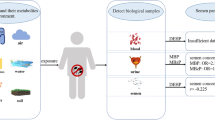
Abstract
Although people are constantly exposed to phthalates little is known about the extent to which PAEs affect sperm. Most studies do not address changes at the single-cell level. Our study concentrated on the examination of donors who were assumed to have been exposed to high levels of phthalate under plateletpheresis. We used Computer-Assisted-Sperm-Analysis to study the association between the most potent phthalate, di-2-ethylhexyl phthalate, and a decrease in sperm motility. In an exploratory in vivo study, we investigated whether plateletpheresis of donors led to an increase in the concentration of active metabolites of DEHP in seminal plasma and whether this had an effect on sperm motility. PAE metabolites and sperm motility parameters of ejaculate donors were analyzed at a single-cell level before and after plateletpheresis. We found an increase in PAE metabolite concentration in the seminal plasma, associated with a decrease in flagellar beat frequency after plateletpheresis. Follow-up analysis showed that this was a transient effect of plateletpheresis in terms of a PAE concentration increase in seminal plasma and a decrease in sperm motility. This study shows that plateletpheresis results in high levels of phthalate exposure and that these are associated with a transient and reversible decrease in sperm motility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility is a global health problem affecting at least 15% of couples (Thoma et al. 2013; Vander Borght and Wyns 2018), with an increasing trend observed in recent years. In Germany, for example, the proportion of unintentionally childless women and men in the 20–50 age group rose from 25% in 2013 to 32% in 2020 (Wippermann 2020). Human infertility has various causes, with approximately 1/3 of infertility due to male factors, 1/3 due to female factors, and in 1/3 of couples no cause can be identified (Isidori et al. 2006; Turner et al. 2020; Wippermann 2020). Apart from genetical causes, one reason for infertility may be exposure to exogenous compounds such as endocrine-disrupting chemicals (EDCs) which potentially affect hormonal pathways involved in the development and function of the male and female reproductive systems (Meeker and Ferguson 2014; Rogers et al. 2013). Phthalates (PAEs) belong to the group of endocrine disruptors (Darbre 2017), which disturb the hormonal balance of organisms, leading to an alteration of the development and function of hormone-dependent structures of the reproductive system (Meeker et al. 2012; Skakkebaek et al. 2016). Further, there is an association between PAE exposure and the development of testicular dysgenesis syndrome, which includes the occurrence of undescended testes, hypospadias, as well as subfertility, infertility, and increased risk of testicular cancer (Main et al. 2010; Skakkebaek et al. 2001, 2016).
In general, PAEs are ortho-phthalic acid (1,2-benzenedicarboxylic acid) diesters which, since the 1930s, have been the main plasticizers used in the polymer industry (Ferguson et al. 1946; Giuliani et al. 2020). To date, more than 24 types of PAEs with metabolites of di(2-ethylhexyl) phthalate (DEHP) (Hosseinzadeh et al. 2021) were the most commonly used phthalates (Wang et al. 2019) associated with male reproductive toxicity (Cha et al. 2018; Wittassek and Angerer 2008). DEHP is metabolized in the liver to its toxic agent monoester mono(2-ethylhexyl)phthalate (MEHP), which is further metabolized to mono(2-ethyl-5-hydroxyhexyl)phthalate (5OH-MEHP), mono(2-ethyl-5-oxohexyl)phthalate (5oxoMEHP) and 5cx-MEPP mono(2-ethyl-5-carboxypentyl)phthalate (Albro et al. 1982; Koch et al. 2005; Peck and Albro 1982; Schmid and Schlatter 1985).
PAEs are used in the production of many products such as food packaging, cosmetics and medical devices (PETROVIČOVÁ, 2014; Schettler 2006; Yen et al. 2011). As they are not covalently attached to their substrates they are constantly released into the environment (Pearson and Trissel 1993; Wang et al. 2019) with the consequence of high exposure for humans across their lifespan (Radke et al. 2018). DEHP and its metabolites have been detected in 98% of the US population (Api 2001; Zota et al. 2016). The absorption of these substances occurs mainly via ingestion, inhalation or intravenous medication administration (Hauser et al. 2004; Johns et al. 2015; Meeker et al. 2009). PAEs are rapidly metabolized in the body and primarily excreted into the urine (Genuis et al. 2012; Schulz and Rubin 1973). Thus, increased DEHP values have been determined in blood or urine samples of patients undergoing extracorporeal membrane oxygenation (ECMO) treatment, plasmapheresis or plateletpheresis (Kaestner et al. 2020; Koch et al. 2005). In addition, PAEs have been detected in blood and breast milk (Genuis et al. 2012; Mortensen et al. 2005), the placenta (Minatoya et al. 2018; Skakkebaek et al. 2016) and in semen (Pant et al. 2014). Several general studies evaluating globally established parameters of sperm motility after exposure to PAEs have been published (Amjad et al. 2021; Axelsson et al. 2015; Hosseinzadeh et al. 2021; Jurewicz et al. 2013; Khasin et al. 2020).
In addition, PAE exposure results in severe male reproductive system toxicity (Hosseinzadeh et al. 2021). There is also evidence that sperm come into contact with unmetabolized DEHP via intravenous medication administration (Hauser et al. 2004; Johns et al. 2015; Meeker et al. 2009). During the platelet and plasmapheresis process, which are the most common apheresis procedures (Zantek et al. 2021), PAEs are released into the donors blood (Buchta et al. 2003, 2005). Apheresis in general is a procedure for separating specific components in an apheresis machine. The technology uses centrifugation, beads or filters to perform separation. Plateletpheresis is a standard procedure for collection of thrombocytes from healthy individuals. The procedure includes harvesting of the whole blood by transferring it into a special machine, equipped with a disposable sterile set of plastic tubing. Next, thrombocytes are concentrated. Non-needed blood components, such as plasma and erythrocytes, are re-transfused to the donor. Platelets are needed for patients who are bleeding (during surgery or accidents) or patients with high risk of bleeding and low platelet counts (e.g. after stem cell transplantation or with hematology disorders). Estimated by the Paul-Ehrlich Institut, over 320.000 platelet concentrates were produced in Germany in 2021.
It has already been shown that both chronic and short-term exposure to DEHP negatively affects sperm motility, chromatin DNA stability, and capacitation, a physiological process leading to hypermotility (Pant et al. 2011; Sumner et al. 2019). However, contact with plasticizers through plateletpheresis has not yet been examined.
This study was designed as an exploratory investigation to demonstrate for the first time how systematic PAE exposure leads to changes in sperm parameters such as motility, velocity and beat frequency. It was a test of the hypothesis that there is a correlation between the aforementioned sperm parameters and PAE exposure, both in vitro and in vivo. In this context, the reversibility of possible altered sperm parameters after PAE exposure was also an intriguing aspect.
Materials and Methods
Reagents and Chemicals
Di(2-ethylhexyl) phthalate (DEHP) and MEHP (mono(2-ethylhexyl)phthalate) were purchased from Merck (Darmstadt, Germany). The three secondary metabolites – the biologically active metabolites forms called mono-(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP), mono-(2-ethyl- 5-oxohexyl) phthalate (5oxo-MEHP) and mono-(2-ethyl-5-carboxypentyl) phthalate (5cx-MEPP) were obtained from LGC Standards (Wesel, Germany).
Animals
Wildtype NMRI mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). The mice were treated in accordance with guidelines approved by the University of Duisburg-Essen Animal Center.
Selection of Donors and Semen Sample Collection
All experiments with human spermatozoa were approved by the Ethics Committee of the University of Duisburg-Essen (14-5748-BO and Addendum 11/2019) and the samples were pseudonymised. All donors signed informed consent forms before sample donation. Prior to donation, sexual abstinence for a period of 48–72 h was required. The same requirement applied to measurements prior to apheresis. All samples were analyzed according to the WHO 2010 classification (5th edition) (WHO 2010).
For in vitro studies, three healthy donors were recruited through flyers posted on the university notice boards. Semen samples were obtained by masturbation at a site chosen by the donor and brought to the Department of Anatomy within one hour.
Platelet donors (N = 25 males, ≥ 18 years) who underwent plateletpheresis up to every four weeks at the Institute for Transfusion Medicine at the University Hospital Essen were recruited for this study via takeaway study information envelopes between December 2015 and March 2016 as well as between June and September 2019. The inclusion criterium was that the participants were male platelet donors without previous urological operations affecting fertility. In addition, all donors had to comply with the regulations for plateletpheresis, e.g. no current infectious disease and 48 h alcohol and THC abstinence. Human semen samples (N = 25) were obtained before (-48 h to -1 h) and after apheresis (1 h), with a minimum period of 28 days since the last apheresis. The average duration of apheresis was 80 min. In addition, samples were also obtained from seven of the 25 platelet donors on the second and seventh day after apheresis. Sperm motility and individual daily phthalate exposure of the respective donor were determined prior to apheresis and provided control data.
Ejaculates were collected in DEHP-free containers – a Weck® 160 ml lintel glass, with a fitting glass cover (Weck® Rundrand Glas 60, Wehr-Öflingen, Deutschland).
Spermatozoa Preparation
After liquefaction at RT for 30–60 min, human sperm were separated from the ejaculates by means of the swim-up procedure as previously described (Muschol et al. 2018). In brief, 0.5 ml of the sperm suspension was carefully layered under 2 ml of HS buffer (in mM: 135 NaCl, 5 KCL, 2 CaCl2, 1 MgCl2, 20 HEPES, 5 glucose, 10 DL-lactic acid, 10 pyruvic acid, pH 7.4, 353 mOsm) in a 50 ml Falcon tube. The swim-up procedure took place at 37 °C with 5% CO2 for 60 min. Subsequently, 1.5 ml of the supernatant containing motile sperm was used for further experiments.
Mouse sperm were subjected to the swim-out procedure for 15 min at 37 °C and 5% CO2 in HSB-buffer (Wiesehofer et al. 2022). After two washing steps (300 g for 5 min), a final concentration was adjusted to 1–2 × 107 cells/ml in HS medium.
Computer-assisted Spermatozoa Analysis (CASA)
A spermiogram was carried out for each donor according to WHO 2010 (WHO 2010) before using sperm samples for single-cell analysis. In brief: After a washing step (300 g, 5 min), the human or murine sperm pellet was re-suspended in pre-warmed HS buffer and different DEHP concentrations (0, 0.07, 6.67 and 66.67 µM). After incubation for 30 min at 37 °C with 5% CO2, 10 µl of the sperm suspension was placed in a pre-warmed Makler® counting chamber (Sefi-Medical Instruments, Haifa, Israel) and motility was examined on a 37 °C stage with a CASA system (MedeaLAB CASA, v. 5.5, Medical Technology GmbH, Altdorf, Germany). CASA was used to measure total motility [%], the proportions according to WHO sperm classifications (≥ 25 μm/s) [%] and the curvilinear velocity (VCL) [µm/s].
Sperm Flagellar Beat Frequency Analysis
The flagellar beat frequency was analyzed as previously described (Mannowetz et al. 2011; Wennemuth et al. 2003). In this study, the beat frequency of human or murine spermatozoa (N = 3, n = 30 sperm) previously incubated for 30 min with different DEHP concentrations (0, 0.07, 6.67 and 66.67 µM) was investigated. In addition, beat frequency analysis of sperm suspension from platelet donors (Npre−post apheresis = 25, npre−post apheresis = 250 sperm; Nlong−term study = 7, nlong−term study = 70) was performed at different time points before and after apheresis (-48 h to -1 h before apheresis, 1 h, 48 and 168 h after apheresis). For the analysis, 10 µl of the respective sperm suspension was transferred to a FluoroDish™ (World Precision Instruments, Sarasota, FL, USA) and the spermatozoa were allowed to adhere to the bottom (2 min) before 3 ml of HS buffer were added. Stop motion flagellar beat frequency analysis was performed using an inverted microscope (Nikon Diaphot 300, Tokyo, Japan) with a 40 × 0.65 N.A. objective and a MotionScope M3-mono fast speed camera (Imaging Solutions, Ehingen, Germany). Images were recorded at 300 frames per second for a total time of 1 s. Pictures were collected using the MotionStudio 64 software (Imaging Solutions, Regensburg, Germany) and stored in TIFF format for subsequent semi-quantitative single-cell flagellar movement analysis. The brightness and contrast of the pictures were adjusted using ImageJ V1.37 (National Institute of Health, Buckinghamshire, Little Chalfont, UK) and regions of interest (single sperm) were selected. Flagellar beat frequency analysis for each individual sperm was conducted using a semi-automated analysis software written in Igor Pro™ (v6.04, Wavemetrics, Lake Oswego OR, US) (Wennemuth et al. 2003).
Plateletpheresis
Healthy apheresis donors who fulfilled the German guidelines and recommendations for cytapheresis were included in the study after providing their written informed consent. Donors with a body weight ≥70 kg and a hemoglobine level of 13.5 g per dl were included in this study. Thrombocyte collection was performed using the Amicus blood cell separator system (Fresenius, Bad Homburg, Germany) with a single-needle procedure in an air-conditioned surrounding. The donors who agreed to participate in the study were automatically assessed using an algorithm which took the donors’ criteria (height, weight, hematocrit and platelet count) into account. To prevent coagulation, donor blood was supplemented with Anticoagulant Citrate Dextrose Solution, Solution A (ACD-A). The average volume of blood processed per donor was 4080 ml (from 1800 to 5700 ml) and the average length of the procedure was 80 min (in a range of 60–90 min).
Quantification of DEHP Metabolites in Seminal Plasma
DEHP metabolite analysis was performed by the Institute and Policlinic for Occupational, Social and Environmental Medicine of the Friedrich-Alexander-University in Erlangen-Nuremberg as previously described (Eckert et al. 2015). In short, the donated ejaculates of platelets were transferred to 8 ml glass tubes (Macherey-Nagel, Düren, Deutschland) with DEHP-free screw caps (Wheaton®, VWR, Darmstadt, Deutschland) and were stored at -20 °C, a temperature at which DEHP remains metabolically stable. For analysis, 1 ml of the ejaculate and 30 µl of the internal standards were transferred into a glass vial. After adding 200 µl ethanol, 1 ml aqueous 0.9% sodium chloride solution and 100 µl aqueous phosphoric acid (phosphoric acid/water, 1/1, v/v), the sample was extracted by liquid-liquid extraction using 2 ml of η-hexane and 1 ml ethyl acetate. For analysis, 5 µl of the solutions were injected into the LC-MS/MS system. The quantitative determination of DEHP in the seminal plasma was performed by using tandem mass spectrometry with electrospray ionization (Sciex API 2000, Applied Biosystems, Langen, Germany) coupled to an Agilent 1100 series HPLC value system (Agilent, Waldbronn, Germany), including a quaternary pump (Agilent G 1311 A), a vacuum degasser (Agilent G 1322 A) and an autosampler (Agilent G 1313 A). The limit of substance detection (LOD) was > 0.25 µg/l and the limit of quantification (LOQ) was 0.5 µg/l.
In this study, the DEHP primary metabolite MEHP (mono(2-ethylhexyl)phthalate) and its three secondary metabolites – the biologically active metabolites forms called mono-(2-ethyl-5-hydroxyhexyl) phthalate (5OH-MEHP), mono-(2-ethyl- 5-oxohexyl) phthalate (5oxo-MEHP) and mono-(2-ethyl-5-carboxypentyl) phthalate (5cx-MEPP) - were determined in the ejaculate samples.
Statistics
Statistical analysis was carried out with GraphPad Prism (Vers. 9, Statcon GmbH, Witzenhausen, Germany) using descriptive statistics. Numerical results are presented as mean with N = number of independent individuals and n = number of determinations. We wish to avoid publication bias by preferential reporting of significant results. Instead, we rather judge the value of our estimates by their precision and validity (Lash 2007; Wasserstein et al. 2019). However, in order to optimize the structure of our research project and to improve the readability of the manuscript, statistical tests were performed as one-way analysis of variance (ANOVA) or Student’s t-test analysis incase of normal distribution. Detailed p-values are written in the corresponding figure legend. Numerical results are presented as mean with N = number of independent individuals and n = number of determinations. Due to the small n-number in the single cell analysis presented in Fig. 6, we did not perform significance tests, but calculated standard errors and assessed the precision of our estimates.
Results
DEHP Decreases the Motility of Mouse and Human Sperm
In vitro studies were carried out on mouse and human spermatozoa to examine the effect of exposure to different DEHP concentrations (0, 0.07, 6.67, 66.7 µM) on sperm motility. The incubation of mouse spermatozoa for 30 min with 0.07 µM DEHP resulted in reduced motility (total motility: 28.33 ± 4.8%, WHOa: 17.00 ± 3.7%, VCL: 70.16 ± 12.5 μm/s) compared to untreated sperm (total motility: mean 32.67 ± 3.3%, WHOa: 18.33 ± 5.0%, VCL: 74.35 ± 14.9 μm/s) (Fig. 1A-C). The treatment of sperm with 6.67 µM DEHP (total motility: mean 31.66 ± 4.0%, WHOa: 19.33 ± 2.6%, VCL: 75.11 ± 16.3 μm/s) caused a velocity nearly as high as that of control sperm without contact to DEHP. Incubation with 66.67 µM DEHP again reduced total sperm motility to 30.00 ± 2.8% (Fig. 1A), whereas the proportions of WHOa classified sperm (18.67 ± 1.9%) were also nearly the same as those of control sperm (18.33 ± 5.0%) (Fig. 1B). In contrast, the VCL of sperm after treatment with 66.67 µM DEHP was 5.3% (78.52 ± 6.2 μm/s) higher than that of control sperm (Fig. 1C). Flagellar beat frequency increased after 0.07 µM DEHP incubation (4.95 ± 1.8 Hz; control: 4.18 ± 1.5 Hz), decreased after 6.67 µM DEHP incubation (4.38 ± 1.6 Hz) and at the higher dose of 66.67 µM DEHP, a stable beat frequency was regained (4.18 ± 1.5 Hz) compared to the control group (4.18 ± 1.5 Hz) (Fig. 1D).
Sperm motility and flagellar beat frequency of murine sperm are affected by DEHP. (A-C) CASA analysis of mouse sperm after 30 min incubation with different DEHP concentrations (0, 0.07, 6.67, 66.67 µM). Statistical analysis of total motility (A), WHOa classified sperm (B) and of their curvilinear velocity (VCL) (C), N = 3 mice, n ≥ 750 sperm/condition. (D) Analysis of the single-sperm beat frequency responses to DEHP, N = 3 mice, n = 30 sperm/condition. All data are shown as mean ± s.e.m
In human sperm all the velocity parameters investigated by CASA were reduced by DEHP at dose-dependent rates. With increasing concentrations total motility decreased from 75.33 ± 7.4% to 54.33 ± 12.5% (Fig. 2A), WHOa classified sperm from 48.67 ± 7.6% to 30.00 ± 15.6% (Fig. 2B), VCL from 69.79 ± 7.0 μm/s to 51.44 ± 5.6 μm/s (Fig. 2C). The beat frequency of the sperm w/o DEHP present in the control group was 9.16 ± 3.2 Hz. When incubated with 0.07, 6.67, and 66.67 µM DEHP, it rose to 9.52 ± 3.7 Hz (+ 3.78%), 10.53 ± 3.9 Hz (+ 13.01%), and 10.96 ± 4.6 Hz (+ 16.42%), respectively.
DEHP decreases human sperm motility. (A-C) Velocity analysis by CASA of human sperm after 30 min incubation with different DEHP concentrations (0, 0.07, 6.67, 66.67 µM). Statistical analysis of total motility (A), WHOa classified sperm (B) and of their curvilinear velocity (VCL) (C), N = 3 donors, n ≥ 750 sperm/condition. (D) Beat frequency after different exposure to different DEHP conditions, N = 3 donors, n = 30 sperm/condition. All data are shown as mean ± s.e.m
Beat Frequency Decreases Immediately after Plateletpheresis
Plateletpheresis over a period of 70–90 min was used to mimic a short-term exposure to phthalates of apheresis tubing. The sperm beat frequency of 25 donors was calculated before (-48 h to -1 h; representing the control) and after apheresis (+ 1 h) (Fig. 3). For each donor, the mean beat frequency of 10 sperm per condition (pre and post apheresis) was calculated (mean valuedonor, pre apheresis, mean valuedonor, post apheresis). A decrease in beat frequency after plateletpheresis was detected in 84% (21/25) of the donors (Fig. 3A). To clarify the effect of plateletpheresis on the beat frequency, the mean valuesdonor, pre apheresis were used to calculate the mean beat frequency value of all donors prior to apheresis (mean valuetotal, pre apheresis) and set to 100% (first scatter blot, thick black line, Fig. 3B). Subsequently, to calculate the percentage of beat frequency alteration (Δ beat frequency) the single mean values pre and post apheresis (mean valuedonor, pre apheresis, mean valuedonor, post apheresis) were set in relation to meantotal, pre apheresis (dots, Fig. 3B). The reduction of the relative mean beat frequency between the two groups was 19.76%.
Apheresis reduces sperm beat frequency of platelet donors. (A) Beat frequency analysis of human sperm before (-48 h to -1 h) and after plateletpheresis (+ 1 h). Examination before plateletpheresis served as control. (B) Beat frequency changes (Δ) of human sperm before and after apheresis (dots). The mean differences in beat frequency of all analyzed donors is shown as a continuous line (mean ± s.e.m.). On average, the beat frequency of sperm after apheresis was reduced by 19,76%, compared to sperm before apheresis, N = 25 platelets donors, n = 10 analyzed sperm/donor/dot
After determining that apheresis leads to a decrease in single cell motility, we additionally determined the concentration of DEHP metabolites in the seminal plasma before and after apheresis. We used the same 25 donors that were used for the analysis in Fig. 3. The primary metabolite of DEHP - MEHP - as well as its three biologically active metabolites – 5OH-MEHP, 5oxo-MEHP and 5cx-MEPP – were measured by mass spectrometry (Fig. 4, S1). In seminal plasma collected prior to plateletpheresis, MEHP was detected in 24 out of 25 donors (Fig. 4A). In contrast, there were no detectable levels of the metabolites 5OH-MEHP (Fig. 4B), 5oxo-MEHP (Fig. 4C), 5cx-MEPP (Fig. 4D) in the majority of samples (5OH-MEHP: 20/25 donors; 5oxo-MEHP: 24/25 donors; 5cx-MEPP: 21/25 donors). An increase of MEHP concentration after apheresis was observed in 76% of the donors (19/25) (Fig. 4A). A rise in the seminal plasma concentration of 5OH-MEHP was documented in 80% (20/25) (Fig. 4B), of 5oxo-MEHP in 72% (18/25) (Fig. 4C) and of 5cx-MEPP in 68% (17/25) of the donors (Fig. 4D).
Increase in active metabolites of DEHP in the seminal plasma after apheresis. (A-D, left) Analysis of concentrations of active DEHP metabolites (MEHP, 5OH-MEHP, 5oxo MEHP, 5cx-MEPP) in the seminal plasma before and after apheresis. (A-D, right) Differences (as Δ) of concentrations of active DEHP metabolites (MEHP, 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP) in the seminal plasma before and after apheresis illustrated by waterfall plots. Examination before plateletpheresis serves as a control. N = 25 platelets donors, n = 1 metabolite value/platelets donor
To characterize the transient effects of plateletpheresis on sperm motility parameters, we analyzed the sperm samples of seven donors before (-48 h to -1 h) and after (1 h) plateletpheresis with additional time points on days 2 (48 h) and 7 (168 h) (Fig. 5A). As before, we used sperm for single cell analysis of the beat frequency (Fig. 5B, S2). A decrease in flagellar beat frequency 1 h (10.60 ± 0.42 Hz) and 48 h (10.25 ± 0.39 Hz) after apheresis was detected in comparison to beat frequency analysis of sperm before apheresis (12.59 ± 0.46 Hz). Flagellar beat frequency analysis 168 h after apheresis revealed a reconstitution of the beat frequency 48 h after apheresis, suggesting a reversible effect (Fig. 5B). Determination of MEHP and its metabolites in the seminal plasma revealed an increased concentration after apheresis (MEHP: 4.46 ± 1.01 µg/l, 5OH-MEHP: 1.46 ± 0.43 µg/l, 5oxo-MEHP: 0.96 ± 0.31 µg/l, 5cx-MEPP: 1.29 ± 0.32 µg/l) compared to determination before apheresis (MEHP: 3.48 ± 0.91 µg/l; 5OH-MEHP, 5oxo-MEHP, 5cx-MEPP: below detection) (Fig. 5C-F, S2). MEHP and all active metabolites of MEHP measured in this study decreased to a level close to the values before apheresis or below detectable concentrations 168 h post apheresis (MEHP: 2.25 ± 0.72 µg/l; 5OH-MEHP, 5oxo-MEHP: below detection; 5cx-MEPP: 0.30 ± 0.04 µg/l).
Reversible effect of apheresis on sperm beat frequency and increase of active DEHP metabolites in the seminal plasma. (A) shows the time sequence of the metabolite and beat frequency evaluation. Measurements took place − 48 to 1 h before apheresis, 1 h, 48 and 168 h after apheresis. For the beat frequency analysis, sperm were collected by swim-up procedure, whereas sample preparation for metabolite analysis was performed by centrifugation. – h = hours before apheresis; + h = hours after apheresis; BF = Beat frequency analysis; M = metabolite analysis. (B) Time dependent beat frequency of human sperm (N = 7 platelets donors, n = 70 sperm/ time point). Examination before plateletpheresis (-48 h to -1 h) served as control. Beat frequency decreased 1 and 48 h after apheresis, increased again between 48 and 168 h, however the value did not regain the initial value. (C-F) Time-dependent concentration of active DEHP metabolites in the seminal plasma. Concentration of MEHP (C), 5OH-MEHP (D), 5oxo-MEHP (E) and 5cx-MEPP (F) increased 1 h after apheresis and continuously decreased afterwards. The time point of apheresis is marked by a dashed line. N = 7 platelets donors, n = 1 metabolite value/platelets donor, mean ± s.e.m
To correlate beat frequency and DEHP metabolites in more detail, we show beat frequency of semen samples of the same 7 donors as in Fig. 5 and their individual concentration of the DEHP metabolite 5OH-MEHP (Fig. 6A-E). Again, samples immediately before and 1 h, 48 and 168 h after plateletpheresis were used. A significant increase of 5OH-MEHP 1 h after apheresis was detected in 5 of 7 samples (meanbefore apheresis: 0.28 ± 0.03 µg/l, meanafter apheresis: 1.77 ± 0.54 µg/l) correlating with a decrease in sperm beat frequency (meanbefore apheresis: 12.36 ± 0.44 Hz, meanafter apheresis: 10.20 ± 0.47 Hz) (Fig. 6A-E).
In two donors, the highest concentration of 5OH-MEHP in the ejaculate was measured 48 h post plateletpheresis (Fig. 6F-G). While one donor showed only a small increase (5OH-MEHPbefore apheresis: not detectable, 5OH-MEHP48 h after apheresis: 0.27 µg/l) correlated with a decrease of the beat frequency from 17.63 Hz to 8.87 Hz (Fig. 6F), a significant increase could be detected in the second donor (5OH-MEHPbefore apheresis: not detectable, 5OH-MEHP48 h after apheresis: 2.77 µg/l) (Fig. 6G). Only this one donor showed an increase in beat frequency after plateletpheresis (1 h: 12.20 ± 1.28 Hz, 48 h: 12.30 ± 0.72, 168 h: 10.2 ± 0.81 Hz) in comparison to the measured beat frequency before apheresis (8.70 ± 1.0 Hz).
Even if the seminal plasma concentration of 5OH-MEHP was higher for one or two days (mean7 donors: 1.70 ± 0.46 µg/l), it did revert to its initial pre-apheresis value (mean 5OH-MEHP7 donors: 0.27 ± 0.02 µg/l) after 168 h. The same tendency was observed in beat frequency. After apheresis, the mean beat frequency of spermatozoa was slightly decreased (mean7 donors: 10.31 ± 0.39 Hz) compared with its value before apheresis (mean beat frequency7 donors: 12.59 ± 0.46 Hz), but increased again after 168 h (mean beat frequency7 donors: 11.23 ± 0.33 Hz).
Single donor analysis of time-dependent effect of apheresis on beat frequency and accumulation of active DEHP metabolite 5OH-MEHP in the seminal plasma. (A-G) Time-dependent beat frequency analysis (mean ± s.e.m.) and 5OH-MEHP accumulation [µg/l] of all seven donors (N = 7 donors, n = 10 sperm, n = 1 metabolite value/measure time). Analysis was conducted − 48 h to -1 h before plateletpheresis, serving as a control and 1 h, 48 and 168 h after apheresis. While the beat frequency of most donors decreased, concentration of 5OH-MEHP increased 1 h after apheresis. The time point of apheresis is marked by a dashed line
Discussion
In vitro experiments were conducted with increasing DEHP concentrations to demonstrate a causal relationship between the plasticizer DEHP and sperm motility. Using CASA, we were able to identify a DEHP dose-dependent reduction of sperm motility parameters in humans. These results are in line with other CASA studies showing a reduction in sperm motility as well as in hyperactivity after exposure to DEHP or its metabolites (Amjad et al. 2021; Hosseinzadeh et al. 2021; Jurewicz et al. 2013).
After showing a DEHP exposure dependent reduction in sperm motility in vitro, we extended this analysis to evaluate the sperm parameters in vivo of healthy donors who underwent plateletpheresis with a presumably substantial load of DEHP. The concept of the study foresaw that donors delivered ejaculates 48 h to 1 h before apheresis and 1 h after apheresis. This resulted in different time intervals between first ejaculation and ejaculation after apheresis between donors (maximum time interval: 49 h; minimum time interval: 2 h). A shorter interval between ejaculations affects sperm concentration, semen volume and sperm count in all motility subgroups, but more sperm with higher velocity, progressiveness and hyperactivation could be identified (Alipour et al. 2017; Carlsen et al. 2004). Therefore, the different time periods between donations are not a determining factor for the single-cell analyses performed in this study.
Increasing concentrations of DEHP in serum and urine samples after plateletpheresis have been shown previously (Buchta et al. 2003; Koch et al. 2005). In our study, mass spectrometric analyses of DEHP, MEHP as well as its three biologically active metabolites - 5OH-MEHP, 5oxo-MEHP and 5cx-MEPP - showed a short-term accumulation of phthalates in seminal plasma one hour after plateletpheresis. Single-cell analysis performed at the same time as mass spectrometric analysis demonstrated a decrease in human sperm beat frequency. Depending on the donor, a return to increased beat frequency is measurable after 2–7 days. These results show for the first time on a single-cell level, a correlation between an increase of phthalate concentration in the seminal plasma and changes in sperm motility. The design of our study does not enable us to prove a causal link between the concentration of plasticizer and a decrease in sperm beat frequency in vivo. However, other reasons for this observation are very unlikely. One could argue that citrate, which is applied to the donors blood during the apheresis procedure, might be responsible for a Ca2+ decrease in the ejaculate, leading to altered sperm motility. A decrease of Ca2+ in the seminal fluid longer than 60 min after apheresis is rather unlikely. Lokhande and co-workers showed that the serum Ca2+ level of platelet donors recovers almost completely 30 min after termination of plateletpheresis (Lokhande et al. 2021). The ejaculates analyzed in this study were obtained more than 60 min after plateletpheresis.
A possible explanation for the decreased beat frequency after plateletpheresis observed in this study could be a negative effect of accumulated PAEs on CatSper, the major calcium channel in sperm, necessary for sperm motility and thus for successful fertilization (Carlson et al. 2003; Ren et al. 2001). It has already been speculated that EDCs may manipulate sperm fertility via CatSper (Schiffer et al. 2014). The arrangement of CatSper nanodomains in long zigzag rows, formed by repeating CatSper units which could coordinate the opening of the entire array of CatSper channels along the flagellar axis, guarantees synchronous Ca2+ along the sperm tail to regulate flagellar bending (Zhao et al. 2022). A misalignment of the CatSper zigzag rows could impede continuous Ca2+ influx, resulting in sperm movement changes (Wiesehofer et al. 2022; Zhao et al. 2022). Further analysis is needed to address the question as to whether EDCs can manipulate human sperm motility via CatSper. A possible approach to this question could be to repeat the current study and analyse sperm motility in patients who have undergone thrombocytapheresis and have a deletion or mutation in the CatSper genes. However, the mouse study conducted by the Lishko group found no significant changes in monovalent CatSper currents after DEHP exposure (Khasin et al. 2020). The differences in the effect on sperm motility parameters in humans and mice as shown in Figs. 1 and 2 could also be explained by differences in CatSper activation in the two species. The Lishko group speculated that it is also possible that the massive production of reactive oxygen species (ROS) impairs sperm fertility (Khasin et al. 2020). It is known that mitochondria are the key producers of ROS (Murphy 2009), which have cytotoxic effects on sperm reproductivity. Phthalate esters and bisphenols trigger oxidative stress through the formation of ROS, as well as through the reduction of enzymatic and non-enzymatic antioxidants in both animal models and seminal plasma of infertile patients (Al-Saleh et al. 2019; Bahrami et al. 2018; Rahman and Pang 2019). Therefore, it is possible that the PAE accumulation measured after plateletpheresis leads to increased ROS production, resulting in the observed sperm motility deficiencies. There is already evidence that acute DEHP exposure results in an excessive ROS production in sperm, leading to an altered capacitation, marked by fast tyrosine phosphorylation (Khasin et al. 2020). Capacitation is a developmental process which sperm undergo in the female reproductive tract (Austin 1951; Chang 1951) and is required for mammalian sperm navigation and successful oocyte penetration (Carlson et al. 2003; Ho et al. 2009; Yanagimachi 1970). Capacitation is characterized by a pattern of hyperactive sperm motility that requires Ca2+ influx via the sperm-specific selective CatSper channel (Qi et al. 2007; Ren et al. 2001). In addition to altered capacitation, inhibition of the acrosome reaction, a Ca2+-dependent process required for oocyte plasma membrane binding and fusion (Breitbart 2002; Yanagimachi 1995), was also observed after DEHP exposure (Khasin et al. 2020). The authors speculate that the inhibition of the acrosome reaction is due to lipid peroxidation, provoked by excessive oxidative stress (Khasin et al. 2020). Based on these results, further work should investigate whether there is an inhibition of the acrosome reaction, possibly through the induction of oxidative stress, triggered by the PAE accumulation in seminal plasma observed in this study after plateletpheresis.
Apart from ROS production, a reduction in ATP levels in spermatozoa due to PAE exposure could explain the observed motility decrease (Amjad et al. 2021). ATP is produced via oxidative phosphorylation in mitochondria and is crucial for sperm motility (Tourmente et al. 2015). A study has already been published on the DEHP-dependent inhibition of the expression of oxidative phosphorylation complex subunits in testes leading to reduced ATP levels and resulting in decreased sperm motility and asthenozoospermia (Li et al. 2014; Pelliccione et al. 2011; Piomboni et al. 2012). Additionally, reduced sperm motility has already been found in DEHP-exposed workers (Huang et al. 2014). Thus, the reduced sperm motility after plateletpheresis observed in this study is possibly due to the reduction of ATP levels and induction of ROS production after DEHP exposure.
Based on the results of this study, we hypothesize that there is a relationship between phthalate exposure due to plateletpheresis and a transient decrease in sperm beat frequency. Analysis of seminal plasma PAE concentration and beat frequency over a 168-hour period showed a reversible effect of apheresis on sperm beat frequency and on the DEHP concentration in seminal plasma. The key question as to whether decreasing motility parameters such as beat frequency also have an effect on the reproductive capacity is difficult to answer. There are many factors which influence the boundaries between fertility and infertility. Therefore, we can only speculate that the decrease in beat frequency represents a measurable change in sperm motility but that it does not inevitably cause infertility. However, this decrease could become clinically relevant if other factors affecting fertility, such as decreased sperm concentration, are also present. We therefore hypothesize that the effects of DEHP on sperm motility may be of greater interest in subfertile men who already show reduced fertility, than in men with no other suspicious sperm parameters. This aspect requires investigation in further studies. However, donors should be informed prior to apheresis of the possible effect with regard to a transient and reversible decrease in their reproductive capacity.
Data Availability
The original contributions presented in the study are included in the article or Supplementary Material. Further inquiries can be directed to the corresponding author.
Abbreviations
- ACD-A:
-
Anticoagulant Citrate Dextrose Solution, Solution A
- BF:
-
Beat frequency analysis
- DEHP:
-
Di-2-ethylhexyl phthalate
- ECMO:
-
Extracorporeal membrane oxygenation
- EDCs:
-
Endocrine-disrupting chemicals
- HPLC:
-
High performance/pressure liquid chromatography
- HS:
-
HEPES-buffered saline solution
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- M:
-
Metabolite analysis
- MEHP:
-
Mono(2-ethylhexyl) phthalate
- 5OH-MEHP:
-
Mono(2-ethyl-5-hydroxyhexyl) phthalate
- 5oxo-MEHP:
-
Mono(2-ethyl-5-oxohexyl) phthalate
- 5cx-MEPP:
-
Mono(2-ethyl-5-carboxypentyl) phthalate
- PAEs:
-
Phthalates
- VCL:
-
Curvilinear velocity
References
Al-Saleh I, Coskun S, Al-Doush I, Al-Rajudi T, Al-Rouqi R, Abduljabbar M, Al-Hassan S (2019) Exposure to phthalates in couples undergoing in vitro fertilization treatment and its association with oxidative stress and DNA damage. Environ Res 169:396–408. https://doi.org/10.1016/j.envres.2018.11.018
Albro PW, Corbett JT, Schroeder JL, Jordan S, Matthews HB (1982) Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect 45:19–25. https://doi.org/10.1289/ehp.824519
Alipour H, Van Der Horst G, Christiansen OB, Dardmeh F, Jorgensen N, Nielsen HI, Hnida C (2017) Improved sperm kinematics in semen samples collected after 2 h versus 4–7 days of ejaculation abstinence. Hum Reprod 32(7):1364–1372. https://doi.org/10.1093/humrep/dex101
Amjad S, Rahman MS, Pang WK, Ryu DY, Adegoke EO, Park YJ, Pang MG (2021) Effects of phthalates on the functions and fertility of mouse spermatozoa. Toxicology 454:152746. https://doi.org/10.1016/j.tox.2021.152746
Api AM (2001) Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol 39(2):97–108. https://doi.org/10.1016/s0278-6915(00)00124-1
Austin CR (1951) Observations on the penetration of the sperm into the mammalian egg. Australian J Sci Res Ser B-Biological Sci 4(4):581–596. https://doi.org/10.1071/Bi9510581
Axelsson J, Rylander L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Giwercman A (2015) Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ Int 85:54–60. https://doi.org/10.1016/j.envint.2015.07.005
Bahrami N, Goudarzi M, Hosseinzadeh A, Sabbagh S, Reiter RJ, Mehrzadi S (2018) Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed Pharmacother 108:515–523. https://doi.org/10.1016/j.biopha.2018.09.044
Breitbart H (2002) Role and regulation of intracellular calcium in acrosomal exocytosis. J Reprod Immunol 53(1–2):151–159. https://doi.org/10.1016/s0165-0378(01)00085-7
Buchta C, Bittner C, Hocker P, Macher M, Schmid R, Seger C, Dettke M (2003) Donor exposure to the plasticizer di(2-ethylhexyl)phthalate during plateletpheresis. Transfusion 43(8):1115–1120. https://doi.org/10.1046/j.1537-2995.2003.00479.x
Buchta C, Bittner C, Heinzl H, Hocker P, Macher M, Mayerhofer M, Schmid R, Seger C, Dettke M (2005) Transfusion-related exposure to the plasticizer di(2-ethylhexyl)phthalate in patients receiving plateletpheresis concentrates. Transfusion 45(5):798–802. https://doi.org/10.1111/j.1537-2995.2005.04380.x
Carlsen E, Petersen JH, Andersson AM, Skakkebaek NE (2004) Effects of ejaculatory frequency and season on variations in semen quality. Fertil Steril 82(2):358–366. https://doi.org/10.1016/j.fertnstert.2004.01.039
Carlson AE, Westenbroek RE, Quill T, Ren DJ, Clapham DE, Hille B, Garbers DL, Babcock DF (2003) CatSper1 required for evoked Ca2 + entry and control of flagellar function in sperm. Proc Natl Acad Sci USA 100(25):14864–14868. https://doi.org/10.1073/pnas.2536658100
Cha S, Jung K, Lee MY, Hwang YJ, Yang E, Lee SH, Jung HI, Cheon YP (2018) Nonmonotonic effects of Chronic Low-Dose Di(2-ethylhexyl) phthalate on gonadal Weight and Reproductive. Dev Reprod 22(1):85–94. https://doi.org/10.12717/DR.2018.22.1.085
Chang MC (1951) Fertilizing Capacity of Spermatozoa Deposited into the fallopian tubes. Nature 168(4277):697–698. https://doi.org/10.1038/168697b0
Darbre PD (2017) Endocrine disruptors and obesity. Curr Obes Rep 6(1):18–27. https://doi.org/10.1007/s13679-017-0240-4
Eckert E, Muller J, Goen T (2015) Simultaneous determination of polyvinylchloride plasticizers di(2-ethylhexyl) phthalate and tri(2-ethylhexyl) trimellitate and its degradation products in blood by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1410:173–180. https://doi.org/10.1016/j.chroma.2015.07.083
Ferguson MS, Graham OH et al (1946) Studies on schistosomiasis japonica; protection experiments against schistosomiasis japonica. Am J Hyg 44(3):367–378. https://doi.org/10.1093/oxfordjournals.aje.a119103
Genuis SJ, Beesoon S, Lobo RA, Birkholz D (2012) Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. ScientificWorldJournal, 2012, 615068. https://doi.org/10.1100/2012/615068
Giuliani A, Zuccarini M, Cichelli A, Khan H, Reale M (2020) Critical review on the Presence of phthalates in Food and evidence of their Biological impact. Int J Environ Res Public Health 17(16). https://doi.org/10.3390/ijerph17165655
Hauser R, Duty S, Godfrey-Bailey L, Calafat AM (2004) Medications as a source of human exposure to phthalates. Environ Health Perspect 112(6):751–753. https://doi.org/10.1289/ehp.6804
Ho K, Wolff CA, Suarez SS (2009) CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Fertil Dev 21(2):345–350. https://doi.org/10.1071/rd08183
Hosseinzadeh A, Mehrzadi S, Siahpoosh A, Basir Z, Bahrami N, Goudarzi M (2021) The ameliorative effect of ellagic acid on di-(2-ethylhexyl) phthalate-induced testicular structural alterations, oxidative stress, inflammation and sperm damages in adult mice. Reprod Biol Endocrinol 19(1):146. https://doi.org/10.1186/s12958-021-00830-0
Huang LP, Lee CC, Fan JP, Kuo PH, Shih TS, Hsu PC (2014) Urinary metabolites of di(2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int Arch Occup Environ Health 87(6):635–646. https://doi.org/10.1007/s00420-013-0905-6
Isidori AM, Pozza C, Gianfrilli D, Isidori A (2006) Medical treatment to improve sperm quality. Reprod Biomed Online 12(6):704–714. https://doi.org/10.1016/s1472-6483(10)61082-6
Johns LE, Cooper GS, Galizia A, Meeker JD (2015) Exposure assessment issues in epidemiology studies of phthalates. Environ Int 85:27–39. https://doi.org/10.1016/j.envint.2015.08.005
Jurewicz J, Radwan M, Sobala W, Ligocka D, Radwan P, Bochenek M, Hawula W, Jakubowski L, Hanke W (2013) Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod Toxicol 42:232–241. https://doi.org/10.1016/j.reprotox.2013.10.001
Kaestner F, Seiler F, Rapp D, Eckert E, Muller J, Metz C, Bals R, Drexler H, Lepper PM, Goen T (2020) Exposure of patients to di(2-ethylhexy)phthalate (DEHP) and its metabolite MEHP during extracorporeal membrane oxygenation (ECMO) therapy. PLoS ONE 15(1):e0224931. https://doi.org/10.1371/journal.pone.0224931
Khasin LG, Della Rosa J, Petersen N, Moeller J, Kriegsfeld LJ, Lishko PV (2020) The impact of Di-2-Ethylhexyl phthalate on sperm fertility. Front Cell Dev Biol 8:426. https://doi.org/10.3389/fcell.2020.00426
Koch HM, Angerer J, Drexler H, Eckstein R, Weisbach V (2005) Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health 208(6):489–498. https://doi.org/10.1016/j.ijheh.2005.07.001
Lash TL (2007) Heuristic thinking and inference from observational epidemiology. Epidemiology 18(1):67–72. https://doi.org/10.1097/01.ede.0000249522.75868.16
Li X, Fang EF, Scheibye-Knudsen M, Cui H, Qiu L, Li J, He Y, Huang J, Bohr VA, Ng TB, Guo H (2014) Di-(2-ethylhexyl) phthalate inhibits DNA replication leading to hyperPARylation, SIRT1 attenuation, and mitochondrial dysfunction in the testis. Sci Rep 4:6434. https://doi.org/10.1038/srep06434
Lokhande T, Thomas S, Kumar G, Bajpai M (2021) The play of citrate infusion with calcium in Plateletpheresis Donors. Indian J Hematol Blood Transfus 37(2):295–301. https://doi.org/10.1007/s12288-020-01339-z
Main KM, Skakkebaek NE, Virtanen HE, Toppari J (2010) Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 24(2):279–289. https://doi.org/10.1016/j.beem.2009.10.003
Mannowetz N, Wandernoth P, Hornung J, Ruffing U, Raubuch M, Wennemuth G (2011) Early activation of sperm by HCO3- is regulated hormonally in the murine uterus. Int J Androl 34(2):153–164. https://doi.org/10.1111/j.1365-2605.2010.01067.x
Meeker JD, Ferguson KK (2014) Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab 99(11):4346–4352. https://doi.org/10.1210/jc.2014-2555
Meeker JD, Calafat AM, Hauser R (2009) Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl 30(3):287–297. https://doi.org/10.2164/jandrol.108.006403
Meeker JD, Calafat AM, Hauser R (2012) Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol 22(4):376–385. https://doi.org/10.1038/jes.2012.7
Minatoya M, Araki A, Miyashita C, Ait Bamai Y, Itoh S, Yamamoto J, Onoda Y, Ogasawara K, Matsumura T, Kishi R (2018) Association between prenatal bisphenol A and phthalate exposures and fetal metabolic related biomarkers: the Hokkaido study on Environment and Children’s Health. Environ Res 161:505–511. https://doi.org/10.1016/j.envres.2017.11.052
Mortensen GK, Main KM, Andersson AM, Leffers H, Skakkebaek NE (2005) Determination of phthalate monoesters in human milk, consumer milk, and infant formula by tandem mass spectrometry (LC-MS-MS). Anal Bioanal Chem 382(4):1084–1092. https://doi.org/10.1007/s00216-005-3218-0
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13. https://doi.org/10.1042/Bj20081386
Muschol M, Wenders C, Wennemuth G (2018) Four-dimensional analysis by high-speed holographic imaging reveals a chiral memory of sperm flagella. PLoS One, 13(6):e0199678.https://doi.org/10.1371/journal.pone.0199678
Pant N, Pant A, Shukla M, Mathur N, Gupta Y, Saxena D (2011) Environmental and experimental exposure of phthalate esters: the toxicological consequence on human sperm. Hum Exp Toxicol 30(6):507–514. https://doi.org/10.1177/0960327110374205
Pant N, Kumar G, Upadhyay AD, Patel DK, Gupta YK, Chaturvedi PK (2014) Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ Sci Pollut Res Int 21(18):11066–11074. https://doi.org/10.1007/s11356-014-2986-5
Pearson SD, Trissel LA (1993) Leaching of diethylhexyl phthalate from polyvinyl chloride containers by selected Drugs and formulation components. Am J Hosp Pharm 50(7):1405–1409. https://www.ncbi.nlm.nih.gov/pubmed/8362871
Peck CC, Albro PW (1982) Toxic potential of the plasticizer Di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect 45:11–17. https://doi.org/10.1289/ehp.824511
Pelliccione F, Micillo A, Cordeschi G, D’Angeli A, Necozione S, Gandini L, Lenzi A, Francavilla F, Francavilla S (2011) Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil Steril 95(2):641–646. https://doi.org/10.1016/j.fertnstert.2010.07.1086
PETROVIČOVÁ IK, PILKA B, Tomáš (2014) The human biomonitoring of occupational exposure to phthalates. Mediterranean J Social Sci 5:101. https://doi.org/10.5901/mjss.2014.v5n19p101
Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V (2012) The role of mitochondria in energy production for human sperm motility. Int J Androl 35(2):109–124. https://doi.org/10.1111/j.1365-2605.2011.01218.x
Qi HY, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L, Kirichok Y, Ramsey IS, Quill TA, Clapham DE (2007) All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA 104(4):1219–1223. https://doi.org/10.1073/pnas.0610286104
Radke EG, Braun JM, Meeker JD, Cooper GS (2018) Phthalate exposure and male reproductive outcomes: a systematic review of the human epidemiological evidence. Environ Int 121(Pt 1):764–793. https://doi.org/10.1016/j.envint.2018.07.029
Rahman MS, Pang MG (2019) Understanding the molecular mechanisms of bisphenol A action in spermatozoa. Clin Exp Reprod Med 46(3):99–106. https://doi.org/10.5653/cerm.2019.00276
Ren DJ, Navarro B, Perez G, Jackson AC, Hsu SF, Shi Q, Tilly JL, Clapham DE (2001) A sperm ion channel required for sperm motility and male fertility. Nature 413(6856):603–609. https://doi.org/10.1038/35098027
Rogers JA, Metz L, Yong VW (2013) Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol 53(4):421–430. https://doi.org/10.1016/j.molimm.2012.09.013
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29(1):134–139 discussion 181 – 135. https://doi.org/10.1111/j.1365-2605.2005.00567.x
Schiffer C, Muller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, Frederiksen H, Waschle B, Kaupp UB, Balbach M, Wachten D, Skakkebaek NE, Almstrup K, Strunker T (2014) Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep 15(7):758–765. https://doi.org/10.15252/embr.201438869
Schmid P, Schlatter C (1985) Excretion and metabolism of di(2-ethylhexyl)phthalate in man. Xenobiotica 15(3):251–256. https://doi.org/10.3109/00498258509045356
Schulz CO, Rubin RJ (1973) Distribution, metabolism, and excretion of di-2-ethylhexyl phthalate in the rat. Environ Health Perspect 3:123–129. https://doi.org/10.1289/ehp.7303123
Skakkebaek NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16(5):972–978. https://doi.org/10.1093/humrep/16.5.972
Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jorgensen N, Swan SH, Sapra KJ, Ziebe S, Priskorn L, Juul A (2016) Male Reproductive disorders and Fertility trends: influences of Environment and genetic susceptibility. Physiol Rev 96(1):55–97. https://doi.org/10.1152/physrev.00017.2015
Sumner RN, Tomlinson M, Craigon J, England GCW, Lea RG (2019) Independent and combined effects of diethylhexyl phthalate and polychlorinated biphenyl 153 on sperm quality in the human and dog. Scientific Reports, 9:3409. https://doi.org/10.1038/s41598-019-39913-9
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Louis B, G. M (2013) Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 99(5):1324–1331e1321. https://doi.org/10.1016/j.fertnstert.2012.11.037
Tourmente M, Villar-Moya P, Rial E, Roldan ER (2015) Differences in ATP Generation Via Glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J Biol Chem 290(33):20613–20626. https://doi.org/10.1074/jbc.M115.664813
Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, Avidor-Reiss T (2020) Male Infertility is a Women’s Health Issue-Research and Clinical Evaluation of Male Infertility Is Needed. Cells 9(4). https://doi.org/10.3390/cells9040990
Vander Borght M, Wyns C (2018) Fertility and infertility: definition and epidemiology. Clin Biochem 62:2–10. https://doi.org/10.1016/j.clinbiochem.2018.03.012
Wang Y, Zhu H, Kannan K (2019) A review of Biomonitoring of Phthalate exposures. Toxics 7(2). https://doi.org/10.3390/toxics7020021
Wasserstein RL, Schirm AL, Lazar NA (2019) Moving to a World Beyond p < 0.05. Am Stat 73(sup1):1–19. https://doi.org/10.1080/00031305.2019.1583913
Wennemuth G, Carlson AE, Harper AJ, Babcock DF (2003) Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development 130(7):1317–1326. https://doi.org/10.1242/dev.00353
WHO (2010) WHO laboratory manual for the Examination and processing of human semen (5 ed.)
Wiesehofer C, Wiesehofer M, Dankert JT, Chung JJ, von Ostau NE, Singer BB, Wennemuth G (2022) CatSper and its CaM-like Ca2 + sensor EFCAB9 are necessary for the path chirality of sperm. Faseb Journal, 36(5):e22288. https://doi.org/10.1096/fj.202101656RR
Wippermann C (2020) Ungewollte Kinderlosigkeit 2020: Leiden - Hemmungen - Lösungen. Bundesministerium für Familie, Frauen und Jugend. https://www.delta-sozialforschung.de/cms/upload/pdf/Ungewollte-kinderlosigkeit-2020.pdf
Wittassek M, Angerer J (2008) Phthalates: metabolism and exposure. Int J Androl 31(2):131–138. https://doi.org/10.1111/j.1365-2605.2007.00837.x
Yanagimachi R (1970) The movement of golden hamster spermatozoa before and after capacitation. J Reprod Infertil 23(1):193–196. https://doi.org/10.1530/jrf.0.0230193
Yanagimachi R (1995) Mammalian fertilization. In e. a. E. Knobil (Ed.), The Physiology of Reproduction (pp. 189–317). Raven Press
Yen TH, Lin-Tan DT, Lin JL (2011) Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J Formos Med Assoc 110(11):671–684. https://doi.org/10.1016/j.jfma.2011.09.002
Zantek ND, Martinez RJ, Johnson AD, Tholkes AJ, Shah S (2021) Apheresis practice patterns in the United States of America: analysis of a market claims database. J Clin Apher 36(5):750–758. https://doi.org/10.1002/jca.21926
Zhao Y, Wang H, Wiesehoefer C, Shah NB, Reetz E, Hwang JY, Huang X, Wang TE, Lishko PV, Davies KM, Wennemuth G, Nicastro D, Chung JJ (2022) 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat Commun 13(1):3439. https://doi.org/10.1038/s41467-022-31050-8
Zota AR, Phillips CA, Mitro SD (2016) Recent fast food consumption and Bisphenol A and Phthalates exposures among the U.S. Population in NHANES, 2003–2010. Environ Health Perspect 124(10):1521–1528. https://doi.org/10.1289/ehp.1510803
Acknowledgements
We thank Kai Wilkens for his expert technical assistance and Ann Soether for language editing of the manuscript.
Funding
No funding was provided.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.W., G.W., N.E.O. designed research; N.E.O, A.M., J.S., M.W., C.T., A.S., J.T.D., C.W. analyzed data; N.E.O, A.M. and J.S. performed experiments; C.W., G.W., N.E.O. drafted the manuscript, N.E.O., A.M., M.W., B.A.H., C.T., A.S., J.T.D., C.W. reviewed and edited the manuscript;. The project was administered and supervised by G.W.
Corresponding author
Ethics declarations
Competing Interests
B.A.H. has had advisory roles for ABX, AAA/Novartis, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen R&D, Lightpoint Medical, Inc., and Pfizer; has received research funding from Astellas, Bristol Myers Squibb, AAA/Novartis, German Research Foundation, Janssen R&D, and Pfizer; and has received compensation for travel from Astellas, AstraZeneca, Bayer and Janssen R&D, all outside the submitted work. N.E.O. has received compensation for travel from Janssen R&D, outside the submitted work. All other authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethics Approval
All experiments with human spermatozoa have been approved by the Ethics Committee of the University of Duisburg-Essen (14-5748-BO and Addendum 11/2019).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
von Ostau, N.E., Martynov, A., Schreiber, J. et al. Transient Decrease in Sperm Motility after Plateletpheresis. Expo Health (2024). https://doi.org/10.1007/s12403-023-00621-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12403-023-00621-5