiology (ESC), Strauss and American Heart Association (AHA).
Results
A total of 177 patients (59 LBBBAVI and 118 LBBBcontrol) were enrolled in the study. LBBBAVI patients had more lateral QRS notching/slurring (100% vs 85%, p = 0.001), included a higher percentage with a QRS duration ≥ 130 ms (98% vs 86%, p = 0.007) and had a less leftward oriented QRS axis (−15° vs −30°, p = 0.013) compared to the LBBBcontrol group. ESC and Strauss criteria were fulfilled in 100% and 95% of LBBBAVI patients, respectively, but only 18% met the AHA criteria. In LBBBcontrol patients, concordance with LBBB definitions was lower than in the LBBBAVI group: ESC 85% (p = 0.001), Strauss 68% (p < 0.001) and AHA 7% (p = 0.035). No differences in electrocardiographic characterisation or concordance with LBBB definitions were observed between LBBBAVI and LBBBcontrol patients with lateral QRS notching/slurring.
Conclusion
Non-uniformity exists among current LBBB definitions concerning the detection of proximal LBBB. LBBBAVI may provide a framework for more consensus on defining proximal LBBB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
Lateral QRS notching/slurring is an essential criterion for diagnosing proximal left bundle branch block (LBBB).
-
The use of different LBBB definitions results in discordance when scoring LBBB.
-
Aortic valve implantation (AVI)-induced LBBB showed the highest concordance with the 2013 European Society of Cardiology and Strauss definitions.
-
AVI-induced LBBB provides a framework for uniform criteria for true proximal LBBB.
Introduction
Left bundle branch block (LBBB) in humans was first recorded electrocardiographically in 1914 [1]. Multiple criteria for LBBB have been proposed since, based on experimental canine studies [1, 2], human case studies [3], electrophysiological data [4] and observations in cardiac resynchronisation therapy (CRT) responders [1, 5,6,7]. However, the electrocardiographic pattern of LBBB has not been fully clarified and various LBBB definitions are currently used [1, 8,9,10], resulting in significant discordance when scoring LBBB in clinical practice [7, 11].
Although conduction block may theoretically occur at any level in the His-Purkinje network, growing evidence suggests that only proximal left bundle branch (LBB) lesions cause ‘true’ LBBB [12] and that only ‘true’ LBBB is considered a strong predictor of CRT response in heart failure patients [6, 13]. Notching or slurring of the QRS complex during LBBB has been linked to a proximal origin of the LBB conduction block and might be considered a key feature of proximal LBBB [14, 15]. A limitation of current LBBB definitions is that they are not exclusively based on electrocardiographic observations in patients with proximal LBBB, which may contribute to the heterogeneity in LBBB definitions.
New-onset LBBB after transcatheter (TAVR) or surgical aortic valve replacement (SAVR) implies a proximal pathogenesis of LBBB and may provide a ‘framework’ towards uniform criteria for proximal LBBB. In this study, we compare the electrocardiographic characteristics and LBBB definitions in aortic valve implantation (AVI)-induced LBBB to a non-procedure-induced LBBB control group.
Methods
Study populations
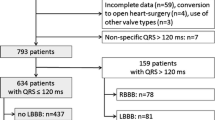
Enrolled in the study were all patients with AVI-induced LBBB (LBBBAVI), including both patients with TAVR- and SAVR-induced LBBB, at Ghent University Hospital between January 2013 and June 2019. All patients with a primary TAVR and SAVR procedure and without pre-existing LBBB were screened. Exclusion criteria were pre-procedural ventricular pacing and peri-procedural permanent pacemaker implant. Presence of acute LBBB was scored within 24 h following TAVR/SAVR.
A control group of LBBB patients (LBBBcontrol) consisted of randomly selected LBBB patients at Ghent University Hospital. LBBBcontrol patients were matched for age, sex, left ventricular ejection fraction (LVEF), history of coronary artery disease (CAD) and acute coronary syndrome to the LBBBAVI group in a 2:1 ratio. In both the AVI and control groups, LBBB was defined according to broad conventional criteria (QRS duration (QRSD) ≥ 120 ms, QS or rS in lead V1 and absence of Q waves in leads V5 and V6) [16]. The study was approved by the ethics committee of Ghent University Hospital.
Electrocardiographic analysis and LBBB definitions
Electrocardiograms (ECGs) were recorded at a sweep speed of 25 mm/s and a calibration of 10 mm/mV, and digitally stored in the MUSE ECG database (GE Healthcare, Chicago, IL, USA). All ECGs were independently reviewed by two investigators and classified according to three currently used LBBB definitions: European Society of Cardiology 2013 (ESC 2013) [9], Strauss et al. [1] and American Heart Association 2009 (AHA 2009) [10] (see Electronic Supplementary Material, Fig. S1). Continuous electrocardiographic characteristics (QRSD, QRS axis, R wave peak time (RWPT)) were digitally analysed by the Marquette 12SL algorithm (GE Healthcare) [17]. RWPT was defined according to the Minnesota Code [18].
Validation of proposed criteria for proximal LBBB
Based on our observations in LBBBAVI, we adapted currently used LBBB criteria and propose a revised definition of LBBB. The revised definition was validated in consecutive LBBB patients (broad criteria) who underwent implantation of a CRT device at Ghent University Hospital according to current guidelines (LVEF ≤ 35%) [9] and who were categorised as CRT super-responders (LBBBCRT) based on improvement in LVEF to > 45% after at least 6 months of CRT therapy at a prospective echocardiographic examination between October 2018 and August 2020. All pre-implant ECGs were reviewed by an investigator blinded to the revised LBBB criteria.
Statistical analysis
Categorical variables are expressed as absolute number (percentage). Continuous variables are expressed as mean (± standard deviation) in the case of Gaussian distribution or median (1st quartile; 3rd quartile) if data are non-Gaussian distributed. Normality was tested using the Shapiro-Wilk test. To compare means/medians of two variables, Student’s t-test and the Mann-Whitney U test were used. Comparison of categorical variables among groups was performed by Fisher’s exact test and chi-square test. Linear regression analysis was used to assess the effects of clinical, echo- and electrocardiographic parameters on QRSD. Statistical significance was set at a two-tailed probability level of < 0.05. All statistical analyses were performed using SPSS software (version 26.0, IBM, Armonk, NY, USA).
Results
Characteristics of patients with new-onset LBBBAVI
A total of 59 LBBBAVI patients (34 TAVR and 25 SAVR patients, median age 82 years, 42% male) were enrolled in the study. The characteristics of the TAVR and SAVR patients are shown in the Electronic Supplementary Material (Table S1). All patients had severe aortic valve stenosis with an aortic valve area < 1.0 cm2 as the indication for TAVR/SAVR. Pre-procedural conduction disease (left anterior/posterior hemiblock or intraventricular conduction delay) was observed in 8 (15%) patients. All patients developed LBBB immediately during implantation or within 24 h post-procedure. Except for age, no significant differences were observed between TAVR and SAVR patients.
With the occurrence of LBBBAVI, QRSD increased from 94 (86;100) ms to 148 (140;160) ms (p < 0.001) (Tab. 1). An LBBB QRSD of ≥ 130 ms was observed in 98% of patients and, with regard to the Strauss definition, 95% of patients met the sex-specific QRSD cut-off (Tab. 2). Notably, 100% of females had a QRSD ≥ 130 ms and 88% of males had a QRSD ≥ 140 ms. QRSD in LBBBAVI males was longer than in females (154 [145;162] ms vs 145 [138;153] ms, p = 0.006). No other electrocardiographic differences were observed between the sexes. In a multivariate linear regression model including age, height, weight, sex, CAD and end-diastolic diameter, only male sex was independently associated with increased QRSD (β = 11.49; p = 0.039). The baseline frontal QRS axis shifted from 9 (−15;45)° to −15 (−37;11)° post-AVI (p = 0.001). In 72% of LBBBAVI patients with a normal QRS axis (90%), a leftward shift was observed.
Electrocardiographic analysis of LBBBAVI and LBBBcontrol
The 59 LBBBAVI patients were matched to 118 LBBBcontrol patients. The characteristics of LBBBAVI and LBBBcontrol patients are shown in Tab. 1. Representative ECGs are shown in Fig. 1. No clinical or echocardiographic differences were observed between the two groups.
a Electrocardiogram of an 85-year-old control patient with non-procedure-induced left bundle branch block (LBBBcontrol) fulfilling none of the European Society of Cardiology (ESC) 2013, Strauss and American Heart Association (AHA) 2009 definitions. b Electrocardiogram of a 72-year-old patient with left bundle branch block following aortic valve implantation (LBBBAVI) fulfilling the ESC 2013 and Strauss definitions. c Electrocardiogram of a 86-year-old LBBBAVI patient fulfilling the ESC 2013, Strauss and AHA 2009 definitions
All LBBBAVI patients presented with QRS notching/slurring in the lateral leads (I, aVL, V5 or V6), whereas this was present in only 85% (100) of the LBBBcontrol group (p = 0.001). Inferior (lead II, III or aVF) and septal (lead V1 or V2) QRS notching/slurring was also more prevalent in the LBBBAVI group (83% vs 70%, p = 0.067 and 20% vs 5%, p = 0.002, respectively).
Overall, QRSD was not significantly different between the two groups, neither was RWPT. However, patients with LBBB QRSD ≥ 130 ms (98% vs 86%, p = 0.007) and patients meeting the Strauss sex-specific QRSD cut-off (95% vs 81%, p = 0.012) were more frequently observed in the LBBBAVI group.
Classification according to current LBBB definitions
Of all LBBBAVI patients, 100% met the ESC 2013 and 95% the Strauss LBBB definition, whereas only 17% of patients met the AHA 2009 definition (Tab. 2, Fig. 2). Low concordance with the AHA definition is explained by the low prevalence of QRS notching/slurring combined in all four lateral leads (54%, Tab. 2). Interestingly, except for one patient, all LBBBAVI patients had QRS notching in at least two lateral leads (I, aVL, V5 or V6). Furthermore, only 27% of patients had an RWPT > 60 ms in both leads V5 and V6, contributing to the low agreement with the AHA 2009 definition. When the analysis was restricted to the first three AHA 2009 criteria only, 48–53% of LBBBAVI patients fulfilled the AHA 2009 definition. The presence of a Q wave in lead aVL minimally reduced adherence to the AHA 2009 definition (Tab. 2).
In the LBBBcontrol group, concordance with the different definitions was significantly lower than in the LBBBAVI group: ESC 2013 85% (p = 0.001) and Strauss 68% (p < 0.001) (Tab. 2, Fig. 2). The lower agreement with the different LBBB definitions is explained by: (1) lower prevalence of lateral notching/slurring (85% vs 100%, p = 0.001) and (2) the higher number of patients with a shorter QRSD (QRSD ≥ 130 ms, 86% vs 98%, p = 0.007) in the LBBBcontrol group. Only 7% of patients fulfilled the AHA 2009 definition (p = 0.035, compared to LBBBAVI). As in the LBBBAVI group, low concordance with the AHA 2009 criteria is caused by a low combined prevalence of QRS notching/slurring in all four lateral leads (31%) and most patients not meeting the RWPT criterion (83%).
Subanalysis of LBBBAVI versus LBBBcontrol with presence of lateral QRS notching
Comparison of LBBBAVI patients and LBBBcontrol patients with lateral QRS notching/slurring is shown in the Electronic Supplementary Material (Table S2).
No differences in frontal QRS axis, nor in QRSD and number of patients with QRSD ≥ 130 ms were observed between the LBBBAVI group and the LBBBcontrol group with lateral notching. Furthermore, concordance with the different LBBB definitions was comparable between LBBBAVI patients and LBBBcontrol patients with lateral notching, but not when comparing LBBBAVI versus LBBBcontrol without lateral notching.
Extrapolation of LBBBAVI features to LBBBCRT
Clinical, echo- and electrocardiographic characteristics of the 33 CRT responders (median age 61 (48;71) years, 52% male) are summarised in Tab. 3. During a median follow-up of 53 (20;77) months, as per definition, LVEF increased from 27 ± 5.9% to 54 ± 7.5% (p < 0.001). Except for an increased QRSD (160 [155;173] ms vs 148 [140;160] ms, p < 0.001) and a higher prevalence of QRS notching/slurring in all four lateral leads (76% vs 54%, p = 0.047) in the LBBBCRT group, no differences in electrocardiographic features or agreement with the LBBB definitions were observed between LBBBAVI and LBBBCRT.
Discussion
Main findings
This study assesses and reviews electrocardiographic features of LBBB in a population with proximal LBBB, i.e. patients with AVI-induced LBBB. As all LBBBAVI patients had lateral QRS notching/slurring, QRS notching/slurring in the lateral leads is fundamental in the diagnosis of proximal LBBB. The LBBBAVI group showed high concordance with ESC 2013 and Strauss definitions, but low agreement with the AHA 2009 definition. Our observations in LBBBAVI were compared to matched LBBB patients from a general population, showing a higher number of patients with QRSD ≥ 130 ms, a higher prevalence of lateral QRS notching/slurring and a higher concordance with LBBB definitions in the LBBBAVI group.
Obstacles in defining LBBB
Current controversy in defining LBBB is primarily related to the difficulty in identifying patients with ‘true’ electrocardiographic LBBB. As studies over the past century have included patients with various types of conduction delay (proximal vs distal, focal vs diffuse), this obviously resulted in heterogeneous LBBB electrocardiographic patterns and criteria. Furthermore, most current LBBB definitions are derived from the same 1985 consensus criteria [19], but with different adaptations and interpretations (Electronic Supplementary Material, Fig. S1).
True LBBB and proximal LBBB: two of a kind?
Although the importance of QRS notching/slurring was acknowledged even in early LBBB definitions [1, 20, 21], CRT was fundamental to the understanding of the relationship between electro-mechanical dyssynchrony in LBBB and a subset of LBBB electrocardiographic patterns with lateral QRS notching/slurring (‘true’ LBBB) [1, 13]. Patients without ‘true’ LBBB morphology were shown to demonstrate less electromechanical dyssynchrony [22], and absence of QRS notching/slurring resulted in less clinical and echocardiographic improvement than in true LBBB patients [6]. Experimental animal studies [14], His bundle pacing [23] and recent mapping studies [15] were able to link these ‘true’ LBBB electrocardiographic patterns with QRS notching to a proximal block in the LBB.
In our procedure-induced LBBB population, QRS notching/slurring was the most distinctive electrocardiographic characteristic of proximal LBBB. Our findings are in line with observational TAVR studies [24, 25] and a recent mapping study by Upadhyay et al., showing that QRS notching had the highest sensitivity and best negative predictive value to diagnose proximal LBBB [15]. Moreover, most proximal LBBBs were correctable by His bundle pacing in their study, indicating that in these patients no distal conduction disease was present. In contrast, LBBB patients without lateral notching demonstrated an intact proximal left conduction system and their LBBB was not correctable by His bundle pacing [15]. These findings suggest that an LBBB pattern without notching/slurring most likely reflects ‘distal block’ [1].
The question remains whether the presence of lateral QRS notching/slurring in a non-AVI-induced LBBB population also corresponds to proximal conduction disease of the left bundle. However, as uniformity was observed among LBBBAVI patients and LBBBcontrol patients with lateral notching, these findings suggest pathophysiological similarities between the two groups and corroborate the evidence of a proximal block in all LBBB patients when notching/slurring is present.
Proposed criteria for proximal LBBB
AVI-induced LBBB implies an unequivocal proximal block and is therefore well suited for defining proximal LBBB. Based on our findings in LBBBAVI, we selected and adapted currently used criteria (Fig. 3).
QRS duration ≥120 ms
A minority of LBBBAVI patients might present with a QRS duration <130 ms. Of interest is that female LBBBAVI patients had a shorter QRSD than male LBBBAVI patients. This is in line with the findings of previous work by our group, showing that female patients show proximal LBBB morphology at shorter QRSD [26].
QS or rS in lead V1 and absent Q waves in leads V5-6
At inclusion, all our patients had a QS or rS in lead V1 and absent Q waves in leads V5‑6. We observed a small Q wave in leads I and aVL in 15% of proximal LBBB patients and therefore recommend against an ‘absent Q wave’ criterion in leads I and aVL.
QRS notching or slurring in ≥ 2 lateral leads
Patients with proximal LBBB always presented QRS notching/slurring in at least two lateral leads. Only half of the LBBBAVI patients had QRS notching/slurring in all four lateral leads, indicating that AHA 2009 requirements may lead to significant underdiagnosis of LBBB [7, 11, 27]. Whether the variable degree of QRS notching/slurring relates to suboptimal detection and/or differences in underlying electro-anatomical myocardial substrate remains unclear [28].
Leftward and superior oriented frontal QRS axis
Our observations in LBBBAVI support those of previous studies [25, 29], which showed that the onset of LBBB causes a variable degree of QRS axis shift in a leftward and superior direction in most patients. A more leftward oriented QRS axis may support the diagnosis of proximal LBBB, but we recommend against absolute cut-off values because of the large range.
Advantages of the revised LBBB definition
The ESC 2013 and Strauss definitions identify most LBBBAVI patients. However, 5–14% of our proximal LBBB patients did not reach the computer-simulation-based Strauss QRSD thresholds of 130 and 140 ms for females and males, respectively. As such, QRS prolongation with a lower limit of 120 ms is preferable to define proximal LBBB. Although the ESC 2013 definition provides excellent sensitivity (100% of LBBBAVI patients fulfilling the definition), the specificity might still be improved by adding the requirement of QRS notching/slurring in at least two lateral leads and the ancillary criterion of a leftward and superior oriented frontal QRS axis: 98% of LBBBAVI patients had QRS notching/slurring in at least two lateral leads and 85% of patients had a QRS axis ≤ 30°. As AVI-induced LBBB patients and CRT super-responders both represent a ‘true’ LBBB electromechanical substrate within the large spectrum of left ventricular dysfunction, excellent compliance (97%) with the proposed criteria among CRT super-responders corroborates our revised LBBB definition.
Limitations
A potential drawback in LBBBAVI patients for studying the characteristics and definition of LBBB may relate to the age and co-morbidity in this particular population. Studying LBBB in a population with an unaffected myocardial substrate could overcome these issues. However, the almost identical observations in LBBBAVI, matched LBBBcontrol with lateral notching and LBBBCRT patients argue against important myocardial substrate differences between these populations. In acute LBBB, as in LBBBAVI, electrical remodelling might affect electrocardiographic characteristics and alter LBBB features over time. However, this mainly involves changes in repolarisation features rather than changes in QRS features [30].
Conclusion
In patients with proximal procedure-induced LBBB, the presence of QRS notching/slurring in the lateral leads seems a sine qua non for proximal LBBB. Non-uniformity exists among current recommendations for the diagnosis of proximal LBBB, with the ESC 2013 and Strauss definitions providing a higher sensitivity than the AHA 2009 definition. The LBBBAVI population may therefore provide a framework for uniform criteria for assessing proximal LBBB.
References
Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34.
Rodriguez MI, Sodi-Pallares D. The mechanism of complete and incomplete bundle branch block. Am Heart J. 1952;44:715–46.
Grant RP, Dodge HT. Mechanisms of QRS complex prolongation in man—left ventricular conduction disturbances. Am J Med. 1956;20:834–52.
Vassallo JA, Cassidy DM, Marchlinski FE, et al. Endocardial activation of left bundle branch block. Circulation. 1984;69:914–23.
Mascioli G, Padeletti L, Sassone B, et al. Electrocardiographic criteria of true left bundle branch block: a simple sign to predict a better clinical and instrumental response to CRT. Pacing Clin Electrophysiol. 2012;35:927–34.
Tian Y, Zhang P, Li X, et al. True complete left bundle branch block morphology strongly predicts good response to cardiac resynchronization therapy. Europace. 2013;15:1499–506.
Caputo ML, van Stipdonk A, Illner A, et al. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int J Cardiol. 2018;269:165–9.
Zareba W, Klein H, Cygankiewicz I, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation. 2011;123:1061–72.
Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace. 2013;15:1070–118.
Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part III: intraventricular conduction disturbances. Circulation. 2009;119:e235–e40.
van Stipdonk AMW, Vanbelle S, ter Horst IAH, et al. Large variability in clinical judgement and definitions of left bundle branch block to identify candidates for cardiac resynchronisation therapy. Int J Cardiol. 2019;286:61–5.
Nguyen UC, Verzaal NJ, van Nieuwenhoven FA, Vernooy K, Prinzen FW. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace. 2018;20:1898–909.
Calle S, Delens C, Kamoen V, De Pooter J, Timmermans F. Septal flash: at the heart of cardiac dyssynchrony. Trends Cardiovasc Med. 2020;30:115–22.
Liu L, Tockman B, Girouard S, et al. Left ventricular resynchronization therapy in a canine model of left bundle branch block. Am J Physiol Heart Circ Physiol. 2002;282:H2238–H44.
Upadhyay GA, Cherian T, Shatz DY, et al. Intracardiac delineation of septal conduction in left bundle-branch block patterns. Circulation. 2019;139:1876–88.
Surkova E, Badano LP, Bellu R, et al. Left bundle branch block: from cardiac mechanics to clinical and diagnostic challenges. Europace. 2017;19:1251–71.
De Pooter J, El Haddad M, Stroobandt R, De Buyzere M, Timmermans F. Accuracy of computer-calculated and manual QRS duration assessments: clinical implications to select candidates for cardiac resynchronization therapy. Int J Cardiol. 2017;236:276–82.
Prineas RJ, Crow RS, Zhang Z. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. London: Springer; 2010.
Willems JL, Robles de Medina EO, Bernard R, et al. Criteria for intraventricular conduction disturbances and pre-excitation. World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol. 1985;5:1261–75.
Scott RC. Left bundle branch block—a clinical assessment. I. Am Heart J. 1965;70:535–66.
The Criteria Committee of the New York Heart Association. Diseases of the heart and blood vessels. Nomenclature and criteria for diagnosis. Boston: Little, Brown and Co; 1964.
Corteville B, De Pooter J, De Backer T, El Haddad M, Stroobandt R, Timmermans F. The electrocardiographic characteristics of septal flash in patients with left bundle branch block. Europace. 2017;19:103–9.
Huang W, Su L, Wu S, et al. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019;105:137–43.
Sundh F, Simlund J, Harrison JK, et al. Incidence of strict versus nonstrict left bundle branch block after transcatheter aortic valve replacement. Am Heart J. 2015;169:438–44.
Alqarawi W, Sadek MM, Golian M, et al. A new electrocardiographic definition of left bundle branch block (LBBB) in patients after transcatheter aortic valve replacement (TAVR). J Electrocardiol. 2020;63:167–72.
De Pooter J, Kamoen V, El Haddad M, et al. Gender differences in electro-mechanical characteristics of left bundle branch block: potential implications for selection and response of cardiac resynchronization therapy. Int J Cardiol. 2018;257:84–91.
van Stipdonk AMW, Hoogland R, Ter Horst I, et al. Evaluating electrocardiography-based identification of cardiac resynchronization therapy responders beyond current left bundle branch block definitions. JACC Clin Electrophysiol. 2020;6:193–203.
Nguyen UC, Potse M, Regoli F, et al. An in-silico analysis of the effect of heart position and orientation on the ECG morphology and vectorcardiogram parameters in patients with heart failure and intraventricular conduction defects. J Electrocardiol. 2015;48:617–25.
Chou TC. Electrocardiology in clinical practice. Philadelphia: Saunders; 2008.
Engels EB, Poels TT, Houthuizen P, et al. Electrical remodelling in patients with iatrogenic left bundle branch block. Europace. 2016;18:iv44–iv52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Calle, M. Coeman, A. Demolder, T. Philipsen, P. Kayaert, M. De Buyzere, F. Timmermans and J. De Pooter declare that they have no competing interests.
Supplementary Information
Fig. S1
A historical overview of electrocardiographic criteria for left bundle branch block [1, 8, 9, 10, 19, 20, 21]. Definitions and subsequent adaptations are connected with arrows. QRSD QRS duration, RWPT R wave peak time
Table S1
Baseline clinical, echo- and electrocardiographic characteristics of TAVR- and SAVR-induced LBBB patients
Table S2
Clinical, echo- and electrocardiographic characteristics of aortic valve implantation-induced LBBB and matched control LBBB patients (extended version)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calle, S., Coeman, M., Demolder, A. et al. Aortic valve implantation-induced conduction block as a framework towards a uniform electrocardiographic definition of left bundle branch block. Neth Heart J 29, 643–653 (2021). https://doi.org/10.1007/s12471-021-01565-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-021-01565-8