Abstract
Arsenic (As) is a toxic metalloid that can enter the food chain through uptake by plants from soils followed by production of plant-based food. While soil–plant transfer of As in crops, especially rice, is relatively well studied, the role of soil microbes in As translocation in maize is not well understood. We performed a greenhouse pot experiment with maize plants grown at different soil As levels to study the role of soil microbes on uptake of different As species by maize. Three soil treatments with varying disturbance of the soil microbes (native soil, sterilized soil, and sterilized soil reconditioned with soil indigenous microbes) were intersected with three levels of As in soils (0, 100 and 200 mg kg−1 spiked As, aged for 8 weeks) in a greenhouse experiment, where maize was grown for 5 months. Compared to uncontaminated soils, maize in high-As soils tended to accumulate more As in stems and less in leaves and grains, proportionally. Arsenic levels in stems were increased in sterilized soils due to the disturbance of the microbiome. The sterilization effects caused a phosphorus and manganese deficiency, leading to a higher As uptake in plants, that increased with rising As levels and resulted in a lower total dry biomass of the plants. In summary, this study highlights the role of soil indigenous microbes in limiting the uptake and translocation of inorganic As into maize. Compared to rice, cultivating maize plants in high-As soils is recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The consumption of crops grown on arsenic (As)-contaminated soils raises serious concerns for human and animal health (Punshon et al. 2017; Ruiz-Chancho et al. 2008). The transfer of As from soils to plants is not only an agronomic problem, as it negatively affects plant development and leads to yield losses (Srivastava 2020), but more a human health problem as consumption of As-containing staple foods will increase human As uptake with consequences to human health (Khan et al. 2022). Maize (Zea mays L.) is the most widely grown cereal in the world with an annual production of more than one billion tons. Maize is an important animal feed and a staple food for many people in South America, Africa, and Asia (Rosas-Castor et al. 2014a). In the maize-producing countries, As in some soils has significantly exceeded the global average background (10 mg kg−1) and the maximum allowable limit for agricultural soils (20 mg kg−1) recommended by the US Environmental Protection Agency (Kabir et al. 2016; Rosas-Castor et al. 2014a). The main pathway of As exposure in humans (> 90%) is the dietary consumption of contaminated foodstuffs and drinking water, making As poisoning a global health issue that affects tens of millions of people worldwide (Anjum et al. 2017). Studying As in agricultural crops is thus crucial to assess the risks to humans.
Understanding As speciation is essential because it determines the bioavailability, mobility, and toxicity of As and thus the health consequences for soils, plants, and humans (Rosas-Castor et al. 2014a). Arsenic occurs in a variety of different inorganic and organic species. Arsenate (AsV) and arsenite (AsIII) are the two most predominant inorganic As species (inAs) in soil and aquatic environments (Bowell 1994; Moreno-Jiménez et al. 2012; Sadee et al. 2023). They can be methylated by soil microbes to organic As species (orgAs), e.g., methylarsonic acid (MMAV), dimethylarsinic acid (DMAV), and trimethylarsine oxide (TMAO). These non-thiolated and pentavalent orgAs are about 10 to 60 times less toxic than in As (Sadee et al. 2023; Thirunavukkarasu et al. 2002). Redox transformation and cycling among different As species characterize its toxicity (Borch et al. 2010; Wagner et al. 2020). In general, the toxicity of As compounds decreases as follows: inAs > DMAV, MMAV > TMAO (Khairul et al. 2017). InAs species are the most abundant species in maize roots, stems, and leaves (Ruiz-Chancho et al. 2008; Yu et al. 2009), whereas orgAs represent only small amounts (< 1%) (Rosas-Castor et al. 2014a; Yu et al. 2009). However, in one study growing maize hydroponically, orgAs was the major As species identified in grains, accounting for 61% of the total As concentration (totAs) (Ci et al. 2012).
Since arsenate and phosphate are chemical analogues, AsV enters plants through phosphate channels in their roots. If phosphorus (P) is increased in system, plant uptake of AsV would be decreased due to their competitive absorption (Wu et al. 2022). Plants have evolved to produce both antioxidant enzymes (e.g., catalase and peroxidase) and non-enzymatic antioxidants (e.g., glutathione (GSH) and proline) (Sharma and Dietz 2009), which act as the first defense line against the radicals generated during As oxidative stress (Guevara-García et al. 2017; Mittler 2002). Irrespective of whether the plants are exposed to AsV or AsIII, AsIII predominates in the plant, showing an efficient reduction of AsV in the plant cells (Mishra et al. 2017). AsIII has a high affinity for the sulfhydryl groups (-SH) of thiol-rich peptides such as phytochelatins (PCs) and GSH and can form metal(loid)-GSH and metal(loid)-PCs complexes, which are sequestered in vacuoles to protect cellular components against As exposure (Garbinski et al. 2019; Schmöger et al. 2000). Because of their storage in vacuoles, these complexes in plant roots reduce As mobility for efflux and long-distance transport (Liu et al. 2010). Therefore, the translocation efficiency of inAs can be affected by the presence of chelating agents such as GSH and PCs in plant roots and stems (Rosas-Castor et al. 2014a; Srivastava et al. 2016; Yadav 2010).
Metal partitioning in the soil–plant system is usually assessed by different factors such as the bioconcentration factor (BCF) and the bioaccumulation coefficient (BAC). In the context of this study, BCF represents the As concentration in roots relative to that in soils. The BAC describes the ratio of As concentration in the aerial parts of plants to that in soils. The translocation factor (TF) describes As translocation in plants, i.e., As concentration in the aerial tissues compared to that in the roots. Tolerant plant species tend to restrict As transfers from soils to root and from root to stem, resulting in significantly less As accumulation in plants (Finnegan and Chen 2012), whereas hyperaccumulators actively take up and translocate metals into the aerial tissues (Yoon et al. 2006). Maize is generally classified as a tolerant plant to heavy metals (BAC < 1) and is an As excluder with a low capacity to translocate metal(loid)s (TF < 1) (Abbas and Abdelhafez 2013; Armienta et al. 2020; Fellet et al. 2007; Rosas-Castor et al. 2014a). However, As can also accumulate in maize roots once taken up (CI et al. 2012; Yang et al. 2021; Zhao et al. 2018). Similar As concentrations are usually observed in maize stems and leaves at around 0.2—5.7% of the As concentration in soils, while in a few cases higher As concentrations are detected in the stems (up to 23% of soil As concentration). The translocation of As to grains is usually low and As concentrations in maize grains range from 0.04 to 5% of the As levels in soils (Cao et al. 2019; Neidhardt et al. 2012; Rosas-Castor et al. 2014a).
Soil sterilization can affect plant growth for both biotic and abiotic reasons in the presence of heavy metals in the soil. The primary consequence of soil sterilization is the elimination of soil indigenous microbes and a reduction of the number of viable microbial cells by two to three orders of magnitude (Blankinship et al. 2014; Li et al. 2023). Nonetheless, after soil sterilization, microbes can rapidly recolonize and build a new microbial community with a lower diversity, which takes up the released nutrients and organic acids (Huang et al. 2012; Mahmood et al. 2014; Marschner and Rumberger 2004; Yan et al. 2020). Soil microbes can help to mitigate the toxic effects of As in soils (Afroz et al. 2019; Cavalca et al. 2019; Hasanuzzaman op. 2018; Li et al. 2009; Li et al. 2018; Pandey et al. 2018; Turpeinen et al. 2002) by influencing the reduction, oxidation, and methylation reactions that leads to the formation of different As species (Jia et al. 2013). Soil microbes can potentially promote As removal from contaminated soils through bioleaching as they possess respiratory arsenate-reductase that reduces AsV to more mobile AsIII (Roychowdhury et al. 2018).
Soil sterilization can have abiotic effects such as modifying the soil physicochemical properties by altering soil structures, e.g., by reducing the proportion of fine and medium sand in soils (Li et al. 2023). Soil sterilization also accelerates the decomposition of soil organic matter and releases cellular compounds of soil microbes, which increases the organic carbon content and promotes plant growth (Berns et al. 2008; Boyd 1971; Dietrich et al. 2020; Schaller et al. 2011). It can decrease soil pH due to the release of organic acids (Guan et al. 2023; Razavi and Lakzian 2007; Skipper and Westermann 1973) and cause nutrient releases such as P (Hu et al. 2020; Strawn 2018). Soil sterilization can also mobilize As by altering its sorption behavior on soils, as phosphate competition on sorption sites increases (Dao et al. 1982; Razavi and Lakzian 2007).
To date, the translocation of As speciation from soils is less well studied in maize plants than in other crops such as rice. We also know little about the effects of soil indigenous microbes and their interaction with plants on the uptake, translocation, and speciation of As in plants. To disentangle the biotic and abiotic effects of soil sterilization, an additional treatment was performed that we reconditioned soils with indigenous microbes after sterilization, so that the role of soil indigenous microbes in As translocation and speciation in plants can be better elucidated. In this experiment, three soil treatments with varying disturbance of the soil microbes (native soil, soil sterilized before the experiment, and sterilized soil reconditioned with soil indigenous microbes) were intersected with three levels of As in soils (0, 100, and 200 mg kg−1 spiked As, aged for 8 weeks). The abiotic sterilization effect, including pH decrease and nutrient increase, etc., was the overall effect of abiotic factors on As, exclusive of the biotic effects of the soil microbes.
In our previous study (Guan et al. 2023), the same experiment was reported with a focus on the interaction effects of soil treatment (sterilization ± microbial conditioning), maize plants, and As treatment on the concentration and speciation of As in the soil water. Both the abiotic sterilization effect and the microbial disturbance effects were found to increase As release into soil water (Guan et al. 2023). The microbial disturbance effect was more pronounced for orgAs, showing the influence of soil microbes involved in As (de-)methylation. The abiotic sterilization effect was more evident in unplanted pots and the microbial disturbance effect was observed only in unplanted pots, suggesting that both effects were mitigated by the presence of maize. Since As uptake in crops is of high relevance for human As exposure, the interactions between soil treatment and As treatment are further investigated on the bioavailability and speciation of As in maize plants. In current study, we aim to further investigate to answer the following research questions: (1) What are the differences in the translocation and accumulation of As species in maize tissues at different soil As concentrations (As treatment)? (2) How does the microbial disturbance effect and (3) the abiotic sterilization effect influence the translocation and accumulation of As species in maize tissues?
Materials and Methods
Greenhouse Pot Experiment
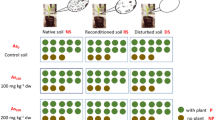
The soil for the experiment (silty loam, pH = 7.0 ± 0.1, organic carbon content = 21.9 ± 0.1 g kg−1; Table S1) was taken from the uppermost 20 cm of an agricultural site in Frauenkappelen, Switzerland, by a soil recycling company (Kästli Bau AG). The soil pile was then stored outside the greenhouse (Ostermundigen, Switzerland) of the Institute of Plant Sciences at the University of Bern. For this greenhouse pot experiment, approximately 800 kg of soil was sampled from different spots of the pile to ensure homogeneity and sieved to 1 cm. This experiment comprised nine experimental groups: three soil treatments (native soil (NS), reconditioned soil (RS) and disturbed soil (DS)) × three As treatments (As0, As100 and As200, addition of 0, 100 and 200 mg As kg−1 soil), with ten replicates in each group (Fig. 1). The soils in the As0 group were without As addition and had a natural As concentration of 2.91 ± 0.54 mg kg−1 and are considered uncontaminated soils. For As100 and As200 groups, around 400 kg of soils were evenly spiked with the solution of sodium arsenate salt (Na2HAsO4·7H2O, ≥ 98.0%; Sigma-Aldrich®, CH) to enrich an additional 100 and 200 mg kg−1 As in soils. After the arsenate solution was applied to the soil 4 times in a stepwise process, all soils were thoroughly turned over with a shovel to ensure thorough mixing (more details in SI). The success of the homogenization of the treatments was confirmed by a standard deviation between 3.5–5 mg kg−1 between the pots in the respective treatments (0–200 mg As kg−1) (Guan et al. 2023). The soils were incubated for two months at room temperature and 50% of the water holding capacity (WHC), allowing for As equilibration between soil water and soil phases (Song et al. 2006).
The soils in the three As treatments were then subdivided into three subgroups for the three soil treatments (NS, RS, and DS). The first subgroup was kept untreated and named as native soil (NS). The second and third subgroups were sterilized by X-ray (25 kGy minimum to 60 kGy maximum at Synergy Health Däniken AG, CH). After soil sterilization, the second subgroup was reconditioned with microbial extracts from NS and designated as reconditioned soil (RS). The third subgroup was sterilized soil without microbial reconditioning, referred to as disturbed soils (DS). Due to the presence of microbes in the greenhouse and potential recolonization, DS was not assumed to be free of microbes but to have a disturbed microbial composition. The microbial extracts for the RS treatment were obtained by entirely mixing 70 kg of native soils with 70 L of Milli-Q water (> 18.2 MΩ∙cm at 25 °C) in a pre-sterilized concrete mixer (pre-sterilized with ethanol and a gas burner; Figure S1). The extracts were left to stand for 2 h to let large soil particles settle and then filtered through a 250 μm stainless sieve and 25 μm filter papers (Whatman®, CH). Lastly, 800 mL of the soil microbial extracts was added sequentially to RS (Hu et al. 2018). The microbial extracts still contained nematodes, arbuscular mycorrhizal spores, and suspended microbes after the filtration (Hu et al. 2018). The soils with microbial extracts were left to stand for 1 h. The detailed characterizations of soil parameters can be found in Table S1.
The sterilization effect was the same in DS and RS, while the microbial disturbance was partly eliminated in the RS treatment due to the reconditioning with microbial extracts. Therefore, it is assumed that the difference between RS and DS showed the microbial disturbance effect, and the difference between NS and RS reflected the abiotic sterilization effect. All soils were adequately homogenized, and each pot (7 L) was filled with 6.5 kg of soils to reach the same height to ensure a uniform bulk density of soils. In the end, 90 pots with maize plants were cultivated from April to September 2019 in a greenhouse, allowing for microbes to recolonize sterilized and reconditioned soils. This led to heavily disturbed soil microbes in the sterilized soil (DS) and to less disturbed soil microbes in the reconditioned soil (RS). More information on the sampling and analysis of soil water can be found in our previous study (Guan et al. 2023).
Maize Cultivation
Maize seeds (Zea mays L., W22 genotype) were soaked for 6 min in a commercial bleach containing 5% active hypochlorite (Potz Javel-Wasser Natur, Migros, CH) followed by 6 washes and 8h soak in autoclaved Milli-Q water (> 18.2 MΩ∙cm at 25 °C). One week after soil sterilization, maize seeds were placed overnight in plastic Petri plates (Petri dish 94 × 16 mm, without vents, sterile, Greiner Bio-One, CH) before sowing with moist filter papers (Rundfilter Sorte 1 Whatman®, 90 mm, Huberlab, CH). Each pot was initially sown with three pre-sterilized maize kernels and only the best performing seedling per pot was kept for further growth. To minimize the difference in growth conditions among treatments, all pots were randomly placed in the greenhouse. In the beginning, maize was watered weekly by weighing pots and adjusting the WHC to 60%. From the third month of growth, maize was watered more frequently. The weekly fertilization of maize plants started with 100 mL of 2 g L−1 complex fertilizer (Plantaktiv Starter 151, Hauert®) supplemented with a 0.25 g of low iron supplement (Sequestrene Rapid, Maag®), increasing to 200 mL complex fertilizer with a 0.5 g of high iron supplement after one month. The complex fertilizer mainly contained 52% phosphate (P2O5), 10% total nitrogen (8.4% NH3-N and 1.4% NO3-N), and 10% potassium oxide (K2O). Maize plants were cultivated in the greenhouse with 14 h of light each day and the temperature of 18—26 °C during the day and 16—24 °C at night. The greenhouse cabin was heated in case of temperatures below 18 °C during the day and below 16 °C at night. The cooling system automatically turned on if the temperature exceeded 26 °C. The ventilation system turned on once the temperature was over 22 °C in the daytime or over 20 °C at night. The humidity ranged from 30 to 60%. After a half-year of growth, maize plants were harvested individually as roots, stems, leaves, and cobs. The root samples were carefully dug out from the soils and washed with Milli-Q water. Grains were peeled from cobs and all maize materials were oven-dried at 70 °C and then weighted for their dry biomass. Afterward, the maize tissues were ground to powder in the Retsch MM400 Mixer Mill (Fisherbrand™, Waltham, MA).
Additionally, a side experiment was conducted to estimate the fresh biomass of maize during growth while maintaining the same WHC in soils by controlling the weight of all pots. In this experiment, 60 maize plants were grown for five months and three of them were harvested weekly to determine their fresh biomass. Maize images were simultaneously recorded to derive the green pixels area of their leaves. Therefore, a linear model could be built between the calculated biomass and the leaf area to estimate the maize’s actual fresh biomass (Figure S2) (Neumann et al. 2015; Valasek and Thomasson 2016). The estimated fresh biomass was then applied to calculate the amount of irrigation water and correct pot weight to retain 50% of WHC.
Analysis of As Concentration and Speciation
0.25 g of milled maize tissue powder (roots, stems, leaves and grains) was mixed with 4 mL of 65% (w/w) nitric acid (HNO3; LC–MS grade, VWR®, FR) and 2 mL of 30% (w/w) peroxide (Suprapur H2O2; Sigma-Aldrich®, CH), left for at least 30 min at room temperature before conducting an open-vessel microwave digestion (95 °C for 30 min; Microwave Digestion System MARS™ 6; CEM GmbH, Kamp-Lintfort, DE) (Norton et al. 2013). After digestion, the solutions were diluted to 50 mL with Milli-Q water and stored at 4 °C and centrifuged at 2500 rpm for 5 min (Multifuge™ X1 Centrifuge Series, Thermo Scientific™, Reinach, CH) before transferring into 15 mL centrifuge tubes for the multielement analysis by inductively coupled plasma mass spectrometer (ICP-MS; 7700 × Agilent Technologies, Santa Clara, CA). The multielement analysis included As (noted totAs in the manuscript) and P, B, Al, V, Cr, Mn, Co, Ni, Cu, Zn, Ga, Se, Rb, Ag, Cd, Cs, Ba, Ti, Pb, U as well as Rh and In used as online Internal Standards. Triplicates of certified reference material (CRM) and blank samples were digested and measured together with the maize samples. The CRM ERM®- CD281 Rye grass and the Standard Reference Material® 1573a Tomato leaves were analyzed (certified As concentrations of 0.042 ± 0.01 mg kg−1 and 0.112 ± 0.004 mg kg−1, respectively). The As recovery was 82 ± 13% (n = 20) for rye grass and 124 ± 15% for tomato leaves (n = 23) (Table S2).
For As speciation analysis, 0.2 g of ground maize tissues was mixed with 4.8 mL of 1% (w/w) nitric acid (HNO3; LC–MS grade, VWR®, FR) and 0.2 mL of 30% (w/w) Suprapur H2O2 (Sigma-Aldrich®, CH), left for at least 30 min at room temperature before conducting the open-vessel microwave digestion as described above (Norton et al. 2013). After extraction, samples were centrifuged at 2500 rpm for 5 min, filtered with a 0.22 μm hydrophilic polytetrafluoroethylene filter (13 mm syringe filter, BGB®, CH), diluted if needed, and stored at 4 °C (less than one week) before the analysis with high-performance liquid chromatography (HPLC; 1260 Infinity, Agilent Technologies, Santa Clara, CA) coupled to ICP-MS (HPLC-ICP-MS). The separation of As species was achieved using a Hamilton PRP-X100 anion-exchange column (4.1 × 50 mm, 5 μm). The column recovery was 96 ± 17% (n = 153). The operating parameters for HPLC were adopted from the literature (Jackson 2015) and listed in Table S3. Due to the addition of H2O2 and HNO3, all trivalent or potential thiolated As species were oxidized and the determined As species were all pentavalent. They included AsV as inAs as well as MMAV, DMAV, and TMAO as orgAs. The identification of TMAO was confirmed by cation-exchange HPLC-ICP-MS (Figure S3). Triplicates of CRMs and blank were extracted together with the maize samples. The CRM ERM®-BC211 rice was utilized, and the recoveries were 101 ± 17% for inAs (n = 26) and 108 ± 9% for DMAV (n = 26) (Table S2).
Statistical Analysis
In this study, all data were processed on a dry weight basis and their statistical analysis was performed with the R software (version 1.2.5033) including the following packages: car, multcomp, emmeans, and vegan. The concentrations of totAs and total dry biomass (Table S4) were Log10-transformed and square-root transformed, respectively, to improve normality when using linear mixed models. Experimental factors, namely soil treatment (three levels: NS, DS, and RS), As treatment (three levels: As0, As100, and As200), tissue types (four levels: roots, stems, leaves, and grains), and their cross-factor interactions, were analyzed for their significance on the concentration of totAs and individual As species in maize (Table S5). For multiple As species (multiple dependent variables), multivariate analysis of variance (MANOVA) was applied to the comparison of multivariate sample means in maize tissues, studying the interaction effects and individual effects of the four experimental factors on individual As species in maize (Table S6).
Moreover, the estimated marginal means (in the emmeans package), which were derived to make model predictions, were calculated during the post hoc analysis. These predictions are typically averaged with equal weights across one or more predictors. Such marginally averaged predictions are helpful in describing the results of fitting a model, particularly when presenting factor effects. The original emmeans data are listed as supplementary documents (Tables S4, S8 and S10-S13). The compact letter display (CLD; in the multcomp package) was used to visually report the significance of pairwise comparisons. Groups with the same CLD letters do not differ significantly, whereas groups that significantly differ have different CLD letters. The conventional (Pearson) correlation was applied to determine the correlations between the individual As species (inAs, MMAV, DMAV, and TMAO) in soil water and in maize tissues.
Results
Arsenic Translocation and Accumulation in Maize
We first analyzed total As (totAs; Table S4) in maize tissues to understand the distribution and translocation of As in maize. In most cases, we found a significant interaction among the three experimental factors (soil treatment, As treatment, and tissue types, Table S5). The interactions suggested that the effect of soil treatment on totAs concentration depended on the levels of As treatment and tissue types and vice versa. The concentration of totAs in the entire maize was significantly increased by soil treatment (F2,66 = 12.157, p < 0.001) and the As treatment (F2,66 = 226.431, p < 0.001) (Fig. 2 and Table S5). The concentration of totAs in maize tissues was significantly affected by the interactions among soil treatment, As treatment and tissue types (F7,195 = 2.727, p = 0.010; Table S5). Significant interactions were also found between soil treatment and tissue types (F7,195 = 3.545, p = 0.002) and between As treatment and tissue types (F5,195 = 20.974, p < 0.001). The concentrations of totAs were higher in some maize tissues grown in RS than in NS, i.e., in stems of the As0 and As100 groups and in leaves of the As200 group (Fig. 3). This showed the abiotic sterilization effect (difference NS-RS). In contaminated soils (As100 and As200 groups), totAs concentration in the plants decreased in the order roots > stems ≥ leaves > grains, while in uncontaminated soils (As0 group), totAs had a higher concentration in the leaves than in the stems in all three soil treatments (Fig. 3).
Total As concentrations (totAs) in maize tissues. Data were emmeans ± standard error. Pairwise comparisons were explored and reported using CLD letters. Different letters in each figure indicated a statistically significant difference between emmeans (p < 0.05). Missing data in grains were due to the inability of maize to bear grains under high As stress and the absence of grains in cobs
The relative translocation of As in leaves and grains, i.e., the As concentration in leaves and grains relative to that in soils, was highest in the As0 group, showing as the values of BAC and BCF (Table S7). The BAC values decreased as follows: leaves > stems ≥ grains in the As0 group, stems ≥ leaves > grains in the As100 group, and stems > leaves ≈ grains in the As200 group (“ > ” indicates significance at α = 0.05). The higher the As levels in soils, the relatively less As was translocated into maize leaves. BCFroot was one order of magnitude greater than BACstem. Arsenic relative translocation in the stems was considerably higher in the As200 group than in the As0 and As100 groups, and the highest As accumulation in the stems reached 31% of the As levels in roots (TF = 0.31 in DS of the As200 group). BACleaf was in the same range as BACstem, while BACgrain was one order of magnitude lower. Upon comparison of the three soil treatments, the relative As translocation to leaves and stems was always lower in NS (lower BAC values) than in RS and DS, regardless of the As levels in soils.
Arsenic Speciation in Maize Tissues
The inAs and three orgAs (MMAV, DMAV and TMAO) were detected in all four maize tissues (roots, stems, leaves, and grains; Figs. 4–6). These three organic species were significantly affected by soil treatment, As treatment, and tissue types, as well as their interactions (p < 0.001; Table S5). The microbial disturbance effect (difference RS-DS) increased inAs concentrations in the maize stems grown in DS compared to RS in the As200 group (Fig. 4). Due to the abiotic sterilization effect (difference NS-RS), higher inAs levels were detected in the tissues of maize grown in RS than in NS: in the stems of the As200 group, in the leaves of the As0 and As100 groups, and in the grains of the As0 group (Fig. 4). Overall, inAs concentrations in different maize tissues followed the same orders as the totAs concentrations shown above.
The concentrations of inorganic As species (inAs) in maize tissues. Data were emmeans ± standard error. Pairwise comparisons were explored and reported using CLD letters. Different letters indicated a statistically significant difference between emmeans (p < 0.05). Missing data in grains were due to the inability of maize to bear grains under high As stress and the absence of grains in cobs
OrgAs was the sum concentrations of MMAV, DMAV, and TMAO (Table S10). The orgAs levels in maize tissues were significantly affected by soil treatment (F2,195 = 36.833, p < 0.001). The microbial disturbance effect increased orgAs levels in the stems of maize grown in DS compared to in RS at 200 mg kg−1 (Fig. 5a). The abiotic sterilization effect enhanced orgAs levels in the maize grown in RS, observed in the stems and leaves of the As200 group (Fig. 5a). Among the three uncontaminated soils (As0 group), orgAs levels in maize tissues showed no significant difference. In contaminated soils (As100 and As200 groups), orgAs levels in maize tissues, except grains, increased with increasing soil microbial disturbance (NS < RS ≤ DS; Fig. 5a). This trend was more pronounced at higher soil As concentrations, except for grain As, where no difference existed. Moreover, orgAs concentration in maize tissues was affected by tissue types (F3,195 = 310.927, p < 0.001), decreasing from roots ≥ leaves > stems > grains. For the individual orgAs, the microbial disturbance effect was only identified for MMAV in the stems of the As200 group with higher concentrations in DS than in RS (Figure S4). The abiotic sterilization effect caused higher concentrations in RS than in NS for all three orgAs in the leaves of the As200 group and for TMAO in the stems of the As200 group.
The a sum concentrations of organic As species (orgAs) and b orgAs% in maize tissues. Data were emmeans ± standard error. Pairwise comparisons were explored and reported using CLD letters. Different letters in each figure indicated a statistically significant difference between emmeans (p < 0.05). Missing data in grains were due to the inability of maize to bear grains under high As stress and the absence of grains in cobs
As soil microbial disturbance increased, orgAs% in maize roots increased (Figs. 6a, b or Figs. 6c, d; Table S10). The orgAs% was exceedingly low in maize roots in the As200 group (Fig. 6c). However, in uncontaminated soils, orgAs% in maize leaves decreased with increasing soil microbial disturbance (Figs. 6e, f). Furthermore, in both roots and leaves, orgAs% decreased sharply because of the increasing soil inAs levels. The orgAs% showed an ascending trend of roots < stems < leaves < grains (Fig. 5b). We discovered the highest orgAs% in maize grains with 4.3 ± 0.9% (Table S13). In maize roots, DMAV % were generally higher than that of MMAV and TMAO. Conversely, TMAO was the primary As species in maize stems and leaves (Fig. 6), although its content was the lowest in grains.
The changes in As species in maize tissues with the increasing soil As levels (from the As0 group to the As200 group) and the increasing soil microbial disturbance (from NS to DS), presenting the percentages of inorganic As species (inAs%) and organic As species (orgAs%, i.e., MMAV, DMAV, and TMAO) in a roots in NS of the As0 group; b roots in DS of the As0 group; c roots in NS of the As200 group; d roots in DS of the As200 group; e leaves in NS of the As0 group; f leaves in DS of the As0 group; g leaves in NS of the As200 group; and h leaves in DS of the As200 group
The link Between As in Soil Water and Maize
The concentrations of totAs in soil water can be found in our previous paper (Guan et al. 2023). TotAs in soil water was strongly positively correlated with totAs concentrations in roots (r = 0.94, p < 0.001), stems (r = 0.97, p < 0.001), and leaves (r = 0.91, p < 0.001), but not in grains (r = 0.48, p > 0.05). There were also strong positive correlations between inAs in soil water and inAs in roots (r = 0.94, p < 0.001), leaves (r = 0.93, p < 0.001), and stems (r = 0.82, p < 0.001), but not in grains (r = 0.59, p > 0.05; Figure S5). TMAO concentration in soil water was positively correlated with that in the leaves (r = 0.56, p = 0.0007; Figure S6), which was not the case for MMAV and DMAV.
Dry Biomass and Its Correlation with As Species in Maize
The dry biomass in both the entire maize and maize tissues was significantly influenced by soil treatment, As treatment, and their interaction effects (p < 0.001; Table S5). The microbial disturbance effect (difference RS-DS) was not observed, while the abiotic sterilization effect (difference NS-RS) significantly reduced the total dry biomass of maize in the As100 and As200 groups (Fig. 7a and Table S8). Maize in uncontaminated soils could buffer the abiotic sterilization effect and showed no changes in the total dry biomass between unsterilized and sterilized soils. At a soil As level of 100 mg kg−1, the total dry biomass of maize grown in NS was at the same level as those in uncontaminated soils. Specifically, the dry biomass of maize grown in RS was, however, reduced by the abiotic sterilization effect in stems, leaves, and cobs of the As100 group and only in stems of the As200 group (Fig. 7b). In comparison with the other tissues, stem biomass was the most reduced by As. The stem biomass of maize in RS and DS decreased sharply due to the abiotic sterilization effect, which was more evident at elevated As levels in soils.
The dry biomass a in the entire maize and b in maize tissues under the soil treatment and As treatment. Pairwise comparisons were explored and reported using CLD letters. Different letters indicated a statistically significant difference between emmeans (p < 0.05). Missing data in grains were due to the inability of maize to bear grains under high As stress and the absence of grains in cobs
Phosphorus Deficiency in Reconditioned and Disturbed Soils
Only the DS and RS were P deficient, with values below the minimum level of P in the dry stem required for adequate plant growth (Kirkby 2012) (Fig. 8). Their P concentrations in the stems were significantly lower than those of NS maize. In all three soil treatments, manganese (Mn) levels in maize stems were below the concentrations required for their adequate growth (50 mg kg−1) (Kirkby 2012). The deficiency in Mn was also found to be greater in DS and RS maize than in NS maize, but only in the highest As group (200 mg kg−1) (Figure S7).
Discussion
Differential As Translocation and Accumulation in Maize at Different Soil As Concentrations
In the present study, As was accumulated mostly in the maize roots. The BCF of the root was an order of magnitude higher than the BAC of the stem, demonstrating significant As uptake by the root. A prior study also found that the BCF of root (2.0) was four orders of magnitude higher than the TF of the stem (0.005) (Gulz et al. 2005). In the As200 group, maize stems had higher BAC and TF values than leaves and grains, showing that the translocation of inAs to leaves and grains was restricted, probably due to an increase in chelating agents and antioxidants in maize roots and stems (Arora and Jha 2019; Yadav 2010). Arsenic translocation to the stem was greater in this work compared to previous studies, which reported lower BAC and TF values in the stem at comparable soil As concentrations (Drličková et al. 2013; Gulz et al. 2005). In the study of (Gulz et al. 2005), although the available As in soil water (4.70 mg kg−1) was higher than in our study (2.16 mg kg−1) as well as the BCF of root (2.0) was higher than ours (0.3, As200 group), the TF of stem (0.005) was significantly lower than ours (0.191, As200 group). Accordingly, our maize plants translocated As more efficiently from roots to stems, accounting for the greatest decrease in stem dry biomass than the other tissues.
Compared to maize plants in the As100 group, As translocation from leaves into grains tended to be lower in the As200 group, resulting in less relative As accumulation in grains. These findings agree well with the literature (Batista et al. 2014; Mallick et al. 2011; Rosas-Castor et al. 2014a). Maize plants can accumulate high levels of As in their roots and have a very slow acropetal transport of As from their roots to stems (Gulz et al. 2005; Requejo and Tena 2006). Maize produces peptides in roots that combine with inAs to sequester As and reduce As mobility (Liu et al. 2010), which is considered as a tolerance mechanism for plants in As-stressed environments (Caporale et al. 2013). The translocation efficiency of As can be lowered at high concentration of soluble As in soils (Rosas-Castor et al. 2014a). As the concentration of soluble As in soils rises, the translocation efficiency of As from maize roots to leaves decreases, as evidenced by their declining translocation values (Mallick et al. 2011). In soils with high As levels, maize plants probably produce higher amounts of chelating agents in roots and stems to more efficiently prevent As translocation and the resulting toxic effects (Batista et al. 2014; Mallick et al. 2011; Rosas-Castor et al. 2014a).
When comparing between our As100 and As200 plants, totAs in their leaves did not significantly increase with increasing As levels in soils. OrgAs in grains also did not change between As0 and As100 plants, while orgAs% in grains even decreased in the NS group. The total dry biomass of the maize plants in the As200 group decreased drastically, especially in the DS and RS plants, which died before the harvest time and stopped growing when they were small and could rarely bear grains. The As100 plants, on the other hand, were still able to grow and bear grains. This indicates that the tolerance mechanism of maize plants worked well at as levels up to 100 mg kg−1 in soils, but at As levels of 200 mg kg−1 or more, this exceeds their defense capability.
Lower orgAs% was found in our maize grown in the contaminated soils compared to uncontaminated soils. Since higher plants lack the ability to methylate As (Jia et al. 2013; Zheng et al. 2013), orgAs in plants originate from As methylation by soil microbes and following uptake by plants from soil water (Lomax et al. 2012). The reduction in orgAs% in our maize on contaminated soils is due to the high input of inAs into the soils upon As addition into the soils and thus to a dilution effect. When comparing between unsterilized and sterilized soils, orgAs% increased in the roots grown in sterilized soils. Our earlier study demonstrated that both microbial and abiotic sterilization effects mobilized orgAs into the soil water, which is then taken up by plants (Guan et al. 2023). On the one hand, the abiotic immobilization of As by sorption to soils is reversible, and the remobilization of adsorbed orgAs may occur if soil physicochemical conditions are changed by sterilization (Wang and Mulligan 2006); on the other hand, the microorganisms killed by sterilization might release orgAs to the soil solution. Soil sterilization may have furthermore inhibited enzymatic and microbial activities that are important for the immobilization and demethylation of orgAs in soils, thereby increasing the orgAs levels in soil water (Guan et al. 2023). These findings agree well with the findings from Kumpiene et al. (2007) who reported that As mobility is negatively correlated with microbial activity. Thus, the increased the orgAs concentration in the soil solution might have caused increased orgAs in plants, because of increased orgAs availability after sterilization.
For maize leaves, however, the situation was different depending on whether the soils were contaminated. In uncontaminated soils, orgAs% in maize leaves was lower in sterilized soils than in unsterilized soils, while it was higher when the sterilized soils were contaminated. This was probably because inAs translocation into the leaves of maize grown in contaminated soils was hindered by the As tolerance mechanisms mentioned above. When contaminated soils were sterilized, a higher percentage of orgAs was mobilized into soil water and taken up by maize plants, being efficiently translocated into maize leaves. On the other hand, soil microbes can also demethylate As (Lehr et al. 2003; Yoshinaga et al. 2011) and the As-demethylating microbes might have been eliminated by soil sterilization, which prevented the conversion of orgAs into inAs, resulting in higher levels of orgAs in the sterilized soil water.
In the current study, orgAs showed the same distribution among maize tissues in both uncontaminated and contaminated soils (roots ≥ leaves > stems > grains). This is probably because orgAs are directly transported through plant stems and form fewer complexes than inAs (Carey et al. 2011). In our study, orgAs% increased from 2.8% in roots to 35.6% in leaves in NS, again, due to their rapid translocation compared to the less efficient translocation of inAs (Awasthi et al. 2017; Carey et al. 2011; Raab et al. 2005; Ye et al. 2010; Zhao et al. 2013). As MMAIII-PC complexes have been detected in sun flower (Raab et al. 2005) and rice root vacuoles (Kerl et al. 2019), this might also explain the lower concentration of MMAV compared to DMAV in our maize tissues. Pentavalent MMA and DMA are less likely to be bound to PCs (Mishra et al. 2017) and only the DMAV-GSH complex has been observed in Brassicaceae plants with a high sulfur content (Raab et al. 2007). Taken together, orgAs can, on the one hand, pass directly through the stems and be readily translocated in maize plants; on the other hand, orgAs complexes appear less likely to be formed, and some of them are very unstable. This might have resulted in the same distribution of orgAs in different tissues of our maize. This is further confirmed by the correlation found between TMAO in soil water and in leaves. TMAO was the primary As species in maize leaves (Fig. 5) and its concentration was positively correlated with that in the soil water (Figure S6).
Microbial Disturbance Effects on Maize
In our study, the microbial disturbance effect was observed in the stems, where maize grown in DS had higher concentrations of inAs, orgAs, and MMAV than maize in RS. Contrary results have been reported, indicating that rice cultivated in sterilized soils accumulates less As than rice in unsterilized soils, which is ascribed to the differences in soil bacterial communities (Huang et al. 2021). Plant growth-promoting microorganisms (PGPMs) are defined as microbes that can colonize the root rhizosphere, survive and multiply in the microhabitats associated with the root surface, and promote plant growth (Kumar 2016). Certain bacterial taxa such as PGPMs are found in higher abundance in sterilized soils, and their relative abundance is negatively correlated with As concentrations in grains (Huang et al. 2021). However, these differences can be due to the different microbial communities and the generally different growth conditions of rice compared to maize. Ochieno (2022) reported that soil sterilization killed some pathogens, but also eliminated beneficial microbes in soils, such as PGPMs, thus impairing their potential beneficial effects on the host plant. Soil sterilization alters microbial composition and significantly inhibits microbial activity (Mahmood et al. 2014; Marschner and Rumberger 2004). The microbial activity is found to be negatively correlated with As mobility, i.e., As release from soils would be higher at a low microbial activity (Kumpiene et al. 2007). ϒ-sterilization can cause damage to proteins, disrupting enzyme activity, and halting microbial exoenzyme production (Blankinship et al. 2014). Therefore, it is suspected in our study that the higher relative abundance and different composition of soil indigenous microbes in unsterilized soils might have contributed to lower As accumulation in maize plants.
Plant–microbe interactions have reciprocal effects on both partners and play an important role in their adaptation and survival in stressed environments (Berg 2009). In our experiment, soil sterilization might have also eliminated beneficial soil microbes such as PGPMs and disrupted their interactions with the host plant, losing their beneficial functions in promoting maize growth and potential resistance to As stress. Eliminating soil indigenous microbes through soil sterilization could disrupt plant–microbe interactions and negate the beneficial effects of soil microbes that promote maize health and assist maize to cope with As stress. However, the reduction in maize dry biomass was not attributable to the microbial disturbance effect, but to the abiotic sterilization effect.
Abiotic Sterilization Effects on Maize
The abiotic sterilization effects decreased the levels of essential nutrients in maize; in particular, a deficiency of P and Mn was observed in maize grown on sterilized soils. We observed indications for P deficiency in the sterilized soils (RS and DS) of all three As groups (Fig. 8). In these groups, the P concentrations in maize stems were below the concentrations required for adequate growth (2000 mg kg−1)(Kirkby 2012). Phosphorus deficiency has a negative impact on plant growth and health due to either a decrease in photosynthesis or an increase in energy investment, which causes various morphological, physiological, and biochemical adaptations (Malhotra et al. 2018). Phosphorus deficiencies might have influenced As translocation and accumulation in maize plants grown on both uncontaminated and contaminated soils. At a high As content in soils (200 mg kg−1), Mn deficiency in maize grown in sterilized soils was greater than in unsterilized soils (Figure S7). This could be ascribed to a possible complex between amorphous hydroxides of Mn and AsV in plants (Rosas-Castor et al. 2014b). The low concentration of Mn in soils can raise As concentrations in plant aerial tissues and increase As transference across the food chain (Rosas-Castor et al. 2014b). The Mn deficiency observed in maize in sterilized soils could have contributed to poor plant health and increased As accumulation in plants, aggravating the harmful effects of As on plant health.
In the present study, maize dry biomass was unaffected in uncontaminated soils but significantly reduced in contaminated soils. The missing effect on the dry biomass of maize grown in uncontaminated soils suggested that maize plants were resilient to P deficiency in the absence of As stress (Fig. 7). In the presence of As, however, P deficiency caused by soil sterilization might have significantly reduced the dry biomass of our maize. Due to similar chemical structures and shared phosphate transporters of As and P, low P can result in high As uptake by plants (Cattani et al. 2015; Long et al. 2021; Wu et al. 2022). Due to the abiotic sterilization effect, higher As accumulation was observed in our maize cultivated in sterilized soils than in unsterilized soils (NS < RS), including totAs and all measured As species. Therefore, in our maize grown in contaminated soils, the induced P deficiency by soil sterilization is coupled to a higher As uptake and the reduction in maize dry biomass.
Compared with other tissues, maize stems were most affected by As. For maize grown in As-contaminated soil, the decrease in stem dry biomass was greater for maize grown in sterilized or reconditioned soils. Crops cultivated in sterilized soils grow much worse than those in unsterilized soils, and the dry biomass production of stems in different crops is significantly depressed at a low P supply, which is particularly noticeable in sterilized soils (Ortas 2003). Besides, As translocation to stems in our study was higher than in previous studies at comparable soil As concentrations, which reported lower values of BACstem and TFstem (Drličková et al. 2013; Gulz et al. 2005). The high As translocation into our maize stems might explain the observed highest reduction in dry biomass of the stems. We observed that both the microbial disturbance and abiotic sterilization effects led to increased orgAs levels in the stems as well as high As translocation into the stems, which might explain why the dry biomass of stems was more reduced than of the other tissues.
Environmental Relevance and Implications
The lowest limit set by the European Union (EU) for As in complete feed and feed materials is 2 mg kg−1 (Adamse et al. 2017). Arsenic levels in the stem and leaves of maize grown in uncontaminated soils were all below this limit. In reconditioned and disturbed microbial treatments, As concentrations in stems and leaves of maize grown in 100 or 200 mg kg−1 soil were consistently higher than 2 mg kg−1, ranging from 3.2 to 16.4 mg kg−1 (Table S11, S12). Arsenic levels in maize grown in soils possessing indigenous microbes were much lower, with levels in stems and leaves (maize grown in 100 mg kg−1) below the safe limits. Some countries and authorities, such as China, the World Health Organization (WHO), and the EU have established limits for As in human food (0.2 mg kg−1 of inAs). The EU advised an even lower concentration of inAs for infants and young children (0.1 mg kg−1) (WHO 2018). The concentrations of inAs in our maize grains were lower than the limit for infants and young children, indicating a low environmental exposure risk to humans when maize plants were grown in soils containing 100 mg As kg−1 soils (data in soils at 200 mg kg−1 remain unknown due to the lack of grains).
A range of benchmark-dose lower confidence limits (BMDL01) values was identified by European Food Safety Authority, indicating 0.3 to 8 μg kg−1 body weight (bw) per day as reference points for the assessment of the carcinogenicity of inAs in humans (EFSA 2009). The inAs data in our maize grains were used to estimate potential dietary risks for people with high maize consumption. The obtained exposure estimate for maize grown at 100 mg kg−1 soils (0.12 µg kgbw−1 per day; Table S13) was below the reference limit for carcinogenicity. Due to low acropetal transport to upper tissues and low As levels in grains, maize is thus considered a suitable crop for As-contaminated farmlands (Cao et al. 2019), as also demonstrated in our study. In comparison with maize as an As excluder (Abbas and Abdelhafez 2013), rice is regarded as an As accumulator (Nath et al. 2014). Therefore, the cultivation of maize plants instead of rice species in high-As soils is recommended.
Conclusion
In As-contaminated soils, soil microbes and maize plants limited the uptake and translocation of inAs from soils into maize upper tissues. The translocation of orgAs was less inhibited, as it has a higher translocation efficiency in maize. In our high-As soils, both the microbial disturbance and abiotic sterilization effects increased As concentrations in maize stems. Interestingly, in the absence of As, soil sterilization had no effects on the dry biomass. In As-contaminated soils, the reduction in dry biomass was greater in maize grown in sterilized than unsterilized soils, which was probably caused by P and Mn deficiency and higher As uptake. Further work, including molecular biology investigations to identify key microbes and the study of other soils with different properties and microbiome, is needed to better understand the mechanisms by which soil microbes protect plants from As uptake and its toxic effects.
Associated Content
Supporting Information
Additional information: I Materials and Methods, including the chemical characterizations of NS and DS (Table S1); the preparation of microbial extracts (Figure S1) and the maize biomass estimating model (Figure S2). II Results, the CRMs data on totAs and As species in maize tissues (Table S2); the operating parameters of HPLC (Table S3); the identification of trimethylarsine oxide (TMAO) by cation-exchange HPLC-ICP-MS (Figure S3); the emmeans values for totAs concentration and dry biomass in the entire maize (Table S4); the p values of ANOVA on totAs concentration, As species concentrations, and dry biomass in maize tissues (Table S5); the p values of MANOVA statistical analysis on As species in maize tissues (Table S6); The bioconcentration factor (BCF), bioaccumulation coefficient (BAC), and translocation factor (TF) in maize tissues under different soil and As treatment (Table S7); the concentrations of individual orgAs (a) MMAV; (b) DMAV; and (c) TMAO in maize tissues (Figure S4); Pearson correlations of totAs and inAs concentrations in soil water (Figure S5); Spearman correlation of TMAO concentrations in soil water and in maize leaves (Figure S6); the concentrations of total manganese (Mn) in maize stems under soil treatment and As treatment (Figure S7); the emmeans of dry biomass in maize tissues (Table S8); Pearson correlation coefficients r between As species concentrations in the entire maize and dry biomass (Table S9); and the emmeans of totAs and As species concentrations in maize roots, stems, leaves and grains (Table S10–S13).
Data Availability
The data used in the manuscript will be made available from the corresponding author of the manuscript on request.
References
Abbas MHH, Abdelhafez AA (2013) Role of EDTA in arsenic mobilization and its uptake by maize grown on an As-polluted soil. Chemosphere 90:588–594. https://doi.org/10.1016/j.chemosphere.2012.08.042
Adamse P, van der Fels-Klerx HJI, de Jong J (2017) Cadmium, lead, mercury and arsenic in animal feed and feed materials—trend analysis of monitoring results. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34:1298–1311. https://doi.org/10.1080/19440049.2017.1300686
Afroz H, Su S, Carey M, Meharg AA, Meharg C (2019) Inhibition of microbial methylation via arsm in the rhizosphere: arsenic speciation in the soil to plant continuum. Environ Sci Technol 53:3451–3463. https://doi.org/10.1021/acs.est.8b07008
Valasek J, Thomasson JA (eds) (2016) Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping. SPIE Proceedings. SPIE
Anjum NA, Gill SS, Tuteja N (2017) Enhancing Cleanup of Environmental Pollutants. Springer International Publishing, Cham
Armienta MA, Beltrán M, Martínez S, Labastida I (2020) Heavy metal assimilation in maize (Zea mays L.) plants growing near mine tailings. Environ Geochem Health 42:2361–2375. https://doi.org/10.1007/s10653-019-00424-1
Arora, Jha (2019). Microbial interventions in agriculture and environment: Volume 2 Rhizosphere, microbiome and agro-ecology. In: Impact of Plant-Associated Microbial Communities on Host Plants Under Abiotic Stresses, Springer, NY
Awasthi S, Chauhan R, Srivastava S, Tripathi RD (2017) The journey of arsenic from soil to grain in rice. Front Plant Sci 8:1007. https://doi.org/10.3389/fpls.2017.01007
Batista BL, Nigar M, Mestrot A, Rocha BA, Barbosa Júnior F, Price AH, Raab A, Feldmann J (2014) Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot 65:1467–1479. https://doi.org/10.1093/jxb/eru018
Berg G (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18. https://doi.org/10.1007/s00253-009-2092-7
Berns PH, Narres H-D, Burauel P, Vereecken H, Tappe W (2008) Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur J Soil Sci 59:540–550. https://doi.org/10.1111/j.1365-2389.2008.01016.x
Blankinship JC, Becerra CA, Schaeffer SM, Schimel JP (2014) Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol Biochem 71:68–75. https://doi.org/10.1016/j.soilbio.2014.01.010
Borch T, Kretzschmar R, Kappler A, van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23. https://doi.org/10.1021/es9026248
Bowell RJ (1994) Sulphide oxidation and arsenic speciation in tropical soils. Environ Geochem Health 16:84. https://doi.org/10.1007/BF00209833
Boyd HW (1971) Manganese toxicity to peanuts in autoclaved soil. Plant Soil 35:133–144. https://doi.org/10.1007/BF01372638
Cao X, Bai L, Zeng X, Zhang J, Wang Y, Wu C, Su S (2019) Is maize suitable for substitution planting in arsenic-contaminated farmlands? Plant Soil Environ. https://doi.org/10.17221/155/2019-PSE
Caporale AG, Pigna M, Sommella A, Dynes JJ, Cozzolino V, Violante A (2013) Influence of compost on the mobility of arsenic in soil and its uptake by bean plants (Phaseolus vulgaris L.) irrigated with arsenite-contaminated water. J Environ Manage 128:837–843. https://doi.org/10.1016/j.jenvman.2013.06.041
Carey A-M, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98. https://doi.org/10.1111/j.1469-8137.2011.03789.x
Cattani I, Beone GM, Gonnelli C (2015) Influence of Rhizophagus irregularis inoculation and phosphorus application on growth and arsenic accumulation in maize (Zea mays L.) cultivated on an arsenic-contaminated soil. Environ Sci Pollut Res 22:6570–6577. https://doi.org/10.1007/s11356-014-3837-0
Cavalca L, Zecchin S, Zaccheo P, Abbas B, Rotiroti M, Bonomi T, Muyzer G (2019) Exploring biodiversity and arsenic metabolism of microbiota inhabiting arsenic-rich groundwaters in Northern Italy. Front Microbiol 10:1480. https://doi.org/10.3389/fmicb.2019.01480
Ci X, Liu H, Hao Y, Zhang J, Liu P, DONG S, (2012) Arsenic distribution, species, and its effect on maize growth treated with arsenate. J Integr Agric 11:416–423. https://doi.org/10.1016/S2095-3119(12)60026-4
Dao TH, Marx DB, Lavy TL, Dragun J (1982) Effect, and statistical evaluation, of soil sterilization on aniline and diuron adsorption isotherms. Soil Sci Soc Am J 46:963–969. https://doi.org/10.2136/sssaj1982.03615995004600050016x
Dietrich P, Cesarz S, Eisenhauer N, Roscher C (2020) Effects of steam sterilization on soil abiotic and biotic properties. Soil Org. https://doi.org/10.25674/so92iss2pp99
Drličková G, Vaculík M, Matejkovič P, Lux A (2013) Bioavailability and toxicity of arsenic in maize (Zea mays L.) grown in contaminated soils. Bull Environ Contam Toxicol 91:235–239. https://doi.org/10.1007/s00128-013-1035-2
EFSA (2009) Scientific opinion on arsenic in food. EFSA J 7:1351. https://doi.org/10.2903/j.efsa.2009.1351
Fellet G, Marchiol L, Perosa D, Zerbi G (2007) The application of phytoremediation technology in a soil contaminated by pyrite cinders. Ecol Eng 31:207–214. https://doi.org/10.1016/j.ecoleng.2007.06.011
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182. https://doi.org/10.3389/fphys.2012.00182
Garbinski LD, Rosen BP, Chen J (2019) Pathways of arsenic uptake and efflux. Environ Int 126:585–597. https://doi.org/10.1016/j.envint.2019.02.058
Guan H, Caggìa V, Gómez-Chamorro A, Fischer D, Coll-Crespí M, Liu X, Chávez-Capilla T, Schlaeppi K, Ramette A, Mestrot A, Bigalke M (2023) The effects of soil microbial disturbance and plants on arsenic concentrations and speciation in soil water and soils. Expo Health. https://doi.org/10.1007/s12403-023-00593-6
Guevara-García ÁA, Juárez K, Herrera-Estrella LR (2017) Heavy metal adaptation, 1st edn. Wiley
Gulz PA, Gupta S-K, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347. https://doi.org/10.1007/s11104-004-5960-z
Hasanuzzaman M (ed) (op. 2018) Mechanisms of arsenic toxicity and tolerance in plants. Springer, Singapore
Hu L, Robert CAM, Cadot S, Zhang X, Ye M, Li B, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M (2018) Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738. https://doi.org/10.1038/s41467-018-05122-7
Hu W, Wei S, Chen H, Tang M (2020) Effect of sterilization on arbuscular mycorrhizal fungal activity and soil nutrient status. J Soil Sci Plant Nutr 20:684–689. https://doi.org/10.1007/s42729-019-00156-2
Huang H, Jia Y, Sun G-X, Zhu Y-G (2012) Arsenic speciation and volatilization from flooded paddy soils amended with different organic matters. Environ Sci Technol 46:2163–2168. https://doi.org/10.1021/es203635s
Huang L, Wang X, Chi Y, Huang L, Li WC, Ye Z (2021) Rhizosphere bacterial community composition affects cadmium and arsenic accumulation in rice (Oryza sativa L). Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2021.112474
Jackson B (2015) Fast ion chromatography-ICP-QQQ for arsenic speciation. J Anal at Spectrom 30:1405–1407. https://doi.org/10.1039/C5JA00049A
Jia Y, Huang H, Zhong M, Wang F-H, Zhang L-M, Zhu Y-G (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47:3141–3148. https://doi.org/10.1021/es303649v
Kabir MS, Salam MA, Paul DNR, Hossain MI, Rahman NMF, Aziz A, Latif MA (2016) Spatial variation of arsenic in soil, irrigation water, and plant parts: a microlevel study. Sci World J 2016:2186069. https://doi.org/10.1155/2016/2186069
Kerl CF, Schindele RA, Brüggenwirth L, Colina Blanco AE, Rafferty C, Clemens S, Planer-Friedrich B (2019) Methylated thioarsenates and monothioarsenate differ in uptake, transformation, and contribution to total arsenic translocation in rice plants. Environ Sci Technol 53:5787–5796. https://doi.org/10.1021/acs.est.9b00592
Khairul I, Wang Q, Jiang Y, Chao W, Naranmandura H (2017) Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 8:23905
Khan MI, Ahmad MF, Ahmad I, Ashfaq F, Wahab S, Alsayegh AA, Kumar S, Hakeem KR (2022) Arsenic exposure through dietary intake and associated health hazards in the middle east. Nutrients. https://doi.org/10.3390/nu14102136
Kirkby E (2012) Introduction, definition and classification of nutrients. Marschner’s Mineral Nutrition of Higher Plants. Elsevier
Kumar, (ed) (2016) Plant Growth-Promoting Microorganisms: Interaction with Plants and Soil. Springer International Publishing, Cham
Kumpiene J, Castillo Montesinos I, Lagerkvist A, Maurice C (2007) Evaluation of the critical factors controlling stability of chromium, copper, arsenic and zinc in iron-treated soil. Chemosphere 67:410–417. https://doi.org/10.1016/j.chemosphere.2006.08.031
Lehr CR, Polishchuk E, Radoja U, Cullen WR (2003) Demethylation of methylarsenic species byMycobacterium neoaurum. Appl Organometal Chem 17:831–834. https://doi.org/10.1002/aoc.544
Li F, Zheng Y-M, He J-Z (2009) Microbes influence the fractionation of arsenic in paddy soils with different fertilization regimes. Sci Total Environ 407:2631–2640. https://doi.org/10.1016/j.scitotenv.2008.12.021
Li J, Sun Y, Jiang X, Chen B, Zhang X (2018) Arbuscular mycorrhizal fungi alleviate arsenic toxicity to medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol Environ Saf 157:235–243. https://doi.org/10.1016/j.ecoenv.2018.03.073
Li H, Liu Le, Li C, Liu X, Ziadi N, Shi Y (2023) Efficiency of different soil sterilization approaches and their effects on soil particle size distribution. J Soil Sci Plant Nutr 23:3979–3990. https://doi.org/10.1007/s42729-023-01315-2
Liu W-J, Wood BA, Raab A, McGrath SP, Zhao F-J, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in arabidopsis. Plant Physiol 152:2211–2221. https://doi.org/10.1104/pp.109.150862
Lomax C, Liu W-J, Wu L, Xue K, Xiong J, Zhou J, McGrath SP, Meharg AA, Miller AJ, Zhao F-J (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193:665–672. https://doi.org/10.1111/j.1469-8137.2011.03956.x
Long J, Chen B, Zhu Y, Li X, Yue X, Zhang N, Xia Y (2021) Mycorrhiza and iron tailings synergistically enhance maize resistance to arsenic on medium arsenic-polluted soils through increasing phosphorus and iron uptake. Bull Environ Contam Toxicol 107:1155–1160. https://doi.org/10.1007/s00128-021-03329-x
Mahmood T, Mehnaz S, Fleischmann F, Ali R, Hashmi ZH, Iqbal Z (2014) Soil sterilization effects on root growth and formation of rhizosheaths in wheat seedlings. Pedobiologia 57:123–130. https://doi.org/10.1016/j.pedobi.2013.12.005
Malhotra H, Vandana SS, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. Plant Nutrients and Abiotic Stress Tolerance. Springer, Singapore, pp 171–190
Mallick S, Sinam G, Sinha S (2011) Study on arsenate tolerant and sensitive cultivars of Zea mays L.: differential detoxification mechanism and effect on nutrients status. Ecotoxicol Environ Saf 74:1316–1324. https://doi.org/10.1016/j.ecoenv.2011.02.012
Marschner P, Rumberger A (2004) Rapid changes in the rhizosphere bacterial community structure during re-colonization of sterilized soil. Biol Fertil Soils 40:1–6. https://doi.org/10.1007/s00374-004-0736-4
Mishra S, Mattusch J, Wennrich R (2017) Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol-complexation to restrict their translocation to shoot. Sci Rep 7:40522. https://doi.org/10.1038/srep40522
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Moreno-Jiménez E, Esteban E, Peñalosa JM (2012) The fate of arsenic in soil-plant systems. Rev Environ Contam Toxicol 215:1–37. https://doi.org/10.1007/978-1-4614-1463-6_1
Nath S, Panda P, Mishra S, Dey M, Choudhury S, Sahoo L, Panda SK (2014) Arsenic stress in rice: redox consequences and regulation by iron. Plant Physiol Biochem 80:203–210. https://doi.org/10.1016/j.plaphy.2014.04.013
Neidhardt H, Norra S, Tang X, Guo H, Stüben D (2012) Impact of irrigation with high arsenic burdened groundwater on the soil-plant system: results from a case study in the Inner Mongolia, China. Environ Pollut 163:8–13. https://doi.org/10.1016/j.envpol.2011.12.033
Neumann K, Klukas C, Friedel S, Rischbeck P, Chen D, Entzian A, Stein N, Graner A, Kilian B (2015) Dissecting spatiotemporal biomass accumulation in barley under different water regimes using high-throughput image analysis. Plant Cell Environ 38:1980–1996. https://doi.org/10.1111/pce.12516
Norton G, Deacon C, Mestrot A, Feldmann J, Jenkins P, Baskaran C, Meharg AA (2013) Arsenic speciation and localization in horticultural produce grown in a historically impacted mining region. Environ Sci Technol 47:6164–6172. https://doi.org/10.1021/es400720r
Ochieno DMW (2022) Soil sterilization eliminates beneficial microbes that provide natural pest suppression ecosystem services against radopholus similis and fusarium oxysporum V5w2 in the endosphere and rhizosphere of tissue culture banana plants. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2022.688194
Ortas I (2003) Effect of selected mycorrhizal inoculation on phosphorus sustainability in sterile and non-sterile soils in the harran plain in South Anatolia. J Plant Nutr 26:1–17. https://doi.org/10.1081/PLN-120016494
Pandey N, Chandrakar V, Keshavkant S (2018) Mitigating arsenic toxicity in plants: role of microbiota. Mechanisms of Arsenic Toxicity and Tolerance in Plants. Springer, Singapore, pp 191–218
Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K, Guerinot ML (2017) Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci Total Environ 581–582:209–220. https://doi.org/10.1016/j.scitotenv.2016.12.111
Raab SH, Meharg AA, Feldmann J (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168:551–558. https://doi.org/10.1111/j.1469-8137.2005.01519.x
Raab A, Wright SH, Jaspars M, Meharg AA, Feldmann J (2007) Pentavalent arsenic can bind to biomolecules. Angew Chem 119:2648–2651. https://doi.org/10.1002/ange.200604805
Razavi Lakzian (2007) Evaluation of chemical and biological consequences of soil sterilization methods. Casp J Environ Sci 5:87–91
Requejo R, Tena M (2006) Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 6(Suppl 1):S156–S162. https://doi.org/10.1002/pmic.200500381
Rosas-Castor JM, Guzmán-Mar JL, Hernández-Ramírez A, Garza-González MT, Hinojosa-Reyes L (2014a) Arsenic accumulation in maize crop (Zea mays): a review. Sci Total Environ 488–489:176–187. https://doi.org/10.1016/j.scitotenv.2014.04.075
Rosas-Castor JM, Guzmán-Mar JL, Alfaro-Barbosa JM, Hernández-Ramírez A, Pérez-Maldonado IN, Caballero-Quintero A, Hinojosa-Reyes L (2014b) Evaluation of the transfer of soil arsenic to maize crops in suburban areas of San Luis Potosi, Mexico. Sci Total Environ 497–498:153–162. https://doi.org/10.1016/j.scitotenv.2014.07.072
Roychowdhury R, Roy M, Rakshit A, Sarkar S, Mukherjee P (2018) Arsenic bioremediation by indigenous heavy metal resistant bacteria of fly ash pond. Bull Environ Contam Toxicol 101:527–535. https://doi.org/10.1007/s00128-018-2428-z
Ruiz-Chancho MJ, López-Sánchez JF, Schmeisser E, Goessler W, Francesconi KA, Rubio R (2008) Arsenic speciation in plants growing in arsenic-contaminated sites. Chemosphere 71:1522–1530. https://doi.org/10.1016/j.chemosphere.2007.11.054
Sadee BA, Galali Y, Zebari SMS (2023) Toxicity, arsenic speciation and characteristics of hyphenated techniques used for arsenic determination in vegetables. A Review RSC Adv 13:30959–30977. https://doi.org/10.1039/d3ra05770d
Schaller J, Weiske A, Dudel EG (2011) Effects of gamma-sterilization on DOC, uranium and arsenic remobilization from organic and microbial rich stream sediments. Sci Total Environ 409:3211–3214. https://doi.org/10.1016/j.scitotenv.2011.05.014
Schmöger ME, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122:793–801. https://doi.org/10.1104/pp.122.3.793
Sharma SS, Dietz K-J (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50. https://doi.org/10.1016/j.tplants.2008.10.007
Skipper HD, Westermann DT (1973) Comparative effects of propylene oxide, sodium azide, and autoclaving on selected soil properties. Soil Biol Biochem 5:409–414. https://doi.org/10.1016/0038-0717(73)90067-9
Song J, Zhao F-J, McGrath SP, Luo Y-M (2006) Influence of soil properties and aging on arsenic phytotoxicity. Environ Toxicol Chem 25:1663–1670. https://doi.org/10.1897/05-480r2.1
Srivastava S (2020) Arsenic in Drinking Water and Food. Springer Singapore, Singapore
Srivastava S, Akkarakaran JJ, Sounderajan S, Shrivastava M, Suprasanna P (2016) Arsenic toxicity in rice (Oryza sativa L.) is influenced by sulfur supply: Impact on the expression of transporters and thiol metabolism. Geoderma 270:33–42. https://doi.org/10.1016/j.geoderma.2015.11.006
Strawn DG (2018) Review of interactions between phosphorus and arsenic in soils from four case studies. Geochem Trans 19:10. https://doi.org/10.1186/s12932-018-0055-6
Thirunavukkarasu O, Viraraghavan T, Subramanian K, Tanjore S (2002) Organic arsenic removal from drinking water. Urban Water 4:415–421. https://doi.org/10.1016/S1462-0758(02)00029-8
Turpeinen R, Pantsar-Kallio M, Kairesalo T (2002) Role of microbes in controlling the speciation of arsenic and production of arsines in contaminated soils. Sci Total Environ 285:133–145
Wagner S, Hoefer C, Puschenreiter M, Wenzel WW, Oburger E, Hann S, Robinson B, Kretzschmar R, Santner J (2020) Arsenic redox transformations and cycling in the rhizosphere of Pteris vittata and Pteris quadriaurita. Environ Exp Bot 177:104122. https://doi.org/10.1016/j.envexpbot.2020.104122
Wang S, Mulligan CN (2006) Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J Hazard Mater 138:459–470. https://doi.org/10.1016/j.jhazmat.2006.09.048
WHO (2018) Arsenic. https://www.who.int/news-room/fact-sheets/detail/arsenic. Accessed 22 Oct 2020
Wu J, Liang J, Björn LO, Li J, Shu W, Wang Y (2022) Phosphorus-arsenic interaction in the ‘soil-plant-microbe’ system and its influence on arsenic pollution. Sci Total Environ 802:149796. https://doi.org/10.1016/j.scitotenv.2021.149796
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yan M, Zeng X, Wang J, Meharg AA, Meharg C, Tang X, Zhang L, Bai L, Zhang J, Su S (2020) Dissolved organic matter differentially influences arsenic methylation and volatilization in paddy soils. J Hazard Mater 388:121795. https://doi.org/10.1016/j.jhazmat.2019.121795
Yang N, Wang H, Wang H, Wang Z, Ran J, Guo S, Peng Y (2021) Screening maize (Zea mays L.) varieties with low accumulation of cadmium, arsenic, and lead in edible parts but high accumulation in other parts: a field plot experiment. Environ Sci Pollut Res 28:33583–33598. https://doi.org/10.1007/s11356-021-12958-y
Ye W-L, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, Feldmann J, Zhao F-J (2010) Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol 154:1505–1513. https://doi.org/10.1104/pp.110.163261
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated florida site. Sci Total Environ 368:456–464. https://doi.org/10.1016/j.scitotenv.2006.01.016
Yoshinaga M, Cai Y, Rosen BP (2011) Demethylation of methylarsonic acid by a microbial community. Environ Microbiol 13:1205–1215. https://doi.org/10.1111/j.1462-2920.2010.02420.x
Yu Y, Zhang S, Huang H, Luo L, Wen B (2009) Arsenic accumulation and speciation in maize as affected by inoculation with arbuscular mycorrhizal fungus glomus mosseae. J Agric Food Chem 57:3695–3701. https://doi.org/10.1021/jf900107y
Zhao F-J, Zhu Y-G, Meharg AA (2013) Methylated arsenic species in rice: geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47:3957–3966. https://doi.org/10.1021/es304295n
Zhao Z, Zhang H, Fu Z, Chen H, Lin Y, Yan P, Li W, Xie H, Guo Z, Zhang X, Tang J (2018) Genetic-based dissection of arsenic accumulation in maize using a genome-wide association analysis method. Plant Biotechnol J 16:1085–1093. https://doi.org/10.1111/pbi.12853
Zheng R, Sun G, Zhu Y (2013) Effects of microbial processes on the fate of arsenic in paddy soil. Chin Sci Bull 58:186–193. https://doi.org/10.1007/s11434-012-5489-0
Acknowledgements
We especially thank Dr. Daniela Fischer and Patrick Neuhaus for their support in the laboratory, Dr. Valentin Gfeller for his guidance in plant cultivation, as well as Dr. Xiaowen Liu, Pia Bergamaschi, Andrea Weber, Florian Enz, and Christina Désirée Widmer for their help with plant incubation and data collection. We would also like to thank Dr. Suyi Hu for his help with data organization and Nichole Wetter for her proofreading.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was financially supported by the University of Bern through the Interfaculty Research Cooperation One Health (https://www.onehealth.unibe.ch/).
Author information
Authors and Affiliations
Contributions
Hang Guan: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—Original draft, Visualization, and Project administration. Veronica Caggìa: Investigation, Writing—Review, and Editing. Andrea Gómez-Chamorro: Formal analysis, Writing—Review, and Editing. Miquel Coll Crespi: Investigation. Teresa Chávez-Capilla: Methodology, Writing—Review, and Editing. Alban Ramette: Conceptualization, Formal analysis, Writing—Review, and Editing. Klaus Schlaeppi: Conceptualization, Resources, Writing—Review, and Editing. Adrien Mestrot: Methodology, Conceptualization, Supervision, Funding acquisition, Writing—Review, and Editing. Moritz Bigalke: Methodology, Conceptualization, Supervision, Writing—Review and Editing, Funding acquisition, and Project administration.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guan, H., Caggìa, V., Gómez-Chamorro, A. et al. Soil Indigenous Microbes Interact with Maize Plants in High-Arsenic Soils to Limit the Translocation of Inorganic Arsenic Species to Maize Upper Tissues. Expo Health (2024). https://doi.org/10.1007/s12403-024-00655-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12403-024-00655-3