Abstract
Tropical xero-epiphytic orchids undergo a photosynthetic mechanism called ‘Crassulacean Acid Metabolism’, a modification of Calvin–Benson cycle. This anabolic process aids in the long-term retention of organically fixed CO2, imprinting them as active sequestrators and fixers of gaseous CO2. The malate decarboxylation competence of enzymes—phosphoenolpyruvate carboxykinase (PEPCK), Nicotinamide Adenine Dinucleotide malic enzyme (NAD-ME) and Nicotinamide Adenine Dinucleotide Phosphate malic enzyme (NADP-ME) in xero-epiphytic orchids that concentrate CO2 by employing RuBisCO to embark on a carboxylation route is defined in this paper. Physiological parameters like photosynthetic rates, and quantum yield, accompanied by histomorphometry were analysed. Our study indicated PEPCK as the dominant decarboxylating enzyme in tropical orchids. The efficiency of PEPCK was reinforced by two additional malic enzymes, which are dependent on cofactors, namely Nicotinamide Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide Phosphate. These results indicated the need to appraise the potential of tropical orchids as strategic plant contenders for CO2 triggered greenhouse effect mitigator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop production, forestry, and resource use account for roughly 30% of worldwide Green House Gas (GHG) emissions (Panchasara et al. 2021; “United States Environmental Protection Agency,” 2020). Increasing food production to meet consumption trends and nutritional needs and a reduction in GHG emissions are imperative if sustainable food security and eco-climate targets are to be met. However, since crop production is both a victim and perpetrator of GHG emissions, this has proved challenging.
Agricultural GHG contribution can be reduced by (a) Improving soil Carbon sequestration and reducing deforestation, (b) Improving N use efficiency, and (c) Water use efficiency of crops (Verge et al. 2007). Carbon sequestration or long-term carbon storage is primarily facilitated by photosynthesis. The enzyme Rubisco supports the key carbon fixing step called the Calvin–Benson cycle. Plants that use only the Calvin cycle for their carbon assimilation are called C3 plants. But the unspecific nature of Rubisco resulted in an uneconomical process called photorespiration which made it rather inefficient. This led to the C3 pathway’s evolutionary anatomical and biochemical modification, giving rise to C4 (Hatch and Slack pathway) plants and CAM (Crassulacean acid metabolism pathway) plants.
Fluctuating CO2 concentration resulted in the molecular evolution of Rubisco within these photosynthetic modifications (Hermida-carrera et al. 2020). C4 plants evolved to overcome the limitations of Rubisco, which are exacerbated in high temperatures, thus bypassing photorespiration. Selective factors for CAM photosynthesis are low daytime CO2 and reduced ambient CO2 concentration in aquatic plants. It is an adaptation to arid environment and proves advantageous in habitats with excess rainfall (Gamisch et al. 2021). The forest canopies and buffer precincts of Western Ghats, a ‘Biodiversity Hotspot’, are rich in diverse and persistent epiphytic and terrestrial orchids.
Epiphytic hydraulics have been shown to affect the forest microclimate evapotranspiration rates by employing various distinctive characteristics. Epiphytes are touted to be models for studying the hydraulic behaviour of plants to help contribute to global sustainable water management (Miller et al. 2021). The orchid family is one of few flowering plant lineages that thrive in epiphytic and terrestrial forms. In the late 1950s, studies on atmospheric CO2 fixation by gas exchange in aerial roots (ARs) of orchids were demonstrated (Hew and Yong 2003). The carbon isotopic ratios indicated that the leaves and aerial roots in orchids rely on Crassulacean acid metabolism (CAM) (Zotz et al. 1997; Aschan and Pfanz 2003) for gaseous CO2 fixation.
The degree of CAM expression is influenced by various environmental and endogenous factors, such as temperature, irradiance, moisture, growth stage and leaf age (Ota et al. 1991; Ehleringer 1994; Luttge 2004; Dayer et al. 2017; Lambers et al. 2019; Yadav et al. 2020). The CAM photosynthetic pathway in these plants is segregated into four phases based on time (Osmond et al. 1999). Nocturnal stomatal opening results in CO2 uptake, followed by PEPC fixation and vacuolar storage as organic acid constitutes phase 1. The highly sensitive phase II involves the continued open phase of stomata in the early light for CO2 uptake. After the remobilisation of the organic acid for decarboxylation and fixation, the more stable phase III presents assimilated carbon behind closed stomata. The transient Phase IV reopens the stomata in late light to replenish CO2, followed by direct assimilation due to depleted organic acid stock. Phase II and IV are transient due to the shift in the enzyme for CO2 uptake. Whilst phase II is PEPC dominated, phase IV is Rubisco dominated C3 photosynthesis (Maxwell et al. 1999).
The characteristic traits that discriminate C4 (Hatch 1987) and CAM plants from C3 species are: (a) high optimal temperature and extraordinary irradiance saturation for maximum leaf photosynthetic rates, (b) reduced O2 fixation and CO2 evolution under high temperature and irradiance (photorespiration) (c) truncated CO2 compensation point, (d) low mesophyll resistance to CO2 diffusion coupled with high stomatal resistance leading to high water use efficiency, (e) higher internal exposed mesophyll surface area per cell volume and (f) ability to recycle respiratory CO2 by illuminated leaves (Ota et al. 1991; Ehleringer 1994; Luttge 2004). These findings laid the foundation for a series of subsequent physiological, anatomical and biochemical studies that assisted in the elucidation of the C4 and CAM photosynthetic pathways. In an anthropologically altered milieu, it is predicted that C4 plants might lose their competitive benefit over C3 plants (Sage and Sage 2009; Silvera et al. 2009), unlike CAM plants.
Surprisingly CAM group of species shares similar features expressed by their C4 counterparts except for temporal and spatial dissimilarity in the localisation of substrates. Therefore, identifying tolerant CAM species with optimal photosynthetic efficiency would complement mitigating greenhouse effects in micro-ecological niches. With this view, orchid species in the Western Ghats were scrutinised for their photosynthetic efficiencies, as their ability to reduce CO2 is less studied. There are no studies in Orchidaceae to date, which reported the decarboxylation efficiency of enzymes, especially Nicotinamide Adenine Dinucleotide Malic Enzyme (NAD-ME) Nicotinamide Adenine Dinucleotide Phosphate Malic Enzyme (NADP-ME) or Phosphoenolpyruvate Carboxykinase (PEPCK). These enzymes play a primary role in decarboxylating malate to pyruvate by releasing CO2 for re-fixation by RuBisCO. Their decarboxylation efficiency is vital to computing the carbon assimilating ability in plants. This is crucial to assess their micro-niche climate re-tuning ability. Histomorphological studies were carried out on aerial roots, leaves and pseudo bulb tissues of the selected plants. We measured the photosynthetic efficiency (A), quantum yield (ФCO2), and RuBisCO activity in intact leaf tissues of all the plants using the photosynthesis measuring system. The study interrelated the parameters above to identify photosynthetically efficient and tolerant tropical orchid candidates that can mitigate CO2-based greenhouse effects in a micro-ecological niche.
Materials and Methods
Plant Material, Collection, and Maintenance
In the present study, we identified the efficiencies of the three critical malate decarboxylation enzymes in eight common tropical orchids. These are Acampe praemorsa, Bulbophyllum neilgherrense, Dendrobium Sonia (white variety), Pholidota pallida, Rhynochostylis retusa, Sarcanthus pauciflorus, Vanda spathulata, Vanilla planifolia and three monocot control species representing C3, C4 and CAM photosynthetic types like Oryza sativa, Spathoglottis plicata, Saccharum officinarum, and Aloe vera respectively. The phenology of orchids (vegetative and reproductive cycles and their abundance with seasonal changes) was studied every three months in a year at four sites in central Western Ghats regions of Karnataka, India. These were Agumbe (13.502712°N, 75.0903191°E), Hebri (13.458111°N, 74.995251°E), Horanadu (13.162679°N, 75.203887°E) and Sringeri (13.419814°N, 75.256738°E). Dendrobium Sonia and Vanilla planifolia were obtained commercially from an Indian Nursery (Fig. 1A). The species identification was done with the help of a taxonomist, Dr A. Radhakrishna Rao, and also by referring to various texts on regional floras (Abraham and Vatsala 1981; Bhat 2003). The inclusion of orchid species for this study was based on the following criteria: (a) high tolerance to seasonal changes, (b) high abundance throughout the year, with the predominant vegetative phase in natural conditions, (c) superior acclimatisation ability to greenhouse conditions and (d) species with CAM morphological characters. We excluded saprophytic orchid species. Four control plants, such as Oryza sativa, Spathoglottis plicata, Saccharum officinarum, and Aloe vera, along with the eight test orchids (Table 1), that served as sources of tissue samples were acclimatised to the greenhouse at Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, India (Fig. 1B). Orchids maintained in pots were well aerated and percolated with charcoal, stones, sand, and soil in 1:1:1:1 proportion. The orchids were grown in the greenhouse for three months to monitor their adaptability before the harvest of tissues for the experiment. The samples and readings were taken at 30 °C at 11am for every reading.
A Map showing the Western Ghats. a Climate variations and orchid prevalence statistics of Western Ghats. b Arrows indicating the collection sites 1-Hebri, 2-Agumbe Ghats, 3-Sringeri, 4-Horanadu of Central Western Ghats where Agumbe is located c Profuse growth of orchids (Cymbidium aloifolium together with Dendrobium herbaceum) found on the crown of Artocarpus heterophyllus, a frequent host at Horanadu d Cymbidium aloifolium found on a senile branch of Mangifera indica at Sringeri. B Orchid and test species in situ and ex situ. The natural habitat of a Acampe praemorsa, b Bulbophyllum neilgherrense, c Dendrobium Sonia in Dhanalaxmi Nursery, Hebri, Karnataka, India. The natural habitat of d Pholidota pallida, e Rhynchostylis retusa, f Sarcanthus pauciflorus, g Spathoglottis plicata acclimatised to the greenhouse, h Vanda spathulata in the wild habitat, i Vanilla planifolia, j Oryza sativa, k Saccharum officinarum, l Aloe vera which is acclimatised to the greenhouse (scale bar = 15 cm) of Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India
Sample Processing for Enzyme Analysis
500 mg of tissue was harvested from illuminated fresh young leaves, tender pseudo bulbs, and aerial roots of the plants. Enzyme extraction was carried out at a temperature of 4 °C in a pre-chilled mortar and pestle. Complete maceration of the tissue sample was ensured before grinding in an appropriate volume of extraction buffer (1 ml). The components of extraction buffer included 50 mM HEPES–KOH (pH 7.3), 2.5 mM MgCl2, 2.5 mM MnCl2, 1 mM Na4–EDTA, 20% (v/v) Glycerol and 10 mM 2-Mercaptoethanol (Ueno et al. 2005). The homogenate was filtered and centrifuged at 9000×g for 10 min at 4 °C, and then the supernatant was collected. The crude extract was stored in ice until assays were performed. The aliquots were used for three separate analyses. The analyses performed were a) activity detection of three malate decarboxylation enzymes, such as NADP-ME, NAD-ME, PEPCK, b) total chlorophyll content and c) total protein content. The substrates and the chemicals were purchased from Sigma-Aldrich, USA.
Malate Oxidative Decarboxylation Efficiency
The enzyme activity was detected in Quartz cuvettes based on the change in the absorbance using UV-1800 UV–VIS Spectrophotometer (Shimadzu Scientific Instruments). The reaction was performed at 28 °C in a total volume of 1 ml of assay buffer in which 10 µl of the crude extract was analysed. The principle of detection of the enzyme activity was the formation of NAD (P) H or the depletion of NADH which was monitored at 340 nm. The stability of each buffer was analysed before the addition of enzymes. Malic enzymes (NADP-ME and NAD-ME) were assayed in decarboxylase direction coupled to the reduction of cofactor NADP+ and NAD+ respectively. The assay medium for NADP-ME comprised 50 mM Tris–HCl (pH 8.2), 1 mM Na4–EDTA, 20 mM MgCl2, 0.5 mM NADP+ and 5 mM Na-malate (Ku et al. 1991). The composition of the assay mix for NAD-ME was slightly modified from the buffer proposed elsewhere (Chen et al. 1993; Muhaidat et al. 2007). NAD-ME buffer mix comprised 25 mM HEPES—KOH (pH 7.2), 0.75 mM Na4-EDTA, 20 mM KCl, 2.5 mM NAD+, 75 µM coenzyme A, 8 mM MnCl2, 5 mM Na-malate. The PEPCK was assayed in the carboxylation direction. In a two-reaction system, the formation of oxaloacetic acid (OAA) from phosphoenolpyruvate (PEP) by PEPCK was monitored by the conversion of OAA to malate by the enzyme malic dehydrogenase (MDH). This reaction oxidises NADH. Therefore, the decrease in the absorbance at 340 nm was detected. The PEPCK assay buffer components include 100 mM HEPES–KOH (pH 7.0), 1 mM ADP,100 mM KCl, 4% (v/v) 2-Mercaptoethanol, 90 mM NaHCO3, 5 mM PEP, 4 mM MgCl2, 10 µM MnCl2, 0.14 mM NADH and 6 units of Porcine Heart MDH (Sigma Aldrich, USA). The production of 1 µmol of NAD (P) H or depletion of 1 µmol of NADH per hour at 28 °C is defined as the 1 unit of enzyme activity, which was later expressed both in µmol.mg total chlorophyll−1 h−1 and also as total protein in µmol. mg total protein−1 h−1.
Estimation of Total Chlorophyll and Total Protein Contents
A crude extract was aliquoted and was re-extracted with an equal volume of 100% acetone, and the absorbance was read using UV–VIS Spectrophotometer. Chlorophyll a showed maximum absorbance at 662 nm, and chlorophyll b showed maximum absorbance at 645 nm. Three concordant readings were taken for each wavelength and values of chlorophyll a and b were calculated based on the following equations: Chlorophyll a [Chl a = 11.75 A662–2.350 A645]; Chlorophyll b [Chl b = 18.61 A645–3.960 A662] (Lichtenthaler 1987). Total chlorophyll content was the additive value of Chlorophyll a and Chlorophyll b, and the expression unit was µg chlorophyll/g tissue of the plant. Total protein concentrations of the crude enzyme extracts were determined in a 96-well plate assay protocol by spectrophotometer using Bradford’s Reagent (Sigma-Aldrich, USA).
Histomorphometry and Calculations for Assessing Photosynthetic Efficiency
Three replicates of young photosynthetic tissues were excised from each plant and were incubated in Farmers’ fixative (3:1 ratio of 100% ethanol to glacial acetic acid). Transverse section of leaves, pseudo bulbs and aerial roots was chosen for freehand sectioning. Sections were stained with 1% safranin O and also with 1% toluidine blue. Sections were viewed using Motic BA400 microscope. Images were captured and measured using Motic Images Plus 2.0. In leaves, a reference frame of 100,000 µm2 was set enclosing a vascular bundle. Due to their broadleaf in Rhynochostylis retusa and Vanilla planifolia, a reference area measured was 150,000 µm2. The entire cross-sectional area of the pseudo bulb and aerial root was measured. The parameters analysed are defined below:
-
1.
Total Photosynthetic Carbon Reductive (PCR) Tissue: PCR tissue stands for the area of the bundle sheath (BSArea), which encircles a vascular bundle (VB). PCR holds a circular dimension denoted as factor ‘X’. Area of bundle sheath was calculated, by measuring the radius of the vascular bundle (R) inclusive of the BS. Then the radius of the vascular bundle excluding BS is measured (r). The area of BS is calculated using a formula:
$$BS = \, (\pi R^{2} - \pi r^{2} )$$ -
2.
Total Photosynthetic Carbon Assimilative (PCA) Tissue: The area of PCA stands for the zone occupied by mesophyll tissue in the case of leaves/zone occupied by the cortical region in the case of stem and pseudo bulb exclusive of the vascular bundle within the reference frame. PCA is calculated using the formula = (Areference − πR2).
-
3.
BS to VB ratio estimates the Photosynthetic Carbon Reductive (PCR) Tissue which is calculated by the formula (πR2 − πr2)/πR2. In case of multiple vascular bundles (for example in aerial roots and pseudo bulbs), the total number of vascular bundles were counted and were subjected to the formula mentioned above.
-
4.
The ratio of the PCA/Area of Reference (RA), is labelled as (PCA/RA = Y). The ratio of the PCR/Area of Reference (RA), is marked as (PCR/RA). The ratio of Y to PCR gives a factor that normalises every tissue (leaf, aerial root, and pseudo bulb) under scrutiny, irrespective of their stage or different dimensions.
-
5.
Mean Leaf Thickness: After cross-sectioning, the leaf thickness was measured in three different samples for each plant, and the mean ± SD was calculated.
Statistical Analysis
To compare and understand the degree of dissimilarity between the quantitative variables, we performed a one-way ANOVA, and the variables were statistically evaluated using post hoc test viz., Bonferroni’s Multiple Comparison test. The mean ± standard deviation & post hoc tests were computed using Graph Pad Prism version 5.01, using the triplicate readings obtained from three independent enzyme isolations and three different cross sections. We also performed a Principal Component Analysis (PCA) using R programme to correlate the activity of three decarboxylating enzymes and to identify the most significant anatomical variable.
Physiological Response Curves
The mobile photosynthesis measuring system used for the present study was LICOR (LI-6400XT Portable Photosynthesis System), USA. The Infrared Gas Analyser (IRGA) was connected first to the console with chemical cylinders in a complete bypass mode. The instrument was set to be in Light control settings and was allowed to warm up for a minimum of 15 min. The reference value set was 380 μmol mol−1 CO2, after which the stabilisation and matching of CO2 were performed.
Light Response Curve
A 6400-02B LED light source was used with a constant CO2 reference set at ambient at 380 μmol CO2 mol−1 and flow fixed at 500 μmol s−1. Temperature control set was at 35ºC for constant leaf temperature. Insertion of the leaf into the chamber was done first after matching CO2 values, and the leaf area was finally adjusted. The stomatal ratio was kept constant at 0.5. Measurements were taken using a light response panel as follows: 2000, 1500, 1000, 600, 200, 50, 0, 10, 100, 400, 600, 1000, 1500 and 2000 μmol m−2 s−1. At each set value, the leaf was allowed to adjust for 10 min. The values recorded were, net photosynthetic rate (A), intercellular CO2 concentration (Ci) and transpiration rate (T), and graphs plotted were for photosynthetic rate and quantum yield. Triplicate readings taken from individuals of each species were used for computing the mean ± standard error.
CO2 Response Curves
The 6400-02B LED light source was used and set at 650 μmol m−2 s−1 with a constant CO2 reference set above ambient at 420 μmol CO2 mol−1 and flow fixed at 300 μmol s−1. The temperature set was at 35ºC. After matching CO2 values, leaf sample was inserted into the chamber. The leaf area and stomatal ratio were kept constant. Measurements were taken using a CO2 response panel of 400, 300, 200, 100, 50, 0, 100, 200, 300, 400 μmol CO2 mol−1. At each set value, the leaf was allowed to adjust for 5 min. The values recorded were the net photosynthetic rate (A), intercellular CO2 concentration (Ci) and A–Ci curve, as well as photosynthetic efficiency (PE) against time, were plotted. Measurements were taken from four individuals of each species in triplicates.
Real-Time Monitoring of Photosynthetic Competence in CAM Plants
To assess the real-time photosynthetic competence in orchids, we used the Farquhar approach (Farquhar et al. 1980) that spectacled a biochemical growth model which computes photosynthesis as a function of demand and obtainability of CO2. The advantage of this model is that photosynthesis is regulated not only by radiation and transpiration but also by air humidity, leaf temperature, CO2 availability and leaf nitrogen content. For a precise performance, driving variables need to be given to the simulation at least once per hour. In this model, the calculation of photosynthesis [A] is computed as mole carbon per leaf area per second. ‘R’ is the RuBisCO (photosynthetic enzyme) or carboxylation limited rate of assimilation, which is a function of light, leaf nitrogen and temperature and soil moisture. Photosynthesis, as per this model, works as a function of internal CO2 concentrations and is calculated accordingly, Eq. (1) for C3 and Eq. (2) for C4.
and
Vmax is the function of the maximum activity of RuBisCO. Cc is the chloroplast CO2 concentration. Γ* is the CO2 compensation point in the light in the absence of mitochondrial respiration. Kc is the Michaelis–Menten constant of RuBisCO for CO2, O is the oxygen concentration in the atmosphere, and Ko is the Michaelis–Menten constant of RuBisCO for O2.
Results
Profiles of Decarboxylation Enzymes Depicting the CO2 Reduction Efficiency of Tropical Orchids
The enzymes NAD-ME, NADP-ME and PEPCK were seen in the C3, C4, and CAM control plants. The C3 control plant Oryza sativa and Spathoglottis plicata have a higher PEPCK activity than NADP-ME and NAD-ME (Fig. 2A). The results indicated that the C3 plant used more PEPCK than NADP-ME and NAD-ME for decarboxylation of malate to pyruvate. In C4 control, Saccharum officinarum, NADP-ME was a dominant enzyme assisted by decarboxylation enzymes PEPCK and NAD-ME. Aloe vera, the CAM control, displayed predominant PEPCK activity closely followed by NAD-ME.
A Malate decarboxylating activity based on total protein content. The enzyme activity of malate decarboxylating enzymes a PEP-CK, b NADP-ME and c NAD–ME, reported in µmol.mg protein hr.Values depicted indicate the mean ± standard deviation. The relative enzyme activity was statistically validated using one-way ANOVA with and the significance between groups was verified using Bonferroni’s multiple comparison tests. The abbreviated letters stand for the parts of the plants selected for enzyme evaluations (L leaf, AR aerial root, PB pseudo bulb). d Principal Component Analysis plot depicting the enzyme dominance based on total protein content. B Malate decarboxylating activity based on total Chlorophyll content. The enzyme activity of malate decarboxylating enzymes a PEP-CK, b NADP-ME and c NAD–ME, reported in µmol.mg chlorophyll hr.Values depicted indicate the mean ± standard deviation. The relative enzyme activity was statistically validated using one-way ANOVA with and the significance between groups was verified using Bonferroni’s multiple comparison tests. The abbreviated letters stand for the parts of the plants selected for enzyme evaluations (L Leaf., AR Aerial root, PB Pseudo bulb). d Principal Component Analysis plot depicting the enzyme dominance based on total chlorophyll content
In chlorophyll-based enzyme activity calculations, highest PEPCK, NADP-ME, and NAD-ME activity were observed for homogenates of Pholidota pseudo bulbs and Vanilla pseudo bulb and leaf (Fig. 2B).
In total protein content-based enzyme activity, Pholidota leaf showed the highest NADP-ME activity, whereas Bulbophyllum pseudo bulb showed higher PEPCK activity. NAD-ME activity was highest in Bulbophyllum leaf and pseudo bulb. Only three tissues found in the eight orchids studied: Bulbophyllum leaf, Dendrobium aerial roots and Vanda spathulata aerial roots showed NAD-ME predominance. The remaining homogenates, when assayed, showed high levels of PEPCK independently or in combination with NADP/NAD-ME.
PEPCK dominance was observed in Rhynchostylis aerial root, Dendrobium leaf, Bulbophyllum pseudobulb, and the aerial root of Rhynchostylis and Vanilla. The PEPCK activity detected in leaf homogenates of Pholidota and Vanilla displayed a similar trend as that of CAM control Aloe. Pseudobulb homogenates of Pholidota and Bulbophyllum exhibited a closer resemblance to Saccharum officinarum (Table 2).
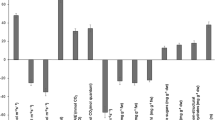
NADP-ME activity in leaf homogenates of Pholidota was on par with Saccharum officinarum. Homogenate of the elongated aerial root of Vanilla showed higher similarity to C4. The PCA plots of enzyme activity based on total protein [Fig. 2A(d)] and total chlorophyll content [Fig. 2B(d)] identified tissue-specific photosynthetic subtypes (Table 3).
Variables in Quantitative Histomorphometry that Aided in the Classification of Photosynthetic Subtypes
The ratio ‘Y/PCR’ could segregate CAM test species closer to C4 and C3 test plants, thus eliminating confounding anatomical dissimilarities (Fig. 4). The leaf thickness assessment and comparison (Fig. 4A) between the plant groups revealed that Sarcanthus has the highest midrib thickness, followed by Acampe and Vanda. All other species were closer to the CAM control plant Aloe vera.
All the test plants, irrespective of the leaf (Fig. 3A), aerial root (Fig. 3B) or the pseudo bulb (Fig. 3C), comprise a tight wadding of cells to diminish the intercellular air spaces. The mesophyll region of the leaf tissue and cortical area of the pseudo bulb and aerial root were packed with large vacuoles for storing the C4 acid (malic acid or oxaloacetic acid). Bundle sheath cells encircling the vascular bundles (Fig. 3D) were highly condensed, indicating their water use efficiency is relatively more efficient.
A Histomorphology of leaves. Leaf cross sections showing the architecture of midrib vascular bundle a Acampe praemorsa, b Bulbophyllum neilgherrense, c Dendrobium Sonia, d Pholidota pallida, e Rhynochostylis retusa, f Sarcanthus pauciflorus g Spathoglottis plicata, h Vanda spathulata, i Vanilla planifolia, j Oryza sativa, k Saccharum officinarum and l Leaf like the modified succulent stem of Aloe vera (scale bar = 100 µm). B Histology of Aerial roots. Aerial root cross section showing the architectures of a, b Acampe praemorsa, c, d Dendrobium Sonia, e Rhynchostylis retusa, f Spathoglottis plicata, g, h Vanda spathulata, I Vanilla planifolia (scale bar = 100 µm). C Histomorphology of Pseudobulbs. Pseudobulb architectures of a-Bulbophyllum neilgherrense, b Pholidota pallida and c-Vanilla planifolia (scale bar = 100 µm) D Histology of Vascular Bundle. Vascular bundle architecture is revealing the nature of bundle sheath cells a succulent stem of Aloe vera, Leaf of b Bulbophyllum neilgherrense, c Dendrobium Sonia, d Pholidota pallida, e Rhynchostylis retusa, f Sarcanthus pauciflorus, g Pseudostem of Spathoglottis plicata, the h aerial root of Vanilla planifolia, a leaf of i Oryza sativa and j Saccharum officinarum
The Y/PCR ratio was the highest for Oryza, followed by Saccharum. The PCA plot could precisely segregate plants with similar histomorphometric features of C3, C4 and CAM control groups (Fig. 4A–F). Acampe (leaf & aerial root), leaf blades of Bulbophyllum, Dendrobium, Pholidota, Rhynochostylis, Sarcanthus, and Vanilla displayed CAM type of histomorphology (Fig. 3A). Pseudo bulbs of Bulbophyllum, Pholidota, Vanilla, aerial roots of Dendrobium, Rhynochostylis, Vanda, Vanilla and leaves of Vanda demonstrated the C4 type of histomorphology [Fig. 3A(c)].
Quantitative analysis of variables about Histology. Histomorphometric variables for quantifying the photosynthetic efficiency of plants A Leaf thickness B The photosynthetic carbon reductive tissue obtained by the ratio of the area of the bundle sheath (BS) enclosing the vascular bundle to the area of the vascular bundle (VB) is used to find the cause behind the photosynthetic efficiency of plants C Ratio of Photosynthetic Carbon Assimilatory Tissue abbreviated as PCA (area of the tissue, excluding the vascular bundle and the bundle sheath) to the fixed reference area (RA) denoted as factor ‘Y’ D Ratio of Photosynthetic Carbon Reductive tissue to a fixed reference area (RA) E Quantitative morphometric variable that gave significant data regarding the photosynthetic efficiency in orchid plants computed as a ratio factor Y to PCR, where Y is Ratio of Photosynthetic Carbon Assimilatory Tissue abbreviated as PCA (area of the tissue excluding the vascular bundle and the bundle sheath) to the fixed reference area and PCR is the Photosynthetic carbon reductive tissue obtained by the ratio of the area of the bundle sheath (BS) enclosing the vascular bundle to the area of the vascular bundle (VB) abbreviated as X. Values depicted indicate mean ± standard deviation. The relative enzyme activity was statistically validated using one-way ANOVA and the significance between groups was verified using Bonferroni’s multiple comparison tests. The abbreviated letters stand for the parts of the plants selected for enzyme evaluations (L leaf, AR aerial root, PB pseudo bulb) F Principal Component Analysis plot depicting the correlation of PCR, PCA/RA, PCR/RA and Y/PCR
Evaluating the Photosynthetic Efficiency of CAM Tropical Orchids Using Light-Responsive Curve Plots
In our study, the typical CAM Aloe vera showed a light saturation point well below Oryza sativa [Fig. 5A(a)]. It is evident from Fig. 5A(a) that the orchids exhibited negative photosynthetic rates. Of these adverse photosynthetic rates cluster, only the C3 orchid control Spathoglottis plicata displayed a positive photosynthetic rate [Fig. 5A(b)], resembling the C3 and C4 control plants. Unusual behaviour was observed for Pholidota pallida, which stood out from the ordinary test plant clusters. Pholidota pallida demonstrated a negative photosynthetic rate.
A Photosynthetic Efficiency in Plants A. Light-Responsive Curves of plants. Comparative photosynthetic light response curve and photosynthetic efficiency plotted by the measurements obtained by the portable measuring system from leaves of selected plant species at three different PAR (Photosynthetically Active Radiation) values viz., 0, 500 and 1000 µ mol m−2 s−1) B Net Photosynthetic rates of tissues. Quantum yield obtained from the portable measuring system by plotting PAR values in X-axis against Net Photosynthetic rate in Y-axis [A] in the Y-axis (Four PAR values selected were 0, 400, 800 and 1200 µ mol m−2 s−1). B Quantum Yield. Slopes are showing the relative quantum yield of all plants indicating that the highest value was for the C4 control plant species Saccharum officinarum. C Relative Quantum Yield. Graph showing the relative quantum yield of all plants indicating that the highest value was for the C4 control plant species Saccharum officinarum. Values depicted in the table as inset indicate mean ± standard error of all plants selected for the study. D Relative Photosynthetic Rate. A-Ci curves of the selected orchid species plotted to find the corresponding photosynthetic performance where the slope gives the RuBisCO activity. E RuBisCO Activity in plant clusters. The RuBisCO activity of plant species with the values indicating the mesophyll conductance (inset tabulated). Values depicted in the table as inset suggests the mean ± standard error of all plants selected for the study
The C4 test plant Saccharum officinarum exhibited high photosynthetic rates in our study. It is clear from Fig. 5B that in C4 type plants, light saturation point is higher than the C3 and the light compensation point for C4 is lower than the C3 plants. Both of these characteristic features relate to the ability of C4 plants to provide an increased amount of CO2 availability to the Calvin–Benson cycle. We surveyed quantum yield in all eight orchid species and four controls of distinct photosynthetic types (Fig. 5B, C) and calculated the light-limited quantum yield from CO2 assimilation. We attempted to eliminate photo inhibitory conditions. The cause of low quantum yield is attributed to photorespiration and alternative electron sinks. Several physiological processes divert reducing power from carbon reduction, which accounts for evident variations in ΦCO2 in the orchid population.
Assessing the Photosynthetic Efficiency of CAM Tropical Orchids Using Quantum Yield
Photosynthetic efficiency is defined as the segment of light energy converted to chemical energy during photosynthesis and is regarded as the rate of net photosynthesis at a particular time point. It is also represented as the net photosynthetic rate against time (Fig. 5A). The linear region of each light response curve varied considerably amongst the species. Thus, the current study determined the range of photosynthetically active radiation (PAR) valid for ΦCO2 measurements by individually evaluating each light response curve. The data are represented in Fig. 5A(a). We plotted PE in the present study using ‘A’ against time, as depicted by [Fig. 5A(b)]. Highest PE was observed in Saccharum officinarum, followed by Oryza sativa and Spathoglottis plicata.
Saturation of CO2 and light beyond 30 min gave a positive value for PE for the C3 plant, reaching a value almost close to C4. However, this was not observed in the CAM cluster. Comparative quantum yield values were represented in a bar diagram (Fig. 5C), where the values were tabulated and displayed as an inset. From the quantum yield statistics, it is evident that Saccharum officinarum (C4) is photosynthetically efficient.
Quantum yield is a confounding factor for photosynthetic competence, and from the C3 control, the C4 control displayed almost a three-fold increment in quantum yield (Fig. 5B). Spathoglottis plicata also showed a similar quantum yield value to C3 Oryza sativa. Spathoglottis plicata was superior in photosynthesis and was distinct from other CAM types. Saccharum officinarum, Oryza sativa and Spathoglottis plicata and the clustering of CAM displayed similar trends in net photosynthetic rates (Fig. 5D), relative quantum yield (Fig. 5 C) and photosynthetic efficiency (PE) [Fig. 5A(b)]. Since the trend is the same in all the three calculations, we overruled underestimation/ overestimation of ΦCO2.
Gauging Net Carbon Assimilation Rate of CAM Tropical Orchids Using A–Ci Curves
A–Ci curves are the function of net carbon assimilatory rate [A] versus calculated sub stomatal CO2 concentration [Ci]. As per the Farquhar model, the action of RuBisCO was calculated as depicted in Fig. 5E. The plants can be grouped into three clusters: one was exhibiting high RuBisCO activity, which includes Oryza sativa, Saccharum officinarum, and Pholidota pallida; the second bunch with a moderate RuBisCO activity displayed by Spathoglottis plicata, Sarcanthus pauciflorus and Aloe vera and the third cluster including the remaining species presenting low RuBisCO activity.
Discussion
Tropical Orchids and the Factors That Contribute to Their Effective Decarboxylation
Malate decarboxylation efficiency in tropical orchids indicated that PEPCK is the dominant decarboxylation enzyme whose efficiency is reinforced by the activity of NADP-ME or NAD-ME. The enzyme analysis proved that Pholidota leaves displayed high decarboxylation efficiency and shared similar traits with Saccharum. Studies suggested that high NADP-ME activity indicates high RuBisCO activity (Ghannoum et al. 2005). We expected that Pholidota leaf, Acampe aerial root, and Vanda leaf would be the most tolerant species to display high photosynthetic efficiency based on the enzyme activity. The quantitative parameters, such as vein density, intercellular airspaces, and epidermal tissue proportions, can discriminate between C3 and C4 species but not within C4 subtypes (Muhaidat et al. 2007). CAM plants always displayed an enigmatic light-responsive curve, occasionally behaving very close to C4 and sporadically falling below C3. The CO2 exchange in CAM species is based on at least four distinct phases of autotrophic and heterotrophic CO2 fixation biochemistry (Osmond 1978). CO2 fixation processes are expected to quicken at the low light intensity and infrequently pose difficulty in computing the light-limited rate of photosynthesis using net CO2 fixation (Osmond et al. 1979).
Indications from Stomatal Behaviour and Quantum Yield
Stomata also tend to be closed during the de-acidification process, resulting in negligible CO2 fluxes. Stomatal closure can lead to negative photosynthetic rates. The diversity in the photosynthetic light response curve in orchids chosen for the study is attributed to various factors. The factors that affect the light-responsive curves include—(a) location of the leaf (horizontal or vertical) and its phyllotaxy, (b) shape of the leaf, (c) area of the leaf (broad or narrow), (d) the number of stomata in the upper (adaxial) and the lower (abaxial) surfaces and (e) photosynthetic enzymes and their behaviour (Evans 1993). Photosynthesis in the intermediate light range (600–700 μmol m−2 s−1) is the most efficient region where the convexity of the photosynthetic light response curve is maximum.
In our study, the quantum yield of photosynthesis is chosen as a measure of photosynthetic efficiency expressed in moles of PAR absorbed per mole of CO2 fixed. Depending on the measurement conditions and techniques adopted, Φ measurements ideally reflect different aspects of the photosynthetic apparatus. The maximum quantum yield (Φ max) was obtained when photosynthesis is light-limited, a state inferred by a linear relationship between photosynthesis and PAR. Theoretically, when Φ max = x, x moles of photons are required to reduce one mole of CO2 without photorespiration. Because there is some cyclic photophosphorylation operational, Φ maxima are inferred to be closer to 0.112 in most plants (Long et al. 1993).
Evidence Obtained from Light Response Curves on CAM Photosynthetic Efficiency
Some plants, predominantly wild genera growing in a compromised milieu, may intrinsically display low photosynthetic efficiency (Singsaas et al. 2001). It is evidenced that the higher the quantum yield, the higher will be the photosynthetic efficiency. Quantum yield was calculated from the photosynthetic light response data using linear regression across three or more points, where net CO2 assimilation was linearly related to PAR (von Caemmerer and Farquhar 1981). Photoinhibition is a light-induced reduction in photosynthesis, which may either result from photodamage or photoprotective chloroplast processes. These could probably be induced by the growth locale of the plant or by the experimental protocol (Bongi and Long 1987; Groom and Baker 1992), often caused by shallow temperatures or excess PAR (Sharkey 1988).
The largest of these sinks is photorespiration, which usually occurs at 10–30% of the photosynthesis rate at 25 °C with 350 μmol mol−1 CO2 at 21% oxygen (Sharkey 1988; Peterson 1990; Yeo et al. 1997). This sink can be eliminated by measuring ΦCO2 under a reduced 1–2% O2 atmosphere. Many plant physiologists suggest that photorespiration cannot adequately define variance in quantum yield. Since photoinhibition and photorespiration cannot clarify the variation in quantum yield, we based our analysis of the photosynthetic light response curve on the calculation of Φ max (Singsaas et al. 2001).
Photorespiration Influences Photosynthetic Energy Transduction
The primary difference between C3 and C4 plants is that photorespiration occurs in C3 plants, even at high levels of CO2. Leverenz (1987) suggested that the light-responsive curve can be subdivided into four regions- the first is the Kok Effect region, where the mitochondrial respiration increases at minimal light. We observed a similar effect in the present study, where we found that < 10 μmol m−2 s−1 PAR affected net photosynthesis and thereby increased the slope of the light-responsive curve. The second is the region, where net photosynthetic rate data points are aligned in linear mode with increasing PAR. The third is the transition region between the linear and light-saturated region, and the fourth and the last are the light saturating region.
When the photosynthetic rate is just about to take a linear phase, it implies that light is restrictive and respiration is constant for calculating Φ max. It is often seen that the inclusion of data points from the Kok Effect region would cause an overestimation of ΦCO2. In contrast, the inclusion of data points from the transition region would cause an underestimation of Φ CO2. It is suggested that improvement in PE played only a minor role in increasing productivity achieved in the last half-century (Zhu et al. 2010). However, assessment of PE remains a crucial factor because it portrays inadequacies in photosynthetic energy transduction. The immediate goal includes improving the demonstration of leaves in flora (notably in crops and threatened species) to evade light saturation and additional investigations on the successful photorespiratory bypass, which demonstrated enhanced productivity in model species.
Variables that Affect CAM Photosynthetic Efficiency
The analysis and interpretation of the A–Ci curve depend on some underlying assumptions (Farquhar et al. 1980). ‘A–Ci’ curve (assimilation rate plotted against intercellular CO2 concentration) can provide some insights into the biochemistry of a leaf or plant. For example, the value of Ci where photosynthesis and respiration are equipoised is designated as the CO2 compensation point. The initial data points of slope deliver an in vivo measure of the activity of RuBisCO in the leaf suggests the plants’ carboxylation efficiency, termed ‘mesophyll conductance.' The reaction function of ‘A–Ci’ curve represents the mechanical basis behind many models reported to date in plant physiology (Manter et al. 2003; Harley et al. 1992). According to Farquhar et al. (1980), carboxylation rates are regulated by three processes. These are (a) amount, activity and kinetics of RuBisCO, (b) rate of RuBP restoration sustained by the electron transport system, and (c) infrequent availability of Triose Phosphate. The A–Ci curve gives values on estimates of the maximum rate of RuBisCO-mediated carboxylation is defined merely as RuBisCO activity.
In the present study, nonlinear regression techniques, based on the equations of Farquhar et al. (1980), which were later modified in some studies (Sharkey 1985; Harley and Sharkey 1991), were used to estimate the maximum rate of RuBisCO activity for each A–Ci curve. Our results indicated that although ‘A’ is a primary confounding factor for RuBisCO activity, it is not the sole variable that controls it. Besides ‘A’, mesophyll conductance (gm) also plays a critical role in determining the RuBisCO activity. Our study indicated low ΦCO2 for the CAM cluster compared to C3 and C4, which could be attributed to photorespiration. We adopted the C3 Farquhar model for computing RuBisCO activity for all plants except Saccharum officinarum-the C4 control. RuBisCO exists both in storage and functional form in plant systems. Since RuBisCO might exist as a storage protein and in inactivated form (Cheng and Fuchigami 2000; Warren et al. 2000; Warren et al. 2003), its activation is probably restricted in CAM plants due to the time the readings were taken. RuBisCO activity is highest during the third phase in CAM plants, whilst the readings were taken in the second phase.
Tropical Orchids' Ability to Mitigate CO2 and the Associated Causes
Fischer and Turner (1978) hypothesised that in dry habitats, natural selection would favour plants with high water use efficiency. The Y/PCR ratio (factor ‘X’) as per the histomorphometric analysis agrees with the photosynthetic measurements and enzyme studies. The thriving ability of CAM orchids, notably in Acampe praemorsa, Bulbophyllum neilgherrense and Vanilla planifolia, is due to its high WUE. These orchids cover the canopies of the forest land and play a crucial role in retaining the fixed CO2 much longer than the C4 groups. The data derived from tissues, such as the aerial root, the pseudo bulb, and the leaf lamina, prove efficient carbon retention by orchids. This feature tags them as right candidates for relocating in areas where mitigation of greenhouse gases is of paramount importance. High adaptability of CAM plants to CO2 enriched areas is termed as ‘Downregulation’ as it negates photosynthetic apparatus, which is visualised as low RuBisCO activity. However, they possess a functional PEPC (Phosphoenolpyruvate carboxylase) which displays a higher affinity for CO2 than RuBisCO. The PEPC-driven CAM mechanism provides them with an intrinsic ability to mitigate CO2 superfluously. CO2 influx results in nocturnal acidification of mesophyll cells, the transpiration ratio is 3–10-fold with an innate ability to evade ROS-mediated damage.
Conclusion
The present study emphasises the significance of the widespread prevalence of tropical orchids in the Western Ghats and also Orchidaceae being the second largest plant family in the globe. There is a demand for increased crop production brought about by the rise in population. Emissions from agronomy necessitate novel strategies to incentivize sustainable agronomic practices (Godfray and Robinson 2015; Carlson et al. 2016). Total CO2 influx through Saccharum crops in an area of 68,541 ha, monitored at 2006/2007 crop season with 5,133,212 tonne of CO2, came to approximately 74.9 tonne of CO2 ha−1 (de Figueiredo et al. 2010). However, emissions from post-harvest burning resulted in CO2 efflux that often exceeds the carbon influx. The present study indicated that propagation of CAM topical orchids, such as Vanilla planifolia, Dendrobium Sonia (white variety), Bulbophyllum neilgherrense, Acampe praemorsa, Pholidota pallida on trees and hedges, would alleviate GHG emissions, notably CO2, in a micro-niche equivalent to Saccharum. Orchids being an ornamental group, there is no apprehension of postharvest carbon efflux as well. Increasing the area apportioned for essential indigenous plant species to prevent competition from non-native plants could help local carbon assimilation. Because of high water use efficiency, propagation of CAM orchids is one of the winning landscaping strategies that ecologists could adopt to mitigate CO2 in a micro-ecological niche showing steady temperature rise.
Data availability
The datasets used and analysed during the current study are available from the corresponding institution on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- Ha:
-
Hectare
- HEPES:
-
(4-(2-Hydroxyethyl) -1-piperezineethanesulfonic acid)
- kPa:
-
Kilopascal
- LED:
-
Light-emitting diode
- UV–Vis:
-
Ultraviolet–visible
References
Abraham A, Vatsala P (1981) An introduction to orchids with illustrations and description of 150 south Indian Orchids. Tropical Botanic Garden Research Institute, Trivandrum, Kerala, India
Aschan G, Pfanz H (2003) Non–foliar photosynthesis – a strategy of additional carbon acquisition. Flora 198:81–97
Bhat KG (2003) Flora of Udupi. Indian Nat. (Regd.) Inchara, Chitpady, Udupi, Karnataka, India
Bongi G, Long SP (1987) Light-dependent damage to photosynthesis in olive leaves during chilling and high-temperature stress. Plant Cell Environ 10:241–249
Carlson KM, Gerber SJ, Mueller DN, Herrero M, MacDonald KG, Brauman AK, Havlik P, O’Connell SC, Johnson AJ, Saatchi S, West CP (2016) Greenhouse gas emissions intensity of global croplands. Nat Clim Change 7:63–68
Chen Z, Silva H, Klessig D (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886
Cheng L, Fuchigami LH (2000) Rubisco deactivation state decreases with increasing nitrogen content in apple leaves. J Exp Bot 51:1687–1694
Dayer S, Peña JP, Gindro K, Torregrosa L, Voinesco F, Martínez L, Prieto JA, Zufferey V (2017) Changes in leaf stomatal conductance, petiole hydraulics and vessel morphology in grapevine (Vitis vinifera cv. Chasselas) under different light and irrigation regimes. Funct Plant Biol 44:679
de Figueiredo EB, Panosso AR, Romão R, La Scala JN, La Scala N (2010) Greenhouse gas emission associated with sugar production in southern Brazil. Carbon Balance Manag 5:3
Ehleringer JR (1994) Variation in gas exchange characteristics among desert plants. Ecophysiology of photosynthesis. Ecol Stud Ser 100:361–392
Evans J (1993) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Aust J Plant Physiol 20:55
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fischer RA, Turner NC (1978) Plant productivity in the arid and semiarid zones. Annu Rev Plant Physiol 29:277–317
Gamisch A, Klaus W, Fischer GA, Comes HP (2021) Evolution of crassulacean acid metabolism (CAM) as an escape from ecological niche conservatism in Malagasy Bulbophyllum (Orchidaceae). BioRxiv. https://doi.org/10.1101/2021.01.09.426039
Ghannoum O, Evans RJ, Chow SW, Andrews JT, Conroy PJ, von Caemmerer S (2005) Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiol 137:638–650
Godfray HCJ, Robinson S (2015) Contrasting approaches to projecting long-run global food security. Oxford Rev Econ Policy 31:26–44
Groom QJ, Baker NR (1992) Analysis of light-induced depressions of photosynthesis in leaves of a wheat crop during the winter. Plant Physiol 100:1217–1223
Harley P, Sharkey T (1991) An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth Res 27:169–178
Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modeling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Hermida-carrera C, Fares MA, Font-carrascosa M, Kapralov MV, Koch MA, Mir A, Galmés J (2020) Exploring molecular evolution of Rubisco in C 3 and CAM orchidaceae and bromeliaceae. BMC Evol Biol 8:1–17
Hew C, Yong J (2003) The physiology of tropical orchids in relation to the industry, 2nd edn. SciTech Book News 28 World Scientific publishing Co., Singapore, pp 93–128
Ku MS, Wu J, Dai Z, Scott RA, Chu C, Edwards GE (1991) Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiol 96:518–528
Lambers H, Oliveira RS, Lambers H, Oliveira RS (2019) Plant Water Relations. Plant Physiol Ecol. Springer International Publishing, pp 187–263
Leverenz JW (1987) Chlorophyll content and the light response curve of shade-adapted conifer needles. Physiol Plant 71:20–29
Lichtenthaler HK (1987) Chlorophylls, and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Long SP, Postl WF, Bolhar-Nordenkampf HR (1993) Quantum yields for uptake of carbon dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta 189:226–234
Luttge U (2004) Ecophysiology of crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Manter DK, Bond BJ, Kavanagh KL, Stone JK, Filip GM (2003) Modelling the impacts of the foliar pathogen, Phaeocryptopus gaeumannii, on Douglas-fir physiology: net canopy carbon assimilation, needle abscission, and growth. Ecol Modell 164:211–226
Maxwell K, Borland AM, Haslam RP, Helliker BR, Roberts A, Griffithsm H (1999) Modulation of rubisco activity during the diurnal phases of the Crassulacean acid metabolism plant Kalanchoe daigremontiana. Plant Physiol 121(3):849–856. https://doi.org/10.1104/pp.121.3.849
Miller G, Hartzell S, Porporato A (2021) Ecohydrology of epiphytes: modelling water balance, CAM photosynthesis, and their climate impacts ecohydrology of epiphytes: modeling water balance, CAM photosynthesis, and their climate impacts. Ecohydrology 14:e2275
Muhaidat R, Sage RF, Dengler NG (2007) Diversity of Kranz anatomy and biochemistry in C4 eudicots. Am J Bot 94:362–381
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol 29:379–414
Osmond CB, Nott DL, Firth PM (1979) Carbon assimilation patterns and growth of the introduced CAM plant opuntia-inermis in Eastern Australia. Oecologia 40:331–350
Osmond C, Maxwell K, Popp M, Robinson SBM, Bryant J, Kruger N (1999) On being thick: fathoming apparently futile pathways of photosynthesis and carbohydrate metabolism in succulent CAM plants. Carbohydrate metabolism in plants. OxfordBIOS Scientific Publishers, pp 183–200
Ota K, Morioka K, Yamamoto Y (1991) Effects of leaf age, inflorescence, temperature, light intensity and moisture conditions on CAM photosynthesis in Phalaenopsis. J Japanese Soc 198:81–97
Panchasara H, Samrat NH, Islam N (2021) Greenhouse gas emissions trends and mitigation measures in australian agriculture sector—a review. Agriculture 11:1–16
Peterson RB (1990) Effects of water vapor pressure deficit on photochemical and fluorescence yields in tobacco leaf tissue. Plant Physiol 92:608–614
Sage TL, Sage RF (2009) The functional anatomy of rice leaves : implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice special issue – mini review. Plant Cell Physiol 50:756–772
Sharkey TD (1985) Photosynthesis in intact leaves of C3 plants: Physics, physiology, and rate limitations. Bot Rev 51:53–105
Sharkey TD (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73:146–152
Silvera K, Santiago LS, Cushman JC, Winter K (2009) Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol 149:1838–1847
Singsaas EL, Ort DR, DeLucia EH (2001) Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 128:15–23
Ueno K, Kinoshita T, Takashi IS, Shimazaki KE (2005) Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol 46:955–963
United States Environmental Protection Agency (2020). https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data. Accessed June 5 2020
Verge XPC, De Kimpe C, Desjardins RL (2007) Agricultural production, greenhouse gas emissions and mitigation potential. Agric for Meteorol 142:255–269
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153:376–387
Warren CR, Adams MA, Chen Z (2000) Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants. Aust J Plant Physiol 27:407–416
Warren C, Livingston N, Turpin D (2003) Responses of gas exchange to reversible changes in whole-plant transpiration rate in two conifer species. Tree Physiol 24:971–979
Yadav SK, Singh H, Nautiyal R, Ginwal HS, Ansari SA, Barthwal S (2020) Modulation of morpho-physiological responses in Populus deltoides by elevated carbon dioxide and temperature. For Sci 66:105–118
Yeo ME, Cuartero J, Flowers TJ, Yeo AR (1997) Gas exchange, water loss and biomass production in rice and wild Oryza species in well-watered and water-limiting growth conditions. Bot Acta 110:32–42
Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261
Zotz G, Tyree MT, Patiño S (1997) Hydraulic architecture and water relations of a flood tolerant tropical tree, Annona glabra. Tree Physiol 17:359–365
Acknowledgements
The authors thank Manipal Multidisciplinary Developmental Research Centre (MMRDC) and the European Union for funding this research program. The authors thank the Former Vice-Chancellor of Manipal Academy of Higher Education, Dr H.Vinod Bhat, for reviewing the progress in the research work, every three months. The authors thank Dr Neeta Inamdar, for facilitating the regular assessments. The authors are thankful to Dr Radhakrishna Rao and (Late) Dr K.G. Bhat, the consultant taxonomists of Manipal School of Life Sciences, Manipal Academy of Higher Education, who helped in confirming the taxonomical identity of tropical orchids. The authors are indebted to Manipal Academy of Higher Education, for providing all the necessary facilities for implementing the Research program.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
VSB and KS: devised the plan of experiments for implementing the study. VSB, NSM and SB: performed the enzyme assays and histomorphometric analyses. VSB, NSM and SK: conducted real-time analyses related to photosynthetic efficiencies using the photosynthesis measuring system. VSB, NSM, and KS: were involved in the phenological studies of orchids both ex situ and in situ. VSB and KS: contributed to the interpretation of the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mukundan, N.S., Banerjee, S., Kumar, S. et al. C4 Equivalent Decarboxylation Competence in Tropical Orchids. J. Plant Biol. 66, 163–180 (2023). https://doi.org/10.1007/s12374-023-09385-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-023-09385-6