Abstract
The conversion of wild caves into tourist sites poses serious threats to the conservation of subterranean environments. Among them, the extensive growth of photosynthetic biofilms induced by artificial lighting—the so-called lampenflora—is of particular concern for cave managers. The identification of cost-effective management actions controlling the growth of lampenflora is therefore required to preserve the environmental and touristic values of show caves. By taking advantage of the closure period imposed to contain the COVID-19 pandemic, we tested whether 6 months of cave closure could be an effective strategy to reduce the concentration of photosynthetic biofilms on speleothems in four geographically close Italian show caves. We compared the concentration of the three main microorganism groups composing lampenflora, i.e., cyanobacteria, diatoms, and green algae, measured in September 2020 with values recorded 6 months after the closure, in May 2021. Although slight variations have been observed across the different sampling sessions, we did not detect any significant effect of the closure period on the overall concentration values of lampenflora. Also, we recorded no significant differences in lampenflora concentration after 4 months of regular tourist use, in September 2021. Our results suggest that management practices based on regulating visits to show caves are not effective strategies to reduce lampenflora. Therefore, management practices aiming at a sustainable use of show caves should focus on the active removal of photosynthetic biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first record of tourists entering a cave is dated back to 1633 in the Vilenica Cave in Slovenia (Cigna and Forti 2013). Since then, and especially during the early 1980s, a number of caves around the world have been converted into touristic attractions, the so-called “show caves,” where paying visitors experience the cave environment and its wonders via constructed trails, guided tours, artificial lighting systems, and regular opening hours (Cigna 2019). However, given their peculiar environmental conditions—ultra-oligotrophic habitats, lack of light and nutrients, spatial confinement, low climatic fluctuations, and low levels of biodiversity (Culver and Pipan 2019)—caves are extremely susceptible to anthropogenic disturbances imposed by their conversion into tourist attractions (Cigna 2016), jeopardizing not only their ecological but also their touristic values. Tourists in caves alter the natural microclimatic conditions (Addesso et al. 2022a) in terms of relative humidity, temperature of air (e.g., Cowan et al. 2013; Šebela et al. 2015) and water (e.g., Šebela and Turk 2014), and CO2 concentration (e.g., Lang et al. 2015; Pla et al. 2016), which may enhance carbonate dissolution and damage speleothems (Fernandes-Cortes et al. 2010). In addition, they bring propagules of external microorganisms inside the cave (Addesso et al. 2022b), such as fungi and bacteria, which may generate outbreaks in the cave air (Martin-Sanchez et al. 2014; Porca et al. 2011), water (Ando and Murakami 2020; Moldovan et al. 2020), soil (Kukla et al. 2018; Mammola et al. 2017), and especially speleothems (Saiz-Jimenez et al. 2012), with potential repercussions on the entire subterranean ecosystem. Also, the installation of artificial lights in show caves is necessary to allow visitors to enjoy the natural wonders in an otherwise completely dark environment, but at the same time, it allows the growth of a photosynthetic community alien to the cave (Mulec 2019). The proliferation of photosynthetic microorganisms represents a severe threat to the natural heritage of show caves because this photobiota induces dramatic physical, chemical, and ecological damages (Baquedano Estevez et al. 2019). More in detail, photosynthetic microorganisms grow as components of complex biofilms, forming thick green, brown, or grayish patinas on cave walls, with consequent aesthetic damage to speleothems (Mulec 2019). Also, phototrophic microorganisms, especially cyanobacteria, produce exopolymeric substances (EPSs), which induce the adsorption of cations and dissolved organic molecules from the mineral surface causing the deterioration of the substrate (Bruno and Valle 2017). This substantial damage has consequent negative repercussions on the economic profits of the local populations, ultimately challenging the general sustainability of show cave tourism (Cigna 2016).
The identification of cost-effective interventions controlling the growth of lampenflora is therefore required to preserve the touristic values of show caves (Baquedano Estevez et al. 2019). Among the possible measures, the implementation of the tourist carrying capacity (Chen et al. 2021; Lobo et al. 2013) or the closure of show caves to the public for some periods (Killing-Heinze et al. 2017) has been demonstrated as effective strategies to re-establish the baseline values of the cave microclimate. Their efficacy is strictly related to the fact that microclimate alterations induced by visitors are temporary and largely depend on cave size (Dominguez-Villar et al. 2010). Conversely, studies conducted in the iconic Lascaux cave demonstrated that microbial invasions are extremely hard to control, and the return of the cave to pristine conditions is compromised even after several years of cave closure (Alonso et al. 2018, 2019; Martin-Sanchez et al. 2014; Porca et al. 2011). However, the effectiveness of cave closure as a control measure for the lampenflora proliferation has never been tested so far.
To fill this gap, we took advantage of the 6-month lockdown period imposed by the Italian government to control the COVID-19 pandemic. During this time, all cultural and natural heritage sites, including show caves, were closed to the public. We compared the total chlorophyll-a (hereafter chl-a) concentration of the three main groups of photosynthetic microorganisms composing lampenflora, i.e., cyanobacteria, diatoms, and green algae, before and after the closure period, as well as after 4 further months of a regular tourist flux, in four geographically close Italian show caves.
Materials and Methods
Sampling Design
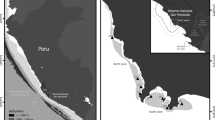
We performed our study in four show caves located at a distance of < 50 km from each other in NW Italy, all opening in limestone rocks, that are Bossea, Caudano, Borgio Verezzi, and Toirano show caves (Fig. 1). Data were collected in three sampling sessions. The first session (hereafter “Control” session) was performed in September 2020, immediately before the cave closure announced by the Italian government to control the COVID-19 pandemic. The second session (hereafter “Closure” session) was performed in May 2021, when all show caves reopened to the public at the end of the national lockdown, for a total of 176 days of closure (closing date = 05/11/2020; opening date = 01/05/2021). We then repeated our measurements 4 months after the cave reopening (hereafter “Opening” session), in September 2021. This third sampling session was included to verify whether the lampenflora concentration has recovered to the values observed during the control session after the summer tourist season.
Map of the four examined show caves with information about their characteristics with respect to geography (elevation), morphology (cave planimetric length), physical parameters (mean annual temperature), and tourism (opening year, tourist flux, length of tourist path, and light type). The tourist flux is reported for the year 2019 (photo credits: https://www.grotteturistiche.it). *LED lights have been installed after this study was concluded, while halogen lamps were present at the time of the study
Data Collection
In each cave, we selected one illuminated speleothem (hereafter “plot”) on average every 50 m from the cave entrance along the touristic path, for a total of 24 plots in Bossea and Caudano, 21 plots in Toirano, and 14 plots in Borgio Verezzi. On each speleothem, we identified one sampling point, consisting in a circle of 20 cm diameter (see Fig. 2 for examples of sampling sites). Sampling points were marked with chalk to be able to perform chl-a measurements exactly on the same site in every sampling session. In each sampling point and in each sampling session, we measured three replicates of lampenflora concentration by means of the BenthoTorch®. This instrument, developed by BBE Moldaenke GmbH (Schwentinental, Germany), is a pulse amplitude modulated (PAM) fluorimeter specifically intended to measure the chlorophyll a density (µg chl-a/cm2) of the three main photosynthetic organisms, i.e., cyanobacteria, diatoms, and green algae, composing biofilms on hard substrates. The density values are obtained by emitting light pulses at three different wavelengths (470, 525, and 610 nm) and recording the response at 690 nm wavelength. Thanks to an inbuilt algorithm, the instrument calculates an instantaneous and in situ measure of chlorophyll a (chl-a) concentration for each of the three photosynthetic groups.
Data Analysis
We retained the median value of the three chl-a replicates collected in each plot for each photosynthetic group to be included in the subsequent statistical analyses. The median value was preferred over the mean because it is less influenced by extreme values (Legendre and Legendre 2012). Given that the distribution of the chl-a measures obtained for the three groups was mainly composed by low values with some extremely high values, we used the median values as a dependent variable in the subsequent analyses in order to obtain robust and unbiased results. The total chl-a concentration for each plot was obtained by summing the median chl-a measures obtained for cyanobacteria, diatoms, and green algae.
All statistical analyses were performed with the R software (R Core Team 2021). In a preliminary step, following the approach proposed by Zuur et al. (2009, 2010), we performed data exploration by checking the distribution of dependent variables and identifying possible outliers with the “hist” and “plot” functions, respectively. We considered as dependent variables the total chl-a concentration and the chl-a concentrations of cyanobacteria, diatoms, and green algae, for a total of four dependent variables. The effect of the sampling session was then tested on the dependent variables by means of Generalized Linear Mixed Models (GLMMs), for a total of four different models. Statistical models were performed with the function “glmmTMB” from the glmmTMB package (version 1.1.2.3, Brooks et al. 2017). Considering that our dependent variables could not assume negative values, but were highly zero-inflated, we specified a ziGamma distribution, with a log link function, which allowed us to perform zero-inflated gamma models, i.e., ZIGs (Mills 2013). According to the ZIG approach, the algorithm ran a binomial-GLMM, which tests the probability that an outcome is a non-zero value, and a gamma-GLMM, which is appropriate for data with strictly continuous non-zero values. The outcome of the model is composed by two different outputs: (i) the output of the binomial model that explains how the sampling session affects the presence or the absence of the target groups and (ii) the output of the gamma model that reports whether there are differences among sampling sessions in terms of chl-a density values. The presence/absence data are obtained by considering the target group as absent in a sampling site when its chl-a density is equal to 0, while it is considered present when its chl-a density is > 0.
Results and Discussion
The average values of total chl-a concentration and the concentrations of the three examined photosynthetic groups showed some fluctuations across the three sampling sessions (Table 1). Although a slight decrease in the observed values was recorded for all the three groups, as well as for the total chl-a concentration, during the cave closure and after the cave re-opening, we could not detect any significant effect of the sampling session in terms of both chlorophyll-a density (Table 2a) and presence/absence of photosynthetic microorganisms (Table 2b) on any of the examined group, i.e., cyanobacteria, diatoms, and green algae, and on their overall growth, i.e., total chl-a (Fig. 3).
The observed lack of significant difference in the lampenflora concentration before (“Control” session) and after the cave closure (“Closure” session) points out that the absence of light experienced during the closure period does not significantly reduce the proliferation of photosynthetic organisms living in subterranean habitats. Our results are corroborated by evidence in literature showing that lampenflora can survive at low light intensities or even in the absence of light (Aley 2004; Mulec and Kosi 2009). More in detail, there are studies demonstrating that cyanobacteria, but also fast-growing green algae, such as Chlorella minutissima, which are usually the first colonizers in cave biofilms (Mulec et al. 2008; Nikolić et al. 2020), can survive even at extremely low values of light intensity (Czerwik-Marcinkowska et al. 2015; Roldán et al. 2004) in some cases even considerably below the photosynthetic compensation point (Bruno and Valle 2017). Similarly, some aerophilous diatom species can be recorded in dim light or in complete darkness, where the relative humidity of the air is sufficiently high (Asencio and Aboal 2000a, b). Thus, based on our results, we can hypothesize that the extreme conditions of subterranean ecosystems likely exert a strong ecological filter selecting only species adapted to tolerate low light regimes or even light absence. Once these species become established, they can tolerate even abrupt changes in environmental conditions, like the absence of light for long periods.
Our study also pointed out no significant differences between concentration values observed after the cave closure (“Closure” session) and after the summer tourist season (“Opening” session) for all examined groups, even if a slight increase can be observed in the “Opening” session. Although lampenflora shows spatial variation in response to local spatial changes in anthropogenic and environmental parameters, such as light intensity, light duration, humidity, and temperature (Borderie et al. 2015; Falasco et al. 2014, 2015; Piano et al. 2015), our results suggest that temporal variations in its concentration are negligible. In other words, photosynthetic biofilms in show caves seem not to be subject to seasonal variations, contrary to what happens in freshwater (Beck et al. 2019; Justus et al. 2021; Piano et al. 2017) and epigean terrestrial ecosystems (Foets et al. 2020) where seasonal changes of ecological drivers, e.g., flow velocity, nutrient availability, and grazer abundance, determine consequent variations in patterns of photosynthetic microbial communities. This lack of seasonality may indicate that the high temporal stability of environmental conditions in subterranean habitats allow the undisturbed growth of photosynthetic organisms to their plateau, without evident changes across the periods of the year.
Although our study is limited to only four show caves, we can reasonably support the hypothesis that scheduling periods of cave closure of less than 6 months does not represent an effective method to reduce the concentration of lampenflora in show caves. Considering this, the implementation of adequate management practices is required to control lampenflora and contain its repercussions on the subterranean ecosystem. Several studies demonstrated that the modulation of light—that is, the environmental factor easiest to control in show caves—may significantly reduce the growth of photosynthetic microorganisms (Borderie et al. 2014; Bruno and Valle 2017; D’Agostino et al. 2015; Havlena et al. 2021; Mulec 2012; Mulec et al. 2008; Piano et al. 2021; Roldán et al. 2006). However, our results pointed out no significant differences in lampenflora concentration when exposed to different lighting regimes—light absence during 6 months of cave closure or regular lighting during the tourist use. Thus, effective mitigation actions aiming at actively removing lampenflora are likely required to guarantee the tourist values of show caves. Multiple methods are currently being used, e.g., bleach or hydrogen peroxide solutions (Faimon et al. 2003; Trinh et al. 2018) and UV-C lights (Borderie et al. 2014; Pfendler et al. 2017). Although little is known about their side effects on the subterranean environment, some evidences in literature suggest that they increase proportionally with their efficiency in eradicating lampenflora (Meyer et al. 2017). Therefore, their use should be limited to the most compromised speleothems, in combination with an overall modulation of lighting in the entire show cave, e.g., by adopting low-temperature LED (Havlena et al. 2021) or reducing light intensity (Piano et al. 2015) and duration (Piano et al. 20212021) and increasing the distance of lamps from surfaces.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Addesso R, Bellino A, Baldantoni D (2022a) Underground ecosystem conservation through high-resolution air monitoring. Environ Manag 69:982–993. https://doi.org/10.1007/s00267-022-01603-0
Addesso R, Pingaro S, Bisceglia B, Baldantoni D (2022b) Sustainable tourism and conservation of underground ecosystems through airflow and particle distribution modeling. Sustainability 14:7979. https://doi.org/10.3390/su14137979
Aley T (2004) Tourist caves: algae and lampenflora. In: Gunn J (ed) Encyclopedia of caves and karst science. Taylor and Francis Routledge, New York, pp 7733–7734
Alonso L, Creuzé-des-Châtelliers C, Trabac T, Dubost A, Moënne-Loccoz Y, Pommier T (2018) Rock substrate rather than black stain alterations drives microbial community structure in the passage of Lascaux Cave. Microbiome 6:1–15. https://doi.org/10.1186/s40168-018-0599-9
Alonso L, Pommier T, Kaufmann B, Dubost A, Chapulliot D, Doré J, Douady CJ, Moënne-Loccoz Y (2019) Anthropization level of Lascaux Cave microbiome shown by regional-scale comparisons of pristine and anthropized caves. Mol Ecol 28:3383–3394. https://doi.org/10.1111/mec.15144
Ando K, Murakami T (2020) Detection of human-associated bacteria in water from Akiyoshi-do Cave, Japan. Wat Environ Res 92:1866–1873. https://doi.org/10.1002/wer.1355
Asencio AD, Aboal M (2000a) A contribution to knowledge of chasmoendolithic algae in cave-like environments. Arch Hydrobiol Suppl 133:133–151
Asencio AD, Aboal M (2000b) Algae from La Serreta cave (Murcia, SE Spain) and their environmental conditions. Algol Stud/ Arch Hydrobiol Suppl 96:59–78. https://doi.org/10.1127/algol_stud/96/2000/59
Baquedano Estevez C, Moreno Merino L, de la Losa RA, Duran Valsero JJ (2019) The lampenflora in show caves and its treatment: an emerging ecological problem. Int J Speleol 48:249–277. https://doi.org/10.5038/1827-806X.48.3.2263
Beck WS, Markman DW, Oleksy IA, Lafferty MH, Poff NL (2019) Seasonal shifts in the importance of bottom–up and top–down factors on stream periphyton community structure. Oikos 128:680–691. https://doi.org/10.1111/oik.05844
Borderie F, Tete N, Cailhol D, Alaoui-Sehmer L, Bousta F, Rieffel D, Aleya L, Alaoui-Sossé B (2014) Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci Tot Environ 484:43–52. https://doi.org/10.1016/j.scitotenv.2014.03.043
Borderie F, Alaoui-Sossé B, Aleya L (2015) Heritage materials and biofouling mitigation through UV-C irradiation in show caves: state-of-the-art practices and future challenges. Environ Sci Pollut Res 22:4144–4172. https://doi.org/10.1007/s11356-014-4001-6
Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Macheler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.3929/ethz-b-00024089
Bruno L, Valle V (2017) Effect of white and monochromatic lights on cyanobacteria and biofilms from Roman Catacombs. Int Biodeterior Biodegradation 123:286–295. https://doi.org/10.1016/j.ibiod.2017.07.013
Chen Y, Chen A, Mu D (2021) Impact of walking speed on tourist carrying capacity: the case of Maiji Mountain Grottoes. China. Tour Manag 84:104273. https://doi.org/10.1016/j.tourman.2020.104273
Cigna AA (2016) Tourism and show caves. Zeitschrift Für Geomorphol Suppl Issues 60:217–233. https://doi.org/10.1127/zfg_supl/2016/00305
Cigna AA (2019) Chapter 108: Show caves. In: White BW, Culver DC, Pipan T (eds) Encyclopedia of Caves, 3rd edn. Academic Press, London, pp 909–921
Cigna AA, Forti P (2013) Caves: the most important geotouristic feature in the world. Tourism Karst Areas 6:9–26
Cowan BD, Osborne MC, Banner JL (2013) Temporal variability of cave-air CO2 in central Texas. J Cave Karst Stud 75:38–50. https://doi.org/10.4311/2011ES0246
Culver DC, Pipan T (2019) The biology of caves and other subterranean habitats, 2nd edn. Oxford University Press, Oxford, pp 276
Czerwik-Marcinkowska J, Wojciechowska A, Massalski A (2015) Biodiversity of limestone caves: aggregations of aerophytic algae and cyanobacteria in relation to site factors. Pol J Ecol 63:481–499. https://doi.org/10.3161/15052249PJE2015.63.4.002
D’Agostino D, Beccarisi L, Camassa M, Febbroriello P (2015) Microclimate and microbial characterization in the Zinzulusa show cave (South Italy) after switching to LED lighting. J Cave Karst Stud 77:133–144. https://doi.org/10.4311/2014EX0123
Dominguez-Villar D, Fairchild IJ, Carraasco RM, Pedraza J, Baker A (2010) The effect of visitors in a touristic cave and the resulting constraints on natural thermal conditions for palaeoclimate studies (Eagle Cave, central Spain). Acta Carsol 39:491–502
Faimon J, Štelcl J, Kubešová S, Zimák J (2003) Environmentally acceptable effect of hydrogen peroxide on cave ‘“lamp-flora”’, calcite speleothems and limestones. Environ Pollut 122:417–422. https://doi.org/10.1016/S0269-7491(02)00309-3
Falasco E, Ector L, Isaia M, Wetzel CE, Hoffmann L, Bona F (2014) Diatom flora in subterranean ecosystems: a review. Int J Speleol 43:231–251. https://doi.org/10.3161/15052249PJE2015.63.4.002
Falasco E, Bona F, Isaia M, Piano E, Wetzel CE, Hoffmann L, Ector L (2015) Nupela troglophila sp. nov., an aerophilous diatom (Bacillariophyta) from the Bossea cave (NW Italy), with notes on its ecology. Fottea 15:1–9
Fernandez-Cortes A, Sanchez-Moral S, Cañaveras JC, Cuevas-Gonzalez J, Cuezva S, Andreu JM (2010) Variations in seepage water geochemistry induced by natural and anthropogenic microclimatic changes: implications for speleothem growth conditions. Geodin Acta 23:1–13. https://doi.org/10.3166/ga.23.1-13
Foets J, Wetzel CE, Teuling AJ, Pfister L (2020) Temporal and spatial variability of terrestrial diatoms at the catchment scale: controls on communities. PeerJ 8:e8296. https://doi.org/10.7717/peerj.8296
Havlena Z, Kieft TL, Veni G, Horrocks RD, Jones DS (2021) Lighting effects on the development and diversity of photosynthetic biofilm communities in Carlsbad Cavern, New Mexico. Appl Environ Microbiol 87:e02695-e2720. https://doi.org/10.1128/AEM.02695-20
Justus BG, Driver LJ, Burge DR (2021) Seasonal periphyton response to low-level nutrient exposure in a least disturbed mountain stream, the Buffalo River. Arkansas. Ecol Ind 121:107150. https://doi.org/10.1016/j.ecolind.2020.107150
Killing-Heinze M, Pflitsch A, Furian W, Allison S (2017) The importance of air temperature as a key parameter to identify climatic processes inside Carlsbad Cavern, New Mexico, USA. J Cave Karst Stud 79:153–167
Kukla J, Holec M, Trögl J, Holcová D, Hofmanová D, Kuráň P, Popelka J, Pacina J, Kříženecká S, Ust’ak S, Honzík R (2018) Tourist traffic significantly affects microbial communities of sandstone cave sediments in the protected landscape area “Labské Pískovce” (Czech Republic): implications for regulatory measures. Sustainability 10:396. https://doi.org/10.3390/su10020396
Lang M, Faimon J, Ek C (2015) The relationship between carbon dioxide concentration and visitor numbers in the homothermic zone of the Balcarka Cave (Moravian Karst) during a period of limited ventilation. Int J Speleol 44:167–176. https://doi.org/10.5038/1827-806X.44.2.6
Legendre P, Legendre L (2012) Numerical ecology. Elsevier, Amsterdam
Lobo HAS, Trajano E, de Alcântara MM, Bichuette ME, Scaleante JAB, Scaleante OAF, Rocha BN, Laterza FV (2013) Projection of tourist scenarios onto fragility maps: framework for determination of provisional tourist carrying capacity in a Brazilian show cave. Tour Manag 35:234–243. https://doi.org/10.1016/j.tourman.2012.07.008
Mammola S, Di Piazza S, Ziotti M, Badino G, Isaia M (2017) Human-induced alterations of the mycobiota in an alpine show cave (Italy, SW-Alps). Acta Carsol 46:111–123. https://doi.org/10.3986/ac.v46i1.2531
Martin-Sanchez PM, Jurado V, Porca E, Bastian F, Lacanette D, Alabouvette C, Saiz-Jimenez C (2014) Airborne microorganisms in Lascaux cave (France). Int J Speleol 43:295–303. https://doi.org/10.5038/1827-806X.43.3.6
Meyer E, Seale LD, Permar B, MacClary A (2017) The effect of chemical treatments on lampenflora and a Collembola indicator species at a popular tour cave in California, USA. Environ Manag 59:1034–1042. https://doi.org/10.1007/s00267-017-0842-3
Mills ED (2013) Adjusting for covariates in zero-inflated gamma and zero-inflate log-normal models for semicontinuous data. Dissertation, University of Iowa. http://ir.uiowa.edu/etd/2583. Accessed 20 June 2022
Moldovan OT, Bercea S, Năstase-Bucur R, Constantin S, Kenesz M, Mirea IC, Petculescu A, Robu M, Arghir RA (2020) Management of water bodies in show caves–a microbial approach. Tour Manag 78:104037. https://doi.org/10.1016/jtourman2019104037
Mulec J (2012) Lampenflora as an accompaniment of mass cave tourism, problems and solutions for Postojnska jama, Slovenia. In: Saiz-Jimenez C (ed) The conservation of subterranean cultural heritage. CRC Press, Taylor & Francis Group, London, pp 253–256
Mulec J (2019) Chapter 75: Lampenflora. In: White BW, Culver DC, Pipan T (eds) Encyclopedia of Caves, 3rd edn. Academic Press, London, pp 909–921
Mulec J, Kosi G (2009) Lampenflora algae and methods of growth control. J Caves Karst Stud 71:109–115
Mulec J, Kosi G, Vrhovšek D (2008) Characterization of cave aerophytic algal communities and effects of irradiance levels on production of pigments. J Caves Karst Stud 70:3–12
Nikolić N, Zarubica N, Gavrilović B, Predojević D, Trbojević I, SubakovSimić G, Popović S (2020) Lampenflora and the entrance biofilm in two show caves: comparison of microbial community, environmental, and biofilm parameters. J Caves Karst Stud 82:69–81. https://doi.org/10.4311/2018EX0124
Pfendler S, Einhorn O, Karimi B, Bousta F, Cailhol D, Alaoui-Sossé L, Alaoui-Sossé B, Aleya L (2017) UV-C as an efficient means to combat biofilm formation in show caves: evidence from the La Glacière Cave (France) and laboratory experiments. Env Sci Pollut Res 24:24611–24623. https://doi.org/10.1007/s11356-017-0143-7
Piano E, Bona F, Falasco E, La Morgia V, Badino G, Isaia M (2015) Environmental drivers of phototrophic biofilms in an Alpine show cave (SW-Italian Alps). Sci Tot Environ 536:1007–1018. https://doi.org/10.1016/jscitotenv201505089
Piano E, Falasco E, Bona F (2017) Mediterranean rivers: consequences of water scarcity on benthic algal chlorophyll a content. J Limnol 76:39–48. https://doi.org/10.4081/jlimnol20161503
Piano E, Nicolosi G, Isaia M (2021) Modulating lighting regime favours a sustainable use of show caves: a case study in NW-Italy. J Nat Cons 64:126075. https://doi.org/10.1016/jjnc2021126075
Pla C, Galiana-Merino JJ, Cuezva S, Fernandez-Cortes A, Cañaveras JC, Benavente D (2016) Assessment of CO2 dynamics in subsurface atmospheres using the wavelet approach: from cavity–atmosphere exchange to anthropogenic impacts in Rull cave (Vall d′ Ebo, Spain). Environ Earth Sci 75:1–16. https://doi.org/10.1007/s12665-016-5325-y
Porca E, Jurado V, Martin-Sanchez PM, Hermosin B, Bastian F, Alabouvette C, Saiz-Jimenez C (2011) Aerobiology: an ecological indicator for early detection and control of fungal outbreaks in caves. Ecol Ind 11:1594–1598. https://doi.org/10.1016/jecolind201104003
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 15 May 2022
Roldán M, Clavero E, Canals T, Gómez-Bolea A, Ariño X, Hernández-Mariné M (2004) Distribution of phototrophic biofilms in cavities (Garraf, Spain). Nova Hedwigia 78:329–351. https://doi.org/10.1127/0029-5035/2004/0078-0329
Roldán M, Oliva F, Gónzalezdel Valle MA, Saiz-Jimenez C, Hernández-Mariné M (2006) Does green light influence the fluorescence properties and structure of phototrophic Biofilms? Appl Environ Microbiol 72:3026–3031. https://doi.org/10.1128/AEM7243026-30312006
Saiz-Jimenez C (2012) Microbiological and environmental issues in show caves. World J Microbiol Biotechnol 28:2453–2464. https://doi.org/10.1007/s11274-012-1070-x
Saiz-Jimenez C, Miller AZ, Martin-Sanchez PM, Hernandez-Marine M (2012) Uncovering the origin of the black stains in Lascaux Cave in France. Environ Microbiol 14:3220–3231. https://doi.org/10.1111/1462-292012008
Šebela S, Turk J (2014) Natural and anthropogenic influences on the year-round temperature dynamics of air and water in Postojna show cave, Slovenia. Tour Manag 40:233–243. https://doi.org/10.1016/jtourman201306011
Šebela S, Pipan TJ, T, (2015) Cave micro-climate and tourism: towards 200 years (1819–2015) at Postojnska jama (Slovenia). Cave Karst Sci 42:78–85
Trinh DA, Trinh QH, Tran N, Guinea JG, Mattey D (2018) Eco-friendly remediation of lampenflora on speleothems in tropical karst caves. J Caves Karst Stud 80:1–12. https://doi.org/10.4311/2017ES0101
Zuur AF, Ieno EN, Walker NJ, Savaliev AA, Smith GM (2009) Mixed effect models and extensions in ecology with R. Springer, Berlin
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Met Ecol Evol 1:3–14. https://doi.org/10.1111/j2041-210X200900001x
Acknowledgements
We are grateful to Benedetta Baroni, Elena Cumino, Prof. Massimo Delfino, and Simone Marzocchi for their help during the fieldwork. We also thank Marta Zunino, Cooperativa Arcadia, Ufficio Turistico Mondolè, and Associazione Alto Corsaglia for providing us the access to the caves.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. This work was realized within the framework of the PRIN SHOWCAVE “A multidisciplinary research project to study, classify and mitigate the environmental impact in tourist caves”—code 2017HTXT2R, funded by the Italian Ministry of Education, University and Research. The grant of EP is co-financed by the PON “Research and Innovation” Programme (Axis IV “Education and Research for recovery”—Action IV.6 “Research contracts on Green themes”).
Author information
Authors and Affiliations
Contributions
Elena Piano and Marco Isaia set the lines of inquiry. Elena Piano performed the statistical analysis and led the manuscript writing. Elena Piano, Marco Isaia, and Giuseppe Nicolosi performed the fieldwork, organized the data, reviewed and edited the first draft of the paper, and provided important improvements to the original text.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piano, E., Nicolosi, G. & Isaia, M. Scheduling Closure Periods Is Not an Effective Management Strategy to Reduce Lampenflora in Show Caves. Geoheritage 15, 20 (2023). https://doi.org/10.1007/s12371-023-00788-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12371-023-00788-y