Abstract
Background
The prevalence of heart failure (HF) is increasing substantially and, despite improvements in medical therapy, HF still carries a poor prognosis. Mechanical circulatory support (MCS) by a continuous-flow left ventricular assist device (cf-LVAD) improves survival and quality of life in selected patients. This holds especially for the short-term outcome, but experience regarding long-term outcome is growing as the waiting time for heart transplantation is increasing due to the shortage of donor hearts. Here we present our results from the University Medical Centre Utrecht.
Methods
Data of all patients with a cf-LVAD implant between March 2006 and January 2018 were collected. The primary outcome was survival. Secondary outcomes included adverse events defined according to the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) definitions, described per patient year.
Results
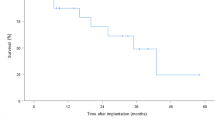
A total of 268 patients (69% male, mean age 50 ± 13 years) received a cf-LVAD. After a median follow-up of 542 (interquartile range 205–1044) days, heart transplantation had been performed in 82 (31%) patients, the cf-LVAD had been explanted in 8 (3%) and 71 (26%) had died. Survival at 1, 3 and 5 years was 83%, 72% and 57%, respectively, with heart transplantation, cf-LVAD explantation or death as the end-point. Death was most often caused by neurological complications (31%) or infection (20%). Major bleeding occurred 0.51 times and stroke 0.15 times per patient year.
Conclusion
Not only short-term results but also 5‑year survival after cf-LVAD support demonstrate that MCS is a promising therapy as an extended bridge to heart transplantation. However, the incidence of several major complications still has to be addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
This is the first study investigating the long-term outcome of mechanical circulatory support (MCS) in The Netherlands.
-
The 1‑, 3‑ and 5‑year survival on MCS was 83%, 72% and 57%, respectively. These results support its use as an extended bridge to heart transplantation, as necessitated by the shortage of donor hearts in our country.
-
Survival in the period 2006–2012 did not differ from that in 2013–2017.
-
Adverse events in terms of major bleeding and stroke occurred 0.51 and 0.15 times per patient year, respectively.
Background
The prevalence of heart failure (HF) is increasing substantially in Western countries. In the Netherlands already 1.3% of the total population (227,300 patients) suffer from HF. This percentage will certainly grow in the coming decades owing to the aging population and better treatment of heart disease in general [1, 2].
Despite a substantial improvement in prognosis resulting from the use of beta blockers, ACE inhibitors/angiotensin receptor blockers, angiotensin receptor-neprilysin inhibitors, aldosterone antagonists, implantable cardioverter defibrillators and resynchronisation therapy, HF still carries a poor prognosis with a 1-year mortality of 26% in patients below the age of 75 years and 56% for those aged above 75 [3].
In patients with end-stage HF refractory to optimal medical therapy, heart transplantation is ‘the gold standard’ [4, 5]. However, because of the severe shortage of donor hearts, only few patients may benefit from this procedure. It is not to be expected that the number of donor hearts will increase substantially, so alternative treatment options need to be considered. Long-term mechanical circulatory support (MCS) by continuous-flow left ventricular assist devices (cf-LVADs) has demonstrated improved life expectancy and quality of life in these patients and may hold promise for the future as a realistic alternative to heart transplantation [6,7,8,9,10]. On the other hand, management of patients on long-term cf-LVADs is still very laborious owing to well-known adverse events, such as infection, bleeding, thrombosis and device malfunction [11].
In our centre cf-LVADs have been implanted since 2006, initially the HeartMate II (HM-II, Abbott, St. Paul, MN, USA), from 2010 the HVAD (Medtronic, Framingham, MA, USA) and, since the end of 2015, the HeartMate 3 (HM 3, Abbott) [12].
Previously, only relatively short-term results of cf-LVAD implantations in the Netherlands have been published [13, 14]. As the duration of MCS is growing, partly caused by the shortage of donor hearts, this study was performed to provide insight into the long-term outcome in terms of survival and adverse events. Furthermore, we were interested whether the clinical situation of patients before cf-LVAD implantation has changed over the years with respect to Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile.
Methods
Study population
Data of all patients in whom the HM-II, HM 3 or the HVAD was implanted at the University Medical Centre Utrecht between March 2006 and January 2018 (end of study) were collected in a central database. The database included baseline clinical characteristics and all adverse events defined according to the INTERMACS definitions [15]. Adverse events were described for the total population. The institutional ethics board approved the study.
cf-LVAD implantation and anticoagulation
cf-LVADs were implanted via a median sternotomy using extracorporeal circulation on the beating heart. In the absence of bleeding, heparin was started within 48 h after surgery if drainage during 3 consecutive hours post-implant did not exceed 50 ml/h. A vitamin K antagonist was started after drain removal and heparin was stopped when the INR reached the lower limit of the therapeutic range as described for each cf-LVAD type below.
A thrombocyte aggregation inhibitor was started after 48 h, in general aspirin 100 mg/day. From March 2006 until August 2009, in HM-II patients warfarin was titrated to an INR of 2–2.5, 7 days after implantation. The INR range was reduced to 1.5–2.0 due to a substantial incidence of bleeding, as reported in the literature [16]. From December 2011 the INR range was increased again to 1.8–2.5 because of increased thrombo-embolic complications. For the HVAD and HM 3, target INR was 2.5–3.5 and 2.0–3.0, respectively, according to the manufacturer’s advice.
Outcome
The primary outcome of the study was survival on cf-LVAD support until a pre-specified end-point, i.e. death, device explantation, heart transplantation or the end of the study. Secondary outcomes included all adverse events defined according to INTERMACS.
Definition of adverse events
All adverse events were defined according to the INTERMACS definitions. Major bleeding was defined as suspected internal or external bleeding, resulting in death, re-operation, hospitalisation and/or transfusion of red blood cells (within the first 7 days after the implantation requiring transfusion ≥4 units of packed red blood cells, or any transfusion beyond 7 days postoperatively). Neurological complications included a transient ischaemic attack and ischaemic/haemorrhagic strokes. Major infection was defined as a clinical infection accompanied by pain, fever, drainage and/or leukocytosis, treated by antimicrobial agents (non-prophylactic).
Major haemolysis included biochemical signs of haemolysis (free plasma haemoglobin >200 mg/l or lactate dehydrogenase >625 U/l), accompanied by at least one of the following symptoms: haemoglobinuria, anaemia, hyperbilirubinaemia and/or pump malfunction. Minor haemolysis comprised asymptomatic biochemical abnormalities.
Major device malfunction included pump thrombosis, high-urgency transplantation, pump replacement, pump explantation, breach of driveline or death. Minor device malfunction included inadequately functioning external components which required repair or replacement. Right heart failure (RHF) was defined as symptoms and signs of persistent right ventricular dysfunction (central venous pressure >18 mm Hg with a cardiac index <2.3 l/min/m2 in the absence of elevated left atrial/pulmonary capillary wedge pressure (>18 mm Hg), tamponade, ventricular arrhythmias or pneumothorax) requiring implantation of a right ventricular assist device, inhaled nitric oxide or inotropic therapy for >1 week at any time after cf-LVAD implantation.
Statistical analysis
We used SAS software (SAS Institute, Cary, NC, USA) for statistical analysis. Kaplan-Meier survival estimation was applied for survival analysis of the entire cohort, and as specified by INTERMACS profile at baseline. Differences in survival were considered statistically significant if the log-rank test showed a p-value <0.05. Comparison of dichotomous variables between implantations from 2006 to 2012 and from 2013 to 2017 was performed by chi-square test or Fisher’s exact test. Continuous variables were compared by the Mann-Whitney U test. In patients who died, the cause of death was retrospectively verified by one researcher and categorised according to the annual INTERMACS reports. Rate of complications was described per patient year.
Results
Baseline
From March 2006 until January 2018, 268 patients underwent cf-LVAD implantation (69% male, mean age 50 (±13) years). In 59% of patients HM-II was implanted, 98.5% of devices as a bridge to transplantation, the remaining 1.5% as destination therapy. Follow-up was completed for all 268 patients for a median period of 542 (interquartile range (IQR): 205–1044) days, resulting in a total experience of 510 patient years (mainly determined by 380 patient years for HM-II).
The clinical profile before cf-LVAD implantation was most often INTERMACS 2 (42%) or 3 (27%), implying a progressive decline on inotropic support and stable but inotrope dependent, respectively. Furthermore, 19% of the patients were on temporary MCS prior to cf-LVAD implantation, mostly by central or peripheral extracorporeal life support, so were originally INTERMACS 1 but stabilised on temporary MCS. Baseline characteristics for the total cohort and per device type are summarised in Tab. 1.
The INTERMACS profile prior to the cf-LVAD implantation has changed over time. Since 2013, patients in higher INTERMACS profiles received implants more frequently than in the first few years. Statistical analysis was performed to compare survival of patients receiving implants between 2006 and 2012 with those between 2013 and 2017. Patients in whom a cf-LVAD was implanted between 2006 and 2012 were significantly younger (p < 0.001) and more frequently in INTERMACS profile 2 (p = 0.008) than those receiving implants between 2013 and 2017 (Electronic Supplementary Material, Table 1).
Primary outcome
Seventy-one (26%) patients (44 HM-II, 24 HVAD and 3 HM 3) died after cf-LVAD implantation after a median of 216 (IQR: 20-807) days. Death was most often caused by neurological complications (22 patients) or infections (14 patients) (Tab. 2). Device malfunction was the cause of death in 7 patients, pump thrombosis in 5 cases and technical failure in 2. Eighty-two (31%) patients underwent a heart transplantation, after a median duration of 674 (IQR: 394–1028) days on cf-LVAD-support. Explantation of the cf-LVAD was possible in 8 (3%) patients after a median period of 529 (IQR: 351–670) days. In these 8 patients, myocarditis and peri-partum cardiomyopathy were the most common aetiologies. In one patient with dilated cardiomyopathy in whom the cf-LVAD was initially explanted following the recovery of left ventricular function, a new device had to be implanted after 144 days due to recurrent HF.
Survival after 1, 3 and 5 years was 83%, 72% and 57%, respectively (Fig. 1). There was a trend towards worse survival in patients with INTERMACS profile 1 in comparison to INTERMACS profile 2 or 3, though not significantly different (Fig. 2, p = 0.24). Neither did survival differ significantly between implants in 2006–2012 in comparison to implants in 2013–2017 (p = 0.44). Because patients receiving implants between 2006 and 2012 were significantly younger and more frequently in INTERMACS 2 in comparison to 2013–2017, correlation between survival and these variables was analysed. Both age and INTERMACS profile 2 were not associated with mortality in this cohort (hazard ratio (HR) 0.98 (95% confidence interval, CI, 0.96–1.01), p = 0.123 and HR 0.91 (95% CI 0.55–1.50), p = 0.702, respectively).
Secondary outcomes
Beside localised infections not specifically related to the MCS, such as urinary tract infections and pneumonias, the three most commonly encountered adverse events were major bleeding, ventricular tachycardia and minor haemolysis with corresponding event rates of 0.51, 0.35 and 0.26 per patient year, respectively, as shown in Tab. 3.
Strokes (haemorrhagic and/or ischaemic) occurred 0.15 times per patient year. RHF occurred 0.23 times per patient year, most often (65%) within the first month after implantation. In 29 patients, RHF developed beyond 30 days after implantation, of whom 8 (28%) also suffered from early RHF.
Discussion
This analysis of 268 patients, resulting in clinical experience of 510 patient years, describes the 5‑year outcome of cf-LVAD patients in a Dutch population, in whom the device was initially implanted as a bridge to transplantation. Survival at 1, 3 and 5 years was 83%, 72% and 57%, respectively, in this selected group of end-stage HF patients. This denotes its use as an extended bridge to heart transplantation, although still with considerable morbidity.
Interpretation of findings
Previously, only a few smaller single-centre studies were performed regarding long-term results of cf-LVAD support. Takeda et al. presented their results in 140 patients, showing a survival rate of 83%, 75% and 61% after 1, 3 and 5 years, respectively [17]. We now confirmed these results in a larger population. In the most recent annual INTERMACS report, survival rates at 1, 3 and 5 years were 83%, 63% and 46%, respectively [18]. With regard to the pre-operative condition, it is known that patients in INTERMACS profiles 1–3 have worse survival rates, especially INTERMACS profile 1 [15, 18]. Our study confirmed the relationship between the initial poor state and the trend towards worse survival of patients in INTERMACS profile 1, in comparison to INTERMACS profile 2 or 3, despite prior stabilisation on short-term MCS, although this was not statistically significant.
Generally, in MCS patient selection is of utmost importance for the outcome. Stewart et al. studied the use of the INTERMACS classification to identify ambulatory patients with advanced HF who may benefit from a cf-LVAD. In that study, patients in INTERMACS profile 4 had a higher mortality rate and needed MCS more often compared to patients in INTERMACS profile 5–7 [19]. In addition, the ROADMAP trial concluded that patients in INTERMACS 4 have better survival, functional capacity and improved quality of life when treated with a cf-LVAD in comparison to optimal medical management [20].
Furthermore, prediction of RHF is important, because this is related to worse survival. Recently, the EUROMACS-RHF risk score was developed, which can be used to predict early RHF [21]. Unfortunately, little is known about risk factors for late RHF, which needs further research in the setting of long-term MCS.
Technical improvements in the HM 3, using a magnetically levitating environment, revealed fewer haemocompatibility-related adverse events (e.g. pump thrombosis) in comparison to HM-II at 2 years, as concluded in the MOMENTUM 3 trial. However, the rate of bleeding events was comparable in both groups [22,23,24]. A personalised anticoagulation regimen could decrease the individual risk for bleeding and thrombosis. Furthermore, the risk for infection could be decreased by the use of cf-LVAD with smaller (or no) external components including the driveline, which is the most frequently encountered location for VAD-related infections.
Strengths and limitations
This is the largest single-centre study reporting on 5‑year outcome in cf-LVAD patients in whom the device was implanted as a bridge to transplantation, reflecting the Dutch results of long-term MCS. Complications were recorded prospectively and systematically in a central database. Patient follow-up was complete in our own centre, minimising the risk of missing data.
However, the single-centre design may indicate that our results cannot be extrapolated directly to other centres. Furthermore, in nearly all patients the cf-LVAD was implanted as a bridge to transplantation. In general these patients appear to have a more favourable outcome in comparison to those receiving this device as destination therapy [15, 18, 25]. Finally it has to be realised that our results are mainly based on the HM-II LVAD, as almost 60% of patients received this device.
Conclusion
In our experience, based on 268 cf-LVADs, the use of cf-LVADs for end-stage HF demonstrated a survival of 57% after 5 years, proving relatively good long-term results. These results support the use of such devices as an extended bridge to heart transplantation, necessitated by the shortage of donor hearts. However, several important adverse effects need to be tackled by further technical improvements. Also risk stratification before cf-LVAD implantation in individual patients is essential. Presently we are only on the verge of acquiring this knowledge [26, 27]. The assessment of a personal risk model could improve individualised therapy, for example the anticoagulation regimen, which is now generally the same for each type of device and for every patient.
References
Buddeke J, van Dis I, Vaartjes I, et al. Hartfalen in Nederland. Den Haag: Hartstichting; 2016.
Engelfriet PM, Hoogenveen RT, Poos MJJC, et al. Hartfalen: epidemiologie, risicofactoren en toekomst. RIVM report. 2012.
Cleland JG, McDonagh T, Rigby AS, Yassin A, et al. The national heart failure audit for England and Wales 2008–2009. Heart. 2011;97:876–86.
Lund L, Edwards L, Kucheryavaya A, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report—2014. J Heart Lung Transplant. 2014;33:996.
Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505–35.
Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43.
Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol. 2011;26(3):232–6.
de Jonge N, Kirkels H, Lahpor JR, et al. Exercise performance in patients with end-stage heart failure after implantation of a left ventricular assist device and after heart transplantation. An outlook for permanent assisting? J Am Coll Cardiol. 2001;37(7):1794–9.
Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96.
Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51.
Felix SE, Martina JR, Kirkels JH, et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012;14(4):351–6.
Lahpor JR, de Jonge N, van Swieten HA, et al. Left ventricular assist device as bridge to transplantation in patients with end-stage heart failure: eight-year experience with the implantable HeartMate LVAS. Neth Heart J. 2002;10(6):267–71.
Lok SI, Martina JR, Hesselink T, et al. Single-centre experience of 85 patients with a continuous-flow left ventricular assist device: clinical practice and outcome after extended support. Eur J Cardiothorac Surg. 2013;44(3):e233–e8.
Haeck ML, Beeres SL, Höke U, et al. Left ventricular assist device for end-stage heart failure: results of the first LVAD destination program in the Netherlands. Neth Heart J. 2015;23(2):102–8.
Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–6.
Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–7.
Takeda K, Takayama H, Kalesan B, et al. Long-term outcome of patients on continuous-flow left ventricular assist device support. Thorac Cardiovasc Surg. 2014;148(4):1606–14.
Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38(2):114–26.
Stewart GC, Kittleson MM, Patel PC, et al. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) profiling identifies ambulatory patients at high risk on medical therapy after hospitalizations for heart failure. Circ Heart Fail. 2016;9(11). https://doi.org/10.1161/CIRCHEARTFAILURE.116.003032
Shah KB, Starling RC, Rogers JG, et al. Left ventricular assist devices versus medical management in ambulatory heart failure patients: an analysis of INTERMACS Profiles 4 and 5 to 7 from the ROADMAP study. J Heart Lung Transplant. 2018;37(6):706–14.
Soliman OII, Akin S, Muslem R, et al. Derivation and validation of a novel right-sided heart failure model after implantation of continuous flow left ventricular assist devices: the EUROMACS (European Registry for Patients with Mechanical Circulatory Support) right-sided heart failure risk score. Circulation. 2018;137(9):891–906.
Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135(21):2003–12.
Krabatsch T, Netuka I, Schmitto JD, et al. Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure—1 year results from the Ce mark trial. J Cardiothorac Surg. 2017;12(1):23.
Mehra MR, Goldstein DJ, Uriel N, et al. MOMENTUM 3 Investigators. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018;378(15):1386–95.
Kirklin JK, Xie R, Cowger J, et al. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant. 2018;37(6):685–91.
Magnussen C, Bernhardt AM, Ojeda FM, et al. Gender differences and outcomes in left ventricular assist device support: The European Registry for Patients with Mechanical Circulatory Support. J Heart Lung Transplant. 2018;37(1):61–70.
Frontera JA, Starling R, Cho SM, et al. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant. 2017;36(6):673–83.
Funding
F.W. Asselbergs is supported by UCL Hospitals NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.E.A. Felix, F.Z. Ramjankhan, M.P. Buijsrogge, K.A. Jacob, F.W. Asselbergs, M.I.F. Oerlemans, J.H. Kirkels, L.W. van Laake, A.M.C. Oppelaar, W.J.L. Suyker and N. de Jonge declare that they have no competing interests.
Caption Electronic Supplementary Material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felix, S.E.A., Ramjankhan, F.Z., Buijsrogge, M.P. et al. Outcome of mechanical circulatory support at the University Medical Centre Utrecht. Neth Heart J 28, 210–218 (2020). https://doi.org/10.1007/s12471-020-01375-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-020-01375-4