Abstract
Artificial illumination in caves visited by tourists (“show caves”) gives rise to growth of photosynthetic biofilms, termed “lampenflora.” Besides being unsightly, these biofilms can damage speleothems, and thus finding a safe and effective means of controlling lampenflora is an important consideration in cave management. A variety of different physical and chemical means of biofilm mitigation have been proposed and tested. Here we tested benzalkonium chloride (BAC), a quaternary ammonium cationic detergent commonly used in pharmaceuticals and cosmetics, and germicidal UV light (UV-C) for lampenflora control. Algae and cyanobacteria derived from Carlsbad Cavern, USA, were cultivated in the lab and inoculated onto smooth calcium carbonate (CaCO3) tiles and incubated under fluorescent lighting to simulate lampenflora; these were then treated with BAC or UV-C in various concentrations and intensities, respectively. A 1–10% BAC solution prevented biofilm growth, and repeated treatments with a 1% solution bleached preformed photosynthetic pigments. Germicidal UV-C (≥ 3200 mJ cm−2) also bleached preformed biofilms. BAC may be especially useful for bleaching thick localized growths, since high concentrations are required and toxicity to non-toxic organisms could be an issue; whereas UV-C could more easily be applied to broader areas, e.g., the tens of square meter areas in the immediate vicinity of the lamps at Carlsbad Cavern and other show caves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caves are normally dark and devoid of photosynthetic organisms, but artificial lighting in caves visited by tourists, i.e., show caves, provides sufficient energy for the proliferation of algae, cyanobacteria, and in severe cases even higher plants. These photosynthetic growths, termed “lampenflora,” are unsightly and they can damage the speleothems on which they reside. Even if they are re not directly damaging to the colonized surfaces, attempts to remove them with chemicals and/or by scrubbing can erode or modify the underlying minerals. Photosynthetic primary production in a normally dark cave also generates organic carbon that would not otherwise be present and that can support various heterotrophs including non-native animals.
This project was undertaken with the aim of testing methods for mitigating lampenflora biofilms specifically in Carlsbad Cavern, Carlsbad Caverns National Park, New Mexico, USA, but with the understanding that the findings could be applicable to other show caves, as well. A previous study characterized the microbial communities in Carlsbad Cavern lampenflora biofilms using a culture-independent DNA sequencing approach (Havlena et al. 2021) and revealed a diversity of cyanobacteria and algae, as well as heterotrophic bacteria. It was also found that diminishing the amount of illumination in the blue range of wavelengths had a significant effect on the extent of lampenflora and their makeup and that distance from the light source, texture of the mineral substrate, and length of time since previous cleaning of the substrate affected biofilm development and diversity. While this study provided useful basic information on Carlsbad lampenflora, it provided minimal information on lampenflora control, aside from finding that lowering color temperature by using less blue light was useful but in itself insufficient to prevent biofilm proliferation.
A range of physical and chemical methods have been proposed and tested for removal and control of lampenflora biofilms (e.g., Mulec and Kosi 2009; Estevez et al. 2019) and also the related problem of phototrophic biofilms on cultural heritage sites, e.g., buildings and monuments (Warscheid and Braams 2000; Ascaso et al. 2002; Young et al. 2008; Sterflinger and Piñar 2013; Pfendler et al. 2018; Romani et al. 2022). Bleach (sodium hypochlorite) is commonly used to kill and decolorize lampenflora in many show caves, including Carlsbad Cavern. Hydrogen peroxide (H2O2) is another strong oxidant with potential for lampenflora control (Grobbelaar 2000; Faimon et al. 2003; Meyer et al. 2017). Herbicides, e.g., atrazine and simazine, have been tested for controlling lampenflora, but with limited success (Grobbelaar 2000). The algicide DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) and bromine compounds were deemed inappropriate by Mulec and Kosi (2009) due to toxicity. Young et al.’s (2008) study on biocides for use on stone heritage sites explored a range of compounds classified as cell permeabilizers, polysaccharide and pigment inhibitors, or photodynamic agents, including compounds such as EDTA, tricyclazole, bismuth dimercaprol (BisBAL), and the dyes methylene blue and nuclear fast red used in conjunction with H2O2. They suggest a polyphasic approach with these compounds, although use of dye compounds in the cave environment is probably inappropriate. Arrhenius et al.’s (2014) study on biocides for use on ships (biofouling preventative agents) investigates a variety of copper, zinc, and commercial algaecidal compounds found copper pyrithione most effective, but the resulting discoloration and copper accumulation is inappropriate for cave use.
Quaternary ammonium compounds (QACs) have been used as disinfectants for a range of applications, including biofilm control (Tezel and Pavlostathis 2015). Benzalkonium chloride (BAC) is among the most widely used QACs (Pereira and Tagkopoulos 2019), and it has even seen used in caves, e.g., to control biofilms in the famous rock art-containing Lascaux Cave in France (Bastian et al. 2010). It’s also been used at a monastery built from carbonate rock, where it was noted to be the most effective compound at reducing cyanobacterial growth (Ascaso et al. 2002). BAC has long been considered relatively non-toxic to humans and animals, having been approved in the USA for pharmaceutical use including nasal sprays and ophthalmic solutions (U.S.E.P.A. 2006) and it can be degraded, especially under aerobic conditions (Tezel and Pavlostathis 2015); however, studies in recent decades point to negative consequences of acute and chronic exposure (Pereira and Tagkopoulos 2019; Barber and Hartmann 2022). Toxicity to nontarget organisms could thus be a concern in cave applications. Toxicity to aquatic invertebrates was found to be in the tens to hundreds of µg/L in acute exposure to water fleas Daphnia and Ceriodaphnia, respectively, and tenfold lower in acute exposure (Lavorgna et al. 2016). Repeated treatments can also select for microbial resistance to BAC (Tandukar et al. 2013; Guo et al. 2014; Pereira and Tagkopoulos 2019 Barber and Hartmann 2022).
UV-C irradiation at 200-–28-nm wavelength, especially germicidal UV near 260 nm, has not been extensively explored in situ against lampenflora growth, but a few studies describe promising results. The concept is advantageous since UV-C is strongly germicidal but leaves no toxic residue (Borderie et al. 2015). First mentioned by Dobat (1963) for use in a cave, UV-C lamps were observed to kill and bleach algae in both the lab and in a cave. UV-C was extensively explored by a group in France that investigated the effects on lampenflora in the lab and in the field (Borderie et al. 2011, 2014a, b, 2015; Pfendler et al. 2017). They describe a bleaching effect on lampenflora and diminished cell viability following the exposure to doses ranging from 150 to 300 kJ/m2; however, they noted recolonization about a year after exposure.
The National Park Service is understandably reluctant to apply any treatment that has a potential to damage speleothems (“cave formations”), that leaves a residue, or that is toxic. Synthetic pesticides are considered undesirable, in part due their persistence in the environment and also due to their possible effects on non-target organisms. These restrictions leave only a short list of alternatives to bleach for lampenflora mitigation. Irradiation with UV-C is an attractive approach, since it’s a purely physical treatment that affects only surface features and leaves no residues, other than dead biomass. BAC, although it’s a synthetic biocide, might be acceptable to the National Park Service since it’s approved for use in the environment, cosmetics, and pharmaceuticals (U.S.E.P.A. 2006); however, any beneficial effects will have to be weighed against risk factors. For this study, UV-C irradiation and BAC were selected to test their biocidal and bleaching effects on photosynthetic biofilms derived from lampenflora at Carlsbad Cavern and growing on calcium carbonate surfaces. The biocidal effects of UV-C and BAC are well known, but the intent here was to quantify the responses to a range of doses and treatment regimes in lab experiments designed to mimic cave biofilms, with an eye to their potential application in Carlsbad Cavern and other show caves.
Materials and Methods
Samples of photosynthetic biofilms were collected from illuminated sites in the Big Room of Carlsbad Cavern using sterile swabs. These were transported to New Mexico Tech and used to inoculate enrichment cultures in BG-11 medium incubated under fluorescent lighting (Fig. 1). Algae and cyanobacteria were identified by DNA sequence analysis following the methods of Havlena et al. (2021). DNA was extracted from the enrichment cultures and sent to the Marine Biological Laboratory, Woods Hole, Massachusetts, USA, for amplicon sequencing of bacterial and eukaryal small subunit rRNA. Results showed a combination of cyanobacteria and eukaryotic algae. The cyanobacteria were dominantly of the genus Nostoc with lesser amounts of the genus Nodularia. Both of these are nitrogen fixers, which makes sense considering that there’s no obvious source of fixed nitrogen in the cavern. Nostoc was also identified during characterization of Carlsbad Cavern lampenflora (Havlena et al. 2021). Eukaryotic algae were members of the family Trebouxiophyceae within the phylum Chlorophyta and members of the family Chysophyceae (golden-brown algae) within the phylum Ochrophyta. These were not identified to the level of genus. Members of both families were also the dominant algae identified in Carlsbad Cavern lampenflora (Havlena et al. 2021).
Initial studies of BAC effects on algae and cyanobacteria were carried out in liquid BG-11 medium treated with various concentrations of BAC (Sigma-Aldrich no. 63249). Cell abundance was quantified by measuring turbidity in a spectrophotometer at 600-nm wavelength. Subsequent experimentation was carried out using lampenflora enrichment cultures inoculated onto flat calcium carbonate tile surfaces to approximate growth surfaces in caves and conduct carefully controlled, reproducible experiments. These calcium carbonate tiles were generously donated by New Mexico Travertine in Belen, New Mexico, USA. The tiles were all from the same quarry in Leuders, Texas, USA, and are nearly 100% CaCO3. The tiles were cut into 5 cm × 5 cm squares and placed into extra deep Petri dishes along with moistened filter paper to maintain a nearly saturated relative humidity (Fig. 2). The tiles were inoculated by spreading 1 ml of enrichment culture onto the upper surface and then they were incubated under fluorescent lighting. Growth of the lampenflora was monitored using a hand-held reflected light spectrophotometer, as described below and/or by taking photographs of the tiles. Experiments using tiles were conducted in triplicate, or in quadruplicate for one experiment, i.e., three (or four) tiles were used for each treatment and each of these was analyzed independently at each time point.
Calcium carbonate tiles in Petri dishes inoculated with lampenflora enrichment cultures for biocide experiments. The Petri dish lids were removed for UV-C irradiation and for photographing. The lids were replaced during incubation under fluorescent lighting. The filter paper was moistened to maintain nearly saturated relative humidity. The relative amount of photopigment was scored 0 through 3
Treatments with UV-C were carried out using an MRL-58 Multiple-Ray lamp (UVP, Inc., Upland, California, USA) equipped with a Sankyo Denki G8TB 8-W, 11-inch germicidal tube that delivers 2.5-W illumination at 254-nm wavelength. An MS-100 Multi-Sense optical radiometer equipped with an MS-125 UVC sensor (UVP, Inc., Upland, California, USA) was used to quantify the radiation flux. Total UV-C irradiation in mJ/cm2 depended on distance from the lamp to the tiles and the time of exposure, e.g., at 25 cm and 1.5 mW/cm2 flux, a 12,800˚mJ/cm2 irradiation was achieved in 2 h and 22 min. In a separate experiment intended to simulate use of UV-C overnight in a show cave from a longer distance (25 cm), the flux was 0.25 mW/cm2 for 6 h, achieving a total dose of 5400 mJ/cm2.
Photopigments were quantified using a reflected light spectrophotometer (Konica Minolta CM-600d), which measures color of a surface in CIELAB (International Commission on Illumination L*a*b*) color space, where L* values represent a light-to-dark scale, a* represents the red-to-green color range, and b* represents the yellow-to-blue color scale. Negative a* values indicate a green color. For one experiment, light reflected in the green range (500–550 nm) was monitored with the reflected light spectrophotometer. Growth of photosynthetic biofilms was also quantified by scoring growth from 0 to 3 in comparison to a rubric, as shown in Fig. 2.
Results and Discussion
BAC Treatments
The effects of BAC as a growth inhibitor were tested using an enrichment culture of algae and cyanobacteria in liquid BG-11 medium. The results showed partial inhibition by as little as 0.001 parts per million and complete inhibition by 10 parts per million (Fig. 3). It should be noted that microbes grown in suspension in a liquid growth medium are generally more susceptible to chemical inhibitors than the same microbes in a biofilm (Barber and Hartmann 2022). This likely explains the sensitivity of these phototrophic microbes to low concentrations of benzalkonium chloride in this experiment.
The effects of various concentrations of benzalkonium chloride (BAC) on growth of an enrichment culture of algae and cyanobacteria derived from Carlsbad Cavern lampenflora. The algae and cyanobacteria were grown in liquid culture. Growth was measured as increase in light absorbance at 600 nm. n = 1 for this liquid culture experiment only
In a first test of BAC on growth of a lampenflora culture on tiles, we found that a single dose with 15 ppm was sufficient to prevent subsequent growth and to cause a decline in green pigmentation, evidenced by increasingly positive a* values in color space as measured by reflected light spectrophotometry (Fig. 4). Further experimentation tested the effects of various concentrations of benzalkonium chloride and various times of treatment (Figs. 5, 6, 7). When the benzalkonium chloride was applied to the tiles immediately after inoculation, concentrations less than or equal to 10 ppm showed no effect, i.e., growth of the photosynthetic biofilms was not slowed. Concentrations of 100 ppm or more prevented or slowed the onset of growth. The 100-ppm treatment delayed growth until day 4 and then the level of growth was nearly as high as on the 0-ppm control tiles. Similarly, the 1000-ppm treatment delayed growth, but then photosynthetic biofilm appeared on day 14. The results of this experiment indicate that benzalkonium chloride can inhibit lampenflora growth, but only at very high concentrations (10,000 ppm or more).
Relative amounts of photopigment vs. time in an experiment in which benzalkonium chloride (BAC) was applied at various concentrations immediately after inoculation of the tiles with lampenflora enrichment culture. All treatments were performed with triplicate tiles. Tiles were scored for photopigment development as shown in Fig. 2. Scores of replicate tiles varied by no more than and usually less than one unit
Relative amounts of photopigment vs. time in an experiment in which benzalkonium chloride (BAC) was applied on day 7 after full photosynthetic biofilm development. All treatments were performed with triplicate tiles. Tiles were scored for photopigment development as shown in Fig. 2. Scores of replicate tiles varied by no more than and usually less than one unit
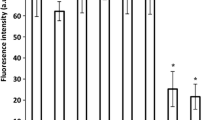
Relative amounts of photopigment vs. time in an experiment in which benzalkonium chloride (BAC) was applied on day 7, day 14, and day 21 after full photosynthetic biofilm development had occurred. All treatments were performed on quadruplicate tiles. Tiles were scored for photopigment development as shown in Fig. 2. Scores of replicate tiles varied by no more than and usually less than one unit
BAC was tested as a means of treating fully developed lampenflora and was found to bleach the photopigments, but high concentrations were necessary to show this effect (Fig. 6). 100,000 ppm (10% weight to volume) benzalkonium chloride nearly completely bleached the biofilm. Lower concentrations also had an effect, with as little as 10 ppm causing a decline in photopigments; however, the biofilms showed some recovery on all of the tiles except those receiving a 10% solution of benzalkonium chloride. Repeated, weekly treatments with benzalkonium chloride at 10,000 ppm (1%) were sufficient to bleach a preformed biofilm and to prevent its regrowth; however, lower concentrations were ineffective (Fig. 7).
It was encouraging to see that BAC not only prevents growth on uncolonized calcium carbonate, it also bleaches preformed photosynthetic biofilms. BAC can be biologically degraded (Boethling 1984; Tezel and Pavlostathis 2015; Fortunato et al. 2019), although as a quaternary ammonium compound, it degrades relatively slowly. For this reason, frequent application of BAC could leave an undesirable residue. Therefore, the most appropriate use will likely be limited to brief exposures on limited areas where the lampenflora are densely formed. Also, as with any biocidal treatment, there is concern over effects on non-target organisms, e.g., cave invertebrates. However, this effect would be limited to the sites of existing lampenflora. Currently the sole cleaning agent used on lampenflora in Carlsbad Cavern and most other show caves is commercial bleach (sodium hypochlorite), a strong oxidant with toxic potential (Slaughter et al. 2019).
UV-C Treatments
A preliminary experiment in which lampenflora algae and cyanobacteria were inoculated onto tile surfaces and then immediately irradiated with UV-C indicated that the biofilms were sensitive to UV-C at dosages as low as 16 mJ/cm2 but that exposure to 512 or 1024 mJ/cm2 had the greatest effect (data not shown). A further experiment simulated a scenario in which lampenflora is irradiated with UV-C during the night when tourists are not present in a cave, alternating with periods during the day when the cave lighting is on. Six hours of UV-C (5400 mJ/cm2) caused a decline in photopigment abundance (Fig. 8). Subsequent exposure to fluorescent lights didn’t promote a resurgence of algae and cyanobacteria, and a second 6-h UV-C treatment at the same radiation flux caused further photopigment decline, also with no subsequent recovery during simulated cave lighting. This experiment suggests that UV-C could be applied during the night on a schedule, perhaps with long intervening intervals of no treatment.
Three replicate tiles inoculated with lampenflora cultures and then UV-C irradiated for 6 h, followed by 18 h of fluorescent light, followed by 6 more hours of UV-C, and then 48 h of fluorescent light. Green color declined throughout. The experiment was intended to simulate 6 h of UV-C treatment on two successive nights when no tourists are present, followed by daytime intervals of cave lighting. Each 6-h UV-C treatment delivered a 5400 mJ/cm2 dose. The UV-C decreased the photopigment abundance and the photopigments did not return, even after 48 more hours under fluorescent light. Error bars represent one standard deviation (n = 3)
In a follow-up experiment in which the UV-C dosages were varied, exposure to 3200 mJ/cm2 or greater caused bleaching of an established biofilm (Fig. 9). The lampenflora showed some recovery after the initial treatment, but subsequent weekly treatments caused further decrease in photopigments. Exposure to 400 or 800 mJ/cm2 caused a partial decrease in lampenflora. Note that these doses are much lower than the 150–300 kJ/m2 (15,000–30,000 mJ/cm2) doses tested by Borderie et al. (2011, 2014a, b, 2015) or the 646 kJ/m2 (64,600 mJ/cm2) irradiation used by Pfendler et al. (2018) for a cultural heritage site.
UV-C treatment of lampenflora enrichment culture that had been allowed to grow for one week. Tiles were treated with various amounts of UV light on day 7, day 14, and day 21. All treatments were in triplicate. Tiles were scored for photopigment development as shown in Fig. 2. Scores of replicate tiles varied by no more than and usually less than one unit
An advantage of using germicidal lamps in the UV-C range is that the lamps could be installed in the same locations as the visible lights used to illuminate the caves since the electrical power supply is already there. The UV treatments could then be applied during hours when there are no visitors in the cave. The main objection to this approach is the cost of installing and maintaining a large number of UV-C lamps. An alternative strategy could be to purchase only a few portable UV-C lamps and to deploy them in sites with the most problematic lampenflora growth, using high doses (e.g., using high-intensity lamps, short distances, long exposure times, repeated exposure) following the conditions used in the experiment shown in Fig. 9. The treatment could be applied during evening hours while the cave is closed to visitors. The lamps could be left in place, but turned off while the cave is open to visitors, with signs to explain their use to visitors.
As with chemical treatments, UV-C has the potential to harm sensitive cave fauna. However, the biocidal effects of UV-C will occur only in the very localized illuminated regions that comprise only a minute fraction of the cave ecosystem. Any animals in the vicinity of the lampenflora are likely under somewhat unnatural selection, drawn to the unnatural organic carbon accumulation. The cave fauna dwelling in the dark or in areas so dimly lit as not to form lampenflora biofilms, will be unaffected.
Conclusions
Benzalkonium chloride is an effective biocide and additionally causes a bleaching of lampenflora; however, high concentrations (1–10% weight/volume) and repeated treatment may be necessary. We recommend that benzalkonium chloride be tested in Carlsbad Cavern with an eye to using it as a spot treatment for highly visible lampenflora, but with care to avoid exposure of cave fauna to BAC. Germicidal UV light (UV-C) is a very attractive lampenflora control measure. Ideally, UV lamps could be installed along with the LEDs and turned on at night when tourists are absent. However, the cost of installation and operation may be problematic, and their long-term impacts on cave invertebrates should be monitored. Simple, relatively inexpensive (< 1 k USD) UV-C lamps could be deployed temporarily to treat especially visible lampenflora biofilms. These could even be left in place during visitor hours, with signage explaining the need for lampenflora mitigation. As with BAC, efforts should be made to minimize exposure of cave fauna to UV-C. Finally, there is not likely to be a single solution to the lampenflora problem. Instead, it will require a combination of mitigation strategies that include not only physical and chemical removal, but also minimizing the duration and intensity of illumination, and avoiding red and blue wavelengths of light.
References
Arrhenius Å, Backhaus T, Hilvarsson A, Wendt I, Zgrundo A, Blanck H (2014) A novel bioassay for evaluating the efficacy of biocides to inhibit settling and early establishment of marine biofilms. Mar Pollut Bull 87:292–299. https://doi.org/10.1016/j.marpolbul.2014.07.011
Ascaso C, Wierzchos J, Souza-Egipsy V, De los Rıos A, Rodrigues JD (2002) In situ evaluation of the biodeteriorating action of microorganisms and the effects of biocides on carbonate rock of the Jeronimos Monastery (Lisbon). Int Biodeterior Biodegrad 49:1–12. https://doi.org/10.1016/S0964-8305(01)00097-X
Barber OW, Hartmann EM (2022) Benzalkonium chloride: A systematic review of its environmental entry through wastewater treatment, potential impact, and mitigation strategies. Crit Rev Environ Sci Technol 52:2691–2719. https://doi.org/10.1080/10643389.2021.1889284
Bastian F, Jurado V, Nováková A, Alabouvette C, Sais-Jimenez C (2010) The microbiology of Lascaux Cave. Microbiol 156:644–652. https://doi.org/10.1099/mic.0.036160-0
Boethling RS (1984) Environmental fate and toxicity in wastewater treatment of quaternary ammonium. Water Res 18:1061–1076. https://doi.org/10.1016/0043-1354(84)90220-3
Borderie F, Laurence AS, Naoufal R, Faisl B, Geneviève O, Dominique R, Badr AS (2011) UV–C irradiation as a tool to eradicate algae in caves. Int Biodeterior Biodegrad 65:579–584. https://doi.org/10.1016/j.ibiod.2014.05.014
Borderie F, Alaoui-Sehmer L, Bousta F, Alaoui-Sossé B, Aleya L (2014) Cellular and molecular damage caused by high UV-C irradiation of the cave-harvested green alga Chlorella minutissima: implications for cave management. Int Biodeterior Biodegrad 93:118–130. https://doi.org/10.1016/j.ibiod.2014.05.014
Borderie F, Tête N, Cailhol D, Alaoui-Sehmer L, Bousta F, Rieffel D, Aleya L, Badr A-S (2014) Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci of the Total Environ 484:43–52. https://doi.org/10.1016/j.scitotenv.2014.03.043
Borderie F, Alaoui-Sehmer L, Aleya L (2015) Heritage materials and biofouling mitigation through UV-C irradiation in show caves: state-of-the-art practices and future challenges. 2015. Environ Sci Pollut Res 22:4144–4172. https://doi.org/10.1007/s11356-014-4001-6
Dobat K (1963) “Höhlenalgen" bedrohen die Eiszeitmalereien von Lascaux. Die Höhle 14:41–45
Estévez CB, Morino-Merino L, Román AD, Valsero JJD (2019) The lampenflora in show caves and its treatment: an emerging ecological problem. Int J Speleol 48:249–277. https://doi.org/10.5038/1827-806X.48.3.2263
Faimon J, Štelcl J, Kubešová S, Zimák J (2003) Environmentally acceptable effect of hydrogen peroxide on cave “lamp-flora”, calcite speleothems and limestones. Environ Pollut 122:417–422. https://doi.org/10.1016/S0269-7491(02)00309-3
Fortunato MS, Baroni S, Gonzalez AJ, Roncancio JDA, Storino A, Parise C, Planes E, Gallego A, Korol SE (2019) Biodegradation and detoxification of benzalkonium chloride in synthetic and industrial effluents in upflow aerobic reactors. Water Air Soil Pollut 230:79. https://doi.org/10.1007/s11270-019-4126-9
Grobbelaar JU (2000) Lithophytic algae: A major threat to the karst formation of show caves. J Appl Phycol 12:309–315. https://doi.org/10.1023/A:1008172227611
Guo W, Cui S, Xu X, Wang H (2014) Resistance mechanism study of benzalkonium chloride selected Salmonella typhimurium mutants. Microb Drug Resist 20:11–16. https://doi.org/10.1089/mdr.2012.0225
Havlena Z, Kieft TL, Veni G, Horrocks RD, Jones DS (2021) Lighting effects on the development and diversity of photosynthetic biofilm communities in Carlsbad Cavern, New Mexico. Appl Environ Microbiol 87:e02695-e2720. https://doi.org/10.1128/AEM.02695-20
Lavorgna M, Russo C, D’Abrosca B, Parrella A, Isidori M (2016) Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ Pollut 210:34–39. https://doi.org/10.1016/j.envpol.2015.11.042
Meyer E, Seale LD, Permar B, McClary A (2017) The effect of chemical treatments on lampenflora and a collembola indicator species at a popular tour cave in California, USA. Environ Manage 59:1034–1042. https://doi.org/10.1007/s00267-017-0842-3
Mulec J, Kosi G (2009) Lampenflora algae and methods of growth control. J Cave Karst Stud 7:109–115
Pereira BMP, Tagkopoulos I (2019) Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl Environ Microbiol 85:e00377-19. https://doi.org/10.1128/AEM.00377-19
Pfendler S, Einhorn O, Karimi B, Bousta F, Cailhol D, Alaoui-Sosse B, Aleya L (2017) UV-C as an effective means to combat biofilm formation in show caves: evidence from in situ and laboratory experiments. Environ Sci Pollut Res 24:24611–24623. https://doi.org/10.1007/s11356-018-1654-6
Pfendler S, Borderie F, Bousta F, Alaoui-Sosse L, Alaoui-Sosse B, Aleya L (2018) Comparison of biocides, allelopathic substances and UV-C as treatments for biofilm proliferation on heritage monuments. J Cult Herit 33:117–124. https://doi.org/10.1016/j.culher.2018.03.016
Romani M, Warscheid T, Nicole L, Marcon L, Di Martino P, Suzuki MT, Lebaron P, Lami R (2022) Current and future chemical treatments to fight biodeterioration of outdoor building materials and associated biofilms: moving away from ecotoxic and towards efficient, sustainable solutions. Sci Total Environ 802:149846. https://doi.org/10.1016/j.scitotenv.2021.149846
Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ (2019) The toxicology of sodium hypochlorite. Clin Toxicol 57:303–311. https://doi.org/10.1080/15563650.2018.1543889
Sterflinger K, Piñar G (2013) Microbial deterioration of cultural heritage and works of art − tilting at windmills? Appl Microbiol Biotechnol 97:9637–9646. https://doi.org/10.1007/s00253-013-5283-1
Tandukar M, Oh S, Tezel U, Konstantinidis KT, Paviostathis SG (2013) Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased microbial resistance. Environ Sci Technol 47:9730–9738. https://doi.org/10.1021/es401507k
Tezel U, Pavlostathis SG (2015) Quaternary ammonium disinfectants: microbial adaptation, degradation and ecology. Curr Opin Biotechnol 33:296–304. https://doi.org/10.1016/j.copbio.2015.03.018
U.S. Environmental Protection Agency (EPA) (2006) Reregistration eligibility decision for alkyl dimethyl benzyl ammonium chloride (ADBAC), vol 71, no 250. Environmental Protection Agency, Office of the Federal Register, National Archives and Records Administration, Washington, DC. https://nepis.epa.gov/Exe/ZyNET.exe/P1005J4P.txt?ZyActionD=ZyDocument&Client=EPA&Index=2006%20Thru%202010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=
Warscheid T, Braams J (2000) Biodeterioration of stone: a review. Int Biodeterior Biodegrad 46:343–368. https://doi.org/10.1016/S0964-8305(00)00109-8
Young ME, Alakomi H-L, Fortune I, Gorbushina AA, Krumbein WE, Maxwell I, McCullagh C, Robertson P, Saarela M, Valero J, Vendrell M (2008) Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ Geol 56:631–641. https://doi.org/10.1007/s00254-008-1455-1
Acknowledgements
We thank the personnel of Carlsbad Caverns National Park for access to Carlsbad Cavern and for help in sampling, especially the Chief of Natural and Cultural Resources, Rod Horrocks.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This research was funded by a grant from the U.S. National Park Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kieft, T.L., Del Curto, D., Havlena, Z. et al. Potential for Mitigation of Cave Lampenflora Using Benzalkonium Chloride or UV-C. Geoheritage 15, 68 (2023). https://doi.org/10.1007/s12371-023-00839-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12371-023-00839-4