Abstract
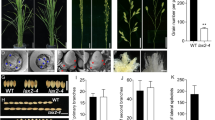
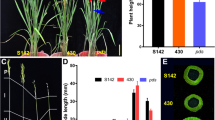
Proper function of the LAX1 gene is required for the development of axillary meristem in rice. Here, we report genetic and phenotypic characters of a novel recessive mutant allele of rice LAX1 gene, lax1-6, which showed abnormal panicle phenotypes with few numbers of elongated primary rachis branches. Beside typical lax mutant phenotype, abnormalities of lax1-6 mutant allele were observed with defect lemma and palea primordial in floral organs. The lax1-6 mutant locus was linked between SSR markers RM7594 and RM5389 on chromosome 1 with 1.02% and 1.0% recombination frequencies, respectively. Molecular analysis revealed that the lax1-6 mutant allele was caused by a transversion mutation of nucleotide T to G substitution that resulted in an amino acid substitution from serine (S) to alanine (A) at the 117th position from amino terminus of a basic helix-loop-helix protein coded by LAX1 gene. Furthermore, we found that the Oryza sativa indica type cv. IRRI347 contained 24 nucleotide deletion in the upstream sequence in the LAX1 gene, but this deletion did not influence panicle morphology, which demonstrated that the deletion is a polymorphism in rice. All together, the lax1-6 mutant is a newly identified allele of LAX1 gene displaying the abnormal axillary meristems and inflorescences in rice.






Similar content being viewed by others
References
Agrawal GK, Abe K, Yamazaki M, Miyao A, Hirochika H (2005) Conservation of the E-function for floral organ identity in rice revealed by the analysis of tissue culture-induced loss-of-function mutants of the OsMADS1 gene. Plant Mol Biol 59:125–135
Araki T (2001) Transition from vegetative to reproductive phase. Curr Opin Plant Biol 4:63–68
Bommert P, Sato-Nagasawa N, Jackson D, Hirano HY (2005) Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol 46:69–78
Bortiri E, Hake S (2007) Flowering and determinacy in maize. J Exp Bot 58:909–916
Bortiri E, Chuck G, Vollbrecht E, Rocheford T, Martienssen R, Hake S (2006) ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18:574–585
Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112:1–20
Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E (1997) Inflorescence commitment and architecture in Arabidopsis. Science 275:80–83
Chuck G, Muszynski M, Kellogg E, Hake S, Schmidt RJ (2002) The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298:1238–1241
Coen ES, Meyerowltz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Coen ES, Nugent J (1994) Evolution of flowers and inflorescences. Dev Suppl 120:107–116
Ellenberger T, Fass D, Arnaud M, Harrison SC (1994) Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev 8:970–980
Furutani I, Sukegawa S, Kyozuka J (2006) Genome-wide analysis of spatial and temporal gene expression in rice panicle development. Plant J 46:503–511
Gallione CJ, Rose JK (1985) A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol 54:374–382
Hong SY, Oh JE, Lee KH (1999) Effect of d-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem Pharm 58:1775–1780
Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, Kapoor S, Tyagi AK, Khurana JP (2007) F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol 143:1467–1483
Jeon JS, Lee S, An G (2008) Intragenic control of expression of a rice MADS box gene OsMADS1. Mol Cells 26:474–480
Jung KH, Han MJ, Lee YS, Kim YW, Hwang I, Kim MJ, Kim YK, Nahm BH, An G (2005) Rice undeveloped tapetum1 is a major regulator of early tapetum development. Plant Cell 17:2705–2722
Kang HG, An G (1997) Isolation and characterization of a rice MADS box gene belonging to the AGL2 gene family. Mol Cells 7:45–51
Kang SG, Hannapel DJ, Suh SG (2003) Potato MADS-box gene POTM1-1 transcripts are temporally and spatially distributed in floral organs and vegetative meristems. Mol Cells 15:48–54
Kaur P, Larson SR, Shaun Bushman B, Wang RR, Mott IW, Hole D, Thimmapuram J, Gong G, Liu L (2008) Genes controlling plant growth habit in Leymus (Triticeae): maize barren stalk1 (ba1), rice lax panicle, and wheat tiller inhibition (tin3) genes as possible candidates. Funct Integr Genomics 8:375–386
Kellogg EA (2007) Floral displays: genetic control of grass inflorescence. Curr Opin Plant Biol 10:26–31
Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124:3045–3054
Kiribuchi K, Jikumaru Y, Kaku H, Minami E, Hasegawa M, Kodama O, Seto H, Okada K, Nojiri H, Yamane H (2005) Involvement of the basic helix-loop-helix transcription factor RERJ1 in wounding and drought stress responses in rice plants. Biosci Biotechnol Biochem 69:1042–1044
Kobayashi K, Maekawa M, Miyao A, Hirochika H, Kyozuka J (2010) PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol 51:47–57
Komatsu M, Maekawa M, Shimamoto K, Kyozuka J (2001) The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev Biol 231:364–373
Komatsu M, Chujo A, Natago Y, Shimamoto K, Kyozuka J (2003) Frizzy panicle is required to prevent the formation of axillary meristems and to establish floral meristem identity in rice spikelets. Development 130:3841–3850
Lander ES, Green P, Abrahmson J, Barloe A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary linkage maps of experimental and natural populations. Genomics 1:174–181
Lee DY, Lee J, Moon S, Park SY, An G (2006) The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J 49:64–78
Levy YY, Dean C (1998) The transition to flowering. Plant Cell 10:1973–1990
Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF (1992) Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360:273–277
Mao L, Begum D, Chuang HW, Budiman MA, Szymkowiak EJ, Irish EE, Wing RA (2000) JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 406:910–913
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xin Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClarck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
McSteen P, Hake S (2001) Barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 128:2881–2891
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8:4321–4325
Nagato Y, Yoshimura A (1998) Report of committee on gene symbolization, nomenclature and linkage map. Rice Genet Newsl 15:13–74
Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L (2000) The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12:1863–1878
Oikawa T, Kyozuka J (2009) Two-step regulation of LAX PANICLE1 protein accumulation in axillary meristem formation in rice. Plant Cell 21:1095–1108
Prasad K, Parameswaran S, Vijayraghavan U (2005) OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43:915–928
Rao NN, Prasad K, Kumar PR, Vijayraghavan U (2008) Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proc Natl Acad Sci U S A 105:3646–3651
Sakamoto W, Ohmori T, Kageyama K, Miyazaki C, Saito A (2001) The Purple leaf (Pl) locus of rice: the Pl w allele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis. Plant Cell Physiol. 42:982-991
Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441:227–230
Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H (1990) Genetic control of flower development by homeotic genes in Antirrhinum majus. Science 250:931–936
Sentoku N, Kato H, Kitano H, Imai R (2005) OsMADS22, an STMADS11-like MADS-box gene of rice, is expressed in non-vegetative tissues and its ectopic expression induces spikelet meristem indeterminacy. Mol Gen Genomics 273:1–9
Theissen G, Saedler H (2001) Plant biology. Floral quartets. Nature 409:469–471
Vissing H, D'Alessio M, Lee B, Ramirez F, Godfrey M, Hollister DW (1989) Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem 264:18265–18267
Vollbrecht E, Springer PS, Goh L, Buckler ES IV, Martienssen R (2005) Architecture of floral branch systems in maize and related grasses. Nature 436:1119–1126
Wang Y, Li J (2008) Molecular basis of plant architecture. Annu Rev Plant Biol 59:253–279
Wang L, Yin H, Qian Q, Yang J, Huang C, Hu X, Luo D (2009) NECK LEAF 1, a GATA type transcription factor, modulates organogenesis by regulating the expression of multiple regulatory genes during reproductive development in rice. Cell Res 19:598–611
Wang J, Gao X, Li L, Shi X, Zhang J, Shi Z (2010) Overexpression of Osta-siR2141 caused abnormal polarity establishment and retarded growth in rice. J Exp Bot 61:1885–1895
Weberling F (1992) Morphology of flowers and inflorescences. Cambridge Univ. Press, Cambridge, p 405
Weigel D (1995) The genetics of flower development: from floral induction to ovule morphogenesis. Annu Rev Genet 29:19–39
Yamagishi J, Miyamoto N, Hirotsu S, Laza RC, Nemoto K (2004) QTLs for branching, floret formation, and pre-flowering floret abortion of rice panicle in a temperate japonica × tropical japonica cross. Theor Appl Genet 109:1555–1561
Yano M, Kojima S, Takahashi Y, Lin H, Sasaki T (2001) Genetic control of flowering time in rice, a short-day plant. Plant Physiol 127:1425–1429
Yi G, Choi JH, Jeong EG, Chon NS, Jena KK, Ku YC, Kim DH, Eun MY, Jeon JS, Nam MH (2005) Morphological and molecular characterization of a new frizzy panicle mutant, “fzp-9(t)”, in rice (Oryza sativa L.). Hereditas 142:92–97
Zhu QH, Hoque MS, Dennis ES, Upadhyaya NM (2003) Ds tagging of BRANCHED FLORETLESS 1 (BFL1) that mediates the transition from spikelet to floret meristem in rice (Oryza sativa L). BMC Plant Biol 3:6–19
Acknowledgements
We would like to thank Professor Suh Hak Soo at Yeungnam University for his assistance. This work was supported by a Yeungnam University Research Grant (208-A-356-041) in 2008 to professor Sang Gu Kang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matin, M.N., Kang, S.G. Genetic and Phenotypic Analysis of lax1-6, a Mutant Allele of LAX PANICLE1 in Rice. J. Plant Biol. 55, 50–63 (2012). https://doi.org/10.1007/s12374-011-9189-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-011-9189-0