Abstract
Purpose of Review
To review the current and future role of cardiovascular magnetic resonance (CMR) assessment of immunotherapy cardiotoxicity.
Recent Findings
In patients who suffer from immune checkpoint inhibitor (ICI) myocarditis, pathologic CMR findings, including myocardial edema, reduced left ventricular ejection fraction (LVEF), late gadolinium enhancement (i.e., fibrosis and/or necrosis), and myocardial strain, are mostly subtle, but fulminant courses have been described. Individual cases of cardiotoxicity in chimeric antigen receptor (CAR) T cell therapy have also already been documented, but there are currently no studies addressing the role of CMR in CAR T cell therapy. There are also classes of immunotherapies for which no cases of cardiotoxicity are known yet, such as cytokines or adjuvants.
Summary
Together with patient symptoms, laboratory markers, electrocardiogram, and echocardiography, CMR is of high value in the diagnostic workup of immunotherapy-associated myocarditis in hemodynamically stable patients, according to recent guidelines. Additionally, quantitative strain analysis and T1 relaxation times with CMR can aid in assessing disease severity, prognosis, and patient outcomes with ICI-associated myocarditis. Future CMR studies on cardiotoxicity in CAR T cell therapy are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Besides traditional cancer treatment such as tumor resection, chemotherapy, and radiation therapy, cancer immunotherapy has become an established method to enable tumor regression in a broad spectrum of cancer types. Cancer immunotherapy utilizes immunomodulators to intervene in signaling pathways, which regulate the immune system’s activity. By targeting either brakes or gas pedals, immunomodulators support or restore the immune system’s ability to find and eliminate cancer cells. Advantages of immunotherapy include the ability to target multiple different tumor entities and the thoroughness in removing microscopic lesions and remaining tumor cells [1]. Additionally, by restoring and improving the immune system, immunotherapy has led to improved survival rates [1].
Unfortunately, with all the new benefits that cancer immunotherapy offers, it also comes with potential drawbacks for patients. With the increasing use of immune checkpoint inhibitors (ICI) a growing number of immune-related adverse events (irAE) has been reported [2]. Common irAE include dermatologic, gastrointestinal, and endocrine toxicities, while neurotoxicity, pulmonary toxicity, and cardiotoxicity are less common but more clinically significant. Cardiotoxicity is the most likely of the irAEs to take a fatal course, though thankfully this remains rare [3]. In addition to many other manifestations of cardiotoxicity such as arrhythmia, acute coronary syndrome, or vasculitis, immunotherapy-associated myocarditis is the most reported cardiac side effect due to its high morbidity and mortality [4]. Major adverse cardiovascular events (MACE) like arrhythmia, acute coronary syndrome, myocardial infarction, or heart failure are documented in up to 40% of patients suffering from ICI myocarditis, and result in death in 15 to 25% of cases [5,6,7]. Importantly, these numbers may be affected by selection bias in the cited studies based on the case series and registry study design. However, it is clear that clinically significant myocarditis must be diagnosed and managed promptly, increasing the importance of cardiovascular magnetic resonance (CMR) and other diagnostic tools. Studies show that MACE are also associated with chimeric antigen receptor (CAR) T cell therapy [8,9,10].
The traditional diagnostic repertoire for diagnosing myocardial inflammation as a side effect of immune therapy traditionally consists of the determination of clinical and anamnestic findings, cardiac biomarkers, electrocardiogram, echocardiography, and endomyocardial biopsy [11,12,13]. Nowadays, CMR is mainly used to noninvasively characterize inflammatory myocardial tissue alterations, analyze involvement patterns, and give important insights into pathological remodeling processes. Previous studies have shown that CMR is an excellent tool for accurately imaging cardiac side effects [14]. Furthermore, it is considered the reference standard for measuring ventricular volumes and function making it ideally suited to assess adverse cardiac remodeling from cancer treatment [15,16,17,18,19]. To confirm the presence of inflammation and to document the extent and pattern of myocardial injury related to cancer immunotherapy, CMR is considered the imaging modality of choice in hemodynamical stable patients to diagnose side effects of cancer immunotherapy [20, 21].
This review provides a comprehensive overview of the role of CMR in patients with immunotherapy-associated cardiotoxicity focusing on ICI and CAR T cell therapy–related side effects.
Cardiotoxic Effects of Immune Checkpoint Inhibitors and CAR T Cell Therapy
Today, a broad spectrum of immunotherapies is available, including targeted antibodies, cancer vaccines, oncolytic virus therapy, adaptive cell therapy, and immunomodulators. The latter includes, among other groups, ICI. CAR T cell therapy belongs to the adaptive cell therapy. Based on current data, this review examines CMR changes associated with immunotherapy cardiotoxicity in ICI as well as early reports in CAR T cell therapy. Table 1 gives an overview of current ICI and CAR T cell therapies and their documented side effects in terms of cardiotoxicity.
Apart from ICI and CAR T cell therapy within the spectrum of immunotherapies, cardiotoxic effects have also been documented in individual cases with Toll-like receptor agonists, a class of immunomodulators [22]. Notably, cardiotoxicity has also been well documented in the context of therapy with monoclonal antibodies, including trastuzumab and alemtuzumab, that can be classified as both targeted therapy and passive immunotherapy [23,24,25,26].
Immune Checkpoint Inhibitors
Over the last years, ICI (i.e., monoclonal antibodies targeting programmed cell death protein 1 (PD-1), its ligand (PD-L1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)) have played an increasingly important role in cancer therapy. ICI have been shown to improve therapy outcomes and overall patient survival [1, 27,28,29]. However, an increasing number of cardiac irAE (including myocarditis, myocardial infarction, heart failure, pericardial disease, and vasculitis) have been reported over the past few years with an incidence up to 1.14% [30, 31]. ICI-induced cardiotoxicity is distinguished by the highest death rate in irAE with 40 to 50% [32, 33], which likely could be overestimated. Nevertheless, early detection and systematic reporting are crucial for therapy and outcome. If ICI myocarditis is suspected, immediate discontinuation of ICI therapy and early initiation of steroid therapy are often essential for patient recovery [11, 34,35,36,37]. However, other etiologies should be considered in parallel. The occurrence of Takotsubo cardiomyopathy in the context of ICI therapy has also been documented [38, 39]. Notably, in many cases of Takotsubo cardiomyopathy, the patients underwent combination therapy consisting of immunotherapy and chemotherapy. In a recent meta-study, ICI was found to account for 9.7% of chemotherapy regimens that were involved in Takotsubo syndrome [40].
CMR for Assessment of Cardiac Adverse Effects in Immune Checkpoint Inhibitors
The suspected diagnosis of ICI myocarditis is initially based on corresponding clinical symptoms, new troponin elevation (associated with cardiovascular symptoms or non-cardiovascular irAE) and/or new abnormalities on electrocardiogram (e.g., tachyarrhythmias) [41, 42]. The European Society of Cardiology guidelines on cardio-oncology recommend that both echocardiography and CMR should be performed in patients with suspected ICI myocarditis [42]. Also, other causes of myocardial injury must be ruled out, e.g., coronary heart disease using coronary angiography. ICI myocarditis is defined by either pathohistological or clinical markers, the latter necessarily involving an increase in troponin accompanied by one major criterion or two minor criteria, illustrated in Table 2 [42]. Pathological inflammatory findings on CMR according to the updated Lake Louise Criteria (LLC) represent a major criterion. The updated LLC including T1 and T2 mapping have been shown to improve the diagnostic performance in comparison to the original LLC [16]. Although T1 relaxation times are a non-specific marker of myocardial disease, they have a high diagnostic performance to detect myocarditis in the appropriate clinical setting [43]. Not only can the occurrence of myocarditis itself be suggested using the T1 relaxation times, but higher native T1 relaxation times have previously correlated with more severe forms of myocardial injury and have been more commonly elevated than T2 values in patients with ICI myocarditis [44]. Studies have shown that abnormal T1 relaxation times in ICI myocarditis are associated with poorer cardiac function, more clinical symptoms, abnormal histopathology, and future development of MACE, suggesting that T1 relaxation time is one of the most powerful CMR outcome parameters [45,46,47, 46].
The presence of LGE in patients with ICI-related cardiotoxicity has varied from 9 to 82% in analyzed studies, excluding case series [5, 6, 20, 21, 47,48,49,50,51]. Not only the presence but also the pattern of myocardial LGE matters for the detection of ICI myocarditis and differentiation from other cardiac pathologies. Cadour et al. compared CMR findings between patients with ICI myocarditis, patients with viral myocarditis, and patients prior to ICI therapy [49•]. LGE in ICI myocarditis patients was predominantly patchy, showed a subepicardial or midwall location, and was mainly septal and lateral [49•]. LGE localized in the ventricular septum was considered to be a possible predictor of MACE, defined as a composite of cardiovascular death, ventricular arrhythmia, complete atrioventricular block, and cardiogenic shock [49•]. Another study showed that LGE was present in 80% of patients with clinically diagnosed ICI myocarditis and was commonly located in the mid-myocardial right ventricular insertion site (75%) [50•]. Although LGE was frequently detected in patients with ICI myocarditis, it did not correlate with other CMR parameters such as volumetry, visual edema, or left ventricular ejection fraction (LVEF) in particular [50•].
Complicating the diagnosis of clinically significant myocarditis, a prospective study of 22 patients undergoing ICI treatment showed a high prevalence of subclinical myocardial inflammation in study participants. Only one patient developed fulminant myocarditis [20•]. An overall decreased LVEF between baseline and follow-up was observed (62% ± 7 vs 59% ± 7, p = 0.048). Additionally, diffuse edema was detected in 9% and slight pericardial effusion was detected in 41% [20•].
In most reported studies of ICI associated myocarditis, an absence of overt left ventricular dysfunction or only mild LVEF impairment has been observed at time of diagnosis [5, 6, 20, 45, 46, 49, 50, 48.]
While documentation of ICI myocarditis cases used to be presumptive and anecdotal, CMR in suspected ICI myocarditis cases has been increasingly performed in clinics in recent years due to growing awareness and standardized guidelines. Another recent clinical study of patients who received ICI for small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC) suggests that ICI myocarditis might be underreported [52]. In the study, 99 patients were systematically screened for the presence of an ICI myocarditis based on electrocardiogram abnormalities, at least a threefold increase in troponin compared to the baseline examination and cardiovascular symptoms. In case of conspicuous screening parameters, CMR, echocardiography, and coronary angiography were performed. A total of three patients were diagnosed with myocarditis, two of whom showed pathological CMR. Thus, the overall ICI myocarditis incidence in this study was about 3%, while the estimated incidence documented in prior studies ranged from 0.01 to 1%. While more patients were diagnosed with non-fulminant myocarditis with a standardized screening algorithm, there are currently no data to help better understand whether these patients would or would not develop fulminant myocarditis if continued on immunotherapy. Given the significant improvement in cancer survival associated with immunotherapy, more studies will be needed to understand how to approach patients with early signs of myocarditis who are otherwise doing well on treatment. The study findings suggest that CMR in combination with systematic screening in cancer patients with ICI therapy could lead to earlier detection of myocarditis. [52]. However, whether patients with subclinical myocarditis should stop immunotherapy treatment or simply have closer monitoring is unknown.
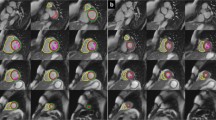
Cardiotoxicity-related CMR findings from the recent literature are summarized in Table 3. The incidence of visual myocardial edema and T1 and T2 mapping alterations, respectively, varied widely among studies. In summary, only subtle CMR abnormalities are often observed in ICI myocarditis, as shown in Fig. 1. Interestingly, similar CMR characteristics are common in patients who were treated with ICI monotherapy and those who were treated with ICI combination therapy [6, 20].
Cardiovascular magnetic resonance in an 84-year-old female patient with metastatic melanoma treated with nivolumab (Opdivo ®), an immune checkpoint inhibitor (ICI) targeting programmed cell death protein 1 (PD-1). Ten weeks after ICI administration, the patient presented with shortness of breath and troponin elevation. Representative images are shown in short axis view. A T2 black blood short tau inversion recovery (STIR) sequence shows focal edema in the basal inferoseptum with a corresponding late gadolinium enhancement lesion in the B phase sensitive inversion recovery (PSIR) late gadolinium enhancment (LGE) sequence (white arrows). C Focal myocardial T1 relaxation times were also elevated. These findings were compatible with immune checkpoint inhibitor myocarditis according to 2022 European Society of Cardiology guidelines on cardio-oncology
CAR T Cell Therapy
CAR T cell therapy is a novel pillar of immunotherapy that has revolutionized the fight against cancer in recent years. For example, in the treatment of leukemia and B cell lymphoma, CAR T cell therapy has led to excellent clinical responses. Nevertheless, there are also major potential life-threatening limitations such as cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome [53].
Cytokine Release Syndrome
CRS is a phenomenon that is triggered by activation of T cells and other immune cells, which leads to elevated blood levels of different cytokines. Clinically, CRS is described as “a disorder characterized by fever, tachypnea, headache, tachycardia, hypotension, rash, and/or hypoxia caused by the release of cytokines” [54]. Recently, the American Society for Transplantation and Cellular Therapy published a consensus grading for CRS [54]. The severity of CRS can be subdivided into 4 grades (see Table 4). In adults with relapsed/refractory B cell lymphoma for example, CRS can occur in 58 to 93% of cases with severe CRS grades of 3 or 4 in 13 to 22% [55, 56]. Although immune effector cell-associated CRS may have a delayed onset, it rarely presents beyond 14 days after initiation of therapy [54]. Low-grade CRS can often be managed with supportive care alone, but in more severe cases, blockade of the IL-6 pathway and/or corticosteroid therapy are recommended [54, 57].
Cardiovascular Complications
Both autoimmune toxicities resulting from antigen-specific T cell infiltration of the heart and cytokine-mediated toxicities are described in literature [9, 58].
Cytokine-associated cardiotoxicities have been described primarily in the context of CRS and might be the cause of most of the cardiovascular adverse reactions observed [9]. A retrospective study including 137 patients investigated cardiovascular events in 137 adults treated with CAR T cell therapy [9]. After a median time of 21 days after CAR T cell therapy initiation, 12% of the patients had clinical apparent MACE. MACE included new-onset arrhythmia and heart failure, as well as decompensated heart failure and cardiovascular death. Elevated troponin levels were found in 54% of the patients, while 28% had a decreased LVEF on echocardiography. Interestingly, all MACE occurred in patients with CRS grade ≥ 2 and troponin elevation was a risk factor for subsequent MACE. Another study examined 150 patients treated with CAR T cell therapy for the occurrence of MACE, including new-onset arrhythmia, symptomatic heart failure, acute coronary syndrome, ischemic stroke, and cardiovascular death [8]. At a median time of 11 days after starting CAR T cell therapy, 21% of the patients experienced MACE. MACE was independently associated with CRS grades of 3 or 4 and baseline creatinine. Overall survival after 1 year was 71% [8]. Another retrospective study analyzing 116 patients with serial echocardiograms after CAR T cell therapy found that 10% of patients developed a decrease in LVEF (average decrease from 58 to 37%) indicating a CAR T cell therapy-associated cardiomyopathy, mostly observed in patients with grade ≥ 2 CRS [59]. A study including 126 patients found that 10% of patients developed MACE after CAR T cell therapy including acute coronary syndrome, myocardial infarction, and new-onset heart failure [10]. In another study, most of the patients experienced new-onset arrhythmia within 30 days after therapy initiation, which was associated with CRS severity and occurrence [60]. MACE was seen in 16% of the patients.
CMR for Assessment of Cardiac Adverse Effects in CAR T Cell Therapy
The pathophysiology and impact of cardiotoxicity in CAR T cell therapy are still insufficiently understood. It is still vague whether cardiotoxicity is just a manifestation of cytokine storm within the scope of CRS, or whether there are more direct cardiotoxic side effects from the CAR T cells themselves. Another assumption is that the observed systolic dysfunction in this setting is comparable to stress-induced (Takotsubo) cardiomyopathy [61]. In this context, physiological stress reactions caused by CRS could trigger Takotsubo cardiomyopathy occurrence.
To date, CMR studies in patients with CAR T cell therapy are lacking. Since pathological CMR findings are known to correlate with troponin values [62], it is likely that CMR may reveal pathological myocardial findings during CRS, which is often accompanied by an increase of troponin [61, 63]. In this context, multiparametric CMR could be used to detect and quantify acute myocardial tissue alterations, such as myocardial edema and fibrosis [18,19,20, 64,65,66,67], as shown in Fig. 2.
CMR before and after CAR T cell therapy in two male patients (left: 68 years old, right: 69 years old) with follicular lymphoma, who underwent CMR before and within 1 week after CAR T cell administration (Tisagenlecleucel, Kymriah®). The first patient did not experience CRS and had normal cardiac biomarkers. Follow-up CMR showed unchanged myocardial T1 relaxation times and regress of bilateral axillary lymphoma manifestations (white arrow). The second patient developed grade 1 CRS and had increased cardiac biomarkers. Follow-up CMR showed signs of diffuse myocardial injury with increased T1 relaxation times and new bilateral pleural effusion
Discussion
The reviewed studies shared mostly subtle CMR abnormalities in ICI-associated cardiotoxicity. Further standardized studies with larger patient collectives are necessary for further characterization. Nevertheless, CMR is already of great importance since it can visualize even minor myocardial abnormalities and, despite higher costs and greater effort, shows a diagnostic superiority compared to echocardiography. In addition, several studies have mentioned the prognostic value of CMR regarding subsequent cardiac function reduction, MACE, and cardiovascular mortality [6, 21, 45, 47, 49, 51, 68].
CMR is an excellent diagnostic tool for the classification of cardiac inflammation or rather immunotherapy-associated myocarditis. But how can the diagnostic value of CMR be further refined to ensure the best possible patient care in the event of a suspected ICI myocarditis? Since abnormal T1 and T2 relaxation times have been described to be the leading CMR finding in the context of ICI myocarditis, they should be included in a standardized CMR protocol in accordance with the updated LLC [6, 20, 45, 46, 49, 50, 6, 11, 34,35,36,37].
Conclusions
CMR findings in ICI myocarditis tend to have a diffuse pattern and may be subtle. Possible findings include prolongation of T1 and T2 relaxation times, diffuse or local edema, fibrosis/necrosis, or LV dysfunction, each with varying degrees and distribution. In particular T1 and T2 mapping should be included in the CMR protocol as they are sensitive parameters for the detection of myocardial edema and inflammation. Diffuse myocardial findings can be further supported by additional reactive changes such as a pericardial effusion. Although individual cases of diffuse myocardial edema in context of CRS after CAR T cell therapy have already been observed, prospective CMR studies assessing myocardial abnormalities after CAR T cell therapy are still lacking and urgently needed.
Abbreviations
- CRS:
-
Cytokine release syndrome
- CAR:
-
Chimeric antigen receptor
- CMR:
-
Cardiovascular magnetic resonance
- irAE:
-
Immune-related adverse events
- ICI:
-
Immune checkpoint inhibitor(s)
- LGE:
-
Late gadolinium enhancement
- LLC:
-
Lake Louise Criteria
- LV:
-
Left ventricle/left ventricular
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiovascular events
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124. https://doi.org/10.1016/j.biopha.2020.109821.
Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20(1):e9. https://doi.org/10.4110/IN.2020.20.E9.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–56. https://doi.org/10.1056/NEJMOA1709684.
Patel RP, Parikh R, Gunturu KS, et al. Cardiotoxicity of immune checkpoint inhibitors. 2021;23:79. https://doi.org/10.1007/s11912-021-01070-6.
Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64. https://doi.org/10.1016/J.JACC.2018.02.037.
• Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41:1733–43. https://doi.org/10.1093/EURHEARTJ/EHAA051. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
Lyon AR, Yousaf N, Battisti NML, et al. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–58. https://doi.org/10.1016/S1470-2045(18)30457-1.
Lefebvre B, Kang Y, Smith AM, et al. Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncol. 2020;2:193–203. https://doi.org/10.1016/J.JACCAO.2020.04.012.
Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–108. https://doi.org/10.1016/J.JACC.2019.10.038.
Qi K, Yan Z, Cheng H, et al. An analysis of cardiac disorders associated with chimeric antigen receptor T cell therapy in 126 patients: a single-centre retrospective study. Front Oncol. 2021;11. https://doi.org/10.3389/fonc.2021.691064.
Palaskas N, Lopez-Mattei J, Durand JB, et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9. https://doi.org/10.1161/JAHA.119.013757/FORMAT/EPUB.
Suresh A, Martens P, Tang WHW. Biomarkers for myocarditis and inflammatory cardiomyopathy. Curr Heart Fail Rep. 2022;19:346–55. https://doi.org/10.1007/S11897-022-00569-8.
Matsuura H, Watanabe N, Shibata Y, Asada Y. Combination of echocardiography and emergency endomyocardial biopsy for suspected myocarditis in the cardiovascular emergency medical care. J Echocardiogr. 2021;19:86–94. https://doi.org/10.1007/S12574-021-00521-0.
Erley J, Beitzen-Heineke A, Tahir E. Cardiooncology-usefulness of cardiac MRI : Inflammation, fibrosis, outcome. Radiologie (Heidelberg, Germany). 2022;62:941–6. https://doi.org/10.1007/S00117-022-01055-X.
Luetkens JA, Petry P, Kuetting D, et al. Left and right ventricular strain in the course of acute myocarditis: a cardiovascular magnetic resonance study. RoFo Fortschr Gebiet Rontgenstrahlen Bildgebenden Verfahren. 2018;190:722–32. https://doi.org/10.1055/a-0585-0271.
Luetkens JA, Faron A, Isaak A, et al. Comparison of original and 2018 Lake Louise Criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging. 2019;1(3). https://doi.org/10.1148/ryct.2019190010.
Luetkens JA, Voigt M, Faron A, et al. Influence of hydration status on cardiovascular magnetic resonance myocardial T1 and T2 relaxation time assessment: an intraindividual study in healthy subjects. J Cardiovasc Magn Reson. 2020;22:1–9. https://doi.org/10.1186/S12968-020-00661-9/FIGURES/5.
Luetkens JA, Homsi R, Sprinkart AM, et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging. 2016;17:154–61. https://doi.org/10.1093/EHJCI/JEV246.
Luetkens JA, Homsi R, Dabir D, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc. 2016;5:e003603. https://doi.org/10.1161/JAHA.116.003603.
• Faron A, Isaak A, Mesropyan N, et al. Cardiac MRI depicts immune checkpoint inhibitor-induced myocarditis: a prospective study. Radiology. 2021;301:602–9. https://doi.org/10.1148/radiol.2021210814. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6:1080–91. https://doi.org/10.1161/CIRCIMAGING.113.000899.
Geller MA, Cooley S, Argenta PA, et al. Toll-like receptor-7 agonist administered subcutaneously in a prolonged dosing schedule in heavily pretreated recurrent breast, ovarian, and cervix cancers. Cancer Immunol Immunother. 2010;59:1877–84. https://doi.org/10.1007/s00262-010-0914-1.
Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016;11:123–40. https://doi.org/10.1007/S11739-015-1362-X/FIGURES/3.
Pondé NF, Lambertini M, De Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1(4):e000073. https://doi.org/10.1136/ESMOOPEN-2016-000073.
Nicolazzi MA, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. 2018;22(7):2175–2185. https://doi.org/10.26355/eurrev_201804_14752.
Hansel TT, Kropshofer H, Singer T, et al. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–38. https://doi.org/10.1038/nrd3003.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974. https://doi.org/10.1200/JCO.2014.59.4358.
Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. https://doi.org/10.1146/annurev-pathol-042020-042741.
Sharma P, Siddiqui BA, Anandhan S, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11:838–57. https://doi.org/10.1158/2159-8290.CD-20-1680.
Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2(4):e000247. https://doi.org/10.1136/ESMOOPEN-2017-000247.
Cau R, Solinas C, De Silva P, et al. Role of cardiac MRI in the diagnosis of immune checkpoint inhibitor-associated myocarditis. Int J Cancer. 2022;151(11):1860–73. https://doi.org/10.1002/ijc.34169.
Russo A, Maurea N, Farmakis D, Giordano A. Cardio-oncology: Management of Toxicities in the Era of Immunotherapy. Springer Nature. 2022.
Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8. https://doi.org/10.1001/JAMAONCOL.2018.3923/.
Moslehi J, Salem JE. Immune checkpoint inhibitor myocarditis treatment strategies and future directions. JACC CardioOncol. 2022;4:704–7. https://doi.org/10.1016/J.JACCAO.2022.11.005.
Zito C, Manganaro R, Ciappina G, et al. Cardiotoxicity induced by immune checkpoint inhibitors: what a cardio-oncology team should know and do. Cancers (Basel). 2022;2022:5403. https://doi.org/10.3390/cancers14215403.
Hu C, Zhao L, Zhou C, et al. Pacemakers and methylprednisolone pulse therapy in immune-related myocarditis concomitant with complete heart block. Open Med (Wars). 2022;17:2109–16. https://doi.org/10.1515/MED-2022-0611.
Li X, Peng W, Wu J, et al. Advances in immune checkpoint inhibitors induced-cardiotoxicity. Front Immunol. 2023;14:1130438. https://doi.org/10.3389/FIMMU.2023.1130438.
Khan NAJ, Pacioles T, Alsharedi M. Atypical Takotsubo cardiomyopathy secondary to combination of chemo-immunotherapy in a patient with non-small cell lung cancer. Cureus. 2020;12(7):e9429. https://doi.org/10.7759/CUREUS.9429.
Airò G, Maffezzoli M, Lazzarin A, et al. Takotsubo syndrome in a patient with metastatic renal cell carcinoma treated with pembrolizumab plus axitinib. Immunotherapy. 2022;14:1297–305. https://doi.org/10.2217/IMT-2022-0013.
Carbone A, Bottino R, Russo V, et al. Takotsubo cardiomyopathy as epiphenomenon of cardiotoxicity in patients with cancer: a meta-summary of case reports. J Cardiovasc Pharmacol. 2021;78:e20–9. https://doi.org/10.1097/FJC.0000000000001026.
Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1714–27. https://doi.org/10.1016/J.JACC.2019.07.079.
Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361. https://doi.org/10.1093/EURHEARTJ/EHAC244.
Wang G, Lee SE, Yang Q, et al. Multicenter study on the diagnostic performance of native-T1 cardiac magnetic resonance of chronic myocardial infarctions at 3T. Circ Cardiovasc Imaging. 2020;13:E009894. https://doi.org/10.1161/CIRCIMAGING.119.009894.
Arcari L, Tini G, Camastra G, et al. Cardiac magnetic resonance imaging in immune check-point inhibitor myocarditis: a systematic review. J Imaging. 2022;8(4):99. https://doi.org/10.3390/jimaging8040099.
• Thavendiranathan P, Zhang L, Zafar A, et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor–associated myocarditis. J Am Coll Cardiol. 2021;77:1503–16. https://doi.org/10.1016/j.jacc.2021.01.050. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
• Wintersperger BJ, Calvillo-Argü Elles O, Lheureux S, et al. Immune checkpoint inhibitor-related myocarditis: an illustrative case series of applying the updated Cardiovascular Magnetic Resonance Lake Louise Criteria. Eur Heart J Case Rep. 2022;6(1):ytab478. https://doi.org/10.1093/ehjcr/ytab478. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
• Zhao Sh, Yun H, Chen Cz, et al. The prognostic value of global myocardium strain by CMR-feature tracking in immune checkpoint inhibitor-associated myocarditis. Eur Radiol. 2022;32:7657–67. https://doi.org/10.1007/S00330-022-08844-X. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
• Liu J, Cao Y, Zhu K, et al. Early evaluation of subclinical cardiotoxicity in patients with lung cancer receiving immune checkpoint inhibitors by cardiovascular magnetic resonance: a prospective observational study. Quant Imaging Med Surg. 2022;12:4771–85. https://doi.org/10.21037/QIMS-22-41/COIF. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
• Cadour F, Cautela J, Rapacchi S, et al. Cardiac MRI features and prognostic value in immune checkpoint inhibitor–induced myocarditis. Radiology. 2022;303:512–21. https://doi.org/10.1148/radiol.211765. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
• Higgins AY, Arbune A, Soufer A, et al. Left ventricular myocardial strain and tissue characterization by cardiac magnetic resonance imaging in immune checkpoint inhibitor associated cardiotoxicity. PLoS One. 2021;16(2):e0246764. https://doi.org/10.1371/journal.pone.0246764. This scientific paper represents relevant literature from the last three years on the role of MRI in ICI myocarditis.
Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–7. https://doi.org/10.1161/CIRCULATIONAHA.117.030571.
Faubry C, Faure M, Toublanc AC, et al. A prospective study to detect immune checkpoint inhibitors associated with myocarditis among patients treated for lung cancer. Front Cardiovasc Med. 2022;9:878211. https://doi.org/10.3389/FCVM.2022.878211/FULL.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. https://doi.org/10.1038/S41408-021-00459-7.
Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38. https://doi.org/10.1016/J.BBMT.2018.12.758.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMOA1707447.
Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. https://doi.org/10.1056/NEJMOA1708566/SUPPL_FILE/NEJMOA1708566_DISCLOSURES.PDF.
Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2017;15(1):47–62. https://doi.org/10.1038/nrclinonc.2017.148.
Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863. https://doi.org/10.1182/BLOOD-2013-03-490565.
Ganatra S, Redd R, Hayek SS, et al. Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-Hodgkin lymphoma. Circulation. 2020;142:1687–90. https://doi.org/10.1161/CIRCULATIONAHA.120.048100.
Steiner RE, Banchs J, Koutroumpakis E, et al. Cardiovascular events in patients treated with chimeric antigen receptor T-cell therapy for aggressive B-cell lymphoma. Haematologica. 2022;107:1555–66.
Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. https://doi.org/10.1182/BLOOD-2014-05-552729.
Lydell CP, Vermes E, Childs HC, et al. Relationship of troponin T to cardiac MRI criteria for acute myocarditis. J Cardiovasc Magn Reson. 2011;13(1):1–1. https://doi.org/10.1186/1532-429X-13-S1-P271.
Cobb DA, Lee DW. Cytokine release syndrome biology and management. Cancer J. 2021;27:119–25. https://doi.org/10.1097/PPO.0000000000000515.
Luetkens JA, Isaak A, Öztürk C, et al. Cardiac MRI in suspected acute COVID-19 myocarditis. Radiol Cardiothorac Imaging. 2021;3(2):e200628. https://doi.org/10.1148/RYCT.2021200628.
Luetkens JA, Doerner J, Thomas DK, et al. Acute myocarditis: multiparametric cardiac MR imaging. Radiology. 2014;273:383–92. https://doi.org/10.1148/RADIOL.14132540.
Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging. 2016;9(3):e004091. https://doi.org/10.1161/CIRCIMAGING.115.004091.
Kravchenko D, Isaak A, Zimmer S, et al. Cardiac MRI in patients with prolonged cardiorespiratory symptoms after mild to moderate COVID-19. Radiology. 2021;301:E419–25. https://doi.org/10.1148/RADIOL.2021211162.
Liu Y, Wu W. Cardiovascular immune-related adverse events: evaluation, diagnosis and management. Asia Pac J Clin Oncol. 2020;16:232–40. https://doi.org/10.1111/AJCO.13326.
Stein-Merlob AF, Rothberg MV, Holman P, Yang EH. Immunotherapy-associated cardiotoxicity of immune checkpoint inhibitors and chimeric antigen receptor T cell therapy: diagnostic and management challenges and strategies. Curr Cardiol Rep. 2021;23:1–11. https://doi.org/10.1007/S11886-021-01440-3/TABLES/2.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marilia B. Voigt, Dmitrij Kravchenko, Alexander Isaak, Annkristin Heine, and Tobias A.W. Holderried declare that they have no conflict of interest. Julian A. Luetkens received payments for lectures from Philips Healthcare and for activities related to the scientific advisory board for BAYER Healthcare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voigt, M.B., Kravchenko, D., Isaak, A. et al. Cardiovascular Magnetic Resonance Assessment of Immunotherapy Cardiotoxicity. Curr Cardiovasc Imaging Rep 16, 103–115 (2023). https://doi.org/10.1007/s12410-023-09584-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-023-09584-2