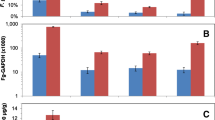
Abstract
Fusarium head blight (FHB) caused by Fusarium graminearum is one of the most serious diseases in wheat (Triticum aestivum) and barley (Hordeum vulgare). Dahongmil is an elite Korean wheat cultivar with relatively high resistance to FHB. To identify differentially expressed genes in the resistant cultivar Dahongmil and the susceptible cultivar Urimil after inoculation of F. graminearum, we used the Affymetrix GeneChip® Wheat Genome Array to identify 328 ESTs that were differentially expressed in inoculated seedling tissues of the two cultivars. From these, we selected 16 induced genes and found that they have defense functions, such as genes encoding pathogen resistance proteins, oxidative stress-related proteins, metabolism, and proteins involved in defense mechanisms. To verify the DNA microarray results, we tested seven of these genes by semiquantitative RT-PCR and confirmed that these defense- and stress-related genes were expressed at much higher levels in the resistant Dahongmil cultivar. We next developed a hypothetical functional gene network and identified 89 interaction pairs mediated by four of the differentially expressed genes in the hypothetical network. We further refined the network by identifying nine genes showing significant up- or down-regulation after FHB challenge in the resistant cultivar and two genes having multiple interactions with queried proteins. We hope that the set of induced genes identified in this study can be used for development of new wheat and barley cultivars with improved resistance to FHB.




Similar content being viewed by others
References
Bai G, Shaner G (2004) Management and resistance in wheat and barley to Fusarium head blight. Annu Rev Phytopathol 42:135–161
Ban T, Suenaga K (2000) Genetic analysis of resistance to Fusarium head blight caused by Fusarium graminearum in Chinese wheat cultivar Sumai 3 and the Japanese cultivar Saikai 165. Euphytica 113:87–99
Bowden RL, Leslie JF (1999) Sexual reproduction in Gibberella zeae. Phytopathology 89:182–188
Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K (2005) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67:171–179
Desmond OJ, Manners JM, Stephens AE, Maclean DJ, Schenk PM, Gardiner DM (2008) The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defense responses in wheat. Mol Plant Pathol 9:435–445
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000) The WRKY superfamily of plant transcription factors. Trends Plant Sci 5:199–206
Foroud NA, Eudes F (2009) Trichothecenes in cereal grains. Int J Mol Sci 10:147–173
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Guldener U, Seong KY, Boddu J, Cho S, Trail F, Xu JR, Adam G, Mewes HW, Muehlbauer GJ, Kistler HC (2006) Development of a Fusarium graminearum Affymetrix GeneChip for profiling fungal gene expression in vitro and in planta. Fungal Genet Biol 43:316–325
Ha YW, Lee SY, Song HS, Heu HY, Nam JH, Sung BR (1992) New early maturing, semi-dwarf, lodging resistant, high yielding and good quality soft wheat variety Urimil. Res Rept RDA 34:1–4 (in Korean)
Hancock JT, Desikan R, Clarke A, Hurst RD, Neill SJ (2002) Cell signalling following plant/pathogen interactions involves the generation of reactive oxygen and reactive nitrogen species. Plant Physiol Biochem 40:611–617
Harris SD (2005) Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia 97:880–887
Hwang IS, Hwang BK (2011) The pepper mannose-binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiol 155:447–463
Joshi CP, Chiang VL (1998) Conserved sequence motifs in plant S-adenosyl-l-methionine-dependent methyltransferases. Plant Mol Biol 37:663–674
Jung KH, Jeon JS, An G (2011) Web tools for rice transcriptome analyses. J Plant Biol 54:65–80
Kolomiets MV, Chen H, Richard J, Gladon RJ, Braun EJ, Hannapel DJ (2000) A leaf lipoxygenase of potato induced specifically by pathogen infection. Plant Physiol 3:1121–1130
Kong L, Anderson JM, Ohm HW (2005) Induction of wheat defense and stress-related genes in response to Fusarium graminearum. Genome 48:29–40
Kumar J, Huckelhoven R, Beckhove U, Nagarajan S, Kogel KH (2001) A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (Teleomorph: Cochliobolus sativus) and its toxins. Phytopathology 91:127–133
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, UK
Mauch F, Dudler R (1993) Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol 102:1193–1201
Morant M, Bak S, Moller BL, Werck-Reichhart D (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotech 14:151–162
Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10:11
Park RK, Suh HS, Lee BH, Suh DY (1980) New wheat variety ‘Milyang 7’. Res Rept RDA 22:114–119 (in Korean)
Parry DW, Jenkinson P, McLeod L (1995) Fusarium ear blight (scab) in small grain cereals-a review. Plant Pathol 44:207–238
Petsch KA, Mylne J, Botella R (2005) Cosuppression of eukaryotic release factor 1–1 in Arabidopsis affects cell elongation and radial cell division. Plant Physiol 139:115–126
Ruepp A, Zollner A, Maier D, Albermann K, Hani J, Mokrejs M, Tetko I, Güldener U, Mannhaupt G, Münsterkötter M, Mewes HW (2004) The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res 32:5539–5545
Rusterucci C, Montillet JL, Agnel JP, Battesti C, Alonso B, Knoll A, Bessoule JJ, Etienne P, Suty L, Blein JP, Triantaphylides C (1999) Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J Biol Chem 274:36446–36455
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuyn M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J (2003) A free open-source system for microarray data management and analysis. Biotechniques 34:374–378
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91(phox) NADPH oxidase: modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97:11655–11660
Schmidt A, Wachtler B, Temp U, Krekling T, Seguin A, Gershenzon J (2010) A bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Picea abies. Plant Physiol 152:639–655
Schroeder HW, Christensen JJ (1963) Factors affecting resistance of wheat scab caused by Gibberella zeae. Phytopathology 53:831–838
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Sutton JC (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Plant Pathol 4:195–209
Trail F, Xu H, Loranger J, Gadoury D (2002) Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94:181–189
Wang L, Pei Z, Tian Y, He C (2004) OsLSD1, a rice zinc finger protein, regulates programmed cell death and callus differentiation. Mol Plant Microbe Interact 5:375–384
Wirdnam C, Motoyama A, Arn-Bouldoires E, Eeden S, Iglesias A, Meins F (2004) Altered expression of an ankyrin-repeat protein results in leaf abnormalities, necrotic lesions, and the elaboration of a systemic signal. Plant Mol Biol 56:717–730
Zwart RS, Muylle H, Bockstaele EV, Ruiz IR (2008) Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor Appl Genet 117:813–828
Acknowledgments
This work was carried out with the support of Cooperative Research Program for Agriculture Science & Technology Development (PJ006662), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sung-Hwan Cho, Jungkwan Lee, and Ki-Hong Jung contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Interaction network mediated by 89 up- and down-regulated genes in response to F. graminearum inoculation using the Cytoscape program (version 2.7). Green nodes indicate down-regulation in Dahongmil and red nodes indicate up-regulation. (JPEG 1773 kb)
ESM Table S1
(DOCX 27 kb)
ESM Table S2
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Cho, SH., Lee, J., Jung, KH. et al. Genome-Wide Analysis of Genes Induced by Fusarium graminearum Infection in Resistant and Susceptible Wheat Cultivars. J. Plant Biol. 55, 64–72 (2012). https://doi.org/10.1007/s12374-011-9190-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-011-9190-7