Abstract
Patients with complex congenital heart disease require serial diagnostic evaluation throughout their lives. Although echocardiography and cardiac MRI are the primary modes of evaluation in the current era, cardiac computed tomography (CT) plays an increasingly important role for specific indications. The high temporal and spatial resolution of the most modern CT scanners used for cardiac imaging allows for rapid, high quality image acquisition. The decreased need for sedation and anesthesia combined with recent developments markedly reducing radiation exposure make it an ideal imaging modality for certain indications in congenital heart disease. This article will briefly review the current use of noninvasive diagnostics in congenital heart disease, focusing on the use of CT for the most common congenital heart lesions referred for surgical intervention. When used appropriately, CT angiography (CTA) can provide critical information necessary to care for patients with congenital heart disease who require evaluation in addition to echocardiography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart disease is the most common congenital anomaly. There have been tremendous advances in surgical and catheter-based intervention since the first ductal ligation in 1937, successful shunt for cyanotic heart disease in 1945, and intra-cardiac repair of an atrial septal defect in 1952. The surgical survival for all forms of congenital heart disease is approximately 95 % in the current era [1]. The majority of patients with even the most complex congenital heart disease now survive to adulthood, and the median age of this population is continually rising [2]. Most patients with congenital heart disease require serial evaluation of their cardiac status throughout their lives. The combination of a growing population of patients with palliated congenital heart disease and a current and expected future shortage of pediatric subspecialists requires that radiologists and adult cardiac imagers develop a proficiency in imaging and understanding of this patient population.
Use of Imaging Modalities in Congenital Heart Disease
When palliative surgical procedures became common in the 1950s and 1960s, cardiac catheterization was the only available diagnostic modality. Catheterization requires central vascular access and sedation or anesthesia for all cases and there is relatively high contrast loads and radiation exposure [3]. It is difficult to opacify different structures simultaneously and invasive angiograms provide 2-dimensional imaging planes, often requiring multiple different injections to properly outline the object of interest. Since the introduction of newer imaging modalities, catheterization is primarily used for intervention, or when pressure measurements are required for clinical decision-making.
Echocardiography has been in routine use since the 1980’s and remains the most commonly used imaging modality for congenital heart disease due to ease of use, wide availability, and excellent diagnostic accuracy for most indications when used by experienced providers [4]. In our practice, echocardiography accounts for 96 % of diagnostic studies. Limitations of echocardiography include degradation of acoustic windows with surgical scarring and obesity, poor vascular-airway definition, poor definition of extra cardiac structures, and poor visualization of distal pulmonary arterial and collateral vessel anatomy. In addition, there is poor reproducibility of right and single ventricular function, poor reproducibility of valvular regurgitation, and poor quantification of intracardiac shunting [5]. When imaging is required after echocardiography, a thorough evaluation of the risk vs benefit of each modality for the specific indication will determine the best test for the patient. For MRI and CT, the anesthesia risk of MRI for very young or critically ill patients and the radiation risk of CT are the most important considerations. In addition, the specific use of an advanced cardiac diagnostic test will depend on the availability of both the technology and the skilled personnel at each institution. As advanced CT technology becomes widely available, cardiac CT will play an increasingly important role in the diagnosis and management of congenital heart disease.
Cardiac MRI overcomes many of the limitations of echocardiography. Because of the lack of ionizing radiation, MRI is the modality first considered, and used most commonly, when imaging is required after echocardiography. It allows reproducible quantification of single and right ventricular systolic function, valvular regurgitant volumes and shunt fraction, and excellent definition of systemic and pulmonary venous and arterial anatomy [6]. Three-dimensional noncontrast sequences based on tissue differentiation as well as gadolinium based angiography provide 3D visualization of complex cardiac structures [7]. Limitations of cardiac MRI include incompatibility with pacemakers and defibrillators, artifact from certain types of metallic coils and implants, requirement for breath holding for most image sequences, and long imaging times that necessitate anesthesia for most patients less than 8 years of age. Most complete MRI scans require 45–70 minutes of scanning [8, 9]. For patients who are critically ill on ventilator support in the ICU, transition to MRI compatible anesthesia equipment is required. MRI with anesthesia has a relatively high relative risk for an adverse event [10, 11]. MRI is also technically challenging in the very smallest patients with the highest heart rates due to limitations in spatial resolution, temporal resolution, and poor signal to noise and contrast to noise ratios.
Cardiac CT has become increasingly used in the assessment of cardiac disease in since the introduction of multidetector CT technology. Recent technical advances in CT technology have resulted in submillimeter spatial resolution and temporal resolution as low as 75 msec. Image acquisition is as fast as a single heart beat for volumetric scanners, is approximately 0.25 seconds for high pitch scanners to cover the typical scan range of a pediatric thorax, or is a single breath hold for ECG gated functional imaging. These advances combined with radiation dose reduction techniques make cardiac CT an excellent modality for certain congenital cardiac indications [12, 13].
Cardiac CT is particularly useful for:
-
(1)
Determination of valvular regurgitation, shunt fraction, or estimation of ventricular function in patients with complex congenital heart disease who are contraindicated for Cardiac MRI, or who are unlikely to have a diagnostic MRI due to artifacts.
-
(2)
Evaluation of complex cardiac anatomy in very young patients who are high risk for adverse event with anesthesia and who could undergo a CT scan with no or limited sedation or anesthesia.
-
(3)
Critically ill patients who are less likely to tolerate the long imaging times required for cardiac MRI.
-
(4)
Coronary artery imaging for evaluation of anomaly, Kawasaki aneurysm, or after surgical reimplantation, or surgical manipulation (s/p Ross procedure, Nikaidoh procedure, arterial switch operation).
-
(5)
Concurrent evaluation of cardiac and extra cardiac structures including airway anatomy and lung parenchyma, or evaluation of sternal anatomy prior to repeat surgical intervention.
-
(6)
Highly complex congenital heart disease where a high resolution 3D or 4D dataset aids in surgical planning, particularly when there is abnormal cardiac situs combined with pulmonary venous anomalies, systemic venous anomalies, and abnormal relationship of the atrioventricular valves to the outflow tracts.
-
(7)
Evaluation of prosthetic valve function or of perivalvular leak.
Figure 1 shows our current institutional use of advanced diagnostics in our congenital heart disease population. Our MRI volume is 2–3 fold higher than our CT volume. Despite the considerable growth of our use of cardiac CT, it accounts for approximately 1 % of our diagnostic studies in this patient population.
Risks of Cardiac CT in the Recent Era
The risk of advanced diagnostics in patients with congenital heart disease is inversely related to their hemodynamic stability, size and age of the patient, and the complexity of their heart disease. The smallest patients with the most complex disease, particularly those who are critically ill, are at highest risk for adverse event with any imaging study.
Radiation Exposure
Radiation exposure is one of the major concerns regarding CT scanning and cardiac catheterization in children. Children are more sensitive to radiation exposure and also have a longer life span over which any adverse effect may manifest. Newer generation CT scanners significantly reduce radiation dose compared with previous generation scanners, and there are many studies that estimate doses less than 1 mSv for many CHD applications even when calculated using a 16 cm phantom size and using age adjusted chest conversion factors [14, 15]. *CT has been shown to deliver 10–15 fold less radiation than cardiac catheterization using current technology [16•]. High doses are still delivered in very large patients or in patients with fast or highly irregular heart rhythms who need an ECG gated scan [17, 18].
Measurement of Radiationon Dose in Pediatric CT
Most adult practitioners calculate estimated radiation dose from a cardiac CT scan by multiplying the scan DLP by a standard chest conversion factor. Calculating age and size specific radiation dose for CT scanning in pediatric patients is more complicated, and multiple methods are proposed [19, 20]. The most common is converting a 32 cm phantom to a 16 cm phantom DLP estimate, and further adjusting this by an age adjusted chest conversion factor to determine dose in millisievert (mSv) [21]. The phantom and age conversions combined give a dose estimate that is approximately 7 times higher than for the same scan output delivered to a larger and older patient [22]. The various methods used to determine radiation dose from CT scanning complicate scanner, sequence, and inter-modality comparisons. In addition, these age and size adjustments are typically not performed for other imaging modalities that use radiation such as cardiac catheterization, fluoroscopy for chest Xray. To compare scan modes and sequences, comparison of DLP for similar size phantoms may be the most reproducible until consensus on this topic is achieved.
The following are general guidelines for reducing scanner output and radiation dose in pediatric cardiac scans. Furthermore, we use aggressive beta blockage if coronary artery imaging is needed to allow use of the highest pitch scan sequence with minimal diastolic padding.
-
(1)
Use the minimal scanner output (kv and mAs) required for a diagnostic scan. We use 80 kv to 60 kg for 0.6 mm collimator, and to 80 kg if using a 1.2 collimator. 70 kv voltage tubes are also now available on some scanner platforms and could further reduce dose for the smallest patients.
-
(2)
Use weight based mAs, with additional online tube current modulation based on localizer images. Additionally, reduce mA 20 %–30 % prospectively when iterative reconstruction is available.
-
(3)
Use the highest pitch scan mode that is diagnostic to minimize both image acquisition time and radiation dose.
-
(4)
For ECG gated scans, use prospective ECG triggering whenever possible, and use minimal padding (systolic and diastolic or systolic intervals only for high heart rates if coronary artery detail is needed), diastolic interval only for heart rate < 60 bpm or at higher heart rates when coronary detail is not required.
-
(5)
Use 1.2 collimator with prospective 20 %–30 % decrease in mA if fine detail is not needed.
-
(6)
Limit the scan range to area of interest.
-
(7)
Use a long contrast injection with image acquisition at the end of the injection to acquire venous and arterial anatomy in the same scan when both are needed (we do second scan on less than 10 % of patients).
Anesthesia Considerations
*Young patients with congenital heart disease in anesthesia class 3 or above are at highest risk for an adverse event with anesthesia [23, 24•]. In a study of 12,000 pediatric anesthetics, the risk of cardiac arrest was higher outside the operating room, and highest in those with single ventricle heart disease. Cardiac CT scans can now be performed without sedation, or with minimal sedation for most indications. Studies have shown very little motion artifact using these techniques [25]. For patients who do require anesthesia for cooperation with a breath hold in the length of anesthesia for MDCT will be much shorter than with MRI or catheterization due to the rapid data acquisition of current generation MDCT scanners. For these indications, a single scan sequence obtained over 1 breath hold will acquire the entire 3 or 4D dataset.
*There is an evolving body of evidence that anesthesia exposure in young patients may adversely affect long term cognitive and behavioral outcomes, particularly those exposed to multiple anesthetics or exposed younger than 2 years of age [26, 27, 28•, 29–33].
IV and Contrast Exposure
Most pediatric cardiac CT scans are performed using a power injector through a peripheral IV or a central line in postoperative patients. The use of power injections in pediatrics is well described and safe [34]. The rate of contrast reaction in the pediatric age range is low and increases with age [35, 36].
Use of CT and Examples for Common Indications
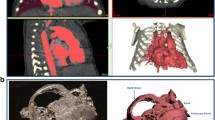
Cardiac CT has been well described to define congenital heart disease. CT is particularly useful for anomalous pulmonary veins, vascular rings, aortic arch pathology, and pulmonary arterial or lung parenchymal pathology [37–41]. Figure 2A shows the images from a patient with an absent left pulmonary artery who also had left lung agenesis. The scan was performed free breathing without sedation, scan DLP 5. Figure 2B is a posterior view of a patient with an AV canal defect and recurrent left lung collapse. The picture shows the vascular airway interaction with compression of the left bronchus between the enlarged pulmonary artery and the aorta. A LeCompte maneuver was performed at the time of AV canal repair.
a, Enlarged right lung (arrow) with left lung and left pulmonary artery agenesis in a 2-day old. The heart is shifted leftward. The scan was performed free breathing without sedation, scan DLP 4. b, This posterior view shows left bronchial compression between an enlarged left pulmonary artery and the aorta. The patient has an AV canal defect with high output congestive heart failure
Planning for Bypass Cannulation and Sternal Entry or Closure
Sternal re-entry can be aided by CT angiography. It readily shows the sternum and the relationship to underlying cardiac and vascular structures. Figure 3A and B show a large pseudoaneurysm from the aortic root in a patient who developed endocarditis after truncal valve repair and VSD closure. Defining the location of the pseudoaneurysm allowed safe sternal re-entry. The central veins were additionally found to be occluded, which changed the plan for venous cannulation. For patients with an open sternum following surgical repair, CT can identify residual lesions that may be addressed at that time of sternal closure. For these critically ill patients in particular the fast acquisition time can limit possible anesthesia adverse events and time away from the intensive care unit. Figure 4 shows images obtained in a 3-day-old with an open sternum and mediastinal chest tubes after repair of total anomalous pulmonary venous return, with a patent vertical vein to the superior vena cava. The time from the first localizer to the helical scan was 3 minutes, helical scan image acquisition was 0.25 seconds, and scan DLP was 9. There was no change in the ICU ventilator management or equipment in preparation for, or during acquisition of the scan. The vertical vein was ligated at the time of delayed sternal closure.
a, The asterisk shows a large truncal valve pseudoaneurysm and the relationship to the sternum (short arrow). A right sided chest tube is seen (long arrow), as are an occluded right subclavian vein, proximal superior vena cava (fat arrow), and innominate vein. The left jugular vein drains via collaterals to the inferior vena cava. b, This sagittal view shows a large pseudoaneurysm (asterisk) from the left ventricular outflow tract and proximal truncal root in a patient with a history of truncus arteriosus after VSD closure and truncal valve repair. The truncal cusp is seen (arrow) with aneurysm extending from both above and below the valve leaflet
a, Two-day old has an open sternum with a mediastinal chest tube after repair of total anomalous pulmonary venous return. Sick patients can be imaged with CTA even prior to sternal closure. b, This coronal view shows a patent vertical vein (asterisk) to the superior vena cava. The vertical vein was ligated at the time of delayed sternal closure
Single Ventricle Heart Disease
Patients with single ventricle heart disease require advanced imaging for interstage evaluation because echocardiography provides for a complete vascular evaluation in only a minority of patients [42]. The traditional modality used for interstage evaluation is cardiac catheterization, which is no longer necessary in low risk patients given the high complication rate without improvement in surgical outcome compared with noninvasive evaluation [43]. MRI has been proposed for interstage evaluation prior to the cavopulmonary anastomosis (Glenn) but has not been widely adopted [44]. Metallic implants decrease the diagnostic quality of MRI for these patients additionally [45]. Cardiac CTA is an alternate modality for assessment of anatomy prior to Glenn cavopulmonary anastomosis with excellent visualization of aortopulmonary shunt or Sano shunt anatomy, pulmonary anatomy, and aortic arch anatomy. High pitch CTA can be obtained free breathing and with minimal or no sedation in this age range. Evaluation of the postoperative Glenn cavopulmonary anastomosis is reliably seen by CTA. Visualization of venous collaterals causing cyanosis after the Glenn procedure is also seen. Some now propose Fontan completion with noninvasive interstage evaluation [46]. Fontan opacification by CT imaging is frequently difficult to achieve and the venous filling is likely dependent on cardiac output. We use either a 2 phase injection with late image acquisition or a delayed scan for this indication to increase the chance of opacifying the inferior vena cava and Fontan pathway. CT imaging has also been proposed as a method of detecting pulmonary embolism and plastic bronchitis in single ventricle patients [47–49]. Figure 5 A‒H show examples of interstage single ventricle palliation through the third stage Fontan.
a, This is an axial view of the distal Sano conduit (asterisk) to the branch pulmonary arteries in a patient with palliated hypoplastic left heart syndrome after a stage 1 procedure. b, This figure shows the length of a modified Blalock Taussig shunt from the innominate artery to the mid-right pulmonary artery in a patient after stage 1 single ventricle palliation. c, This axial view shows the small mitral valve (arrow) and left ventricle in a patient with hypoplastic left heart syndrome. The atrial septum is patent in the area of prior septecomy. d, This posterior view shows bilateral super vena cava (asterisk), bilateral modified Blalock taussig shunts (arrows) to the branch pulmonary arteries, ipsilateral pulmonary venous drainage (short arrows), and a reconstructed aorta prior to the Glenn procedure. e, This shows the superior vena cava to right pulmonary artery anastamosis in a patient after second stage single ventricle palliation with a cavopulmonary anastamosis (Glenn). There is a narrowing at the site of anastamosis (arrow). f, A right arm injection (coronal MIP) is performed to evaluate the cause of cyanosis in a patient after the Glenn procedure. The right internal jugular vein and right subclavian vein are occluded with decompression through the azygous vein (asterisk) to the inferior vena cava. g, This axial 2D image shows an extracardiac Fontan conduit (asterisk) in a patient with hypopastic syndrome (mitral and aortic atresia). The patient also has ventricular dysfunction and elevated pulmonary artery pressures. Opacification of the extracardiac conduit can be difficult given that it carries unopacified IVC blood following an arm injection. h, This patient with tricuspid valve atresia (double asterisk) has an interatrial Fontan (asterisk). Note the epicardial pacer lead (long arrow) and the patent atrial communication (short arrow)
Tetralogy of Fallot
Advanced imaging plays a crucial role in the long-term management of patients with Tetralogy of Fallot. This includes defining optimal timing for pulmonary valve replacement for patients with significant pulmonary insufficiency, and the assessment the branch pulmonary arteries and collateral anatomy for all stages of repair in those with pulmonary atresia [50]. Most all centers index right ventricular end diastolic and end systolic volumes to body surface area along with assessment of ejection fraction to determine the need for repeat intervention on the right ventricular outflow tract in the presence of pulmonary insufficiency [50, 51]. An ECG gated CT scan can be used to obtain this same functional information for patients with repair of Tetralogy of Fallot who are contraindicated for MRI or who have imaging artifacts [52]. CT evaluation of tetralogy of Fallot has been shown to be highly specific for native pulmonary artery anatomy, aorto-pulmonary collateral anatomy, postoperative shunt anatomy and coronary artery assessment [53–61]. A 2006 study directly compared cardiac catheterization and CT angiography in a small number of patients with pulmonary artery atresia and found complete agreement between modalities for definition of pulmonary artery and aorto-pulmonary collateral anatomy [62]. One hundred patients with Tetralogy of Fallot were evaluated by CT preoperatively and there was 100 % sensitivity and specificity for coronary artery anomalies compared with surgical findings in patients with a median age of 6.8 months [53]. In patients who have had placement of a RVOT conduit, transcatheter valve replacement is now an option when repeat intervention is needed. Defining the dimensions of the proximal and distal conduit, the branch pulmonary arteries, anomalous muscle bundles in the right ventricular outflow tract that could affect catheter course and complete definition of the coronary arteries is required prior to catheterization or surgical intervention. Figure 6A shows an enlarged right ventricle in a pacemaker dependent patient with Tetralogy of Fallot. Figure 6B shows aorto-pulmonary collateral arteries in a neonate with pulmonary atresia and the relationship of the collateral vessels to the bronchus that helps guide future surgical dissection.
a, This adult patient has a transvenous pacer (arrow) after repair of Tetralogy of Fallot. Note the right atrial and right ventricular enlargement, even after replacement of the pulmonary valve for severe pulmonary insufficiency. b, This 3D coronal VRT shows the collateral vessels (asterisk) arising from the descending aorta in a patient with Tetralogy of Fallot, pulmonary atresia with aortopulmonary collateral vessels
Transposition Complexes
Atrial Switch
Patients with transposition of the great arteries primarily underwent an atrial switch operation until the technical details of the coronary transfer allowed for survival after the arterial switch operation in 1980s. The systemic venous baffles, pulmonary venous baffles, and biventricular systolic function are well seen by CT imaging when using a biventricular injection protocol. Valvular regurgitant fraction may be calculated by assessing stroke volume differences. Figure 7 shows transposition of the great arteries with an anomalous and interarterial left main coronary artery in a patient who presented with a ventricular fibrillation arrest 15 years after an atrial baffle procedure.
Arterial Switch/Rastelli Procedure/Nikaidoh Procedure
Since the 1980s, the arterial switch operation is the procedure of choice for patients with transposition of the great arteries. The neo-pulmonary root, neo-aortic root, and coronary arteries all subsequently need long-term follow-up with serial evaluation. The mid-term results for the arterial switch are good, but a certain percentage of patients will develop coronary artery problems, particularly if there was a high risk coronary artery pattern such as a commissural malalignment complicating coronary transfer, surgical concern regarding the coronary anastamosis, or a difficult postoperative course [63]. Additionally supravalvular pulmonary stenosis is relatively common at the site of the neo-pulmonary anastamosis (Fig. 8). It is recommended that all patients with reimplanted coronary arteries undergo angiography either prior to adulthood or if symptomatic [1]. In patients with transposition of the great vessels with pulmonary stenosis, the pulmonary conduit placed during the Rastelli operation also requires repeat evaluation to determine the timing of eventual replacement. The Nikaidoh procedure is a newer surgical approach for d-TGA with pulmonary stenosis, consisting of aortic translocation with reconstruction of the right ventricular outflow tract. Figure 9 shows right coronary artery stenosis in a 3 year old following the Nikaidoh procedure, confirmed at the time of therapeutic coronary angioplasty.
This 3D VRT shows supravalvular pulmonary stenosis (asterisk) in a patient after the arterial switch with LeCompte maneuver for d-TGA. Note the position of the main and branch pulmonary arteries anterior to the aorta, and a patent right coronary artery (arrow) after reimplantation at the time of arterial switch
Double Switch
In patients with physiologically corrected transposition (also called L-TGA, (S,L,L) (I,D,D), a concomitant atrial baffle and arterial switch are needed to restore normal cardiac physiology. Pre-operative evaluation for this lesion includes visualization of the coronary arteries, great artery relationships, and the relationship of the VSD to the right ventricular outflow tract for visualization of baffles. Some patients have a pulmonary artery band placed to increase right ventricular afterload prior to a double switch to increase left ventricular afterload. An ECG gated functional scan allows visualization of the interventricular septal configuration and also quantification of left ventricular myocardial mass. Figure 10 shows a narrowed pulmonary venous baffle to the anterior left ventricle in a patient after the double switch procedure.
Prosthetic Valves
Many pediatric patients develop a progressive gradient through a mechanical valve. This may be a result of pannus formation with limitation of valve leaflet mobility, patient size mismatch with growth, or thrombus.
For bileaflet mechanical valves, the end systolic and end diastolic angles can be determined from an ECG gated scan. In patients requiring valve replacement, definition of coronary artery relationship to the AV valve can be determined. Figure 11A shows a prosthetic mitral valve with progressive left ventricular outflow tract gradient thought secondary to the valve position after catheterization. CT showed tissue extending from the valve onto the aortic valve that could be removed lowering the LVOT gradient postoperatively, without replacing the mitral valve. Figure 11B shows a diastolic image of an obstructed prosthetic tricuspid valve that partially responded to thrombolytic therapy.
Perivalvular leaks can also be visualized and regurgitant volume quantified. Figure 12 shows the location of a large mitral perivalvular leak in a patient with severe mitral regurgitation and left ventricular enlargement following valve replacement.
Coronary Artery Abnormalities
Coronary artery imaging is recommended in pediatric patients with symptomatic anomalous coronary arteries with reimplanted coronary arteries, and prior to right ventricular outflow tract intervention [1, 64–66]. Coronary CTA is considered superior to conventional angiography in delineating the ostial origin and proximal path of an anomalous coronary artery. Catheter manipulation may mechanically alter the coronary ostium and the intrinsic 3D noninvasive nature of CT allows simultaneous visualization of the coronary artery, aorta and pulmonary artery [67, 68]. CT evaluation of coronary artery course is possible even in young patients [69]. Recently, the proximal course and length of the intramural segment of an anomalous right coronary artery from the left facing sinus may help risk stratify these lesions [70, 71]. In a study directly comparing cardiac CT angiography and catheterization in patients after the arterial switch operation, there was complete agreement regarding the detection of a significant coronary stenosis [72].
Conclusions
The combination of improved temporal and spatial resolution, rapid image acquisition, and radiation dose reduction techniques makes CT an ideal imaging modality for certain indications in patients with congenital heart disease. It is critical to pay careful attention to all of the details of scan acquisition and radiation dose reduction techniques in order to maintain diagnostic image quality while minimizing risk. As this patient population grows and modern CT technology becomes more accessible, it will play an increasingly important role in the diagnosis and management of congenital heart lesions.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118:714–833.
Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115.
Verghese GR, McElhinney DB, Strauss KJ, Bergersen L. Characterization of radiation exposure and effect of a radiation monitoring policy in a large volume pediatric cardiac catheterization lab. Catheter Cardiovasc Interv. 2012;79:294–301.
Tworetzky W, McElhinney DB, Brook MM, Reddy VM, Hanley FL, Silverman NH. Echocardiographic diagnosis alone for the complete repair of major congenital heart defects. J Am Coll Cardiol. 1999;33:228–33.
Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, et al. Pediatric Heart Network Investigators: comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. 2009;104:419–28.
Rajiah P, Kanne JP. Cardiac MRI: part 1, cardiovascular shunts. Am J Roentgenol. 2011;197:W603–20.
Greil GF, Powell AJ, Gildein HP, Geva T. Gadolinium-enhanced 3-dimensional magnetic resonance angiography of pulmonary and systemic venous anomalies. J Am Coll Cardiol. 2002;39:335–41.
Brown DW, Gauvreau K, Powell AJ, Lang P, Colan SD, Del Nido PJ, et al. Cardiac magnetic resonance vs routine cardiac catheterization before bidirectional glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation. 2007;116:2718–25.
Tsai-Goodman B, Geva T, Odegard KC, Sena LM, Powell AJ. Clinical role, accuracy, and technical aspects of cardiovascular magnetic resonance imaging in infants. Am J Cardiol. 2004;94:69–74.
Girshin M, Shapiro V, Rhee A, Ginsberg S, Inchiosa MA Jr. Increased risk of general anesthesia for high-risk patients undergoing magnetic resonance imaging. J Comput Assist Tomogr. 2009;33:312–5.
Dorfman AL, Odegard KC, Powell AJ, Laussen PC, Geva T. Risk factors for adverse events during cardiovascular magnetic resonance in congenital heart disease. J Cardiovasc Magn Reson. 2007;9:793–8.
Hughes Jr D, Siegel MJ. Computed tomography of adult congenital heart disease. Radiol Clin North Am. 2010;48:817–35.
Lee EY, Siegel MJ. MDCT of tracheobronchial narrowing in pediatric patients. J Thorac Imaging. 2007;22:300–9.
Paul JF, Rohnean A, Elfassy E, Sigal-Cinqualbre A. Radiation dose for thoracic and coronary step-and-shoot CT using a 128-slice dual-source machine in infants and small children with congenital heart disease. Pediatr Radiol. 2011;41:244–9.
Han BK, Lindberg J, Grant K, Schwartz RS, Lesser JR. Accuracy and safety of high pitch computed tomography imaging in young children with complex congenital heart disease. Am J Cardiol. 2011;107:1541–6.
• Watson TG, Mah E, Joseph Schoepf U, King L, Huda W, Hlavacek AM. Effective radiation dose in computed tomographic angiography of the chest and diagnostic cardiac catheterization in pediatric patients. Pediatr Cardiol. 2013;34:518–24.
Weustink AC, Neefjes LA, Kyrzopoulos S, van Straten M, Neoh Eu R, Meijboom WB, et al. Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology. 2009;253:672–80.
Hoffmann A, Engelfriet P, Mulder B. Radiation exposure during follow-up of adults with congenital heart disease. Int J Cardiol. 2007;118:151–3.
Huda W, Ogden KM. Computing effective doses to pediatric patients undergoing body CT examinations. Pediatr Radiol. 2008;38:415–23.
Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008;248:995–1003.
Siegel MJ, Schmidt B, Bradley D, Suess C, Hildebolt C. Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology. 2004;233:515–22.
Khursheed A, Hillier MC, Shrimpton PC, Wall BF. Influence of patient age on normalized effective doses calculated for CT examinations. Br J Radiol. 2002;75:819–30.
Odegard KC, DiNardo JA, Kussman BD, Shukla A, Harrington J, Casta A, et al. The frequency of anesthesia-related cardiac arrests in patients with congenital heart disease undergoing cardiac surgery. Anesth Analg. 2007;105:335–43.
• Ramamoorthy C, Haberkern CM, Bhananker SM, Domino KB, Posner KL, Campos JS, et al. Anesthesia-related cardiac arrest in children with heart disease: data from the Pediatric Perioperative Cardiac Arrest (POCA) registry. Anesth Analg. 2010;110:1376–82.
Lell MM, May M, Deak P, Alibek S, Kuefner M, Kuettner A, et al. High-pitch spiral computed tomography: effect on image quality and radiation dose in pediatric chest computed tomography. Invest Radiol. 2011;46:116–23.
Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:1053–61.
Hansen TG, Flick R. Anesthetic effects on the developing brain: insights from epidemiology. Anesthesiology. 2009;110:1–3.
• Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364:1387–90.
DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91.
Cauldwell C. Anesthesia risks associated with pediatric imaging. Pediatr Radiol. 2011;41:949–50.
Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg. 2010;110:442–8.
Hays SR, Deshpande JK. Newly postulated neurodevelopmental risks of pediatric anesthesia. Curr Neurol Neurosci Rep. 2011;11:205–10.
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804.
Amaral JG, Traubici J, BenDavid G, Reintamm G, Daneman A. Safety of power injector use in children as measured by incidence of extravasation. Am J Roentgenol. 2006;187:580–3.
Dillman JR, Strouse PJ, Ellis JH, Cohan RH, Jan SC. Incidence and severity of acute allergic-like reactions to i.v. nonionic iodinated contrast material in children. Am J Roentgenol. 2007;188:1643–7.
Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: incidence in large urban children's hospital–retrospective analysis of data in 12,494 patients. Radiology. 2009;250:674–81.
Siegel MJ. Multiplanar and 3-dimensional multi-detector row CT of thoracic vessels and airways in the pediatric population. Radiology. 2003;229:641–50.
Siegel MJ, Bhalla S, Gutierrez FR, Billadello JB. MDCT of postoperative anatomy and complications in adults with cyanotic heart disease. Am J Roentgenol. 2005;184:241–7.
Siegel MJ. Cardiac CTA: congenital heart disease. Pediatr Radiol. 2008;38 Suppl 2:S200–4.
Dillman JR, Hernandez RJ. Role of CT in the evaluation of congenital cardiovascular disease in children. Am J Roentgenol. 2009;192:1219–31.
Hlavacek AM. Imaging of congenital cardiovascular disease: the case for computed tomography. J Thorac Imaging. 2010;25:247–55.
Stern KW, McElhinney DB, Gauvreau K, Geva T, Brown DW. Echocardiographic evaluation before bidirectional Glenn operation in functional single-ventricle heart disease: comparison to catheter angiography. Circ Cardiovasc Imaging. 2011;4:498–505.
Brown DW, Gauvreau K, Moran AM, Jenkins KJ, Perry SB, del Nido PJ, et al. Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2003;126:272–81.
Brown DW, Gauvreau K, Powell AJ, Lang P, Colan SD, Del Nido PJ, et al. Cardiac magnetic resonance vs routine cardiac catheterization before bidirectional glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation. 2007;116:2718–25.
Garg R, Powell AJ, Sena L, Marshall AC, Geva T. Effects of metallic implants on magnetic resonance imaging evaluation of Fontan palliation. Am J Cardiol. 2005;95:688–91.
Fogel MA, Pawlowski TW, Whitehead KK, Harris MA, Keller MS, Glatz AC, et al. Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to fontan: a comparison of 3 groups: pre-fontan CMR vs cath evaluation. J Am Coll Cardiol. 2012;60:1094–102.
Goo HW, Jhang WK, Kim YH, Ko JK, Park IS, Park JJ, et al. CT findings of plastic bronchitis in children after a fontan operation. Pediatr Radiol. 2008;38:989–93.
Grewal J, Al Hussein M, Feldstein J, Kiess M, Ellis J, Human D, et al. Evaluation of silent thrombus after the fontan operation. Congenit Heart Dis. 2013;8:40–7.
Varma C, Warr MR, Hendler AL, Paul NS, Webb GD, Therrien J. Prevalence of "silent" pulmonary emboli in adults after the Fontan operation. J Am Coll Cardiol. 2003;41:2252–8.
Geva T. Repaired Tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9.
Ammash NM, Dearani JA, Burkhart HM, Connolly HM. Pulmonary regurgitation after tetralogy of Fallot repair: clinical features, sequelae, and timing of pulmonary valve replacement. Congenit Heart Dis. 2007;2:386–403.
Raman SV, Cook SC, McCarthy B, Ferketich AK. Usefulness of multidetector row computed tomography to quantify right ventricular size and function in adults with either Tetralogy of Fallot or transposition of the great arteries. Am J Cardiol. 2005;95:683–6.
Vastel-Amzallag C, Le Bret E, Paul JF, Lambert V, Rohnean A, El Fassy E, et al. Diagnostic accuracy of dual-source multislice computed tomographic analysis for the preoperative detection of coronary artery anomalies in 100 patients with Tetralogy of Fallot. J Thorac Cardiovasc Surg. 2011;142:120–6.
Lin MT, Wang JK, Chen YS, Lee WJ, Chiu HH, Chen CA, et al. Detection of pulmonary arterial morphology in Tetralogy of Fallot with pulmonary atresia by computed tomography: 12 years of experience. Eur J Pediatr. 2012;171:579–86.
Westra SJ, Hill JA, Alejos JC, Galindo A, Boechat MI, Laks H. Three-dimensional helical CT of pulmonary arteries in infants and children with congenital heart disease. Am J Roentgenol. 1999;173:109–15.
Westra SJ, Hurteau J, Galindo A, McNitt-Gray MF, Boechat MI, Laks H. Cardiac electron-beam CT in children undergoing surgical repair for pulmonary atresia. Radiology. 1999;213:502–12.
Rajeshkannan R, Moorthy S, Sreekumar KP, Ramachandran PV, Kumar RK, Remadevi KS. Role of 64-MDCT in evaluation of pulmonary atresia with ventricular septal defect. Am J Roentgenol. 2010;194:110–8.
Maeda E, Akahane M, Kato N, Hayashi N, Koga H, Yamada H, et al. Assessment of major aortopulmonary collateral arteries with multidetector-row computed tomography. Radiat Med. 2006;24:378–83.
Hayabuchi Y, Inoue M, Watanabe N, Sakata M, Nabo MM, Kitagawa T, et al. Assessment of systemic-pulmonary collateral arteries in children with cyanotic congenital heart disease using multidetector-row computed tomography: comparison with conventional angiography. Int J Cardiol. 2010;138:266–71.
Hayabuchi Y, Inoue M, Kagami S. Rare venous connection causing severe hypoxia after Fontan operation. Interact Cardiovasc Thorac Surg. 2008;7:718–9.
Wang XM, Wu LB, Sun C, Liu C, Chao BT, Han B, et al. Clinical application of 64-slice spiral CT in the diagnosis of the Tetralogy of Fallot. Eur J Radiol. 2007;64:296–301.
Greil GF, Schoebinger M, Kuettner A, Schaefer JF, Dammann F, Claussen CD, et al. Imaging of aortopulmonary collateral arteries with high-resolution multidetector CT. Pediatr Radiol. 2006;36:502–9.
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35.
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association: Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young. Am Heart Assoc Pediatrics. 2004;114:1708–33.
Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology., the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010;4:407.
Legendre A, Losay J, Touchot-Koné A, Serraf A, Belli E, Piot JD, et al. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003;108 Suppl 1:186–90.
Kim SY, Seo JB, Do KH, Heo JN, Lee JS, Song JW, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. Radiographics. 2006;26:317–33. discussion 33–4.
Karaca M, Kirilmaz A, Oncel G, Oncel D, Yilmaz H, Tamci B, et al. Contrast-enhanced 64-slice computed tomography in detection and evaluation of anomalous coronary arteries. Tohoku J Exp Med. 2007;213:249–59.
Han BK, Lindberg J, Overman D, Schwartz RS, Grant K, Lesser JR. Safety and accuracy of dual-source coronary computed tomography angiography in the pediatric population. J Cardiovasc Comput Tomogr. 2012;6:252–9.
Lee HJ, Hong YJ, Kim HY, Lee J, Hur J, Choi BW, et al. Anomalous origin of the right coronary artery from the left coronary sinus with an interarterial course: subtypes and clinical importance. Radiology. 2012;262:101–8.
Kaushal S, Backer CL, Popescu AR, Walker BL, Russell HM, Koenig PR, et al. Intramural coronary length correlates with symptoms in patients with anomalous aortic origin of the coronary artery. Ann Thorac Surg. 2011;92:986–91. discussion 91–2.
Ou P, Celermajer DS, Marini D, Agnoletti G, Vouhé P, Brunelle F, et al. Safety and accuracy of 64-slice computed tomography coronary angiography in children after the arterial switch operation for transposition of the great arteries. JACC Cardiovasc Imaging. 2008;1:331–9.
Acknowledgments
We would like to acknowledge David, Allison, and Lynn for their excellent CT technical support, and Drs Dasseko, McCormick and their anesthesia colleagues for their excellent anesthesia support. Both are critical for safe and successful imaging of pediatric cardiac patients.
Conflict of Interest
B. Kelly Han and John R Lesser receive honoraria and grant support from Siemens Medical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, B.K., Lesser, J.R. Cardiac CT in the Diagnosis and Postoperative Assessment of Congenital Heart Disease. Curr Cardiovasc Imaging Rep 6, 240–250 (2013). https://doi.org/10.1007/s12410-013-9195-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-013-9195-3